
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 27 September 2023
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1260375
This article is part of the Research TopicNew Insights in Leprosy (Hansen's Disease)View all 12 articles
 Louise Pierneef1
Louise Pierneef1 Paritosh Malaviya2
Paritosh Malaviya2 Anouk van Hooij1
Anouk van Hooij1 Shyam Sundar2
Shyam Sundar2 Abhishek Kumar Singh2
Abhishek Kumar Singh2 Rajiv Kumar3
Rajiv Kumar3 Danielle de Jong4
Danielle de Jong4 Maaike Meuldijk1
Maaike Meuldijk1 Awnish Kumar3
Awnish Kumar3 Zijie Zhou1
Zijie Zhou1 Kristien Cloots5
Kristien Cloots5 Paul Corstjens4
Paul Corstjens4 Epco Hasker5
Epco Hasker5 Annemieke Geluk1*
Annemieke Geluk1*Background: It has been amply described that levels of IgM antibodies against Mycobacterium leprae (M. leprae) phenolic glycolipid I (PGL-I) correlate strongly with the bacterial load in an infected individual. These findings have generated the concept of using seropositivity for antibodies against M. leprae PGL-I as an indicator of the proportion of the population that has been infected. Although anti-PGL-I IgM levels provide information on whether an individual has ever been infected, their presence cannot discriminate between recent and past infections. Since infection in (young) children by definition indicates recent transmission, we piloted the feasibility of assessment of anti-PGL-I IgM seroprevalence among children in a leprosy endemic area in India as a proxy for recent M. leprae transmission.
Material and methods: A serosurvey for anti-PGL-I IgM antibodies among children in highly leprosy endemic villages in Bihar, India, was performed, applying the quantitative anti-PGL-I UCP-LFA cassette combined with low-invasive, small-volume fingerstick blood (FSB).
Results: Local staff obtained FSB of 1,857 children (age 3–11 years) living in 12 leprosy endemic villages in Bihar; of these, 215 children (11.58%) were seropositive for anti-PGL-I IgM.
Conclusion: The anti-PGL-I seroprevalence level of 11.58% among children corresponds with the seroprevalence levels described in studies in other leprosy endemic areas over the past decades where no prophylactic interventions have taken place. The anti-PGL-I UCP-LFA was found to be a low-complexity tool that could be practically combined with serosurveys and was well-accepted by both healthcare staff and the population. On route to leprosy elimination, quantitative anti-PGL-I serology in young children holds promise as a strategy to monitor recent M. leprae transmission in an area.
Leprosy, caused by Mycobacterium leprae (M. leprae) or M. lepromatosis, is a debilitating neglected tropical disease (NTD) still predominantly forming a health threat for poor and marginalized populations from over 120 countries (1–3). It is a chronic infectious disease that can cause long-term nerve damage and often results in both physical and social disabilities (4, 5). M. leprae is believed to be transmitted via aerosol droplets from the respiratory system, during repeated and close contact with untreated patients (6). Only approximately 5% of persons infected with M. leprae develop disease symptoms (4). However, it is assumed that infected, asymptomatic individuals carrying sufficient amounts of the mycobacterium contribute to its transmission (7, 8).
Multidrug therapy (MDT) effectively kills M. leprae and provides an effective cure if treatment is initiated timely. Following MDT's introduction in 1981, leprosy prevalence has significantly dropped. Yet, transmission of M. leprae remains, reflected by over 9,000 new child cases detected in 2022 worldwide (9). It is also believed that large numbers go undetected as a result of the drop in leprosy-focused healthcare following the declaration of leprosy elimination on the global level (10).
The WHO's Global Leprosy Strategy 2021–2030 aims to significantly reduce the number of new cases with grade 2 disability and new child cases by focusing on early detection of disease and interruption of transmission. To achieve the latter, it is vital to identify and treat sources of infection, e.g., multibacillary (MB) cases with high bacillary loads that are highly likely to transmit bacteria (11, 12). Detecting M. leprae-infected individuals lacking clinical symptoms who can transmit the bacterium or develop leprosy themselves remains a major challenge for dedicated control programs. Monitoring leprosy elimination is currently conducted by evaluating the proportion of new child cases (below age 15) among the total number of new cases detected (9, 13, 14). As only a small percentage of M. leprae-infected individuals progress to disease and it can take many years before symptoms of leprosy manifest (15), using new cases to monitor elimination does not provide sufficiently accurate and up-to-date information with respect to (elimination of) transmission.
Household contacts of MB leprosy cases have been reported to be most vulnerable to contracting disease (16, 17). Levels of IgM antibodies against phenolic glycolipid I (PGL-I), a specific cell wall component of M. leprae, correlate strongly with the bacterial index (BI) of M. leprae-infected individuals (8, 18, 19). Moreover, based on a literature review covering reports on serology for leprosy from 1987 to 2020 worldwide, we showed that quantitative anti-PGL-I serology in young children as a measure for M. leprae infection provides the potential for assessing recent transmission rates in a community (20, 21). These findings, on top of the observation that MB cases are more likely to transmit bacteria, provide a rationale for identifying seropositive individuals to study transmission. Although the presence of antibodies cannot discriminate between recent and past infection, infection in young children by definition indicates recent transmission. Therefore, the assessment of seropositivity in children is recommended by the WHO “Task Force on definitions, criteria and indicators for interruption of transmission and elimination of leprosy” as an indicator to monitor elimination (9) and offers a tool to study the effects of interventions including post-exposure prophylaxis (PEP).
Over the past years, we have developed a robust, user-friendly test that detects anti-PGL-I IgM antibodies using the unique upconverting reporter particle (UCP) technology in a low-cost lateral flow-based assay (LFA) format [(18, 22–26) and Pierneef et al. (manuscript in preparation)]. The anti-PGL-I UCP-LFA offers a sensitive, low-complexity rapid test to quantitatively determine anti-PGL-I IgM levels in capillary and venous blood (24, 27).
In India, the new leprosy child case detection rate was approximately 10 per 1,000,000 child population in 2020 (28). Of the new cases of all ages registered in India, 15 to 20% (16,000–20,000 individuals each year) are located in Bihar (29), a socio-economically poor state in the eastern part of the country with an estimated population of over 100 million people (30). In this study, we report for the first time the application of the fully integrated anti-PGL-I UCP-LFA cassette in a larger serosurvey using fingerstick blood (FSB). We aimed to assess the use of the anti-PGL-I UCP-LFA as a tool for measuring M. leprae infection among children as a proxy for transmission in Bihar, India.
Study participants were consenting individuals living in a Health and Demographic Surveillance System (HDSS) site in Muzaffarpur district, Bihar, India.
As part of the Tropical Medicine Research Center (TMRC) study on leishmaniasis in the already-existing Muzaffarpur HDSS, a door-to-door screening was performed by the staff of the Banaras Hindu University (BHU; Varanasi, India) (31, 32). The screening started in August 2020 and was completed in January 2021. FSB from children (n = 1,857; 987 male/870 female) between 3 and 11 years of age living in Bihar was collected (Table 1). The inclusion criteria were all children without any signs of leprosy or other diseases residing permanently in one of the following villages in the old HDSS area (32): Singar Phulkahan, Madhopur Chhapra, Godai Phulkahan, Godai Jamal, Vishwanathpur, Raksha North, Raksha North Chauk, Raksha South West, Raksha South, Raksha Deah, Nariyar Nawada, and Arizpur Kothi. Excluded were children below 2 years of age. BCG status was not recorded in this study but has been above 84% and almost uniform across India since the time of the study (2020) (33).
In addition, the following biobank samples collected during the TMRC study in Bihar on leishmaniasis were included.
Serum samples from leishmaniasis patients from the same area were collected, including visceral leishmaniasis (VL; age 12–52; n = 20) and post-kala-azar dermal leishmaniasis (PKDL; age 12–62; n = 20). VL patients were rK39 antibody/splenic aspirate positive and displayed clinical symptoms of active VL. PKDL patients were rK39 antibody or skin slit smear/PCR positive and had a history of VL (34).
Serum samples from asymptomatic individuals (ASY; age 10–70) were collected. ASY were individuals without any clinical symptoms of leishmaniasis, who were seropositive for rK39 antibodies in the Direct Agglutination Test (DAT; cutoff titer for positivity ≥1:1,600) (34).
Serum samples from endemic controls (ECs; age 8–50; n = 20) were collected as part of the NIH-TMRC project. ECs were individuals who tested seronegative for rK39 antibodies/DAT (34).
FSB was collected using disposable 20 μl Minivette® collection tubes (Heparin coated; Sarstedt) and directly mixed with 980 μl high salt finger stick (HSFS) buffer: 100 mM Tris pH 8.0, 270 mM NaCl, 1% (v/v) Triton X-100, and 1% (w/v) BSA. FSB was applied to the anti-PGL-I UCP-LFA cassette on the spot immediately after collection.
For samples that were used as positive and negative controls throughout the study, venous blood samples were collected, in 4 ml plain BD vacutainer serum tubes (BD, Franklin Lakes, NJ, USA). Tubes were centrifuged at an RCF of 500 xg for 10 min and sera were subsequently aliquoted and frozen (−80°C) until use.
Fully integrated and individually packaged UCP-LFA cassettes for the detection of human anti-PGL-I IgM antibodies were produced by MaximBio (Rockville, MD, USA; schematic overview shown in Figure 1). The air-tight pouches with test cassettes contained silica dry packs allowing extended shelf life and protection against humidity. The Test (T) line on the LF strip comprised 100 ng of synthetic PGL-I, phenolic trisaccharide functionalized with a hexanoic acid linker for conjugation to BSA [NPT1-H-BSA; Leiden, the Netherlands (35)]. The Flow-Control (FC) line comprised 100 ng rabbit anti-goat IgG [G4018; Sigma-Aldrich, Inc., St. Louis, MO, USA). Goat IgG specific for anti-human IgM (I0759; Sigma-Aldrich, Inc., St. Louis, MO, USA] was conjugated to polyacrylic acid functionalized UCPs [200 nm, NaYF4:Yb3+, Er 3+; Intelligent Material Solutions Inc. (IMS); Princeton, NJ, USA MS] according to previously described protocols at a concentration of 50 μg antibody per mg UCP (23). Stock solutions were kept at 4°C until use. To dry the UCPs onto the glass fiber conjugate-release pad, the material was diluted in a buffer containing 100 mM Tris pH 8.0, 270 mM NaCl, 10% (w/v) sucrose, 1% (w/v) BSA, 0.5% Tween-20, and striped at a density of 100 ng/mm. Components were mounted on plastic backing cards which were cut into LF strips of 4.8 mm width by 6 cm length, added to an appropriate cassette, and individually sealed in a pouch together with a silica dry pack.
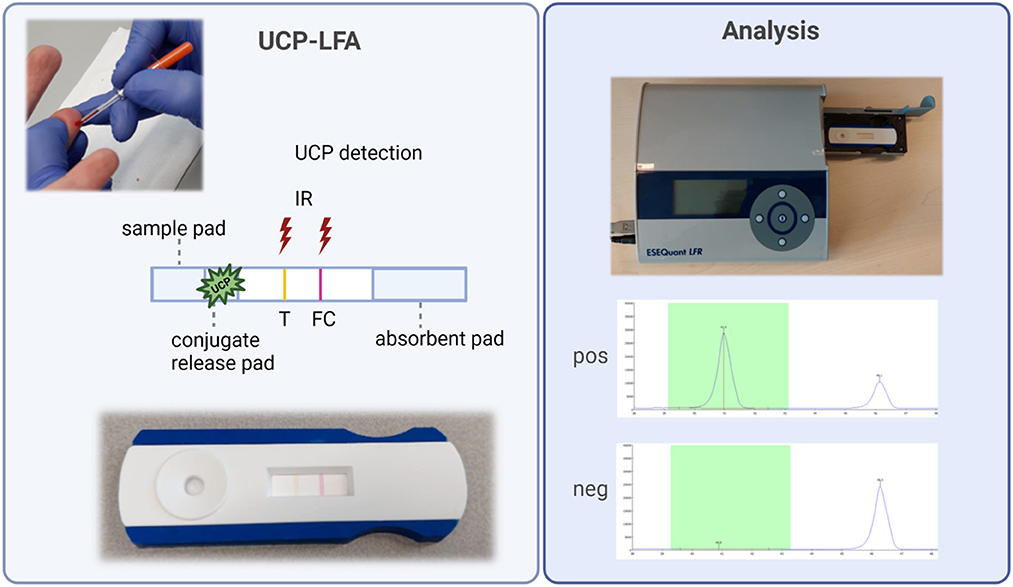
Figure 1. Fully integrated anti-PGL-I UCP-LFA cassette and analysis. Left: FSB is collected with a minivette (20 μl). To initiate LF, a diluted FSB sample is added to the cassette onto the sample pad. Hydration of the anti-IgM UCP-conjugate will allow the binding of anti-PGL-I IgM antibodies from the sample to the conjugate and sequential binding to the T line with synthetic PGL-I. The FC line can bind UCP conjugate not bound to the T line. Color-coded T and FC lines visible by the eye disappear upon flow. Right: UCP signals are detected with a reader upon excitation with 980 IR light generating 540 nm emission. Results for positive and negative samples are shown. Results are calculated as R-value, with the T signal divided by the FC signal. This figure was created with BioRender.com. FC, flow control line; FSB, fingerstick blood; IgM, immunoglobulin M; IR, infrared excitation; PGL-I, phenolic glycolipid I; R, ratio value, result of the UCP-LFA; T, test line; UCP-LFA, upconverting reporter particle lateral flow assay.
To initiate LF, 50 μl of the 50-fold diluted FSB or serum sample was added to the test cassette. Cassettes were analyzed using a UCP-adapted portable reader (LFR; Qiagen, Hilden, Germany; Figure 1). The results were calculated as the ratio value (R) between T and FC signals based on relative fluorescence units (RFUs) measured at the respective lines. The cutoff for positivity (R ≥ 0.12) for the UCP-LFA batch used in this study was based on the median of a sextuple test of a standard control serum sample (+) performed in India plus the standard deviation (SD). Measurements in India yielded an identical cutoff level as in Leiden using aliquots of the same control serum sample (+). In addition, one highly anti-PGL-I IgM-positive serum sample (++) and one negative serum sample (–) were tested as control samples in sextuple to monitor the reproducibility of the test.
This study was performed in accordance with the Helsinki Declaration (7th revision, 64th Meeting, 2013, Fortaleza). This study was a component of Muzaffarpur NIH-TMRC HDSS for which ethics approval was received from the institutional review boards of the Institute of Medical Sciences, Banaras Hindu University (BHU; reference number Dean/2017/EC/185; Dated 24/10/2017), Varanasi, India, and the review committee of the U.S. National Institutes of Health (NIH). The institutional review board of BHU has received accreditation from the National Institutes of Health in the United States. Ethics approval was also obtained by the institutional review board of the Institute of Tropical Medicine, Antwerp (reference number 1305/19), and the Ethics Committee of the University Hospital of Antwerp (reference number 19/28/342), Belgium. All data were anonymized. Participants were informed about the study objectives, sampling protocol, and their right to refuse to take part or withdraw from the study without consequences for their treatment at any point in time. The refusal rate was lower than 5%. Written informed consent was obtained from a parent, guardian, or village head before enrollment. Consent forms were kept in a secure file cabinet at Kala-azar Medical Research Center (KAMRC), Muzaffarpur, Bihar, India.
GraphPad Prism version 9.0.1 for Windows (GraphPad Software, San Diego, CA, USA) and RStudio version 4.2.1 (Boston, MA, USA) were used to perform statistical analysis. The distribution of anti-PGL-I data was checked for normality by plotting a histogram. Data were then log-transformed based on the natural logarithm of the anti-PGL-I R-value plus 1. Mann–Whitney U-tests and Kruskal–Wallis tests were performed to determine the statistical significance between two and three independent groups, respectively. A logistic regression analysis was performed to assess the association between age and anti-PGL-I positivity.
This was the first time applying the anti-PGL-I UCP-LFA cassette format at a larger scale in a field setting. A quality control protocol (as described in the Methods) was performed to monitor reproducibility (data not shown). The anti-PGL-I UCP-LFA was considered feasible and accepted by both healthcare staff and the population.
To assess seroprevalence for anti-PGL-I IgM in a leprosy endemic area, FSB samples of 1,857 children living in the state of Bihar, India, were obtained during a field serosurvey and screened using the anti-PGL-I UCP-LFA cassette. Among these children, 215 (11.58%) tested positive for anti-PGL-I IgM (Figure 2A; Supplementary Table 1). Anti-PGL-I R values ranged from 0 to 0.84 with a median of 0.04 (IQR 0.02–0.07). The distribution appeared unimodal with a mode of 0.01 and strongly skewed to the right. Upon log transformation (based on the natural logarithm of the anti-PGL-I R-value plus 1), the distribution was still very skewed with a small dip around 0.11, followed by a much lower second mode. The log-transformed value of 0.11 corresponds with 0.12 on the original scale, and this confirms the cutoff value of R ≥ 0.12 used, as shown by the second peak in the histogram (Figure 2B). From the seropositive children with R-values >0.2 (median of seropositive children plus SD of seronegative children; n = 61), 51 could be followed up in October 2021 and examined for signs and symptoms of leprosy. One child (male, 9 years old) was diagnosed at follow-up at the primary healthcare center as a new paucibacillary (PB) leprosy case 1 year after the serosurvey (test performed in October 2020; R-value 0.21), showing a lesion on his left upper arm.
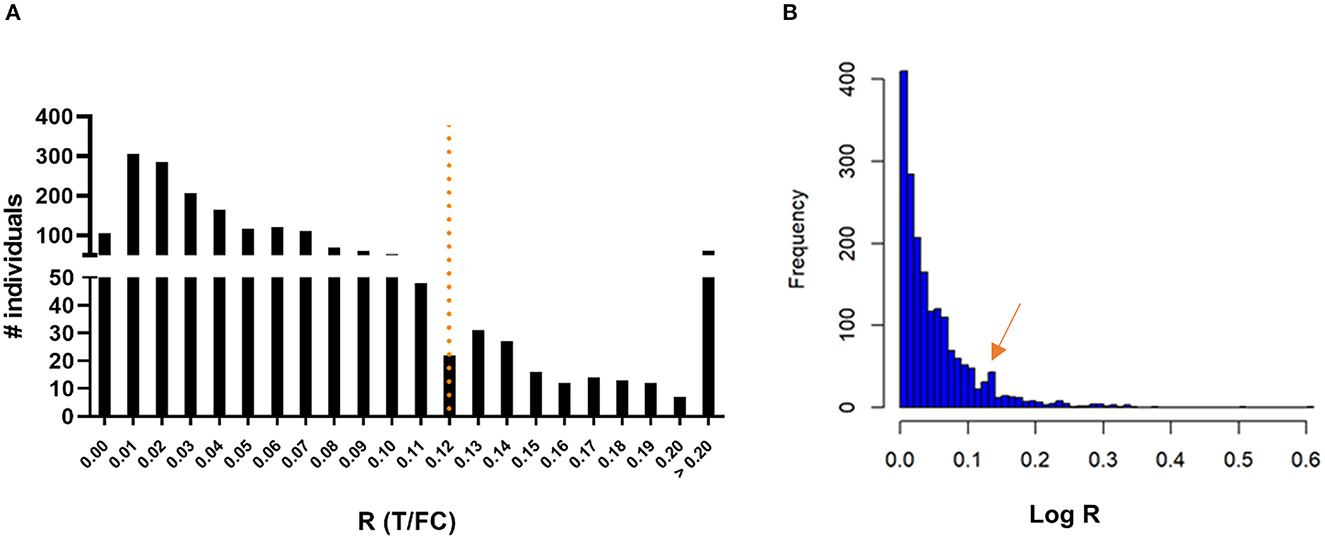
Figure 2. Presence of anti-PGL-I IgM antibodies in FSB of 1,857 children 3–11 years old in Bihar, India. UCP-LFA cassettes were used to obtain a quantitative R-value (T/FC) indicating the presence of anti-PGL-I IgM antibodies using a UCP reader. (A) Histogram with a mode of 0.01 for R: the highest R-value detected was 0.84 (n = 1). The cutoff for positivity (R ≥ 0.12) for the UCP-LFA batch used in this study is indicated by the dotted line and was based on the median of a sextuple test performed in India of a standard control serum sample (+) plus its standard deviation (SD). (B) Histogram of log-transformed R-values. FC, flow control line; IgM, immunoglobulin M; PGL-I, phenolic glycolipid I; R, ratio value, result of the UCP-LFA; T, test line.
For all participants, information on age, gender, and place of origin was recorded at the time of sampling FSB. The ages of the children in this cohort ranged from 3 to 11, with the majority of the children being 5 (n = 410), 6 (n = 353), 7 (n = 392), 8 (n = 430), or 9 (n = 268) years of age. For children aged 5–9 years, the percentage of seropositive children showed an increasing trend with age: from 7.32% at age 5 to 14.55% in age group 9 (Figures 3A, B; Supplementary Table 2). The odds of testing positive for anti-PGL-I IgM in this age range (5–9) increased by 18% per year (logistic regression; p = 0.002).
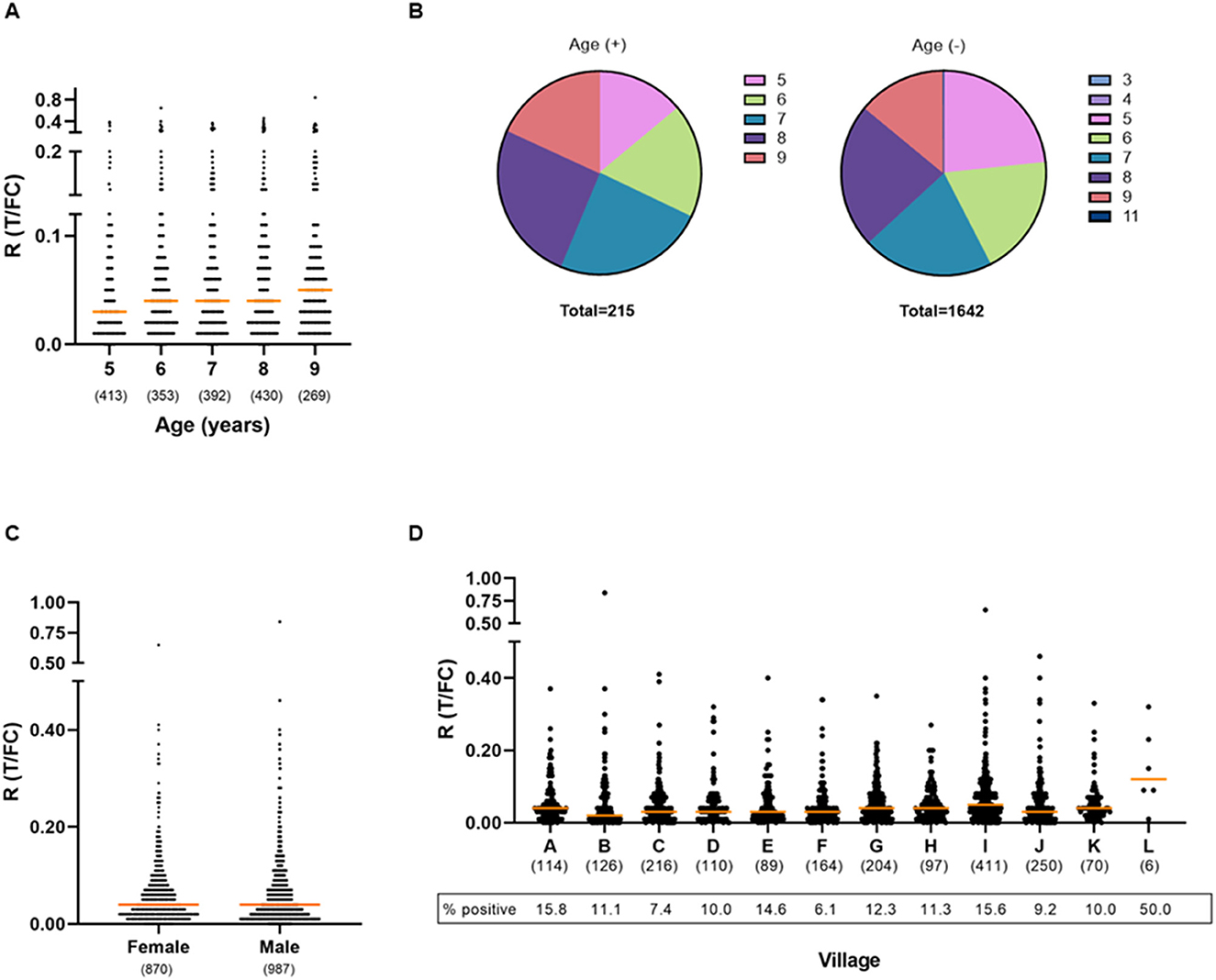
Figure 3. Presence of anti-PGL-I IgM antibodies in FSB of children in relation to age, gender, and place of residence. UCP-LFA cassettes were used to obtain a quantitative ratio (R) value (T/FC) indicating the presence of anti-PGL-I IgM using a UCP reader. Median values for each group are indicated by horizontal bars. The cutoff for positivity (R ≥ 0.12) for the UCP-LFA batch used in this study was based on the median of a sextuple test performed in India of a standard control serum sample (+) plus its standard deviation (SD). The numbers of individuals per group are given in parentheses. (A) Anti-PGL-I IgM R values per age group. Two children aged 3 years and one child aged 4 years were included in the 5-year-old age group and one child aged 11 years was included in the 9-year-old age group. (B) Venn diagrams displaying the proportion of children per age category for anti-PGL-I IgM-positive (+) and -negative (–) children. Colors indicate the age groups. (C) Anti-PGL-I IgM R values per gender. (D) Anti-PGL-I IgM R values per village. A: Singar Phulkahan, B: Madhopur Chhapra, C: Godai Phulkahan, D: Godai Jamal, E: Vishwanathpur, F: Raksha North, G: Raksha North Chauk, H: Raksha South West, I: Raksha South, J: Raksha Deah, K: Nariyar Nawada, L: Arizpur Kothi. FC, flow control line; IgM, immunoglobulin M; PGL-I, phenolic glycolipid I; R, ratio value, result of the UCP-LFA; T, test line.
Ages were represented equally among the 987 male and 870 female participants (Table 2). No correlation was found between gender and the presence of anti-PGL-I IgM antibodies (Mann–Whitney U-test; p = 0.2314; Figure 3C). For female and male participants, almost identical seropositivity percentages of 11.61 and 11.55% were found, respectively.
Children from 12 different villages were included (Supplementary Figure 1). The majority of the children were resident in Raksha South (n = 411). In general, seropositivity rates among the villages were similar (Figure 3D; Supplementary Table 3). However, a slight increase in levels of antibodies (R-values) among Raksha South with villages Madhopur Chhapra, Godai Phulkahan, Godai Jamal, Raksha North, and Raksha Deah was observed (Kruskal–Wallis; p = 0.0003 to p = 0.0154).
In addition to being endemic for leprosy, the area of Bihar is also endemic for leishmaniasis (32, 36). As leishmaniasis is a differential diagnosis of leprosy (37), although not the main purpose of this study, biobanked serum samples from individuals from exactly the same area with VL (n = 20), PKDL (n = 20), asymptomatic Leishmania donovani (L. donovani) infection (n = 20), and endemic controls (n = 20) were tested with the anti-PGL-I UCP-LFA cassette as well. Two of the 60 individuals with confirmed L. donovani infection tested positive (3.33%) for anti-PGL-I IgM (Table 3): one (R = 0.12) PKDL case and one person with an asymptomatic L. donovani infection (R = 0.26). None of the endemic controls (age 8–50) from the same area tested positive for anti-PGL-I IgM (Table 4). Moreover, plasma samples of young children (age 1–6; n = 37) from a non-endemic area (the Netherlands) all tested negative using the anti-PGL-I UCP-LFA cassette (Pierneef et al., unpublished data).
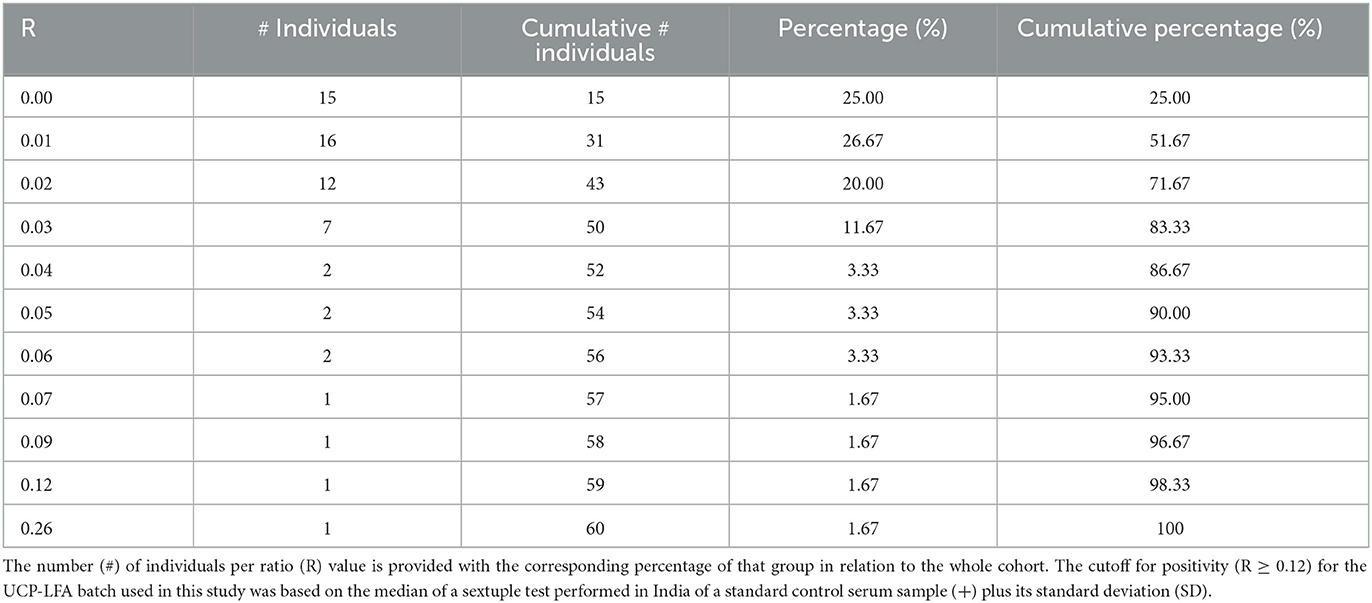
Table 3. Ratio values for anti-PGL-I IgM measured in individuals infected with L. donovani in Bihar, India.
Measuring seroprevalence among young children represents a potential tool to monitor the intensity of recent transmission, particularly when antibody levels are assessed quantitatively. Changes in seroprevalence measured in repeated cross-sectional surveys among young children of a certain age group in an area can indicate the rate of transmission as well as the effect of control measures or interventions in an area. On route to leprosy elimination, an indicator for the intensity of transmission would be highly valuable, as the proportion of child cases, which is currently applied by the WHO, does not provide information swiftly as it can take years for an infected individual to develop leprosy. Moreover, it is not an accurate representation of infection as only a minority of the infected individuals develop the disease (9).
In this study, using the anti-PGL-I UCP-LFA, we found seropositivity of 11.58% among children in Bihar, a leprosy endemic state in India, with a prevalence rate of 17.1 per 10,000 population in 2019 (31), which is above the elimination threshold of 1 per 10,000 population defined by the WHO (38). In agreement with previous research, this percentage falls in line with the seroprevalence (median 14.9%) found in studies from endemic areas—mostly Brazil, India, and Indonesia (20). Seropositivity slightly increased with age (between 5 and 9 years of age). Previous reports describe a similar increase with age, followed by a decrease in antibodies after the age of 20 years (20, 39). Children of school-going age would thus be a suitable target group for sensitive assessment of recent M. leprae infection.
The slightly higher R-values (individual anti-PGL-I IgM levels) found in Raksha South compared to five other villages could not be explained by differences in the age and/or gender of the children as compared to the other villages. However, group sizes were not equal and Raksha South had a notably higher number of participants (n = 411), possibly affecting the analysis.
Previous studies reported that seroprevalence was stable over time if leprosy incidence in an area remained unchanged (20). A significant limitation hindering direct comparison of previous seroprevalence results from endemic areas (mostly from India, Brazil, and Indonesia) was the use of different assays measuring anti-M. leprae-specific antibodies. Measurements were performed using either FSB or serum with variable dilutions, and target antigens varied from native to synthetic PGL-I recognized by various IgM, IgG, or IgA isotypes. In addition, cutoff values were often chosen arbitrarily. However, analysis of data available from China showed that if the same method was used, a decrease in disease prevalence or new case detection rate corresponded to a decrease in anti-M. leprae antibody seroprevalence in children (21), adding the potential of measuring antibodies in children as a tool for recent transmission.
Timely diagnosis and treatment can prevent disabilities from developing and the mycobacterium from spreading (40). Active case-finding approaches by healthcare workers are a proven method to identify cases at early stages (41). In addition to the use of serology to monitor transmission, large-scale screenings have the potential to early identify new cases. Although the presence of anti-PGL-I IgM does not predict disease, seropositive individuals are at an increased risk of developing leprosy (42). In this study, 51 children were followed up and screened for clinical symptoms. One boy aged nine (anti-PGL-I IgM R-value 0.21) was diagnosed at follow-up with PB leprosy indicating the additional benefit of community seroscreening in children even for PB leprosy. As we took a cost-efficient approach, it was outside the scope of this project to follow-up all seropositive children for clinical examination which limits the additional impact of the study. In future studies, follow-up of all seropositive children as well as a subgroup of randomly selected seronegative children should be included besides additional screening of contacts of seropositive children to identify the source of transmission.
Single-dose rifampicin (SDR) PEP is a preventive treatment for leprosy which can decrease the risk of developing disease among (household) contacts of leprosy cases (43). Large-scale, international research proves that SDR-PEP is safe and the WHO recommends its use in the combat against leprosy (44). As many activities including PEP administration are ongoing worldwide and over 175,000 individuals have already received this regimen (44), it is of interest to study the effect over time on M. leprae transmission (measured by seroprevalence rates in children) of such prophylactic interventions and to compare the effect of PEP on transmission between countries. Crucial would be the use of one standardized, quantitative assay, which is preferentially field-friendly and easy to use at a large scale. Therefore, the low-complexity UCP-LFA cassette—which is more robust and easier to perform than the ELISA—quantitatively detecting anti-PGL-I IgM in FSB that was field-tested in this study offers a particularly suitable format for this purpose.
Since PKDL is a differential diagnosis of leprosy (37), although not the main aim of this study, we assessed the potential of the anti-PGL-I UCP-LFA to discriminate between the two infections. We found two out of 60 (3.33%) individuals infected with L. donovani to test positive for anti-PGL-I IgM. However, while those individuals are living in an area where both diseases are endemic, previous exposure to M. leprae cannot be excluded. Furthermore, in an area not endemic for leprosy, young children all tested negative in the anti-PGL-I UCP-LFA cassette, arguing for the specificity of the test. The HDSS team is experienced in conducting studies for leishmaniasis in the field, and this provides opportunities for combining leishmaniasis with leprosy research (32). Performing serosurveillance to monitor the transmission of multiple NTDs simultaneously by pooling expertise into one joint operation could save cost as well as time. To this end, the design of a (combined) rapid test for the detection of antibodies against L. donovani (and M. leprae) could also be valuable and is considered for future research (45).
Screening for quantitative assessment of anti-PGL-I IgM levels in children identified 11.58% seropositivity in 12 villages in Bihar, India. These data are in line with seroprevalence data reported (20, 21) for other endemic areas without PEP in the past decades. There was a slight, significant increase with age, but no difference in seropositivity between genders was observed. Anti-PGL-I IgM antibodies in young children are a useful indicator for M. leprae infection, thereby serving as a proxy for recent transmission in an area and thus can be used as a tool for monitoring the reduction of transmission when new cases are scarce. A follow-up study 5 to 10 years later, again assessing the anti-PGL-I IgM antibodies in these villages in Bihar in the same age groups, would provide insight into the changes in transmission in this area. In addition, similar studies should be conducted in areas where leprosy is less endemic or not endemic anymore to further validate this tool for monitoring the elimination of transmission.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Institutional Review Boards of the Institute of Medical Sciences, Banaras Hindu University (BHU; reference number Dean/2017/EC/185; Dated 24/10/2017), Varanasi, India, and the review committee of the U.S. National Institutes of Health (NIH). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Conceptualization: AG. Data curation: LP, AH, MM, and ZZ. Formal analysis: LP, AH, EH, and AG. Funding acquisition: PM, SS, EH, and AG. Investigation: LP, PM, AH, SS, AS, RK, DJ, MM, AK, and ZZ. Methodology: EH and AG. Supervision: PC and AG. Visualization: LP, PM, AH, PC, EH, and AG. Writing—original draft: LP and AG. Writing—review and editing: LP, PM, AH, KC, PC, EH, and AG.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Muzaffarpur-TMRC HDSS was formally set up in 2007 in the framework of the project Visceral Leishmaniasis in Bihar, funded by the National Institute of Health (NIH), USA, under its Tropical Medicine Research Centers (TMRC) grants. The National Institute of Health—National Institute of Allergic and Infectious Diseases provided TMRC grant no. 1P50AI074321 to establish this HDSS. Intelligent Material Solutions Inc. (IMS; Princeton, NJ, USA) contributed anti-PGL-I UCP-LFA cassettes for this study. This study was made possible thanks to a grant from NIH, a Seeding grant from the Leprosy Research Initiative (LRI), and the Q. M. Gastmann-Wichers Foundation (AG).
The authors thank the members of the WHO (Global Leprosy Programme) Task Force on Criteria for Elimination of Leprosy (TFCEL) for critical discussions and feedback. Plasma samples of young children from a non-endemic area (the Netherlands) were provided by Dr. S. Jochems (LUMC).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1260375/full#supplementary-material
Supplementary Figure 1. Map of the research area indicating the included villages in Bihar, India, and the seropositivity percentages measured in children. The locations of 10 villages (A–J) in Bihar, India, are provided with the corresponding percentage of children testing positive for anti-PGL-I IgM. Note that two villages (K, L) were not shown on this map, as they were located too far away. UCP-LFA cassettes were used to obtain a quantitative ratio (R) value (T/FC) indicating the presence of anti-PGL-I IgM using a UCP reader. The cutoff for positivity (R ≥ 0.12) for the UCP-LFA batch used in this study was based on the median of a sextuple test performed in India of a standard control serum sample (+) plus its standard deviation (SD). A, Singar Phulkahan; B, Madhopur Chhapra; C, Godai Phulkahan; D, Godai Jamal; E, Vishwanathpur; F, Raksha North; G, Raksha North Chauk; H, Raksha South West; I, Raksha South; J, Raksha Deah; K, Nariyar Nawada; L, Arizpur Kothi. Anti-PGL-I, anti-phenolic glycolipid I; FC, flow control line; IgM, immunoglobulin M; R, ratio value, result of the UCP-LFA; T, test line.
Supplementary Table 1. Ratio values for anti-PGL-I IgM measured in children aged 3–11 in Bihar, India.
Supplementary Table 2. Anti-PGL-I IgM positivity in children aged 5–9 in Bihar.
Supplementary Table 3. Place of residence of the children aged 3–11 in Bihar and corresponding percentage of anti-PGL-I IgM positive children.
1. Blok DJ, de Vlas SJ, Richardus JH. Global elimination of leprosy by 2020: are we on track? Trop Med Int Health. (2015) 20:40. doi: 10.1186/s13071-015-1143-4
2. World Health Organization. Leprosy. Available online at: https://www.who.int/news-room/fact-sheets/detail/leprosy (accessed August 18, 2023).
3. Deps P, Collin SM. Mycobacterium lepromatosis as a second agent of Hansen's disease. Front Microbiol. (2021) 12:698588. doi: 10.3389/fmicb.2021.698588
4. Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev. (2006) 19:338. doi: 10.1128/CMR.19.2.338-381.2006
5. Geluk A. Correlates of immune exacerbations in leprosy. Semin Immunol. (2018) 39:111–8. doi: 10.1016/j.smim.2018.06.003
6. Fine PEM, Sterne JAC, Ponnighaus JM, Bliss L, Saul J, Chihana A, et al. Household and dwelling contact as risk factors for leprosy in northern Malawi. Am J Epidemiol. (1997) 146:91–102. doi: 10.1093/oxfordjournals.aje.a009195
7. Araujo S, Freitas LO, Goulart LR, Goulart IM. Molecular evidence for the aerial route of infection of Mycobacterium leprae and the role of asymptomatic carriers in the persistence of leprosy. Clin Infect Dis. (2016) 63:1412–20. doi: 10.1093/cid/ciw570
8. Tio-Coma M, Avanzi C, Verhard EM, Pierneef L, van Hooij A, Benjak A, et al. Genomic characterization of mycobacterium leprae to explore transmission patterns identifies new subtype in Bangladesh. Front Microbiol. (2020) 11:1220. doi: 10.3389/fmicb.2020.01220
9. World Health Organization. Global leprosy (Hansen disease) update, 2021: moving towards interruption of transmission. (2022).
10. Smith WC, van BW, Gillis T, Saunderson P, Richardus JH. The missing millions: a threat to the elimination of leprosy. PLoS Negl Trop Dis. (2015) 9:e0003658. doi: 10.1371/journal.pntd.0003658
11. Sales AM, Ponce de Leon A, Duppre NC, Hacker MA, Nery JA, Sarno EN, et al. Leprosy among patient contacts: a multilevel study of risk factors. PLoS Negl Trop Dis. (2011) 5:e1013. doi: 10.1371/journal.pntd.0001013
12. Smith WCS, Aerts A. Role of contact tracing and prevention strategies in the interruption of leprosy transmission. Leprosy Rev. (2014) 85:2–17. doi: 10.47276/lr.85.1.2
13. World Health Organization. Leprosy Elimination Monitoring (LEM): Guidelines for monitors. (2000).
14. Ogunsumi DOO, Lal V, Puchner KP, van Brakel W, Schwienhorst-Stich EM, Kasang C, et al. Measuring endemicity and burden of leprosy across countries and regions: A systematic review and Delphi survey. Plos Neglect Trop D. (2021) 15:e0009769. doi: 10.1371/journal.pntd.0009769
15. Taggart M, Kelly A, Steil R, Chu E. Multibacillary leprosy with an incubation period exceeding 50 years. Bmj Case Rep. (2022) 15:e250835. doi: 10.1136/bcr-2022-250835
16. Moet FJ, Meima A, Oskam L, Richardus JH. Risk factors for the development of clinical leprosy among contacts, and their relevance for targeted interventions. Leprosy Rev. (2004) 75:310–26. doi: 10.47276/lr.75.4.310
17. Quilter EEV, Butlin CR, Singh S, Alam K, Lockwood DNJ. Patients with skin smear positive leprosy in Bangladesh are the main risk factor for leprosy development: 21-year follow-up in the household contact study (COCOA). Plos Neglect Trop D. (2020) 14:e0008687. doi: 10.1371/journal.pntd.0008687
18. van Hooij A, Fat EMTK, van den Eeden SJF, Wilson L, da Silva MB, Salgado CG, et al. Field-friendly serological tests for determination of M. leprae-specific antibodies. Sci Rep-Uk. (2017) 7:8868. doi: 10.1038/s41598-017-07803-7
19. Zenha EMR, Ferreira MAN, Foss NT. Use of anti-PGL-1 antibodies to monitor therapy regimes in leprosy patients. Braz J Med Biol Res. (2009) 42:968–72. doi: 10.1590/S0100-879X2009001000016
20. Pierneef L, van Hooij A, Taal A, Rumbaut R, Nobre ML, van Brakel W, et al. Detection of anti-M. leprae antibodies in children in leprosy-endemic areas: A systematic review. Plos Neglect Trop D. (2021) 15:e0009667. doi: 10.1371/journal.pntd.0009667
21. Zhou Z, Pierneef L, van Hooij A, Geluk A. Detection of anti-M. leprae antibodies in healthy children in China: A systematic review of Chinese literature. Front Trop Dis. (2022) 3:963674. doi: 10.3389/fitd.2022.963674
22. Corstjens PLAM, van Hooij A, Fat EMTK, Alam K, Vrolijk LB, Dlamini S, et al. Fingerstick test quantifying humoral and cellular biomarkers indicative for M-leprae infection. Clin Biochem. (2019) 66:76–82. doi: 10.1016/j.clinbiochem.2019.01.007
23. van Hooij A, Fat EMTK, da Silva MB, Bouth RC, Messias ACC, Gobbo AR, et al. Evaluation of Immunodiagnostic Tests for Leprosy in Brazil, China and Ethiopia. Sci Rep. (2018) 8:17920. doi: 10.1038/s41598-018-36323-1
24. van Hooij A, Fat EMTK, de Jong D, Khatun M, Soren S, Chowdhury A, et al. Prototype multi-biomarker test for point-of-care leprosy diagnostics. Iscience. (2021) 24:102006. doi: 10.1016/j.isci.2020.102006
25. van Hooij A, van den Eeden S, Richardus R, Fat ETK, Wilson L, Franken KLMC, et al. Application of new host biomarker profiles in quantitative point-of-care tests facilitates leprosy diagnosis in the field. Ebiomedicine. (2019) 47:301–8. doi: 10.1016/j.ebiom.2019.08.009
26. Zhou ZJ, Pena M, van Hooij A, Pierneef L, de Jong D, Stevenson R, et al. Detection and monitoring of Mycobacterium leprae infection in nine banded armadillos (Dasypus novemcinctus) using a quantitative rapid test. Front Microbiol. (2021) 12:763289. doi: 10.3389/fmicb.2021.763289
27. Corstjens PLAM, Zuiderwijk M, Tanke HJ, Schip JJVV, Ottenhoff THM, Geluk A, et al. User-friendly, highly sensitive assay to detect the IFN-gamma secretion by T cells. Clin Biochem. (2008) 41:440–4. doi: 10.1016/j.clinbiochem.2007.12.015
28. World Health Organization. The Global Health Observatory. Leprosy - New leprosy child case detection rate (less than 15 years of age) per 1,000,000 child population (2022).
29. Wagh AN, Mugudalabetta S, Gutierrez NO, Padebettu K, Pandey AK, Pandey BK, et al. Does appreciative inquiry decrease false positive diagnosis during leprosy case detection campaigns in Bihar, India? An operational research study. Plos Neglect Trop D. (2018) 12:7004. doi: 10.1371/journal.pntd.0007004
30. Technical Group on Population Projections. Census of India 2011: Population Projections for India and States 2011–2036. New Delhi: Ministry of Health & Family Welfare (2019).
31. Hasker E, Malaviya P, Scholar VK, de Koning P, Singh OP, Kansal S, et al. Post kala azar dermal leishmaniasis and leprosy prevalence and distribution in the Muzaffarpur health and demographic surveillance site. Plos Neglect Trop D. (2019) 13:e0007798. doi: 10.1371/journal.pntd.0007798
32. Malaviya P, Picado A, Hasker E, Ostyn B, Kansal S, Singh RP, et al. Health & demographic surveillance system profile: the Muzaffarpur-TMRC health and demographic surveillance system. Int J Epidemiol. (2014) 43:1450–7. doi: 10.1093/ije/dyu178
34. Singh OP, Tiwary P, Kushwaha AK, Singh SK, Singh DK, Lawyer P, et al. Xenodiagnosis to evaluate the infectiousness of humans to sandflies in an area endemic for visceral leishmaniasis in Bihar, India: a transmission-dynamics study. Lancet Microbe. (2021) 2:E23–31. doi: 10.1016/S2666-5247(20)30166-X
35. van Dijk JHM, van Hooij A, Groot LM, Geboers J, Moretti R, Verhard-Seymonsbergen E, et al. Synthetic phenolic glycolipids for application in diagnostic tests for leprosy. Chembiochem. (2021) 22:1487–93. doi: 10.1002/cbic.202000810
36. Kumar V, Mandal R, Das S, Kesari S, Dinesh DS, Pandey K, et al. Kala-azar elimination in a highly-endemic district of Bihar, India: A success story. Plos Neglect Trop D. (2020) 14:e0008254. doi: 10.1371/journal.pntd.0008254
37. Trindade MAB, Silva LLD, Braz LMA, Amato VS, Naafs B, Sotto MN. Post-kala-azar dermal leishmaniasis and leprosy: case report and literature review. BMC Infect Dis. (2015) 15:543. doi: 10.1186/s12879-015-1260-x
38. World Health Organization. Leprosy Resolution WHA 44.9: Forty-fourth World Health Assembly (1991).
39. Ulrich M, Smith PG, Sampson C, Zuniga M, Centeno M, Garcia V, et al. IgM antibodies to native phenolic glycolipid-I in contacts of leprosy patients in Venezuela: epidemiological observations and a prospective study of the risk of leprosy. Int J Lepr Other Mycobact Dis. (1991) 59:405–15.
40. Chen KH, Lin CY, Su SB, Chen KT. Leprosy: a review of epidemiology, clinical diagnosis, and management. J Trop Med-Us. (2022) 2022:8652062. doi: 10.1155/2022/8652062
41. Saunderson P. Improving early case detection in leprosy: Reports from recent workshops. Leprosy Rev. (2022) 93:292–7. doi: 10.47276/lr.93.4.292
42. Richardus RA, van der Zwet K, van Hooij A, Wilson L, Oskam L, Faber R, et al. Longitudinal assessment of anti-PGL-I serology in contacts of leprosy patients in Bangladesh. Plos Neglect Trop D. (2017) 11:e0006083. doi: 10.1371/journal.pntd.0006083
43. Mieras LF, Taal AT, van Brakel WH, Cambau E, Saunderson PR, Smith WCS, et al. An enhanced regimen as post-exposure chemoprophylaxis for leprosy: PEP plus. BMC Infect Dis. (2018) 18:506. doi: 10.1186/s12879-018-3402-4
44. Global Partnership for Zero Leprosy I ITC. Factsheet on SDR-PEP: The latest research on single-dose rifampicin post-exposure prophylaxis (2022).
Keywords: children, leprosy, anti-M. leprae PGL-I antibodies, infection, diagnostics, serosurvey, transmission, upconversion
Citation: Pierneef L, Malaviya P, van Hooij A, Sundar S, Singh AK, Kumar R, de Jong D, Meuldijk M, Kumar A, Zhou Z, Cloots K, Corstjens P, Hasker E and Geluk A (2023) Field-friendly anti-PGL-I serosurvey in children to monitor Mycobacterium leprae transmission in Bihar, India. Front. Med. 10:1260375. doi: 10.3389/fmed.2023.1260375
Received: 17 July 2023; Accepted: 24 August 2023;
Published: 27 September 2023.
Edited by:
Sebastian Vernal, University of São Paulo, BrazilReviewed by:
Mariane Martins de Araújo Stefani, Universidade Federal de Goiás, BrazilCopyright © 2023 Pierneef, Malaviya, van Hooij, Sundar, Singh, Kumar, de Jong, Meuldijk, Kumar, Zhou, Cloots, Corstjens, Hasker and Geluk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Annemieke Geluk, QS5HZWx1a0BsdW1jLm5s
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.