
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 21 November 2023
Sec. Family Medicine and Primary Care
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1258536
Backgrounds: Non-vitamin K antagonist oral anticoagulants (NOACs) have been recommended as the first choice over warfarin for non-valvular atrial fibrillation (AF). However, there is limited data about their usage in mainland China.
Methods: Prescriptions of patients diagnosed with AF and containing OACs were extracted from Hospital Prescription Cooperation Project from January 2016 to March 2021. The primary outcome was the changing percentage of different OACs. The secondary outcomes were frequencies as well as factors with the choice of different OACs and dosage of NOACs. Univariate and Multivariate logistic regressions were conducted to explore possible factors. All statistical analyses were performed using SAS software (Version 9.4).
Results: Among the 220,083 distinct prescriptions diagnosed with AF and prescribed with OACs, the percentage of NOACs increased over years, exceeding warfarin in 2018. Until March 2021, 83.53% of included patients were prescribed with NOACs. Rivaroxaban (62.25%) and dabigatran (37.65%) were the most commonly prescribed NOACs. Low dosage was common for NOACs (44.54%), this was mainly driven by rivaroxaban, 67.98% of which were low dosage. Multivariate logistic regression indicated that several factors were positively associated with the preference of low dosage, including outpatients (OR 1.32, 95% CI 1.26–1.39), patients with hypertension (OR 1.49, 95% CI 1.40–1.58), acute coronary syndrome (OR 1.17, 95% CI 1.12–1.22), stroke (OR 1.42, 95% CI 1.33–1.52), and kidney disease (OR 1.63, 95% CI 1.34–1.97), as well as concomitantly using antiplatelet agents (OR 1.52, 95% CI 1.40–1.66), and steroids (OR 1.76, 95% CI 1.50–2.07). On the contrary, they were less common in health insurance holder (OR 0.79, 95% CI 0.75–0.84), patients taking apixaban (vs. rivaroxaban, OR 0.39, 95% CI 0.18–0.81), dabigatran (vs. rivaroxaban, OR 0.01, 95% CI 0.01–0.01), edoxaban (vs. rivaroxaban, OR 0.36, 95% CI 0.23–0.55), diagnosed with heart failure (OR 0.87, 95% CI 0.81–0.93), deep vein thrombosis (OR 0.36, 95% CI 0.29–0.46), pulmonary embolism (OR 0.35, 95% CI 0.28–0.43), and peripheral artery disease (OR 0.68, 95% CI 0.55–0.85).
Conclusion: The usage of OACs for AF was overall complying with updated guidelines. Low dosage was common for NOACs, further studies were warranted to verify its effectiveness and explore the underlying mechanism.
Atrial fibrillation (AF) is a leading risk factor of arterial thromboembolic events including stroke and systemic embolism (1, 2), and the oral anticoagulants (OACs) have a fundamental role in the management of AF (3). Non-vitamin K antagonist oral anticoagulants (NOACs) have shown non-inferiority to warfarin in the prevention of stroke/systemic embolism (4, 5) and a more favorable risk-benefit profile (6) for non-valvular atrial fibrillation (NVAF). Furthermore, patients with NOACs demonstrate higher persistence compared with warfarin (7). However, though the Chinese guideline has also recommended NOACs as the first choice over warfarin in NVAF in 2018 (8, 9), it is not known whether this has been applied in general practice.
At the same time, low-dose of NOACs is reported to be frequently used in daily clinical practice (10). Though meta-analysis based on phase III clinical trials suggested that NOACs were more effective and safer in Asian than non-Asian (11), this phenomenon seems to be more common in Asia (12, 13). Moreover, there is limited data about the low-dose of NOACs from mainland of China.
As the prescribing patterns and factors driving treatment choice may be evolving, the present cross-sectional study of prescription data has the following objectives: firstly, to describe the prescription pattern of OACs from January 2016 to March 2021 and the factors driving these changes, and secondly, to assess the prevalence of low-dose NOACs and explore the possible associated factors for AF patients.
Data was extracted from the Hospital Prescription Cooperation Project database, which aimed to promote the rational use of medicines in hospitals of China (14). The database consisted of prescriptions of randomly selected 10 workdays each month from contracted hospitals. Until 2021, there were a total of 134 hospitals from 9 cities (Beijing, Chengdu, Guangzhou, Hangzhou, Shanghai, Tianjin, Zhengzhou, Shenyang and Harbin) enrolled in this project. The study was approved by the Clinical Research Ethics Committee of Beijing Friendship Hospital (Ethics approval number: 2021-P2-218-01). Patients’ names and IDs were deleted during data analysis.
The study included prescriptions of patients diagnosed with AF and prescribed with OACs between January 2016 and March 2021 ignoring the age. In this study, OACs included warfarin and all approved NOACs in China, involving dabigatran, rivaroxaban, apixaban and edoxaban.
We also collected the following information for analysis: identification number of patients, sex, age, city, date of prescription, health insurance (with or without), type of visit (outpatient or inpatients), level of hospitals, diagnoses as well as generic names, dosage and administration of all agents in these prescriptions. As the database was recorded quarterly, patients with different OACs owing to the changeover are counted more than once.
The primary outcome was the changing percentage of different OACs. The secondary outcomes were frequencies as well as factors with the choice of different OACs and dosage of NOACs.
The type of health insurance was classified into two classes according to whether they have or not, without considering the insurance payer. To analyze the effect of age on the predefined outcomes, we classified the age into five groups: 0–17, 18–59, 60–74, 75–89 as well as ≥90 years old and age above 125 were deemed as mistyping and deleted from the related analysis. Prescriptions with missing data on age, health insurance or gender were excluded from related subgroup analysis, but counted in the overall analyze when these factors were not needed. In this study, we defined the dosage of NOACs in Supplementary Table 1 according to the updated guidelines (9). The low dose referred to the low daily dose, which meant that even though rivaroxaban was recommended as 20 mg QD, the “10 mg BID” was also deemed as standard dose. The dosage of warfarin was not analyzed in this study because the intensity of warfarin was determined by INR, which was not available in this database. Concomitantly used drugs assessed in this study includes antiplatelet agents, non-steroidal anti-inflammatory drugs (NSAIDs), antacids as well as steroids.
All continuous variables were presented as mean with standard deviation and compared by t-test when normally distributed, or reported as medians with interquartile ranges (IQRs) and compared with the Wilcoxon test when skewed. Categorical variables were shown as frequencies and percentages, and evaluated by either chi-square tests. Multivariate logistic regressions were conducted to examine factors associated with the choice of OACs and dosage of NOACs, results were shown with odds ratios (OR) and 95% confidence intervals (CIs). All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, North Carolina). The two-sided P < 0.05 was considered statistically significant.
Between January 2016 and March 2021, there were 220,083 distinct prescriptions diagnosed with AF patients and prescribed with OACs. Figure 1 displayed the changing trends of OACs: the portion of NOACs increased, while that of warfarin decreased over years. The percentage of NOACs exceeded the warfarin from 2018 Q1, and warfarin accounted for only 16.47% of all included AF patients in 2021 Q1. Table 1 showed the baseline characteristics for all included patients by treatment group. Patients prescribed with NOACs and warfarin were significantly different in age, gender, source of data, health insurance, levels of hospitals, comorbidities, concomitant use of drugs as well as the geographical location of patients. The percentage changes of NOACs over time under different conditions can be seen in Supplementary Figure 1.
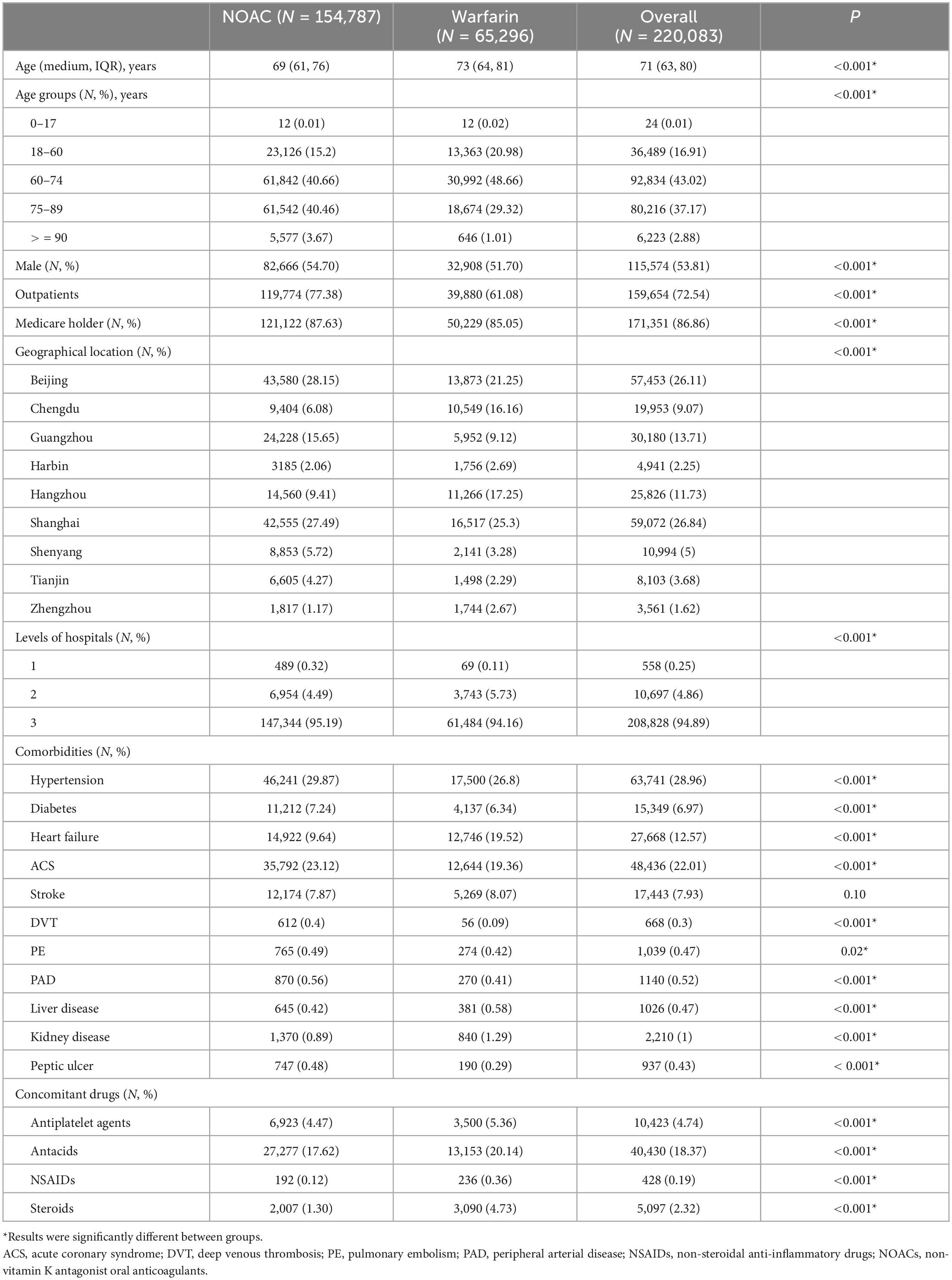
Table 1. Baseline characteristics of included atrial fibrillation patients with oral anticoagulants.
Multi-variate logistic regression suggested that the usage of NOAC was more frequent than warfarin for the male (vs. female, OR 1.17, 95% CI 1.14–1.19), the elderly (P < 0.001 among age groups), outpatients (vs. inpatients, OR 2.17, 95% CI 2.11–2.23), health insurance holder (vs. without, OR 1.23, 95% CI 1.19–1.27), patients treated in the primary hospitals (vs. tertiary hospitals OR 1.54, 95% CI 1.19–1.99), and diagnosed with ACS (OR 1.09, 95% CI 1.05–1.12), stroke (OR 1.25, 95% CI 1.20–1.30), heart failure (OR 1.27, 95% CI 1.22–1.33), deep venous thrombosis (DVT, OR 3.47, 95% CI 2.55–4.72), diabetes (OR 1.11, 95% CI 1.08–1.14), peripheral arterial disease (PAD, OR 1.43, 95% CI 1.21–1.68), pulmonary embolism (PE, OR 1.20, 95% CI 1.02–1.40), liver disease (OR 1.22, 95% CI 1.04–1.41), and co-administration of antacids (OR 1.57, 95% CI 1.52–1.62). On the contrary, NOACs were less common in patients diagnosed with hypertension (OR 0.47, 95% CI 0.46–0.49), kidney disease (OR 0.72, 95% CI 0.65–0.80), and concomitant administration of antiplatelet agents (OR 0.79, 95% CI 0.75–0.83), NSAIDs (OR 0.44, 95% CI 0.34–0.56) as well as steroids (OR 0.34, 95% CI 0.31–0.36) (Figure 2).
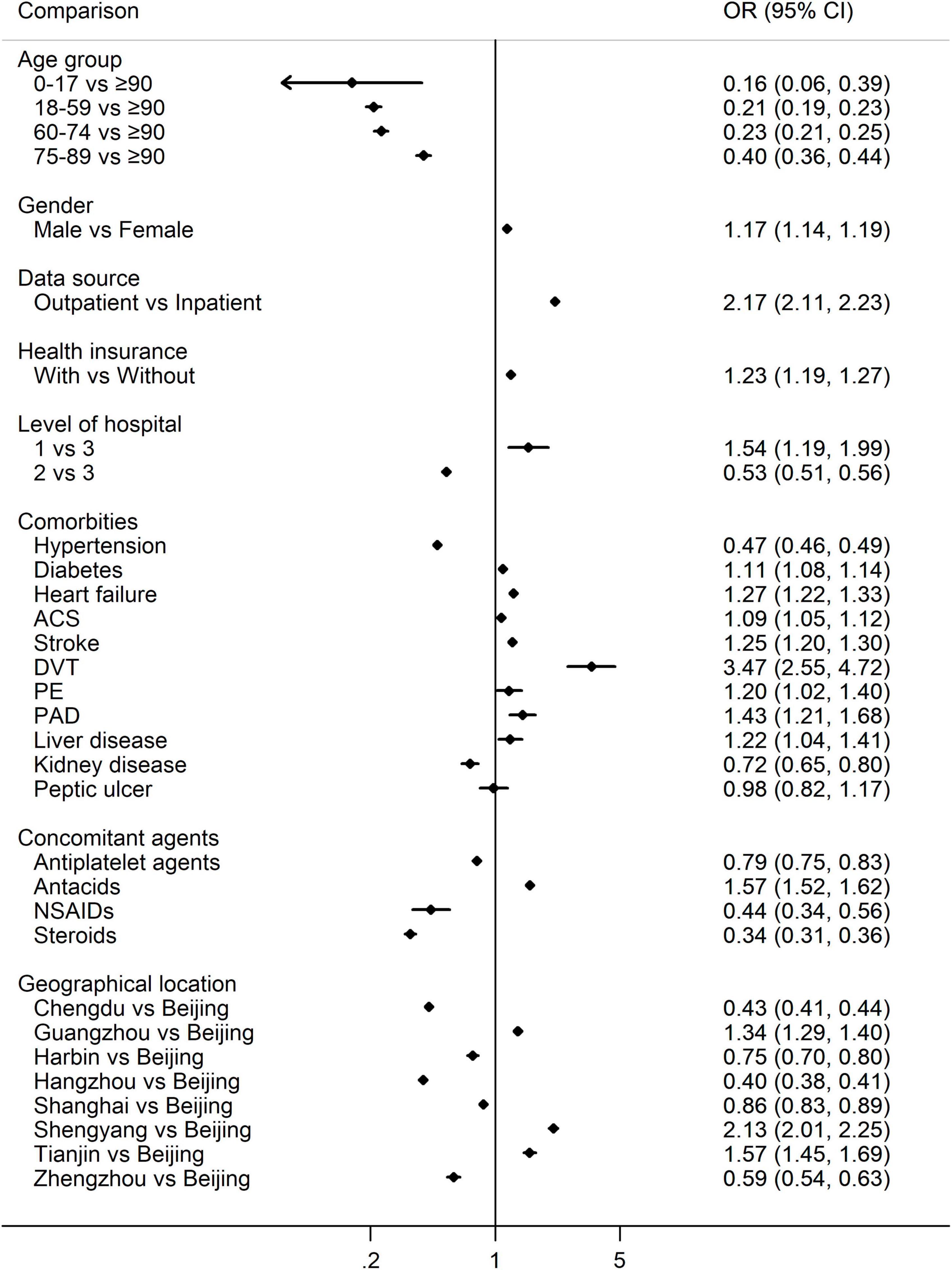
Figure 2. Multivariate logistic regression for the percentage of NOACs vs. warfarin. OR > 1 indicates more NOACs, while OR < 1 indicates more warfarin. NOACs, non-vitamin K antagonist oral anticoagulants; OR, odds ratio; ACS, acute coronary syndrome; DVT, deep venous thrombosis; PE, pulmonary embolism; PAD, peripheral arterial disease; NSAIDs, non-steroidal anti-inflammatory drugs.
For patients prescribed with NOACs, the frequencies of apixaban, rivaroxaban, edoxaban and dabigatran were also different in age, gender, source of data, health insurance holder, levels of hospitals, comorbidities, concomitant use of drugs as well as the geographical location of patients, details can be seen in Supplementary Table 2. Rivaroxaban (62.25%) and dabigatran (37.65%) were the most commonly used, accounting for 99.9% of all NOAC prescriptions.
When considering the intensity of NOACs as predefined, we found that 44.54% of patients were prescribed low-dose of NOACs, and the changing trends over years were demonstrated in Supplementary Figure 2. The choice of low vs. standard dose of NOACs was affected by age, gender, source of data, health insurance, levels of hospitals, comorbidities, concomitant use of drugs as well as the geographical location of patients, details can be seen in Supplementary Table 3. Multivariate logistic regression taking these factors into account found that the frequency of low-dose of NOACs increased with age, and was significantly different among different geographic locations, and levels of hospitals (P < 0.001 within groups, Figure 3). Low-dose of NOACs were more in the outpatients (OR 1.32, 95% CI 1.26–1.39), suffering from hypertension (OR 1.49, 95% CI 1.40–1.58), ACS (OR 1.17, 95% CI 1.12–1.22), stroke (OR 1.42, 95% CI 1.33–1.52) and kidney disease (OR 1.63, 95% CI 1.34–1.97), and concomitantly using antiplatelet agents (OR 1.52, 95% CI 1.40–1.66) and steroids (OR 1.76, 95% CI 1.50–2.07). On the contrary, low-dose of NOACs were less common in the male (OR 0.78, 95% CI 0.75–0.81), health insurance holder (OR 0.79, 95% CI 0.75–0.84), patients taking apixaban (vs. rivaroxaban, OR 0.39, 95% CI 0.18–0.81), dabigatran (vs. rivaroxaban, OR 0.01, 95% CI 0.01–0.01), edoxaban (vs. rivaroxaban, OR 0.36, 95% CI 0.23–0.55), diagnosed with heart failure (OR 0.87, 95% CI 0.81–0.93), DVT (OR 0.36, 95% CI 0.29–0.46), PE (OR 0.35, 95% CI 0.28–0.43), and PAD (OR 0.68, 95% CI 0.55–0.85) (Figure 3).
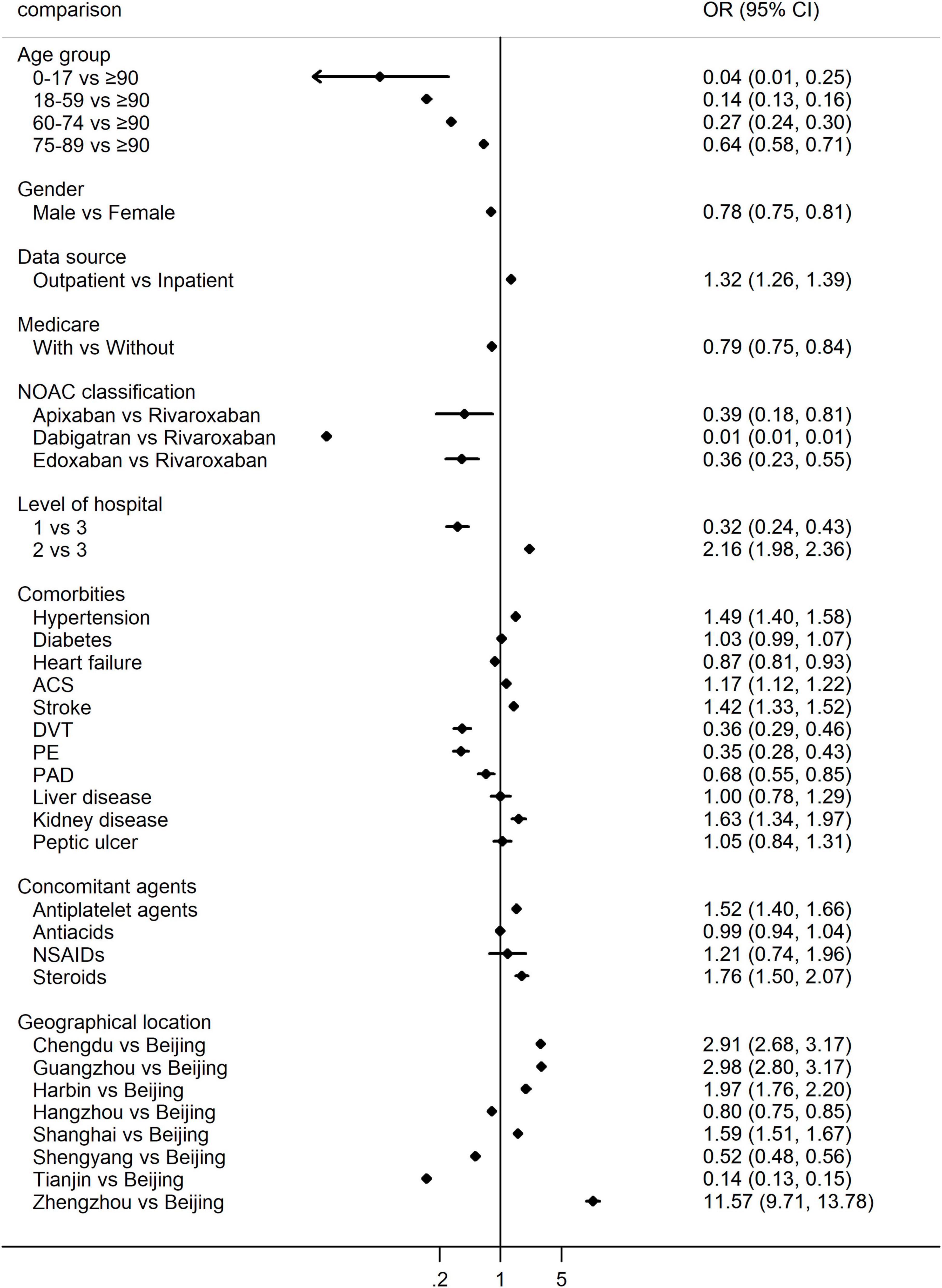
Figure 3. Multivariate logistic regression for the percentage of low-dose vs. standard dose of NOACs. OR > 1 indicates more low-dose of NOACs, while OR < 1 indicates more standard dose of NOACs. NOACs, non-vitamin K antagonist oral anticoagulants; OR, odds ratio; ACS, acute coronary syndrome; DVT, deep venous thrombosis; PE, pulmonary embolism; PAD, peripheral arterial disease; NSAIDs, non-steroidal anti-inflammatory drugs.
The majority of patients (87.48%) were taking standard dose of dabigatran, with 110 mg BID and 150 mg BID taking account for 76.31 and 11.10%, respectively. Among these patients, some (0.07%) took the standard daily dose of 220 or 300 mg once a day. The low dosages included 110 mg QD (3.51%), 150 mg QD (0.85%) and other low-dose strategies such as 55 mg BID, 75 mg BID, etc. (Figure 4A).
As Figure 4B showed, the low dosage was much more frequent for patients taking rivaroxaban (67.98%). The low daily dose varied from 1 to 15 mg, in which 10 mg QD and 15 mg QD were mostly used, taking account for 36.07 and 29.96% of all rivaroxaban patients, respectively. A total of 25.29% of patients took the standard daily dose of rivaroxaban. Among them, a small proportion (1.93%) took the dosage twice a day.
In this analysis of national electronic prescriptions between 2016 and 2021, we found that among patients with OACs, the percentage of NOACs increased over years, and reached 83.53% of all OAC patients. The prescription of NOACs vs. warfarin was affected by age, gender, health insurance, geographical distributions, comorbidities as well as co-administrated drugs. The low dosage was common for NOACs, especially for rivaroxaban, of which the low dosage accounted for 67.98%.
Though warfarin has been used for many years for the prevention of stroke for NVAF patients (15), its effect is easily affected by food and drugs (16). The time within the therapeutic range (TTR), the time spent achieving an international normalized ratio (INR) of 2-3.0, is used to estimate the quality of anticoagulation during warfarin therapy (4, 5). Some data suggested that if the TTR was less than 60%, the benefit of warfarin may lose (17). The reported TTR during general practice was 25–65%, and varied among patients with different physician specialties, geographical regions and population groups (5, 18). The estimated mean TTR from a tertiary hospital was 49.8% in China (19). As the evidence of NOACs accumulated, NOACs were recommended for NVAF patients and became the first-line strategy over warfarin since 2018 in China (9). From the increasing trend of NOACs and the reversal of percentage vs. warfarin in 2018, we can speculate that Chinese physicians can follow the guideline timely and closely.
It is reported that, compared with men, women have older age, more comorbidities and worse outcomes of stroke (20). Even worse, the female were reported to spend more time to arrive hospitals and receive acute stroke treatments (21–23) though possessing better knowledge of major stroke symptoms and stroke risk factors (24). In this study, we found that the female was less likely to be prescribed with NOACs than warfarin. The higher risk of stroke and lower percentage of NOAC usage calls for more attempts to improve the management of women with NVAF in the future.
In a nationwide cohort study of AF patients ≥75 years in Norway, researchers found that compared with warfarin, both standard and reduced dose of NOACs were associated with similar risks of stroke/systemic embolism as well as lower or similar risks of bleeding (25). The prescription of NOACs increased with age, indicating that physicians in China were accepting NOACs as safe options for elderly patients. Consistent with the worldwide observational cohort study (26), the percentage of NOACs was highest in community hospitals, namely primary hospitals in this study, and demonstrated significant geographic variability.
In this cross-sectional study, we found patients with antiplatelet agents were more prescribed warfarin. For NVAF patients undergoing percutaneous coronary intervention, though NOACs were recommended over VKA as the OAC agent in the US (27), the updated Chinese guideline didn’t give specific recommendations on OAC class owing to the lack of evidence for Chinese (28). Interestingly, we found that NSAIDs and steroids, which can cause gastrointestinal damage, were more co-administrated with warfarin. On the contrary, antacids, which were used to ameliorate gastrointestinal symptoms or injuries, were more co-administrated with NOACs. Further studies were needed to investigate the possible mechanism.
In this study, we found that patients with kidney disease were prescribed less percentage of NOACs vs. warfarin. This might be explained by the fact that the usage of NOACs is contraindicated for patients suffering from severe renal dysfunction during general practice and they were also excluded from trials (3, 4, 29). Though the high incidence of renal impairment during heart failure exacerbation or treatment (30, 31), single-/high-dose of NOACs were reported to reduce the risk of stroke or systemic embolic events by 14% (32) for patients with heart failure. In our study, AF patients suffering from heart failure were more commonly prescribed with NOACs.
In this study, rivaroxaban and dabigatran were the most commonly selected NOACs. Though the ARISTOTLE trial demonstrated its superiority to warfarin in hemorrhagic stroke as well as intracranial and major bleeding (5), it was not approved for stroke prevention for AF patients in China until now. The low percentage of edoxaban might be because it was approved in December 2018, much later than the other two NOACs. As there are no trials to compare different NOACs head-to-head, physicians need to choose one NOAC agent under comprehensive consideration of patients’ conditions such as personal will, age, bleeding risk and dyspepsia symptoms (33).
Though growing evidence showed that low-dose of NOACs was associated with increased risk for adverse events (10, 34, 35), it is commonly used in daily practice, especially for the Asian (12, 36). Similarly, nearly half of patients in this study are receiving low-dose of NOACs. This prescribing pattern might be caused by the lack of effective testing methods to verify the effectiveness of NOACs (37) and the fear of bleeding. Though NOACs have predictable pharmacokinetic and pharmacodynamic profiles, physicians may prefer to adjust doses to protect patients against bleeding risk in some “complicated” situations. Furthermore, we have to note that the Xa antidote was not available in China, once major bleeding occurs, patients have to use prothrombin complex concentrates or recombinant factor VIIa (38, 39), which is very expensive. Idarucizumab, the monoclonal antibody to reverse the anticoagulant effect of dabigatran (40), is also expensive in China.
Consistent with the previous study (36), we found that the low dosage was extremely common for rivaroxaban. This might be caused that rivaroxaban is a regular formulation and can be divided as needed. On the contrary, the oral bioavailability of dabigatran etexilate increases by 75% when the pellets are taken without the capsule shell compared to the intact capsule formulation. And the label of dabigatran declares that PRADAXA capsules should not be broken, chewed, or opened before administration (41). However, we can see that there were still a small proportion of patients prescribed with 55 mg BID or 75 mg BID with the 110 mg and 150 mg strengths, respectively. More efforts should be taken to improve the physician’s knowledge about medication.
In this study, we found that the predictors of low-dose of NOACs were female sex, outpatients, without health insurance, concomitantly use of antiplatelet agents and steroids, diagnosed with hypertension, ACS, stroke and kidney disease. This is not the same with results from GARFIELD-AF, the global prospective cohort study, in which diabetes was also detected to be a predictor of low-dose of NOACs (42).
To our knowledge, this is the first study to describe the prescribing pattern of OACs and their associated factors for AF patients until 2021 in mainland of China. Of course, we acknowledge the following limitations: First of all, the CHA2DS2-VASc score was not calculated in this study. However, we have assessed the affection of all involved factors in the CHA2DS2-VASc score on the selection of OACs and dosage of NOACs. On the other hand, the CHA2DS2-VASc score based on outpatient prescriptions might be underestimated because of the missing data. Secondly, we cannot predict the rate of AF patients taking OACs because patients may not need to prescribe OACs for each visit. Similarly, owing to the lack of continuous drug use by patients in this database, we cannot assess the interchange among OACs. Thirdly, because of the nature of this database, we didn’t use the ICD10 code to extract diagnosis information. However, we have got all the possible expressions for each diagnose from the database organizer to reduce omissions. At the same time, we didn’t specify the NVAF and valve AF because we are afraid of data omission.
In conclusion, the percentage of NOACs increase over years for patients taking OACs between 2016 and 2021. The prescription of NOACs vs. warfarin was overall complying with updated evidence and affected by age, gender, health insurance, geographic distributions, comorbidities as well as concomitant drugs. Low-dose of NOACs was common in general practice, especially for rivaroxaban. Further studies were needed to assess the possible factors.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Clinical Research Ethics Committee of Beijing Friendship Hospital (Ethics approval number: 2021-P2-218-01). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
ML: Data curation, Software, Writing – original draft. SZ: Investigation, Methodology, Writing – original draft. LW: Methodology, Software, Writing – review and editing. LH: Investigation, Writing – original draft. DL: Data curation, Validation, Writing – review and editing. JL: Methodology, Project administration, Writing – review and editing. YL: Supervision, Writing – review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
We thank Wei Cheng from the Hospital Prescription cooperation project for providing the raw data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1258536/full#supplementary-material
1. Germanova O, Smirnova D, Usenova A, Tavormina G, Cumming P, Galati G. Cryptogenic stroke in the context of pandemic-related stress: the role of arterial hemodynamics. Psychiatr Danub. (2022) 34:256–61.
2. Germanova O, Galati G, Germanov A, Stefanidis A. Atrial fibrillation as a new independent risk factor for thromboembolic events: hemodynamics and vascular consequence of long ventricular pauses. Minerva Cardiol Angiol. (2023) 71:175–81. doi: 10.23736/S2724-5683.22.06000-8
3. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehaa612
4. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N England J Med. (2009) 361:1139–51. doi: 10.1056/NEJMoa0905561
5. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. (2011) 365:883–91. doi: 10.1056/NEJMoa1009638
6. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. (2014) 383:955–62. doi: 10.1016/S0140-6736(13)62343-0
7. Yao X, Abraham NS, Alexander GC, Crown W, Montori VM, Sangaralingham LR, et al. Effect of Adherence to Oral Anticoagulants on Risk of Stroke and Major Bleeding Among Patients With Atrial Fibrillation. J Am Heart Assoc. (2016) 5:e003074. doi: 10.1161/JAHA.115.003074
8. Huang C, Zhang S, Huang D, Camm AJ, Daubert JC, Allessie M, et al. Current knowledge and management recommendations of atrial fibrillation:2018. Chin J Cardiac Arrhythm. (2018) 22:279–346. doi: 10.3760/cma.j.issn.1007-6638.2018.04.002
9. Cardioelectrophysiology and Pacing Branch of the Chinese Medical Association RPCotCMA, the Working Committee of Atrial Fibrillation Prevention and Treatment. Experts of the China Association of Atrial Fibrillation Centers. Current knowledge and management of atrial fibrillation: consensus of Chinese experts 2021. Chinese J Cardiac Arrhythm. (2022) 26:15–88. doi: 10.3760/cma.j.cn113859-20211224-00264
10. Steinberg BA, Shrader P, Thomas L, Ansell J, Fonarow GC, Gersh BJ, et al. Off-Label Dosing of Non-Vitamin K Antagonist Oral Anticoagulants and Adverse Outcomes: The ORBIT-AF II Registry. J Am Coll Cardiol. (2016) 68:2597–604. doi: 10.1016/j.jacc.2016.09.966
11. Wang K, Lip GY, Lin S, Chiang C. Non-Vitamin K Antagonist Oral Anticoagulants for Stroke Prevention in Asian Patients With Nonvalvular Atrial Fibrillation: Meta-Analysis. Stroke. (2015) 46:2555–61. doi: 10.1161/STROKEAHA.115.009947
12. Chan Y, Chao T, Chen S, Lee H, Yeh Y, Huang Y, et al. Off-label dosing of non-vitamin K antagonist oral anticoagulants and clinical outcomes in Asian patients with atrial fibrillation. Heart Rhythm. (2020) 17:2102–10. doi: 10.1016/j.hrthm.2020.07.022
13. Chan Y, See L, Tu H, Yeh Y, Chang S, Wu L, et al. Efficacy and Safety of Apixaban, Dabigatran, Rivaroxaban, and Warfarin in Asians With Nonvalvular Atrial Fibrillation. J Am Heart Assoc. (2018) 7:8150. doi: 10.1161/JAHA.117.008150
14. Hou W, Li D, Shen S, Lin J, Lou A, Wen A. Frequency and Patterns of Prescribing Antihypertensive Agents in Outpatient Kidney Transplant Recipients Among Six Cities in China from 2011 to 2018. Clin Ther. (2021) 43:602–12. doi: 10.1016/j.clinthera.2021.01.013
15. Hart R, Pearce L, Aguilar M. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. (2007) 146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007
16. Hirsh J, Dalen J, Deykin D, Poller L, Bussey H, Ansell J, et al. Oral anticoagulants. Mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. (1995) 108:231S–46S. doi: 10.1378/chest.108.4_supplement.231s
17. Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. (2008) 118:2029–37. doi: 10.1161/CIRCULATIONAHA.107.750000
18. Pokorney SD, Simon DN, Thomas L, Fonarow GC, Kowey PR, Chang P, et al. Patients’ time in therapeutic range on warfarin among US patients with atrial fibrillation: Results from ORBIT-AF registry. Am Heart J. (2015) 170:141–8. doi: 10.1016/j.ahj.2015.03.017
19. Li X, Sun S, Wang Q, Chen B, Zhao Z, Xu X. Assessment of patients’ warfarin knowledge and anticoagulation control at a joint physician- and pharmacist-managed clinic in China. Patient Prefer Adherence. (2018) 12:783–91. doi: 10.2147/PPA.S156734
20. Cordonnier C, Sprigg N, Sandset EC, Pavlovic A, Sunnerhagen KS, Caso V, et al. Stroke in women - from evidence to inequalities. Nat Rev Neurol. (2017) 13:521–32. doi: 10.1038/nrneurol.2017.95
21. The Lancet Neurology. Sex differences and stroke prevention. Lancet Neurol. (2014) 13:339. doi: 10.1016/S1474-4422(14)70057-2
22. Foerch C, Misselwitz B, Humpich M, Steinmetz H, Neumann-Haefelin T, Sitzer M, et al. Sex disparity in the access of elderly patients to acute stroke care. Stroke. (2007) 38:2123–6. doi: 10.1161/STROKEAHA.106.478495
23. Mandelzweig L, Goldbourt U, Boyko V, Tanne D. Perceptual, social, and behavioral factors associated with delays in seeking medical care in patients with symptoms of acute stroke. Stroke. (2006) 37:1248–53. doi: 10.1161/01.STR.0000217200.61167.39
24. Stroebele N, Müller-Riemenschneider F, Nolte CH, Müller-Nordhorn J, Bockelbrink A, Willich SN. Knowledge of risk factors, and warning signs of stroke: a systematic review from a gender perspective. Int J Stroke. (2011) 6:60–6. doi: 10.1111/j.1747-4949.2010.00540.x
25. Rutherford OW, Jonasson C, Ghanima W, Söderdahl F, Halvorsen S. Effectiveness and safety of oral anticoagulants in elderly patients with atrial fibrillation. Heart. (2022) 108:345–52. doi: 10.1136/heartjnl-2020-318753
26. Bayer V, Kotalczyk A, Kea B, Teutsch C, Larsen P, Button D, et al. Global Oral Anticoagulation Use Varies by Region in Patients With Recent Diagnosis of Atrial Fibrillation: The GLORIA-AF Phase III Registry. J Am Heart Assoc. (2022) 11:e023907. doi: 10.1161/JAHA.121.023907
27. Angiolillo DJ, Bhatt DL, Cannon CP, Eikelboom JW, Gibson CM, Goodman SG, et al. Antithrombotic Therapy in Patients With Atrial Fibrillation Treated With Oral Anticoagulation Undergoing Percutaneous Coronary Intervention: A North American Perspective: 2021 Update. Circulation. (2021) 143:583–96. doi: 10.1161/CIRCULATIONAHA.120.050438
28. Chinese Society of Cardiology of Chinese Medical Association, Editorial Board of Chinese Journal of Cardiology. 2019 Chinese Society of Cardiology (CSC) guidelines for the diagnosis and management of patients with ST?segment elevation myocardial infarction. Chin J Cardiol. (2019) 47:766–83. doi: 10.3760/cma.j.issn.0253-3758.2019.10.003
29. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. (2013) 369:2093–104. doi: 10.1056/NEJMoa1310907
30. Rossignol P, Dobre D, McMurray JJ, Swedberg K, Krum H, Veldhuisen DJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail. (2014) 7:51–8. doi: 10.1161/CIRCHEARTFAILURE.113.000792
31. Löffler AI, Cappola TP, Fang J, Hetzel SJ, Kadlec A, Astor B, et al. Effect of renal function on prognosis in chronic heart failure. Am J Cardiol. (2015) 115:62–8. doi: 10.1016/j.amjcard.2014.09.055
32. Xiong Q, Lau YC, Senoo K, Lane DA, Hong K, Lip GY. Non-vitamin K antagonist oral anticoagulants (NOACs) in patients with concomitant atrial fibrillation and heart failure: a systemic review and meta-analysis of randomized trials. Eur J Heart Fail. (2015) 17:1192–200. doi: 10.1002/ejhf.343
33. Hammersley D, Signy M. Navigating the choice of oral anticoagulation therapy for atrial fibrillation in the NOAC era. Ther Adv Chronic Dis. (2017) 8:165–76. doi: 10.1177/2040622317720106
34. Marzona I, Proietti M, Colacioppo P, Foresta A, Baviera M. Effectiveness and safety of high and low dose NOACs in patients with atrial fibrillation. Eur J Intern Med. (2021) 88:118–22. doi: 10.1016/j.ejim.2021.01.031
35. Kotalczyk A, Guo Y, Wang Y, Lip GY. Are low doses of non-vitamin K antagonists effective in Chinese patients with atrial fibrillation? A report from the Optimal Thromboprophylaxis in Elderly Chinese Patients with Atrial Fibrillation (ChiOTEAF) registry. Int J Stroke. (2021) 2021:17474930211053140. doi: 10.1177/17474930211053140
36. Godino C, Bodega F, Melillo F, Rubino F, Parlati AL, Cappelletti A, et al. Inappropriate dose of nonvitamin-K antagonist oral anticoagulants: prevalence and impact on clinical outcome in patients with nonvalvular atrial fibrillation. J Cardiovasc Med. (2020) 21:751–8. doi: 10.2459/JCM.0000000000001043
37. Jose SP, Banzato A, Carraro P, Haleh A, Rossi K, Nante G, et al. Point of Care Testing (POCT) to assess drug concentration in patients treated with non-vitamin K antagonist oral anticoagulants (NOACs). Thromb Res. (2018) 163:100–4. doi: 10.1016/j.thromres.2018.01.044
38. Majeed A, Agren A, Holmstrom M, Bruzelius M, Chaireti R, Odeberg J, et al. Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood. (2017) 130:1706–12. doi: 10.1182/blood-2017-05-782060
39. Schultz NH, Tran HT, Bjørnsen S, Henriksson CE, Sandset PM, Holme PA. The reversal effect of prothrombin complex concentrate (PCC), activated PCC and recombinant activated factor VII against anticoagulation of Xa inhibitor. Thromb J. (2017) 15:6. doi: 10.1186/s12959-017-0129-1
40. Pollack CV, Reilly PA, Ryn J v, Eikelboom JW, Glund S, Bernstein RA, et al. Idarucizumab for Dabigatran Reversal - Full Cohort Analysis. N Engl J Med. (2017) 377:431–41. doi: 10.1056/NEJMoa1707278
41. U.S. Approval.PRADAXA® (dabigatran etexilate) capsules, for oral use Initial. Washington, DC: U.S. Approval (2010).
Keywords: NOACs, OACs, prescription pattern, AF, influencing factors
Citation: Zhu S, Li M, Wang L, Hou L, Li D, Liu J and Lu Y (2023) Real-world national trends and influencing factors preference of non-vitamin K antagonist oral anticoagulants in China. Front. Med. 10:1258536. doi: 10.3389/fmed.2023.1258536
Received: 14 July 2023; Accepted: 30 October 2023;
Published: 21 November 2023.
Edited by:
Redhwan Ahmed Al-Naggar, National University of Malaysia, MalaysiaReviewed by:
Dushyant Damania, Albert Einstein College of Medicine, United StatesCopyright © 2023 Zhu, Li, Wang, Hou, Li, Liu and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiuhong Liu, d2pqYXNvbl9sQDEyNi5jb20=; Yanxia Lu, bHl4XzY3Mzg2QHNpbmEuY29t
†These authors have contributed equally to this work
‡These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.