
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med. , 06 October 2023
Sec. Family Medicine and Primary Care
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1253951
This article is part of the Research Topic Occupational Immunology: Current Knowledge and Future Perspectives View all 7 articles
Background: Physical activity (PA) is beneficial for preventing several conditions associated with underlying chronic inflammation, e. g., cardiovascular disease (CVD) and cancer. While an active lifestyle appears to have anti-inflammatory effects, high levels of occupational PA (OPA) were associated with inflammation and elevated mortality risks. We aimed to summarize the current knowledge (1) on the association between inflammation and OPA and (2) its implications for health and mortality.
Methods and results: This mini-review summarized relevant literature published before January 2023 using established scientific databases and sources. For the primary outcome, observational studies (S) reporting immunological effects (O) in subjects (P), with high (I) vs. low OPA (C), were included. For secondary outcomes, i.e., morbidity and mortality associated with inflammatory processes, (systematic) reviews were included. While “active” occupations and “moderate” OPA appear to have beneficial effects, low (particularly sedentary) and “high-intensity” OPA (particularly including heavy lifting tasks) were associated with inflammation and (CVD and cancer-related) mortality; higher leisure-time PA has been almost consistently associated with lower proinflammatory markers and all-cause mortality risks. Workplace interventions appear to counter some of the observed health effects of unfavorable work strain.
Conclusion: The few studies addressing OPA “intensity” and inflammatory markers are largely heterogeneous regarding OPA classification and confounder control. Sedentary and “heavy” OPA appear to promote proinflammatory effects. In addition to targeted management of work-related physical strain and hazardous environmental co-factors, occupational health providers should focus on employer-initiated exercise interventions and the promotion of leisure-time PA.
The benefits of physical activity (PA) for health and longevity have been well established. PA has been associated with the prevention and management of excessive body weight, chronic disabilities, and health conditions [e.g., cardiovascular disease (CVD), metabolic syndrome, cancer], strengthening of the musculoskeletal system, and improvement of cognitive functioning and mental health (1). Chronic (low-grade) inflammation is involved in the pathogenesis of several conditions mentioned, such as atherosclerosis, metabolic dysfunction, the development and promotion of cancer, and autoimmune and neurodegenerative diseases (2). Although the exact mechanisms are largely undefined, the growing field of exercise immunology describes how PA is involved in immune modulation and how an active lifestyle mediates anti-inflammatory and antioxidant states, refining dysbiosis of the immunologically highly active gut microbiome and countering the development of chronic health conditions as well as immunosenescence (3). A history of physical inactivity has been associated with an increased “immune risk profile” based on biomarkers predicting morbidity and mortality in the elderly (4). Accelerated aging, cognitive decline, and impaired vascular and immune functions have been associated with an inactive and particularly sedentary lifestyle (5). Immune-specific findings of physical inactivity and sedentary lifestyle include in vitro observations regarding the shortening of leukocyte telomere length, proinflammatory immune mediator production, and impairing innate and adaptive immune cell activity, as well as in vivo responses to inflammatory processes (4). While widely unspecific, circulating inflammatory markers, such as high-sensitivity C-reactive protein levels [(hs)CRP], have been associated with pre-diabetic status (6) and have emerged as more reliable indicators of atherosclerosis than classical lipid markers, e.g., low-density lipoprotein (LDL) cholesterol (7) in CVD. Biomarkers of chronic and systemic inflammatory responses have also been identified as independent prognostic factors, particularly in CVD and cancer-related mortality risk (8). In this context, PA was found to exert beneficial effects on mortality risk associated with a high systemic immune-inflammation index.
A meta-analysis including data from over 122,000 participants (60 years+ of age) reported a curvilinear relationship between overall weekly moderate to vigorous PA [based on metabolic equivalents of task (METs)] and all-cause mortality, with a steep initial increase in benefits and a hereafter linear reduction of mortality from medium to high doses of moderate to vigorous PA. The authors described the strong inverse relationship as related to reduced CVD and, to a lesser extent, reduced cancer-related mortality (9). In 2018, however, a meta-analysis including data from 193,696 participants reported that, compared to low levels of occupational PA, high levels of occupational PA significantly increased mortality risk in men (10). These findings intensified the debate over the existence of a “physical activity paradox,” which indicates that higher levels of leisure-time PA but not occupational PA are beneficial to health, an effect possibly mediated through proinflammatory processes associated with high levels of occupational PA, specifically (11). This mini-review aimed to summarize what is currently known about inflammation in the context of occupational physical activity (OPA) and the potentially associated implications for health, morbidity, and mortality.
This mini-review aims to identify available evidence on the immune effects of occupational physical activity (12) via PubMed (MEDLINE), Embase, and the Cochrane Library using the keywords (“occupation*” OR “work-related”) AND (“physical activity”) AND (“inflammation” OR “immune”). All article types (e.g., original articles, reviews, meta-analyses, editorials, and letters) from inception to 15 January 2023 were screened by abstract regardless of language. Studies were included and evaluated according to the PICOS framework: any observational (cohort, case-control, cross-sectional) studies (S) reporting immune effects (O) in (currently or previously) working subjects (P), with high occupational physical activity (I) vs. controls with low physical activity (C), were included for evidence analysis. The PICOS tool, endorsed by the Cochrane Collaboration, focuses on (P)opulation, (I)ntervention, (C)omparison, (O)utcomes, and (S)tudy design in quantitative research; it is conducive to the identification of relevant components of clinical evidence in scientific reviews (13). Relevant studies were further identified by screening (systematic) reviews and meta-analyses. Nine studies reporting changes in inflammatory markers in relation to OPA activity levels were identified (11, 14–21) and included in the narrative analysis.
Secondary outcomes of interest were morbidity and mortality, particularly outcomes linked to chronic diseases associated with underlying sustained inflammatory processes; here, systematic reviews were preferred in view of limitations on the number of references in a mini-review.
Exercise, but also non-exercise PA, such as occupational or household work, has been reported to result in lower levels of circulating CRP, interleukin 6 (IL-6), and tumor necrosis factor-alpha (TNF-α) in the elderly (15). Moderate weekly PA, regardless of leisure or occupational context, was found to correlate with the modulation of circulating inflammatory markers (16). Total PA was associated with lower proinflammatory TNF-α expression and higher anti-inflammatory IL-10 expression in breast tissues of physically active women, as compared to women in the lowest PA categories; higher OPA was inversely associated with a pro- to anti-inflammatory mediator ratio among premenopausal women in an adjusted model, with a particularly low expression of both IL-6 and TNF-α in women with high OPA (17). Higher levels of overall PA, including occupational activity, were associated with reduced circulating CRP and malnutrition-inflammation scores in hemodialysis patients (18).
Moderate and higher OPA resulted in decreased ischemic heart disease risk and were associated with lower CVD mortality rates in women (22) and men (23, 24). An “active” job showed modest associations with a reduced total incidence of stroke, specifically with a lower ischemic stroke risk in both sexes combined; in this context, active commuting by bicycle or on foot (≥30 min per day) also showed a moderate protective effect. Combining moderate and “active” OPA vs. low OPA resulted in reduced adjusted stroke risk, even after controlling for leisure time and commuting PA (25). Previous smaller studies did not find an association between stroke risk and OPA (26, 27). Moderate to vigorous OPA was significantly associated with a lower diabetes type II risk after adjustment for common risk factors and general (commuting and leisure-time) PA (28). In chronic inflammation-based conditions, such as inflammatory bowel disease (IBD), occupations characterized by higher OPA had protective effects (29) regarding the risk of contracting IBD compared to those with less active OPA (30). A meta-analysis further showed that a significantly reduced colon cancer risk was associated with higher leisure-time PA and higher OPA in men, whereas in women, only leisure-time PA was associated with a reduced colon cancer risk (31). While in another meta-analysis, higher levels of OPA were associated with decreased bladder cancer risk, this finding only reached statistical significance after combining risk estimates with leisure-time PA (32). A protective effect of an “active” job was also hypothesized for breast cancer (33, 34); circulating leukocyte telomere length, a marker for cell aging of innate immune cells involved in anti-cancer responses, was positively associated with overall PA and OPA, respectively (35).
A sedentary lifestyle in combination with elevated hs-CRP appears to have a particularly high all-cause, CVD, and cancer-related mortality risk (14). An inactive lifestyle, i.e., a sedentary job and no recreational activity, was associated with the highest CRP levels and a higher risk of future coronary artery disease in a large case-control study, compared to higher OPA levels partially even without recreational activity (21).
In a prospective study including over 1,500 participants, sedentary OPA was described as a risk factor for all-cause mortality with an adjusted hazard ratio of 1.16 and independent of overall PA, with the lowest cumulative survival rates in occupational groups with the highest sedentary time compared to more active OPA groups (36). Association with mortality remained after adjustment for total PA and common confounders such as age, sex, body mass index, total cholesterol levels, and (systolic) blood pressure. These findings were observed in both genders but did not reach statistical significance for women (36). Low levels of OPA and leisure-time PA were independently associated with higher mortality risk in a Belgian Physical Fitness Study in 1,456 men (37); a sedentary lifestyle was proposed to be more harmful to health in workers with lower overall physical fitness (37). One study validated reported occupational sitting time applying accelerometry and found that sedentary behavior at work was not associated with CVD events and/or mortality, a risk when adjusted for confounders (38). In this study (36), sedentary OPA was significantly associated with CVD-related but not cancer-related mortality, whereas a meta-analysis described a positive association between occupational sedentary time and colon cancer risk, specifically (39). Low OPA, particularly sedentary OPA (40), was also associated with a higher risk of developing IBD in contrast to more active jobs. Not surprisingly, a proinflammatory diet was associated with sedentary OPA in a cohort of Croatian workers (41).
Increased levels of hs-CRP were recently associated with higher OPA and lower levels of leisure-time PA, suggesting an association between systemic inflammation and the “physical activity paradox” (11). The highest levels of OPA were proposed to be directly associated with higher circulating hsCRP and thus an increase in systemic inflammation (19); workers engaging in “higher-intensity” OPA had significantly higher hsCRP levels compared to those with lower OPA and high levels of leisure-time activity, with sex-stratified models again showing more significant outcomes in men than in women. Another study found no statistically significant relationship between “work-related strain” and inflammatory markers (20) but between OPA and CVD risk.
The highest levels of OPA were reported to increase the risk of all-cause mortality, particularly in men (42). Associated increases in blood pressure, prolonged elevation of heart rate (43), and sustained levels of inflammation (44) were proposed as mediating risk factors for CVD in “high-intensity OPA” (45, 46). A significant increase in mortality was observed in workers with high OPA, based on job type and kilocalories per working hour, and low leisure-time PA after adjustment for potential confounders, particularly in those with lower overall physical fitness (37). Strenuous OPA, but particularly repetitive and heavy lifting at work, was associated with an increased myocardial infarction risk (47); “high-intensity” OPA was associated with CVD risk in women (48). A positive association between OPA “intensity” and stroke/transient ischemic attack (TIA) was described after controlling for confounding factors. An increased risk was found for “partially standing” and “high-intensity work,” with a dose-response relationship between stroke and exposure at the longest-held job but also between TIA and current job; leisure-time PA was again described as protective against stroke and TIA (49).
Particularly, heavy lifting tasks are a special aspect of more intensive OPA and are commonly ascribed to the highest OPA categories. Musculoskeletal pain, overuse injuries, and knee osteoarthritis have also been associated with high levels of OPA, particularly heavy lifting and repetitive movements (50). While circulating inflammatory markers are released following tissue damage (51, 52), potentially predicting overuse injuries, some inflammation is considered necessary in the initiation of favorable tissue repair and physiological adaptations to increased working demands (53). Interestingly, proinflammatory mediators, such as IL-6 and IL-8, are increased with higher body fat percentage and in response to lifting work, with low-frequency high-resistance tasks associated with higher systemic inflammatory responses and greater cumulative spinal moments compared to high-frequency low-resistance tasks (51).
While overall PA and an “active” occupation appear to be associated with improved inflammatory markers and related morbidity and mortality risks, the outcomes for very low and sedentary OPA are inconclusive or unfavorable, especially when combined with low leisure-time PA. Emerging evidence points toward the proinflammatory effects of “high-intensity” OPA. Overall, due to the heterogeneity in the design of the few available studies (11, 14–21), still very little is known about the association between OPA “intensity” and inflammatory markers, which are easily impacted by pre-existing comorbidities and immune-modulating medication, but also by (blood) sampling timepoints in relation to diurnal rhythm and hours since PA (54, 55).
Proposed reasons for the frequently reported lack of health benefits of OPA in contrast to leisure-time PA are the suboptimal design of OPA for improving cardiorespiratory fitness, particularly in terms of (potentially harmful) working posture, intensity, or duration of working tasks; furthermore, uncontrolled elevation of 24 h heart rate and blood pressure (particularly by heavy lifting work), lack of sufficient recovery time between intensive tasks, and limited control over work-associated physical and psychosocial stressors (45) are potential proinflammatory hazards. Interestingly, <35 working hours per week were associated with lower all-cause mortality, independent of gender and OPA intensity (56). In addition to long working hours and shift work, which are found in all OPA categories, other working environment factors with proinflammatory potential, such as heat strain, are more common in “high-intensity” OPA jobs (57–59). Lack of sufficient recovery time (60) in combination with work-related and work-independent stressors may indeed support sustained inflammation and promote the development of chronic diseases, such as CVD and cancer. Age, gender, and potentially race are factors associated with differences in the expression of inflammatory markers (61) and probably also with the extent of health benefits derived from PA in general (10).
Valid criticism (62) has also been raised against the occupational “physical activity paradox,” including the often imprecise and usually self-reported determination of “active” and/or “high activity” occupational tasks using questionnaires only. One review summarizing device-measured PA at work reported that while office workers had primarily sedentary OPA, they still were most active during their day compared to “more active” professions such as healthcare workers and laborers (63). Furthermore, the differentiation between OPA “intensity” varies between most studies, and categories of OPA are sometimes merged to obtain larger subgroups. Here, a consistent classification (64), ideally based on objective work intensity data including METs (65) and accounting for work-specific conditions, e.g., heavy lifting tasks and working environment, should be agreed upon for the design of further studies. At this point, we also need to add that OPA is always “physical” work. This should be taken into consideration because, from the perspective of physical work, in OPA, there is currently no differentiation between workload intensity, volume, or density. Although the use of METs makes sense for the objective representation of workload, we still do not understand “high intensity” OPA well enough. Is it truly work “intensity,” and therefore the very high workload concerning the worker's maximum strength, which leads to detrimental health outcomes? Or is it work “volume” and therefore the extensive sum of cumulative workloads? Or is it maybe work “density” and therefore the lack of sufficient rest periods between phases of increased physical work?
The choice of relevant co-variates has also been argued as a potential bias, e.g., the gradation of confounders such as smoking (62). Additionally, commonly controlled confounders are imprecise, such as BMI, which does not adequately reflect muscle vs. fat-free mass, which again makes a significant difference in terms of inflammatory potential and physical resilience. Lastly, synergistic and potentiating effects of confounders, such as multiple unhealthy lifestyle behaviors, including proinflammatory diet consumption at the workplace, commonly associated with, e.g., social class, cannot be fully controlled for and might affect certain “heavy-work” OPA groups more than other OPA categories (66). While workplace safety has been considerably improved over the last decades, studies addressing all-cause mortality among heavy workers should also consider specific hazards of the working environment, e.g., the impact of occupational diseases and fatal accidents (67).
Several strategies for improving health in potentially hazardous high OPA environments have been proposed (45), such as regulating task intensity and recovery time throughout the working day, using mechanical support equipment to avoid heavy manual lifting, and delivering activity interventions through the workplace (Figure 1). Leisure-time PA has been repeatedly described as protective against potentially inflammation-mediated morbidity and mortality, even in workers with unfavorable (sedentary or “high-intensity”) OPA. Simple occupational health promotion, such as activity and walking interventions and even unsupervised employer-initiated PA (68), workplace stressor handling, including a healthy diet and reduction of hazardous stressors, as well as the introduction of mechanical handling aids in the case of heavy OPA (Figure 1), may sustainably improve inflammatory profiles (69) and potentially reduce CVD risk (70).
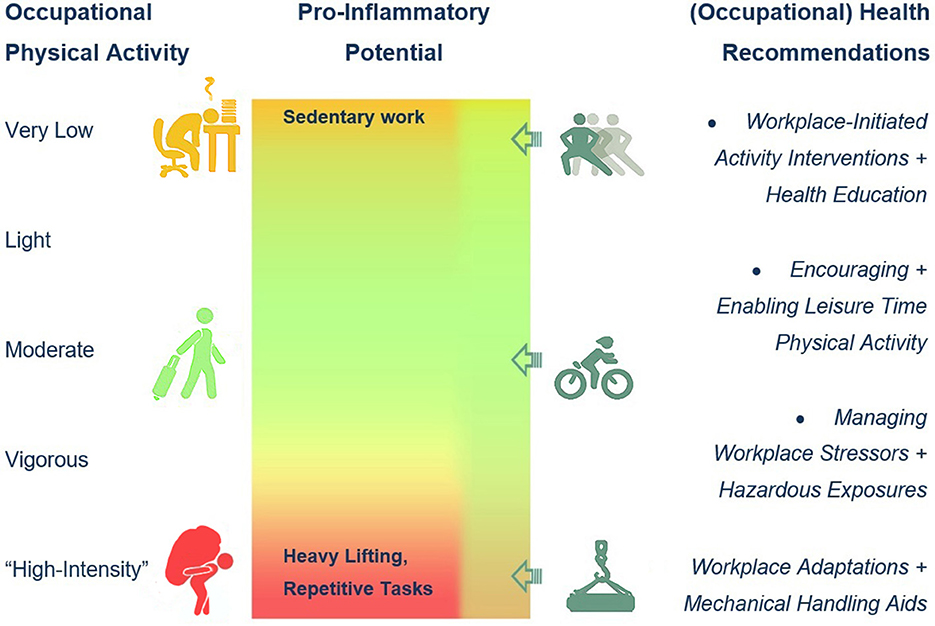
Figure 1. Occupational physical activity (OPA) levels, suspected proinflammatory effects, and occupational health recommendations.
While proinflammatory effects have been originally attributed to very low and sedentary OPA, “high-intensity” OPA is emerging as a risk factor for chronic inflammation and potentially associated mediated morbidity and mortality risks. There is still very little known about the link between OPA “intensity” and inflammatory markers, mainly due to the heterogeneity of OPA classification and confounder control in the available studies. Although the existence of the “physical activity paradox” is still debated, a consensus regarding the necessity for (a workplace-mediated) encouragement of leisure-time PA is evident throughout the scientific literature and arguably for an ever-continuing optimization of workplace conditions in accordance with occupational health care and promotion (Figure 1).
GJ: Writing—original draft, Writing—review and editing. TH: Writing—review and editing. MS: Writing—review and editing. EJ-J: Writing—review and editing. RC: Writing—review and editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Warnberg J, Cunningham K, Romeo J, Marcos A. Physical activity, exercise and low-grade systemic inflammation. Proc Nutr Soc. (2010) 69:400–6. doi: 10.1017/S0029665110001928
3. Nieman DC, Pence BD. Exercise immunology: future directions. J Sport Health Sci. (2020) 9:432–45. doi: 10.1016/j.jshs.2019.12.003
4. Simpson RJ, Guy K. Coupling aging immunity with a sedentary lifestyle: has the damage already been done?–a mini-review. Gerontology. (2010) 56:449–58. doi: 10.1159/000270905
5. Archer T, Fredriksson A, Schutz E, Kostrzewa RM. Influence of physical exercise on neuroimmunological functioning and health: aging and stress. Neurotox Res. (2011) 20:69–83. doi: 10.1007/s12640-010-9224-9
6. Lin J, Zhang M, Song F, Qin J, Wang R, Yao P, et al. Association between C-reactive protein and pre-diabetic status in a Chinese Han clinical population. Diabetes Metab Res Rev. (2009) 25:219–23. doi: 10.1002/dmrr.923
7. Swastini DA, Wiryanthini IAD, Ariastuti NLP, Muliantara A. Atherosclerosis prediction with high sensitivity C-Reactive Protein (hs-CRP) and related risk factor in patient with dyslipidemia. Open Access Maced J Med Sci. (2019) 7:3887–90. doi: 10.3889/oamjms.2019.526
8. Li H, Wu X, Bai Y, Wei W, Li G, Fu M, et al. Physical activity attenuates the associations of systemic immune-inflammation index with total and cause-specific mortality among middle-aged and older populations. Sci Rep. (2021) 11:12532. doi: 10.1038/s41598-021-91324-x
9. Hupin D, Roche F, Gremeaux V, Chatard JC, Oriol M, Gaspoz JM, et al. Even a low-dose of moderate-to-vigorous physical activity reduces mortality by 22% in adults aged >/=60 years: a systematic review and meta-analysis. Br J Sports Med. (2015) 49:1262–7. doi: 10.1136/bjsports-2014-094306
10. Coenen P, Huysmans MA, Holtermann A, Krause N, van Mechelen W, Straker LM, et al. Do highly physically active workers die early? a systematic review with meta-analysis of data from 193 696 participants. Br J Sports Med. (2018) 52:1320–6. doi: 10.1136/bjsports-2017-098540
11. Feinberg JB, Møller A, Siersma V, Bruunsgaard H, Mortensen OS. Physical activity paradox: could inflammation be a key factor? Br J Sports Med. (2022) bjsports-2022-105429. doi: 10.1136/bjsports-2022-105429.
12. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. (2018) 18:143. doi: 10.1186/s12874-018-0611-x
13. Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. (2014) 14:579. doi: 10.1186/s12913-014-0579-0
14. Lee JY, Ryu S, Cheong E, Sung KC. Association of physical activity and inflammation with all-cause, cardiovascular-related, and cancer-related mortality. Mayo Clin Proc. (2016) 91:1706–16. doi: 10.1016/j.mayocp.2016.08.003
15. Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the health, aging and body composition Study. J Am Geriatr Soc. (2004) 52:1098–104. doi: 10.1111/j.1532-5415.2004.52307.x
16. Sellami M, Gasmi M, Denham J, Hayes LD, Stratton D, Padulo J, et al. Effects of acute and chronic exercise on immunological parameters in the elderly aged: can physical activity counteract the effects of aging? Front Immunol. (2018) 9:2187. doi: 10.3389/fimmu.2018.02187
17. Hanna M, Dumas I, Orain M, Jacob S, Tetu B, Diorio C. Association between physical activity and the expression of mediators of inflammation in normal breast tissue among premenopausal and postmenopausal women. Cytokine. (2018) 102:151–60. doi: 10.1016/j.cyto.2017.08.007
18. Santos CP, Silva LF, Lopes MB, Martins MTS, Kraychete AC, Silva FA, et al. Associations of physical activity energy expenditure with nutritional-inflammatory markers in hemodialysis patients. Int J Artif Organs. (2017) 40:670–5. doi: 10.5301/ijao.5000632
19. Lee J, Kim HR, Jang TW, Lee DW, Lee YM, Kang MY. Occupational physical activity, not leisure-time physical activity, is associated with increased high-sensitivity C reactive protein levels. Occup Environ Med. (2021) 78:86–91. doi: 10.1136/oemed-2020-106753
20. Rothenbacher D, Hoffmeister A, Brenner H, Koenig W. Physical activity, coronary heart disease, and inflammatory response. Arch Intern Med. (2003) 163:1200–5. doi: 10.1001/archinte.163.10.1200
21. Boekholdt SM, Sandhu MS, Day NE, Luben R, Bingham SA, Peters RJ, et al. Physical activity, C-reactive protein levels and the risk of future coronary artery disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Eur J Cardiovasc Prev Rehabil. (2006) 13:970–6. doi: 10.1097/01.hjr.0000209811.97948.07
22. Andersen LB, Schnohr P, Schroll M, Hein HO. All-cause mortality associated with physical activity during leisure time, work, sports, and cycling to work. Arch Intern Med. (2000) 160:1621–8. doi: 10.1001/archinte.160.11.1621
23. Hu G, Jousilahti P, Antikainen R, Tuomilehto J. Occupational, commuting, and leisure-time physical activity in relation to cardiovascular mortality among finnish subjects with hypertension. Am J Hypertens. (2007) 20:1242–50. doi: 10.1016/j.amjhyper.2007.07.015
24. Hu G, Jousilahti P, Borodulin K, Barengo NC, Lakka TA, Nissinen A, et al. Occupational, commuting and leisure-time physical activity in relation to coronary heart disease among middle-aged Finnish men and women. Atherosclerosis. (2007) 194:490–7. doi: 10.1016/j.atherosclerosis.2006.08.051
25. Hu G, Sarti C, Jousilahti P, Silventoinen K, Barengo NC, Tuomilehto J. Leisure time, occupational, and commuting physical activity and the risk of stroke. Stroke. (2005) 36:1994–9. doi: 10.1161/01.STR.0000177868.89946.0c
26. Menotti A, Keys A, Blackburn H, Aravanis C, Dontas A, Fidanza F, et al. Twenty-year stroke mortality and prediction in twelve cohorts of the seven countries study. Int J Epidemiol. (1990) 19:309–15. doi: 10.1093/ije/19.2.309
27. Evenson KR, Rosamond WD, Cai J, Toole JF, Hutchinson RG, Shahar E, et al. Physical activity and ischemic stroke risk. the atherosclerosis risk in communities study. Stroke. (1999) 30:1333–9. doi: 10.1161/01.STR.30.7.1333
28. Hu G, Qiao Q, Silventoinen K, Eriksson JG, Jousilahti P, Lindstrom J, et al. Occupational, commuting, and leisure-time physical activity in relation to risk for Type 2 diabetes in middle-aged Finnish men and women. Diabetologia. (2003) 46:322–9. doi: 10.1007/s00125-003-1031-x
29. Rasmussen NF, Bech BH, Rubin KH, Andersen V. Associations between participation in, intensity of, and time spent on leisure time physical activity and risk of inflammatory bowel disease among older adults (PA-IBD): a prospective cohort study. BMC Public Health. (2021) 21:634. doi: 10.1186/s12889-021-10492-7
30. Sonnenberg A. Occupational distribution of inflammatory bowel disease among German employees. Gut. (1990) 31:1037–40. doi: 10.1136/gut.31.9.1037
31. Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis. (2005) 7:204–13. doi: 10.1111/j.1463-1318.2005.00747.x
32. Keimling M, Behrens G, Schmid D, Jochem C, Leitzmann MF. The association between physical activity and bladder cancer: systematic review and meta-analysis. Br J Cancer. (2014) 110:1862–70. doi: 10.1038/bjc.2014.77
33. Friedenreich CM, Thune I, Brinton LA, Albanes D. Epidemiologic issues related to the association between physical activity and breast cancer. Cancer. (1998) 83:600–10. doi: 10.1002/(sici)1097-0142(19980801)83:3+<600::aid-cncr2>3.3.co;2-0
34. Gammon MD, John EM, Britton JA. Recreational and occupational physical activities and risk of breast cancer. J Natl Cancer Inst. (1998) 90:100–17. doi: 10.1093/jnci/90.2.100
35. Ennour-Idrissi K, Tetu B, Maunsell E, Poirier B, Montoni A, Rochette PJ, et al. Association of telomere length with breast cancer prognostic factors. PLoS ONE. (2016) 11:e0161903. doi: 10.1371/journal.pone.0161903
36. Sakaue A, Adachi H, Enomoto M, Fukami A, Kumagai E, Nakamura S, et al. Association between physical activity, occupational sitting time and mortality in a general population: an 18-year prospective survey in Tanushimaru, Japan. Eur J Prev Cardiol. (2020) 27:758–66. doi: 10.1177/2047487318810020
37. Clays E, Lidegaard M, Bacquer DDe, Van Herck K, Backer GDe, Kittel F, et al. The combined relationship of occupational and leisure-time physical activity with all-cause mortality among men, accounting for physical fitness. Am J Epidemiol. (2014) 179:559–66. doi: 10.1093/aje/kwt294
38. Garcia JM, Duran AT, Schwartz JE, Booth 3rd JN, Hooker SP, Willey JZ, et al. Types of sedentary behavior and risk of cardiovascular events and mortality in blacks: the jackson heart study. J Am Heart Assoc. (2019) 8:e010406. doi: 10.1161/JAHA.118.010406
39. Schmid D, Leitzmann MF. Television viewing and time spent sedentary in relation to cancer risk: a meta-analysis. J Natl Cancer Inst. (2014) 106:dju098. doi: 10.1093/jnci/dju098
40. Bøggild H, Tüchsen F, Ørhede E. Occupation, employment status and chronic inflammatory bowel disease in Denmark. Int J Epidemiol. (1996) 25:630–7. doi: 10.1093/ije/25.3.630
41. Kendel Jovanovic G, Pavicic Zezelj S, Klobucar Majanovic S, Mrakovcic-Sutic I, Sutic I. Metabolic syndrome and its association with the Dietary Inflammatory Index (DII)(R) in a Croatian working population. J Hum Nutr Diet. (2020) 33:128–37. doi: 10.1111/jhn.12695
42. Holtermann A, Burr H, Hansen JV, Krause N, Søgaard K, Mortensen OS. Occupational physical activity and mortality among Danish workers. Int Arch Occup Environ Health. (2012) 85:305–10. doi: 10.1007/s00420-011-0668-x
43. Palatini P, Julius S. Elevated heart rate: a major risk factor for cardiovascular disease. Clin Exp Hypertens. (2004) 26:637–44. doi: 10.1081/CEH-200031959
44. Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. (2005) 45:1563–9. doi: 10.1016/j.jacc.2004.12.077
45. Holtermann A, Krause N, van der Beek AJ, Straker L. The physical activity paradox: six reasons why occupational physical activity (OPA) does not confer the cardiovascular health benefits that leisure time physical activity does. Br J Sports Med. (2018) 52:149–50. doi: 10.1136/bjsports-2017-097965
46. Shala R. 'I'm active enough in my job.' why is occupational physical activity not enough? Br J Sports Med. (2022) 56:897–8. doi: 10.1136/bjsports-2021-104957
47. Fransson E, Faire UDe, Ahlbom A, Reuterwall C, Hallqvist J, Alfredsson L. The risk of acute myocardial infarction: interactions of types of physical activity. Epidemiology. (2004) 15:573–82. doi: 10.1097/01.ede.0000134865.74261.fe
48. Allesoe K, Holtermann A, Aadahl M, Thomsen JF, Hundrup YA, Sogaard K. High occupational physical activity and risk of ischaemic heart disease in women: the interplay with physical activity during leisure time. Eur J Prev Cardiol. (2015) 22:1601–8. doi: 10.1177/2047487314554866
49. Hall C, Heck JE, Sandler DP, Ritz B, Chen H, Krause N. Occupational and leisure-time physical activity differentially predict 6-year incidence of stroke and transient ischemic attack in women. Scand J Work Environ Health. (2019) 45:267–79. doi: 10.5271/sjweh.3787
50. Sobti A, Cooper C, Inskip H, Searle S, Coggon D. Occupational physical activity and long-term risk of musculoskeletal symptoms: a national survey of post office pensioners. Am J Ind Med. (1997) 32:76–83. doi: 10.1002/(sici)1097-0274(199707)32:1<76::aid-ajim9>3.0.co;2-p
51. Brancaccio P, Lippi G, Maffulli N. Biochemical markers of muscular damage. Clin Chem Lab Med. (2010) 48:757–67. doi: 10.1515/CCLM.2010.179
52. Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. (2000) 529:237–42. doi: 10.1111/j.1469-7793.2000.00237.x
53. Beharriell TH, Mavor MP, Ramos W Jr, Mauger JF, Imbeault P, Graham RB. Beyond the mechanical lens: systemic inflammatory responses to repetitive lifting under varying loads and frequencies. Appl Ergon. (2020) 89:103199. doi: 10.1016/j.apergo.2020.103199
54. Petrovsky N, McNair P, Harrison LC. Diurnal rhythms of pro-inflammatory cytokines: regulation by plasma cortisol and therapeutic implications. Cytokine. (1998) 10:307–12. doi: 10.1006/cyto.1997.0289
55. Cerqueira E, Marinho DA, Neiva HP, Lourenco O. Inflammatory effects of high and moderate intensity exercise-a systematic review. Front Physiol. (2019) 10:1550. doi: 10.3389/fphys.2019.01550
56. Pearce M, Strain T, Wijndaele K, Sharp SJ, Mok A, Brage S. Is occupational physical activity associated with mortality in UK Biobank? Int J Behav Nutr Phys Act. (2021) 18:102. doi: 10.1186/s12966-021-01154-3
57. Main LC, Wolkow AP, Tait JL, Della Gatta P, Raines J, Snow R, et al. Firefighter's acute inflammatory response to wildfire suppression. J Occup Environ Med. (2020) 62:145–8. doi: 10.1097/JOM.0000000000001775
58. Puttonen S, Viitasalo K, Harma M. Effect of shiftwork on systemic markers of inflammation. Chronobiol Int. (2011) 28:528–35. doi: 10.3109/07420528.2011.580869
59. Tsai SS, Lai CH, Shih TS, Lin MH, Liou SH. High job strain is associated with inflammatory markers of disease in young long-haul bus drivers. J Occup Health Psychol. (2014) 19:336–47. doi: 10.1037/a0036600
60. Boscolo P, Gioacchino MDi, Reale M, Muraro R, Giampaolo LDi. Work stress and innate immune response. Int J Immunopathol Pharmacol. (2011) 24:51s–4.
61. Majka DS, Chang RW, Vu TH, Palmas W, Geffken DF, Ouyang P, et al. Physical activity and high-sensitivity C-reactive protein: the multi-ethnic study of atherosclerosis. Am J Prev Med. (2009) 36:56–62. doi: 10.1016/j.amepre.2008.09.031
62. Shephard RJ. Is there a 'recent occupational paradox' where highly active physically active workers die early? or are there failures in some study methods? Br J Sports Med. (2019) 53:1557–9. doi: 10.1136/bjsports-2018-100344
63. Prince SA, Elliott CG, Scott K, Visintini S, Reed JL. Device-measured physical activity, sedentary behaviour and cardiometabolic health and fitness across occupational groups: a systematic review and meta-analysis. Int J Behav Nutr Phys Act. (2019) 16:30. doi: 10.1186/s12966-019-0790-9
64. Steeves JA, Tudor-Locke C, Murphy RA, King GA, Fitzhugh EC, Harris TB. Classification of occupational activity categories using accelerometry: NHANES 2003-2004. Int J Behav Nutr Phys Act. (2015) 12:89. doi: 10.1186/s12966-015-0235-z
65. Tudor-Locke C, Ainsworth BE, Washington TL, Troiano R. Assigning metabolic equivalent values to the 2002 census occupational classification system. J Phys Act Health. (2011) 8:581–6. doi: 10.1123/jpah.8.4.581
66. Holme I, Helgeland A, Hjermann I, Leren P, Lund-Larsen PG. Physical activity at work and at leisure in relation to coronary risk factors and social class. a 4-year mortality follow-up. the oslo study. Acta Med Scand. (1981) 209:277–83. doi: 10.1111/j.0954-6820.1981.tb11591.x
67. Morris JN, Heady JA. Mortality in relation to the physical activity of work: a preliminary note on experience in middle age. Br J Ind Med. (1953) 10:245–54. doi: 10.1136/oem.10.4.245
68. Lunde LK, Skare O, Aass HCD, Mamen A, Einarsdottir E, Ulvestad B, et al. Physical activity initiated by employer induces improvements in a novel set of biomarkers of inflammation: an 8-week follow-up study. Eur J Appl Physiol. (2017) 117:521–32. doi: 10.1007/s00421-016-3533-5
69. Skogstad M, Lunde LK, Ulvestad B, Aass HCD, Clemm T, Mamen A, et al. The effect of a leisure time physical activity intervention delivered via a workplace: 15-month follow-up study. Int J Environ Res Public Health. (2018) 15:264. doi: 10.3390/ijerph15020264
Keywords: inflammation, proinflammatory markers, high activity work, sedentary work, occupational health
Citation: Jordakieva G, Hasenoehrl T, Steiner M, Jensen-Jarolim E and Crevenna R (2023) Occupational physical activity: the good, the bad, and the proinflammatory. Front. Med. 10:1253951. doi: 10.3389/fmed.2023.1253951
Received: 06 July 2023; Accepted: 11 September 2023;
Published: 06 October 2023.
Edited by:
Dragan Mijakoski, Institute of Occupational Health of RNM, North MacedoniaReviewed by:
Dragana Bislimovska, Institute of Occupational Health of RNM, North MacedoniaCopyright © 2023 Jordakieva, Hasenoehrl, Steiner, Jensen-Jarolim and Crevenna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Galateja Jordakieva, Z2FsYXRlamEuam9yZGFraWV2YUBtZWR1bml3aWVuLmFjLmF0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.