- 1Seoul National University College of Medicine, Seoul, Republic of Korea
- 2Department of Dermatology, Seoul National University College of Medicine, Seoul, Republic of Korea
- 3Department of Dermatology, Seoul National University Hospital, Seoul, Republic of Korea
Introduction: Lentigo maligna (LM) and lentigo maligna melanoma (LMM) are rare in Asian countries. The histopathological diagnosis of LM is often challenging, and misdiagnosis is common. Although histopathologic features of LM/LMM are known, statistical analysis of them were scarcely reported. In this study, we aimed to investigate the histopathological characteristics of LM/LMM in Korean patients and identify key histopathological clues distinguishing LM from benign lentigo.
Methods: We performed a retrospective study of the clinical and histopathological features of patients diagnosed with LM/LMM at our center between 2011 and 2022. We assessed the histopathological features in each case based on 16 pathological criteria according to previous literature. Pathologically confirmed cases of benign lentigo were analyzed for comparison.
Results: Twenty-one patients (10 with LM and 11 with LMM) were analyzed. Several statistically significant difference existed between the features of LM and benign lentigo (N = 10), including asymmetry of overall structure (p < 0.001), cytologic atypia (p < 0.001), predominant single-cell proliferation (p < 0.001), melanocytic nests (p = 0.033), melanocytes forming rows (p = 0.003), pagetoid spread of melanocytes (p < 0.001), and hair follicle invasion by atypical melanocytes (p < 0.001). Degree of solar elastosis was more severe in group “Age ≥ 60” (p = 0.015), and group “Diameter ≥ 20 mm” (p = 0.043). Presence of elongated rete ridges were less common in the older than 60 age group (p = 0.015) and group “Diameter ≥ 20 mm.” Invasion was associated with mitosis (p = 0.001, OR 49.285), multinucleated cells (p = 0.035, OR 17.769), and degree of lymphocyte infiltration (p = 0.004).
Conclusion: This study investigated the clinical and histopathologic characteristics of LM and LMM in Koreans. Although histopathological diagnosis is challenging, especially in the early stages of LM, our data showed essential histopathological changes in architectural, cytological, and dermal patterns. Considering the potential aggressiveness of LM/LMM, it is essential to recognize its histopathological features and provide timely management.
1 Introduction
Lentigo maligna/lentigo maligna melanoma (LM/LMM) is the most common facial melanoma subtype (1). There are significant racial differences in incidence of LM/LMM. According to one study reporting racial differences of epidemiology of melanoma subtypes, age-adjusted incidence of LMM in non-Hispanic white population was 1.87 (1.83–1.90) per 100,000 person-years, while that of Asians/pacific islanders was 0.06 (0.05–0.08) per 100,000 person-years (2).
To the best of our knowledge, there have been few reports on the histopathological features of LM/LMM in Asian patients. In this study, we investigated the clinical and histopathological characteristics of LM and LMM in Korean patients at our center. In addition, we explored the key histopathological clues for the differential diagnosis of LM/LMM in the early stages by comparing LM and benign lentigo.
2 Materials and methods
We retrospectively analyzed the clinical and histopathological features of patients diagnosed with LM/LMM at Seoul National University Hospital between 2011 and 2022. Patients with facial and scalp LM/LMM and available histopathological findings were included. The assessed demographic and clinical factors included sex, age at onset, disease duration, lesion location, lesion multiplicity, diameter, depth, presence or absence of metastasis, sentinel lymph node biopsy, clinical impression, presence or absence of symptoms, presence or absence of previous biopsy/laser therapy, previous history of skin cancer, underlying diseases, operation date, and last follow-up date.
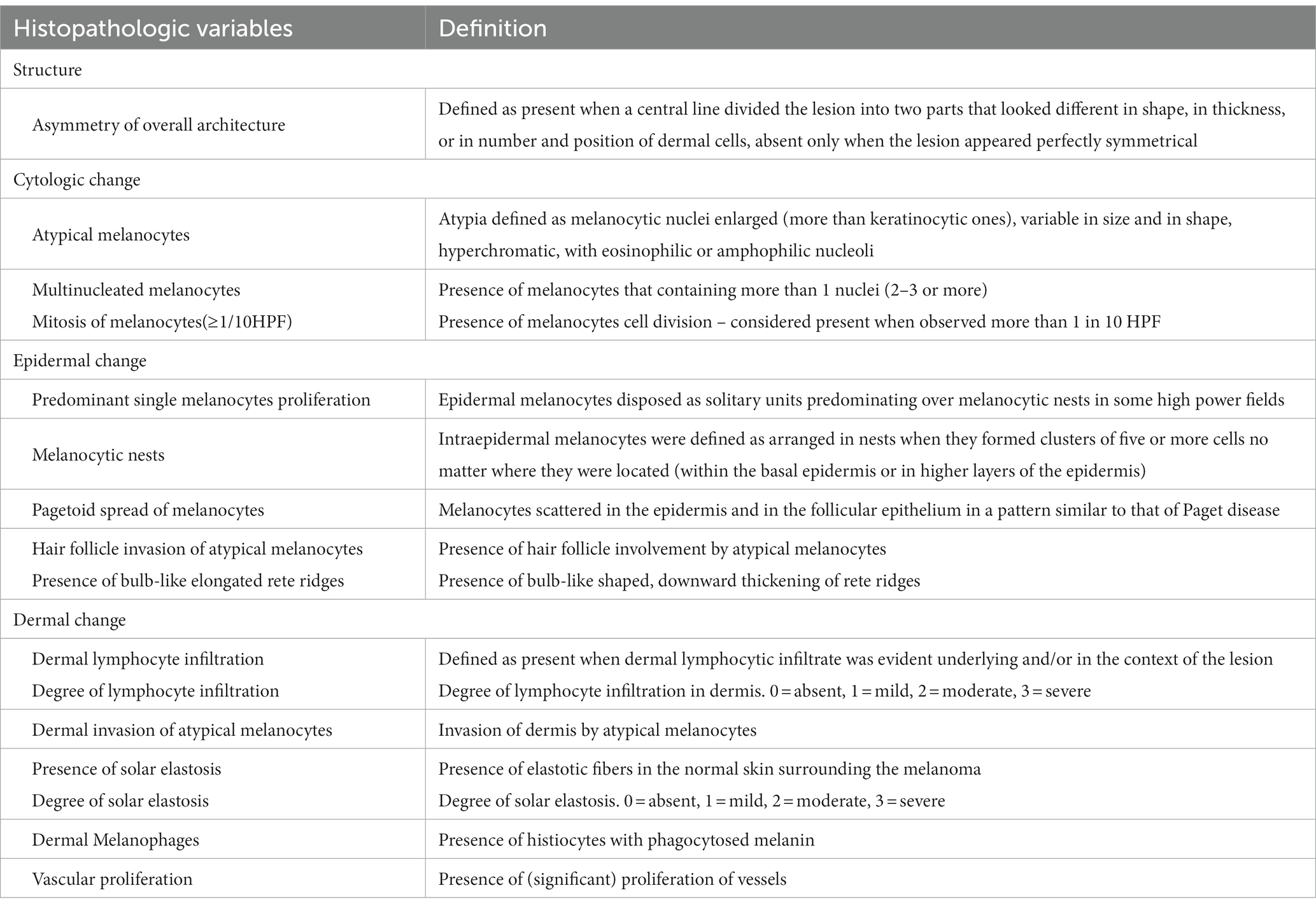
We assessed the histopathological features in each case based on 16 pathological criteria according to previous literature (Table 1) (3–5). They consist of one structural change (asymmetry of overall architecture), three cytologic changes (atypical melanocytes, multinucleated melanocytes, and mitosis), seven epidermal patterns (predominant single melanocyte proliferation, melanocytic nests, melanocyte-forming rows, pagetoid spread of melanocytes, hair follicle invasion of atypical melanocytes, sub-epidermal cleft, and the presence of elongated rete ridges), and five dermal changes (dermal lymphocyte infiltration, dermal invasion of atypical melanocytes, solar elastosis, dermal melanophages, and vascular proliferation). We graded the dermal invasion of atypical melanocytes and solar elastosis into three levels (grades 1–3). All the specimens were evaluated by two authors (CP and JHM). Disagreements were resolved by a consensus meeting involving other evaluators (DHK and KH) based on the above definitions in Table 1. Cases of histopathologically confirmed benign lentigo on the face acquired from our database were included as a control group to compare the histological features with those of LM. This study was approved by the Institutional Review Board of the Seoul National University Hospital.
Continuous variables are presented as means ± standard deviation, and categorical variables are presented as frequencies with percentages. Statistical analyzes were conducted using SPSS Statistics 26 software (IBM Corp., Armonk, NY, United States). Fisher’s exact test and the Mann–Whitney U test were used to determine statistically meaningful correlations between demographic and histopathological variables. Statistical significance was set at p < 0.05. Odds ratios were calculated using 2 × 2 tables and Haldane’s correction was applied for cells with zero (6).
3 Results
3.1 Demographics and clinical characteristics of patients with LM/LMM
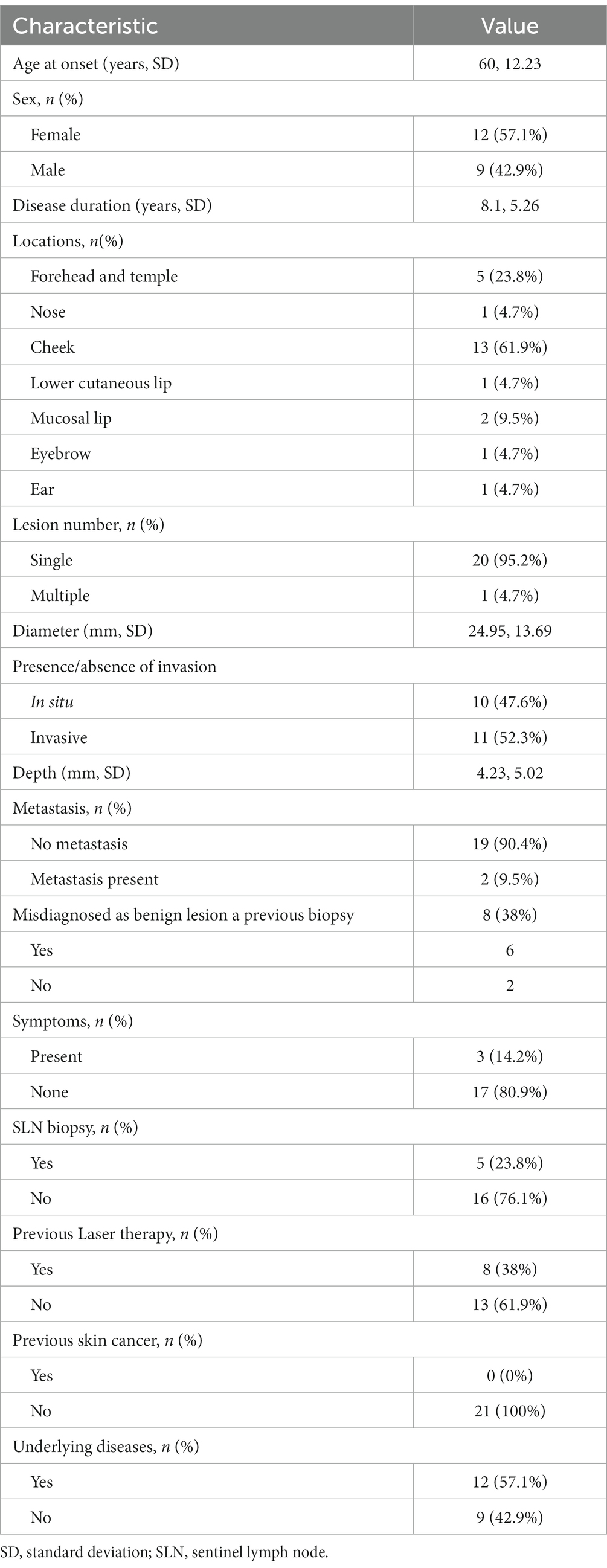
A total of 21 patients (12 women [57.1%]) with LM/LMM were included in this study (Table 2). Mean age at diagnosis was 68.1 ± 10.63 (range, 49–84). Mean duration of the disease was 8.1 ± 5.26 years (range, 1–20). The most frequent location of the lesions was the cheek in 13 patients (61.9%). Twenty (95.2%) patients had a single lesion, whereas one patient with xeroderma pigmentosum presented with multiple lesions. One patient (4.7%) experienced recurrence after surgery and 20 patients were diagnosed first.
Mean lesion diameter was 24.95 ± 13.69 mm (range, 5–60). Ten (47.6%) patients had in situ melanoma (LM), and 11 (52.4%) had invasive melanoma (LMM). Mean Breslow thickness of the invasive cases was 4.23 ± 5.02 mm (range, 0.3–16). The majority of patients were asymptomatic, while three (14.2%) patients had symptoms such as itching, bleeding, or pain. Notably, eight (38%) patients had a history of misdiagnosis as a benign lesion. Among them, six (75.0%) had undergone skin biopsy at local dermatology clinics but were diagnosed with benign lesions, including junctional nevi or lentigo. The patient had no history of skin cancer. Twenty (95.2%) patients were treated with surgical excision, and one patient (4.8%) was successfully treated with topical imiquimod. Among the patients who underwent surgical treatment, one (5.0%) experienced recurrence. Metastasis was found in two (9.5%) cases. Mean follow-up period was 33.88 ± 21.48 months (range, 2.13 ~ 71.86).
3.2 Histopathology features of LM/LMM
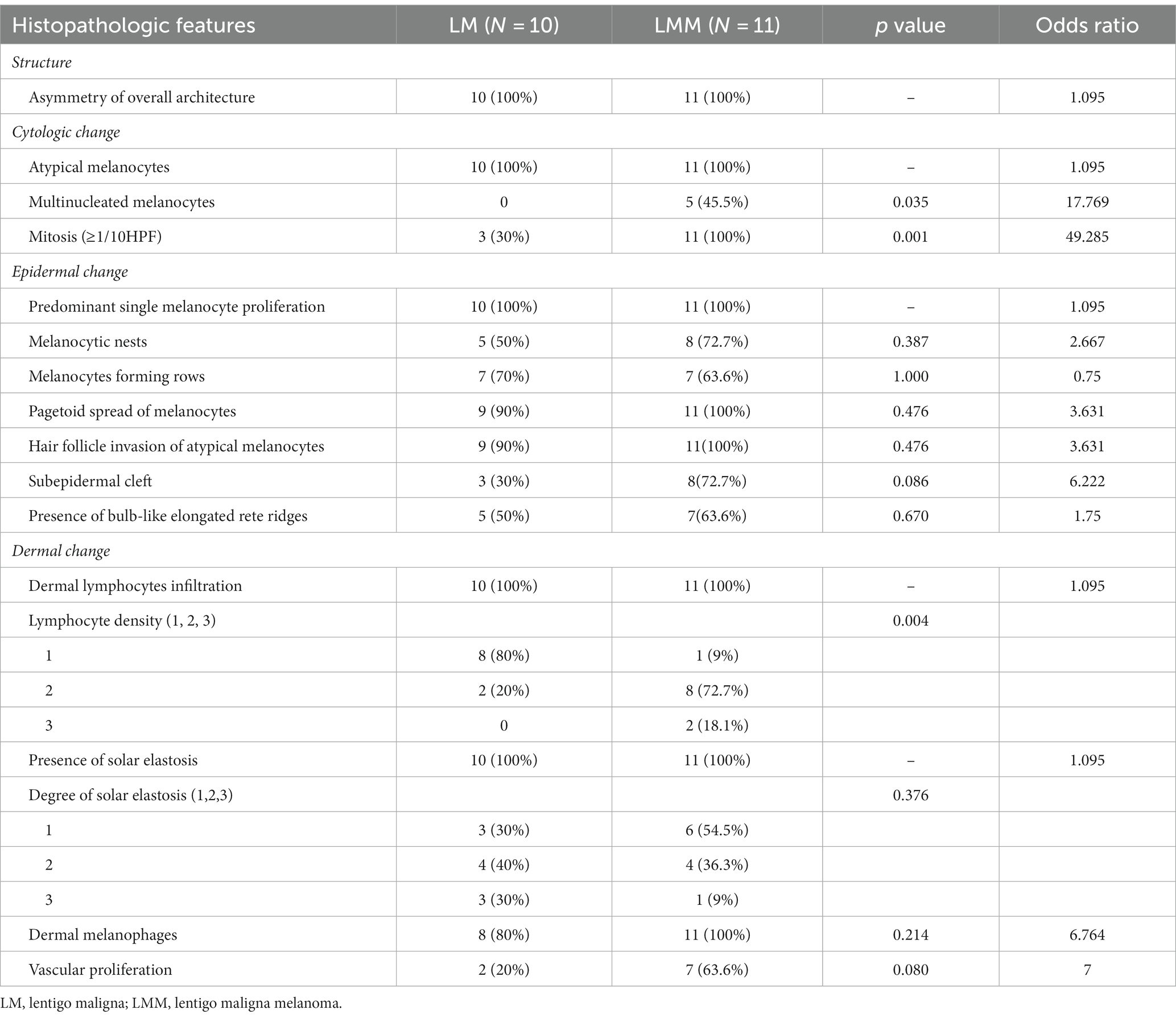
Asymmetry of the overall architecture was observed in all the cases (100%). Atypical melanocytes, multinucleated melanocytes, and mitosis were observed in 21 (100%), five (23.8%), and 14 (66.7%) cases, respectively. For epidermal changes, all specimens had a predominant single melanocyte proliferation pattern, while hair follicle invasion of atypical melanocytes, pagetoid spread of melanocytes, melanocyte-forming rows, melanocytic nests, presence of bulb-like elongated rete ridges, and subepidermal clefts were found in 20 (95.2%), 20 (95.2%), 14 (66.7%), 13 (61.9%), 11 (52.3%), and 11 cases (52.3%), respectively. Lymphocyte infiltration was observed in all the cases. When the density of lymphocyte infiltration was graded from 1 to 3 (1 = mild, 2 = moderate, and 3 = severe), grades 1, 2, and 3 were observed in nine (42.8%), ten (47.6%), and two (9.5%) cases, respectively. Dermal invasion of atypical melanocytes was observed in 11 specimens (52.3%). All specimens exhibited solar elastosis, which was graded from 1 to 3 (1 = mild, 2 = moderate, and 3 = severe). Among the 21 specimens, nine were grade 1, eight were grade 2, and four were grade 3. Nineteen (90.4%) patients had dermal melanophages and nine (42.8%) showed vascular proliferation (Table 3).
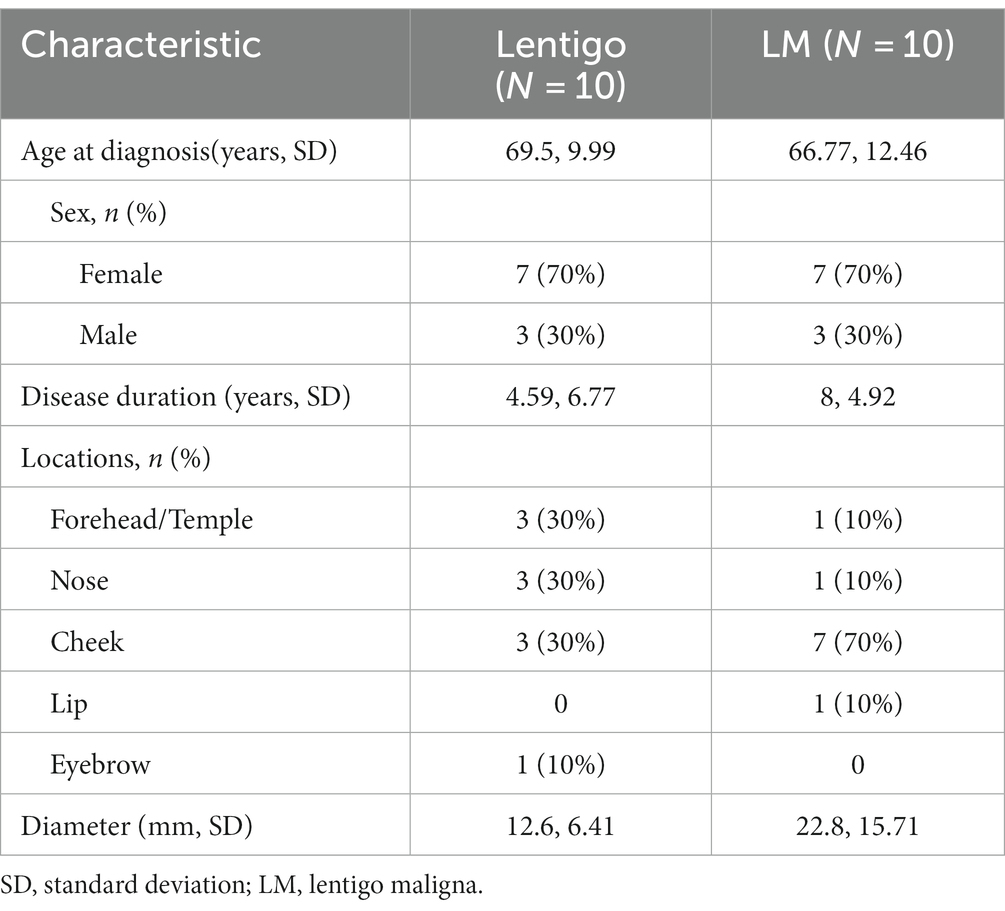
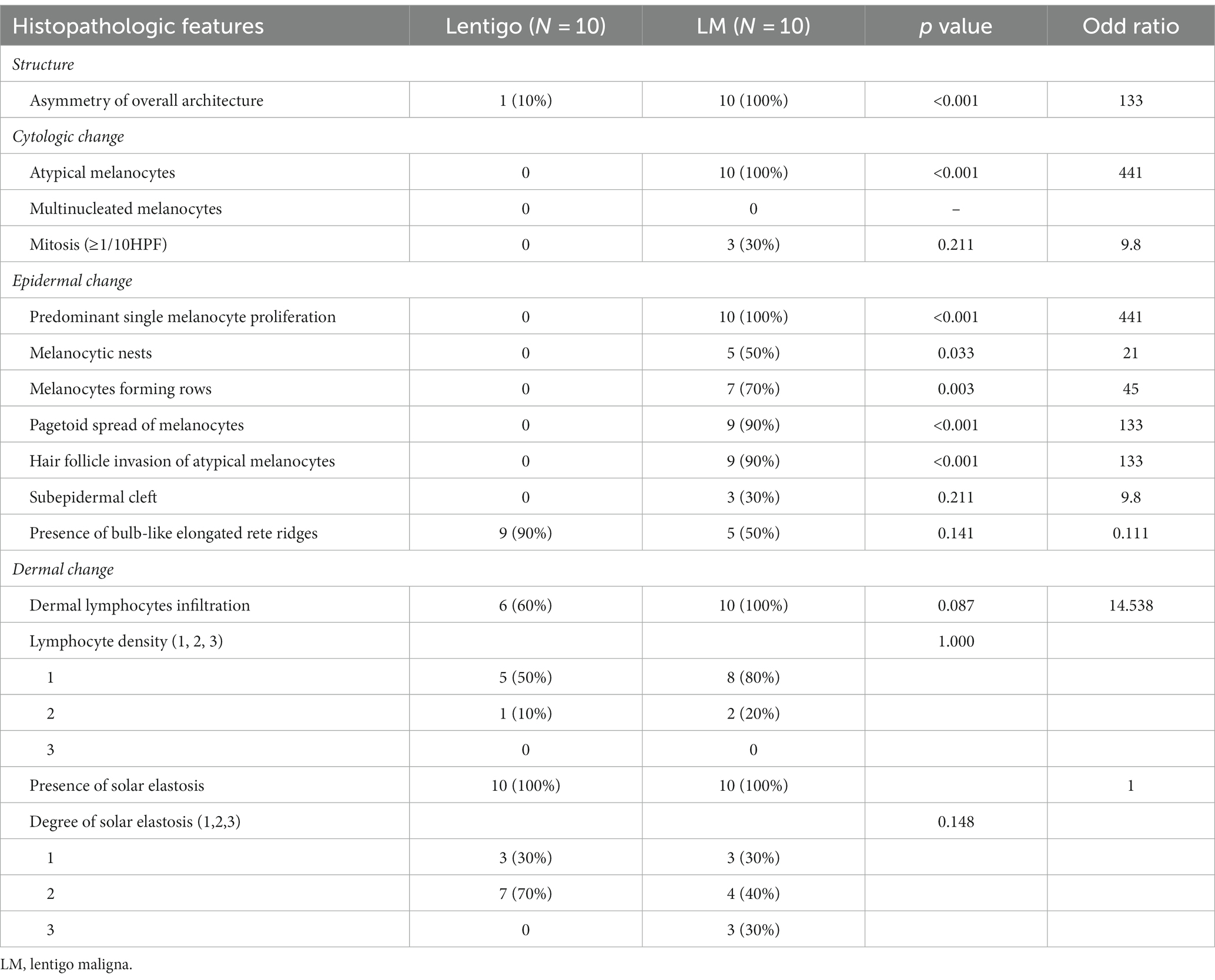
To identify key histopathologically distinguishing points in the early stages of LMM, we further compared the clinical and histopathological features of benign lentigo (N = 10) with those of LM (N = 10; Tables 4, 5). Several histopathological features were statistically relevant, including asymmetry of the overall structure (p < 0.001, odds ratio [OR] 133), cytologic atypia (p < 0.001, OR 441), predominant single-cell proliferation (p < 0.001, OR 441), melanocytic nests (p = 0.033, OR 21), melanocyte-forming rows (p = 0.003, OR 45), pagetoid spread of melanocytes (p < 0.001, OR 133), and hair follicle invasion by atypical melanocytes (p < 0.001, OR 133).
Additional analyzes were performed to evaluate the association between the histopathological findings and clinical features, including age, sex, tumor diameter, and presence of invasion. The degree of solar elastosis was more severe in patients aged >60 years (p = 0.015), and elongated rete ridges were more common in patients aged <60 years (p = 0.015). In large lesions (diameter > 20 mm), the degree of solar elastosis was more severe (p = 0.043), and elongated rete ridges were less common (p = 0.005). Mitosis (p = 0.001, OR 49.285) and multinucleated cells (p = 0.035, OR 17.769) were significantly more common in invasive cases than in in-situ melanoma. The degree of lymphocyte infiltration was higher in the invasive disease group than in the noninvasive disease group (p = 0.004).
4 Discussion
LM refers to melanoma in situ arising on chronically sun-damaged skin (7), and the term “LMM” is used to describe the invasive form of LM (8). LM has the potential to progress to LMM, an invasive tumor with aggressive behavior (9). Therefore, recognizing lesions at an early stage is crucial to minimize the risk of metastasis. The accurate diagnosis of LM/LMM is often challenging, particularly in countries where the incidence of LM/LMM is low. In this study, 38% of the patients were initially misdiagnosed with benign lesions, such as junctional nevi or lentigines, despite previous histopathological examination by skin biopsy. This suggests that it is difficult for pathologists to accurately diagnose LM in its early stages. The lack of experience among pathologists in diagnosing LM and unclear pathologic criteria may contribute to diagnostic delay.
Although several histopathological features of LM/LMM are known (10), essential features for differentiation from benign lentigo have rarely been explored. In this study, the comparison between LM and benign lentigo revealed several statistically significant features, including asymmetry of overall structure, cytologic atypia, predominant single-cell proliferation, melanocytic nests, melanocyte-forming rows, pagetoid spread of melanocytes, and hair follicle invasion by atypical melanocytes. The presence of bulb-like, elongated rete ridges is a characteristic feature of solar lentigo. Although it was observed in the majority of lentigo cases (90% [9/10]), 5 LM (50%) and 6 LMM (54.6%) cases had this pattern. This indicates that the presence of bulb-like elongated rete ridges does not exclude a diagnosis of LM or LMM.
Moreno et al. (3) reported a study of 96 patients with LM/LMM and analyzed the relationship between various histological features and the presence of invasive lesions. In their study, the presence of melanocyte rows (p = 0.02, OR 11.5), subepidermal cleft (p = 0.049, OR 2.8), melanocytic nests (p = 0.04, OR 3.0), and a lower degree of solar elastosis (p = 0.07, OR 0.4) were associated with invasive lesions. However, in our study, mitosis (p = 0.001, OR 49.285), multinucleated cells (p = 0.035, OR 17.769), and a severe degree of lymphocyte infiltration (p = 0.004) were associated with invasion. However, the presence of melanocyte rows (p = 1.000, OR 0.75), subepidermal cleft (p = 0.086, OR 6.222), melanocytic nests (p = 0.387, OR 2.667), and the degree of solar elastosis were not significantly associated with invasion. This difference between the two studies may have originated from different sample sizes or ethnicities of the patient groups. This suggests the necessity for further studies regarding the differences in LM/LMM between Western and Asian patients.
The presentation of LM or LMM has several differences between Asian and Western patients. Invasive lesions were more common in Korean patients. In addition, they had larger lesions compared to Western patients. In a study that analyzed patients in the United States with histologically confirmed LM (Navarrete-Dechent, Cristian et al.) (11), the mean overall LM clinical diameter was 11.4 mm (SD, 8.3; range, 2–56 mm). In our study, it was 24.95 mm (standard deviation [SD], 13.69; range: 5–60 mm). The ratio of in situ lesions (LM) to invasive lesions (LMM) in the Western study was 2.70, while that in our study was 0.91. Our results were consistent with a previous study of clinical and histologic features of 19 Korean patients (12), in which the majority of cases were invasive, as the ratio of LM/LMM was 0.35 (5 in situ, 14 invasive). Compared to that study (12), our data showed a shorter mean disease duration, suggesting some improvement in early diagnosis of LM/LMM. Melanoma overdiagnosis has become a significant global concern, including in the United States (13–15). Data indicate that the incidence of melanoma in situ is currently 50 times higher than in 1975 (25 vs. 0.5 per 100,000 population), and the incidence of invasive melanoma has also increased from 7.9 to 25.4 per 100,000 population over the same period (13). However, in South Korea, the incidence of melanoma did not show an exponential increase, and the number of invasive melanoma cases appears to be higher than in situ cases. According to data from the Korea Central Cancer Registry, the age-standardized incidence rate of cutaneous melanoma has only mildly increased from 0.51 in 1999–2002 to 0.67 in 2011–2014 among men (average annual percentage change [AAPC], 3.0 [95% CI, 0.8 to 5.3]), and from 0.43 in 1999–2002 to 0.60 in 2011–2014 among women (AAPC, 3.5 [95% CI, 2.4 to 4.6]) (16). Considering previous reports and our data, we believe that the diagnosis of LM/LMM is underreported in South Korea. Given the aggressiveness of melanoma and the management challenges, along with the scarring tendency, primarily when it manifests on the face, that often follows surgical treatment, we believe early diagnosis remains an essential component for effective treatment.
The limitations of our study include its retrospective design and small sample size. However, considering the rarity of LM/LMM in Asians, our study analyzed the clinical and histopathological features of the largest number of Korean patients with LM/LMM. As our patient group consisted only of East Asian patients, further studies are necessary to determine whether there are differences between Western and Asian patients with LM and LM/LMM.
In summary, this study analyzed the clinical and histopathologic characteristics of LM in Korean patients. Compared to Western data, the lesion size was larger, and the ratio of the in situ stage (LM) to the invasive stage (LMM) was lower in our study. Although histopathological diagnosis is challenging, especially in the early stages of LM, our data showed essential histopathological changes in architectural, cytological, and dermal patterns. Considering the aggressiveness of LM/LMM, it is important to recognize its histopathological features and provide timely management.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the Seoul National University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because retrospective study design, minimal risks to subjects, anonymous data.
Author contributions
CP, DK, and J-HM conceived and designed the study. CP performed the statistical analyzes. CP and J-HM wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We wish to thank all the patients included in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pralong, P, Bathelier, E, Dalle, S, Poulalhon, N, Debarbieux, S, and Thomas, L. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. (2012) 167:280–7. doi: 10.1111/j.1365-2133.2012.10932.x
2. Wang, Y, Zhao, Y, and Ma, S. Racial differences in six major subtypes of melanoma: descriptive epidemiology. BMC Cancer. (2016) 16:691. doi: 10.1186/s12885-016-2747-6
3. Moreno, A, Manrique-Silva, E, Virós, A, Requena, C, Sanmartín, O, Traves, V, et al. Histologic features associated with an invasive component in lentigo maligna lesions. JAMA Dermatol. (2019) 155:782–8. doi: 10.1001/jamadermatol.2019.0467
4. Gonzalez, ML, Young, ED, Bush, J, McKenzie, K, Hunt, E, Tonkovic-Capin, V, et al. Histopathologic features of melanoma in difficult-to-diagnose lesions: a case-control study. J Am Acad Dermatol. (2017) 77:543–548.e1. doi: 10.1016/j.jaad.2017.03.017
5. Urso, C, Rongioletti, F, Innocenzi, D, Batolo, D, Chimenti, S, Fanti, PL, et al. Histological features used in the diagnosis of melanoma are frequently found in benign melanocytic naevi. J Clin Pathol. (2005) 58:409–12. doi: 10.1136/jcp.2004.020933
6. Valenzuela, C . 2 solutions for estimating odds ratios with zeros. Rev Med Chil. (1993) 121:1441–4.
7. Tiodorovic-Zivkovic, D, Argenziano, G, Lallas, A, Thomas, L, Ignjatovic, A, Rabinovitz, H, et al. Age, gender, and topography influence the clinical and dermoscopic appearance of lentigo maligna. J Am Acad Dermatol. (2015) 72:801–8. doi: 10.1016/j.jaad.2015.01.030
8. Martínez-Leboráns, L, Garcías-Ladaria, J, Oliver-Martínez, V, and Alegre de Miquel, V. Extrafacial lentigo maligna: a report on 14 cases and a review of the literature. Actas Dermosifiliogr. (2016) 107:e57–63. doi: 10.1016/j.ad.2015.10.018
9. Iznardo, H, Garcia-Melendo, C, and Yélamos, O. Lentigo maligna: Clinical presentation and appropriate management. Clin Cosmet Investig Dermatol. (2020) 13:837–55. doi: 10.2147/CCID.S224738
10. Juhász, ML, and Marmur, ES. Reviewing challenges in the diagnosis and treatment of lentigo maligna and lentigo-maligna melanoma. Rare Cancers Ther. (2015) 3:133–45. doi: 10.1007/s40487-015-0012-9
11. Navarrete-Dechent, C, Aleissa, S, Connolly, K, Hibler, BP, Dusza, SW, Rossi, AM, et al. Clinical size is a poor predictor of invasion in melanoma of the lentigo maligna type. J Am Acad Dermatol. (2021) 84:1295–301. (in English). doi: 10.1016/j.jaad.2020.10.023
12. Hong, WJ, Jang, HS, Lee, SH, Lee, SE, Chung, KY, and Roh, MR. Retrospective review of 19 patients with Lentigo Maligna melanoma. Korean J Dermatol. (2016) 54:769–75. doi: 10.1159/000499689
13. Welch, HG, Mazer, BL, and Adamson, AS. The rapid rise in cutaneous melanoma diagnoses. N Engl J Med. (2021) 384:72–9. doi: 10.1056/NEJMsb2019760
14. Bjørch, MF, Gram, EG, and Brodersen, JB. Overdiagnosis in malignant melanoma: a scoping review. BMJ Evid Based Med. (2023) 4:bmjebm-2023-112341. doi: 10.1136/bmjebm-2023-112341
15. Kittler, H . How to combat over, diagnosis of melanoma. Dermatol Pract Concept. (2023) 13:e2023248. doi: 10.5826/dpc.1304a248
Keywords: Hutchinson’s melanoma, freckle, lentigo maligna, lentigo maligna melanoma, melanoma, pathology, pigmented skin lesions
Citation: Park C, Kim DH, Hur K and Mun J-H (2024) Clinical and histopathological features of lentigo maligna and lentigo maligna melanoma: a retrospective analysis in Korea. Front. Med. 10:1249796. doi: 10.3389/fmed.2023.1249796
Edited by:
Bahar Dasgeb, The State University of New Jersey, United StatesReviewed by:
Montserrat Fernández Guarino, Ramón y Cajal University Hospital, SpainAda Girnita, Karolinska Institutet (KI), Sweden
Copyright © 2024 Park, Kim, Hur and Mun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Je-Ho Mun, amVob211bkBnbWFpbC5jb20=
 Chanyong Park1
Chanyong Park1 Je-Ho Mun
Je-Ho Mun