- 1Laboratory of Histology-Embryology, Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 2Laboratory of Pathology, George Papanikolaou Hospital, Thessaloniki, Greece
- 3Pulmonary Department, Medical School, Aristotle University of Thessaloniki, George Papanikolaou Hospital, Thessaloniki, Greece
Introduction
Underlying pathologies of asthma include inflammation and airway remodeling with structural changes including increased smooth muscle mass, thickening of the airway wall, mucus hypersecretion, angiogenesis and alterations in the extracellular matrix (1). The structural changes affect both large and small airways and can lead to airflow imitation, impaired clearance of inflammatory cells and mucus, and altered responsiveness of the airways to triggers, all of which contribute to the persistence of inflammation (2). A feedback cycle of cytokines and growth factors constantly promotes inflammation and remodeling in the respiratory mucosa (3).
In addition, airway remodeling begins early in the age of 2–4 years old, before clinical diagnosis of asthma and it has been related to all stages of asthma severity (4). According to most animal models, airway remodeling and inflammation are two processes that might occur in parallel and in particular from 1 year to school age whereas from school age and later life, it has been reported that remodeling is stable and inflammation continues (4). However, there is no answer to whether airway remodeling is increasing overtime. What we know is that inflammation and remodeling persist even in those whose symptoms have been resolved.
Remodeling and inflammation relationship
Little data exist and there is no consensus about the temporal and even causative relationship between airway remodeling and inflammation (5). According to the conventional hypothesis, repeated cycles of acute chronic inflammation lead to airway remodeling, thus, targeting the inflammation is a widely accepted, an evidence-based strategy and an effective approach in the treatment of asthma. It is important to note that while targeting airway inflammation is crucial in asthma management, asthma is a complex and heterogeneous disease. Besides, what if airway remodeling acts as a protective response to limit bronchoconstriction and systemic absorption of inflammatory mediators from the airway lumen? Therefore, a comprehensive approach that considers all aspects of asthma pathophysiology is necessary for effective asthma management.
Furthermore, an alternative challenging point of view of this conventional hypothesis is that inflammation and remodeling might be triggered by the same underlying problem but progress independently (5). Thus, if we hypothesize that chronic inflammation does not lead to airway remodeling, and they act independently, the origin of airway remodeling is not the issue and the need for novel therapies for directing airway remodeling is at need.
As the most prominent feature of airway remodeling is airway muscle mass, bronchial thermoplasty has the role to target it as an irreversible surgical treatment that treats the large airways. A follow-up of three randomized controlled trials has concluded the safety and effectiveness of bronchial thermoplasty after 10 years in persistent asthma (6). However, until more questions are answered, this non-pharmaceutical procedure might be having the greatest application to Th-2 low asthmatics.
Discussion
Until further alternatives exist to directly target airway remodeling, the question remains: can airway remodeling be reversed? A way to reverse airway remodeling is speculated to occur by targeting airway inflammation. Regarding this research hypothesis, inhaled corticosteroids and other inflammatory treatments have inconsistent results based mostly in in vivo models which are not always reliable since most remodeling characteristics are spontaneously reversed (1). Furthermore, limited studies exist on bronchial biopsies regarding the assessment of human airway remodeling after biologic agents' treatment in asthmatic patients. Biologics such as mepolizumab (anti-IL-5), benralizumab (anti-IL-5R) and omalizumab (anti-igE) may have the potential to promote disease modification (6). Interestingly, biologics have shown some effects on the reduction of the characteristics of airway remodeling (7). In specific, the first study to evaluate the effect of a biologic agent on remodeling was by Flood-Page et al. (8), studying the effect of anti-IL-5 treatment in 24 atopic asthmatics after 3 mepolizumab infusions. They concluded that the expression of extracellular matrix proteins was significantly reduced, proposing a mechanism of action due to the reduction of eosinophils producing TGF-β1. In another study, a computational model predicted a significant reduction of airway smooth muscle mass after benralizumab treatment (9). More recently, in a study on 13 severe allergic asthmatics after omalizumab treatment led to modest reductions up to 5% decrease in the percent wall area (10) whereas tezepelumab (anti- thymic stromal lymphopoietin) treatment had no significant effect on basic membrane thickness and epithelial integrity in a double-blind, randomized, placebo-controlled trial (11). Recently, in the European Respiratory Society International Congress 2023, we presented preliminary data of MESILICO study (NCT04612556) showing that one year of mepolizumab treatment resulted in the improvement of airway remodeling in patients with late onset severe eosinophilic asthma and fixed obstruction (12). In specific, in our study, anti-IL-5 treatment reduced basement membrane thickness, smooth muscle area, epithelial damage and tissue eosinophil numbers (12).
Up until now, which features of airway remodeling might be related to certain phenotypes or endotypes is not known and further research is at need (13). Upon this concept, monitoring airway remodeling during clinical trials is crucial for severe clinical phenotypes such as severe asthmatics with fixed airflow obstruction, asthmatics with severe eosinophilia or late onset asthmatics, speculated to be related to airway remodeling driven by chronic inflammation (14, 15). Indeed, “fixed” airflow obstruction, unresponsive to current therapies, even with high-dose systemic glucocorticoids, can be distinguished from a “reversible” one as demonstrated by lung function normalization by the introduction of biological therapies (2).
Thus, targeting airway inflammation appears to impact airway remodeling through the modification effects of biologic agents that target markers of inflammation. Evidence indicates that in addition to targeting eosinophils, IL-5 and anti-IL-5 biologics may have a direct role on airway epithelial cells (16). Toward these directions, registered clinical trials investigate the impact of biological agents on airway remodeling to shed more light. Busse et al. (17) suggest that studies investigating disease-modifying potential of biologics should consider appropriate end-point selection such as change in airway structural abnormalities, appropriate length of trial, age of study population and comorbidities in the patient population. Clinical trials that are currently evaluating the reversing effect of biologics on airway remodeling include mepolizumab (NCT04612556, NCT03797404, NCT05708300), benralizumab (NCT04365205, NCT03953300) and tezepelumab (NCT05651841).1
Overall, is targeting inflammation or remodeling the right way to treat asthma (Figure 1)? Currently, there are not enough data to show us the right way. Until we know more, biologics seem to be the answer in the treatment of severe asthma regarding both inflammation and remodeling. Although limited data exist on the proven effects of biological therapies on airway remodeling in clinical trials, beneficial effects have been reported. Future clinical trials will shed more light by investigating not only the drug effects on inflammation and remodeling but also the evolution of these two processes on a long-term basis.
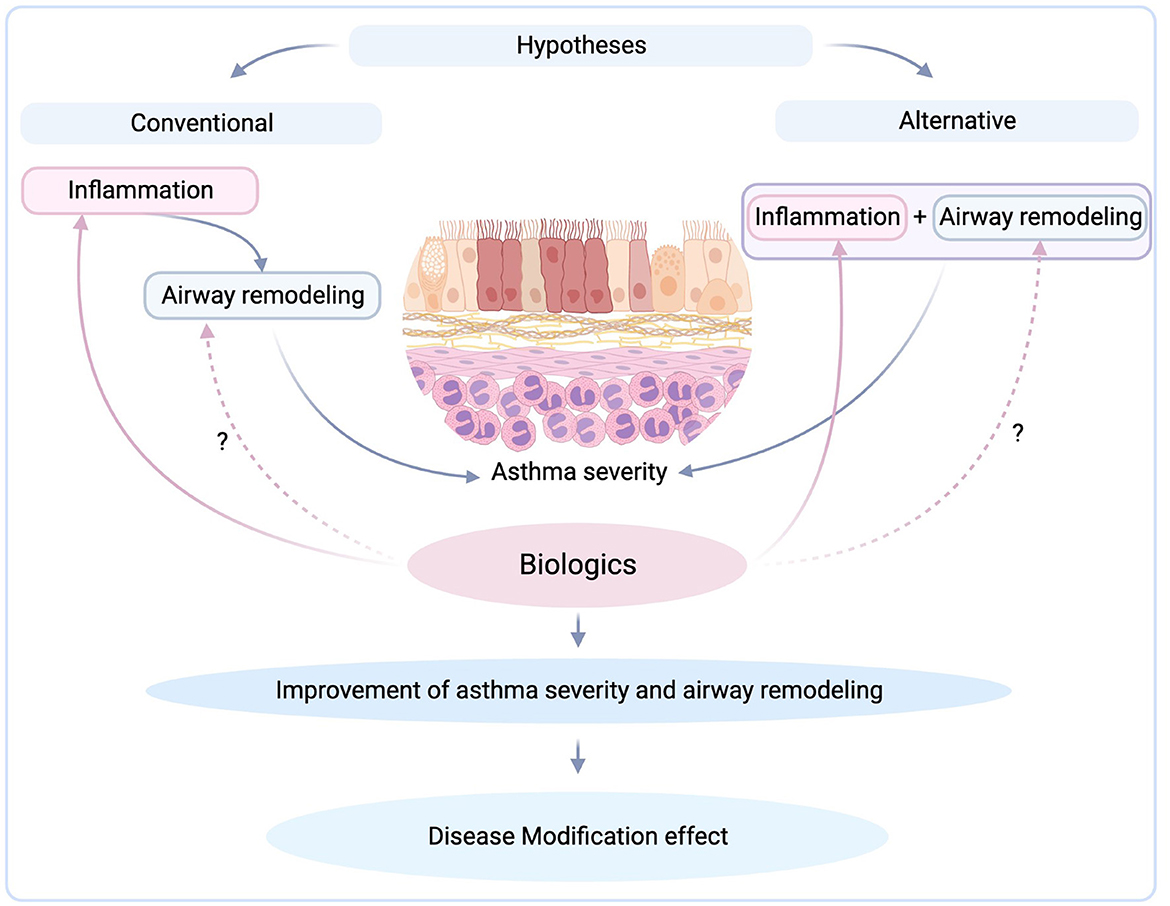
Figure 1. Targeting inflammation by biologics and their possible modification effect (created with BioRender.com).
Author contributions
KD and KP: conceptualization and writing and editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnote
1. ^Available online at: https://clinicaltrials.gov/search?cond=Severe%20Asthma&term=Airway%20Remodeling&aggFilters=status:not%20rec.
References
1. Hsieh A, Assadinia N, Hackett TL. Airway remodeling heterogeneity in asthma and its relationship to disease outcomes. Front Physiol. (2023) 14:1113100. doi: 10.3389/fphys.2023.1113100
2. Varricchi G, Ferri S, Pepys J, Poto R, Spadaro G, Nappi E, et al. Biologics and airway remodeling in severe asthma. Allergy. (2022) 77:3538–52. doi: 10.1111/all.15473
3. Osei ET, Booth S, Hackett TL. What have in vitro co-culture models taught us about the contribution of epithelial-mesenchymal interactions to airway inflammation and remodeling in asthma? Cells. (2020) 9:1694. doi: 10.3390/cells9071694
4. Castro-Rodriguez JA, Saglani S, Rodriguez-Martinez CE, Oyarzun MA, Fleming L, Bush A. The relationship between inflammation and remodeling in childhood asthma: a systematic review. Pediatr Pulmonol. (2018) 53:824–35. doi: 10.1002/ppul.23968
5. Wang KCW, Donovan GM, James AL, Noble PB. Asthma: Pharmacological degradation of the airway smooth muscle layer. Int J Biochem Cell Biol. (2020) 126:105818. doi: 10.1016/j.biocel.2020.105818
6. Chaudhuri R, Rubin A, Sumino K, Lapa ESJR, Niven R, Siddiqui S, et al. Safety and effectiveness of bronchial thermoplasty after 10 years in patients with persistent asthma (BT10+): a follow-up of three randomised controlled trials. Lancet Respir Med. (2021) 9:457–66. doi: 10.1016/S2213-2600(20)30408-2
7. Kardas G, Kuna P, Panek M. Biological therapies of severe asthma and their possible effects on airway remodeling. Front Immunol. (2020) 11:1134. doi: 10.3389/fimmu.2020.01134
8. Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. (2003) 112:1029–36. doi: 10.1172/JCI17974
9. Chachi L, Diver S, Kaul H, Rebelatto MC, Boutrin A, Nisa P, et al. Computational modelling prediction and clinical validation of impact of benralizumab on airway smooth muscle mass in asthma. Eur Respir J. (2019) 54:5. doi: 10.1183/13993003.00930-2019
10. Zastrzezynska W, Przybyszowski M, Bazan-Socha S, Gawlewicz-Mroczka A, Sadowski P, Okon K, et al. Omalizumab may decrease the thickness of the reticular basement membrane and fibronectin deposit in the bronchial mucosa of severe allergic asthmatics. J Asthma. (2020) 57:468–77. doi: 10.1080/02770903.2019.1585872
11. Diver S, Khalfaoui L, Emson C, Wenzel SE, Menzies-Gow A, Wechsler ME, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. (2021) 9:1299–312. doi: 10.1016/S2213-2600(21)00226-5
12. Domvri K, Tsiouprou I, Bakakos P, Rovina N, Stiropoulos P, Voulgaris A. Effect of Mepolizumab on airways remodeling in patients with late-onset severe eosinophilic asthma and fixed obstruction (preliminary data of the MESILICO study). Eur Res J. (2023) 62:OA3152. doi: 10.1183/13993003.congress-2023.OA3152
13. Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res. (2017) 367:551–69. doi: 10.1007/s00441-016-2566-8
14. Bakakos A, Vogli S, Dimakou K, Hillas G. Asthma with fixed airflow obstruction: from fixed to personalized approach. J Pers Med. (2022) 12:333. doi: 10.3390/jpm12030333
15. Chung KF, Dixey P, Abubakar-Waziri H, Bhavsar P, Patel PH, Guo S, et al. Characteristics, phenotypes, mechanisms and management of severe asthma. Chin Med J. (2022) 135:1141–55. doi: 10.1097/CM9.0000000000001990
16. Barretto KT, Brockman-Schneider RA, Kuipers I, Basnet S, Bochkov YA, Altman MC, et al. Human airway epithelial cells express a functional IL-5 receptor. Allergy. (2020) 75:2127–30. doi: 10.1111/all.14297
Keywords: asthma, inflammation, airway remodeling, biologics, modification effect
Citation: Domvri K and Porpodis K (2023) Targeting inflammation or remodeling in asthma? Is there a right way? Front. Med. 10:1241920. doi: 10.3389/fmed.2023.1241920
Received: 17 June 2023; Accepted: 06 November 2023;
Published: 23 November 2023.
Edited by:
Dawei Yang, Fudan University, ChinaReviewed by:
Konstantinos Bartziokas, Independent Researcher, Trikala, GreeceCopyright © 2023 Domvri and Porpodis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kalliopi Domvri, a2RvbXZyaWRAYXV0aC5ncg==
 Kalliopi Domvri
Kalliopi Domvri Konstantinos Porpodis
Konstantinos Porpodis