
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 17 July 2023
Sec. Intensive Care Medicine and Anesthesiology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1231570
This article is part of the Research Topic Pediatric Anesthesia and Surgery: Prophylaxis, Managements, and Rehabilitation of Short-term and Long-term Complications of CNS during Perioperative Period View all 8 articles
Objective: The aim of this study was to systematically review the efficacy and safety of parecoxib and flurbiprofen axetil for perioperative analgesia in children through Bayesian network meta-analysis.
Methods: We systematically searched PubMed, Embase, Cochrane Library, Web of Science, Sinomed, CNKI, VIP, and Wanfang Data databases on 18 July 2022 to obtain randomized controlled trials comparing perioperative parecoxib or flurbiprofen with placebo or standard treatment for pediatric analgesia. The outcomes were the postoperative pain score and the incidence of adverse events. The Gemtc package of R-4.0.3 and Stata 17.0 were used for Bayesian network meta-analysis.
Results: We retrieved 942 articles and 49 randomized controlled trials involving 3,657 patients who met the inclusion criteria. Compared with children who received placebo treatment, those who received flurbiprofen axetil had lower pain sores at each time point within 24 h postoperatively, and those who received parecoxib had lower pain sores at each time point within 12 h postoperatively. Compared with children who received tramadol treatment, both the children who received flurbiprofen axetil or parecoxib had lower pain scores at 8 h postoperatively. The ranking results demonstrated that flurbiprofen axetil had significant superiority in reducing pain scores at 2, 4, and 12 h postoperatively, and parecoxib had significant superiority in reducing pain scores at 0, 0.5, 1, 6, 8, and 24 h postoperatively. In terms of safety, compared with children who received placebo, those who received flurbiprofen axetil or parecoxib had a lower incidence of total adverse events and postoperative agitation. Compared with tramadol, flurbiprofen axetil and parecoxib both significantly reduced the incidence of total adverse events and postoperative nausea and vomiting. Compared with flurbiprofen axetil or fentanyl, parecoxib significantly reduced the incidence of postoperative nausea and vomiting. The ranking results showed that parecoxib was advantageous in decreasing the incidence of total adverse events and postoperative nausea and vomiting.
Conclusion: Flurbiprofen axetil was most effective at reducing pain scores at 2, 4, and 12 h postoperatively. Parecoxib had an advantage in terms of reducing pain scores at 0, 0.5, 1, 6, 8, and 24 h postoperatively, as well as the incidence of total adverse events and postoperative nausea and vomiting.
Systematic trial registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=348886, PROSPERO (CRD42022348886).
Nearly half of the children still report moderate to severe pain during postoperative hospitalization (1). The results of a survey conducted in China showed that 42 out of 66 hospitals (63.64%) implemented pain management in children, but only eight (12.12%) achieved full coverage of pain assessment for children, including all outpatients and hospitalized children (2). Pain in early childhood that is inadequately treated, unrecognized, or poorly managed can persist into adulthood and lead to severe and persistent negative consequences (3). On the one hand, it can turn into chronic postoperative pain, which can cause functional impairment, disability, or depression (4). On the other hand, it may affect children's future healthcare experience, deepen unpleasant memories and psychological burdens, and generate anxiety and fear (5). Therefore, perioperative analgesia in children is essential.
There are limited options for analgesics for children. Ethical constraints in pediatric clinical trials have led to inadequate clinical evidence for many analgesics in children compared with adults, and the labels of most analgesics clearly state that they are not recommended for children (6). Opioids are still the cornerstone of multimodal analgesic regimens for children with moderate to severe acute pain, but their adverse events have been a frequent problem (7). The most common opioid complications, such as nausea, dizziness, and vomiting, occurred at the rates of 18.9, 18.4, and 13.4%, respectively (8). The incidence of pruritus caused by intravenous morphine injection ranges 2–10% (9). Therefore, a multimodal analgesic regimen based on non-steroidal anti-inflammatory drugs is recommended for effective perioperative pain control and the reduction of opioid consumption. Non-steroidal anti-inflammatory drugs are recommended as part of multimodal analgesia in the perioperative period in children by consensus and guidelines in several countries (10–12).
Flurbiprofen axetil is an injectable, non-selective cyclooxygenase (COX) inhibitor. Owing to the novel drug carrier system of lipid microspheres, flurbiprofen axetil can be targeted to inflammatory sites and surgical incisions and control the release of the encapsulated drug for a longer-lasting effect (13). Flurbiprofen axetil also readily penetrates into the cerebrospinal fluid, where it can reach concentrations of up to seven times higher than unconjugated plasma concentrations, contributing to the analgesic effect in the central nervous system (14). Parecoxib is the first injectable selective COX-2 inhibitor that is rapidly converted to its active metabolite, valdecoxib, after hepatic enzymatic hydrolysis in vivo (15). Parecoxib has a highly selective inhibitory effect on COX-2 and reduces peripheral and central sensitization by blocking the arachidonic acid cascade and inhibiting prostaglandin synthesis, thus providing an analgesic effect. It has no inhibitory effect on COX-1 at the recommended dose and has little impact on the normal physiological function of the gastric mucosa and platelets (16).
Clinical evidence for the use of parecoxib and flurbiprofen ester for perioperative analgesia in children has emerged as multimodal analgesia regimens and is increasingly advocated. However, only one meta-analysis published in 2015 systematically evaluated the effect of perioperative parecoxib on postoperative pain in children, demonstrating that, compared with placebo, parecoxib reduced pain scores and the incidence of adverse events (17). The efficacy and safety of flurbiprofen axetil in children remain unclear, and few studies have directly compared the drugs. Therefore, we performed a Bayesian network meta-analysis that integrated as much data as possible from direct and indirect comparative evidence to systematically evaluate the efficacy and safety of parecoxib and flurbiprofen axetil in pediatric perioperative analgesia.
This network meta-analysis was registered at PROSPERO (CRD42022348886), and the work was conducted following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) Statement. We systematically searched PubMed, Embase, Cochrane Library, Web of Science, Sinomed, CNKI, VIP, and Wanfang Data databases on 18 July 2022, using “parecoxib,” “flurbiprofen axetil,” “child, preschool,” “adolescent,” “postoperative period,” “perioperative period,” “intraoperative period,” “preoperative period,” “surgical procedures,” “operative,” and “general surgery” as keywords and MeSH terms combined with Boolean operators. The search equations can be scrutinized in Supplementary Appendix S1. Two reviewers independently screened all titles and abstracts, excluded duplicate studies, and selected works for final analysis via full-text analysis. Any disagreements were resolved by discussion with the third author.
The included studies were limited to those published in English or Chinese. The participants were children or adolescents (younger than 18 years) who underwent elective surgery with general anesthesia. The study groups received parecoxib or flurbiprofen axetil, and the control groups received placebo or standard treatment (fentanyl or tramadol) for perioperative analgesia. The outcomes evaluated were postoperative pain at 0, 0.5, 1, 2, 4, 6, 8, 12, and 24 h and the incidence of total adverse events, nausea and vomiting, and agitation. We excluded guidelines, reviews, comments, conference abstracts without full text and nonrandomized controlled studies.
Two reviewers extracted the data independently. Disagreements were resolved by consensus through discussion and consultation with a third reviewer. The name of the first author, year of publication, sample size, patient age, surgery type, study design, and outcome measures were extracted from each study. In multi-arm trials, we only extracted the data from the arm related to our research.
The quality of the included articles was evaluated by two reviewers using the Cochrane risk-of-bias tool for randomized controlled trials (RCTs) in the following domains: random sequence generation, concealment of allocation sequence, blinding of patients or outcome assessors, incomplete outcome data, selective reporting, and other types of bias. Disagreements were resolved through discussion or by the third reviewer. The risk of bias in each RCT was graded as high, low, or unclear.
The gemtc package of R 4.0.3, based on the Markov Chain Monte Carlo method, was used for Bayesian network meta-analysis. The mean difference with 95% confidence intervals (CIs) was used as the treatment effect estimator for continuous variables, while for dichotomous variables, it was the odds ratio. An MD of <0 indicated that the experimental group had a smaller value. The 95% CIs that did not include 0 were considered to show a significant difference between the two groups, which were compared. The odds ratio of <1 indicated that the incidence in the experimental group was lower. The 95% CIs that did not include 1 were considered to show a significant difference between the two groups. Global heterogeneity was assessed by I2 and of >50% indicated significant heterogeneity. We used the node-splitting analysis to evaluate the inconsistency between the direct and indirect comparisons. If the P-value was >0.05, it indicated that direct and indirect comparisons tended to be consistent. We used random-effect consistency models within a Bayesian framework. The potential scale reduction factor proposed by Brooks et al. was used to diagnose the convergence degree of the model, and if the value tended to 1, the convergence was good (18). We plotted the ranking probability diagrams based on the surface under the cumulative ranking curve. Funnel plots were generated by Stata 17.0 software to evaluate the publication bias of outcome indicators.
We retrieved 942 articles, and after the duplicates were removed, 437 articles were screened through titles and abstracts, and the full texts of 167 articles were retrieved to assess eligibility. A total of 49 RCTs involving 3,657 patients were included in the final analysis (19–67). The PRISMA flow diagram is shown in Figure 1.
The characteristics of the included studies are shown in Supplementary Table 1. A total of 23 RCTs studied tonsillectomy/adenoidectomy; 14 studied abdominal surgery/laparoscopic surgery; and three each studied plastic surgery, circumcision, strabismus surgery, and orthopedic surgery. The following interventions were compared in these RCTs: 30 compared flurbiprofen axetil with placebo; 5 flurbiprofen axetil with fentanyl; 7 flurbiprofen axetil with tramadol; 1 flurbiprofen axetil with parecoxib; 13 parecoxib with placebo; 2 parecoxib with fentanyl; and 6 parecoxib with tramadol.
The quality assessment results of the included RCTs are summarized in Figure 2. The overall study quality grading on individual parameters was low. A total of 12 articles described specific methods of random sequence generation: only 2 claimed allocation concealment and nine blinded the participants or evaluators. A total of 3 RCTs were evaluated as having a high risk of bias, and 46 were identified as having an unclear risk of bias.
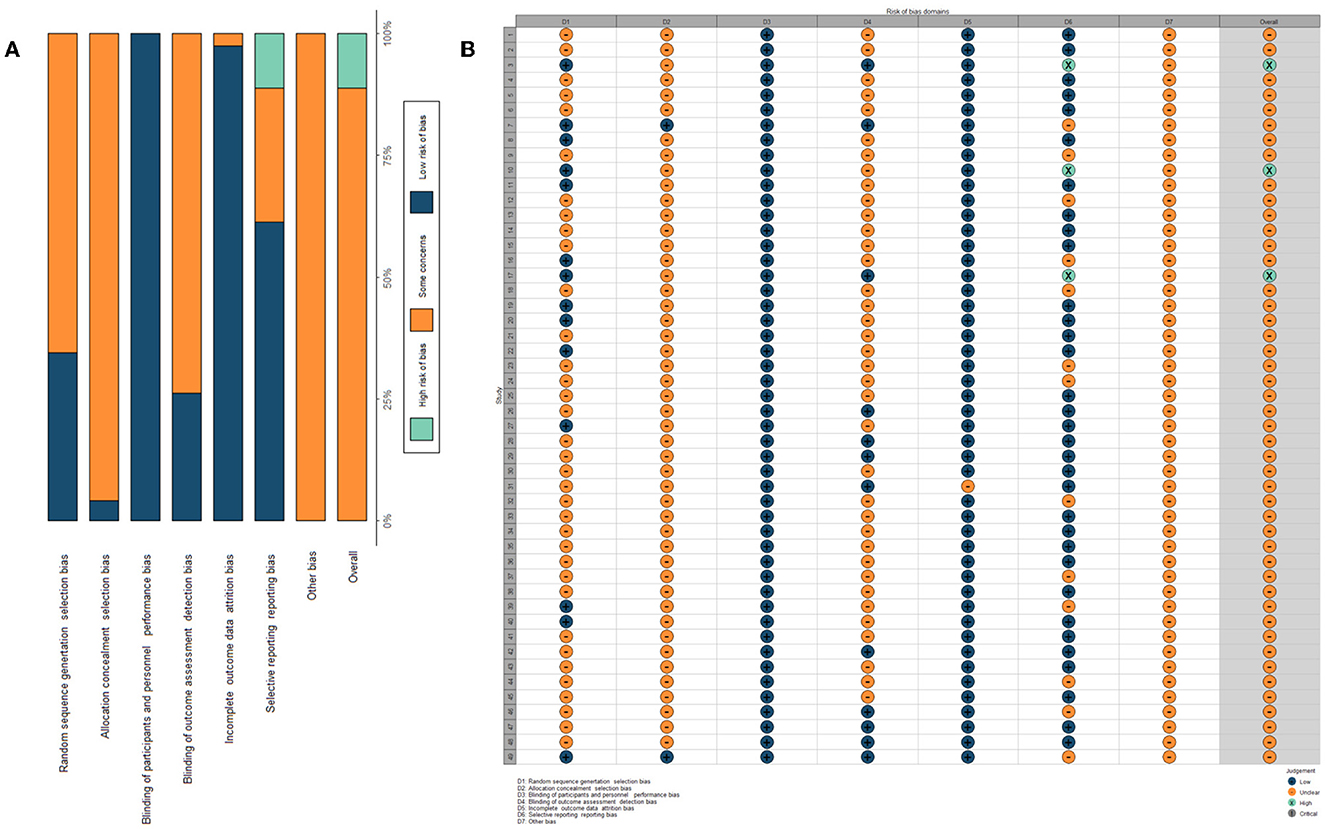
Figure 2. Assessment of the risk of bias. (A) Risk of bias summary table: it is a summary table of review authors' judgments for each risk of bias entry for each study. (B) Assessment of risk of bias within each trial: it is the distribution of judgments (low, high, and unclear) across studies for each risk of bias entry.
A total of 9 RCTs reported pain scores at postoperative 0 and 0.5 h and 8 direct comparisons were established in the network model; 27 trials reported pain scores at postoperative 1 h and 10 direct comparisons were established in the network model; 30 trials reported pain scores at postoperative 2 h and 10 direct comparisons were established in the network model; 31 trials reported pain scores at postoperative 4 h and 10 direct comparisons were established in the network model; 17 trials reported pain scores at postoperative 6 h and 10 direct comparisons were established in the network model; 28 trials reported pain scores at postoperative 8 h and 9 direct comparisons were established in the network model; 28 trials reported pain scores at postoperative 12 h and 8 direct comparisons were established in the network model; and 26 trials reported pain scores at postoperative 24 h and 9 direct comparisons were established in the network model (Figure 3).
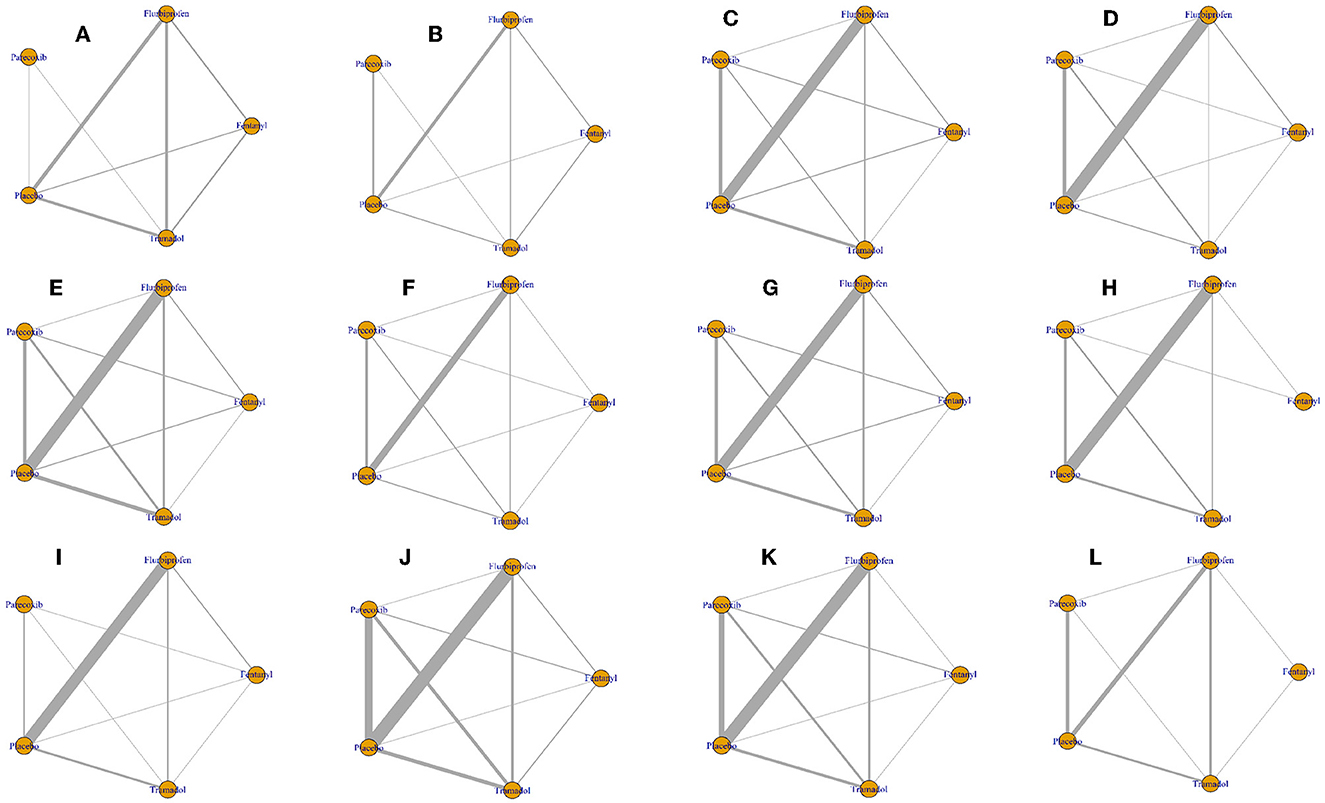
Figure 3. Network plot of all outcomes. The nodes represent the drugs involved, and the size of every node is proportional to the number of randomized participants. The lines between the nodes represent direct comparisons between drugs, and the width of the lines indicates the cumulative number of RCTs for each pairwise comparison. (A) Pain scores at postoperative 0 h. (B) Pain scores at postoperative 0.5 h. (C) Pain scores at postoperative 1 h. (D) Pain scores at postoperative 2 h. (E) Pain scores at postoperative 4 h. (F) Pain scores at postoperative 6 h. (G) Pain scores at postoperative 8 h. (H) Pain scores at postoperative 12 h. (I) Pain scores at postoperative 24 h. (J) Incidence of total adverse events. (K) Incidence of postoperative nausea and vomiting. (L) Incidence of agitation after surgery.
At postoperative 0 h, flurbiprofen axetil and tramadol reduced the pain scores compared with placebo (Table 1). At postoperative 0.5, 1, 2, 4, 6, 8, and 12 h, compared with placebo, fentanyl, flurbiprofen axetil, parecoxib, and tramadol all reduced the pain scores (Table 1). At postoperative 24 h, the pain score of the flurbiprofen axetil group was significantly lower than that of the placebo group (Table 1). Compared with the tramadol group, the postoperative 8-h pain scores of the flurbiprofen axetil or parecoxib groups were significantly lower (Table 1). The ranking results demonstrated that flurbiprofen axetil had significant superiority in reducing pain scores at 2, 4, and 12 h postoperatively, and parecoxib was the highest-ranking drug to reduce pain scores at 0, 0.5, 1, 6, 8, and 24 h postoperatively (Supplementary Table 3, Figure 4).
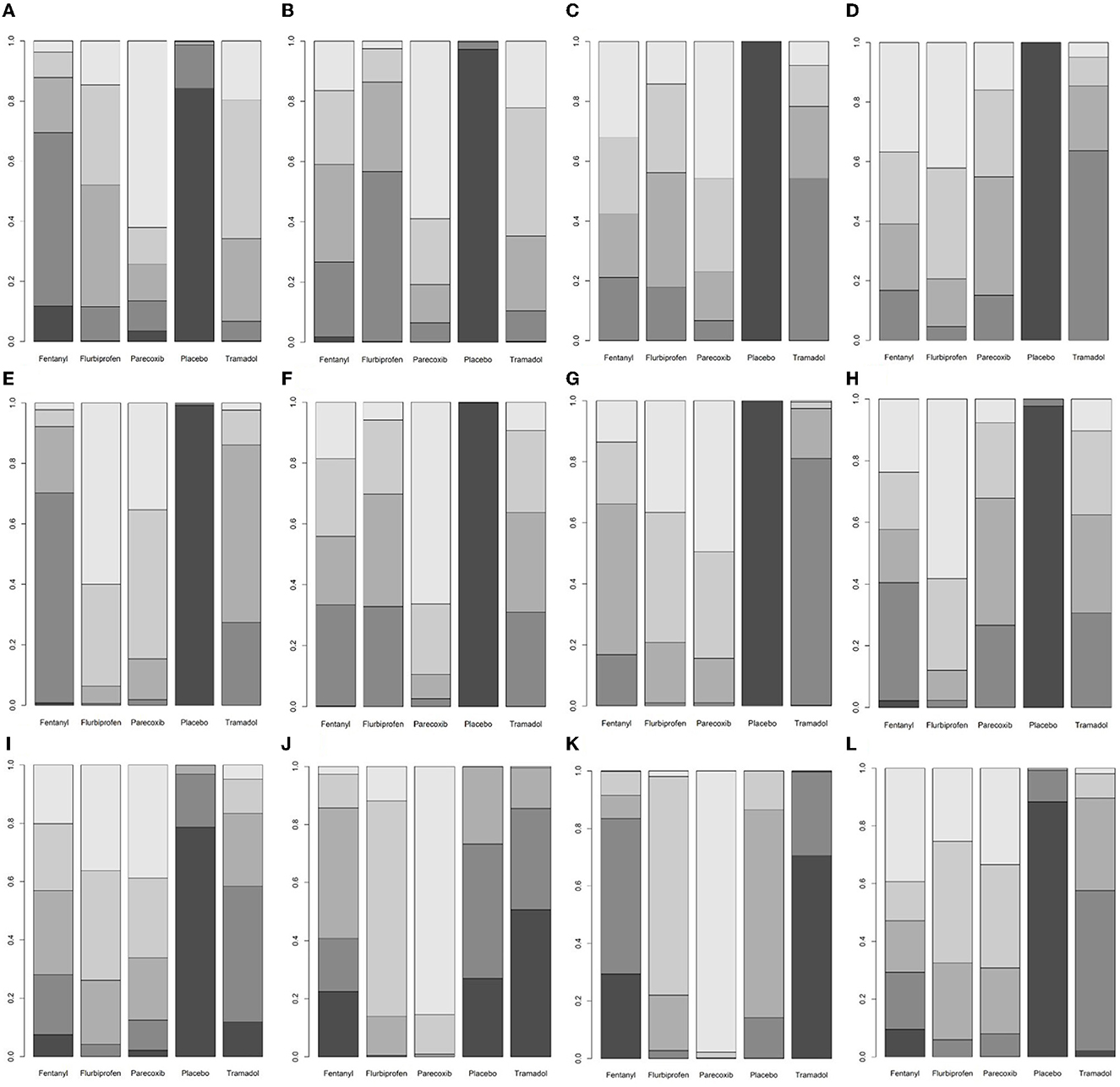
Figure 4. A ranking plot based on the probabilities of drugs. The horizontal axis shows the different interventions, and the vertical axis shows the ranking probability. The lighter the color of the block, the higher the ranking, and the darker the color of the block, the lower the ranking. (A) Pain scores at postoperative 0 h. (B) Pain scores at postoperative 0.5 h. (C) Pain scores at postoperative 1 h. (D) Pain scores at postoperative 2 h. (E) Pain scores at postoperative 4 h. (F) Pain scores at postoperative 6 h. (G) Pain scores at postoperative 8 h. (H) Pain scores at postoperative 12 h. (I) Pain scores at postoperative 24 h. (J) Incidence of total adverse events. (K) Incidence of postoperative nausea and vomiting. (L) Incidence of agitation after surgery.
A total of 40 RCTs reported the incidence of total adverse events and 10 direct comparisons were established in the network model; 34 trials reported the incidence of postoperative nausea and vomiting and 10 direct comparisons were established in the network model; and 13 trials reported the incidence of postoperative agitation and 7 direct comparisons were established in the network model (Figure 3).
Compared with the placebo group, flurbiprofen axetil and parecoxib reduced the incidence of total adverse events and postoperative agitation (Table 2). Compared with the tramadol group, flurbiprofen axetil and parecoxib reduced the incidence of total adverse events and postoperative nausea and vomiting (Table 2). The incidence of postoperative nausea and vomiting was significantly lower in the parecoxib group than in the flurbiprofen axetil, fentanyl, and placebo groups (Table 2). There was no significant difference between the other comparisons. The ranking results showed that parecoxib was relatively more advantageous in decreasing the incidence of total adverse events and postoperative nausea and vomiting (Supplementary Table 3, Figure 4).
Across all outcomes, the assessment of inconsistency always supported the assumption of consistency between direct and indirect evidence. The results of the node splitting models are shown in Supplementary Figure 1. The potential scale reduction factor (PSRF) was used to diagnose the convergence degree of the model and the PSRF value tends to 1 indicates that the convergence is good (Supplementary Figure 2). The global heterogeneity and pairwise comparisons of heterogeneity are shown in Supplementary Figure 3. The comparison-adjusted funnel plots of all outcomes are shown in Supplementary Figure 4, and figures with poor symmetry indicate the possibility of publication bias.
Our analysis included 49 RCTs with 3,657 children aged 6 months to 14 years, and the aim of this study was to systematically evaluate the efficacy and safety of parecoxib and flurbiprofen axteil in perioperative analgesia in children using a Bayesian network meta-analysis. The results demonstrated that the pain scores of the flurbiprofen axetil group were significantly lower than those in the placebo group at all time points in the 24-h postoperative period, and the pain scores at all time points during the 12-h postoperative period of the parecoxib group were significantly lower than those in the placebo group. From the potential rankings, flurbiprofen axetil was superior to other drugs in reducing pain scores at 2, 4, and 12 h postoperatively, while parecoxib had some advantages in reducing pain scores at 0, 0.5, 1, 6, 8, and 24 h postoperatively.
Few meta-analyses have been conducted to investigate the efficacy and safety of parecoxib or flurbiprofen axetil for perioperative analgesia in children, but evidence in adults is constantly being updated. A previous meta-analysis by Li et al. (68) reported that low to moderate evidence indicated that parecoxib relieved postoperative orthopedic pain while sparing opioid analgesic consumption without increasing the incidence of adverse events (68). Sun et al. (69) reported that the perioperative administration of flurbiprofen was effective in reducing postoperative pain, nausea, and vomiting in Chinese surgical patients (69). Another meta-analysis comparing flurbiprofen axetil vs. parecoxib found that parecoxib had an advantage over flurbiprofen axetil for patients with postoperative pain beyond 6 h (70). It has been reported that intravenous administration of parecoxib (1.0 mg/kg to a maximum of 40 mg) in children resulted in free valdecoxib concentrations above the IC50 for COX-2 for at least 12 h, and the area under the valdecoxib concentration-time curve was similar to that in adults (40 mg) (71). A population pharmacokinetic study of intravenous flurbiprofen axetil in Chinese patients with postoperative pain discovered that between-subject variability for flurbiprofen was associated with body height and weight (72). In children aged 3 months to 13 years, the estimated clearance rate was 0.96 L/h/70 kg and the volume of distribution at steady state was 8.1 L/70 kg, which was significantly lower than that in adults (14). Therefore, the efficacy and safety of parecoxib and flurbiprofen axetil for perioperative analgesia in children need to be confirmed, as the results of the adult studies cannot be directly extrapolated to children because of the limitations of individual differences between adults and children.
In a previous network meta-analysis, parecoxib for analgesia after tonsillectomy in children was found to be better than fentanyl, and flurbiprofen axetil was superior to tramadol (73), but it only reported one pain score and did not specify the pain scores in children at different time points. Another meta-analysis found that the perioperative use of parecoxib in children was associated with less acute postoperative pain and adverse events compared with placebo or standard treatment (fentanyl or tramadol) (17), but that study lacked an evaluation of the analgesic effects of parecoxib beyond 12 h postoperatively. Our network meta-analysis systematically evaluated the efficacy and safety of parecoxib and flurbiprofen axetil for perioperative analgesia in children while adding more recent studies to provide a more reliable evidence-based analysis.
In terms of safety, compared with children who received placebo, those who received flurbiprofen axetil or parecoxib had a lower incidence of total adverse events and postoperative agitation. Compared with tramadol, flurbiprofen axetil and parecoxib both significantly reduced the incidence of total adverse events and postoperative nausea and vomiting. Compared with flurbiprofen axetil or fentanyl, parecoxib significantly reduced the incidence of postoperative nausea and vomiting. The ranking results showed that parecoxib was more advantageous in decreasing the incidence of total adverse events and postoperative nausea and vomiting.
Because few adverse events were reported in the included trials, such as respiratory depression, pruritus, headache, dizziness, and urinary retention, only total adverse events, postoperative nausea and vomiting, and agitation were analyzed in this study because of insufficient data. A multicenter retrospective study involving 3,542 patients found that postoperative administration of parecoxib resulted in a 0.2% incidence of adverse events, primarily hypotension, nausea, vomiting, pruritus, and rash (74). Another study discovered that flurbiprofen axetil accounted for the largest number of adverse reactions (686/5597, 12.26%), with the most common being nausea, rash, vomiting, pruritus, and dizziness (75). Several studies have revealed that, compared with tramadol, parecoxib and flurbiprofen axetil both resulted in less postoperative nausea and vomiting in adults (76–78). Our study also found in children that the incidence of postoperative nausea and vomiting was lower for both parecoxib and flurbiprofen axetil than for tramadol. Bu et al. (17) found that, when compared to placebo or standard treatment, perioperative parecoxib administration for children resulted in a lower incidence of postoperative nausea, vomiting, and agitation (17). Li et al. (67) also discovered that the incidence of postoperative nausea and vomiting was significantly higher in the placebo group (11/30, 37%) than in the parecoxib group (4/30, 13%) (67). These results suggested that parecoxib was an effective and safe analgesic for children, particularly in reducing postoperative nausea and vomiting, which is consistent with the results of our study. The included trials demonstrated that none of the children had bleeding, gastrointestinal injury, renal injury, or cardiovascular events. In a pooled analysis of 28 randomized, double-blind, placebo-controlled clinical trials, patients in the parecoxib and placebo groups had similar frequencies of gastrointestinal events, renal failure and impairment, and cardiovascular embolic and thrombotic events (79). Therefore, this study considered that both parecoxib and flurbiprofen axetil decreased the risk of total adverse events compared with placebo and tramadol and that parecoxib was more advantageous in reducing postoperative nausea and vomiting.
There were a few limitations to this study. First, the included trials were associated with high risks of bias because most of them only mentioned “random” without specifying the method of generating the random sequence or describing the allocation concealment in detail. Second, most studies only recorded pain scores within the first 24 h after surgery, paying little attention to 48 or 72 h. Furthermore, we did not analyze other outcomes, such as opioid consumption or proportions of patients requiring rescue analgesia, which may have more clinical implications. Third, data on direct head-to-head comparisons of flurbiprofen axteil and parecoxib within the network were insufficient. Finally, there were some differences between studies in the type of surgery, perioperative care protocol, study drug dose, and timing of administration, which could explain the heterogeneity in our results.
Based on the available clinical evidence, our network meta-analysis reached the following conclusions. Perioperative administration of flurbiprofen axetil and parecoxib were both effective in reducing postoperative pain scores and total adverse events compared with placebo. Flurbiprofen axetil and parecoxib also caused fewer total adverse events and postoperative nausea and vomiting compared with tramadol. Flurbiprofen axetil was most effective at reducing pain scores at 2, 4, and 12 h postoperatively. Parecoxib had an advantage in terms of reducing pain scores at 0, 0.5, 1, 6, 8, and 24 h postoperatively, as well as reducing the incidence of total adverse events and postoperative nausea and vomiting.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
XiC helped with study design, the selection of included articles, data extraction, quality assessment, data analysis and interpretation, and writing the manuscript. PC and QH helped with the study design, selection of included articles, data extraction, quality assessment, data analysis and interpretation, and the final review. XiaC, MH, and KT helped with the study design and the final review. All authors contributed to the article and approved the submitted version.
This study was financially supported by the Clinical Drug Research Fund of Guangdong Province (2023JZ07).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1231570/full#supplementary-material
1. Groenewald CB, Rabbitts JA, Schroeder DR, Harrison TE. Prevalence of moderate-severe pain in hospitalized children. Paediatr Anaesth. (2012) 22:661–8. doi: 10.1111/j.1460-9592.2012.03807.x
2. Shen Q, Zheng XL, Lin Z, Li X, Leng HY. Survey of current status of children's pain management practice in 66 hospitals in China. Chin Nursing Management. (2019) 19:187–93. doi: 10.3969/j.issn.1672-1756.2019.02.007
3. Eccleston C, Fisher E, Howard RF, Slater R, Forgeron P, Palermo TM, et al. Delivering transformative action in paediatric pain. The Lancet Child Adol Health. (2021) 5:47–87. doi: 10.1016/S2352-4642(20)30277-7
4. Correll D. Chronic postoperative pain: recent findings in understanding and management. F1000Res. (2017) 6:1054. doi: 10.12688/f1000research.11101.1
5. Pancekauskaite G, Jankauskaite L. Paediatric pain medicine: pain differences, recognition and coping acute procedural pain in paediatric emergency room. Medicina. (2018) 54:94. doi: 10.3390/medicina54060094
6. Wright JA. An update of systemic analgesics in children. Anaesthes Int Care Med. (2019) 20:324–9. doi: 10.1016/j.mpaic.2019.03.010
7. Rosen DM, Alcock MM, Palmer GM. Opioids for acute pain management in children. Anaesth Intensive Care. (2022) 50:81–94. doi: 10.1177/0310057X211065769
8. Chen LQ, Wu B, Hong AM, Xu CF, Zhai ZX Li J. A survey of the current status of acute postoperative pain. J Clin Anesthesiol. (2021) 37:1200–3.
9. Faerber J, Zhong W, Dai D, Baehr A, Maxwell LG, Kraemer FW, et al. Comparative safety of morphine delivered via intravenous route vs. patient-controlled analgesia device for pediatric inpatients. J Pain Sympt Manage. (2017) 53:842–50. doi: 10.1016/j.jpainsymman.2016.12.328
10. Vittinghoff M, Lönnqvist P-A, Mossetti V, Heschl S, Simic D, Colovic V, et al. Postoperative pain management in children: guidance from the pain committee of the european society for paediatric anaesthesiology (espa pain management ladder initiative). Pediatr Anaesth. (2018) 28:493–506. doi: 10.1111/pan.13373
11. Chou R, Gordon DB, Leon-Casasola OA de, Rosenberg JM, Bickler S, Brennan T. Management of postoperative pain: a clinical practice guideline from the American pain society, the American society of regional anesthesia and pain medicine, and the American society of anesthesiologists' committee on regional anesthesia, executive committee, and administrative council. The J Pain. (2016) 17:131–57. doi: 10.1016/j.jpain.2015.12.008
12. Zuo YX, Wu XM, Lian QQ, Wang YW. Consensus on Pediatric Postoperative Analgesia (2017 Edition). (2017) Available online at: https://csahq.cma.org.cn/guide/detail_378.html (accessed March 20, 2023).
13. Ohmukai O. Lipo-NSAID preparation. Adv Drug Deliv Rev. (1996) 20:203–7. doi: 10.1016/0169-409X(95)00123-O
14. Kumpulainen E, Välitalo P, Kokki M, Lehtonen M, Hooker A, Ranta V-P, et al. Plasma and cerebrospinal fluid pharmacokinetics of flurbiprofen in children. Br J Clin Pharmacol. (2010) 70:557–66. doi: 10.1111/j.1365-2125.2010.03720.x
15. Dalpiaz AS, Peterson D. Parecoxib: a shift in pain management? Expert Rev Neurother. (2004) 4:165–77. doi: 10.1586/14737175.4.2.165
16. Cheer SM, Goa KL. Parecoxib (Parecoxib Sodium). Drugs. (2001) 61:1133–41. doi: 10.2165/00003495-200161080-00010
17. Bu X, Yang L, Zuo Y. Efficacy and safety of perioperative parecoxib for acute postoperative pain treatment in children: a meta-analysis. Front Med. (2015) 9:496–507. doi: 10.1007/s11684-015-0414-y
18. van Valkenhoef Gert, Tervonen Tommi, Zwinkels Tijs, de Brock Bert, Hillege Hans. ADDIS: A decision support system for evidence-based medicine. Decision Support Systems (2013) 55:459–475. doi: 10.1016/j.dss.2012.10.005
19. Li H, Lian QQ. Different doses of flurbiprofen axetil for post-tonsillectomy analgesia in pediatric patients. J Clin Anesthesiol. (2011) 27:189–90. doi: 10.1007/s10008-010-1224-4
20. Li XF, Gong HY, Zhang YQ, Liu J, Zhang K, Zhang JF, et al. Analgesic effect of different dose of flurbiprofen axetil on postoperative unilateral indirect inguinal herniorrhaphy. J Med College. (2014) 31:278–81. doi: 10.7683/xxyxyxb.2014.04.011
21. Sun CL, Zhao TX Li XZ. A comparison of the anesthetic effects of dexmedetomodine, parry celebrex sodium and tramadol for children during tonsillectomy. J Med Theor Prac. (2014) 27:1127–9. doi: 10.19381/j.issn.1001-7585.2014.09.004
22. Zhang R, Liu JP, Dong ZF, Ni RF, Wu J, Liu GD. Effect of preemptive analgesia on emergence agitation after sevoflurane anesthesia in pediatric patients. Xingjiang Med J. (2017) 47:256–60.
23. Kong L, Zhang XM, Wang SY. The effect of preemptive analgesia for postoperative pain in children undergoing tonsillectomy and adenoidectomy. Int J Anesthesiol Resuscitation. (2012) 2:443–447.
24. Zhang R, Liu JP, Dong ZF, Ni RF, Wu J, Liu GD, et al. Preemptive analgesia for pediatric laparoscopic surgery. China J Emerg Resus Disaster Med. (2015) 10:1054–7. doi: 10.3969/j.issn.1673-6966.2015.11.014
25. Zhang YZ, He J, Li H. Effect of preemptive analgesia in children undergoing plastic surgery. Chinese J Aesth Med. (2014) 23:351–3.
26. Wang RM, Cai XQ, Chen QZ. The preemptive analgesia of dezocine in combination with parecoxib in children undergoing tonsillectomy. Anhui Medical J. (2014) 35:1483–5. doi: 10.3969/j.issn.1000-0399.2014.11.003
27. Lin L, Chen ZY, Wang YB. Comparison of flurbiprofen axetil, tramadol and fentanyl preemptive analgesia in children undergoing tonsillectomy. Fujian Med J. (2011) 33:116118. doi: 10.3969/j.issn.1002-2600.2011.06.062
28. Li WX, Luo JW, Zhang HY. Effect of flurbiprofen ester, tramadol and fentanyl preemptive analgesia on postoperative pain in children undergoing tonsillectomy. J North Pharmacy. (2016) 13:36–7.
29. Lu HR, Mao GQ, Zhou ZW. Clinical observation of preemptive analgesia of flurbiprofen axetil in children circumcision. J Chin Phys. (2010) 2:1420–1421.
30. Peng ZH Li Y, Zhou L. The clinical observation of the apply of preemptive analgesia of flurbiprofen axetil injection on children tonsilloadenoidectomy. Nat Med Front China. (2011) 6:59–60. doi: 10.3969/j.issn.1673-5552.2011.06.0030
31. Zeng RF, Ni YF, Wang JG, Li J, Lian QQ. Clinical observation of flurbiprofen axetil injection preemptive analgesia in children undergoing tonsillectomy. J Prac Med. (2008) 5:2488–2490. doi: 10.3969/j.issn.1006-5725.2008.14.056
32. Yang Y, Sun HM, Cui EH. Flurbiprofen axetil preemptive analgesia for pediatric short and minor surgical analgesia. Modern J Int Trad Chin Western Med. (2011) 20:3324–5. doi: 10.3969/j.issn.1008-8849.2011.26.051
33. Peng SJ, Liu WY, Qin YH, Xu KQ. Clinical observation on flurbiprofen axetil for preemptive analgesia in children undergoing adenoidectomy. J Gannan Med Univ. (2011) 31:33–4. doi: 10.3969/j.issn.1001-5779.2011.01.018
34. Yang PC, Zeng ZD, Yang SH. Preventive effects of preemptive analgesia of flurbiprofen axetil injection on emergence agitation in children. J North Pharmacy. (2015) 12:118–9.
35. Zhang H, Liu QN, Zhou RS. Preemptive analgesic of flurbinprofen axetil in children orthopedic surgery. J China-Japan Friendship Hospital. (2012) 26:90–2. doi: 10.3969/j.issn.1001-0025.2012.02.008
36. Long FY, Lu KS, Wang CL. Flurbiprofen axetil preemptive analgesia in pediatric surgery. Modern J Int Trad Chin Western Med. (2008) 2:5509–5510.
37. Zeng RF, He W, Wang JG, Li J, Lian QQ. Flurbiprofen axetil preemptive analgesia in pediatric hiatal hernia surgery. Modern Prac Med. (2008) 20:976–7. doi: 10.3969/j.issn.1671-0800.2008.12.031
38. Tian H, Xu KL, Yang D, Liu JH, Wei LX, Deng XM. Flurbiprofen axetil preemptive analgesia in pediatric plastic surgery. Chinese J Med. (2011) 46:71–2. doi: 10.3969/j.issn.1008-1070.2011.07.030
39. Ma SM, Sun Y, Zhang XZ, Zhou Q, Song JN, Liang XD. The pre-analgesic efficacy of flurbiprofen axetil in children undergoing lower abdomen surgery. Beijing Med J. (2013) 35:675–7. doi: 10.15932/j.0253-9713.2013.08.040
40. Huang XQ, Zhang ZG Li BF, Chen Y. Clinical observation of flurbiprofen axetil injection postopetative analgesia in children undergoing circumcision. Modern Med J. (2012) 40:167–70. doi: 10.3969/j.issn.1671-7562.2012.02.009
41. Lei S, Mo H, Wu XF, He Y. Efficacy of flurbiprofen axetil for postoperative analgesia in pediatric tonsillectomy and adenoidectomy. Chin J Clin Rat Drug Use. (2011) 4:67–8. doi: 10.15887/j.cnki.13-1389/r.2011.09.084
42. Zhu TQ. Clinical observation of flurbiprofen axetil for pediatric tonsillar surgery. China Rural Health. (2020) 12:30–1.
43. He YJ, Tan XH, Jiang ZX. The clinical study of postoperative analgesia with flurbiprofen axetil in children undergoing strabismus. J Snake. (2011) 23:251–7.
44. Li Y, Hong BY Li SJ, Zhang Y, Wang HB, Yang CX. Influence of preadministration of flurbiprofen axetil on postoperative analgesia efficacy and inflammatory cytokine expression of children undergoing tonsillectomy. Int Med Health Guidance News. (2014) 20:451–4. doi: 10.3760/cma.j.issn.1007-1245.2014.04.003
45. Mo H, Zong ZJ, Yang L. Flurbiprofen axetil in postoperative analgesia in pediatric general anesthesia. J Clin Res. (2009) 26:1757–8. doi: 10.3969/j.issn.1671-7171.2009.09.071
46. Yang H, Han XM, Tan L. Clinical observation on the analgesic effect of flurbiprofen axetil in pediatric tonsillectomy and adenoidectomy. J Front Med. (2014) 21:155–156. doi: 10.3969/j.issn.2095-1752.2014.17.145
47. Wei PH, Yang ZQ, Ma GF. The effect dose of flurbiprofen for relieving postoperative pain in patients undergone tonsillectomy. J Taishan Med College. (2010) 31:499–500. doi: 10.3969/j.issn.1004-7115.2010.07.005
48. Li H, Liang FQ, Chen L, Zeng K. Application of flurbiprofen axetil injection in post-circumcision analgesia in pediatric patients. J Fujian Med Univ. (2017) 51:256–8.
49. Chen Q, Tan YES, Li D, Wu G. Analgesic effects of flurbiprofen axetil and tramadol in children undergoing tonsillar and adenoid surgery. West China Medical J. (2010) 25:1883–5.
50. Ma SM, Sun Y, Song JN, Zhang XZ, Zhou Q, Liang XD. Comparison of ketamine and flurbiprofen ester for preemptive analgesia in pediatric hernia repair. J Inner Mongol Med Univ. (2013) 35:334–6. doi: 10.16343/j.cnki.issn.2095-512x.2013.s2.134
51. Zeng QG Li DF, Cen XR, Yang YY, Li YY, Chen XQ. The clinical observation of Parecoxibin preemptive analgesia in children undergoing tonsillectomy. Jilin Med J. (2017) 38:2033–6. doi: 10.3969/j.issn.1004-0412.2017.11.013
52. Ma XG, Yan XT. The clinical observation of Parecoxibin preemptive analgesia in the control of adenoid and tonsil surgery. J Hub Univ Nationalit. (2013) 30:53–5.
53. Miao YL, Shi WZ, Guo WZ, Zhong J, Fang WW, Liu J, et al. Effect of preemptive analgesia with Parecoxib sodium in children undergoing endoscopic adenoidectomy and tonsillectomy of snoring disease. Clinical J Med Officers. (2012) 40:300–2. doi: 10.3969/j.issn.1671-3826.2012.02.018
54. Fan WJ, Wu N, Niu L. Clinical study of parecoxib sodium preemptive analgesia in pediatric suburethral cleft surgery. Chinese J Clin Res. (2013) 26:1358–9.
55. Li XZ Li W, Xia Q. Clinical study of preemptive analgesia with parecoxib sodium in children undergoing upper limb orthopedic surgery. West China Med J. (2011) 26:1189–91.
56. Li D, Kang Y, Tan Y, Zhu CY, Chen Q, Wu G. A comparison of parecoxib sodium and flurbiprofen axetil in children undergoing endoscopic adenoidectomy and tonsillectomy. Int J Anesthesiol Resusc. (2014) 35:1098–108. doi: 10.3760/cma.j.issn.1673-4378.2014.12.008
57. Yuan YX Li WB, Gao RY, Dong YJ. Parecoxib sodium combined with tramadol in the prevention of emergence agitation in children after general anesthesia. J Qiqihar Univ Med. (2010) 31:1729–30. doi: 10.3969/j.issn.1002-1256.2010.11.029
58. Xiu MY, Xu DM, Luan HF. Analgesic effect of parecoxib sodium and desocine in preemptive analgesia after laparoscopic hernia surgery in children. Modern Med Health Res. (2019) 3:54–6.
59. Zhang YZ, Li Q, Liu QX. Effect of preemptive analgesia with parecoxib sodium and tramadol in children undergoing laparoscopic hernia operation. J Clin Pediatr Surg. (2014) 2:250–2. doi: 10.3969/j.issn.1671-6353.2014.03.024
60. Ye WL, Jiang WN. Comparison of parecoxib sodium and tramadol for postoperative analgesia in pediatric strabismus correction. Strait Pharm J. (2013) 25:164–5. doi: 10.3969/j.issn.1006-3765.2013.07.083
61. Li K, Pan JZ. Parecoxib prevents restlessness during waking-up from sevoflurane anesthesia in children. Mod Hosp. (2011) 11:30–2. doi: 10.3969/j.issn.1671-332X.2011.02.012
62. He JG, Yuan LY, Chen L, Jiang J. Preemptive analgesic effect of parecoxib sodium in laparoscopic appendectomy in children. Chin J Clin Pharmacol Therap. (2017) 22:705–8.
63. Yi SH, Chen ZH, Hu SY, Zhong JF, Li J. Effect of preoperative dexmedetomidine combined with flurbiprofen axetil on agitation after tonsillectomy in children. Chin J Clin Pharmacol Therap. (2013) 18:1044–8.
64. Sun YY, Yuan XR, Liu Y. Flurbiprofen axetil injection for postoperative pain in children undergoing tonsillectomy and adenoidectomy or not. J China-Japan Friendship Hosp. (2007) 2:207–209.
65. Tao J, Chen FF, Zhou JL, Wang H, Liang QS. The effect of pre-injection of parecoxib sodium on emergence agitation in children with sevoflurane anesthesia. J Bengbu Med College. (2016) 41:1431–5. doi: 10.13898/j.cnki.issn.1000-2200.2016.11.008
66. Mikawa K, Nishina K, Maekawa N, Shiga M, Obara H. Dose-response of flurbiprofen on postoperative pain and emesis after paediatric strabismus surgery. Can J Anesth. (1997) 44:95. doi: 10.1007/BF03014332
67. Li X, Zhou M, Xia Q, Li J. Parecoxib sodium reduces the need for opioids after tonsillectomy in children: a double-blind placebo-controlled randomized clinical trial. J Can Anesth. (2016) 63:268–74. doi: 10.1007/s12630-015-0560-3
68. Li X, Zhou P, Li Z, Tang H, Zhai S. Intravenous parecoxib for pain relief after orthopedic surgery: a systematic review and meta-analysis. Pain Ther. (2022) 54:1–14. doi: 10.1007/s40122-022-00400-1
69. Sun M, Cong X, Chang E, Miao M, Zhang J. Efficacy of flurbiprofen for postoperative pain in chinese surgical patients: a meta-analysis. J Surg Res. (2020) 252:80–8. doi: 10.1016/j.jss.2019.11.032
70. Shi XL, Ye RM, Yang H, Yang H, Jia W. Efficacy and safety of parecoxib and flurbiprofen in treatment of pain after postoperative: a meta-analysis. Drug Eval Res. (2020) 43:1414–20. doi: 10.7501/j.issn.1674-6376.2020.07.041
71. Hullett B, Salman S, O'Halloran SJ, Peirce D, Davies K, Ilett KF. Development of a population pharmacokinetic model for parecoxib and its active metabolite valdecoxib after parenteral parecoxib administration in children. Anesthesiology. (2012) 116:1124–33. doi: 10.1097/ALN.0b013e31825154ef
72. Zhang J, Zhang H, Zhao L, Gu J, Feng Y, An H. Population pharmacokinetic modeling of flurbiprofen, the active metabolite of flurbiprofen axetil, in Chinese patients with postoperative pain. J Pain Res. (2018) 11:3061–70. doi: 10.2147/JPR.S176475
73. Li X, Zheng XL, Shen Q, Lin Z. Effect of different drugs on analgesia after tonsillectomy in children: a network meta-analysis. Chin J Pain Med. (2018) 24:697–704. doi: 10.3969/j.issn.1006-9852.2018.09.013
74. Zhang B, Jin Y, Gong H, Mei D, Zhong MK, Tang Y, et al. A retrospective, multi-center survey of parecoxib prescribing practice in 3542 surgical inpatients. Chin Pharm J. (2013)48:1005–9. doi: 10.11669/cpj.2013.12.015
75. Wang JX, Kong XH, Guo DH, Yuan YH, Liu SY, Zhang B, et al. Analysis of 5597 reports of adverse reactions to NSAIDs. Chin J Pharmacoepidemiol. (2021) 30:457–61. doi: 10.19960/j.cnki.issn1005-0698.2021.07.006
76. Zhou LW, Zhao XY, Qiu EY, Xu LJ, Shan YF. A meta-analysis of flurbiprofen axetil versus tramadol in postoperative analgesia effect and adverse drug reactions. J Wenzhou Med Univ. (2017) 47:206–10. doi: 10.3969/j.issn.2095-9400.2017.03.011
77. Liang GS, Du JB, Xia DT, Wu XF. Analgesic effect of flurbiprofen axetil, parecoxib and tramadol after orthopedic trauma surgery. J North Pharmacy. (2015) 12:76–7.
78. Jiang Y, Duan YM, Wu G, Yang ZH, Wang LF, Liu Q, et al. Effects of flurbiprofen axetil, parecoxib and tramadol on postoperative analgesia after orthopedic trauma surgery. Hebei Medicine. (2011) 17:1206–8. doi: 10.3969/j.issn.1006-6233.2011.09.028
79. Schug SA, Parsons B, Li C, Xia F. The safety profile of parecoxib for the treatment of postoperative pain: a pooled analysis of 28 randomized, double-blind, placebo-controlled clinical trials and a review of over 10 years of postauthorization data. J Pain Res. (2017) 10:2451–9. doi: 10.2147/JPR.S136052
Keywords: NSAIDs, parecoxib, flurbiprofen axetil, children, perioperative analgesia
Citation: Chen X, Chen P, Chen X, Huang M, Tang K and He Q (2023) Efficacy and safety of parecoxib and flurbiprofen axetil for perioperative analgesia in children: a network meta-analysis. Front. Med. 10:1231570. doi: 10.3389/fmed.2023.1231570
Received: 30 May 2023; Accepted: 22 June 2023;
Published: 17 July 2023.
Edited by:
Cong Yu, Chongqing Medical University, ChinaReviewed by:
Yang Hui, First Affiliated Hospital of Chongqing Medical University, ChinaCopyright © 2023 Chen, Chen, Chen, Huang, Tang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiuyi He, c3lzdV9oQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.