- 1Liver Cirrhosis Study Group, Department of Gastroenterology, General Hospital of Northern Theater Command, Shenyang, China
- 2Postgraduate College, China Medical University, Shenyang, China
- 3Chinese People’s Liberation Army General Hospital, Chinese People’s Liberation Army Medical School, Beijing, China
- 4Postgraduate College, Shenyang Pharmaceutical University, Shenyang, China
- 5Postgraduate College, Dalian Medical University, Dalian, China
Background and aims: Hepatitis B virus (HBV) infection is the most common cause of liver cirrhosis. Portal venous system thrombosis (PVST) is a major complication of liver cirrhosis. Recently, it has been shown that C-type lectin-like receptor 2 (CLEC-2) and galectin-1 participate in the activation and aggregation of platelets, thereby promoting the development of thrombosis. This cross-sectional study aims to evaluate the association of serum CLEC-2 and galectin-1 levels with PVST in patients with HBV-related liver cirrhosis.
Methods: Overall, 65 patients with HBV-related liver cirrhosis were included, of whom 23 had PVST and 42 did not have. Serum CLEC-2 and galectin-1 levels were measured using enzyme-linked immunosorbent assay kits. PVST was assessed by contrast-enhanced computed tomography and/or magnetic resonance imaging scans. Subgroup analyses were conducted according to the degree and location of PVST.
Results: Patients with PVST had significantly higher serum CLEC-2 (p = 0.006) and galectin-1 (p = 0.009) levels than those without. Patients with partial/complete PVST or fibrotic cord (p = 0.007; p = 0.002), but not those with mural PVST (p = 0.199; p = 0.797), had significantly higher serum CLEC-2 and galectin-1 levels than those without PVST. Patients with superior mesenteric vein thrombosis had significantly higher serum CLEC-2 (p = 0.013) and galectin-1 (p = 0.025) levels than those without PVST. Patients with main portal vein thrombosis had higher serum CLEC-2 (p = 0.020) and galectin-1 (p = 0.066) levels than those without PVST, but the difference in serum galectin-1 level was not significant between them.
Conclusion: Serum CLEC-2 and galectin-1 levels may be associated with the presence of PVST in HBV-related cirrhotic patients, but this association should be dependent upon the degree of PVST.
1. Introduction
Hepatitis B virus (HBV) infection is the most common etiology of liver cirrhosis (1). Portal venous system thrombosis (PVST) is one of major complications of liver cirrhosis (2). Its prevalence ranges from 1 to 26%, which increases with the severity of liver disease (3, 4). In addition, PVST can increase the risk of long-term death, portal hypertension-related bleeding, ascites, and acute kidney injury in cirrhotic patients (5). Therefore, it is necessary to explore the mechanism regarding the development of PVST in HBV-related liver cirrhosis.
C-type lectin-like receptor 2 (CLEC-2) is a type II transmembrane receptor composed of an extracellular ligand-binding C-type lectin-like domain, stalk region, single transmembrane helix, and short cytoplasmic tail (6). It is encoded by the gene CLEC1B located on 12p13.31 with a molecular weight of 32-40 kDa (7). It is highly expressed in megakaryocytes and platelets and slightly expressed in myeloid cell subsets (8, 9). It has been shown that CLEC-2 mediates the activation and aggregation of platelets (10). Moreover, deep venous thrombosis is significantly inhibited in CLEC-2-deficient mice, suggesting that CLEC-2, especially platelet CLEC-2, should be critical for the development of venous thrombosis (11).
Galectin-1 is the firstly discovered member of the galectin family and belongs to the prototype galectin containing a carbohydrate-binding domain, which can exist as monomers or dimers (12). It is a 14.5 kDa protein encoded by the gene LSGALS1 located on 22q12 (13). Due to the lack of signal peptides, galectin-1 is secreted through a non-classical secretion pathway that translocate directly across the plasma membrane, which performs important biological functions both inside and outside cells (14). Galectin-1 knockout mice have a significantly longer median tail bleeding time than wild-type mice (15). Furthermore, galectin-1-deficient platelets exhibit impairment in fibrinogen adhesion and clot retraction, suggesting that galectin-1 should contribute to hemostasis and involve in thrombosis (15).
Some animal studies have demonstrated a relationship of CLEC-2 and galectin-1 with venous thrombosis, but human studies are lacking (10, 15–17). On the other hand, their association with PVST has never been explored yet. For this reason, this cross-sectional study aimed to evaluate the relationship between CLEC-2/galectin-1 and PVST in patients with HBV-related liver cirrhosis, and further explore the impact of degree and location of PVST on their relationship.
2. Materials and methods
2.1. Study design
This study followed the Declaration of Helsinki and was approved by the Medical Ethical Committee of the General Hospital of Northern Theater Command (approval number: Y2023-008). All patients who were admitted to the Department of Gastroenterology of the General Hospital of Northern Theater Command between January 2020 and August 2022 were selected. Inclusion criteria were as follows: (1) patients were diagnosed with HBV-related liver cirrhosis; (2) patients underwent contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI); and (3) patients had already agreed to donate their blood samples and their blood samples remained. Exclusion criteria were as follows: (1) patients with a diagnosis of malignancy or history of splenectomy, splenic artery embolization, transjugular intrahepatic portosystemic shunt, or liver transplantation, which are well-known local risk factors of PVST; (2) patients with other thrombotic diseases; (3) patients with coronary heart disease (18) or acute ischemic stroke (19), which may affect serum CLEC-2/galectin-1 level; (4) patients took anticoagulants and antiplatelet drugs within one month prior to their admissions; and (5) PVST could not be accurately determined. Repeated admissions were not excluded.
2.2. Diagnosis and definition
Diagnosis of liver cirrhosis was based on medical history, clinical manifestations, cirrhosis-related complications, laboratory tests, imaging, and histology. HBV-related liver cirrhosis would be defined, if cirrhotic patients were diagnosed with HBV infection or had positive hepatitis B surface antigens. Based on the contrast-enhanced CT/MRI imaging, PVST was defined as thrombosis within portal venous system vessels, including left portal vein, right portal vein, main portal vein (MPV), superior mesenteric vein (SMV), splenic vein (SV), or the confluence of SMV and SV (20). Based on the most severe thrombosis in any vessel of the portal venous system, the degree of thrombosis was divided into mural thrombosis (<50%), partial thrombosis (50–80%), complete thrombosis (>80%), and fibrotic cord (20).
2.3. Clinical data collection
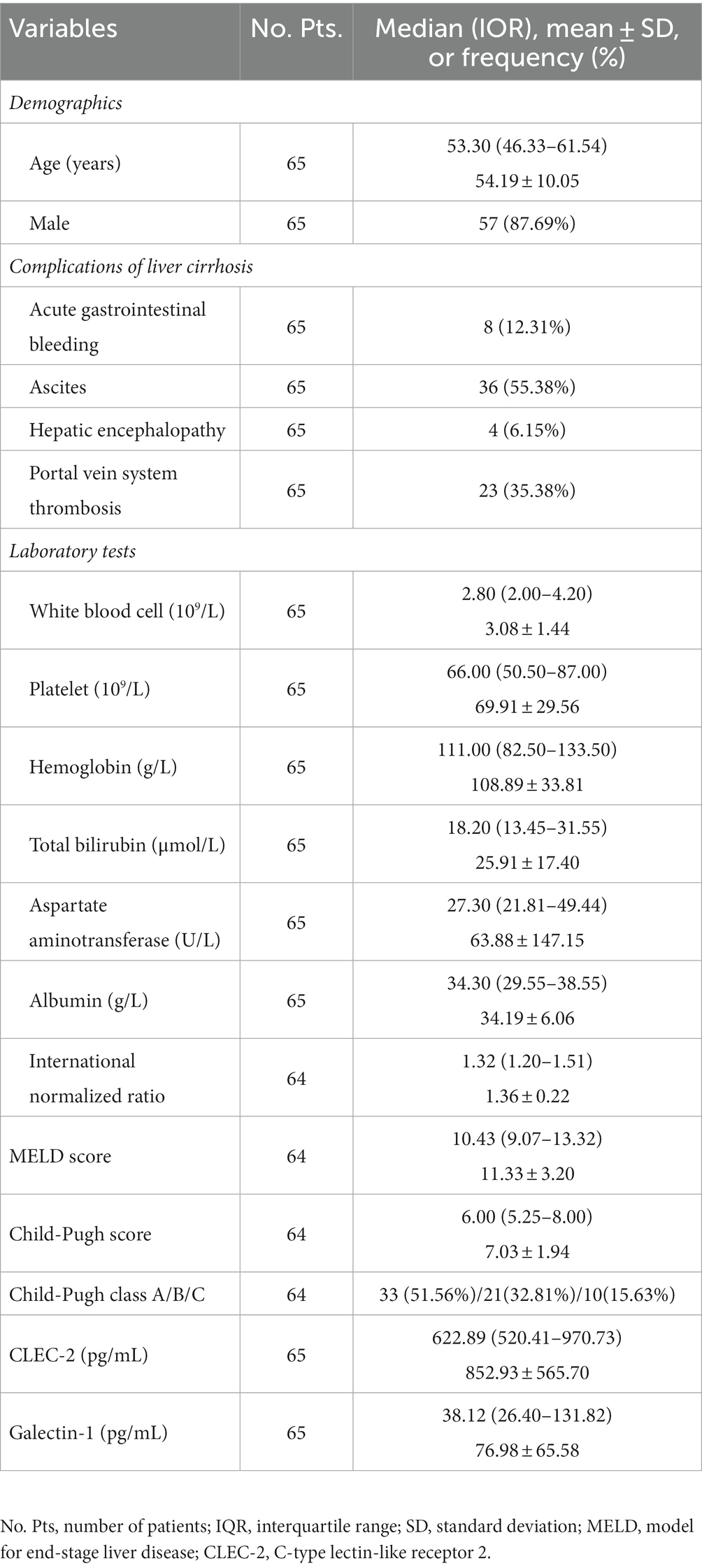
Demographics, laboratory tests (i.e., white blood cell, platelet, hemoglobin, total bilirubin, aspartate aminotransferase, albumin, and international normalized ratio), and major complications of liver cirrhosis (i.e., acute gastrointestinal bleeding, ascites, and hepatic encephalopathy) at admission were collected. Child-Pugh score and model for end-stage liver disease (MELD) score at admission were calculated (21). Data accuracy was checked by two researchers (XX and JC).
2.4. Measurement of CLEC-2 and galectin-1
Blood samples used in the present study were obtained from the remaining samples of cirrhotic patients prospectively collected by our group. After fasting for 12 h, venous blood samples were collected by gel-procoagulant tubes from cirrhotic patients and centrifuged at 3000 rpm for 10 min at room temperature. The supernatant was carefully collected to obtain serum and stored at a −80°C refrigerator until further analyses. Serum CLEC-2 (MM-2468H1, Meimian, Shanghai, China) and galectin-1 (MM-51147H1, Meimian, Shanghai, China) levels were measured using enzyme-linked immunosorbent assay kits according to the manufacturer’s instructions.
2.5. Statistical analyses
Continuous variables were expressed as median (interquartile range, [IQR]) and mean ± standard deviation, and their differences between groups were compared with the Mann–Whitney U test (if non-normal distribution) or T-test (if normal distribution). Categorical variables were expressed as frequency (percentage), and their differences between groups were compared with the Chi-square test or Fisher’s exact test. Subgroup analyses were conducted according to the degree and location of PVST. A two-tailed p < 0.05 was considered statistically significant. SPSS version 20.0 (IBM, Armonk, New York, United States) and GraphPad Prism version 8.0.1 (GraphPad software Inc., San Diego, California, USA) were used for all statistical analyses.
3. Results
3.1. Patients’ characteristics
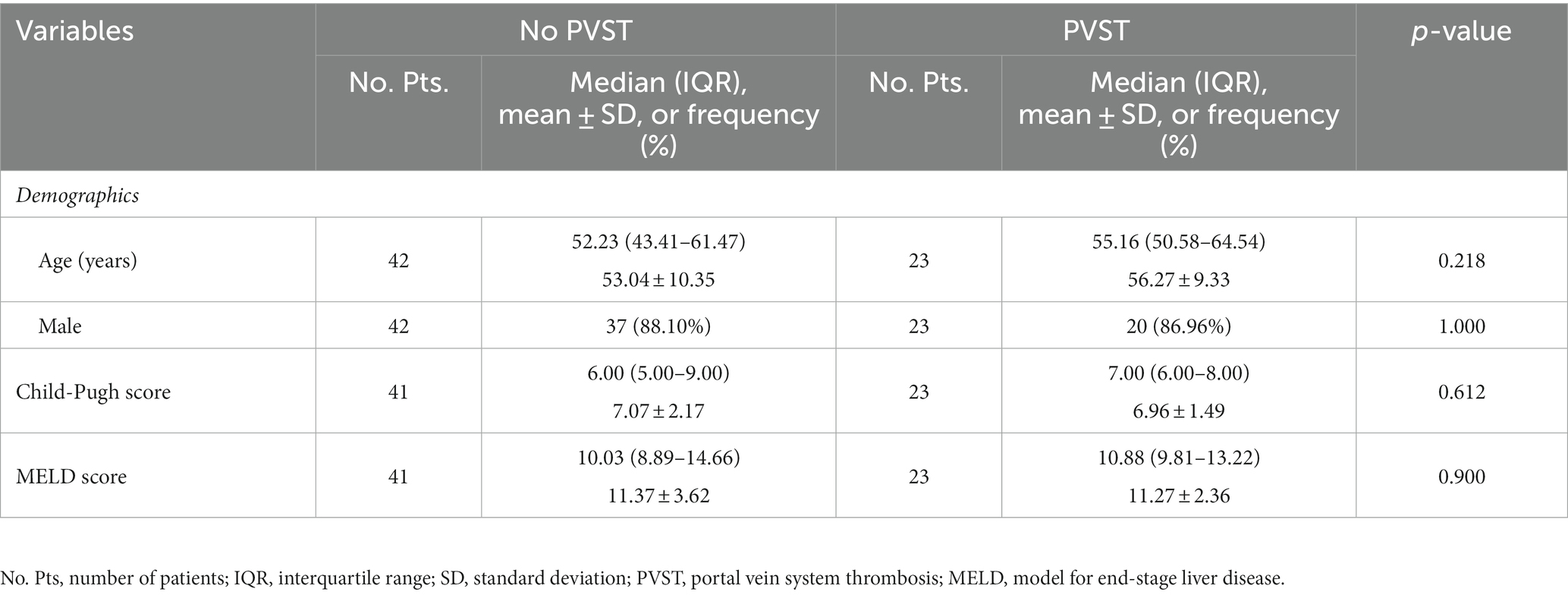
Overall, 65 patients were included (Figure 1). Their median age was 53.30 years (IQR: 46.33–61.54) and 57 (87.69%) patients were male. The median Child-Pugh score and MELD score were 6.00 (IQR: 5.25–8.00) and 10.43 (IQR: 9.07–13.32), respectively (Table 1). Twenty-three patients (35.38%) had PVST (Figure 2A). Among patients with PVST, seven (30.43%) had mural PVST and 16 (69.57%) had partial/complete PVST or fibrotic cord (Figure 2B). The most common location of PVST was MPV (17/23, 73.91%), followed by SMV (13/23, 56.52%) (Figure 2C).
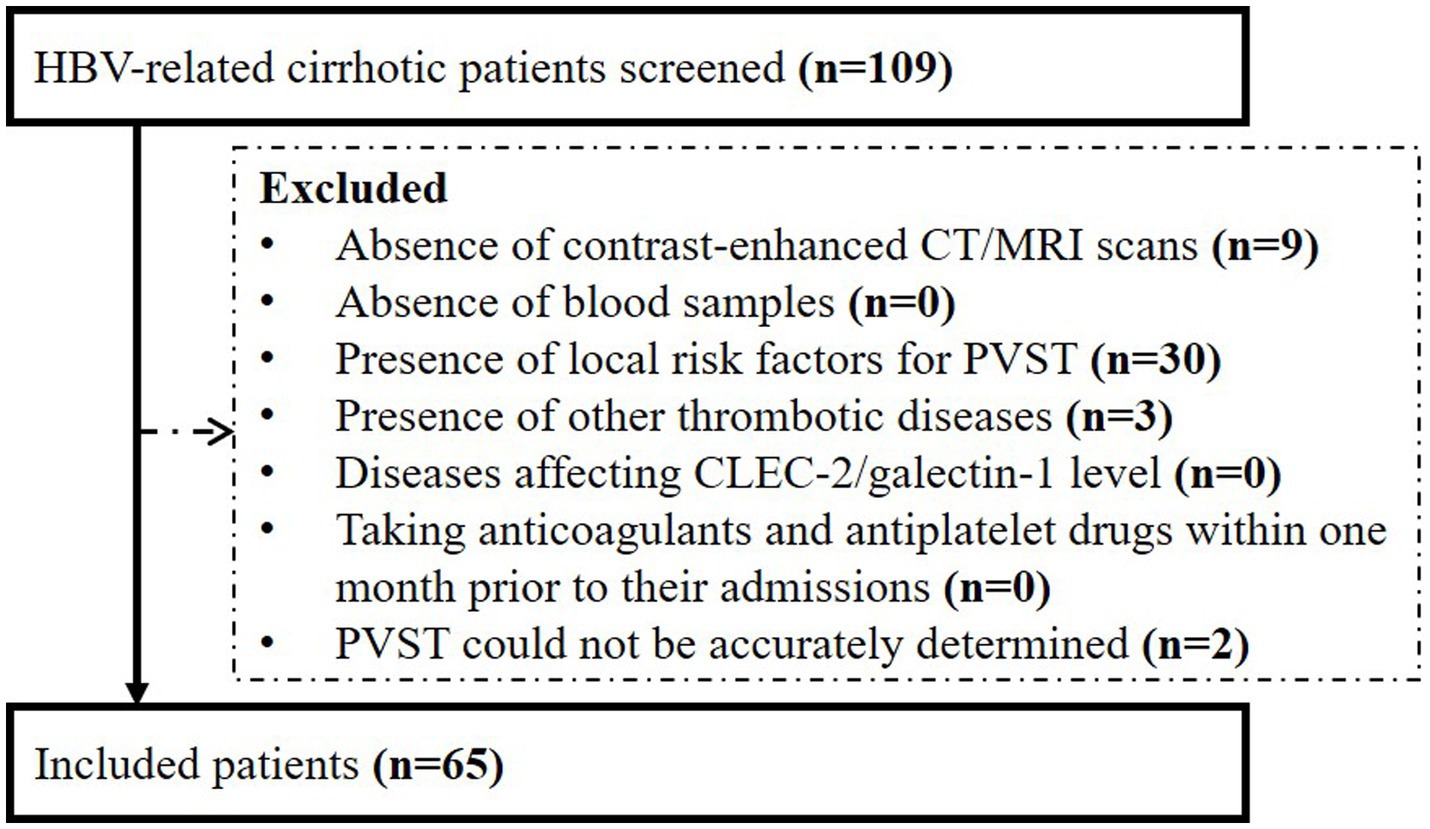
Figure 1. Flow chart of patients’ selection. HBV, hepatitis B virus; CT, computed tomography; MRI, magnetic resonance imaging; PVST, portal venous system thrombosis; CLEC-2, C-type lectin-like receptor 2.
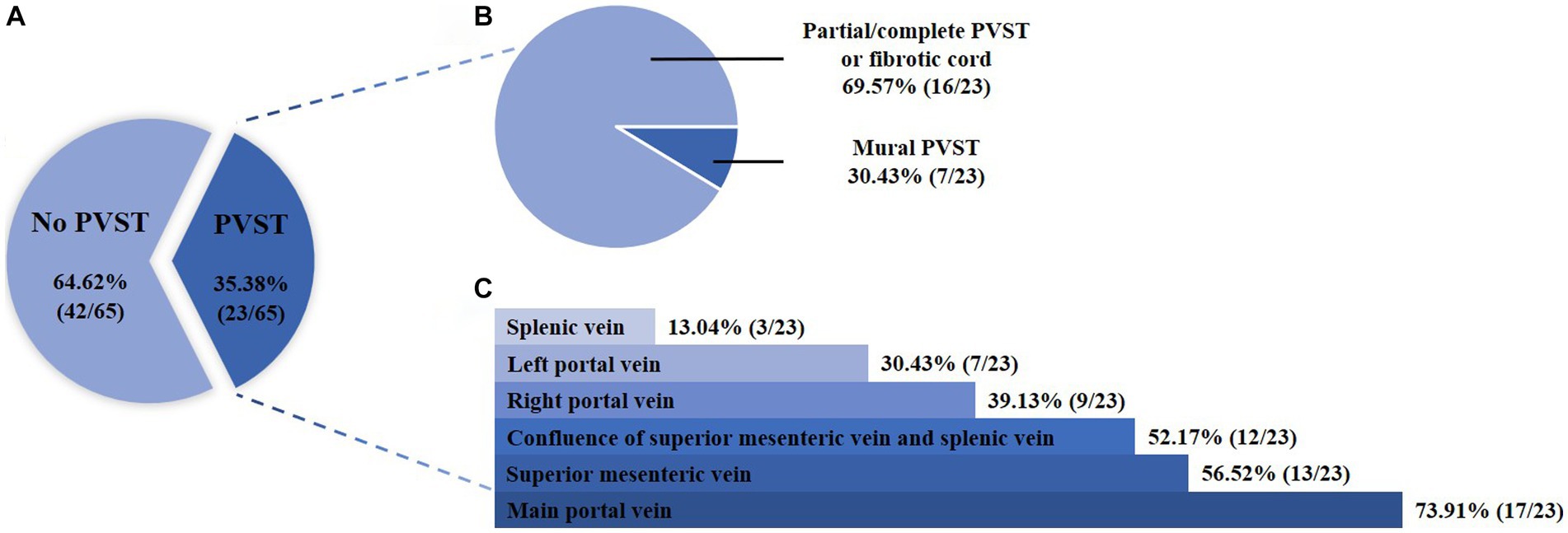
Figure 2. Proportion of patients according to the presence (A), degree (B), and location (C) of PVST. PVST, portal venous system thrombosis.
3.2. Difference according to the presence of PVST
Child-Pugh score (6.96 ± 1.49 vs. 7.07 ± 2.17, p = 0.612) and MELD score (11.27 ± 2.36 vs. 11.37 ± 3.62, p = 0.900) were statistically similar between patients with and without PVST (Table 2). Patients with PVST had significantly higher serum CLEC-2 (1035.17 ± 630.93 pg/mL vs. 753.14 ± 507.06 pg/mL, p = 0.006; Figure 3A) and galectin-1 (104.42 ± 73.37 pg/mL vs. 61.95 ± 56.32 pg/mL, p = 0.009; Figure 4A) levels than those without.
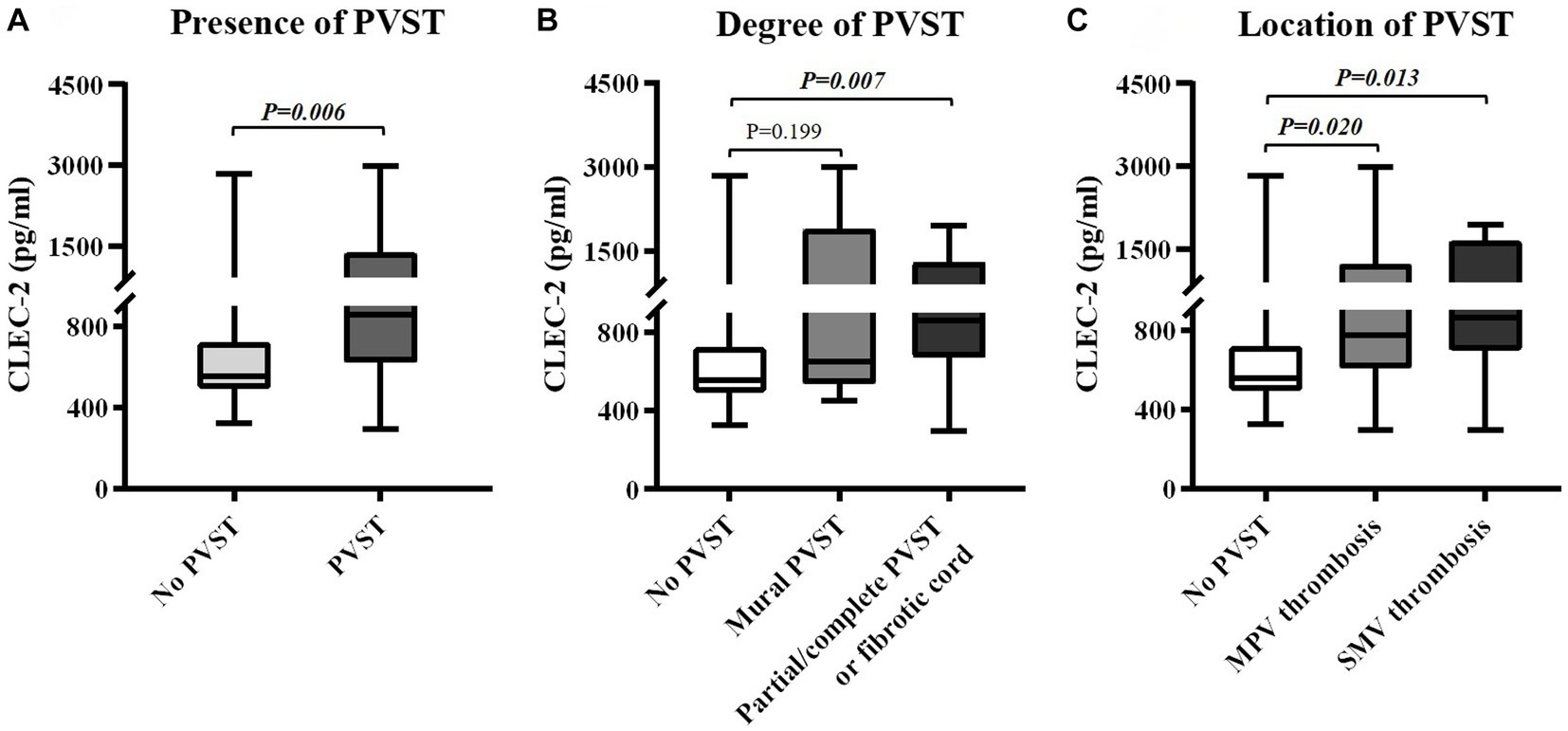
Figure 3. Differences in serum CLEC-2 levels between groups. (A) Differences in serum CLEC-2 levels between patients with versus without PVST. (B) Differences in serum CLEC-2 levels between patients with mural PVST and patients with partial/complete PVST or fibrotic cord versus those without PVST. (C) Differences in serum CLEC-2 levels between patients with MPV thrombosis and patients with SMV thrombosis versus those without PVST. CLEC-2, C-type lectin-like receptor 2; PVST, portal venous system thrombosis; MPV, main portal vein; SMV, superior mesenteric vein.
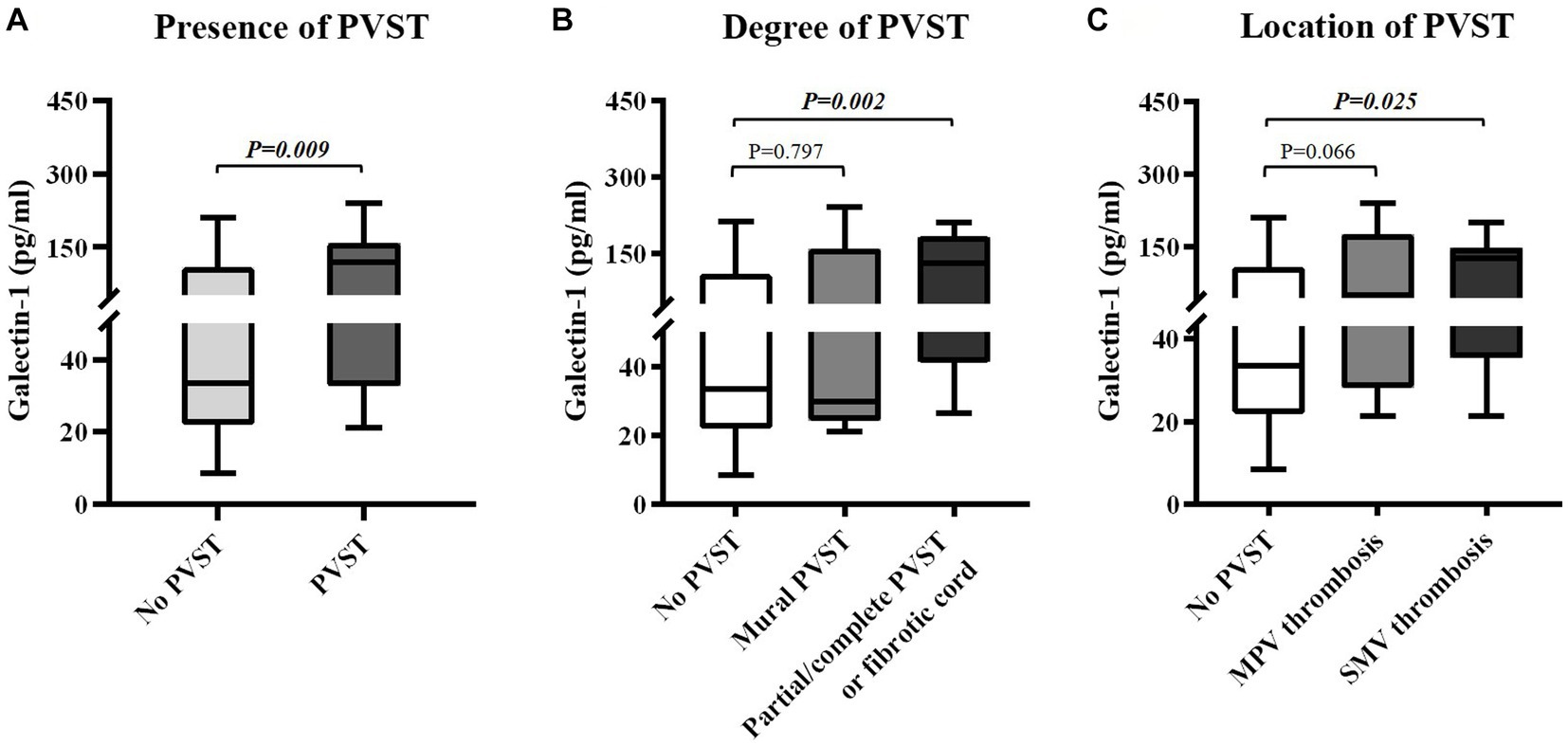
Figure 4. Differences in serum galectin-1 levels between groups. (A) Differences in serum galectin-1 levels between patients with versus without PVST. (B) Differences in serum galectin-1 levels between patients with mural PVST and patients with partial/complete PVST or fibrotic cord versus those without PVST. (C) Differences in serum galectin-1 levels between patients with MPV thrombosis and patients with SMV thrombosis versus those without PVST. PVST, portal venous system thrombosis; MPV, main portal vein; SMV, superior mesenteric vein.
3.3. Difference according to the degree of PVST
Child-Pugh score (6.71 ± 1.38 vs. 7.07 ± 2.17, p = 0.940) and MELD score (10.76 ± 3.24 vs. 11.37 ± 3.62, p = 0.681) were statistically similar between patients with mural PVST and those without PVST (Table 3). Serum CLEC-2 (1147.65 ± 951.76 pg/mL vs. 753.14 ± 507.06 pg/mL, p = 0.199; Figure 3B) and galectin-1 (76.83 ± 87.03 pg/mL vs. 61.95 ± 56.32 pg/mL, p = 0.797; Figure 4B) levels were not significantly different between patients with mural PVST and those without PVST.
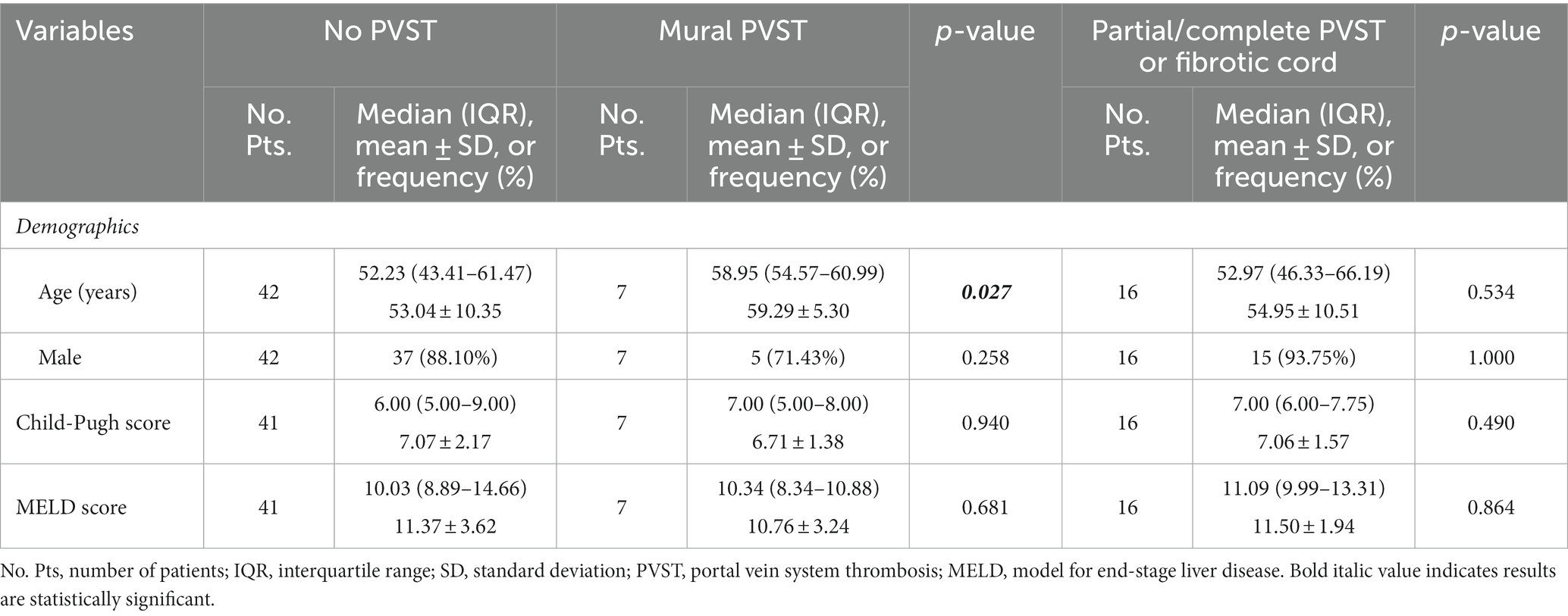
Table 3. Comparison between patients with mural PVST and those with partial/complete PVST or fibrotic cord versus those without PVST.
Child-Pugh score (7.06 ± 1.57 vs. 7.07 ± 2.17, p = 0.490) and MELD score (11.50 ± 1.94 vs. 11.37 ± 3.62, p = 0.864) were statistically similar between patients with partial/complete PVST or fibrotic cord and those without PVST (Table 3). Patients with partial/complete PVST or fibrotic cord had significantly higher serum CLEC-2 (985.96±461.53 pg/mL vs. 753.14±507.06 pg/mL, p = 0.007; Figure 3B) and galectin 1 116.49±65.98 pg/mL vs. 61.95±56.32 pg/mL, p =0.002; Figure 4B) levels than those without PVST.
3.4. Difference according to the location of PVST
Child-Pugh score (6.88 ± 1.62 vs. 7.07 ± 2.17, p = 0.834) and MELD score (10.85 ± 2.37 vs. 11.37 ± 3.62, p = 0.522) were statistically similar between patients with MPV thrombosis and those without PVST (Table 4). Patients with MPV thrombosis had higher serum CLEC-2 (1012.55 ± 674.39 pg/mL vs. 753.14 ± 507.06 pg/mL, p = 0.020; Figure 3C) and galectin-1 (96.67 ± 79.43 pg/mL vs. 61.95 ± 56.32 pg/mL, p = 0.066; Figure 4C) levels than those without PVST. The difference in serum CLEC-2 level was statistically significant between them, but not serum galectin-1 level.
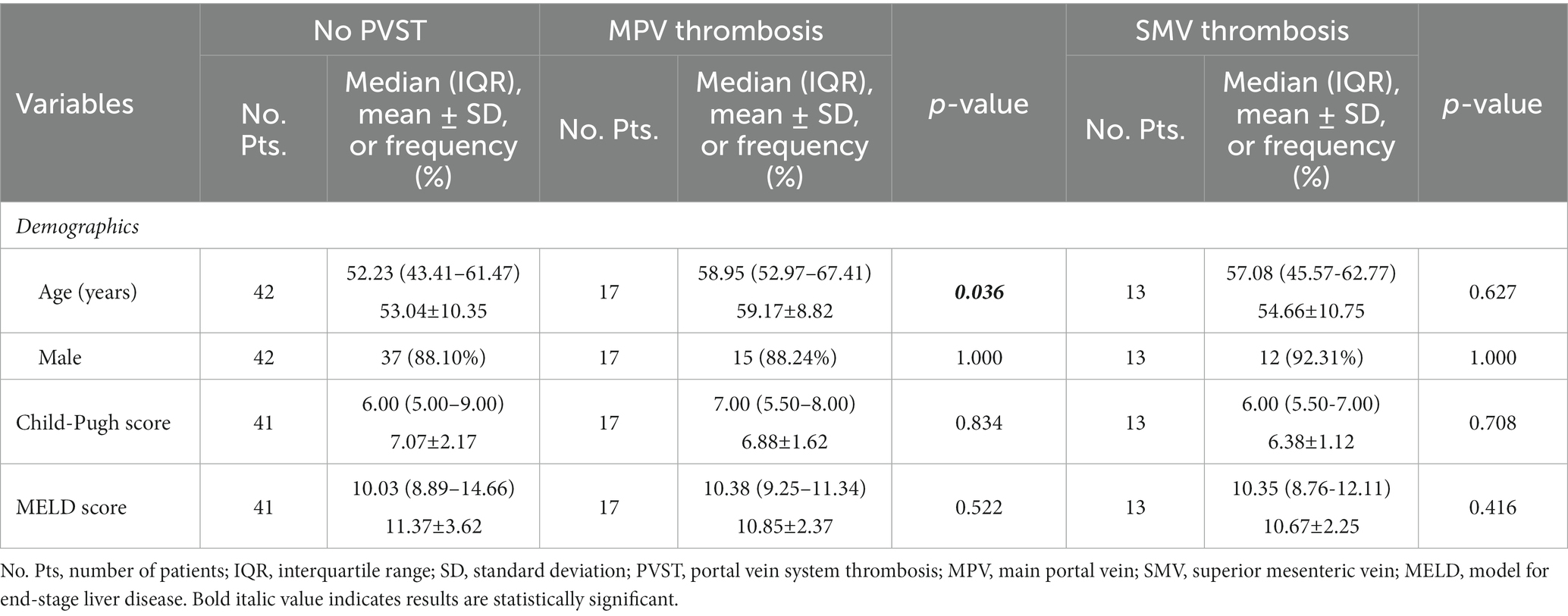
Table 4. Comparison between patients with MPV thrombosis and those with SMV thrombosis versus those without PVST.
Child-Pugh score (6.38 ± 1.12 vs. 7.07 ± 2.17, p = 0.708) and MELD score (10.67 ± 2.25 vs. 11.37 ± 3.62, p = 0.416) were statistically similar between patients with SMV thrombosis and those without PVST (Table 4). Patients with SMV thrombosis had significantly higher serum CLEC-2 (1084.65 ± 558.48 pg/mL vs. 753.14 ± 507.06 pg/mL, p = 0.013; Figure 3C) and galectin-1 (98.64 ± 61.17 pg/mL vs. 61.95 ± 56.32 pg/mL, p = 0.025; Figure 4C) levels than those without PVST.
4. Discussion
Some major findings of this study were as follows: (1) serum CLEC-2/galectin-1 level was positively associated with the presence of PVST in HBV-related cirrhotic patients; (2) patients with partial/complete PVST or fibrotic cord, but not those with mural PVST, had significantly higher serum CLEC-2 and galectin-1 levels than those without PVST; (3) patients with MPV or SMV thrombosis had a significantly higher serum CLEC-2 level than those without PVST; and (4) patients with SMV thrombosis, but not those with MPV thrombosis, had a significantly higher serum galectin-1 level than those without PVST.
Platelets are denucleated blood cells from megakaryocytes in the bone marrow (22). Thrombocytopenia, a common complication of liver cirrhosis, is mainly due to decreased synthesis of thrombopoietin in the liver and increased breakdown of platelets in the spleen (23). However, the coagulation system can be often compensated in people with liver disease (24). A reduction in the number of platelets can induce an increase in von Willebrand factor and a decrease in plasma metalloproteinase ADAMTS13, compensatively promoting platelets function (24). On the other hand, platelets adhesion, dissemination, and aggregation are essential for venous thrombosis (25). They also express phosphatidylserine, which provides procoagulant surface for prothrombinase complexes to activate coagulation cascade, and exert pro-inflammatory effects by inducing neutrophil extracellular trap formation, enhancing leukocyte recruitment, and secreting granular contents to activate coagulation system, which further promote the development of venous thrombosis (26, 27). Taken together, it should be reasonable to speculate that the change of platelets function in cirrhotic patients contributes to the development of PVST.
CLEC-2 is an important platelet-activating receptor (9). Its ligand, podoplanin, expresses near the lumen side of endothelial cell in the vascular wall, and the expression of podoplanin is increased in the presence of inflammation and blood flow obstruction (11). Systemic and local inflammation caused by viral hepatitis and increased portal pressure can damage vascular endothelial cells, allowing circulating platelet CLEC-2 to interact with subendothelial podoplanin and possibly with some other unidentified ligands (11, 28, 29). CLEC-2 binds to its ligand to induce phosphorylation of tyrosine residues in a single YXXL motif in the CLEC-2 intracellular domain (30, 31). Splenic tyrosine kinase is activated by binding its tandem Src homologous 2 domain to two tyrosine-phosphorylated YXXL motifs, subsequently triggering downstream signaling pathways that ultimately promote platelets activation and aggregation (7, 17, 32).
Extracellular galectin-1 can induce platelets activation by binding to different surface receptors on platelets (15). As potent platelets agonist, it activates the “inside-out” signal transduction pathway to promote a conformational change in the integrin αIIbβ3 on the platelets surface, changing from a low-affinity/resting state to a high-affinity/active state, exposing the high-affinity binding sites of fibrinogen (15, 33–35). At the same time, galectin-1 can also directly bind to platelets surface integrin αIIbβ3 through αIIb subunit, trigger the “outside-in” signal transduction pathway, and then promote platelets activation and aggregation (15, 34). Moreover, platelets can also express galectin-1, and galectin-1 contained in platelets may play a role in platelets activation, which requires further studies (15, 35).
Our study has for the first time demonstrated a relationship between CLEC-2/galectin-1 level and PVST in cirrhotic patients, which may be dependent upon the degree of PVST. Similarly, an animal study also showed that the progression to stable large thrombosis in CLEC-2-deficient mice was almost completely eliminated compared to the control group, suggesting the critical role of CLEC-2 in pathologically occlusive thrombosis (10). At present, both the Chinese consensus (5) and Baveno VII consensus (36) indicate that the timing of anticoagulation should be related to the degree of PVST. Therefore, both CLEC-2 and galectin-1 levels may be valuable for deciding the initiation of anticoagulant therapy.
Our study also demonstrated that serum galectin-1 level was higher in patients with MPV thrombosis than those without PVST, but this difference was not statistically significant. There were two possible explanations for this unexpected phenomenon. First, Xu et al. (37) showed that galectin-1 level in the peripheral blood of mice decreased with its age. Indeed, the mean age was significantly higher in our patients with MPV thrombosis than those without PVST, suggesting that age might weaken the difference in serum galectin-1 level between the two groups. Second, the sample size of this study was relatively insufficient, thereby compromising the statistical power.
There were some limitations in our study. First, the number of patients included was limited, thus large-scale studies are needed to further verify our results. Second, only patients with HBV-related liver cirrhosis were selected, thus further validation is needed in liver cirrhosis secondary to other etiology.
5. Conclusion
Our current findings support a relationship between CLEC-2/galectin-1 and PVST in patients with HBV-related liver cirrhosis. Thus, CLEC-2/galectin-1 may predict the risk of more severe PVST, and they should be considered as potential targets for the prevention and treatment of PVST. In future, the mechanism regarding how CLEC-2/galectin-1 influences the development of PVST needs to be explored.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study followed the Declaration of Helsinki and was approved by the Medical Ethical Committee of the General Hospital of Northern Theater Command (approval number: Y2023-008).
Author contributions
XQ: conception and design, administrative support. YZ, XX, SX, JC, and XQ: provision of study materials or patients. YZ, XX, SX, and JC: collection and assembly of data. YZ and XQ: data analysis and interpretation. YZ, XZ, XX, XG, SX, SM, JC, and XQ: manuscript writing. YZ, XZ, XX, XG, SX, SM, JC, and XQ: final approval of manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was partially supported by National Natural Science Foundation of China (8227034094).
Acknowledgments
The authors are indebted to our study team, including XX, Yang An, SX, Xiaojie Zheng, Xiaoting Song, and JC, of whom all had worked for our study group for establishing and updating the database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HBV, hepatitis B virus; PVST, portal venous system thrombosis; CLEC-2, C-type lectin-like receptor 2; CT, computed tomography; MRI, magnetic resonance imaging; MPV, main portal vein; SMV, superior mesenteric vein; SV, splenic vein; MELD, model for end-stage liver disease; IQR, interquartile range.
References
1. Romanelli, RG, and Stasi, C. Recent advancements in diagnosis and therapy of liver cirrhosis. Curr Drug Targets. (2016) 17:1804–17. doi: 10.2174/1389450117666160613101413
2. Pan, J, Wang, L, Gao, F, An, Y, Yin, Y, Guo, X, et al. Epidemiology of portal vein thrombosis in liver cirrhosis: a systematic review and meta-analysis. Eur J Intern Med. (2022) 104:21–32. doi: 10.1016/j.ejim.2022.05.032
3. Intagliata, NM, Caldwell, SH, and Tripodi, A. Diagnosis, development, and treatment of portal vein thrombosis in patients with and without cirrhosis. Gastroenterology. (2019) 156:1582–99.e1. doi: 10.1053/j.gastro.2019.01.265
4. Mantaka, A, Augoustaki, A, Kouroumalis, EA, and Samonakis, DN. Portal vein thrombosis in cirrhosis: diagnosis, natural history, and therapeutic challenges. Ann Gastroenterol. (2018) 31:315–29. doi: 10.20524/aog.2018.0245
5. Hepatobiliary Disease Study Group, Chinese Society of Gastroenterology, Chinese Medical Association . Consensus for management of portal vein thrombosis in liver cirrhosis (2020, Shanghai). J Dig Dis. (2021) 22:176–86. doi: 10.1111/1751-2980.12970
6. Martin, EM, Zuidscherwoude, M, Morán, LA, Di, Y, García, A, and Watson, SP. The structure of CLEC-2: mechanisms of dimerization and higher-order clustering. Platelets. (2021) 32:733–43. doi: 10.1080/09537104.2021.1906407
7. Suzuki-Inoue, K, Inoue, O, and Ozaki, Y. The novel platelet activation receptor CLEC-2. Platelets. (2011) 22:380–4. doi: 10.3109/09537104.2011.556274
8. Bourne, JH, Smith, CW, Jooss, NJ, Di, Y, Brown, HC, Montague, SJ, et al. CLEC-2 supports platelet aggregation in mouse but not human blood at arterial shear. Thromb Haemost. (2022) 122:1988–2000. doi: 10.1055/a-1896-6992
9. Meng, D, Luo, M, and Liu, B. The role of CLEC-2 and its ligands in Thromboinflammation. Front Immunol. (2021) 12:688643. doi: 10.3389/fimmu.2021.688643
10. May, F, Hagedorn, I, Pleines, I, Bender, M, Vögtle, T, Eble, J, et al. CLEC-2 is an essential platelet-activating receptor in hemostasis and thrombosis. Blood. (2009) 114:3464–72. doi: 10.1182/blood-2009-05-222273
11. Payne, H, Ponomaryov, T, Watson, SP, and Brill, A. Mice with a deficiency in CLEC-2 are protected against deep vein thrombosis. Blood. (2017) 129:2013–20. doi: 10.1182/blood-2016-09-742999
12. Malik, RK, Ghurye, RR, Lawrence-Watt, DJ, and Stewart, HJ. Galectin-1 stimulates monocyte chemotaxis via the p44/42 MAP kinase pathway and a pertussis toxin-sensitive pathway. Glycobiology. (2009) 19:1402–7. doi: 10.1093/glycob/cwp077
13. Camby, I, Le Mercier, M, Lefranc, F, and Kiss, R. Galectin-1: a small protein with major functions. Glycobiology. (2006) 16:137r–57r. doi: 10.1093/glycob/cwl025
14. Nickel, W . Unconventional secretory routes: direct protein export across the plasma membrane of mammalian cells. Traffic (Copenhagen, Denmark). (2005) 6:607–14. doi: 10.1111/j.1600-0854.2005.00302.x
15. Romaniuk, MA, Croci, DO, Lapponi, MJ, Tribulatti, MV, Negrotto, S, Poirier, F, et al. Binding of galectin-1 to αIIbβ₃ integrin triggers "outside-in" signals, stimulates platelet activation, and controls primary hemostasis. FASEB J. (2012) 26:2788–98. doi: 10.1096/fj.11-197541
16. Hughes, CE, Pollitt, AY, Mori, J, Eble, JA, Tomlinson, MG, Hartwig, JH, et al. CLEC-2 activates Syk through dimerization. Blood. (2010) 115:2947–55. doi: 10.1182/blood-2009-08-237834
17. Izquierdo, I, Barrachina, MN, Hermida-Nogueira, L, Casas, V, Morán, LA, Lacerenza, S, et al. A comprehensive tyrosine Phosphoproteomic analysis reveals novel components of the platelet CLEC-2 Signaling Cascade. Thromb Haemost. (2020) 120:262–76. doi: 10.1055/s-0039-3400295
18. Fei, M, Xiang, L, Chai, X, Jin, J, You, T, Zhao, Y, et al. Plasma soluble C-type lectin-like receptor-2 is associated with the risk of coronary artery disease. Front. Med. (2020) 14:81–90. doi: 10.1007/s11684-019-0692-x
19. Zhang, X, Zhang, W, Wu, X, Li, H, Zhang, C, Huang, Z, et al. Prognostic significance of plasma CLEC-2 (C-type lectin-like receptor 2) in patients with acute ischemic stroke. Stroke. (2019) 50:45–52. doi: 10.1161/STROKEAHA.118.022563
20. Wang, L, Guo, X, Bai, Z, Yin, Y, Xu, S, Pan, J, et al. Impact of asymptomatic superior mesenteric vein thrombosis on the outcomes of patients with liver cirrhosis. Thromb Haemost. (2022) 122:2019–29. doi: 10.1055/s-0042-1756648
21. Peng, Y, Qi, X, and Guo, X. Child-Pugh versus MELD score for the assessment of prognosis in liver cirrhosis: a systematic review and Meta-analysis of observational studies. Medicine. (2016) 95:e2877. doi: 10.1097/MD.0000000000002877
22. Jurk, K, and Kehrel, BE. Platelets: physiology and biochemistry. Semin Thromb Hemost. (2005) 31:381–92. doi: 10.1055/s-2005-916671
23. Hugenholtz, GGC, Porte, RJ, and Lisman, T. The platelet and platelet function testing in liver disease. Clin Liver Dis. (2009) 13:11–20. doi: 10.1016/j.cld.2008.09.010
24. Lisman, T, and Porte, RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. (2010) 116:878–85. doi: 10.1182/blood-2010-02-261891
25. Koupenova, M, Clancy, L, Corkrey, HA, and Freedman, JE. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ Res. (2018) 122:337–51. doi: 10.1161/CIRCRESAHA.117.310795
26. Zhao, L, Bi, Y, Kou, J, Shi, J, and Piao, D. Phosphatidylserine exposing-platelets and microparticles promote procoagulant activity in colon cancer patients. J Exp Clin Cancer Res. (2016) 35:54. doi: 10.1186/s13046-016-0328-9
27. Stevens, H, and McFadyen, JD. Platelets as central actors in thrombosis-reprising an old role and defining a new character. Semin Thromb Hemost. (2019) 45:802–9. doi: 10.1055/s-0039-1698829
28. Squizzato, A, and Gerdes, VE. Viral hepatitis and thrombosis: a narrative review. Semin Thromb Hemost. (2012) 38:530–4. doi: 10.1055/s-0032-1305783
29. Li, MX, Zhang, XF, Liu, ZW, and Lv, Y. Risk factors and clinical characteristics of portal vein thrombosis after splenectomy in patients with liver cirrhosis. Hepatob Panc Dis Int. (2013) 12:512–9. doi: 10.1016/S1499-3872(13)60081-8
30. Watson, AA, Christou, CM, James, JR, Fenton-May, AE, Moncayo, GE, Mistry, AR, et al. The platelet receptor CLEC-2 is active as a dimer. Biochemistry. (2009) 48:10988–96. doi: 10.1021/bi901427d
31. O'Callaghan, CA . Thrombomodulation via CLEC-2 targeting. Curr Opin Pharmacol. (2009) 9:90–5. doi: 10.1016/j.coph.2008.11.001
32. Rayes, J, Watson, SP, and Nieswandt, B. Functional significance of the platelet immune receptors GPVI and CLEC-2. J Clin Invest. (2019) 129:12–23. doi: 10.1172/JCI122955
33. Romaniuk, MA, Negrotto, S, Campetella, O, Rabinovich, GA, and Schattner, M. Identification of galectins as novel regulators of platelet signaling and function. IUBMB Life. (2011) 63:521–7. doi: 10.1002/iub.483
34. Li, Z, Delaney, MK, O'Brien, KA, and Du, X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. (2010) 30:2341–9. doi: 10.1161/ATVBAHA.110.207522
35. Pacienza, N, Pozner, RG, Bianco, GA, D'Atri, LP, Croci, DO, Negrotto, S, et al. The immunoregulatory glycan-binding protein galectin-1 triggers human platelet activation. FASEB J. (2008) 22:1113–23. doi: 10.1096/fj.07-9524com
36. de Franchis, R, Bosch, J, Garcia-Tsao, G, Reiberger, T, and Ripoll, C. Baveno VII - renewing consensus in portal hypertension. J Hepatol. (2022) 76:959–74. doi: 10.1016/j.jhep.2021.12.022
Keywords: portal venous system thrombosis, C-type lectin-like receptor 2, galectin-1, hepatitis B virus infection, liver cirrhosis
Citation: Zhang Y, Zhang X, Xu X, Guo X, Xu S, Ma S, Chen J and Qi X (2023) Association of C-type lectin-like receptor 2 and galectin-1 with portal vein system thrombosis in HBV-related liver cirrhosis. Front. Med. 10:1228636. doi: 10.3389/fmed.2023.1228636
Edited by:
Carlos Jerjes-Sanchez, Tecnológico de Monterrey, MexicoCopyright © 2023 Zhang, Zhang, Xu, Guo, Xu, Ma, Chen and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingshun Qi, eGluZ3NodW5xaUAxMjYuY29t
†These authors share first authorship
 Yiyan Zhang1,2†
Yiyan Zhang1,2† Shixue Xu
Shixue Xu Xingshun Qi
Xingshun Qi