- 1Department of Dermatology, George Emil Palade University of Medicine, Pharmacy, Science and Technology of Targu Mures, Targu Mures, Romania
- 2CMI Dermamed Private Medical Office, Targu Mures, Romania
- 3Department of Electrical Engineering and Information Technology, George Emil Palade University of Medicine, Pharmacy, Science and Technology of Targu Mures, Targu Mures, Romania
- 4National Institute of Public Health, Regional Center for Public Health, Targu Mures, Romania
- 5Doctoral School, George Emil Palade University of Medicine, Pharmacy, Science and Technology of Targu Mures, Targu Mures, Romania
Introduction: Embolia cutis medicamentosa or Nicolau syndrome is a rare drug reaction associated with the administration of various injectable medications. The pathogenesis of the disease is unknown, though intra and periarterial injection of the drug is a possible cause. The aim of this study was to describe and analyze the clinical characteristics of Nicolau syndrome in patients examined in daily dermatological practice.
Methods: We performed a retrospective chart review, between January 2011 and December 2020, in patients diagnosed with Nicolau syndrome, from the cases of a private dermatology medical office in Târgu Mureș, Romania.
Results: During the 10-year period, 7 patients were diagnosed with Nicolau syndrome. Of these, 4 (57%) patients were males and 3 (43%) were females, The male to female ratio was 1.33. The median age was 64 (interquartile range, IQR, 62–71), with the youngest patient being diagnosed at age 61 and the oldest at age 74. Regarding the drugs classes that caused Nicolau syndrome, these were intravenous antibiotics in 57%, and non-steroidal anti-inflammatory drugs in 43% of cases.
Conclusion: All patients healed in a period of 6 to 8 weeks. No complications occurred. In conclusion, Nicolau syndrome is a rare side effect of injectable drug administration.
1. Introduction
Nicolau syndrome was first described in the early 1920s by Nicolau as an adverse effect of using intramuscular injections of bismuth salts in the treatment of syphilis (1). Since then, several case reports of this disease occurring after intramuscular, intra-articular, intravenous, and subcutaneous injections, especially in an oily or suspension form, have appeared in the literature associated with a large variety of drugs (2). The pathogenesis of the disease is unknown, though intra and periarterial injection of the drug is a possible cause (3). Stimulation of the sympathetic nerve due to periarterial injection causes spasms and consequent ischemia. Inadvertent intra-arterial injections may cause artery and branch embolization and occlusion, associated with artery wall irritation. Lipophilic drugs can produce fat embolization and cytotoxic drugs may produce inflammation with tissue necrosis. An acute ischemia syndrome of the segmental skin area can occur. The extent and severity of the lesions are closely related to the size of the affected artery. Necrosis ensues in this stage, with possible ulceration (4, 5).
The purpose of this retrospective review was to investigate and chronicle the clinical and disease progression of Nicolau syndrome in patients encountered during routine practice in order to uncover shared characteristics that might foster the medical understanding of the disease.
2. Materials and methods
We performed a retrospective chart review between January 2011 and December 2020, in patients diagnosed with Nicolau syndrome, from the cases of a private dermatology medical office in Târgu Mureș, Romania. All patients were Caucasian. Written informed consent of the patients was obtained at the moment of consultation. When making the decision to write this paper, some of the patients were personally invited to the office, and others were contacted by phone to have the study explained and reconfirm the informed consent on the processing of patient personal data. The diagnosis was established by dermatological clinical examination. One investigator evaluated patients and collected data (general data, disease onset, clinical aspect, and evolution, relevant personal history, and present comorbidities). Patients were followed-up during treatment until healing by clinical examination and /or telephone interview. Other investigators, who were blinded to the clinical cases, performed data analysis and interpreted the results.
3. Results
Table 1 presents the history and clinical findings. During the 10-year period, 7 patients were diagnosed with Nicolau syndrome. From these, 4 (57%) patients were males and 3 (43%) were females. The male to female ratio was 1.33. The median age was 64 (IQR 62–71) years, with the youngest patient being diagnosed at age 61 and the oldest at age 74.
The disease manifested at a median of 24 (IQR 24–36) hours after injection. In 4 out of 7 cases, the lesions appeared within 24 h, while in 3 cases they appeared 36 h after injection. In 4 cases, the lesions were situated on the anterior area of the forearm, the rest on the dorsal area of the fist. Regarding the clinical appearance of the lesions, all of them were red-purple plaques, between 5 and 8 cm in diameter, with a livedoid aspect, centrally necrotic, very painful, with well-defined edges and geographic contours. The lesions were round-oval, apart from one case in which they were rectangular, after using an intravenous branula (Figure 1).
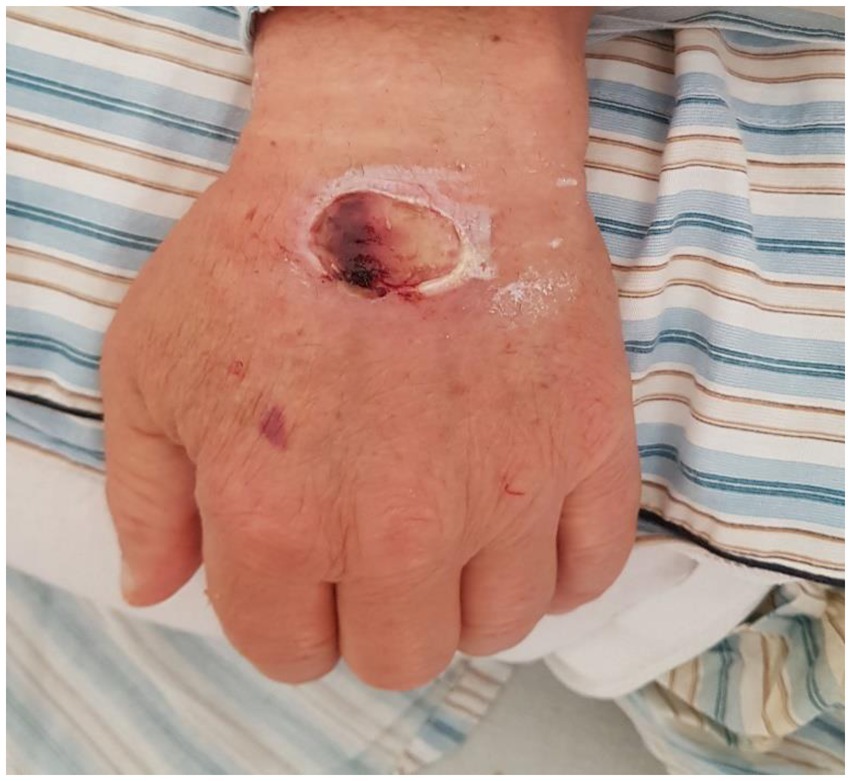
Figure 1. A case of Nicolau syndrome showing a well-defined, oval ulcer with necrotic base on the dorsum of the left hand.
For the verification of outliers, we have applied the Grubbs test (6, 7), which did not detect any outlier.
For all patients the treatment was topical, and consisted of the use of antibiotics and epithelializing ointments, with good results. In some cases, however, surgical debridement was necessary. All patients recovered within a period of 6 to 8 weeks. No complications occurred. All patients suffered from multiple morbid conditions, which was the reason for prescribing injectable, intravenous treatments. Regarding the drugs that caused Nicolau syndrome, in 4 cases it was intravenous antibiotics, and in 3 cases non-steroidal anti-inflammatory drugs. In the group of antibiotics, we found 3 cases of Cefuroxim 1 g as a causative agent 2x1g /day, used intravenously, and in one case Ciprofloxacin 400 mg, 2×400 mg/ day intravenously. Regarding the group of non-steroidal anti-inflammatory drugs in all the 3 cases, the drug was Diclofenac 75 mg, used intravenously (Table 1).
Table 2, row labeled “time to resolution,” presents a descriptive statistic of the healing time. For the verification of outliers, we used the Grubbs test, which did not detect any outlier.
4. Discussions and conclusion
Embolia cutis medicamentosa or Nicolau syndrome is a rare drug reaction associated with the administration of various injectable medications. It is a rare disease, and the true incidence is unknown. The disease can occur at any age, depending on the need to administer intravenous, muscular, or intra-arterial drugs, being linked to the presence of severe comorbidity. The patient’s data analyzed in this study was carefully collected and recorded in a database during the 10-year period. Over this time, we detected 7 patients, 4 males and 3 females, median age 64 years, having suffered from Nicolau syndrome.
Mojjarad et al., analyzing 135 cases from multiple databases on Nicolau syndrome and concluded that the disease can occur mainly in females of any age group, but mainly in children and the age group 31–40 years (8). Regarding the onset of the disease due to the appearance of pain at the level of injection, in our cases, the latency was between 24 and 36 h, which corresponds to the data from meta-analytical studies (8). The clinical appearance of the skin lesions is identical in each case, regardless of the cause, the site of injection being a single round oval lesion with a diameter between 5 and 8 cm, well delimited, with ulceration and necrosis on the surface. In our retrospective case series, the healing period was between 6 and 8 weeks, regardless of the causative agent or location. The prescribed treatments were similar in these cases and the disease course was favorable. Local treatments included antibiotics, epithelisants, magistral prescriptions with Silver nitrate, and Peru Balsam used in difficult-to-heal ulcers (9). In our cases, previously reported complications such as fasciitis, superinfections, amputations, or deaths did not occur (10–13). A multitude of drugs can cause this disease, among which the most important groups of drugs are non-steroidal anti-inflammatory drugs and antibiotics. In a review assessing 145 articles, Mojjarad et al. found that the most common causes of the syndrome are diclofenac (35 articles, 24%) and penicillins (32 articles, 22%) (8). In our cases, the cause of the injection was Diclofenac in 3 patients; Cefuroxime in 3 patients and Ciprofloxacin in 1 patient. According to previously published literature, Gentamicin (14) and Penicillins (15, 16) are among the most frequently reported antibiotics causing Nicolau syndrome. Among the non-steroidal anti-inflammatory drugs, Diclofenac is the most frequently reported drug, followed by other drugs such as Naltroxene, Etofenamate, and Ketofrofenid (17–20). Cases of Nicolau syndrome caused by dermatocosmetic procedures such as hyaluronic acid injections (21), meso, and sclerotherapy (22, 23) have been described. Other drug classes have been mentioned as causative factors such as: triamcinolone (24), glatiramer acetate (25), terlipressin (26), bortezomib (27), hydroxyzine (28), interferon alfa (29), etc. At the same time, it may occur as a local reaction after the administration of vaccines such as the hexavalent vaccine (30), or the DTP vaccine (31). In conclusion, Nicolau syndrome is a rare side effect of injectable drugs which can be severe.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Dermamed private office ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the participant/patient(s) for the publication of this article.
Author contributions
GF, LF, and JF: conceptualization, methodology, validation, formal analysis, investigation, and writing—review and editing. LI: software, data curation, and writing—original draft preparation. GF and LF: resources. GF, LF, JF, and LI: visualization. GF: supervision, project administration and funding acquisition. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank the Research Center on Artificial Intelligence, Data Science, and Smart Engineering (ARTEMIS) for the research infrastructure support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nicolau, S. Dermite livédoïde et gangréneuse de la fesse, consécutive aux injections intra-musculaires, dans la syphilis. A propos d’un cas d’embolie artérielle bismuthique, Annales des maladies vénériennes. (1925) 20:321–39.
2. Agarwal, A, Kabra, A, Jain, R, and Bhargava, G. Nicolau’s Syndrome (Embolia Cutis Medicamentosa). J Assoc Physicians India. (2018) 66:85.
3. Brachtel, R, and Meinertz, T. Local skin necroses after intramuscular injection-experimental animal studies. Arch Dermatol Res. (1977) 258:281–8. doi: 10.1007/BF00561131
5. Gulseren, D, Sahin, EB, Bozdogan, O, and Artuz, F. An avoidable adverse drug reaction: Nicolau syndrome. Int Wound J. (2017) 14:440–1. doi: 10.1111/iwj.12663
6. Grubbs, FE. Procedures for detecting outlying observations in samples. Technometrics. (1969) 11:1–21. doi: 10.1080/00401706.1969.10490657
7. Stefansky, W. Rejecting outliers in factorial designs. Technometrics. (1972) 14:469–79. doi: 10.1080/00401706.1972.10488930
8. Mojarrad, P, Mollazadeh, H, Barikbin, B, and Oghazian, MB. Nicolau syndrome: a review of case studies. Pharm Sci. (2022) 28:27–38. doi: 10.34172/PS.2021.32
9. Dixon, D, and Edmonds, M. Managing diabetic foot ulcers: pharmacotherapy for wound healing. Drugs. (2021) 81:29–56. doi: 10.1007/s40265-020-01415-8
10. Stanescu, AMA, Totan, A, Mircescu, D, Diaconescu, S, Bratu, OG, Fekete, L, et al. Assessment of suicidal behavior in dermatology (review). Exp Ther Med. (2020) 20:73–7. doi: 10.3892/etm.2019.8145
11. Kim, KK, and Chae, DS. Nicolau syndrome: A literature review. World J Dermatol. (2015) 4:103–7. doi: 10.5314/wjd.v4.i2.103
12. Masthan, SD, Reddy, KC, and Sridevi, L. Nicolau syndrome. Indian J Dermatol Venereol Leprol. (2002) 68:45–6.
13. Tierce, ML, Schultz, SM, and Lanier, BQ. Tissue loss with subcutaneous immunotherapy--Nicolau syndrome. J Allergy Clin Immunol Pract. (2016) 4:154–5. doi: 10.1016/j.jaip.2015.07.014
14. Kim, DH, Ahn, HH, Kye, YC, and Choi, JE. Nicolau syndrome involving whole ipsilateral limb induced by intramuscular administration of gentamycin. Indian J Dermatol Venereol Leprol. (2014) 80:96. doi: 10.4103/0378-6323.125516
15. Lopes, L, Filipe, P, Alves, A, Guerreiro, F, and Pires, S. Nicolau syndrome after benzathine penicillin treated with hyperbaric oxygen therapy. Int J Dermatol. (2015) 54:e103–6. doi: 10.1111/ijd.12751
16. Noaparast, M, Mirsharifi, R, Elyasinia, F, Parsaei, R, Kondori, H, and Farifteh, S. Nicolau syndrome after intramuscular benzathine penicillin injection. Iran J Med Sci. (2014) 39:577–9.
17. Perli, D, Martone, C, and Rapose, A. Naltrexone-induced Nicolau syndrome masquerading as cutaneous abscess. BMJ Case Rep. (2012) 2012:bcr2012007785. doi: 10.1136/bcr-2012-007785
18. Malik, MH, Heaton, H, and Sloan, B. Nicolau syndrome following intramuscular naltrexone injection. Dermatol Online J. (2020) 26:17. doi: 10.5070/D3267049568
19. Ozlu, E, Baykan, A, Ertas, R, Ulas, Y, Ozyurt, K, and Avci, A. Case report: Nicolau syndrome due to etofenamate injection. F1000Res. (2017) 6:867. doi: 10.12688/f1000research.11705.1
20. Lardelli, PF, Jermini, LMM, Milani, GP, Peeters, GGAM, Ramelli, GP, Zgraggen, L, et al. Nicolau syndrome caused by non-steroidal anti-inflammatory drugs: systematic literature review. Int J Clin Pract. (2020) 74:e13567. doi: 10.1111/ijcp.13567
21. Andre, P, and Haneke, E. Nicolau syndrome due to hyaluronic acid injections. J Cosmet Laser Thera. (2016) 18:239–44. doi: 10.3109/14764172.2016.1157260
22. Zaragoza, J, Delaplace, M, Benamara, M, and Esteve, E. A rare side effect of mesotherapy: Nicolau syndrome. Ann Dermatol Venereol. (2013) 140:713–7. doi: 10.1016/j.annder.2013.07.009
23. Bieleveld, LM, and Aldenzee, MJ. A young woman with skin necrosis after sclerotherapy. Ned Tijdschr Geneeskd. (2015) 159:A9074.
24. Grover, C, Kharghoria, G, Daulatabad, D, and Bhattacharya, SN. Nicolau syndrome following intramatricial triamcinolone injection for nail lichen planus. Indian Dermatol Online J. (2017) 8:350–1. doi: 10.4103/idoj.IDOJ_333_16
25. Ciprian, S, Lava, SAG, Milani, GP, Bianchetti, MG, Consolascio, D, and Lardelli, PF. Nicolau syndrome caused by Glatiramer. Mult Scler Relat Disord. (2022) 57:103365. doi: 10.1016/j.msard.2021.103365
26. Polychronis, GG, Stephanos, K, Panayiotides, IG, Dimitriadis, GD, and Konstantinos, T. Embolia cutis medicamentosa: an unusual adverse reaction to terlipressin. Ann Gastroenterol. (2017) 30:700–3. doi: 10.20524/aog.2017.0158
27. Almudimeegh, A, Pelletier, F, and Dupin, N. Nicolau syndrome secondary to subcutaneous bortezomib injection. J Eur Acad Dermatol Venereol. (2014) 30:348–50. doi: 10.1111/jdv.12759
28. Gayken, J, Westanmo, A, Knutsen, A, Ahrenholz, DH, Mohr, IIIWJ, and Solem, LD. Livedoid dermatitis and severe necrosis (Nicolau’s syndrome) after intramuscular hydroxyzine injection. J Burn Care Res. (2006) 27:541–4. doi: 10.1097/01.BCR.0000225917.09339.03
29. Rasokat, H, Bendick, C, Wemmer, U, and Steigleder, GK. Aseptic skin necrosis after subcutaneous injection of interferon-alfa. (Aseptische hautnekrose nach subkutaner injektion von interferon-alpha.). Dtsch Med Wochenschr. (1989) 114:458–60. doi: 10.1055/s-2008-1066618
30. Stefano, PC, Garello, M, Nolte, MF, Lamy, P, Giglio, N, and Castellano, V. Nicolau syndrome induced by intramuscular injection of a hexavalent vaccine in a 6-month-old girl. Arch Argent Pediatr. (2017) 115:e13–6. doi: 10.5546/aap.2017.e13
Keywords: adverse drug reaction, Nicolau syndrome, embolia cutis, cutaneous gangrene, rare drug reaction
Citation: Fekete GL, Iantovics LB, Fekete JE and Fekete L (2023) Embolia cutis Medicamentosa (Nicolau syndrome): case series. Front. Med. 10:1216781. doi: 10.3389/fmed.2023.1216781
Edited by:
Lawrence Chukwudi Nwabudike, Nutrition and Metabolic Diseases N. Paulescu, RomaniaReviewed by:
Alin Laurentiu Tatu, Dunarea de Jos University, RomaniaSebastiano A. G. Lava, Centre Hospitalier Universitaire Vaudois (CHUV), Switzerland
Copyright © 2023 Fekete, Iantovics, Fekete and Fekete. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laszlo Barna Iantovics, YmFybmEuaWFudG92aWNzQHVtZnN0LnJv
†These authors have contributed equally to this work
 Gyula Laszlo Fekete
Gyula Laszlo Fekete Laszlo Barna Iantovics
Laszlo Barna Iantovics Júlia Edit Fekete4†
Júlia Edit Fekete4†