
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 31 May 2023
Sec. Obstetrics and Gynecology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1211070
This article is part of the Research Topic Update on Diagnostic and Prognostic Biomarkers for Women's Cancers View all 12 articles
Background: Primary ovarian insufficiency (POI) leads to not only infertile but several adverse health events to women. Traditional treatment methods have their own set of limitations and drawbacks that vary in degree. Application of human umbilical cord mesenchymal stem cell (hUCMSC) is a promising strategy for POI. However, there is a lack of literatures on application of hUCMSC in human. Animal experimental model, however, can reflect the potential effectiveness of this employment. This study aimed to evaluate the curative effect of hUCMSC on animals with POI on a larger scale.
Methods: To gather data, Pubmed, Embase, and Cochrane Library were searched for studies published up to April 2022. Various indices, including the animals' estrous cycle, serum sex hormone levels, and follicle number in the ovary, were compared between the experimental group and those with Premature Ovarian Insufficiency (POI).
Results: The administration of human umbilical cord-derived mesenchymal stem cells (hUCMSC) has been shown to significantly improve the estrous cycle (RR: 3.32, 95% CI: [1.80, 6.12], I2 = 0%, P = 0.0001), but robustly decrease its length (SMD: −1.97, 95% CI: [−2.58, −1.36], I2 = 0%, P < 0.00001). It can also strikingly increase levels of serum estradiol (SMD: 5.34, 95% CI: [3.11, 7.57], I2 = 93%, P < 0.00001) and anti-müllerian hormone (SMD: 1.92, 95% CI: [0.60, 3.25], I2 = 68%, P = 0.004). Besides, it lowers levels of serum follicle-stimulating hormone (SMD: −3.02, 95% CI: [−4.88, −1.16], I2 = 93%, P = 0.001) and luteinising hormone (SMD: −2.22, 95% CI: [−3.67, −0.76], I2 = 78%, P = 0.003), and thus collectively promotes folliculogenesis (SMD: 4.90, 95% CI: [3.92, 5.88], I2 = 0%, P < 0.00001).
Conclusions: Based on the presented findings, it is concluded that the administration of hUCMSC in animal models with POI can result in significant improvements in several key indicators, including estrous cycle recovery, hormone level modulation, and promotion of folliculogenesis. These positive outcomes suggest that hUCMSC may have potential as a treatment for POI in humans. However, further research is needed to establish the safety and efficacy of hUCMSC in humans before their clinical application.
Systematic review registration: https://inplasy.com/inplasy-2023-5-0075/, identifier: INPLASY202350075.
Primary ovarian insufficiency (POI), also known as premature ovarian failure (POF), is a syndrome characterized by reduced or absent ovarian function (hypogonadism) and elevated levels of gonadotropins, specifically luteinising hormone (LH) and follicle-stimulating hormone (FSH) (hypergonadotropic) (1, 2). This occurs due to the lack of negative sex-steroid and inhibin feedback. Therefore, POI is also referred to as hypergonadotropic hypogonadism. The condition is diagnosed when oocytes and the surrounding support cells are lost before the age of 40 years, along with serum FSH levels above the threshold range of 30–40 mIU/mL twice (at least 1 month apart). POI is a systemic disease that can lead to various effects. Recent research has summarized the long-term health consequences of POI, including an increased risk of cardiovascular disease (CVD), decreased bone mineral density, significantly reduced fertility, psychological distress, vulvovaginal atrophy, neurological effects, and overall reduced life expectancy (3). While the incidence of POI is not peculiar, the underlying causes of this condition remain largely unknown (4). Despite extensive research, the etiology of POI is still not fully elucidated, and it is considered a complex and multifactorial condition. Genetic disorders, such as chromosomal abnormalities, are among the most prevalent causes of POI (5). These disorders can lead to early ovarian failure and an increased risk of POI. However, other factors like autoimmune diseases, iatrogenic injuries, and infectious diseases can also contribute to the onset of POI (6–8). In some cases, autoimmune disorders like systemic lupus erythematosus or Hashimoto's thyroiditis can trigger the body's immune system to attack ovarian tissue, leading to POI (9). Additionally, with the increasement of gynaecologic cancer, medical treatments like chemotherapy, radiation therapy, or surgical removal of the ovaries can also cause damage to the ovarian tissue, leading to POI (10). Infections, such as mumps, tuberculosis, or sexually transmitted diseases like gonorrhea, can also contribute to POI by damaging the ovaries or disrupting their function (11). Given the complex and multifactorial nature of POI, early detection and timely intervention are crucial to help manage the condition and improve the quality of life of affected individuals. Therefore, a better understanding of the factors contributing to POI and advancements in diagnostic methods can aid in developing effective treatments and management strategies for this condition (12). Currently, traditional therapy for POI is limited. To patients without desire for pregnancy, hormone replacement therapy (HRT) is appropriate. HRT can significantly relieve POI symptoms and decrease bone fracture and CVD risks. It can even help fertility for those females who still have ovarian follicle reserve (13). Infertility treatment is another therapeutic aspect for POI. Oocyte donation is a traditional but useful way to help delivery, but is limited in many countries and regions. A way to preserve fertility is the cryopreservation of oocytes, embryos and ovarian tissues. For those who undergo radiotherapy, GnRH analog can help protect fertility, but some data are conflicting. Furthermore, a new method called in vitro activation (IVA) of dormant follicles can help patients with POI conceive as well (14). However, all of these therapies can be conducive to helping a small proportion of patients with POI. Human umbilical cord mesenchymal stem cell (hUCMSC) is mesenchymal stem cells derived from Wharton's jelly of a fetal umbilical cord. These cells have multiple differentiation potentials. They can generate cell types such as adipocytes, osteocytes and cartilage. In addition, neurons, astrocytes, glial cells, liver and islet cells are the potential lineage of hUCMSC (15). Stem cell therapy has been proposed for a long time. Some clinical trials have tried to understand the therapeutic effect of hUCMSC in POI. Evidence revealed follicular activated, estradiol (E2) increased and FSH decreased after hUCMSC transplantation in patients with POI (16, 17). Collagen scaffold with hUCMSC is another stem cell delivery approach that has shown a therapeutic effect. In an in vivo study, hUCMSC activated primordial follicles by phosphorylating FOXO3a and FOXO1 (17). Apart from clinical trials, many studies tested the therapeutic effect of hUCMSC on the ovary of animals. For instance, hUCMSC introduction led to an atretic follicle decrease and a healthy antral follicle increase in mice. Granulosa cell (GC) apoptosis induced by POI was inhibited. Based on molecular analysis, the expression of SOD2, CAT and Bcl2 mRNA increased, whereas Bax mRNA expression declined (18). Given that these genes are associated with oxidation and apoptosis, hUCMSC infusion may influence the antioxidative and antiapoptotic procedures of the ovary. Furthermore, in vivo cell culture found that hUCMSC can secrete VEGF, IGF-1 and HGF (19). Through Sirius red and Masson trichrome staining of the ovary tissue, researchers found that fibrosis developed in POI rats, but after hUCMSC treatment, the fibrosis area was significantly reduced. The TGF-β1 signaling pathway is a crucial immune regulative factors (20), also reportedly involved in hUCMSC regulation. The hUCMSC can inhibit the expression of TGF-β1 and p-smad3 in the ovary, thereby depressing the differentiation of stromal cells into inner theca cells (TCs) and consequently inhibiting fibrosis in POI rats (21). However, only few integrated analyses have been found. Thus, this study aimed to summarize the results of animal studies investigating on hUCMSC and POI, form more valid evidence and confirm the therapeutic effect of hUCMSC on experimental animals compared with the POI model by analyzing the estrous cycle, serum sex hormone and ovarian follicles in the two groups.
This systematic meta-analysis appraises the association between employment of hUCMSC and the indices of ovarian reserve function in experimental animal models. We followed the preferred reporting items for meta-analysis and systematic review (PRISMA) 2020 guidelines and putting forward the research question using the PECOS format. We have registered at International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY). Registration number is INPLASY202350075.
We searched the Pubmed, Embase and Cochrane Library databases. Specific search strategy is “((Primary Ovarian Insufficiency) OR (Premature Ovarian Failure) OR (Gonadotropin-Resistant Ovary Syndrome) OR (Hypergonadotropic Ovarian Failure)) AND ((Stem cell) OR (Progenitor Cell))”. To conclude, we used MeSH terms and their typical synonyms and combined them with “OR.” Then, we combined the results of “primary ovarian insufficiency” and “stem cell” with “AND.” All results from the date of database establishment to 1 April 2022 were included.
Initially, we excluded all duplicated studies. Subsequently, we collected studies that met the following criteria: female animals; hUCMSC; successful POF model establishment; and serum hormone, follicle count and estrous cycle as the outcome. Furthermore, the following five study types were excluded: reviews and meta-analysis, studies that are not associated with stem cell or POI, non-animal studies, case reports and animal studies without hUCMSC application. After selecting studies related to hUCMSC and POI, we thoroughly read the full text and further excluded studies that we failed to collect the exact data and studies with no outcomes that we aimed.
Data were extracted and qualifiedly assessed by using “SYRCLE animal experiment bias risk assessment form.” We used risk ratios (RRs) with 95% confidence intervals (CI) for categorical data, and standardized mean difference (SMD) for numerical data to combine studies. If the heterogeneity test showed I2 > 50%, we used random effects model. Otherwise, we used fixed effects model. All statistical data were analyzed on RevMan 5.4 (22). The extracted data from each study included the first author, country or region, publishing year, experiment animal, POI model establishing method, hUCMSC intervention situation, group situation and outcome data. During the analysis, we firstly tested the heterogeneity of the studies and selected the effects model, as mentioned before. Then, we divided the studies according to unit, injection location, hUCMSC concentration, transplantation time and follicle type for the subgroup analyses. Sensitivity was assessed by eliminating studies one by one. We also used funnel plot to determine the publication bias. All statistical significances were defined at P < 0.05.
We identified 446, 335 and 16 studies from Pubmed, Embase and Cochrane Library, respectively. Among them, 175 duplicate studies, 223 reviews or meta-analyses, 204 studies that are not associated with stem cell or POI, 26 animal studies, 44 case reports and 95 animal studies without hUCMSC used were excluded. Conclusively, 30 animal studies remained, and they were all correlated to hUCMSC and POI. After full text reading, we further excluded 16 studies because we failed to obtain the exact data, and three studies because they lack our aimed outcome. Ultimately, 11 studies were analyzed (Figure 1) (23–33).
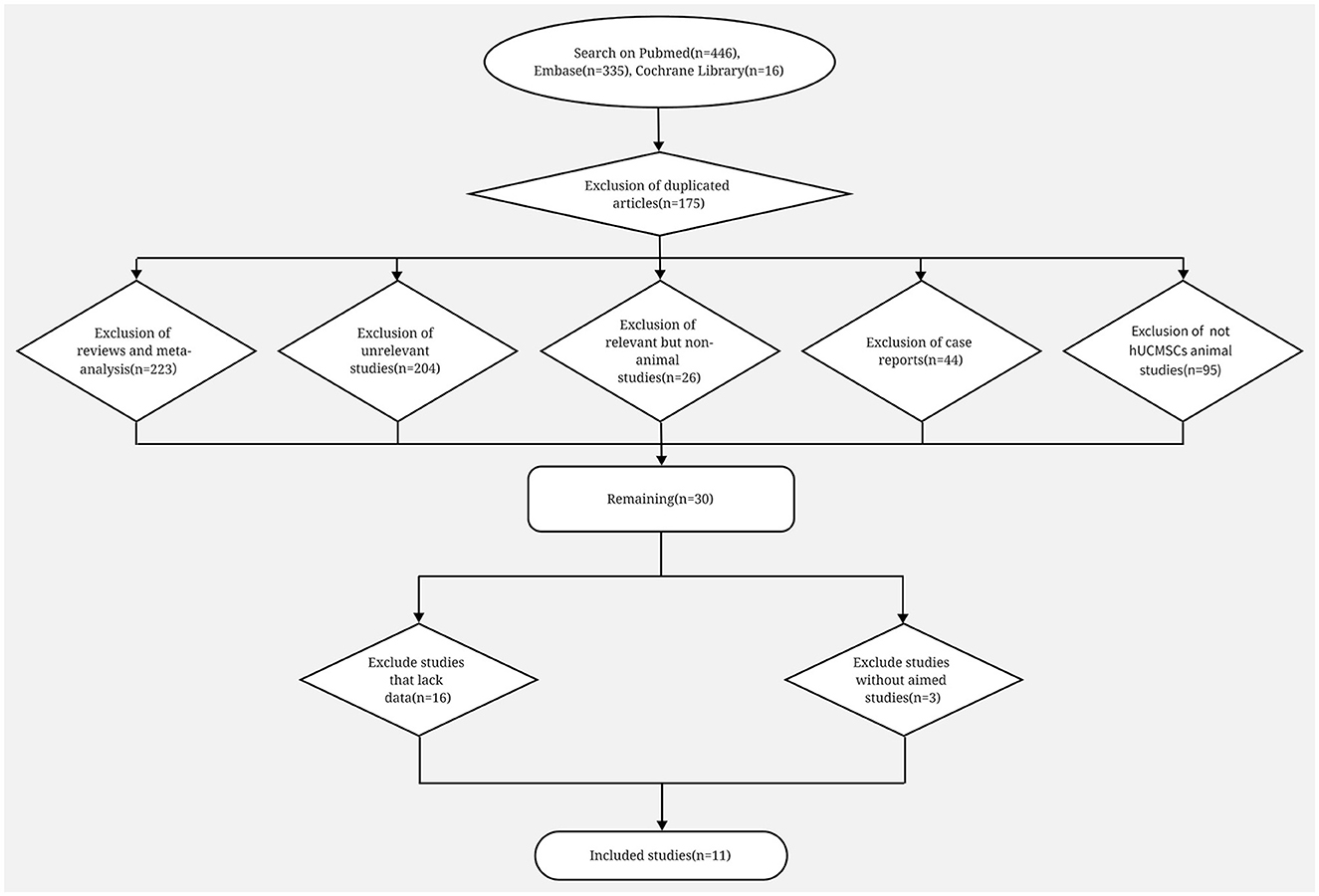
Figure 1. Flow diagram on search procedure of Pubmed, Embase and Cochrane Library and the exclusion criteria.
We extracted the data on the first author, country, publication year, experiment animal number and situation, model establishment situation, group situation, some outcomes and web link. We included nine studies from China (23, 24, 26, 28–33), one from Saudi Arabia (25) and one from Iran (27). A total of 158 stem cell–treated animals and 155 POI model animals were included. Eight studies infused hUCMSC by tail vein (23, 24, 27–31, 33), whereas four injected hUCMSC directly into the ovary (25, 26, 29, 32); in addition, one study compared the effects of these two methods (29). Stem cell concentrations varied, ranging from 1 × 105 to 5 × 106. However, the concentration units in some studies were unclear; thus, we only conducted a subgroup analysis by stem cell concentration in hormone analyses. Transplantation time also varied. Some studies set a series of observation time to better show the effect of hUCMSC. To simplify our analysis, we only chose the data at the end of the study for our meta-analysis (Tables 1, 2). In order to identify the effect of different transplantation time, we conducted a subgroup analysis as well.
Five studies reported estrous cycle situation (23, 26, 28, 30, 32). Two outcomes were used to divide them into two analyses. Of the five studies, two (23, 30) calculated the proportion of animals with normal estrous cycle. Based on different cell concentrations, four groups were included in one analysis. The three other studies (26, 28, 32) measured the length of the estrous cycle in animals. Results showed that hUCMSC significantly improved the proportion of animals with normal estrous cycle (RR: 3.32, 95% CI: [1.80, 6.12], I2 = 0%, P = 0.0001; Figure 2A) and shortened the estrous cycle length (SMD: −1.97, 95% CI: [−2.58, −1.36], I2 = 0%, P < 0.00001; Figure 2B). Based on the location of stem cell injection, the subgroup analysis showed that estrous cycle improvement is independent of the injection site (Table 3).
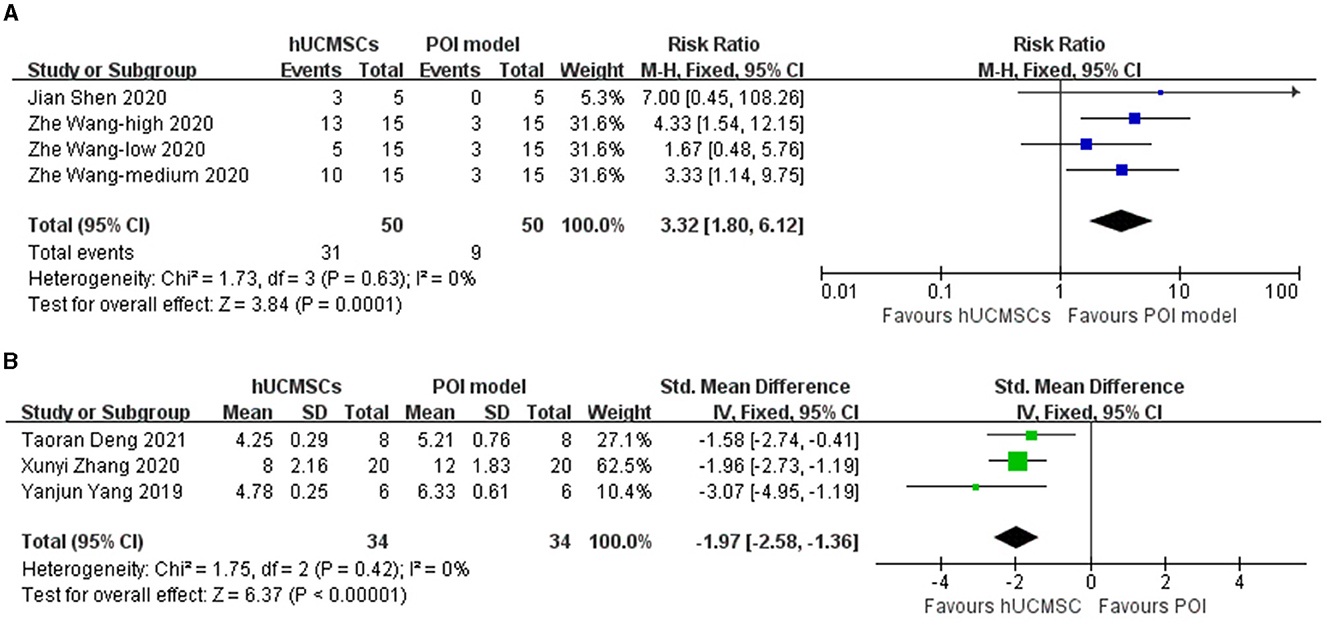
Figure 2. Forest plot of animals' estrous cycle situation in the hUCMSC group versus the POI model group. (A) Numerous animals in the hUCMSC group recovered normal estrous cycle compared with those in the POI model group (RR: 3.32, 95% CI: [1.80, 6.12], I2 = 0%, P = 0.0001). (B) The cycle length significantly decreased in the hUCMSC group compared with that in the POI model group (SMD: −1.97, 95% CI: [−2.58, −1.36], I2 = 0%, P < 0.00001).
Eight studies reported serum E2 levels (23–29, 32). Based on the injection location, nine groups were included in the analysis. Serum E2 significantly increased in the hUCMSC group compared with that in the POI model group (SMD: 5.34, 95% CI: [3.11, 7.57], I2 = 93%, P < 0.00001; Figure 3A). We conducted a subgroup analysis according to the statistical units, stem cell injection location and stem cell concentration. Besides, as some included studies compared several transplantation time, we also conducted a subgroup analysis based on it. The elevation of serum E2 level was not significant when calculated by ng/mL or when the stem cell concentration was 2 × 10e6. However, the effect of hUCMSC on the serum E2 level of animals was independent of the injection location. Significance was observed in all intervention time subgroup, indicating hUCMSC can increase serum E2 level at the beginning of 2 weeks (Table 3).
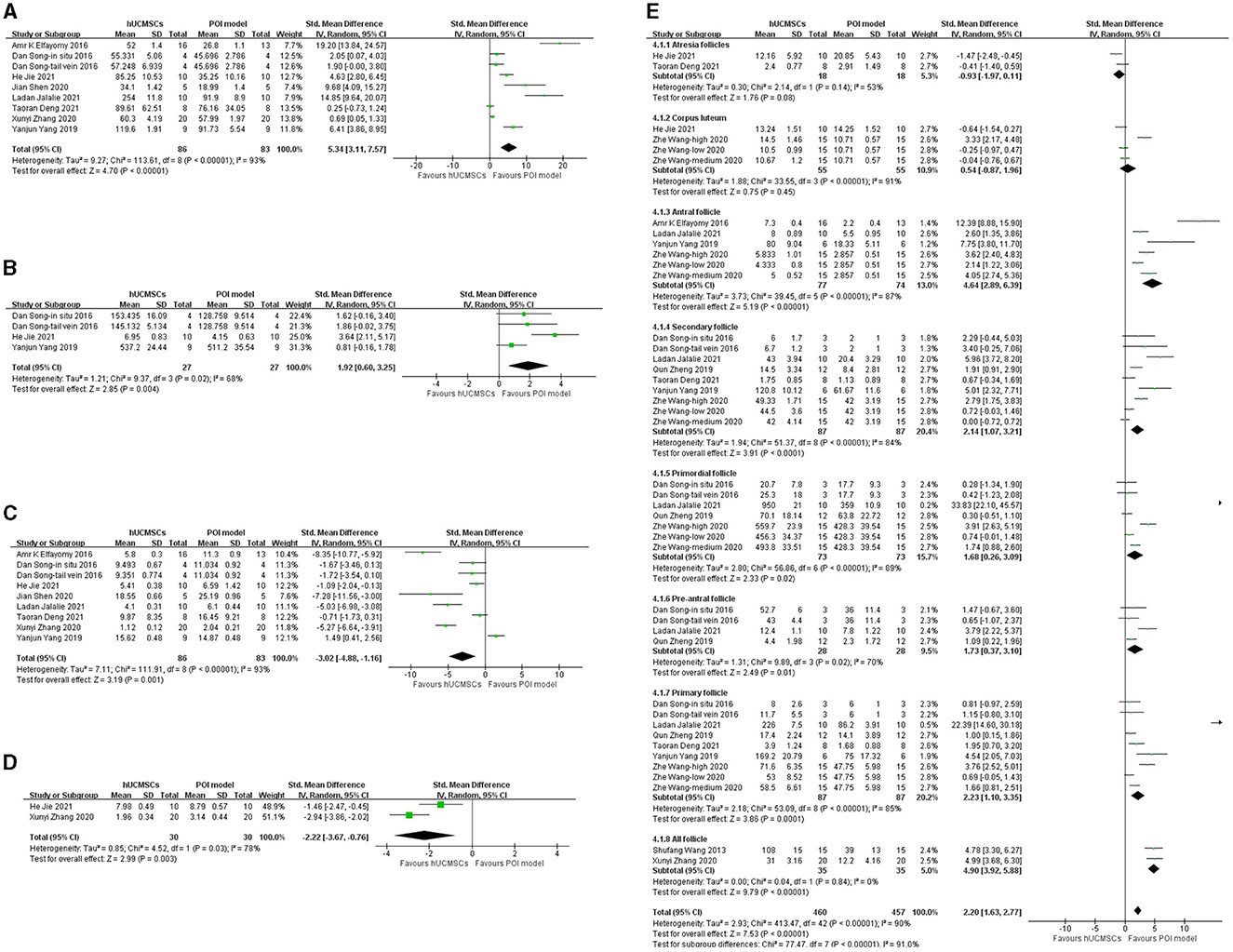
Figure 3. Forest plot of animals' serum hormone level and follicle number in the hUCMSC group vs. the POI model group. (A) Serum E2 concentrations significantly increased in the hUCMSC group compared with those in the POI model group (SMD: 5.34, 95% CI: [3.11, 7.57], I2 = 93%, P < 0.00001). (B) Serum AMH concentrations significantly increased in the hUCMSC group compared with those in the POI model group (SMD: 1.92, 95% CI: [0.60, 3.25], I2 = 68%, P = 0.004). (C) Serum FSH concentrations significantly decreased in the hUCMSC group compared with those in the POI model group (SMD: −3.02, 95% CI: [−4.88, −1.16], I2 = 93%, P = 0.001). (D) Serum LH concentrations significantly decreased in the hUCMSC group compared with those in the POI model group (SMD: −2.22, 95% CI: [−3.67, −0.76], I2 = 78%, P = 0.003). (E) Ovarian follicle count comparison between the hUCMSC group versus the POI model group. Antral follicles (SMD: 4.64, 95% CI: [2.89, 6.39], I2 = 87%, P < 0.00001), pre-antral follicles (SMD: 1.73, 95% CI: [0.37, 3.10], I2 = 70%, P = 0.01), secondary follicles (SMD: 2.14, 95% CI: [1.07, 3.21], I2 = 84%, P < 0.0001), primary follicle (SMD: 2.23, 95% CI: [1.10, 3.35], I2 = 85%, P = 0.0001), primordial follicle (SMD: 1.68, 95% CI: [0.26, 3.09], I2 = 89%, P = 0.02) and all follicles (SMD: 4.90, 95% CI: [3.92, 5.88], I2 = 0%, P < 0.00001) increased significantly in the hUCMSC group compared with those in the POI model group.
Three studies reported serum AMH (24, 29, 32). Based on the injection location, four groups were included in the analysis. Serum AMH significantly increased in the hUCMSC group compared with that in the POI model group (SMD: 1.92, 95% CI: [0.60, 3.25], I2 = 68%, P = 0.004; Figure 3B). The subgroup analysis revealed that serum AMH was independent of the statistical units and injection location. However, when the stem cell concentration was 2 × 10e5 and 2 × 10e6, no significant change was observed between the model and hUCMSC groups (Table 3).
Eight studies reported serum FSH levels (23–29, 32). Based on the injection location, nine groups were included in the analysis. Compared with the POI model group, the hUCMSC group showed a significant reduction in serum FSH (SMD: −3.02, 95% CI: [−4.88, −1.16], I2 = 93%, P = 0.001; Figure 3C). According to the statistical units, stem cell injection location, stem cell concentration and transplantation time, the subgroup analysis showed that no significant change was observed when hUCMSC was injected in situ and when the stem cell concentration was 2 × 10e6. However, in most cases, the FSH level decreased significantly after hUCMSC injection. Meanwhile, FSH decreases significantly 2 weeks after hUCMSC injection, indicating the effect of hUCMSC works at the beginning of 2 weeks (Table 3).
Two studies reported serum LH (24, 26). Given that they used different calculation units, injection location and stem cell concentration, we conducted a subgroup analysis. Compared with the POI model group, the hUCMSC group showed a significant decrease in serum LH (SMD: −2.22, 95% CI: [−3.67, −0.76], I2 = 78%, P = 0.003; Figure 3D). Subgroup analysis results showed that the treatment effect on LH concentration was independent of the calculation unit, injection location and stem cell concentration (Table 3).
Ten studies determined the follicle count in animals (24–33). However, considering the various follicle types, we only conducted a subgroup analysis based on the follicle types (Figure 3E). All subgroups, except the atresia follicles (P = 0.08) and corpus luteum (P = 0.45), showed significant differences. After hUCMSC injection, the antral follicles (SMD: 4.64, 95% CI: [2.89, 6.39], I2 = 87%, P < 0.00001), secondary follicles (SMD: 2.14, 95% CI: [1.07, 3.21], I2 = 84%, P < 0.0001), primordial follicles (SMD: 1.68, 95% CI: [0.26, 3.09], I2 = 89%, P = 0.02), pre-antral follicles (SMD: 1.73, 95% CI: [0.37, 3.10], I2 = 70%, P = 0.01), primary follicles (SMD: 2.23, 95% CI: [1.10, 3.35], I2 = 85%, P = 0.0001) and all follicles (SMD: 4.90, 95% CI: [3.92, 5.88], I2 = 0%, P < 0.00001) significantly increased compared with those in the POI model group.
By picking out studies one by one, we conducted a sensitivity analysis on five outcomes of the estrous cycle, E2, FSH, AMH and LH. Results of the estrous cycle, E2, FSH and LH were stable, but after picking out Jie et al. (24) or Yang et al. (32), the heterogeneity of the AMH outcome reduced significantly (Jie et al.: I2 = 0%, P = 0.53; Yang et al.: I2 = 43%, P = 0.17). The data unit, transplantation time and study animals may explain the heterogeneity; larger data are needed to determine the origin (Table 4).
Funnel plots of the normal estrous cycle proportion, E2, FSH and follicle number are asymmetric, whereas those of the estrous cycle length, AMH and LH are symmetric. Publication bias likely existed, especially in the results of the estrous cycle, E2, FSH and follicle number (Figure 4).
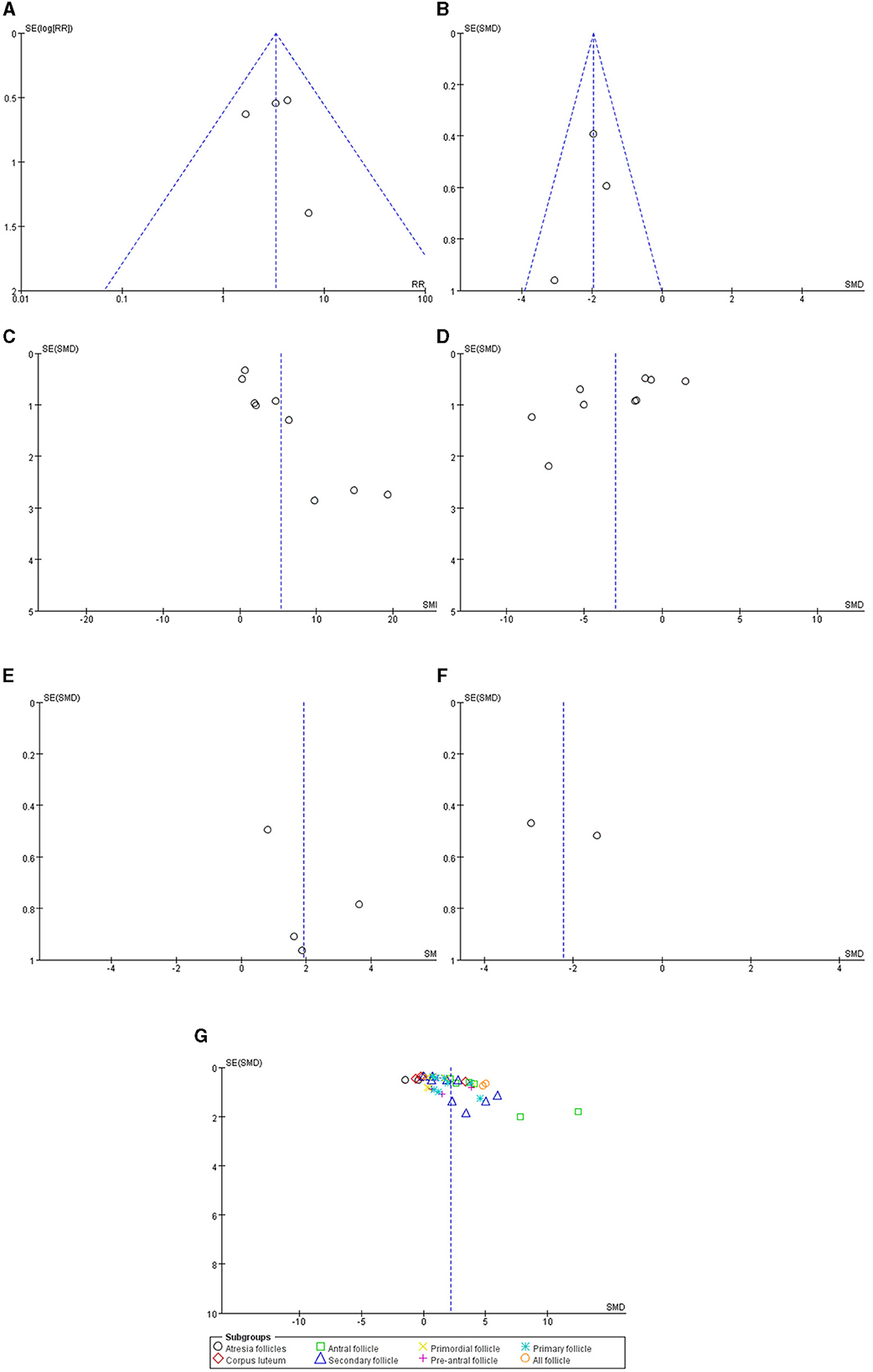
Figure 4. Funnel plots of the (A) normal estrous cycle, (B) estrous cycle length, (C) serum E2, (D) serum FSH, (E) serum AMH, (F) serum LH and (G) follicle count.
In the year 1942, POI was initially described as a mysterious ailment that perplexed medical practitioners. As the medical community's focus began to shift toward unraveling its elusive nature, POI gradually gained notoriety and its prevalence surged to an alarming 1% (35). Chemical injury is a common method to establish POI model and a main cause of POI clinically apart from genetic disorders (12, 36). But the pathophysiological change of POI is similar regardless of etiology. According to our results, the reduced ovarian function and elevated gonadotropins is reversed by hUCMSC therapy. Follicles are stimulated as well. Therefore, we think the therapeutic effect of hUCMSC is adaptable to all causes. The traditional treatment for POI is HRT, but it only relieves symptoms. The ovarian function remains poor and bring many clinical adverse events about in many patients. In a retrospective cohort study, only 3 of 20 patients achieved pregnancy by assisted reproductive technology (ART) (37). Besides, patients with HRT are more likely to develop sleep problems (38). Given these limitations, stem cell therapy has gained considerable attention recently. Currently, hUCMSC has two other different forms to apply, that is, the microvesicles (34) and extracellular vesicles (39) derived from such cells. Stem cells in the umbilical cord are easy to acquire and do not cause extra donor injury compared with the other stem cell types. Considering their proliferative ability, multidifferentiation and safety, hUCMSC has been researched in the treatment of respiratory (40), cardiovascular (41), liver (42), central nervous system (43, 44) and autoimmune (45) diseases as well as diabetes (46). AMH is crucial in POI diagnosis. In our search on POI, AMH research has gained a large proportion. Physiologically, AMH is secreted by primary ovarian follicles and can negatively regulate the progression of earlier resting follicles into active and progressive ones (47). Considering that AMH secretion slightly varies in menstrual cycle and only healthy follicles secrete it, AMH is considered as a stable marker of ovarian reserve and POI. Our results showed a significant increase of AMH in the hUCMSC group, possibly because of the cytokine secreted by hUCMSC. However, the follicle number increased after stem cell transplantation; the proliferation of healthy follicles may be the cause of the AMH increase. Further research is needed to elucidate the underlying mechanism.
Many factors contribute to the development of infertility. And the gene expressions of different cases are various according to the diseases as a bioinformatic analysis showed (48). As a result, there are only some general treatments of infertility like IVF-ET and artificial insemination. Etiology based therapy of infertility is rare. Infertility therapies are mainly ART, aiming at gaining embryo directly (49). Our meta-analysis confirms folliculogenesis of hUCMSC in animal models, providing an etiology-specific therapy of POI. Apart from efficacy, safety is another important factors under evaluation. Although our result does not cover safety concerns, several phase 1/2 trials have been conducted for hUCMSC in other diseases. General outcomes for safety consideration include immediate infusion related adverse events, blood test like hepatic and renal function and blood cell count, inflammatory cytokine level, hypersensitive, infection, tumorigenesis (50–52). No adverse event is observed in these studies.
Although many studies tried to determine the mechanism of hUCMSC, the specific target remains unclear. Apoptosis regulation was often observed in many animal studies. This result may be derived from some signaling pathways. In a previous study, the expression of CK 8/18, TGF-ß and PCNA increased, while that of CASP-3 decreased (25). Other candidate molecular signals include Bcl-2 (53) and PI3K-Akt (54). Some researchers also hypothesized that angiogenesis can explain the anti-apopotic effect of hUCMSC (32). Further studies should be conducted to determine the exact mechanism and guide the clinical application of hUCMSC. However, hUCMSC can definitely promote ovarian function. Not only AMH but also E2, FSH and LH showed significant changes. Estrogen is mainly produced in the ovarian follicle, and LH and FSH play a crucial role. In addition, GCs and their aromatase convert androgen into estrogen (55). Our results proved the therapeutic effect of hUCMSC on E2, FSH and LH. We also found some pioneering clinical trials, and their results are optimistic. They investigate antral follicle number and sex hormone of patients to evaluate their ovary function. After UCMSC transplantation, patients showed significant recovery of sex hormone, with decreased level of FSH and increased number of antral follicle (16, 17). Further pregnancy follow up showed that UCMSC transplantation does not affect genetic source of fetus (16). Ovarian volume increases after hUCMSC transplantation with significance, but no significance was observed in collagen scaffold group (17). Nevertheless, compared with animal studies, number of high-quality randomized controlled trials (RCTs) is little. Considering the differences between animals and humans, our meta-analysis result cannot fully support stem cell therapy in POI in human, but can provide a preclinical evidence. However, given that hUCMSC is proven to be effective in POI animals, researchers may pay more attention to RCTs. With abundant and valid RCT evidence, further application of stem cells can be discussed in the future. According to quality assessment table (Table 2), major problems are insufficient randomization and blinding. Thus, it is necessary for researchers to exactly illustrate their randomization settings and blinding measures to prove the function of hUCMSC especially in clinical stage. Traditionally, ovarian follicle number is thought to be fixed and decreases by age (56). So folliculogenesis is traditionally thought as an irreversible procedure. Besides, traditional therapy such as HRT can only relieve symptoms. Fertility preservation is indeed hard to achieve. But recently, the discovery of stem cell in ovary gives a new hope for ovarian regeneration. Germline stem cell (GSC) is identified and isolated from human ovarian cortex. The property of isolated stem cell is proved to be stable after cell culture. DDX4, OCT4. IFITM3 and BLIMP-1 are confirmed to be expressed by the GSC (57). Moreover, it can promote ovarian function recovery in sterile animals and achieve pregnancy (58). As our result shows a recovery of ovarian follicle after hUCMSC transplantation. Given the genetic origin of offspring is not from hUCMSC donor as clinical trial proves (16), new experiments can pay some attentions to the effect of hUCMSC on ovarian GSC to explore the mechanism. Many genes can regulate folliculogenesis. Genes, cells or molecules such as SP1, mTOR, Ube2i, YAP1, C1QTNF3, GPR173, ovarian fat pad factors, α-SNAP, CD11c+ cells, M1 MΦs and DCs all play a role in folliculogenesis (59). Some studies have tried to find the association between these genes and the hUCMSC treatment effect. For example, Lu Xueyan et al. found that hUCMSC can inhibit the autophagy of theca-interstitial cells via the AMPK/mTOR signaling pathway (60). Depending on our results, folliculogenesis is a promising direction. Considering that folliculogenesis is tightly connected to anti-apoptosis in GCs (61), some cytokines that can affect GCs may be the target of future mechanism research.
Finally, this study has some limitations that should not be ignored. Clinical study of hUCMSC is rare, and this is also one reason for us to conduct this meta-analysis. Preclinical study is an important part of scientific experiment. Because of limited number of clinical data, it is not the optimal time to conduct a clinical meta-analysis. The meta-analysis of preclinical animal study can promote the development of clinical trails. The relatively insufficient included study, medium quality, high diversity and heterogeneity restrict the application of conclusion. Though random effects model was applied in the analysis, the impact cannot be fully eliminated. All included studies are of medium-quality studies, and higher-quality studies are needed in future research. Due to the limited number of studies, publication bias likely exists in this meta-analysis. However, according to current results, hUCMSC is able to recover ovarian function of POI animals. The result is not affected by limitation. The mentioned limitation should be considered when our conclusion serves as an evidence for clinical study. Thus, we hold a conservative but optimistic view and think more studies are needed in the future to further support the results. Considering the characteristic table, we can observe that a standard animal study procedure has not been formed yet. Future research may focus on a suitable stem cell concentration and transplantation time to eliminate heterogeneity.
The transplantation of hUCMSC has the potential to restore the estrous cycle, increase E2 and AMH levels, decrease FSH and LH levels, and promote folliculogenesis in female rodent models. The results strongly support the use of this therapeutic strategy with a promising outlook. It is important to evaluate the safety and effectiveness of hUCMSC in clinical trials. Randomized controlled trials should also be approached with caution, and safety and adverse effects of hUCMSC should be thoroughly examined in future studies.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
XW and TL conducted study collection, indentification, data extraction, and statistical disposal. XW drafted the manuscript. TL polished the manuscript. XB, YZ, and MZ collected the relevant references and participated in the discussion. LW designed this meta-analysis and revised the manuscript. All authors contributed to this manuscript. All authors read and approved the final manuscript.
This work was supported by Department of Science and Technology of Zhejiang Province, Lingyan Project (2022C03097).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AMH, anti-mullerian hormone; ART, assisted reproductive technology; CI, confidence interval; CVD, cardiovascular disease; E2, estradiol; FSH, follicle stimulating hormone; GC, granulosa cell; GSC, germline stem cell; GnRH, gonadotropin-releasing hormone; HRT, hormone replacement therapy; hUCMSC, human umbilical cord mesenchymal stem cell; IVA, in vitro activation; IVF-ET, in vitro fertilization and embryo transfer; LH, luteinizing hormone; MeSH, medical subject heading; POF, premature ovarian failure; POI, primary ovarian insufficiency; PRISMA, preferred reporting items for meta-analysis and systematic review; RCT, randomized clinical trail; RR, risk ratio; SMD, standardized mean difference; TC, theca cell.
1. Han Q, Chen ZJ, Du Y. Dietary supplementation for female infertility: recent advances in the nutritional therapy for premature ovarian insufficiency. Front Microbiol. (2022) 13:1001209. doi: 10.3389/fmicb.2022.1001209
2. Liu M, Zhang D, Li W, Xu B, Feng HL. Editorial. Ovarian aging and reproduction. Front Endocrinol. (2022) 13:1081348. doi: 10.3389/fendo.2022.1081348
3. Tsiligiannis S, Panay N, Stevenson JC. Premature ovarian insufficiency and long-term health consequences. Curr Vasc Pharmacol. (2019) 17:604–9. doi: 10.2174/1570161117666190122101611
4. Li M, Zhu Y, Wei J, Chen L, Chen S, Lai D. The global prevalence of premature ovarian insufficiency: a systematic review and meta-analysis. Climacteric. (2023) 26:95–102. doi: 10.1080/13697137.2022.2153033
5. Ruth KS, Day FR, Hussain J, Martínez-Marchal A, Aiken CE, Azad A, et al. Genetic insights into biological mechanisms governing human ovarian ageing. Nature. (2021) 596:393–7. doi: 10.1038/s41586-021-03779-7
6. Domniz N, Meirow D. Premature ovarian insufficiency and autoimmune diseases. Best Pract Res Clin Obstet Gynaecol. (2019) 60:42–55. doi: 10.1016/j.bpobgyn.2019.07.008
7. Cattoni A, Parissone F, Porcari I, Molinari S, Masera N, Franchi M, et al. Hormonal replacement therapy in adolescents and young women with chemo- or radio-induced premature ovarian insufficiency. Practical recommendations. Blood Rev. (2021) 45:100730. doi: 10.1016/j.blre.2020.100730
8. Wilkins J, Al-Inizi S. Premature ovarian insufficiency secondary to COVID-19 infection. An original case report. Int J Gynaecol Obstet. (2021) 154:179–80. doi: 10.1002/ijgo.13719
9. Bakalov VK, Gutin L, Cheng CM, Zhou J, Sheth P, Shah K, et al. Autoimmune disorders in women with turner syndrome and women with karyotypically normal primary ovarian insufficiency. J Autoimmun. (2012) 38:315–21. doi: 10.1016/j.jaut.2012.01.015
10. Yi M, Li T, Niu M, Luo S, Chu Q, Wu K. Epidemiological trends of women's cancers from 1990 to 2019 at the global, regional, and national levels: a population-based study. Biomark Res. (2021) 9:55. doi: 10.1186/s40364-021-00310-y
11. Jin M, Yu Y, Huang H. An update on primary ovarian insufficiency. Sci China Life Sci. (2012) 55:677–86. doi: 10.1007/s11427-012-4355-2
12. Ishizuka B. Current understanding of the etiology, symptomatology, and treatment options in premature ovarian insufficiency (POI). Front Endocrinol. (2021) 12:626924. doi: 10.3389/fendo.2021.626924
13. Sullivan SD, Sarrel PM, Nelson LM. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil Steril. (2016) 106:1588–99. doi: 10.1016/j.fertnstert.2016.09.046
14. Meczekalski B, Maciejewska-Jeske M, Podfigurna A. Reproduction in premature ovarian insufficiency patients—From latest studies to therapeutic approach. Prz Menopauzalny. (2018) 17:117–9. doi: 10.5114/pm.2018.78554
15. Ding DC, Chang YH, Shyu WC, Lin SZ. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. (2015) 24:339–47. doi: 10.3727/096368915X686841
16. Yan L, Wu Y, Li L, Wu J, Zhao F, Gao Z, et al. Clinical analysis of human umbilical cord mesenchymal stem cell allotransplantation in patients with premature ovarian insufficiency. Cell Prolif. (2020) 53:e12938. doi: 10.1111/cpr.12938
17. Ding L, Yan G, Wang B, Xu L, Gu Y, Ru T, et al. Transplantation of UC-MSCs on collagen scaffold activates follicles in dormant ovaries of POF patients with long history of infertility. Sci China Life Sci. (2018) 61:1554–65. doi: 10.1007/s11427-017-9272-2
18. Zhang J, Xiong J, Fang L, Lu Z, Wu M, Shi L, et al. The protective effects of human umbilical cord mesenchymal stem cells on damaged ovarian function. A comparative study. Biosci Trends. (2016) 10:265–76. doi: 10.5582/bst.2016.01125
19. Zhu SF, Hu HB, Xu HY, Fu XF, Peng DX, et al. Human umbilical cord mesenchymal stem cell transplantation restores damaged ovaries. J Cell Mol Med. (2015) 19:2108–17. doi: 10.1111/jcmm.12571
20. Yi M, Wu Y, Niu M, Zhu S, Zhang J, Yan Y, et al. Anti-TGF-β/PD-L1 bispecific antibody promotes T cell infiltration and exhibits enhanced antitumor activity in triple-negative breast cancer. J Immunother Cancer. (2022) 10:5543. doi: 10.1136/jitc-2022-005543
21. Cui L, Bao H, Liu Z, Man X, Liu H, Hou Y, et al. hUMSCs regulate the differentiation of ovarian stromal cells via TGF-beta1/Smad3 signaling pathway to inhibit ovarian fibrosis to repair ovarian function in POI rats. Stem Cell Res Ther. (2020) 11:386. doi: 10.1186/s13287-020-01904-3
23. Shen J, Cao D, Sun JL. Ability of human umbilical cord mesenchymal stem cells to repair chemotherapy-induced premature ovarian failure. World J Stem Cells. (2020). doi: 10.4252/wjsc.v12.i4.277
24. Jie H, Jinxiang W, Ye L, Jing Z, Xiangqing Z, Jinxiu H, et al. Effects of umbilical cord mesenchymal stem cells on expression of CYR61, FSH and AMH in mice with premature ovarian failure. Cell Mol Biol. (2022) 67:358–66. doi: 10.14715/cmb/2021.67.5.41
25. Elfayomy AK, Almasry SM, El-Tarhouny SA, Eldomiaty MA. Human umbilical cord blood-mesenchymal stem cells transplantation renovates the ovarian surface epithelium in a rat model of premature ovarian failure. Possible direct and indirect effects. Tissue Cell. (2016) 48:370–82. doi: 10.1016/j.tice.2016.05.001
26. Zhang X, Zhang L, Li Y, Yin Z, Feng Y, Ji Y. Human umbilical cord mesenchymal stem cells (hUCMSCs) promotes the recovery of ovarian function in a rat model of premature ovarian failure (POF). Gynecol Endocrinol. (2021) 37:353–7. doi: 10.1080/09513590.2021.1878133
27. Jalalie L, Rezaee MA, Rezaie MJ, Jalili A, Raoofi A, Rustamzade A. Human umbilical cord mesenchymal stem cells improve morphometric and histopathologic changes of cyclophosphamide-injured ovarian follicles in mouse model of premature ovarian failure. Acta Histochem. (2021) 123:151658. doi: 10.1016/j.acthis.2020.151658
28. Deng T, He J, Yao Q, Wu L, Xue L, Wu M, et al. Human umbilical cord mesenchymal Stem cells improve ovarian function in chemotherapy-induced premature ovarian failure mice through inhibiting apoptosis and inflammation via a paracrine mechanism. Reprod Sci. (2021) 28:1718–32. doi: 10.1007/s43032-021-00499-1
29. Song D, Zhong Y, Qian C, Zou Q, Ou J, Shi Y, et al. Human umbilical cord mesenchymal stem cells therapy in cyclophosphamide-induced premature ovarian failure rat model. Biomed Res Int. (2016) 2016:2517514. doi: 10.1155/2016/2517514
30. Wang Z, Wei Q, Wang H, Han L, Dai H, Qian X, et al. Mesenchymal stem cell therapy using human umbilical cord in a rat model of autoimmune-induced premature ovarian failure. Stem Cells Int. (2020) 2020:3249495. doi: 10.1155/2020/3249495
31. Wang S, Yu L, Sun M, Mu S, Wang C, Wang D, et al. The therapeutic potential of umbilical cord mesenchymal stem cells in mice premature ovarian failure. Biomed Res Int. (2013) 2013:690491. doi: 10.1155/2013/690491
32. Yang Y, Lei L, Wang S, Sheng X, Yan G, Xu L, et al. Transplantation of umbilical cord-derived mesenchymal stem cells on a collagen scaffold improves ovarian function in a premature ovarian failure model of mice. In Vitro Cell Dev Biol Anim. (2019) 55:302–11. doi: 10.1007/s11626-019-00337-4
33. Zheng Q, Fu X, Jiang J, Zhang N, Zou L, Wang W, et al. umbilical cord mesenchymal stem cell transplantation prevents chemotherapy-induced ovarian failure via the NGF/TrkA pathway in rats. Biomed Res Int. (2019) 2019:6539294. doi: 10.1155/2019/6539294
34. Yang Z, Du X, Wang C, Zhang J, Liu C, Li Y, et al. Therapeutic effects of human umbilical cord mesenchymal stem cell-derived microvesicles on premature ovarian insufficiency in mice. Stem Cell Res Ther. (2019) 10:250. doi: 10.1186/s13287-019-1327-5
35. European Society for Human R, Embryology Guideline Group on POI, Webber L, Davies M, Anderson R, Bartlett J, et al. ESHRE guideline: management of women with premature ovarian insufficiency. Hum Reprod. (2016) 31:926–37. doi: 10.1093/humrep/dew027
36. McGlacken-Byrne SM, Conway GS. Premature ovarian insufficiency. Best Pract Res Clin Obstet Gynaecol. (2022) 81:98–110. doi: 10.1016/j.bpobgyn.2021.09.011
37. Sato T, Kusuhara A, Kasahara Y, Haino T, Kishi H, Okamoto A. Follicular development during hormone replacement therapy in patients with premature ovarian insufficiency. Reprod Med Biol. (2021) 20:234–40. doi: 10.1002/rmb2.12375
38. Benetti-Pinto CL, Menezes C, Yela DA, Cardoso TM. Sleep quality and fatigue in women with premature ovarian insufficiency receiving hormone therapy: a comparative study. Menopause. (2019) 26:1141–5. doi: 10.1097/GME.0000000000001379
39. Liu C, Yin H, Jiang H, Du X, Wang C, Liu Y, et al. Extracellular vesicles derived from mesenchymal stem cells recover fertility of premature ovarian insufficiency mice and the effects on their offspring. Cell Transplant. (2020) 29:963689720923575. doi: 10.1177/0963689720923575
40. Hao Y, Ran Y, Lu B, Li J, Zhang J, Feng C, et al. Therapeutic effects of human umbilical cord-derived mesenchymal stem cells on canine radiation-induced lung injury. Int J Radiat Oncol Biol Phys. (2018) 102:407–16. doi: 10.1016/j.ijrobp.2018.05.068
41. Fang Z, Yin X, Wang J, Tian N, Ao Q, Gu Y, et al. Functional characterization of human umbilical cord-derived mesenchymal stem cells for treatment of systolic heart failure. Exp Ther Med. (2016) 12:3328–32. doi: 10.3892/etm.2016.3748
42. Zhang GZ, Sun HC, Zheng LB, Guo JB, Zhang XL. In vivo hepatic differentiation potential of human umbilical cord-derived mesenchymal stem cells. Therapeutic effect on liver fibrosis/cirrhosis. World J Gastroenterol. (2017) 23:8152–68. doi: 10.3748/wjg.v23.i46.8152
43. Tian DZ, Deng D, Qiang JL, Zhu Q, Li QC, Yi ZG. Repair of spinal cord injury in rats by umbilical cord mesenchymal stem cells through P38MAPK signaling pathway. Eur Rev Med Pharmacol Sci. (2019) 23:47–53. doi: 10.26355/eurrev_201908_18627
44. Wang S, Cheng H, Dai G, Wang X, Hua R, Liu X, et al. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res. (2013) 1532:76–84. doi: 10.1016/j.brainres.2013.08.001
45. Wang D, Niu L, Feng X, Yuan X, Zhao S, Zhang H, et al. Long-term safety of umbilical cord mesenchymal stem cells transplantation for systemic lupus erythematosus. A 6-year follow-up study. Clin Exp Med. (2017) 17:333–40. doi: 10.1007/s10238-016-0427-0
46. Kong D, Zhuang X, Wang D, Qu H, Jiang Y, Li X, et al. Umbilical cord mesenchymal stem cell transfusion ameliorated hyperglycemia in patients with type 2 diabetes mellitus. Clin Lab. (2014) 60:1969–76. doi: 10.7754/Clin.Lab.2014.140305
47. Lew R. Natural history of ovarian function including assessment of ovarian reserve and premature ovarian failure. Best Pract Res Clin Obstet Gynaecol. (2019) 55:2–13. doi: 10.1016/j.bpobgyn.2018.05.005
48. Huang X, Yu Q. Bioinformatic analysis confirms differences in circular RNA expression profiles of cumulus cells between patients with ovarian and peritoneal endometriosis-associated infertility. Front Endocrinol. (2023) 14:1137235. doi: 10.3389/fendo.2023.1137235
49. Carson SA, Kallen AN. Diagnosis and management of infertility. A review. Jama. (2021) 326:65–76. doi: 10.1001/jama.2021.4788
50. Wang L, Huang S, Li S, Li M, Shi J, Bai W, et al. Efficacy and safety of umbilical cord mesenchymal stem cell therapy for rheumatoid arthritis patients. a prospective phase I/II study. Drug Des Devel Ther. (2019) 13:4331–40. doi: 10.2147/DDDT.S225613
51. Zang L, Li Y, Hao H, Liu J, Cheng Y, Li B, et al. Efficacy and safety of umbilical cord-derived mesenchymal stem cells in Chinese adults with type 2 diabetes. A single-center, double-blinded, randomized, placebo-controlled phase II trial. Stem Cell Res Ther. (2022) 13:180. doi: 10.1186/s13287-022-02848-6
52. Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome. A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. (2021) 10:660–73. doi: 10.1002/sctm.20-0472
53. Nassauw LV, Tao L, Harrisson F. Distribution of apoptosis-related proteins in thequail ovary during folliculogenesis. BCL-2, BAX, and CPP32. Acta Histochemica. (1999) 101:103–12. doi: 10.1016/S0065-1281(99)80010-5
54. Zhao Y, Ma J, Yi P, Wu J, Zhao F, Tu W, et al. Human umbilical cord mesenchymal stem cells restore the ovarian metabolome and rescue premature ovarian insufficiency in mice. Stem Cell Res Ther. (2020) 11:466. doi: 10.1186/s13287-020-01972-5
55. Findlay JK BK, Kerr JB, O'Donnell L, Jones ME, Drummond AE, Simpson ER. The road to ovulation the role of oestrogens. Reprod Fertil Dev. (2001) 3:71. doi: 10.1071/RD01071
56. Woods DC, Tilly JL. An evolutionary perspective on adult female germline stem cell function from flies to humans. Semin Reprod Med. (2013) 31:24–32. doi: 10.1055/s-0032-1331794
57. Ding X, Liu G, Xu B, Wu C, Hui N, Ni X, et al. Human GV oocytes generated by mitotically active germ cells obtained from follicular aspirates. Sci Rep. (2016) 6:28218. doi: 10.1038/srep28218
58. Zhang C, Wu J. Production of offspring from a germline stem cell line derived from prepubertal ovaries of germline reporter mice. Mol Hum Reprod. (2016) 22:457–64. doi: 10.1093/molehr/gaw030
59. Gershon E, Dekel N. Newly Identified Regulators of Ovarian Folliculogenesis and Ovulation. Int J Mol Sci. (2020) 21:4565. doi: 10.3390/ijms21124565
60. Lu X, Bao H, Cui L, Zhu W, Zhang L, Xu Z, et al. hUMSC transplantation restores ovarian function in POI rats by inhibiting autophagy of theca-interstitial cells via the AMPK/mTOR signaling pathway. Stem Cell Res Ther. (2020) 11:268. doi: 10.1186/s13287-020-01784-7
Keywords: primary ovarian insufficiency, human umbilical cord mesenchymal stem cells, animal model, meta-analysis, estrous cycle, hormone level, folliculogenesis
Citation: Wang X, Li T, Bai X, Zhu Y, Zhang M and Wang L (2023) Therapeutic prospect on umbilical cord mesenchymal stem cells in animal model with primary ovarian insufficiency: a meta-analysis. Front. Med. 10:1211070. doi: 10.3389/fmed.2023.1211070
Received: 24 April 2023; Accepted: 12 May 2023;
Published: 31 May 2023.
Edited by:
Yujiao Deng, The Second Affiliated Hospital of Xi'an Jiaotong University, ChinaReviewed by:
Shengnan Yu, First Affiliated Hospital of Chongqing Medical University, ChinaCopyright © 2023 Wang, Li, Bai, Zhu, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Wang, MjE5NjA0MkB6anUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.