
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 16 August 2023
Sec. Rheumatology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1210170
 Chwan-Li Shen1,2,3*
Chwan-Li Shen1,2,3* John W. Newman4,5,6
John W. Newman4,5,6 Moamen M. Elmassry7
Moamen M. Elmassry7 Kamil Borkowski6
Kamil Borkowski6 Ming-Chien Chyu2,8
Ming-Chien Chyu2,8 Chanaka Kahathuduwa2,3,9,10
Chanaka Kahathuduwa2,3,9,10 Volker Neugebauer2,3,11,12
Volker Neugebauer2,3,11,12 Bruce A. Watkins5*
Bruce A. Watkins5*Background: Tai Chi (TC) controls pain through mind–body exercise and appears to alter inflammatory mediators. TC actions on lipid biomarkers associated with inflammation and brain neural networks in women with knee osteoarthritic pain were investigated.
Methods: A single-center, pre- and post-TC group (baseline and 8 wk) exercise pilot study in postmenopausal women with knee osteoarthritic pain was performed. 12 eligible women participated in TC group exercise. The primary outcome was liquid chromatography tandem mass spectrometry determination of circulating endocannabinoids (eCB) and oxylipins (OxL). Secondary outcomes were correlations between eCB and OxL levels and clinical pain/limitation assessments, and brain resting-state function magnetic resonance imaging (rs-fMRI).
Results: Differences in circulating quantitative levels (nM) of pro-inflammatory OxL after TC were found in women. TC exercise resulted in lower OxL PGE1 and PGE2 and higher 12-HETE, LTB4, and 12-HEPE compared to baseline. Pain assessment and eCB and OxL levels suggest crucial relationships between TC exercise, inflammatory markers, and pain. Higher plasma levels of eCB AEA, and 1, 2-AG were found in subjects with increased pain. Several eCB and OxL levels were positively correlated with left and right brain amygdala-medial prefrontal cortex functional connectivity.
Conclusion: TC exercise lowers pro-inflammatory OxL in women with knee osteoarthritic pain. Correlations between subject pain, functional limitations, and brain connectivity with levels of OxL and eCB showed significance. Findings indicate potential mechanisms for OxL and eCB and their biosynthetic endogenous PUFA precursors that alter brain connectivity, neuroinflammation, and pain.
Clinical Trial Registration: ClinicalTrials.gov, identifier: NCT04046003.
Knee osteoarthritis (OA), a progressive disease characterized by joint degeneration and inflammation (1), is manifested by movement limitations and chronic pain. Alleviating the pain and improving dysfunction in patients with knee OA are public health priorities. Symptomatic treatment is the aim of pharmacological management for knee OA pain relief.
While the etiology of knee OA pain and underlying causes are complex, compelling evidence suggests that oxylipins (OxL) and endocannabinoids (eCB) are involved in the progression of knee OA pain. The endocannabinoid system (ECS) has emerged as a possible therapeutic target for OA pain reduction (2, 3). The ECS is composed of eCB, at least 2 cannabinoid receptors [namely, cannabinoid receptor type 1 (CB1R) and cannabinoid receptor type 2 (CB2R)], their endogenous ligands [anandamide (AEA), 2-arachidonoylglycerol (2-AG)], other structurally related compounds, and the enzymes responsible for eCB biosynthesis and inactivation. eCBs are lipid mediators biosynthesized from polyunsaturated fatty acids (PUFA) and produced in the brain and peripheral tissues that mimic the action of Δ9-tetrahydrocannabinol (4). The ECS participates in pathophysiological processes such as pain, emotion, and memory function (3, 5, 6). In addition, the ECS can mediate pain and modulate knee OA symptoms (3, 5). The ECS may exert analgesic effects on OA pain as the eCB are found in cells of the nervous system responsible for pain processing as well as in immune cells that regulate the neuro-immune interactions to convey inflammatory hyperalgesia (7). CB1R and CB2R are found in the central nervous system (CNS) (8) including multiple sites in the brain (9); they are targets for neuroinflammation and neuropathic pain (7).
Many of the eCB and OxL are biosynthesized from PUFA of the essential omega-6 and omega-3 families (10), although endocannabinoid-like compounds are derived from saturated fatty acids. The dietary levels of the different families of omega-6 and omega-3 PUFA directly influence the extent of which eCB and OxL are synthesized, thereby influencing the levels of pro-inflammatory or less inflammatory OxL (11).
Expression of CB2R protein by both neurons and microglia in the spinal cord was significantly increased in an OA pain model, suggesting that targeting eCB may have potential in treating OA (12). In human studies, individuals with OA pain compared with healthy individuals without OA pain had increased levels of 2-AG in plasma and CB1R and CB2R gene expression in peripheral blood lymphocytes, and these changes were correlated with pain, emotion, and cognitive symptoms (5).
OxLs are recognized lipid-derived mediators of inflammation and immune cell function in patients with OA pain (13). For example, relative to those without OA pain, individuals with OA pain had higher levels of circulating cyclooxygenase-2 (COX-2) products, 8-iso-PGF2α (14), 15-keto-dihydroPGF2α (14), PGE2 (15), and 15-LOX product 15-HETE (15), suggesting higher systemic inflammation and OxL production.
The eCB system (ECS) is a strategic factor for synaptic plasticity and homeostatic processes of the nervous system (9) including the receptor ligands (AEA and 2-AG), that participate in synaptic plasticity (16) and neuroinflammation. The eCBs affect mood, alter neurotrophin levels, restore mPFC output cognitive function, and inhibit pain (10, 17, 18). The actions of eCB and OxL which modulate pain and inflammation are driven at least in part by their actions on the amygdala (18). In contrast, moderate-intensity exercise increases eCB in the brain and blood and are thought to contribute to athletic wellbeing (10, 11) and play a role as retrograde synaptic messengers (16) for pain reduction.
Tai Chi (TC) exercise, a form of mind–body moderate-intensity exercise, affords both physical benefits for knee OA (physical function, balance, and muscle strength) (19) and a cognitive component promoting psychological wellbeing, life satisfaction, and improved perceptions of health (20, 21). A recent meta-analysis evaluated the effects of TC on walking function and posture control in elderly with knee OA and reported that (i) the length of the TC intervention time ranged from 8 to 24 weeks and the types of TC included Yang-style and Sun-style; and (ii) TC is effective and safe for knee OA (19). The authors conclude that TC can be used as an adjuvant, reliable physical training strategy to improve walking function and balance control (19). Similar to the recent review (19), the present study employed 24-form Yang-style TC for 8 weeks in older women with knee OA and measured clinical outcomes of pain, stiffness, and functional limitation using Western Ontario and McMasters Universities Osteoarthritis Index (WOMAC), Osteoarthritis Index, Visual Analog Scale (VAS), and Brief Pain Inventory (BPI) (22). Different from the review of previous studies (19), our investigation determined the effects of TC on circulating levels of eCB and OxL and their correlations with our reported clinical outcomes and amygdala-medial prefrontal cortex (mPFC) connectivity by resting-state functional magnetic resonance imaging (rs-fMRI) and diffusion tensor imaging (DTI) (22).
Chronic pain is thought to alter brain reward circuitry (23, 24) to include effects on shifting emotional states associated with pain chronification (25). Understanding this relationship between TC and reducing pain measurements of eCB and OxL can help advance knowledge to treat knee OA. Therefore, the objectives of this new study were (i) to explore if 8 weeks of TC exercise would modify plasma eCB and OxL levels and (ii) to conduct secondary analysis on correlations between TC-associated pre-post changes between eCB and OxL levels, clinical outcomes (pain, stiffness, and functional limitation), and amygdala mPFC connectivity changes. We hypothesize that changes in eCB levels and pattern as well as reduced inflammatory OxL mediators are, in part, responsible for the beneficial effects of TC on knee OA pain reduction and related changes in brain connectivity.
The present study was based on a single group pre-test and post-test design in 12 subjects to examine the effects of an 8 week TC exercise intervention on circulating eCB and OxL levels in postmenopausal women with knee OA. Sex is a strong risk factor for knee OA. After the age of 50, there is steep increase in incidence of knee OA in women compared with men, leading to a higher prevalence in women (26). Also, women with knee OA are more often accompanied by knee OA-associated pain and disability compared with men (26). Therefore, postmenopausal women with knee OA were recruited for this study.
Blood samples were collected at baseline and after 8 weeks of TC intervention. After centrifugation, plasma samples were obtained and stored at −80°C for later eCB and OxL analyses. This study was approved by the Institutional Review Board at the Texas Tech University Health Sciences Center (ClinicalTrials.gov Identifier: NCT04046003). In the correlation analyses of bioactive lipids and clinical outcomes 9 subjects were used. The criteria of knee OA exhibited symptoms was based on American College of Rheumatology clinical classification criteria for OA. Correlations between eCB and OxL levels and clinical outcomes (pain, stiffness, and functional limitation) data and between eCB and OxL levels and rs-fMRI data were also evaluated. Subjects were requested to maintain their current diets, medication, and physical activity, and not take any new supplements or vitamins.
In brief, postmenopausal women (≥50 years old) with knee pain were recruited from clinics and community centers in the Lubbock, Texas (United States) area by flyers and advertisements through newspaper (22). Inclusion criteria included (1) Postmenopausal women, (2) WOMAC pain score with at least 2 items out of 5 items are rated as moderate, severe, or extreme, respectively, (3) English literacy, (4) able to undergo an MRI scan for subjects having MRIs, (5) current pain in the knee, and (6) medical diagnosis of knee OA or knee(s) exhibited symptoms based on American College of Rheumatology clinical classification criteria for OA [as previously described Shen et al. (22)]. Exclusive criteria included (1) Prior experience with mind–body practice (e.g., TC, Qi Gong, yoga, or acupuncture) or physical therapy programs for knee OA within the past 3 months, (2) Severe medical limitations (i.e., dementia, symptomatic heart or vascular disease, or recent stroke) precluding full participation, (3) Medical/neurological or other systemic diseases affecting the musculoskeletal systems (i.e., polio/Parkinson’s/multiple sclerosis, rheumatoid arthritis, uncontrolled gout, etc. in addition to cerebral vascular accident or stroke) and diabetes with peripheral neuropathy affecting their sensory/balance, (4) intra-articular steroid injection or reconstructive surgery on most severely affected knee in the past 3 months, (5) intra-articular hyaluronic acid injections on most severely affected knee in the past 6 months, and (6) inability to walk without an assistive device.
In this study the participants were enrolled in an 8 week 24-form Yang style TC program (22). Instructor led TC group consisted of 3 classes per week on 3 nonconsecutive days for 60 min at the Gym for the Department of Kinesiology and Sport Management, Texas Tech University, Lubbock. Compliance of TC classes was assessed by attendance records maintained by a Master TC instructor for the entire period. The Master TC instructor and assistant closely observed all participants and insured that each aspect of TC was performed correctly. The subjects did not have any TC practice experience prior to the study.
Details of the patient recruitment and screening, TC intervention and compliance, self-reported clinical outcomes assessed by Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC: 5 items for pain, 2 items for stiffness, and 17 items for functional limitation), Visual Analog Scale (VAS), Brief Pain Inventory (BPI), and rs-fMRI data have been published previously (22).
Twenty four plasma samples (50 μL) (baseline and after TC) were subjected to protein precipitation in the presence of isotopically labeled analytical surrogates, filtered, and stored at −20°C until analysis as previously reported (27). After sample re-randomization, analytical targets were separated by UPLC and detected by electrospray ionization with positive/negative switching and multiple reaction monitoring on an API 6500 QTrap (AB Sciex, Framingham, MA, United States). Analytes were quantified using surrogate/analyte response ratios and 6- to 10-point calibration curves (27). Data were processed with AB Sciex MultiQuant v 3.0.1. All auto-integrations were reviewed and adjusted as necessary by an experienced analyst blinded to the sample key and reviewed by the project quality assurance officer. Data below the lowest calibration point or with >25% blank contribution were flagged and manually inspected, but all values were reported if signal was observed in >15% of the analyzed samples. Samples were processed in a single experimental batch along with two method blanks, and one plasma standard reference materials (NIST-1950; Sigma-Aldrich, St. Louis, MO), and accuracy was evaluated by comparison to laboratory historical measures of these materials (26). All values are quantitative in nM. It should be noted that monoacylglycerol isomerization occurs under these conditions, and the sum of the 1- and 2-isomers should only be considered as a surrogate measure of changes in 2-AG tone.
Descriptive statistics were calculated to inspect the distributional properties of eCB and OxL. Further, values for eCB and OxL are also presented as standardized differences calculated from the difference between values of treatment and control, divided by the pooled SEM (28, 29). All analyses were conducted using R statistical software version 4.0.5 (30). Wilcoxon signed-rank tests were performed to examine the changes between pre- and post-TC intervention for eCB and OxL metabolites. Statistical significance was determined at 0.05 alpha level and an effect size was computed for each comparison. Furthermore, Spearman’s correlation analyses were performed using these parameter estimates between pre- vs. post-intervention changes in eCB and OxL and the corresponding changes in clinical measures (i.e., WOMAC, VAS, BPI, and fMRI). A final analysis of Spearman’s correlation of data was performed for the eCB and OxL values for all clinical measures and fMRI. Partial least squares discriminate analysis (PLS-DA) was used to visualize metabolite differences in women using endocannabinoid and oxylipin data at baseline (week 0) and after (week 8) TC exercise. Analyses were performed using auto-scaled data after the imputation of metabolites missing was detected in >70% of study participants (31). All metabolites were used to perform the analysis. Metabolites with variable importance in projections (VIP) >1 were considered significant in group discrimination. VIP scores were correlated with the t-test value of ps.
Table 1 shows the demographic information and health status of all participants as previously reported (22). The subjects were recruited locally around the city of Lubbock Texas in the United States. The women had no adverse effects from TC exercise.
Values for plasma levels of eCB AEA, 1,2-AG, and DHEA were lower based on standardized differences after TC exercise compared to baseline (Table 2). Relative to baseline, the subjects had lower plasma levels for LEA after 8 week TC. Notably, AEA was not higher after TC a condition of moderate exercise in contrast to other studies in subjects (10). It is important to note that a dietary record was not obtained from the women in this investigation, yet the baseline and after TC measurements of relevant eCB and OxL precursor substrate PUFAs did not differ (value area ratio means ± SD, baseline and post-TC, arachidonic acid 5.64 and 5.47 ± 2.20, eicosapentaenoic acid 5.20 and 5.92 ± 3.76, docosahexaenoic acid 5.36 and 5.75 ± 3.96 area ratios). Several eCB and OxL levels were highly positively correlated with both left and right brain amygdala-mPFC functional connectivity in subjects.
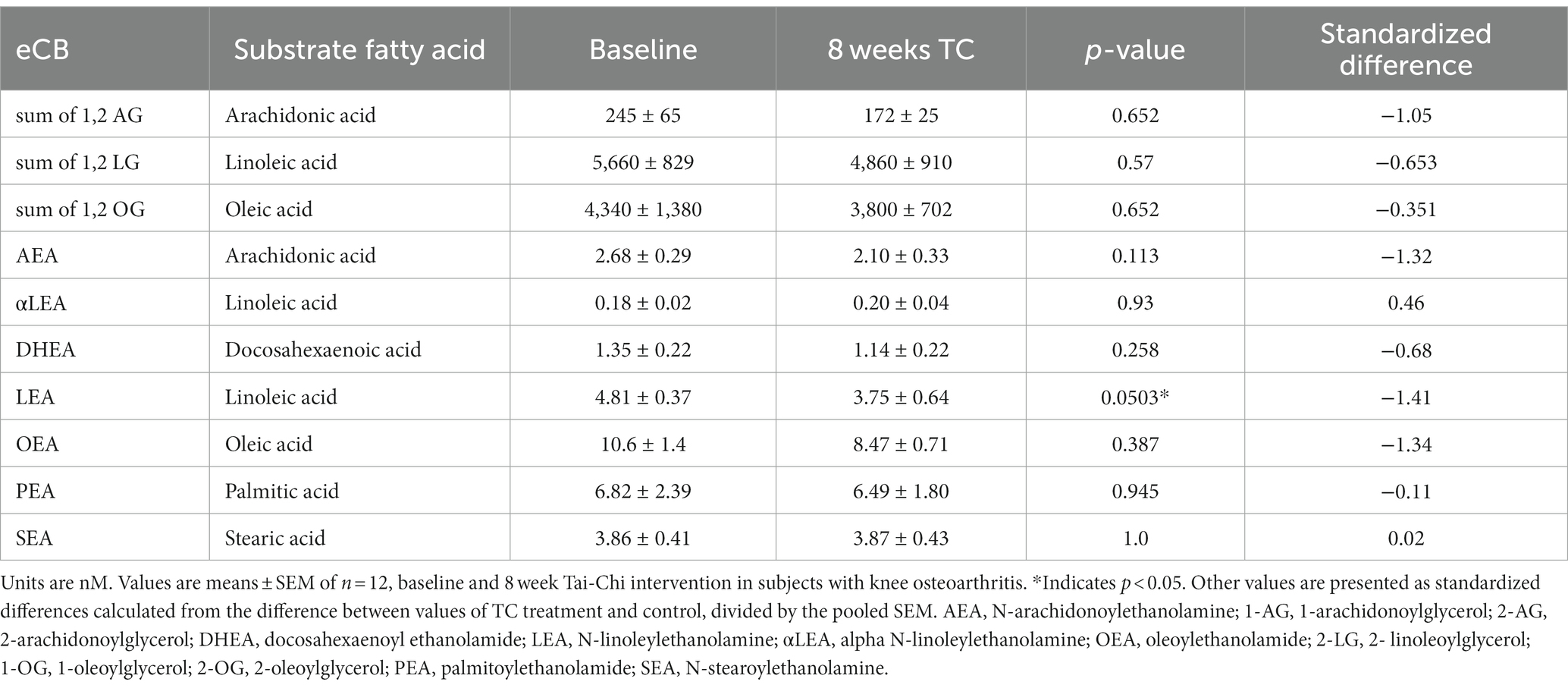
Table 2. Effect of TC on endocannabinoids (eCB) and related-compounds levels in plasma of women with OA.
TC resulted in significant changes for many OxL compared to pre-TC (baseline) in women (Table 3). OxL derived from arachidonic acid, prostanoids PGE1 and PGE2, were reduced after TC. The reduced level of pro-inflammatory PGE2 is a clear indication that TC reduced this potent pro-inflammatory OxL. The levels of 12-HEPE and 12,13-DiHODE (metabolites of eicosapentaenoic and linolenic acids, respectively) as well as 12-HETE, 20-HETE, and LTB4 (metabolites of arachidonic acid) in subjects were higher post TC. The increase in LTB4 could be due to higher inflammation or macrophage activation with TC (32). The not significant values of other identified OxL are presented in Supplementary Table S1.
In general, many products of arachidonic acid can support pro-inflammatory actions compared to those derived from the substrate eicosapentaenoic acid. Interestingly TC resulted in lower LEA compared to baseline. The higher blood levels of 12-HETE and 12-HEPE likely indicate greater 12-LOX pathway flux supported by inflammation (33). The full names and metabolic pathways for the synthesis of OxL are described elsewhere (31, 34).
Figure 1 is the partial least squares discriminate analysis (PLS-DA) for all eCB and OxL data from subjects at baseline week 0 (Wk 0) and after week 8 (Wk 8) of TC exercise. All values are numerically presented in Supplementary Table S2. The PLS-DA shows that values in the upper left quadrant are of high variable importance, VIP above 1 (p ≤ 0.05) and negative relationship with TC exercise. The positive values associated with TC exercise appear in the right upper quadrant of Figure 1. Thus, the VIP values show that TC reduced OxL PGE2, 8,9-DiHeTrE, and 8,15-DiHETE and eCB AEA, which are all derivatives of arachidonic acid, but led to higher 12-HEPE (EPA derivative), and LTB4 and 12-HETE (both arachidonate derivatives). Moreover, for the measured variables many of the eCB and OxL showed strong VIP values, negative or positive relationships. Several other eCB and OxL measurements revealed weaker VIP values. The weaker VIP values suggest a lower confidence in their apparent actions in TC exercise, but do not mean a lesser role of TC on subjects and relationships to pain and well-being. It is interesting that eCB DHEA derived from DHA showed a VIP score of greater than one and might suggest that DHA could help attenuate inflammation or serve in another capacity during TC. Based on VIP values, numerous OxL-derived from long-chain n-6 and n-3 PUFA were changed and thus may play a role in mediating the effects of TC exercise.
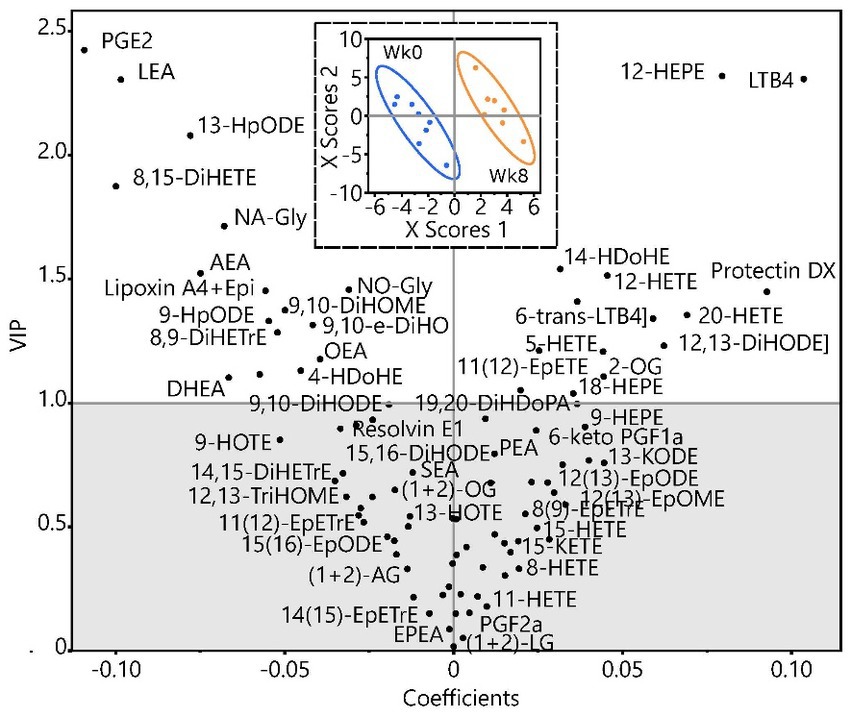
Figure 1. Partial least squares discriminate analysis of endocannabinoid and oxylipin data in women at baseline week 0 (Wk 0) and after 8 weeks (Wk 8) of TC exercise.
Analyses in Figure 1 were performed on data (in nM) after imputation of missing values by visit for metabolites with >60% of measured metabolites, followed by transformation to normal distributions. The analysis scores plot (inset) shows group discrimination with data for each group, baseline, and post-TC, bound by bivariate normal ellipses (p = 0.95). The variable coefficient plot shows metabolite strength in discrimination. Metabolites with variable importance in projections (VIP) >1 are considered to have discriminating power. Due to the low subject number, leave one out cross validation was used to build the model. Group discrimination was achieved with a minimum of 2 dimensions as shown (Q2 = 0.45; r2x = 0.24, r2y = 0.96) but was improved considering a third dimension (Q2 = 0.64; r2x = 0.36; r2y = 0.99). A complete list of metabolites, their concentration group means, VIP scores, and value of ps from 2-tailed t-tests are included in Supplementary Table S2.
Another analysis revealed significant correlations (Spearman’s correlation coefficient) between pain assessment and circulating levels of OxL and eCB, suggesting important relationships between inflammatory/anti-inflammatory markers and pain assessment as shown in Table 4. The correlation comparisons are all measurements for pre- and post-TC. All pain assessments [BPI, brief pain inventory; VAS, visual analog scale; Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)] correlations for OxL and eCB and eCB-like compounds were significant based on Spearmen’s correlation coefficient. In general, most OxL values from pre- and post-TC exercise showed a negative correlation to pain-related assessments. Therefore, differences for pre- and post-TC values indicate a decline in most OxL that potentially plays a role in inflammation. However, positive correlations were found for 6-keto PGE2,15-keto PGE2, LTB4 (BPI_3: pain level at its worst in the past 24 h) with the pain assessments. In contrast, higher plasma eCB levels, such as DHEA (product of docosahexaenoic acid, omega-3 PUFA) were associated with less pain (BPI_9C: in the past 24 h, pain has interfered your walking ability) as were higher plasma levels of eCB-like compound LEA (BPI_5: pain level on average, BPI_9G: in the past 24 h, pain has interfered your enjoyment of life, BPI_9H: in the past 24 h, pain has interfered your ability to concentrate; VAS_3: what is the overall amount of pain you have experienced in the last week in your knee).
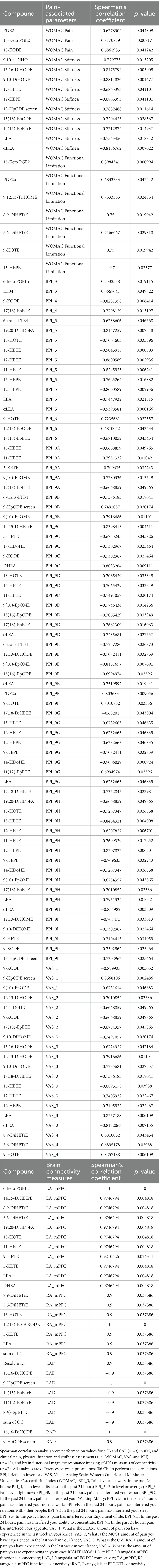
Table 4. Correlations between plasma OxL and eCB with clinical outcomes and functional aspects of brain connectivity.
Several positive correlations were found between brain left and right amygdala-mPFC functional connectivity (LA_mPFC and RA_mPFC) and OxL and eCB (Table 4). For example, the 8,9-DiHETrE, 5,6-DiHETrE, and 5-KETE (arachidonic acid products) showed a positive correlation for both LA_mPFC and RA_mPFC functional connectivity. The eCB DHEA and LEA revealed positive correlations for LA_mPFC, and a positive relationship was found for LEA and sum of LG with RA_mPFC. Negative relationships were found for OxL and LAD. The higher blood levels of 12-HETE and 12-HEPE associated with WOMAC-stiffness and BPI-5: pain level on average, could suggest greater 12-LOX pathway flux supported by inflammation (33).
Additional correlations for changes in OxL and eCB were done as individual pre- and post-TC scatter plots to illustrate the effects of TC as these parameters relate to pain and brain connectivity. Figure 2 shows some examples, specifically differences after TC revealed negative correlations between pain and OxL. Interestingly, the plots show robust TC physiological adaptations for changing OxL levels (e.g., PGE2, 5-KETE) and decreasing subject variability for these compounds.
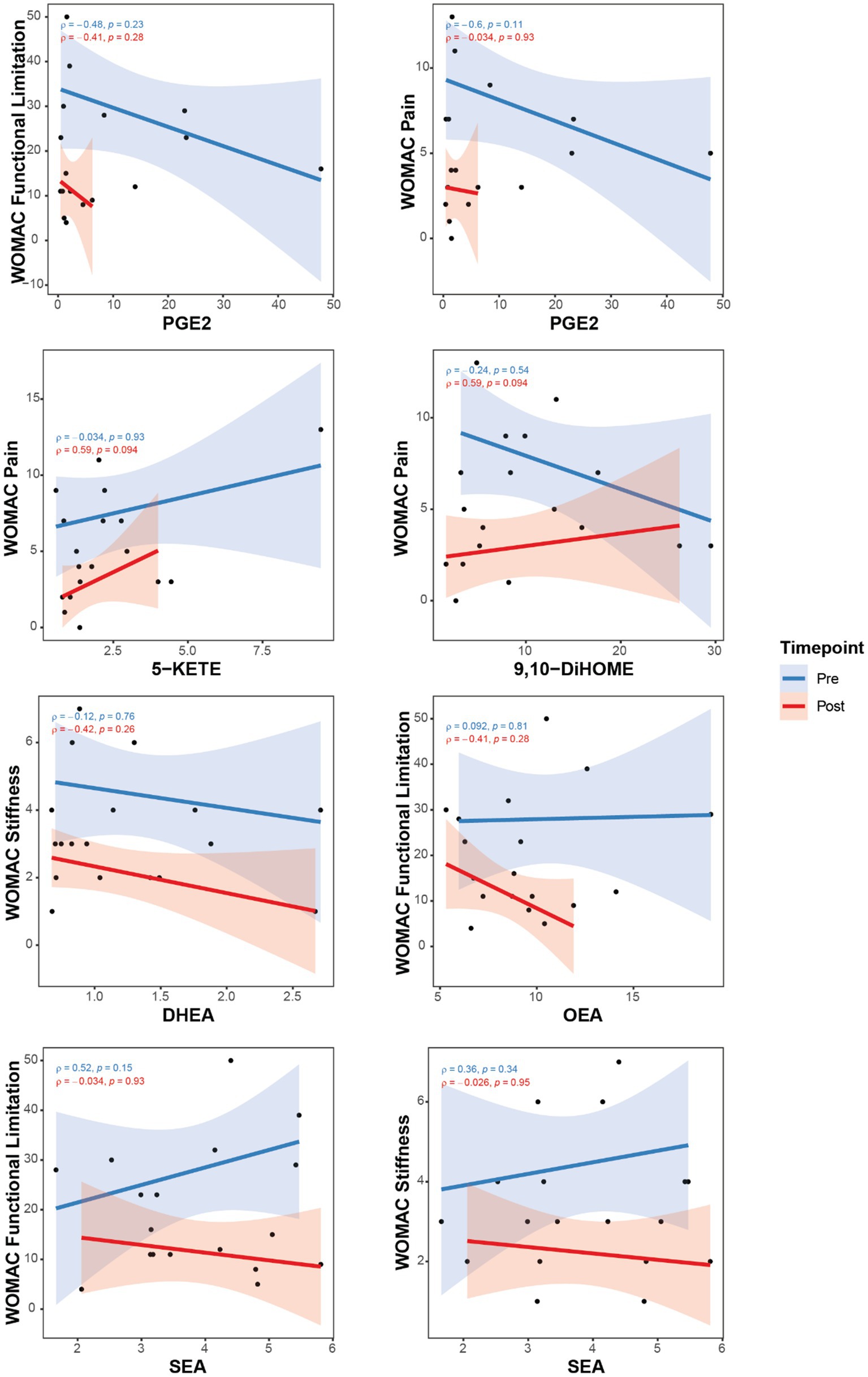
Figure 2. Correlations between pain assessments with OxL and eCB and related compounds for subjects pre- and post-TC exercise. Oxylipins, (PGE2, 5-KETE, and 9,10DiHOME); Endocannabinoid DHEA, docosahexaenoyl ethanolamide; Endocannabinoid-like OEA, oleoylethanolamide; PEA, palmitoylethanolamide; SEA, N-stearoylethanolamine. Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC).
Consistent with significant correlations for differences in values presented in Table 4, the individual plots (Figures 2, 3) indicate a physiologic effect of TC to establish a lower threshold level of OxL in homeostasis. This new chemical threshold for OxL is likely responsible for lower WOMAC-pain values. With respect to eCB and eCB-like compounds, DHEA values were less variable in subjects after TC, which reflects a new level threshold change for the physiological adaptation from TC exercise (Figure 2). Also, OEA showed a tightening of values and negative slope for WOMAC-functional limitations and WOMAC-pain, as well as BPI-3: pain level at its worst in the past 24 h. SEA and WOMAC-functional limitation and stiffness showed an opposite slope post-TC compared to pre-TC or baseline (Figure 2).
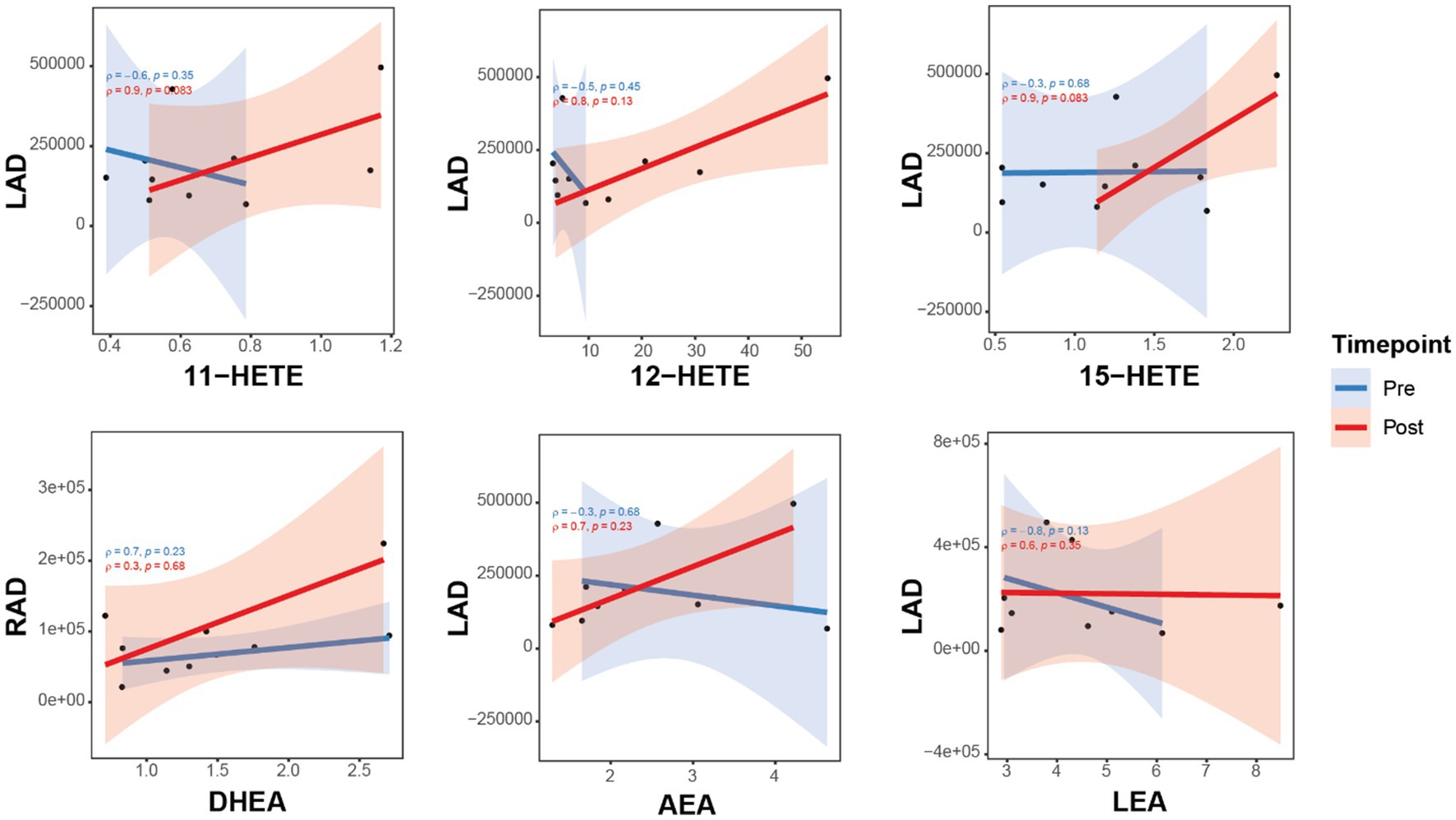
Figure 3. Correlations between brain connectivity with OxL and eCB and related compounds for subjects pre- and post-TC exercise. Brain connectivity measured as resting-state functional magnetic resonance imaging (rs-fMRI). Oxylipins (11-, 12-, 15-HETE, products of arachidonic acid). AEA, N-arachidonoylethanolamine; DHEA, docosahexaenoyl ethanolamide; LEA, N-linoleylethanolamine. LA_mPFC, L/amygdala-mPFC functional connectivity; LAD, L/amygdala-mPFC DTI connectivity; RA_mPFC, R/amygdala-mPFC functional connectivity; RAD, R/amygdala-mPFC DTI connectivity.
The relationships of three HETE (11-, 12-, and 15-products of arachidonic acid) show a positive correlation with brain connectivity after TC (Figure 2). Moreover, the upward slope of all three HETE would suggest a strong association with changes in brain connectivity for LAD. This upward slope was also observed with the eCB DHEA and AEA for RAD and LAD, respectively (Figure 3). In contrast, the eCB-like compound LEA seems to change little with LAD values for brain connectivity after TC. However, LEA level was lower after TC (Table 2).
This pilot study is the first to examine the effects of 8 week TC intervention on circulating eCB and OxL levels in postmenopausal women with knee OA pain. The self-reported pain reduction and its impact on physical functioning results of WOMAC and BPI in OA subjects is evidence that 8 weeks of TC intervention diminishes pain, stiffness, and functional limitations that are linked to some extent with pro-inflammatory OxL biomarkers. The changes in lipid-derived eCB and OxL after TC are associated with improved well-being (11), which is important to pain perception, brain emotional circuits (25), and loss of gray matter density (35). Interestingly TC also resulted in lower LEA compared to baseline and may seem to lower activation of Transient Receptor Potential Vanilloid 1 (TRPV1) and pain sensation (36).
Oxidative stress and systemic low-grade inflammation are upregulated in OA (37). Thus, studies such as ours that focus on plasma eCB and OxL analyses are key to identifying biomarkers connected with OA progression. While previous studies (22, 38) examined the health benefits of TC intervention on mitigation of oxidative stress and inflammation in knee OA patients, no investigation has undertaken a systemic biological/physiological approach to determine the effects of TC on systemic levels of OxL and eCB. Herein, integration of the targeted metabolomics data and correlations with brain functional/structural connectivity and clinical data on pain, stiffness, and functional limitation assessment of our study affords new insights on TC as an intervention for pain management in OA patients. Further, the eCB and their lipid analogues are reported to influence oxidative stress (39). Our investigation is the first to examine the effect of TC exercise on an extensive plasma OxL and eCB lipid biomarker analyses, along with brain functional and structural connectivity in knee OA patients. Our results serve as a foundation for future mechanistic studies.
Our hypothesis examined an important knowledge gap on how TC improves pain associated with knee OA by measuring eCB and OxL as targets to alter inflammation in the peripheral and central nervous systems (11). We establish that TC changes circulating eCB and OxL levels, and amygdala mPFC connectivity, and reduces pain and stiffness, resulting in improved physical function. The effects of TC on OA patients have been evaluated using brain fMRI as described by Shen et al. (22) and Liu et al. (40, 41). Liu et al. reported that all exercises (Tai chi, Baduanjin, and stationary cycling) (i) significantly decreased right periaqueductal grey rs-functional connectivity with the medial orbital prefrontal cortex, and decreased rs-functional connectivity associated with improvements in knee pain; and (ii) significantly increased grey matter volume in the medial orbital prefrontal cortex. There was also significantly decreased rs-functional connectivity between the left ventral tegmental area and the medial orbital prefrontal cortex only in the Tai Chi and Baduanjin groups (40). From the same study, relative to the control group, all exercise groups (i) showed decreased rs-functional connectivity of the dorsolateral prefrontal cortex-supplementary motor area, (ii) increased rs-functional connectivity between the dorsolateral prefrontal cortex and anterior cingulate cortex, and (iii) increased grey matter volume in the supplementary motor area (41). Studies on TC and OA suggest TC can simultaneously modulate the resting-state functional connectivity of the descending pathway and reward/motivation system and blood inflammation markers (40, 41). TC is associated with a strengthening of functional and structural connectivity between the medial prefrontal cortex and left and right amygdala (22). Thus, TC effects are likely, in part, mediated by OxL and eCB as shown in the current study.
Another aspect of our clinical research is the relationship between dietary PUFA, which serves as substrate for the different groups of eCB and OxL, and as a consequence level of these inflammatory bioactive lipids to impact pain. We also found that the eCB DHEA which is derived from DHA showed a VIP score of greater than one, suggesting that dietary DHA could help attenuate inflammation or serve in another capacity during TC. Dietary fat is reported to influence plasma and intestinal concentrations of eCB, N-acylethanolamines (NAEs), and their precursors N-acylphosphatidylethanolamines (NAPEs) (42). Moreover, specific families of PUFA have long been recognized as substrate of pro-inflammatory and less inflammatory OxL (11). Thus, changing the amounts and types of n-3 and n-6 families of PUFA can lead to changes in less pro-inflammatory OxL, by replacing arachidonic acid with eicosapentaenoic acid to modulate inflammatory responses (10, 11, 43, 44). With respect to OxL in this study, the arachidonic acid product PGE2 was significantly lower and associated with lower pain post-TC, and the values for this OxL were less variable within subject group after TC. The higher levels of 12-HEPE (EPA derivative), and LTB4 and 12-HETE (both arachidonate derivatives) observed after TC are important since these OxL afford anti-inflammatory activity and modulate inflammatory response, respectively, via the PUFA substrate (33).
The effect of TC appears to stabilize OxL levels to a new physiological threshold for homeostasis. Considering these findings, future clinical research must include dietary PUFA and TC interactions on the amounts and types of circulating OxL and eCB (and eCB-like compounds) and on pain and brain connectivity. In support of our findings with TC, the actions of stretching were described to reduce inflammatory mediators and oxidative stress to lower pain (45). The authors emphasized the need to explain the anti-inflammatory properties of stretching for OA recovery, which is consistent with our hypothesis to understand the effects of TC and potential benefits causing changes in OxL and eCB levels post TC exercise to lower OA pain.
A limitation of this study is the small subject group; however, each subject was included for baseline and post TC treatment for analysis of eCB and OxL levels. The duration of TC was 8 weeks from baseline as two time points for the measurements. Nevertheless, this study confirms differences in pre- and post-TC exercise changes of the same OA subjects for a comprehensive panel of eCB and OxL levels in blood. These novel findings afford a new approach to understand exercise benefits on clinical biomarkers for OA. The application is two aspects of improving pain and wellbeing utilizing patient pain assessment and brain connectivity. TC altered levels of OxL derived from n-3 and n-6 PUFA family substrate and decreased pro-inflammatory states. The changes in brain connectivity post-TC and correlations with plasma eCB and OxL levels are noteworthy and suggest an important relationship with neuroinflammation and brain neuroplasticity for these lipid biomarkers under mind–body exercise. Future studies should examine the effects of dietary n-3 PUFA and include dietary records to ascertain if n-3 PUFA substrates alter the types of eCB, and further change pro-inflammatory OxL.
Our findings identified an anti-neuroinflammatory role of TC exercise which is linked to modified blood levels of specific OxL, a reduction in pain and stiffness, and improved physical function in subjects with knee osteoarthritis pain. TC appears to serve as a viable means for non-pharmaceutical treatment to modify systemic production of pro-inflammatory OxL compounds. Controlled clinical trials are warranted to confirm our findings and expand on the specific changes for both OxL and eCB following TC exercise. Moreover, TC altered levels of OxL derived from n-3 and n-6 PUFA family substrate and decreased pro-inflammatory states. The changes in brain connectivity post-TC and correlations with plasma eCB and OxL levels are noteworthy and suggest an important relationship with neuroinflammation and brain neuroplasticity for these lipid biomarkers under mind–body exercise. Future studies should examine the effects of dietary n-3 PUFA and include dietary records to ascertain if n-3 PUFA substrates alter the types of eCB during TC, and further change pro-inflammatory OxL during TC in a randomized placebo-controlled trial with a larger sample size for an adequate power. Dietary approaches or supplements that lower substrate for arachidonic acid with n-3 PUFA or fish oil may work synergistically to help reduce pain with TC exercise. In this scenario, emphasis must be placed on brain connectivity and plasticity. Hence, we do support the practice of TC to help control pain in women with knee osteoarthritis given the changes in eCB and OxL.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Texas Tech University Health Sciences Center Lubbock IRB. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
C-LS, JN, M-CC, CK, VN, and BW: conceptualization and methodology. JN, ME, KB, and CK: data collection and analysis. C-LS, JN, ME, and BW: writing-original draft preparation. C-LS, JN, ME, M-CC, CK, KB, VN, and BW: writing-review and editing. C-LS, M-CC, and VN: supervision, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
This study was supported by NIH/NINDS R01 NS038261 (VN), Texas Tech University Neuroimaging Institute, Texas Tech University, Lubbock, TX (CLS) and the Center of Excellence for Translational Neuroscience and Therapeutics, Texas Tech University Health Sciences Center, Lubbock, TX (CLS and VN). Additional support was provided by USDA Intramural Projects 2032-51530-025-00D (JN). The USDA is an equal opportunity employer and provider. The funders had no influence on the conceptualization, data collection, analysis, or interpretation of data presented within the manuscript.
We thank all subjects for their participation in this study. We thank Ami Knox and TTUHSC Clinical Research Institute staff for their support in data collection and project coordination, and Kasey Rieken for performing fMRI on subjects. We also thank Jeff Roark for offering Tai Chi sessions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1210170/full#supplementary-material
1. Bhatia, D, Bejarano, T, and Novo, M. Current interventions in the management of knee osteoarthritis. J Pharm Bioallied Sci. (2013) 5:30–8. doi: 10.4103/0975-7406.106561
2. Bryk, M, Chwastek, J, Kostrzewa, M, Mlost, J, Pedracka, A, and Starowicz, K. Alterations in anandamide synthesis and degradation during osteoarthritis progression in an animal model. Int J Mol Sci. (2020) 21:7381. doi: 10.3390/ijms21197381
3. Mlost, J, Wasik, A, and Starowicz, K. Role of endocannabinoid system in dopamine signalling within the reward circuits affected by chronic pain. Pharmacol Res. (2019) 143:40–7. doi: 10.1016/j.phrs.2019.02.029
4. Battista, N, Di Tommaso, M, Bari, M, and Maccarrone, M. The endocannabinoid system: an overview. Front Behav Neurosci. (2012) 6:9. doi: 10.3389/fnbeh.2012.00009
5. Chadwick, VL, Rohleder, C, Koethe, D, and Leweke, FM. Cannabinoids and the endocannabinoid system in anxiety, depression, and dysregulation of emotion in humans. Curr Opin Psychiatry. (2020) 33:20–42. doi: 10.1097/YCO.0000000000000562
6. Zhou, D, Li, Y, Tian, T, Quan, W, Wang, L, Shao, Q, et al. Role of the endocannabinoid system in the formation and development of depression. Pharmazie. (2017) 72:435–9. doi: 10.1691/ph.2017.7474
7. Donvito, G, Nass, SR, Wilkerson, JL, Curry, ZA, Schurman, LD, Kinsey, SG, et al. The endogenous cannabinoid system: a budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology. (2018) 43:52–79. doi: 10.1038/npp.2017.204
8. Kaur, I, Behl, T, Bungau, S, Zengin, G, Kumar, A, El-Esawi, MA, et al. The endocannabinoid signaling pathway as an emerging target in pharmacotherapy, earmarking mitigation of destructive events in rheumatoid arthritis. Life Sci. (2020) 257:118109. doi: 10.1016/j.lfs.2020.118109
9. Kendall, DA, and Yudowski, GA. Cannabinoid receptors in the central nervous system: their Signaling and roles in disease. Front Cell Neurosci. (2016) 10:294. doi: 10.3389/fncel.2016.00294
10. Watkins, BA. Endocannabinoids, exercise, pain, and a path to health with aging. Mol Asp Med. (2018) 64:68–78. doi: 10.1016/j.mam.2018.10.001
11. Park, Y, and Watkins, BA. Dietary PUFAs and exercise dynamic actions on endocannabinoids in brain: consequences for neural plasticity and neuroinflammation. Adv Nutr. (2022) 13:1989–2001. doi: 10.1093/advances/nmac064
12. Anthony, AT, Rahmat, S, Sangle, P, Sandhu, O, and Khan, S. Cannabinoid receptors and their relationship with chronic pain: a narrative review. Cureus. (2020) 12:e10436. doi: 10.7759/cureus.10436
13. Lundstrom, SL, Yang, J, Brannan, JD, Haeggstrom, JZ, Hammock, BD, Nair, P, et al. Lipid mediator serum profiles in asthmatics significantly shift following dietary supplementation with omega-3 fatty acids. Mol Nutr Food Res. (2013) 57:1378–89. doi: 10.1002/mnfr.201200827
14. Wang, K, Wang, Z, Cui, R, and Chu, H. Polysaccharopeptide from Trametes versicolor blocks inflammatory osteoarthritis pain-morphine tolerance effects via activating cannabinoid type 2 receptor. Int J Biol Macromol. (2019) 126:805–10. doi: 10.1016/j.ijbiomac.2018.12.212
15. Attur, M, Krasnokutsky, S, Statnikov, A, Samuels, J, Li, Z, Friese, O, et al. Low-grade inflammation in symptomatic knee osteoarthritis: prognostic value of inflammatory plasma lipids and peripheral blood leukocyte biomarkers. Arthritis Rheumatol. (2015) 67:2905–15. doi: 10.1002/art.39279
16. Lu, HC, and Mackie, K. An introduction to the endogenous cannabinoid system. Biol Psychiatry. (2016) 79:516–25. doi: 10.1016/j.biopsych.2015.07.028
17. Kiritoshi, T, Ji, G, and Neugebauer, V. Rescue of Impaired mGluR5-driven endocannabinoid Signaling restores prefrontal cortical output to inhibit pain in arthritic rats. J Neurosci. (2016) 36:837–50. doi: 10.1523/JNEUROSCI.4047-15.2016
18. Woodhams, SG, Chapman, V, Finn, DP, Hohmann, AG, and Neugebauer, V. The cannabinoid system and pain. Neuropharmacology. (2017) 124:105–20. doi: 10.1016/j.neuropharm.2017.06.015
19. You, Y, Liu, J, Tang, M, Wang, D, and Ma, X. Effects of tai chi exercise on improving walking function and posture control in elderly patients with knee osteoarthritis: a systematic review and meta-analysis. Medicine. (2021) 100:e25655. doi: 10.1097/MD.0000000000025655
20. Yang, GY, Sabag, A, Hao, WL, Zhang, LN, Jia, MX, Dai, N, et al. Tai chi for health and well-being: a bibliometric analysis of published clinical studies between 2010 and 2020. Complement Ther Med. (2021) 60:102748. doi: 10.1016/j.ctim.2021.102748
21. Hu, L, Wang, Y, Liu, X, Ji, X, Ma, Y, Man, S, et al. Tai chi exercise can ameliorate physical and mental health of patients with knee osteoarthritis: systematic review and meta-analysis. Clin Rehabil. (2021) 35:64–79. doi: 10.1177/0269215520954343
22. Shen, CL, Watkins, BA, Kahathuduwa, C, Chyu, MC, Zabet-Moghaddam, M, Elmassry, MM, et al. Tai chi improves brain functional connectivity and plasma Lysophosphatidylcholines in postmenopausal women with knee osteoarthritis: an exploratory pilot study. Front Med. (2021) 8:775344. doi: 10.3389/fmed.2021.775344
23. Apkarian, AV. Pain perception in relation to emotional learning. Curr Opin Neurobiol. (2008) 18:464–8. doi: 10.1016/j.conb.2008.09.012
24. Navratilova, E, and Porreca, F. Reward and motivation in pain and pain relief. Nat Neurosci. (2014) 17:1304–12. doi: 10.1038/nn.3811
25. Hashmi, JA, Baliki, MN, Huang, L, Baria, AT, Torbey, S, Hermann, KM, et al. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. (2013) 136:2751–68. doi: 10.1093/brain/awt211
26. Szilagyi, IA, Waarsing, JH, Schiphof, D, van Meurs, JBJ, and Bierma-Zeinstra, SMA. Towards sex-specific osteoarthritis risk models: evaluation of risk factors for knee osteoarthritis in males and females. Rheumatology. (2022) 61:648–57. doi: 10.1093/rheumatology/keab378
27. Pedersen, TL, Gray, IJ, and Newman, JW. Plasma and serum oxylipin, endocannabinoid, bile acid, steroid, fatty acid and nonsteroidal anti-inflammatory drug quantification in a 96-well plate format. Anal Chim Acta. (2021) 1143:189–200. doi: 10.1016/j.aca.2020.11.019
28. Horia, E, and Watkins, BA. Comparison of stearidonic acid and alpha-linolenic acid on PGE2 production and COX-2 protein levels in MDA-MB-231 breast cancer cell cultures. J Nutr Biochem. (2005) 16:184–92. doi: 10.1016/j.jnutbio.2004.11.001
29. Horia, E, and Watkins, BA. Complementary actions of docosahexaenoic acid and genistein on COX-2, PGE2 and invasiveness in MDA-MB-231 breast cancer cells. Carcinogenesis. (2007) 28:809–15. doi: 10.1093/carcin/bgl183
30. Team RC. A language environment for statistical computing.: R foundation for statistical computing; (2019) Available at: https://www.R-project.org/.
31. Watkins, BA, Friedman, AN, Kim, J, Borkowski, K, Kaiser, S, Fiehn, O, et al. Blood levels of endocannabinoids, oxylipins, and metabolites are altered in hemodialysis patients. Int J Mol Sci. (2022) 23:9781. doi: 10.3390/ijms23179781
32. Li, P, Oh, DY, Bandyopadhyay, G, Lagakos, WS, Talukdar, S, Osborn, O, et al. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat Med. (2015) 21:239–47. doi: 10.1038/nm.3800
33. Kulkarni, A, Nadler, JL, Mirmira, RG, and Casimiro, I. Regulation of tissue inflammation by 12-lipoxygenases. Biomol Ther. (2021) 11:717. doi: 10.3390/biom11050717
34. Watkins, BA, Kim, J, Kenny, A, Pedersen, TL, Pappan, KL, and Newman, JW. Circulating levels of endocannabinoids and oxylipins altered by dietary lipids in older women are likely associated with previously identified gene targets. Biochim Biophys Acta. (2016) 1861:1693–704. doi: 10.1016/j.bbalip.2016.07.007
35. Apkarian, AV, Sosa, Y, Sonty, S, Levy, RM, Harden, RN, Parrish, TB, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. (2004) 24:10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004
36. Benitez-Angeles, M, Morales-Lazaro, SL, Juarez-Gonzalez, E, and Rosenbaum, T. TRPV1: structure, endogenous agonists, and mechanisms. Int J Mol Sci. (2020) 21:3421. doi: 10.3390/ijms21103421
37. Tootsi, K, Martson, A, Kals, J, Paapstel, K, and Zilmer, M. Metabolic factors and oxidative stress in osteoarthritis: a case-control study. Scand J Clin Lab Invest. (2017) 77:520–6. doi: 10.1080/00365513.2017.1354255
38. Zhuang, SZ, Chen, PJ, Han, J, and Xiao, WH. Beneficial effects and potential mechanisms of tai chi on lower limb osteoarthritis: a biopsychosocial perspective. Chin J Integr Med. (2021) 29:368–76. doi: 10.1007/s11655-021-3529-9
39. Gallelli, CA, Calcagnini, S, Romano, A, Koczwara, JB, de Ceglia, M, Dante, D, et al. Modulation of the oxidative stress and lipid peroxidation by endocannabinoids and their lipid analogues. Antioxidants. (2018) 7:93. doi: 10.3390/antiox7070093
40. Liu, J, Chen, L, Chen, X, Hu, K, Tu, Y, Lin, M, et al. Modulatory effects of different exercise modalities on the functional connectivity of the periaqueductal grey and ventral tegmental area in patients with knee osteoarthritis: a randomized multimodal magnetic resonance imaging study. Br J Anaesth. (2019) 123:506–18. doi: 10.1016/j.bja.2019.06.017
41. Liu, J, Chen, L, Tu, Y, Chen, X, Hu, K, Tu, Y, et al. Different exercise modalities relieve pain syndrome in patients with knee osteoarthritis and modulate the dorsolateral prefrontal cortex: a multiple mode MRI study. Brain Behav Immun. (2019) 82:253–63. doi: 10.1016/j.bbi.2019.08.193
42. Tagliamonte, S, Gill, CIR, Pourshahidi, LK, Slevin, MM, Price, RK, Ferracane, R, et al. Endocannabinoids, endocannabinoid-like molecules and their precursors in human small intestinal lumen and plasma: does diet affect them? Eur J Nutr. (2021) 60:2203–15. doi: 10.1007/s00394-020-02398-8
43. Rajamani, A, Borkowski, K, Akre, S, Fernandez, A, Newman, JW, Simon, SI, et al. Oxylipins in triglyceride-rich lipoproteins of dyslipidemic subjects promote endothelial inflammation following a high fat meal. Sci Rep. (2019) 9:8655. doi: 10.1038/s41598-019-45005-5
44. Gabbs, M, Leng, S, Devassy, JG, Monirujjaman, M, and Aukema, HM. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv Nutr. (2015) 6:513–40. doi: 10.3945/an.114.007732
45. Krol, M, Kupnicka, P, Bosiacki, M, and Chlubek, D. Mechanisms underlying anti-inflammatory and anti-cancer properties of stretching – a review. Int J Mol Sci. (2022) 23:10127. doi: 10.3390/ijms231710127
Keywords: Tai Chi, oxylipins, postmenopausal women, knee osteoarthritis, endocannabinoids, brain
Citation: Shen C-L, Newman JW, Elmassry MM, Borkowski K, Chyu M-C, Kahathuduwa C, Neugebauer V and Watkins BA (2023) Tai Chi exercise reduces circulating levels of inflammatory oxylipins in postmenopausal women with knee osteoarthritis: results from a pilot study. Front. Med. 10:1210170. doi: 10.3389/fmed.2023.1210170
Received: 21 April 2023; Accepted: 25 July 2023;
Published: 16 August 2023.
Edited by:
Cong-Qiu Chu, Oregon Health and Science University, United StatesReviewed by:
Qingguang Zhu, Shanghai University of Traditional Chinese Medicine, ChinaCopyright © 2023 Shen, Newman, Elmassry, Borkowski, Chyu, Kahathuduwa, Neugebauer and Watkins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chwan-Li Shen, bGVzbGllLnNoZW5AdHR1aHNjLmVkdQ==; Bruce A. Watkins, YmF3YXRraW5zQHVjZGF2aXMuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.