
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 13 June 2023
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1209656
This article is part of the Research Topic Translational Research in Severe COVID-19 and Long-Term Symptoms Post-COVID-19, Volume II View all 4 articles
The neurologic manifestations of coronavirus disease 2019 (COVID-19) may range from mild symptoms such as headache or confusion to profound encephalopathy with variable outcomes and sequelae. Here, we reported a case of fatal COVID-19-associated encephalitis with acute fulminant cerebral edema, presenting first with visual hallucination and then a rapid progression into comatose status in a few hours. Serial brain computed tomography depicted cerebral edematous changes from bilateral ventral temporal lobe to the whole brain leading to brain herniation. Multiple cytokines in serum and cerebrospinal fluid (CSF) were increased, with a more prominent rise in the CSF. Therefore, we postulated a hypothesis regarding the mechanism of this fulminant encephalitis that the SARS-CoV-2 virus attacked ventral temporal lobes initially, triggered a severe cytokine storm, and then led to subsequent disruption of the blood-brain barrier, diffuse brain edema, and brain herniation. The trend of cytokine profiles over time may aid in diagnosing and evaluating the severity and prognosis of COVID-19-associated encephalitis.
The coronavirus disease 2019 (COVID-19), starting from a national outbreak in Wuhan, China, rapidly became a pandemic and caused huge disease burdens and mortalities worldwide. While respiratory symptoms are the most common clinical presentations, neurologic symptoms such as confusion, agitation, or seizure are also common (1). The neurologic manifestations could range from self-limited symptoms to fatal presentations such as fulminant encephalitis. Here, we report a case of severe COVID-19-associated encephalitis with acute fulminant cerebral edema, mainly to address the distinct clinical presentation and cytokines profile.
This 10-year-old previously healthy and COVID-19 naive boy who had not received vaccination for SARS-CoV-2 presented to our emergency department (ED) with a new-onset seizure and conscious disturbance. He had intermittent fever, dizziness, and emesis since the day before presenting to ED. His family members got fever and respiratory symptoms and were diagnosed with COVID-19 a few days before. A few hours before arriving at ED, he claimed he saw a monster in the bathroom and on his family member's shoulders and was terrified. Then, he developed upward gazing, generalized clonic convulsion, and impaired awareness lasting 5 min. Another two similar seizure episodes followed within a half hour, and he failed to regain alertness entirely after the last episode. Throughout the course, there was no involuntary movement, smell or taste change, focal weakness or sensory change. He was taken to our ED for further evaluation and management.
At arrival, the patient was alert but had decreased awareness. He was oriented to place, people but not to time, and his response was slower than his usual status. The temperature was 40.1°C, the blood pressure 105/50 mm Hg, the pulse 130 beats per min, the respiratory rate 22 breaths per min, and the oxygen saturation 98% in ambient air. The pupils were equal in size and reactive to light stimuli. Extraocular movements were intact without nystagmus or involuntary eye movements. Muscle strength was symmetrically full, and deep tendon reflexes were 2+ throughout with flexor plantar reflexes. The neck was supple, and Brudzinski and Kernig signs were negative. The result of a finger–nose–finger test was normal, and the performance of tandem gait was steady without deviation. The remaining physical examination was normal.
Computed tomography (CT) of the head with contrast medium (Figures 1A–G), performed 4 h after arrival, depicted bilateral mild cortical swelling, especially bilateral ventral temporal lobes, slightly pushing into tentorial incisura, indicating possible acute encephalitis. The laboratory data showed elevated alanine aminotransferase (91 U/L), aspartate aminotransferase (210 U/L), pro-calcitonin (11.85 ng/mL), and interleukin-6 (IL-6) level [0.271 ng/mL, by IL-6 test strip combined with a spectrum-based optical reader (2, 3)]. The cerebrospinal fluid (CSF) analysis later showed no pleocytosis, but a significantly high total protein (387 mg/dL) and interleukin-6 (IL-6) (5.5 ng/mL, by IL-6 test strip) level. He was positive for COVID-19 by reverse transcriptase polymerase chain reactive (PCR) on a nasopharyngeal swab, but the virus was not detected in the CSF by PCR. Other laboratory data was presented in Supplementary Table.
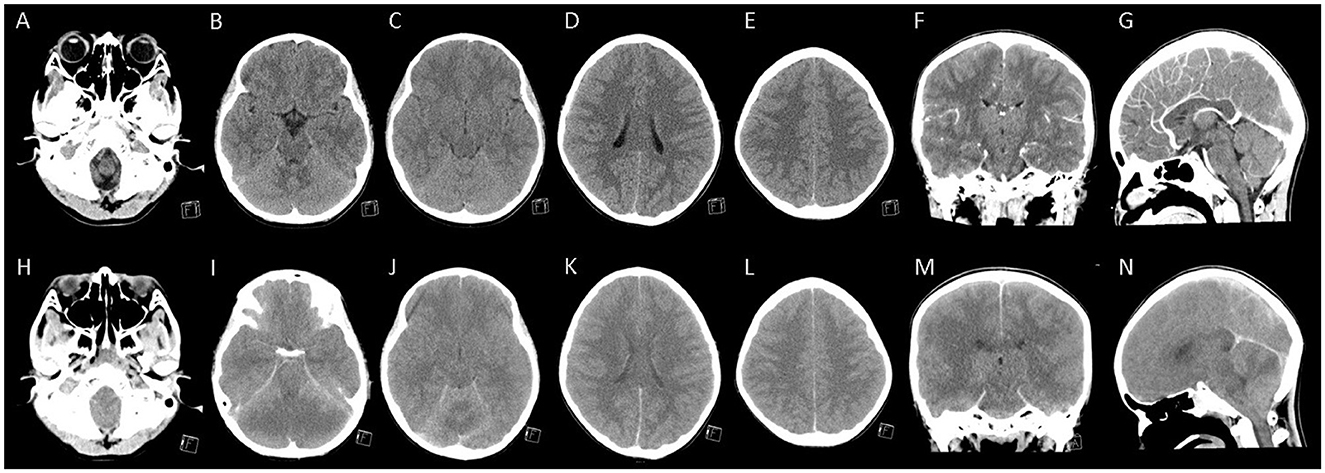
Figure 1. The brain computed tomography of the patient. (A–G) Represented contrast-enhanced computed tomography (CT) images performed 4 h after admission showed mild cortical swelling of bilateral ventral temporal lobes that slightly pushed into tentorial incisura. In contrast, the gray-white matter differentiation was preserved in fronto-pareital lobes. (H–N) Represented contrast-enhanced CT images performed 16 h after admission (6 h after cardiopulmonary cerebral resuscitation) showed diffuse cerebral and cerebellar swelling and bilateral uncal herniation with the inferior displaced brainstem.
Under the diagnosis of COVID-19-associated acute encephalitis, mannitol (0.5 g per kilogram) was given to reduce intracranial pressure and methylprednisolone pulse therapy (30 mg per kilogram) for anti-inflammation. Initially, this patient could still obey simple commands and responded slowly with few verbal outputs. However, he developed junctional tachycardia, then ventricular tachycardia and soon progressed to asystole 10 h after his arrival. After 15 min cardiopulmonary cerebral resuscitation (CPCR), the patient had a return of spontaneous circulation (ROSC) but became unresponsive, had no spontaneous breathing, and fixed dilated pupils since then. In considering COVID-19-related fulminant encephalitis, intravenous remdesivir (at doses of 5 mg per kilogram on the first day and 2.5 mg per kilogram on the subsequent days), tocilizumab (with a single dose of 12 mg per kilogram), and immunoglobulin (at a dose of 2 grams per kilogram) were administered successively. After ROSC, he was treated with therapeutic hypothermia at a target temperature of 33.5°C during the subsequent 3 days. Six hours after CPCR, another head CT (Figures 1H–N) showed diffuse cerebral and cerebellar swelling and bilateral uncal herniation with the inferior displaced brainstem. Mannitol was administered at a dose of 0.5 g per kilogram every 6 h.
Despite these therapeutic measures, in the subsequent 4 days, the patient remained unresponsive with fixed dilated pupils and had unstable hemodynamic status with systolic hypotension, central diabetes insipidus, and acute kidney injury with anuria. Laboratory examination showed hyperlactatemia, elevated serum creatinine level, transaminitis, hyperferritinemia and coagulopathy (Supplementary Table). Due to the progressive multi-organ failure that occurred in an order of acute respiratory distress syndrome, circulatory shock, hepatic dysfunction, acute renal failure, disseminated intravascular coagulation, and finally cardiac failure complicated by sustained ventricular tachycardia, he passed away on the morning of the fifth day of admission. The PCR for SARS-CoV-2 was negative from a post-mortem tissue biopsy of the lung, heart, liver, spleen, and kidney. The CSF obtained soon after death was negative for SARS-CoV-2, but still has a very high IL-6 level (17.978 ng/mL, by IL-6 test strip). The clinical course and the dynamic changes in inflammatory markers were summarized in Figure 2A. Multiple cytokines were tested from serum and CSF using a cytokine array, Multiplex ELISA Kit For Human Cytokine Release Syndrome (16-Plex) (Bosterbio, Pleasanton, California, USA). The results, presented in Table 1, showed an increase in multiple cytokines including GM-CSF, IL-1β, IL-10, IL-12p70, MIP-1α, IL-2Rα, IL-1Rα, IL-6, IL-8, and MCP-1 from both serum and CSF, with a more prominent rise in the CSF than in the serum. The trend of the cytokines over time is presented in Figures 2B, C.
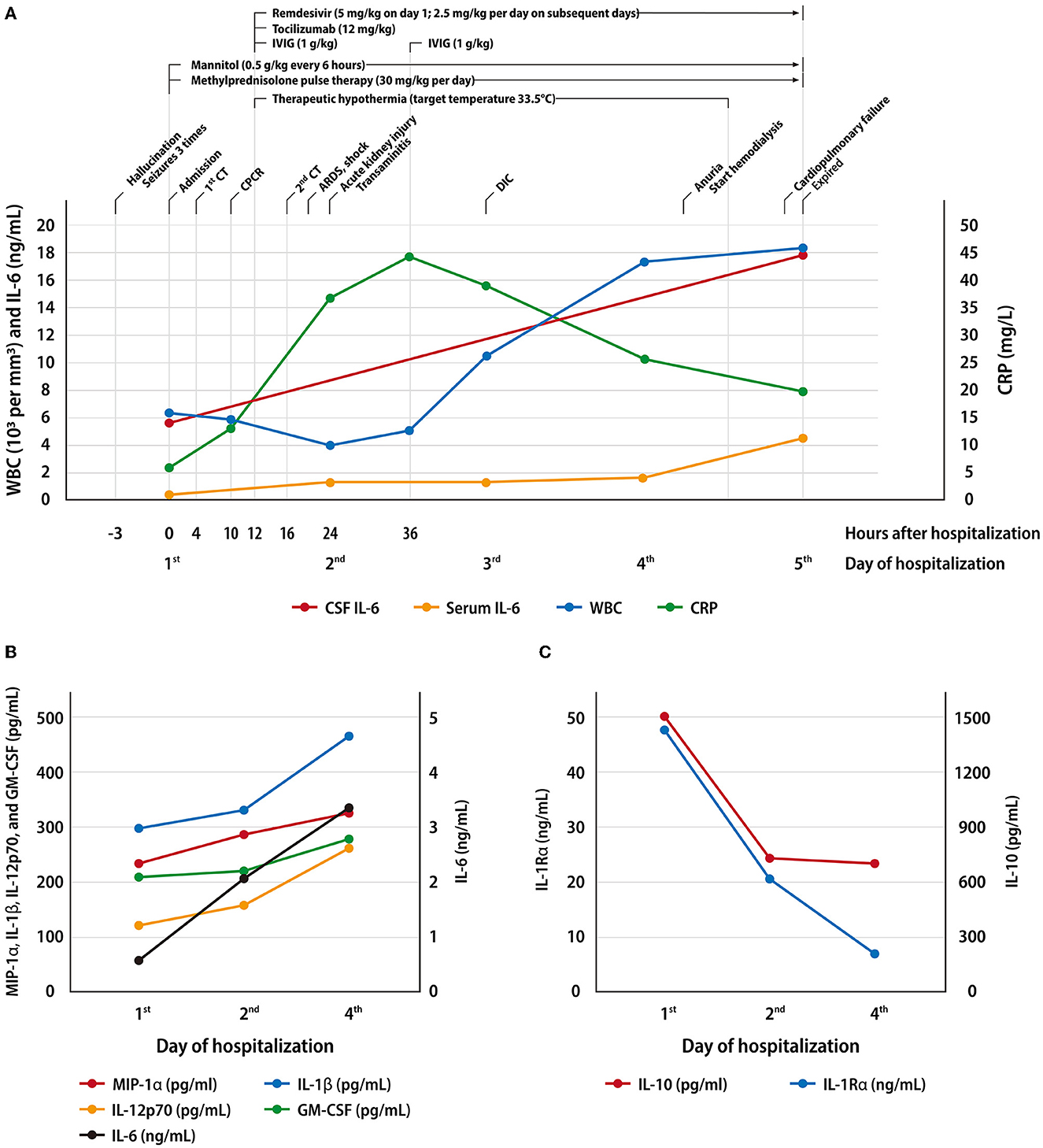
Figure 2. Serial biochemical data and cytokines profile in serum and cerebrospinal fluid. (A) The clinical course and the dynamic changes in inflammatory markers of this patient. (B, C). The serum cytokine profile that showed an escalating trend (B) and a falling trend (C).
Other pathogens survey included human herpesvirus 6, cytomegalovirus, herpes simplex virus, Epstein-Barr virus, hepatitis B and C, Mycoplasma pneumoniae, multiplex PCR using BioFire FilmArray respiratory panel from throat swab, and BioFire FilmArray meningitis/encephalitis panel (BioFire Diagnostics, Salt Lake City, Utah, USA) from CSF. The FilmArray respiratory panel detects pathogens, including adenovirus, human rhinovirus/enterovirus, influenza virus A (A, A/H1, A/H1-2009, A/H3), influenza virus B, respiratory syncytial virus, parainfluenza viruses 1-4, human metapneumovirus, Coronavirus 229E, HKU1, OC43 and NL63, SARS-CoV-2, Chlamydia pneumoniae, Bordetella pertussis, Bordetella parapertussis, and Mycoplasma pneumoniae, and the FilmArray meningitis/encephalitis panel detects pathogen including Escherichia coli K1, Haemophilus influenza, Listeria monocytogenes, Neisseria meningitides, Streptococcus agalactiae, Streptococcus pneumoniae, cytomegalovirus, enterovirus, herpes simplex virus 1 and 2, human herpesvirus 6, human parechovirus, varicella zoster virus, Cryptococcus neoformans/gattii. All these results were negative except SARS-CoV-2 detected from the FilmArray respiratory panel.
We reported a case of fulminant COVID-19-associated encephalitis that is distinct in its extraordinary rapid progression of brain edema within hours and the initial presentation with visual hallucination. We performed a thorough cytokines investigation among serum and CSF to provide prognostic prediction and treatment guidance.
Around one-fourth of children with COVID-19 had neurologic involvement (4). The reported neurologic symptoms of COVID-19 are disorientation/confusion, loss of consciousness, headache, and seizure predominantly. Only a small portion of patients have hallucinations (5, 6). One-tenth of those with neurologic involvement may develop life-threatening conditions, including acute encephalopathy (4). Among those with COVID-19-associated acute encephalopathy, acute fulminant cerebral edema is a recently recognized phenotype and comprises around 2% of them. Fever, seizures, coma, and Asian/Pacific Islander are significant risk factors in children with COVID-19-associated acute encephalopathy, and mortality is significantly higher with this fulminant phenotype (5, 6).
The first neurological manifestation of our patient was a visual hallucination (a monster) with a fear sensation. The initial corresponding brain CT showed trivial cortical swelling of the bilateral ventral temporal lobe. Previous studies examining patients with visual hallucinations by functional magnetic resonance imaging had demonstrated that ventral temporal lobes are associated with hallucinations of costumed figures (7). In addition, due to its proximity to the amygdala, the hallucinations are often related to unpleasant experiences and emotions (7). This evidence supported that the initial preference of the SARS-CoV-2 virus in the central nervous system might be the ventral temporal lobes and amygdala.
The pathogenic mechanisms of COVID-19-associated acute encephalopathy are still uncertain. Up to now, there are two hypotheses about the mechanism of brain damage, one being a direct invasion of the virus and the other being a severe inflammatory process caused by an extensive release of pro-inflammatory cytokines. The excessive pro-inflammatory response theory was supported by the evidence of significant increases in pro-inflammatory cytokines without detecting SARS-CoV-2 virus in CSF (1). This result is consistent with another severe virus-associated encephalopathy, acute necrotizing encephalopathy, in which a significant elevation of IL-6 and other cytokines is detected among CSF without direct identification of viral particles (8). These pro-inflammatory cytokines, in a hypothesis, cause endothelial dysfunction and cytokine storms that further damage the blood-brain barrier (BBB), resulting in the entry of these cytokines into brain parenchyma and the subsequent insults of neurons (1). Hypercytokinemia induces proteolytic destruction of BBB through trypsin and the activation of matrix metalloprotease-9, which causes an increase in vascular permeability resulting in brain edema (9). A considerably higher serum and CSF cytokine level was observed in our patient than in the control group, which might indicate that the higher cytokine level may link to a greater probability of developing cerebral edema. Most interestingly, we observed a more prominent rise of cytokines in the CSF than in serum in our patient, suggesting that the primary site of inflammation and release of cytokines might be the brain itself. Some possible mechanisms have been proposed that SARS-CoV-2 virus might be direct invasion through the olfactory bulb and the cribriform plate of ethmoid bone (10), or a route similar to enteroviral rhombencephalitis that the virus invades the motor neuron of the anterior horn cells of the spinal cord, and then ascends via a neuronal route to the brain (11). Although we did not detect the SARS-CoV-2 virus by PCR among the CSF in this patient, evidence had shown that PCR in the CSF has relatively low sensitivity (1). Studies demonstrate the presence of SARS-CoV-2 virus in the brain tissue on autopsy (12, 13), suggesting the possibility of brain invasion by viral itself.
Previous studies have found that IL-6, IL-1β, IL-8, tumor necrosis factor-alpha (TNF-α), monocyte chemotactic protein-1 (MCP-1), and granulocyte-macrophage colony-stimulating factor (GM-CSF) was highly elevated either in serum or CSF of patients with COVID-19 related encephalitis (1, 14, 15). In addition to the cytokines mentioned above, we also observed a rise in IL-10, IL-12p70, MIP-1α, IL-2Rα, and IL-1Rα among paired samples of CSF and serum (Table 1). IL-6 was deemed as a prognostic factor and potential treatment target of severe COVID-19 cases (16). Among these cytokines tested in serum, we observed an initial peak with a falling trend over time in IL-10 and IL-1Rα, and a rising trend in IL-6, MIP-1α, IL-1β, IL-12p70, and GM-CSF (Figures 2B, C). We propose that the former group might serve as an early diagnostic indicator of fulminant cerebral edema due to its rapid peaking and considerably higher level in cases with cerebral edema than in those without cerebral edema among patients with COVID-19-associated encephalitis. Early identification of hypercytokinemia could hint clinicians at a possibly fulminant course. The latter group of cytokines might be regarded as a prognostic factor which, in cases with an increasing trend, indicates a poor outcome. Although these cytokines are not tissue-specific, severe neurologic disease should be considered if the level of hypercytokinemia is higher in the CSF than in the serum, as seen in our case. This hypothesis needs to be validated further.
In our case, it is likely that the SARS-CoV-2 virus attacked ventral lobes initially, triggered a severe cytokine storm, and then led to subsequent cascades, including disruption of the blood-brain barrier, fulminant brain edema, and brain herniation. The strength of our study lies in the comprehensive dynamics of the pro-inflammatory (mainly IL-1β, IL-6, IL-8, TNF-α) and anti-inflammatory (IL-10) cytokines over time and their comparison with non-severe COVID-19 cases as supporting evidence. The interplay of these cytokines during disease progression might help to elicit underlying immune mechanism and serves as an early biomarker. Nevertheless, the limitation is that the magnetic resonance imaging scan is not available in the presented case due to his rapidly progressing course and critical status. Future study with larger cases population is needed to validate this result.
In conclusion, we report a case of a boy with severe fatal COVID-19-associated encephalitis complicated by acute fulminant cerebral edema. The distinct visual hallucination and its corresponding ventral temporal lobes swelling on brain imaging might be a clue to the primary site of COVID-19-associated encephalitis that subsequently spread to the whole brain. The trend of serum cytokine levels may serve as diagnostic and prognostic biomarkers. The relatively high IL-6 level in the CSF hints that the primary inflammation site is the brain itself, leading to fulminant brain edema.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The Institutional Review Board of National Cheng Kung University Hospital reviewed and approved the study protocol (IRB No. B-EC-112-004). Written informed consent to participate in this study was provided by the participants' legal guardian. Written informed consent was obtained from the participant's legal guardian for the publication of any potentially identifiable images or data included in this article.
Y-MC, C-FS, and Y-FT were responsible for conceptualization. J-NW, C-MC, C-HC, and C-FS were responsible for data curation. Y-MC was responsible for the original drafting. Y-FT and C-FS for reviewing and editing. All authors have read and agreed to the published version of the manuscript.
This work was supported by grants from the Clinical Medical Research Center, National Cheng Kung University Hospital, Taiwan (NCKUH-11202012) and Ministry of Science and Technology, Taiwan (110-2923-B-006-001-MY4).
We thank all the clinician for their devoted efforts in clinical are and providing valuable medical information. We thank Shih-Wei Wang, Hui-Chin Shih, and Hui-Feng Lee for clinical sample collection and processing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1209656/full#supplementary-material
1. Siahaan YMT, Puspitasari V, Pangestu A. COVID-19-associated encephalopathy: systematic review of case reports. J Clin Neurol. (2022) 18:194–206. doi: 10.3988/jcn.2022.18.2.194
2. Wang YC, Lin SW, Wang IJ, Yang CY, Hong C, Sun JR, et al. Interleukin-6 test strip combined with a spectrum-based optical reader for early recognition of COVID-19 patients with risk of respiratory failure. Front Bioeng Biotechnol. (2022) 10:796996. doi: 10.3389/fbioe.2022.796996
3. Lin SW, Shen CF, Liu CC, Cheng CM. A paper-based IL-6 test strip coupled with a spectrum-based optical reader for differentiating influenza severity in children. Front Bioeng Biotechnol. (2021) 9:752681. doi: 10.3389/fbioe.2021.802615
4. LaRovere KL, Riggs BJ, Poussaint TY, Young CC, Newhams MM, Maamari M, et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. (2021) 78:536–47. doi: 10.1001/jamaneurol.2021.0504
5. Krishnan P, Glenn OA, Samuel MC, Sheriff H, Foster-Barber A, Sejvar JJ, et al. Acute fulminant cerebral edema: a newly recognized phenotype in children with suspected encephalitis. J Pediatric Infect Dis Soc. (2021) 10:289–94. doi: 10.1093/jpids/piaa063
6. Lan SY, Lin JJ, Hsia SH, Wang HS, Chiu CH, Lin KL. Analysis of fulminant cerebral edema in acute pediatric encephalitis. Pediatr Neonatol. (2016) 57:402–7. doi: 10.1016/j.pedneo.2015.11.002
7. Santhouse AM, Howard RJ, Ffytche DH. Visual hallucinatory syndromes and the anatomy of the visual brain. Brain. (2000) 123:2055–64. doi: 10.1093/brain/123.10.2055
8. Lin YY, Lee KY, Ro LS, Lo YS, Huang CC, Chang KH. Clinical and cytokine profile of adult acute necrotizing encephalopathy. Biomed J. (2019) 42:178–86. doi: 10.1016/j.bj.2019.01.008
9. Wu X, Wu W, Pan W, Wu L, Liu K, Zhang HL. Acute necrotizing encephalopathy: an underrecognized clinicoradiologic disorder. Mediators Inflamm. (2015) 2015:792578. doi: 10.1155/2015/792578
10. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. (2020) 11:995–8. doi: 10.1021/acschemneuro.0c00122
11. Wang SM, Lei HY, Huang KJ, Wu JM, Wang JR, Yu CK, et al. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: roles of cytokines and cellular immune activation in patients with pulmonary edema. J Infect Dis. (2003) 188:564–70. doi: 10.1086/376998
12. Maiese A, Manetti AC, Bosetti C, Del Duca F, La Russa R, Frati P, et al. SARS-CoV-2 and the brain: a review of the current knowledge on neuropathology in COVID-19. Brain Pathol. (2021) 31:e13013. doi: 10.1111/bpa.13013
13. Duarte-Neto AN, Caldini EG, Gomes-Gouvêa MS, Kanamura CT, de Almeida Monteiro RA, Ferranti JF, et al. An autopsy study of the spectrum of severe COVID-19 in children: from SARS to different phenotypes of MIS-C. EClinicalMedicine. (2021) 35:100850. doi: 10.1016/j.eclinm.2021.100850
14. Li Y, Li H, Fan R, Wen B, Zhang J, Cao X, et al. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. (2016) 59:163–9. doi: 10.1159/000453066
15. Bodro M, Compta Y, Llansó L, Esteller D, Doncel-Moriano A, Mesa A, et al. Increased CSF levels of IL-1β, IL-6, and ACE in SARS-CoV-2-associated encephalitis. Neurol Neuroimmunol Neuroinflamm. (2020) 7:821. doi: 10.1212/NXI.0000000000000821
Keywords: COVID-19, SARS-CoV-2, acute fulminant cerebral edema, encephalitis, cytokine, cerebral edema
Citation: Chang Y-M, Chen C-H, Wang J-N, Cheng C-M, Tu Y-F and Shen C-F (2023) Case report: Distinct neurologic manifestation and cytokine profile of a child with COVID-19-associated acute fulminant encephalitis. Front. Med. 10:1209656. doi: 10.3389/fmed.2023.1209656
Received: 21 April 2023; Accepted: 23 May 2023;
Published: 13 June 2023.
Edited by:
Ramcés Falfán-Valencia, National Institute of Respiratory Diseases-Mexico (INER), MexicoReviewed by:
Karen Martinez-Espinosa, National Institute of Respiratory Diseases-Mexico (INER), MexicoCopyright © 2023 Chang, Chen, Wang, Cheng, Tu and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ching-Fen Shen, czk4MTA0MDIyQGdzLm5ja3UuZWR1LnR3; Yi-Fang Tu, bmNrdXR1QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.