
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 18 July 2023
Sec. Intensive Care Medicine and Anesthesiology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1208570
This article is part of the Research TopicInsights in Intensive Care Medicine and Anesthesiology: 2023View all 22 articles
Introduction: This study aimed to explore the relationship between neuraxial labor analgesia and intrapartum fever and to demonstrate the influence of maternal fever on perinatal outcomes within 6 weeks after birth.
Methods: This was a secondary analysis of a multicenter prospective cohort study that enrolled women with single- and full-term cephalic pregnancy in northern China. Intrapartum maternal fever was defined as the highest axillary temperature during labor ≥37.5°C. Data on baseline characteristics, maternal variables, and neonatal outcomes were all collected. The association between neuraxial labor analgesia and intrapartum maternal fever was analyzed with logistic regression models, and the cutoff point was identified by the receiver operating characteristic curve.
Results: Of 577 parturients, 74 (12.8%) developed intrapartum fever. Neuraxial analgesia was associated with an increased risk of maternal intrapartum fever with or without adjusting for confounding factors (adjusted OR = 2.68; 95% CI: 1.32–5.47; p = 0.007). Further analysis showed that neuraxial analgesia of <5 h did not increase the risk of intrapartum fever compared with no analgesia (OR = 1.52; 95% CI: 0.63–3.64; p = 0.35), and longer neuraxial labor analgesia time (over 5 h) significantly increased the risk of fever (OR = 3.38; 95% CI: 1.63–7.01; p = 0.001). Parturients with intrapartum fever suffered more maternal adverse outcomes compared with those without fever (p < 0.001). Neonates of women with intrapartum fever had slightly higher rates of composite adverse neonatal outcomes compared with those without fever; however, the difference was not statistically significant (p = 0.098).
Conclusion: In women with low-risk pregnancies, a longer time of neuraxial labor analgesia was associated with an increased risk of intrapartum maternal fever. Intrapartum fever was related to adverse maternal outcomes but did not significantly affect neonatal outcomes within 6 weeks after delivery.
Intrapartum maternal fever affects up to one-third of all labors, which might be associated with increased risks of maternal complications, such as intrauterine infection, dystocia, and emergency cesarean delivery (1–3). Moreover, it is also reported to be a potential risk factor for adverse neonatal outcomes, including low Apgar scores, respiratory distress, hypotonia, neonatal brain injury, and even cerebral palsy (4). Multiple obstetric factors, such as intrapartum infection, premature rupture of membranes, Group B streptococcus positive, and prolonged duration of labor, might be involved in the process of maternal fever during labor (5). Moreover, it has been hypothesized that neuraxial analgesia (i.e., epidural-related maternal fever, ERMF) might play a role in intrapartum maternal fever (6, 7).
Neuraxial labor analgesia, including epidural analgesia and combined spinal–epidural analgesia, has been widely used as an effective method to relieve labor pain for decades (8). Many studies have investigated the association between neuraxial labor analgesia and intrapartum maternal fever in the past few years. A randomized clinical trial conducted in Brazil reported that the use of combined spinal and epidural anesthesia was associated with a significant increase in maternal temperature during vaginal delivery (9). The result of a recently published prospective observational study also showed significant associations between epidural labor analgesia and intrapartum maternal fever in all stages of labor (2). However, negative results were also reported (10). Considering the potential adverse effect of intrapartum maternal fever on perinatal outcomes (3), more investigations are still needed to provide further evidence on this issue. The objective of this study was to explore the association between neuraxial labor analgesia and intrapartum maternal fever and to further demonstrate the influence of maternal fever on birth outcomes in low-risk populations of full-term pregnant women.
The study protocol was approved by the Clinical Research Ethics Committees in Peking University First Hospital (2014 [714]) and the institutional review boards of other participating centers and was registered with the Chinese Clinical Trial Registry (www.chictr.org.cn; ChiCTR-OCH-14004888) and ClinicalTrials.gov (NCT02823418). Written informed consent was obtained from all participants before enrollment.
This study was a secondary analysis of multicenter prospective cohort research in northern China, which was conducted from 1 August 2014 to 29 May 2015 in Peking University First Hospital (a tertiary general hospital), Beijing Obstetrics and Gynecology Hospital (a tertiary specialized hospital) and Haidian Maternal and Child Health Hospital (a secondary specialized hospital) in Beijing, China. Detailed inclusion and exclusion criteria of the participants have been described previously (11). In brief, pregnant women were eligible for inclusion if they were nulliparae with full-term single-term cephalic pregnancy (>7 weeks) and prepared for vaginal delivery, who were clinically considered as women with low-risk pregnancies. Patients were excluded if they were younger than 18 years or older than 35 years, had a history of psychiatric disease, had contraindications to neuraxial analgesia, or were admitted to the delivery room outside the daytime working hours (from 5 p.m. to next 8 a.m.).
In this study, the decision of whether to receive neuraxial labor analgesia or not was made by the parturients themselves. As a clinical routine, 40 mg methylprednisolone was administered to parturients via intravenous infusion or bolus injection before lumber puncture in the participating medical centers to prevent nausea and vomiting. Epidural analgesia or combined spinal–epidural analgesia was performed on women who requested neuraxial analgesia when the cervix was dilated to 1 cm or more. For epidural analgesia, a loading dose of 10 ml mixture (0.1% ropivacaine and 0.5 μg/ml sufentanil) was administered through the epidural catheter. An additional dose of 5 ml mixture was administered 10 min later if the numeric rating scale (NRS, an 11-point scale where 0 = no pain and 10 = the worst pain) pain score remained at least 4. A patient-controlled epidural analgesia (PCEA) pump was connected 30 min later, which was established with a mixture of 0.1% ropivacaine (AstraZeneca AB, Södertälje, Sweden) plus 0.5 μg/ml sufentanil (EuroCept BV, Ankeveen, Netherlands) and programmed to deliver a 6 ml bolus with a 15-min lockout interval. For combined spinal–epidural analgesia, 2 to 3 ml of 0.1% ropivacaine was administered intrathecally. A PCEA pump was connected later, which was established with a mixture of 0.1% ropivacaine and 0.5 μg/ml sufentanil, programmed to deliver a 5 ml bolus with a 15-min lockout interval and a 5 m/h background infusion. Obstetric management during labor, such as oxytocin administration, intramuscular meperidine administration, forceps assistance, and cesarean delivery, were decided by the obstetricians as clinical routine.
For all recruited subjects, data on baseline characteristics, maternal variables, and neonatal variables were collected. The detail shows as follows:
(1) Baseline characteristics included sociodemographic information, previous medical history, and obstetrical data of the present pregnancy.
(2) Maternal variables included the use of neuraxial labor analgesia, medications during labor (including oxytocin, meperidine, and antibiotic administration), durations of all stages of labor, body temperature during labor (including baseline temperature, highest temperature, and postpartum temperature), mode of delivery, and estimated blood loss.
(3) Neonatal variables included gender, birthweight, Apgar Scores at 1 and 5 min after delivery, the occurrence of intrauterine fetal distress, assisted ventilation, and admission to the neonatal ward within 1 day postpartum. We also recorded the mode of infant feeding and other health-related problems through a face-to-face follow-up on the first day after delivery and a telephone interview at 6 weeks postpartum.
The occurrence of intrapartum maternal fever was defined as the highest axillary temperature during labor more than or equal to 37.5°C. Axillary temperature was measured by midwives with mercury thermometers every 2 h or when considered necessary since delivery room admission. Baseline temperature and postpartum temperature were defined as the temperature on admission to the delivery room and 2 h after delivery, respectively.
Data were summarized as mean ± standard deviation, number (proportions), or median (interquartile range). The independent t-test, Mann–Whitney U-test, and chi-squared (χ2) test were used to test statistical significance between groups of continuous (normal and non-normal distribution data) and categorical variables, respectively. A logistic regression model was applied to determine the association between neuraxial labor analgesia and intrapartum maternal fever. Variables that showed significant differences between the two groups (p < 0.05) as well as variables that might potentially affect intrapartum temperature based on clinical grounds were adjusted in the above model. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were estimated. The receiver operating characteristic (ROC) curve was used to identify the cutoff point of the association between the duration time of neuraxial labor analgesia and intrapartum fever. Exploratory analyses were performed to assess differences in the primary outcome in subgroups. Treatment-by-covariate interactions were assessed separately for each subgroup factor using logistic regression. Two-tailed p-values of < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 22.0 (IBM SPSS Inc., Chicago, IL, USA).
In total, 1,420 parturients were screened; of these, 793 were eligible, 599 were enrolled and 577 completed the study (Figure 1). There were no significant differences in baseline information between enrolled and not enrolled patients (Supplementary Table S1). All 577 parturients were included in this study with an age range between 22 and 34 years. Of these, 74 (12.8%) developed intrapartum fever (cases), and 503 (87.2%) did not develop a fever (controls). The baseline variables of all parturients are shown in Table 1. There were no significant differences between the two groups with regard to sociodemographic characteristics, medical comorbidity, and obstetrical data. The incidence of fever was higher among people without gynecological diseases (p = 0.035).
Parturients with intrapartum fever had higher rates of neuraxial labor analgesia (p = 0.003) and intrapartum antibiotic administration (p < 0.001), had longer durations of the first and second labor stages (p < 0.001 and p = 0.011, respectively), developed higher postpartum temperature (p = 0.001), and underwent more lateral episiotomy (p = 0.011). The rates of instrumental and cesarean deliveries were significantly higher in women who suffered from intrapartum fever (p < 0.001). Moreover, patients with fever had more blood loss during labor (p = 0.029) and longer lengths of postpartum hospital stay (p = 0.004) compared with those without fever. Overall, the prevalence of composite adverse maternal outcomes was almost 2-fold in the fever group (59.5% vs. 35.0%, p < 0.001). In addition, the length of postpartum hospital stay was significantly longer in parturients with intrapartum fever than those without (p = 0.004). All the detailed information on perinatal maternal variables is shown in Table 2.
Based on logistic regression models, a univariate analysis was used to demonstrate the association between neuraxial labor analgesia and intrapartum maternal fever; six potential confounders (i.e., gynecological diseases before pregnancy, obstetric complications, premature rupture of membranes, baseline temperature, intrapartum meperidine administration, and birthweight of newborns) were adjusted in the adjusted logistic regression model. The result showed that intrapartum maternal fever was associated with neuraxial labor analgesia with or without adjustment for potential confounders (adjusted OR = 2.68; 95% CI: 1.32–5.47; p = 0.007) (Table 3).
ROC curve was applied to investigate the cutoff points of the association between the duration time of neuraxial labor analgesia and intrapartum fever. The result suggested that 5 h was the cutoff point (Supplementary Figure S1). As the duration of neuraxial labor analgesia increased, the risk of intrapartum maternal fever increased. Compared with those parturients without neuraxial labor analgesia, the risk of intrapartum maternal fever in parturients with shorter neuraxial labor analgesia time (< 5 h) and parturients with longer neuraxial labor analgesia time (over 5 h) increased by 52% (OR = 1.52; 95% CI: 0.63–3.64; p = 0.35) and 238.0% (OR = 3.38; 95% CI: 1.63–7.01; p = 0.001), respectively. All of the above results are shown in Table 3.
Subgroup analyses of factors related to intrapartum maternal fever were performed to evaluate the consistency of the effect of neuraxial labor analgesia on intrapartum maternal fever (Figure 2). The results of the subgroup analysis did not show the heterogeneity of risk of incident intrapartum maternal fever from neuraxial labor analgesia besides the subgroup of parturients whether spontaneous delivery or not.
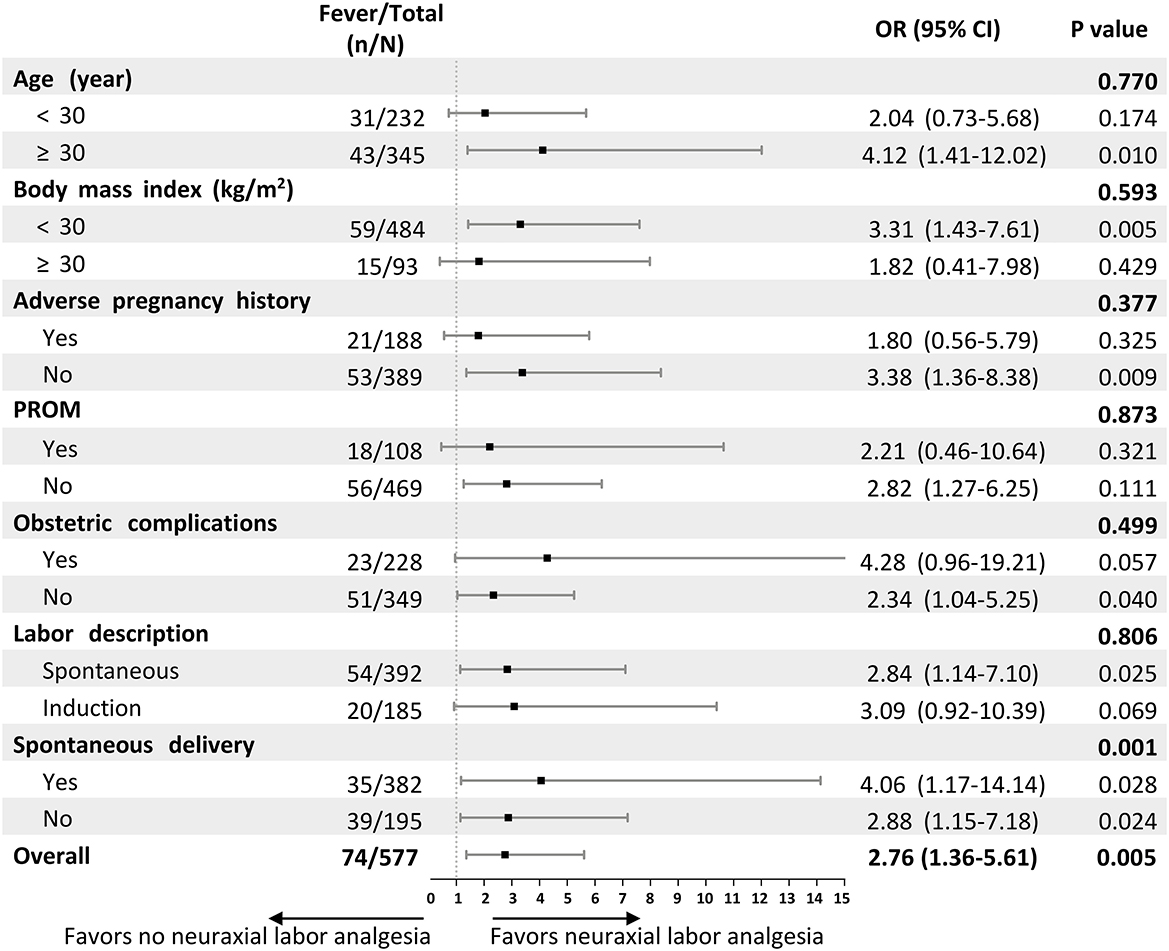
Figure 2. Forest plot assessing interactions between subgroups and the associations between neuraxial labor analgesia and intrapartum maternal fever. ORs and interim-adjusted 95% CIs are shown. The estimated overall OR was derived from a logistic regression model adjusted for the confounder variable in this study including gynecological diseases before pregnancy, obstetric complications, PROM, baseline temperature, and intrapartum meperidine administration. For the subgroup analyses, we assessed the treatment-by-covariate interaction on the association between neuraxial labor analgesia and intrapartum maternal fever, adjusting for the same variables. OR, Odds ratio; CI, confidence interval; PROM, premature rupture of membrane.
For neonatal information, the birthweight of a newborn was significantly heavier in parturients with intrapartum fever (p = 0.016) (Table 4). There were no significant differences in rates of fetal distress, low 1 and 5 min Apgar scores, ventilation support, neonatal ward admission, neonatal infection, and readmission to hospital within 6 weeks after birth between the two groups. In total, the proportion of composite adverse neonatal outcomes was slightly higher in infants with febrile mothers, although the difference was not statistically significant (15 [20.3%] vs. 66 [13.1%], p = 0.098) (Table 4).
Our study demonstrated that, among young nulliparous women with single and full-term cephalic pregnancy, the use of neuraxial analgesia during labor, especially duration of neuraxial analgesia of more than 5 h, was associated with an increased risk of intrapartum fever. Moreover, intrapartum fever was related to maternal adverse outcomes but did not significantly affect the short-term outcomes of neonates.
Maternal intrapartum fever, also called intrapartum hyperthermia, is commonly defined as a temperature more than 37.5°C or 38°C during labor in previous studies (3). The incidence of intrapartum hyperthermia has varied strikingly from 1 to 37% due to different populations and diagnostic criteria (12, 13). Although 38°C was used mostly in previous studies as the definition of maternal fever, we still used 37.5°C as the threshold of clinical fever because the association between low-grade fever (≥37.5°C) and adverse neonatal outcomes has been previously reported (13, 14). It is worth mentioning that the incidence of maternal fever in our study was lower than in other works of literature among Chinese women (15). It is possible that the relatively low concentration of neuroblockade we used for neuraxial analgesia and also different obstetric practices, such as methylprednisolone, meperidine, and antibiotic administrations, might be involved in the lower incidence of intrapartum fever. Goetzl et al. reported that the administration of high-dose corticosteroids during labor resulted in a 90% reduction of maternal fever in parturients with epidural analgesia, suggesting that steroids might decrease the risk of epidural intrapartum fever (16). Thus, we speculated that intrapartum steroid treatment of parturients in this study might be associated with the reduction of intrapartum fever to some extent, and the underlying relationship needs to be further explored.
Our results showed that in women with low-risk pregnancies, neuraxial analgesia was significantly associated with an increased incidence of mild maternal fever, which was in line with previous studies (2, 15). In fact, the etiology of epidural-related maternal fever remains elusive and the sterile inflammation process was considered to be a possible mechanism (17). A randomized controlled trial with prophylactic antibiotics before epidural analgesia showed no difference in the rate of maternal fever between groups, supporting the non-infectious inflammation hypothesis of epidural fever (18). A study reported that acetaminophen prophylaxis did not prevent maternal hyperthermia or fever secondary to epidural analgesia, also suggesting a non-infectious inflammatory process (19). In addition, local anesthetics used in epidural were also reported to trigger non-infectious inflammation pathways via immunomodulation and cell injury (20, 21). Overall, non-infectious inflammation might be involved in the ERMF and the exact mechanisms need to be further clarified.
Our study also found that the risk of intrapartum fever increased significantly in parturients with neuraxial labor analgesia time of more than 5 h. A recently published observational study also reported weak time and dose-dependent correlations between PCEA and intrapartum fever, and an analgesic time over 6.3 h increased the risk of maternal intrapartum fever (22). However, inconsistent results also existed. Wang et al. found that although the duration of analgesia in the early PCEA group was significantly longer than that in the late PCEA group, the average maternal temperature and the incidence of fever were not significantly different, suggesting that the duration of epidural analgesia might not be an important determinant of epidural-related fever (23, 24). Further research is still needed to further clarify the time and dose-dependent correlations.
Numerous studies have demonstrated that intrapartum hyperthermia was associated with a series of adverse obstetric outcomes in mothers. Dior et al. found that maternal fever was significantly associated with adverse maternal outcomes, including postpartum hemorrhage, labor dystocia, and cesarean section (25). The study of Lange et al. also reported that parturients who developed fever were more likely to have prolonged durations of labor and required cesarean delivery (26). Results of this study showed that febrile parturients underwent more cesarean section or instrumental delivery, suffered more postpartum blood loss, and had a longer length of postpartum hospital stay, further demonstrating that intrapartum fever was associated with adverse maternal outcomes. It is possible that the elevation of body temperature is an early indicator of potential obstetric abnormalities, and early attention and interventions should be taken to decrease the potential adverse events followed by intrapartum fever.
In this study, we evaluated neonatal outcomes of term infants and found no significant association between intrapartum maternal fever and adverse neonatal outcomes within 6 weeks after birth. However, the effects of intrapartum fever on newborns remain controversial and conflicting evidence existed in this field (13, 27, 28). A retrospective cohort study reported that adverse neonatal outcomes including assisted ventilation, Apgar scores <7, and NICU admission increased infants' exposure to maternal hyperthermia (1). A recently published meta-analysis including 41 studies suggested that maternal intrapartum fever of any cause was associated with neonatal brain injury (3). It is possible that the definition of maternal fever in this study was over 37.5°C, less than that of some other studies, resulting in fewer influences on intrauterine fetuses. In addition, the inclusion of low-risk parturients, who were young and with single-term pregnancies in our study, might lead to relatively low-risk infants. Moreover, obstetric and pediatric management in participating medical centers might influence the birth outcomes to some extent. Further studies with larger sample sizes and longer time of follow-ups are still needed to explore the long-term effect of maternal fever on neonatal outcomes.
There are strengths and also some limitations in this analysis. Intrapartum maternal fever is a prevalent occurrence, affecting approximately one-third of all labors, which is associated with an increased risk of maternal complications and has the potential to impact neonatal outcomes. However, the impact of epidural-related maternal fever on maternal and neonatal outcomes within 6 weeks following childbirth remains inconclusive. Our data contribute additional evidence on this issue, particularly focusing on primiparous women with low-risk and term pregnancies in the Asia population. Furthermore, many previous studies investigating perinatal outcomes of epidural analgesia were conducted without an opioid-free control group, which may have introduced interference when comparing results to those involving opioids. In this study, the use of opioids was relatively conservative, aligning with local clinical routine, and the proportion of administration of opioid drugs in the control group was very low. Therefore, our study offers insights into the effects of neuraxial analgesia, to some extent, without the confounding influence of opioids. Meanwhile, the study also has some limitations. First, the maternal temperature during labor was not continuously monitored, which might lead to a potential misdiagnose of intrapartum fever. Second, as an observational study, it is inevitable that unidentified confounding factors might influence the results. Although a multivariate regression model was adopted to adjust for potential confounders of maternal fever, we cannot neglect the possible interference of perinatal factors. Third, as routine screening for inflammatory parameters and placental pathology was not performed during hospitalization, data collection before and/or after the neuraxial procedures was not feasible. Fourth, the neonatal outcomes may not be comprehensive enough. Future studies should consider adding more neonatal parameters, such as fetal acidosis, antibiotic treatment, and admission to the neonatal intensive care unit in the neonatal outcome assessment. Finally, as a secondary analysis of a multicenter research database, the sample size might be not enough to establish a causal relationship.
Despite limitations, we found that in nulliparae with single- and full-term cephalic pregnancy, a longer time of neuraxial labor analgesia was associated with an increased risk of intrapartum maternal fever. Intrapartum fever was related to adverse maternal outcomes but did not significantly affect the neonatal outcomes of low-risk mothers within 6 weeks after delivery.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Clinical Research Ethics Committees in Peking University First Hospital. The patients/participants provided their written informed consent to participate in this study.
ZZ and C-MD contributed to the study design, data collection, and manuscript writing. J-HM contributed to the study design, data analysis, and manuscript writing. SL and BL helped to recruit patients and collected the clinical data. TD contributed to the study design, manuscript writing, and revision. All authors read and approved the final manuscript.
This study was supported by the Capital Characteristic Clinic Project (Z151100004015160, TD) and National High Level Hospital Clinical Research Funding (Multi-center Clinical Research Project of Peking University First Hospital) (2022CR56, TD). The study sponsors had no role in the study design, collection, analysis, interpretation of data, and writing of the report.
The authors thank Drs. Si-Chao Xu and Shu-Ting He (MD, Department of Anesthesiology and Critical Care Medicine, Peking University First Hospital, Beijing, China), Ming-Jun Xu (MD, Department of Anesthesiology, Beijing Obstetrics, and Gynecology Hospital, Capital Medical University, Beijing, China), and Lei Wang (MD, Department of Anesthesiology, Haidian Maternal & Child Health Hospital, Beijing, China) for their help in collecting data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1208570/full#supplementary-material
Supplementary Figure S1. Receiver operating characteristic curve of the duration time of neuraxial labor analgesia and intrapartum fever (sensitivity = 0.813, specificity = 0.382, the area under the ROC = 0.577, and 95%CI: 0.504–0.650).
1. Ashwal E, Salman L, Tzur Y, Aviram A, Ben-Mayor Bashi T, Yogev Y, et al. Intrapartum fever and the risk for perinatal complications—The effect of fever duration and positive cultures. J Maternal-Fetal Neonatal Med J Eur Assoc Peri Med Fed Asia Oceania Perinatal Soc Int Soc Perinatal Obstet. (2018) 31:1418–25. doi: 10.1080/14767058.2017.1317740
2. Zhao B, Li B, Wang Q, Song X. The relationship between epidural analgesia and intrapartum maternal fever and the consequences for maternal and neonatal outcomes: a prospective observational study. J Maternal-Fetal Neonatal Med J Eur Assoc Peri Med Fed Asia Oceania Perinatal Soc Int Soc Perinatal Obstet. (2022) 35:5354–62. doi: 10.1080/14767058.2021.1879042
3. Morton S, Kua J, Mullington CJ. Epidural analgesia, intrapartum hyperthermia, and neonatal brain injury: a systematic review and meta-analysis. Br J Anaesth. (2021) 126:500–15. doi: 10.1016/j.bja.2020.09.046
4. Impey LW, Greenwood CE, Black RS, Yeh PS, Sheil O, Doyle P. The relationship between intrapartum maternal fever and neonatal acidosis as risk factors for neonatal encephalopathy. Am J Obst Gynecol. (2008) 198:49.e1–6.doi: 10.1016/j.ajog.2007.06.011
5. Burgess APH, Katz JE, Moretti M, Lakhi N. Risk factors for intrapartum fever in term gestations and associated maternal and neonatal sequelae. Gynecol Obstet Invest. (2017) 82:508–16. doi: 10.1159/000453611
6. Wang H, Yang Z, Wei S, Xia L, Li Y, Wu X, et al. Perinatal outcomes and risk factors for epidural analgesia-associated intrapartum maternal fever: a retrospective study. J Maternal-Fetal Neonatal Med J Eur Assoc Peri Med Fed Asia Oceania Perinatal Soc Int Soc Perinatal Obstet. (2023) 36:2179383. doi: 10.1080/14767058.2023.2179383
7. Sultan P, Segal S. Epidural-related maternal fever: still a hot topic, but what are the burning issues? Anesth Analg. (2020) 130:318–20. doi: 10.1213/ane.0000000000004576
9. de Orange FA, Passini R. Jr., Amorim MM, Almeida T, Barros A. Combined spinal and epidural anaesthesia and maternal intrapartum temperature during vaginal delivery: a randomized clinical trial. Br J Anaesth. (2011) 107:762–8. doi: 10.1093/bja/aer218
10. Frölich MA, Esame A, Zhang K, Wu J, Owen J. What factors affect intrapartum maternal temperature? A prospective cohort study: maternal intrapartum temperature. Anesthesiology. (2012) 117:302–8. doi: 10.1097/ALN.0b013e31825a30ef
11. Deng CM, Ding T, Li S, Lei B, Xu MJ, Wang L, et al. Neuraxial labor analgesia is associated with a reduced risk of postpartum depression: a multicenter prospective cohort study with propensity score matching. J Affect Disord. (2021) 281:342–50. doi: 10.1016/j.jad.2020.12.027
12. Segal S. Labor epidural analgesia and maternal fever. Anesth Analg. (2010) 111:1467–75. doi: 10.1213/ANE.0b013e3181f713d4
13. Greenwell EA, Wyshak G, Ringer SA, Johnson LC, Rivkin MJ, Lieberman E. Intrapartum temperature elevation, epidural use, and adverse outcome in term infants. Pediatrics. (2012) 129:e447–54. doi: 10.1542/peds.2010-2301
14. Impey L, Greenwood C, MacQuillan K, Reynolds M, Sheil O. Fever in labour and neonatal encephalopathy: a prospective cohort study. BJOG Int J Obstet Gynaecol. (2001) 108:594–7. doi: 10.1111/j.1471-0528.2001.00145.x
15. Yin H, Hu R, A. cohort study of the impact of epidural analgesia on maternal and neonatal outcomes. J Obstet Gynaecol Res. (2019) 45:1435–41. doi: 10.1111/jog.13988
16. Goetzl L, Zighelboim I, Badell M, Rivers J, Mastrangèlo MA, Tweardy D, et al. Maternal corticosteroids to prevent intrauterine exposure to hyperthermia and inflammation: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol. (2006) 195:1031–7. doi: 10.1016/j.ajog.2006.06.012
17. Patel S, Ciechanowicz S, Blumenfeld YJ, Sultan P. Epidural-related maternal fever: incidence, pathophysiology, outcomes, and management. Am J Obst Gynecol. (2023) 228:S1283–304. doi: 10.1016/j.ajog.2022.06.026
18. Sharma SK, Rogers BB, Alexander JM, McIntire DD, Leveno KJ, A. randomized trial of the effects of antibiotic prophylaxis on epidural-related fever in labor. Anesth Analg. (2014) 118:604–10. doi: 10.1213/ANE.0b013e3182a5d539
19. Goetzl L, Rivers J, Evans T, Citron DR, Richardson BE, Lieberman E, et al. Prophylactic acetaminophen does not prevent epidural fever in nulliparous women: a double-blind placebo-controlled trial. J Perinatol J Calif Peri Assoc. (2004) 24:471–5. doi: 10.1038/sj.jp.7211128
20. Del Arroyo AG, Sanchez J, Patel S, Phillips S, Reyes A, Cubillos C, et al. Role of leucocyte caspase-1 activity in epidural-related maternal fever: a single-centre, observational, mechanistic cohort study. Br J Anaesth. (2019) 122:92–102. doi: 10.1016/j.bja.2018.09.024
21. Wohlrab P, Boehme S, Kaun C, Wojta J, Spittler A, Saleh L, et al. Ropivacaine activates multiple proapoptotic and inflammatory signaling pathways that might subsume to trigger epidural-related maternal fever. Anesth Analg. (2020) 130:321–31. doi: 10.1213/ane.0000000000004402
22. Zhao BS Li B, Wang QN, Jia JX, Song XR. Time- and dose-dependent correlations between patient-controlled epidural analgesia and intrapartum maternal fever. BMC Anesthesiol. (2021) 21:31. doi: 10.1186/s12871-021-01249-1
23. Wang F, Shen X, Guo X, Peng Y, Gu X. Epidural analgesia in the latent phase of labor and the risk of cesarean delivery: a 5-year randomized controlled trial. Anesthesiology. (2009) 111:871–80. doi: 10.1097/ALN.0b013e3181b55e65
24. Wang LZ, Chang XY, Hu XX, Tang BL, Xia F. The effect on maternal temperature of delaying initiation of the epidural component of combined spinal-epidural analgesia for labor: a pilot study. Int J Obstet Anesth. (2011) 20:312–7. doi: 10.1016/j.ijoa.2011.06.002
25. Dior UP, Kogan L, Eventov-Friedman S, Gil M, Bahar R, Ergaz Z, et al. Very high intrapartum fever in term pregnancies and adverse obstetric and neonatal outcomes. Neonatology. (2016) 109:62–8. doi: 10.1159/000440938
26. Lange EMS, Segal S, Pancaro C, Wong CA, Grobman WA, Russell GB, et al. Association between intrapartum magnesium administration and the incidence of maternal fever: a retrospective cross-sectional study. Anesthesiology. (2017) 127:942–52. doi: 10.1097/aln.0000000000001872
27. Jansen S, Lopriore E, Naaktgeboren C, Sueters M, Limpens J, van Leeuwen E, et al. Epidural-related fever and maternal and neonatal morbidity: a systematic review and meta-analysis. Neonatology. (2020) 117:259–70. doi: 10.1159/000504805
Keywords: neuraxial labor analgesia, intrapartum fever, perinatal outcomes, full-term pregnancy, perinatal period
Citation: Zhang Z, Deng C-M, Ma J-H, Li S, Lei B and Ding T (2023) Effects of neuraxial labor analgesia on intrapartum maternal fever in full-term pregnancy and its influence on birth outcomes. Front. Med. 10:1208570. doi: 10.3389/fmed.2023.1208570
Received: 19 April 2023; Accepted: 27 June 2023;
Published: 18 July 2023.
Edited by:
Ata Murat Kaynar, University of Pittsburgh, United StatesReviewed by:
Reza Aminnejad, Qom University of Medical Sciences, IranCopyright © 2023 Zhang, Deng, Ma, Li, Lei and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Ding, ZGluZ3Rpbmcxc3RAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.