
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 21 June 2023
Sec. Gastroenterology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1208489
This article is part of the Research Topic Clinical Risk Assessment and Intervention of Gastrointestinal Tumors Driven by Big-Data View all 6 articles
 Ji Hyun Song1†
Ji Hyun Song1† Ji Yeon Seo1†
Ji Yeon Seo1† Eun Hyo Jin1
Eun Hyo Jin1 Goh Eun Chung1
Goh Eun Chung1 Young Sun Kim1
Young Sun Kim1 Jung Ho Bae1
Jung Ho Bae1 Sunmie Kim2
Sunmie Kim2 Kyung-Do Han2*‡
Kyung-Do Han2*‡ Sun Young Yang1*‡
Sun Young Yang1*‡Background and aims: The incidence of early-onset colorectal cancer (EO-CRC, diagnosed before 50 years of age) has increased in recent decades. The aim of this study was to investigate the association between changes in obesity status and EO-CRC risk.
Methods: From a nationwide population-based cohort, individuals <50 years old who participated in the national health checkup program in both 2009 and 2011 were included. Obesity was defined as a body mass index ≥25 kg/m2. Abdominal obesity was defined as a waist circumference ≥ 90 cm in men and ≥ 85 cm in women. Participants were classified into 4 groups according to the change in obesity (normal/normal, normal/obese, obese/normal, persistent obese) and abdominal obesity (normal/normal, normal/abdominal obesity, abdominal obesity/normal, persistent abdominal obesity) status. Participants were followed up until 2019 and censored when they became 50 years old.
Results: Among 3,340,635 participants, 7,492 patients were diagnosed with EO-CRC during 7.1 years of follow-up. The risk of EO-CRC was higher in the persistent obesity and persistent abdominal obesity groups than in the normal/normal groups (hazard ratio (HR) [95% confidence interval (CI)] = 1.09 [1.03–1.16] and 1.18 [1.09–1.29], respectively). Participants with both persistent obesity and abdominal obesity had a higher EO-CRC risk than those in the normal/normal groups for both [HR (95% CI) = 1.19 (1.09–1.30)].
Conclusion: Persistent obesity and persistent abdominal obesity before the age of 50 are associated with a slightly increased risk of EO-CRC. Addressing obesity and abdominal obesity in young individuals might be beneficial in reducing the risk of EO-CRC.
The incidence of early-onset colorectal cancer (EO-CRC) in individuals diagnosed with colorectal cancer (CRC) before 50 years of age has increased over the past three decades (1). EO-CRC now accounts for 10–12% of all newly diagnosed CRCs (2). Recently, the US Preventive Service Task Force expanded colorectal cancer screening recommendations to recommend screening for adults aged 45 years with and average risk profile (3). In a cohort study investigating the clinical characteristics of EO-CRC, it was found that the majority of patients exhibited symptoms such as rectal bleeding or abdominal pain (4). Additionally, left-sided cancer and advanced stages of the disease were commonly observed, and more than half of the patients were overweight or obese (4).
Obesity is known to be a risk factor for several major cancers, including CRC (5, 6). Recently, it was reported that not only overall obesity but also abdominal obesity are associated with an increased risk of colon cancer (CC) (7), cancer-related mortality (8), and all-cause mortality (8, 9).
Weight gain can increase the colorectal adenoma recurrence rate (10). A previous study reported that an increased waist circumference (WC) from early to later adulthood was associated with an increased risk of advanced colorectal neoplasia (11). Several studies have suggested that weight gain and an increased WC are associated with a higher risk of CRC (12, 13). The European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study showed that losing weight and entering a lower body mass index (BMI) category in middle adulthood, between 40 and 70 years of age, lowered the risk of CC (5).
A previous study in Korea also demonstrated an association between EO-CRC risk and metabolic syndrome (MetS) and showed that a higher BMI and larger WC are important risk factors for EO-CRC (14).
We aimed to evaluate whether changes in BMI and WC are associated with EO-CRC risk and suggest how to manage them to prevent EO-CRC.
All Koreans join the National Health Insurance Service (NHIS), and universal medical coverage is provided in Korea. The data of all Koreans in the NHIS are managed by the National Health Insurance Corporation (NHIC). This patient database includes all kinds of medical information, such as demographic data, disease diagnoses, prescribed medicines, received treatments, and hospital visitation records. The NHIS also provides national health checkups every 2 years for all Koreans aged ≥40 years or any employees aged >20 years (15). Data from these health checkups include anthropometric data measured by licensed medical staff, lifestyle questionnaire data from self-surveys, blood and urine test results, and previous medical history. Since these data are obtained every 2 years, we could determine serial changes in one person. Therefore, the occurrence and diagnosis date of CRC were determined by reviewing the NHIC database.
The flow of the enrollment process is displayed in Figure 1. Among the participants who participated in the national health screening program in both 2009 (S1) and 2011 (S2), those aged 20–49 years were included. Participants who had incomplete records (n = 221,944) or a previous history of cancer (n = 35,233) were excluded. Additionally, participants who were newly diagnosed with CRC or died from any cause within 1 year of follow-up (n = 2,758) were excluded, as the potential for preexisting CRC could not be considered. Ultimately, 3,340,635 participants were included in the study.
This study was approved by the Ethics Committee of Seoul National University Hospital (IRB No. E-2201-011-1,286) and conformed to the ethical guidelines of the World Medical Association’s Declaration of Helsinki. Since deidentified data were collected and used in this study, the requirement for informed consent from individual participants was waived.
BMI was calculated as weight divided by height2 (kg/m2). According to the Asia-Pacific definition of the World Health Organization (WHO), a BMI ≥25 kg/m2 was defined as obesity, and a BMI <25 kg/m2 was defined as nonobesity (16). WC was measured by tape at the midpoint between the lower costal margin and anterior superior iliac crest (17). Abdominal obesity was defined as a WC ≥ 90 cm in men and ≥ 85 cm in women, according to the definition of the Korean Society for the Study of Obesity (18).
Lifestyle factors were recorded according to a self-survey. For smoking status, participants were considered to have never smoked or to have smoked in the past (no smoking group) or to currently smoke (smoking group). Alcohol intake was divided into no heavy drinking and heavy (average of ≥30 g of alcohol/day) drinking. Regular exercise was defined as performing moderate exercise ≥5 days per week or vigorous exercise ≥3 days per week. Low income was defined as an income in the lowest income quartile or receiving medical aid.
Hypertension was defined as a blood pressure ≥ 140/90 mmHg or a history of receiving antihypertensive medications. Diabetes was defined as a fasting glucose level ≥ 126 mg/dL or a history of receiving glucose-lowering agents. Dyslipidemia was defined as a total cholesterol level ≥ 240 mg/dL after a 12-h fast or a history of receiving dyslipidemia medication. MetS was defined when 3 or more of the following 5 criteria were satisfied: (1) a WC ≥90 cm for men or ≥ 85 cm for women; (2) a blood pressure ≥ 130/85 mm Hg or drug treatment for hypertension; (3) a fasting plasma glucose level ≥ 100 mg/dL or drug treatment for diabetes; (4) a serum triglyceride level ≥ 150 mg/dL or drug treatment for elevated triglycerides; and (5) a serum high-density lipoprotein cholesterol (HDL-C) level < 40 mg/dL in men or < 50 mg/dL in women.
Participants were classified by two methods according to obesity and abdominal obesity status, and follow-up results were analyzed separately. A change in obesity status was defined as a change in BMI measured at S1 and S2. Participants were divided into 4 groups: participants who did not have obesity at either examination (NNo), participants who did not have obesity at the first examination but had obesity at the second examination (NO), participants who had obesity at the first examination but did not have obesity at the second examination (ON), and participants who had obesity at both examinations (OO). A change in abdominal obesity status was defined as a change in WC measured at S1 and S2. Similar to participants with a change in obesity status, those with a change in abdominal obesity status were grouped into 4 categories: participants who did not have abdominal obesity at either examination (NNa), participants who did not have abdominal obesity at the first examination but had abdominal obesity at the second examination (NA), participants who had abdominal obesity at the first examination but did not have abdominal obesity at the second examination (AN), and participants who had abdominal obesity at both examinations (AA).
The primary outcome of this study was new EO-CRC cases, which were diagnosed before 50 years of age. CRC was diagnosed according to the International Classification of Diseases Tenth Revision (ICD-10). The ICD-10 codes used in this study were as follows: C18-C20 for a CRC diagnosis, C18-19 for a CC diagnosis, C20.0 for a rectal cancer (RC) diagnosis, and the registration code for cancer [V193]. Registration of the cancer diagnosis and location was performed by a licensed doctor when the cancer was first diagnosed. Participants were followed until they were diagnosed with CRC or censored when they became older than 50 years.
Variables are expressed as the means ± standard deviations or numbers (percentages). To compare baseline characteristics among groups, ANOVA was used for continuous variables, and the χ2 test was applied for categorical variables. The incidence rates of CRC are presented as the number of events divided by 1,000 person-years. To evaluate EO-CRC risk, a multivariable Cox proportional hazard model was used. Participants were divided into 4 groups according to changes in obesity and abdominal obesity status. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated without adjustment (Model 1), after adjustment for sex and age (Model 2), and after adjustment for sex, age, smoking status, alcohol consumption, regular exercise, income status, and MetS (Model 3). The correlation between the obesity group and abdominal obesity group was assessed using Cremér’s V. Sensitivity analyzes according to CRC subsites were performed. Finally, a Kaplan–Meier curve of EO-CRC was drawn after adjusting for sex, age, smoking status, alcohol consumption, regular exercise, income status, and MetS. Variables with p values <0.05 were considered statistically significant. Statistical analyzes were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, United States).
A total of 3,340,635 participants who received national health screenings in 2009 (S1) and 2011 (S2) were included in this study. During the mean 7.1-year follow-up period, 7,492 patients were diagnosed with CRC. The demographic and clinical characteristics of the group with changes in obesity and abdominal obesity status are shown in Table 1. The data presented in the tables were collected in S2.
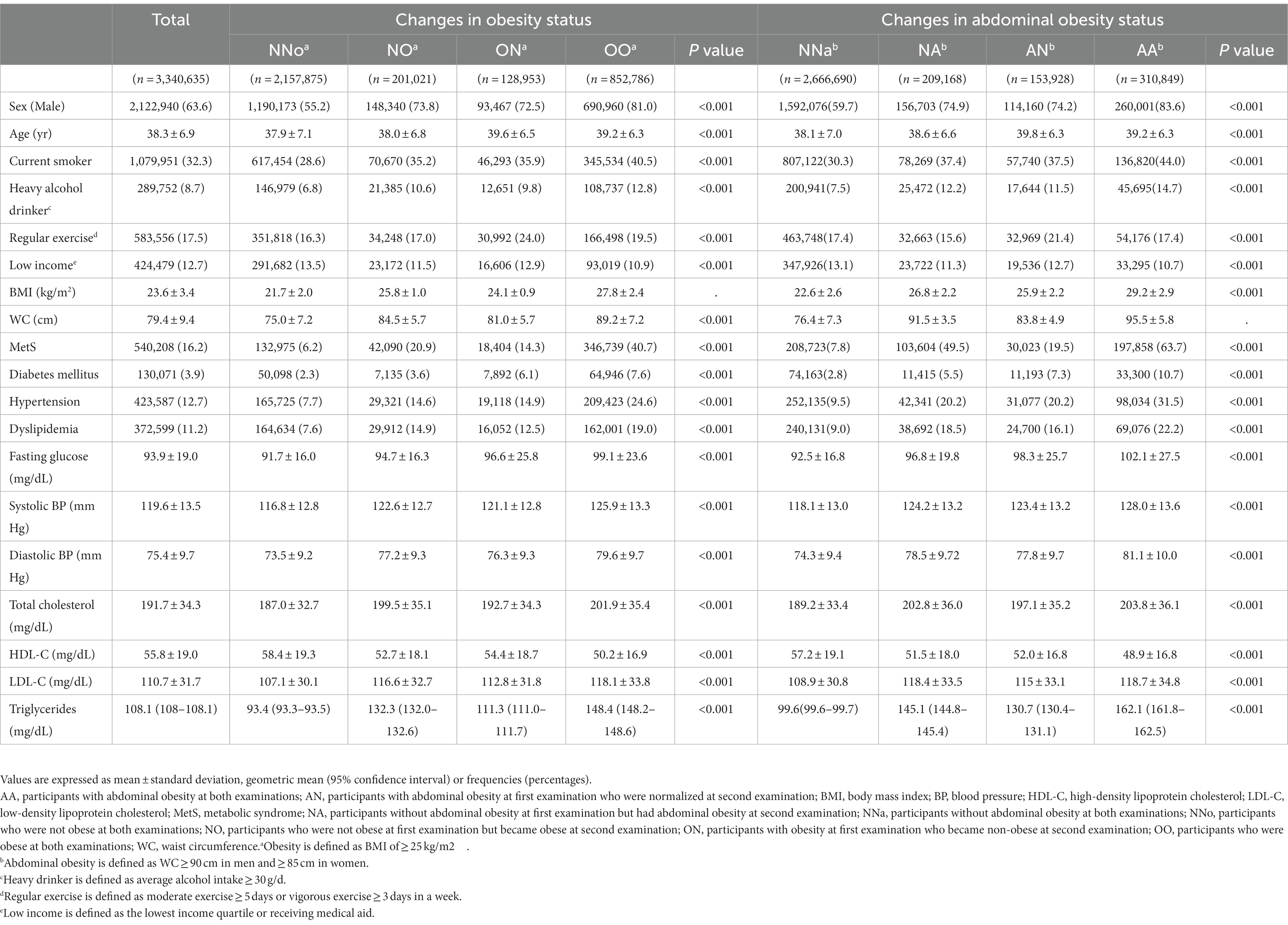
Table 1. Baseline characteristics of the group with changes in obesity and abdominal obesity status.
EO-CRC risk was stratified according to changes in obesity and abdominal obesity status (Table 2). Changes in obesity and abdominal obesity showed a moderate correlation (Cramér’s V = 0.39). After adjusting for sex, age, smoking status, alcohol consumption, regular exercise, income status, and MetS, EO-CRC risk was 9% higher in the OO group than in the NNo group. In the AA group, the increase in EO-CRC risk was even higher, up to 18%.
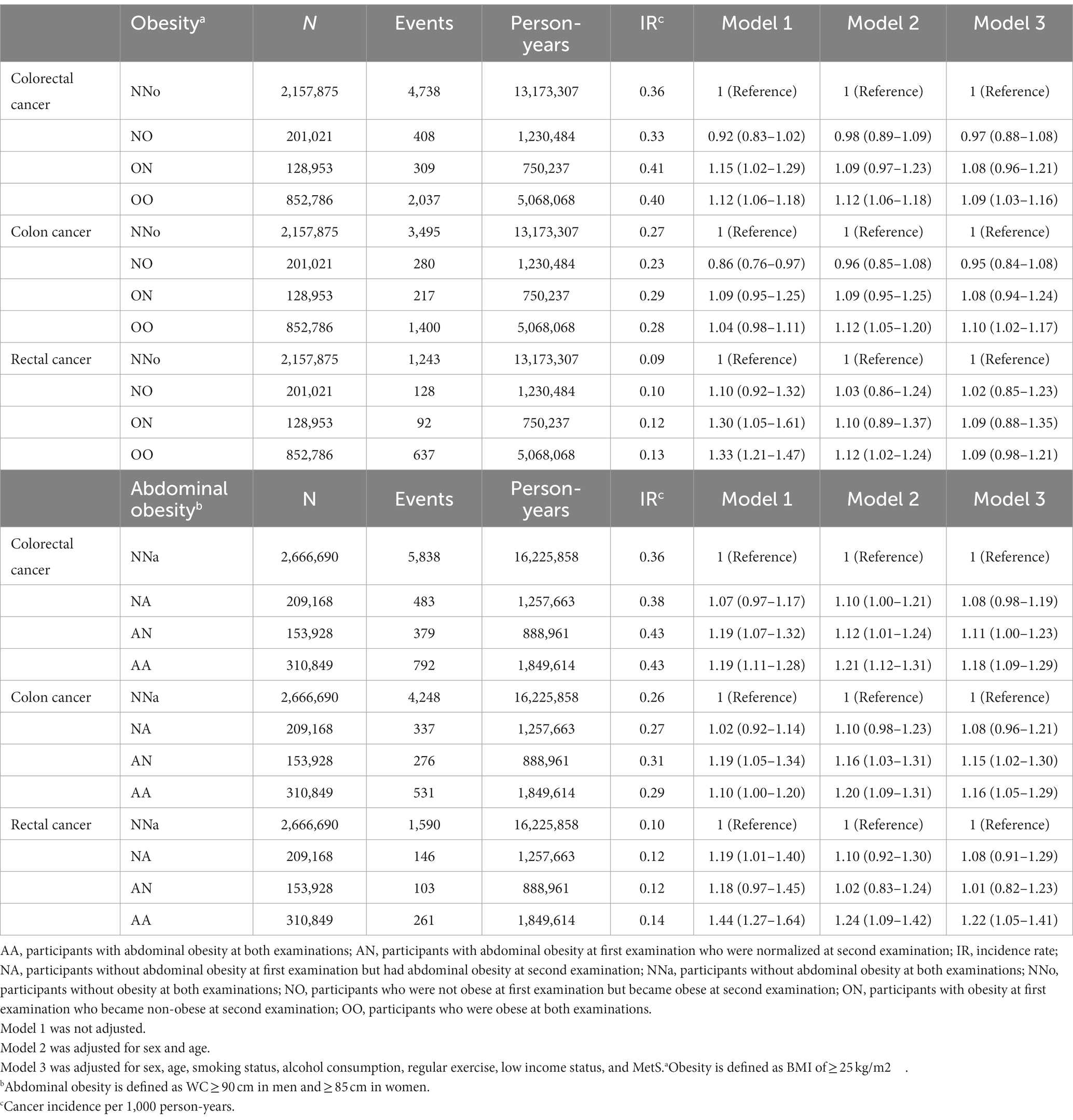
Table 2. Risk of earlier-onset colorectal cancer according to changes in obesity and abdominal obesity status.
The cumulative incidence of EO-CRC according to obesity and abdominal obesity status is shown in Figure 2. Calculating EO-CRC risk according to the cumulative obesity burden (the number of participants diagnosed with obesity or abdominal obesity in S1 and S2), participants with cumulative obesity and abdominal obesity burdens of 2 had a higher incidence of EO-CRC compared with those with burdens of 0 (Figure 2). The incidence of EO-CRC, EO-CC, and EO-RC in participants with a cumulative abdominal obesity burden of 1 was drawn between 0 and 2.
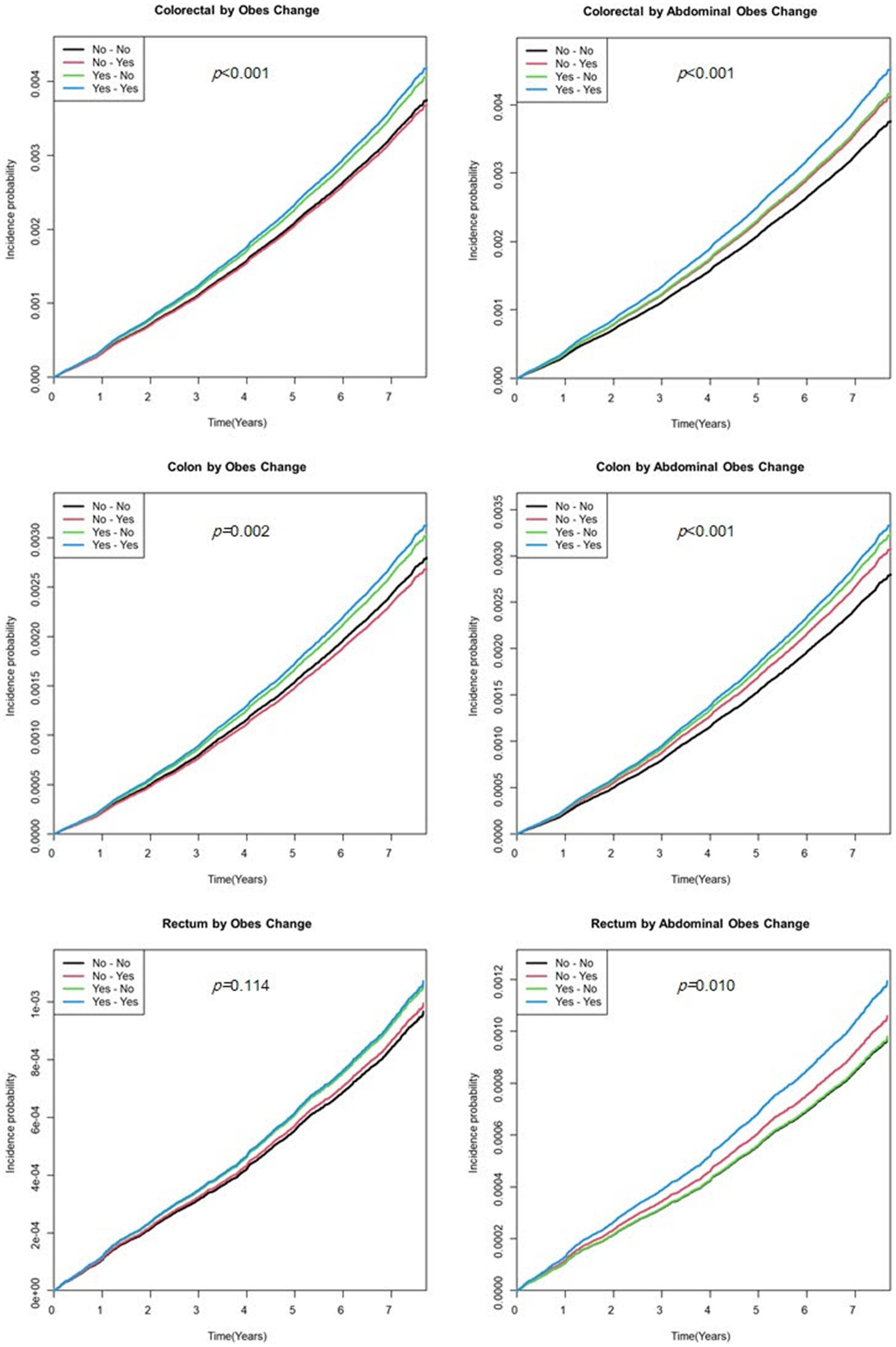
Figure 2. Kaplan–Meier curve of earlier-onset colorectal cancer by the cumulative obesity burden. Model was adjusted for sex, age, smoking status, alcohol consumption, regular exercise, and income status.
Participants were subdivided into 16 groups according to changes in obesity and abdominal obesity status (Table 3). Compared to the NNo-NNa group, the OO-AA group had a 19% higher EO-CRC risk. Interestingly, EO-CRC risk was significantly higher in participants whose BMIs became normal after being diagnosed with obesity but who newly developed abdominal obesity [ON NA, HR (95% CI) = 1.69 (1.04–2.77)] or persistent abdominal obesity [ON AA, HR (95% CI) = 1.87 (1.17–2.97)].
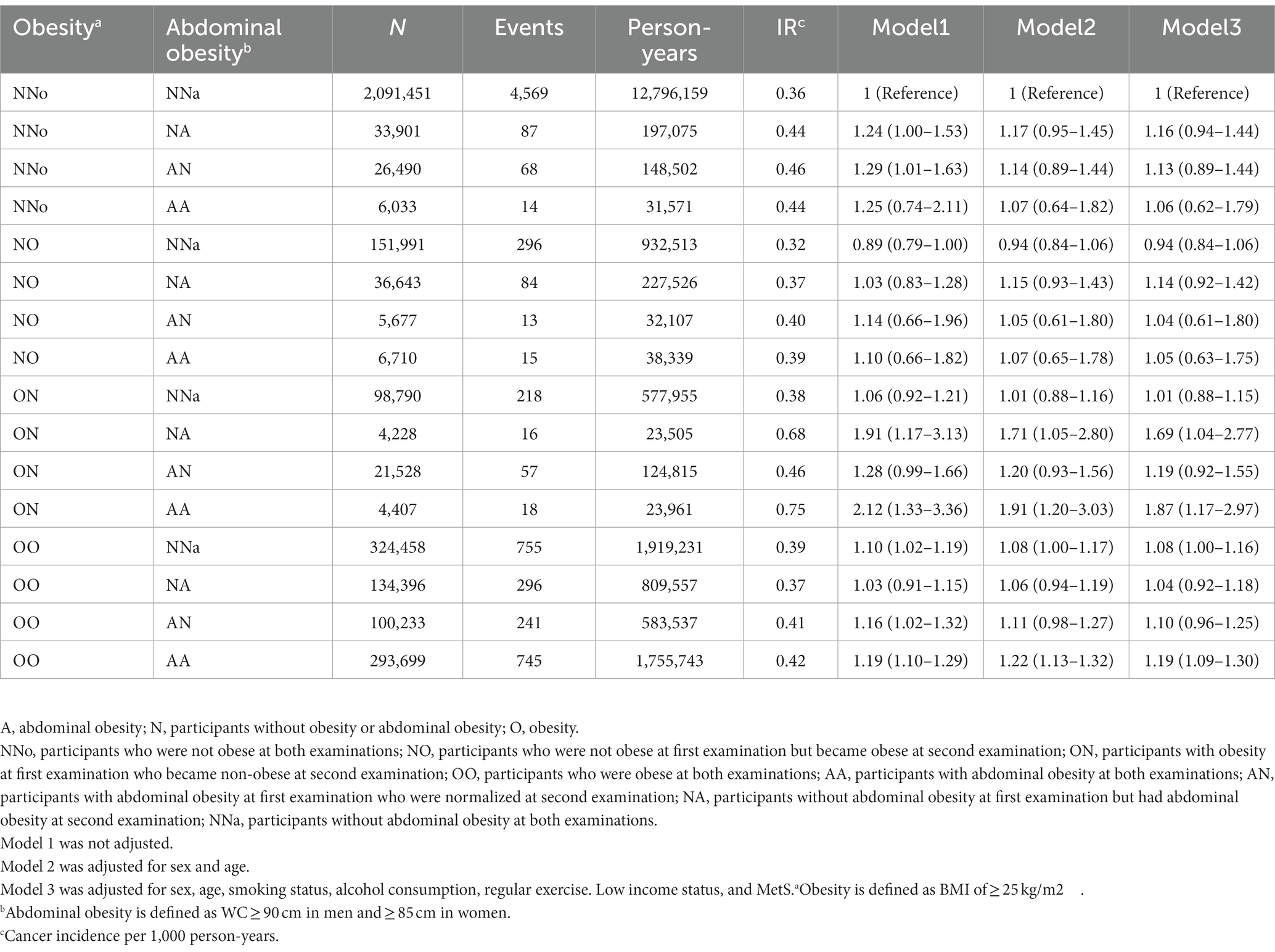
Table 3. The risk of earlier onset colorectal cancer when subdivided according to changes in obesity and abdominal obesity status.
Sensitivity analyzes evaluating the risk factors for EO-CRC according to changes in obesity and abdominal obesity status were performed (Table 4). Males and females showed significantly different risks of EO-CC according to changes in obesity status (P for interaction = 0.005). Heavy drinkers in the AA group had a significantly higher risk of EO-CRC and EO-CC [HR (95% CI) = 1.30 (1.07–1.58) and 1.35 (1.06–1.72), respectively].
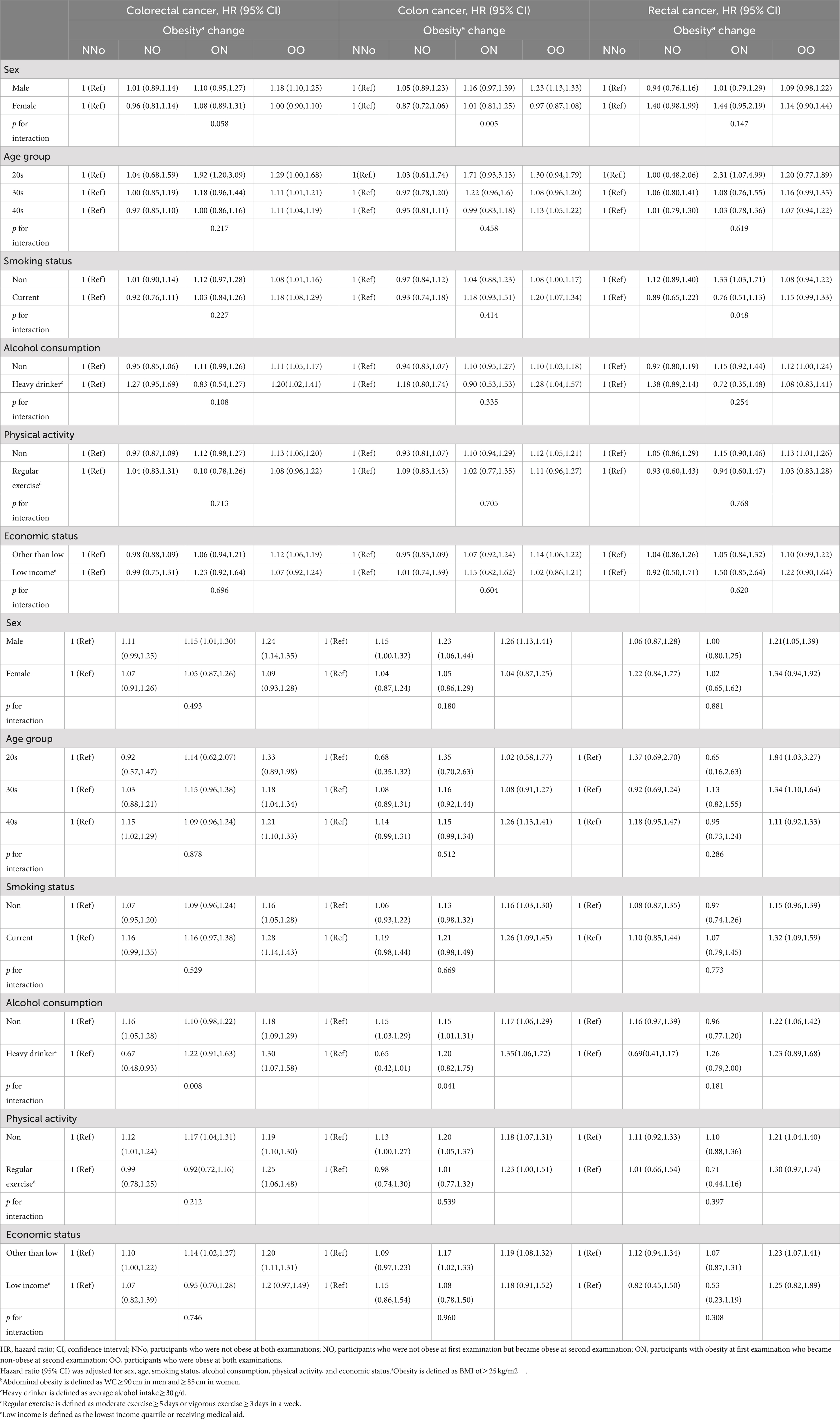
Table 4. Sensitivity analysis of risk of earlier-onset colorectal cancer according to changes in obesity and abdominal obesity status.
According to this large nationwide population-based cohort study, the incidence of EO-CRC showed a slight increase in individuals with persistent obesity (9%) or persistent abdominal obesity (18%). Having both persistent obesity and abdominal obesity further increased EO-CRC risk (19%).
To our knowledge, this is the first study to clarify the association between changes in obesity status and EO-CRC risk. We also compared the effect of changes in obesity and abdominal obesity status. A prospective cohort study evaluating CRC risk according to the degree of weight change revealed that an increased WC and weight during middle adult life were not associated with CRC risk (19). Another prospective cohort study showed that abdominal adiposity was associated with an increased CRC risk in men (13). These studies differ from our study in that the ages of the subjects ranged from 40 to 69 years, or the average participant was in their 60s.
Referring to the baseline characteristics, the percentages of NNo, NO, ON, and OO were 64.6, 6.0, 3.9, and 25.5%, respectively, for changes in obesity status in this study. Although it was a short time interval, the groups changed for only a small number of participants. This means that lean people remain lean and obese people remain obese. A previous meta-analysis showed that obesity persisted from childhood to adulthood (20). Childhood obesity may affect CRC risk later in life (21, 22). The importance of childhood obesity should be emphasized and interventions should be provided.
Interestingly, it was observed that even in cases of weight loss, the risk of EO-CRC was higher when abdominal obesity newly developed (ON NA, HR 1.69) or persisted (ON AA, HR 1.87). These findings suggest that abdominal obesity may have a greater influence on the development of EO-CRC compared to obesity measured by BMI. There have been several studies consistent with these results. A study reported that abdominal fat, independent of overall obesity, was associated with an increased CRC risk in men (13). Another study suggested that a WC change might be a better predictor of advanced colorectal neoplasia than a BMI change (11). Recently, another study reported that a large WC was associated with colorectal neoplasia risk even with a normal weight (23). The plausible mechanisms by which abdominal obesity increases CRC risk include insulin resistance, hyperinsulinemia, chronic inflammation, and altered levels of growth factors, adipocytokines and steroid hormones (24). However, it should be noted that the number of participants in these groups (ON NA, ON AA) was relatively small, which limits the certainty of these conclusions. Further studies with a larger sample size are needed to provide more conclusive evidence.
In the sensitivity analyzes to evaluate the risk factors for EO-CRC according to changes in obesity status, persistent obesity increased EO-CC risk in men but not women. These results were similar to those of previous studies on CRC (25). In the presence of persistent abdominal obesity, participants who reported heavy drinking had a 30% higher risk of developing EO-CRC. A previous meta-analysis of risk factors for EO-CRC showed that high levels of alcohol consumption increased EO-CRC risk (26). However, there has been no report on the effect of alcohol consumption on EO-CRC risk according to changes in abdominal obesity status.
There are several limitations in this study. First, this was a retrospective study, and the follow-up period was relatively short because of the age restriction of the outcome. Second, the diagnostic criteria of obesity (16) and abdominal obesity (18) have been defined for Asians and are different from the WHO criteria. Last, the gap between S1 and S2 was too short to reflect long-term changes in obesity status. To evaluate EO-CRC risk in normal-to-obese and obese-to-normal groups, it is necessary to follow up changes over a period of 2 years or more. Intervals greater than 2 years were not investigated in this study due to insufficient data. Further studies are needed to determine the long-term effects of changes in obesity and abdominal obesity status.
Nevertheless, this study is significant in that changes in obesity and abdominal obesity status were analyzed, which are modifiable risk factors among EO-CRC patients in a large population. Although CRC screening guidelines recommend starting colonoscopy at the age of 50 years, initial colonoscopy at an earlier age should be recommended for younger people who have persistent obesity or are lean but have abdominal obesity. To overcome the limitations of this study, we plan to study how long-term changes in obesity and abdominal obesity status over more than 5 years affect EO-CRC patients.
In conclusion, individuals below the age of 50 who have persistent obesity or persistent abdominal obesity are at a slightly higher risk of developing EO-CRC compared to non-obese individuals. Moreover, the risk of EO-CRC increases with the number of cumulative diagnoses of obesity and abdominal obesity. Therefore, monitoring and intervening to address obesity and abdominal obesity in young individuals may be beneficial in reducing the risk of EO-CRC.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Seoul National University Hospital (IRB No. E-2201-011-1286). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JHS and JYS: conceptualization, interpretation of data, and writing–original draft. EHJ, GEC, YSK, JHB, and SK: interpretation of data. K-DH: conceptualization, formal analysis, interpretation of data, methodology, resources, software, and supervision. SYY: conceptualization, interpretation of data, supervision, and writing–review and editing. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BMI, body mass index; CC, colon cancer; CI, confidence interval; CRC, colorectal cancer; EO, early onset; HbA1C, glycated hemoglobin level; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; ICD-10, International Classification of Diseases Tenth Revision; MetS, metabolic syndrome; NHIC, National Health Insurance Corporation; NHIS, National Health Insurance Service; RC, rectal cancer; S1, national health screening performed in 2009; S2, national health screening performed in 2011; WC, waist circumference; WHO, World Health Organization; NNa, participants who did not have abdominal obesity (WC <90 cm in men and < 85 cm in women) at either examinations; NA, participants who did not have abdominal obesity at the first examination but had abdominal obesity at the second examination; AN, participants who had abdominal obesity at the first examination but did not have abdominal obesity at the second examination; AA, participants who had abdominal obesity at both examinations; NNo, participants who did not have obesity (BMI < 25 kg/m2) at either examinations; NO, participants who did not have obesity at the first examination but had obesity at the second examination; ON, participants who had obesity at the first examination but did not have obesity at the second examination; OO, participants who had obesity at both examinations.
1. Burnett-Hartman, AN, Lee, JK, Demb, J, and Gupta, S. An update on the epidemiology, molecular characterization, diagnosis, and screening strategies for early-onset colorectal Cancer. Gastroenterology. (2021) 160:1041–9. doi: 10.1053/j.gastro.2020.12.068
2. Stoffel, EM, and Murphy, CC. Epidemiology and mechanisms of the increasing incidence of Colon and Rectal cancers in young adults. Gastroenterology. (2020) 158:341–53. doi: 10.1053/j.gastro.2019.07.055
3. Klimkiewicz, P. USPSTF colorectal cancer screening update 2021: a review of evidence. Nurse Pract. (2022) 47:37–42. doi: 10.1097/01.NPR.0000884892.06046.34
4. Park, L, O'Connell, K, Herzog, K, Chatila, W, Walch, H, Palmaira, RLD, et al. Clinical features of young onset colorectal cancer patients from a large cohort at a single cancer center. Int J Color Dis. (2022) 37:2511–6. doi: 10.1007/s00384-022-04286-5
5. Christakoudi, S, Pagoni, P, Ferrari, P, Cross, AJ, Tzoulaki, I, Muller, DC, et al. Weight change in middle adulthood and risk of cancer in the European prospective investigation into Cancer and nutrition (EPIC) cohort. Int J Cancer. (2021) 148:1637–51. doi: 10.1002/ijc.33339
6. Lauby-Secretan, B, Scoccianti, C, Loomis, D, Grosse, Y, Bianchini, F, Straif, K, et al. Body fatness and Cancer--viewpoint of the IARC working group. N Engl J Med. (2016) 375:794–8. doi: 10.1056/NEJMsr1606602
7. Moore, LL, Bradlee, ML, Singer, MR, Splansky, GL, Proctor, MH, Ellison, RC, et al. BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham study adults. Int J Obes Relat Metab Disord. (2004) 28:559–67. doi: 10.1038/sj.ijo.0802606
8. Jang, H, Kim, R, Lee, JT, Lee, DH, Giovannucci, EL, and Oh, H. Overall and abdominal obesity and risks of all-cause and cause-specific mortality in Korean adults: a pooled analysis of three population-based prospective cohorts. Int J Epidemiol. (2023) 2023:dyac242. doi: 10.1093/ije/dyac242
9. Kim, YH, Kim, SM, Han, KD, Jung, JH, Lee, SS, Oh, SW, et al. Waist circumference and all-cause mortality independent of body mass index in Korean population from the National Health Insurance Health Checkup 2009(−)2015. J Clin Med. (2019) 8:72. doi: 10.3390/jcm8010072
10. Jung, YS, Park, JH, Park, DI, Sohn, CI, and Choi, K. Weight change and obesity are associated with a risk of adenoma recurrence. Dig Dis Sci. (2016) 61:2694–703. doi: 10.1007/s10620-016-4194-2
11. Gathirua-Mwangi, WG, Monahan, P, Song, Y, Zollinger, TW, Champion, VL, Stump, TE, et al. Changes in adult BMI and waist circumference are associated with increased risk of advanced colorectal neoplasia. Dig Dis Sci. (2017) 62:3177–85. doi: 10.1007/s10620-017-4778-5
12. Chen, Q, Wang, J, Yang, J, Jin, Z, Shi, W, Qin, Y, et al. Association between adult weight gain and colorectal cancer: a dose-response meta-analysis of observational studies. Int J Cancer. (2015) 136:2880–9. doi: 10.1002/ijc.29331
13. Song, M, Hu, FB, Spiegelman, D, Chan, AT, Wu, K, Ogino, S, et al. Long-term status and change of body fat distribution, and risk of colorectal cancer: a prospective cohort study. Int J Epidemiol. (2016) 45:871–83. doi: 10.1093/ije/dyv177
14. Jin, EH, Han, K, Lee, DH, Shin, CM, Lim, JH, Choi, YJ, et al. Association between metabolic syndrome and the risk of colorectal Cancer diagnosed before age 50 years according to tumor location. Gastroenterology. (2022) 163:637–648.e2. doi: 10.1053/j.gastro.2022.05.032
15. Cheol Seong, S, Kim, YY, Khang, YH, Heon Park, J, Kang, HJ, Lee, H, et al. Data resource profile: the national health information database of the national health insurance service in South Korea. Int J Epidemiol. (2017) 46:dyw253–800. doi: 10.1093/ije/dyw253
16. James, PT, Leach, R, Kalamara, E, and Shayeghi, M. The worldwide obesity epidemic. Obes Res. (2001) 9:228S–33S. doi: 10.1038/oby.2001.123
17. World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. (2000) 894:1–253.
18. Lee, SY, Park, HS, Kim, DJ, Han, JH, Kim, SM, Cho, GJ, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. (2007) 75:72–80. doi: 10.1016/j.diabres.2006.04.013
19. Karahalios, A, Simpson, JA, Baglietto, L, MacInnis, RJ, Hodge, AM, Giles, GG, et al. Change in weight and waist circumference and risk of colorectal cancer: results from the Melbourne collaborative cohort study. BMC Cancer. (2016) 16:157. doi: 10.1186/s12885-016-2144-1
20. Simmonds, M, Burch, J, Llewellyn, A, Griffiths, C, Yang, H, Owen, C, et al. The use of measures of obesity in childhood for predicting obesity and the development of obesity-related diseases in adulthood: a systematic review and meta-analysis. Health Technol Assess. (2015) 19:1–336. doi: 10.3310/hta19430
21. Hidayat, K, Yang, CM, and Shi, BM. Body fatness at an early age and risk of colorectal cancer. Int J Cancer. (2018) 142:729–40. doi: 10.1002/ijc.31100
22. Garcia, H, and Song, M. Early-life obesity and adulthood colorectal cancer risk: a meta-analysis. Rev Panam Salud Publica. (2019) 43:1–8. doi: 10.26633/RPSP.2019.3
23. Jung, YS, Kim, NH, Yang, HJ, Park, SK, Park, JH, Park, DI, et al. Association between waist circumference and risk of colorectal neoplasia in normal-weight adults. J Gastroenterol Hepatol. (2020) 35:43–9. doi: 10.1111/jgh.14767
24. Jochem, C, and Leitzmann, M. Obesity and colorectal Cancer. Recent Results Cancer Res. (2016) 208:17–41. doi: 10.1007/978-3-319-42542-9_2
25. Bardou, M, Rouland, A, Martel, M, Loffroy, R, Barkun, AN, and Chapelle, N. Review article: obesity and colorectal cancer. Aliment Pharmacol Ther. (2022) 56:407–18. doi: 10.1111/apt.17045
Keywords: early onset colorectal cancer, obesity, abdominal obesity, obesity change, cohort study
Citation: Song JH, Seo JY, Jin EH, Chung GE, Kim YS, Bae JH, Kim S, Han K-D and Yang SY (2023) Association of changes in obesity and abdominal obesity status with early-onset colorectal cancer risk: a nationwide population-based cohort study. Front. Med. 10:1208489. doi: 10.3389/fmed.2023.1208489
Received: 19 April 2023; Accepted: 05 June 2023;
Published: 21 June 2023.
Edited by:
Nan Zhang, The First Hospital of Jilin University, ChinaReviewed by:
Hexiao Wang, Memorial Sloan Kettering Cancer Center, United StatesCopyright © 2023 Song, Seo, Jin, Chung, Kim, Bae, Kim, Han and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyung-Do Han, aGtkOTE3QG5hdmVyLmNvbQ==; Sun Young Yang, c3l5YW5nQHNudWgub3Jn
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.