- 1Department of Internal Medicine, Member of the German Center for Lung Research (DZL), Justus-Liebig-University Giessen, Universities of Giessen and Marburg Lung Center (UGMLC), Giessen, Germany
- 2Department of Internal Medicine, Member of the German Center for Lung Research (DZL), Institute for Lung Health (ILH), Cardio-Pulmonary Institute (CPI), Universities of Giessen and Marburg Lung Center (UGMLC), Giessen, Germany
Background: Cardiac interactions with organs such as the liver or kidneys have been described in different cardiovascular diseases. However, the clinical relevance of hepatorenal dysfunction in chronic thromboembolic pulmonary hypertension (CTEPH) remains unclear. We determined the association of hepatorenal dysfunction (measured using the Model for End-stage Liver Disease Sodium [MELDNa] score) with right heart function and survival in patients with CTEPH.
Methods: We analyzed all patients with CTEPH in the Giessen Pulmonary Hypertension Registry who had available MELDNa scores and were not taking vitamin K antagonists. The MELDNa score was calculated as MELD score − serum Na − (0.025 * MELD score * (140 − serum Na)) + 140; the MELD score was calculated as 10*(0.957*ln(creatinine)+0.378*ln(bilirubin)+1.12*ln(International Normalized Ratio))+6.43.
Results: Seventy-two patients were included (74% female; median [Q1, Q3] MELDNa: 9 [6, 11]). MELDNa correlated well with right atrial and ventricular function and pulmonary hemodynamics. Forward regression analysis revealed that hepatorenal dysfunction mainly depends on right atrial strain and tricuspid regurgitation, but not right ventricular systolic dysfunction. Hepatorenal dysfunction predicted mortality at baseline and follow-up (adjusted hazard ratios [95% confidence intervals] per unit increase of MELDNa: 1.6 [1.1, 2.4] and 1.8 [1.1, 2.9], respectively). Changes in hepatorenal function also predicted mortality.
Conclusion: Hepatorenal dysfunction in CTEPH is primarily associated with venous congestion rather than cardiac forward failure. As a surrogate parameter for hepatorenal dysfunction, MELDNa is a simple method to identify at-risk patients at baseline and follow-up.
1. Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a progressive and life-threatening disease that ultimately leads to right heart insufficiency and premature death. Owing to the pathophysiological nature of the disease (which is characterized by pulmonary artery obstructions), right ventricular (RV) afterload and wall tension are increased, and the right ventricle seeks to counteract these changes with cardiac remodeling. First, homeometric adaptation according to the Laplace law leads to concentric hypertrophy and increased wall thickness. A further increase in RV afterload can then lead to heterometric (mal)adaptation with right heart dilatation, RV insufficiency, and venous congestion (1).
Clinical studies have focused on the right ventricle and its systolic (dys)function (2–4). However, an increase in RV stiffness can also lead to diastolic dysfunction, even before RV systolic failure is obvious. RV diastolic dysfunction may cause vena cava backflow during contraction of the right atrium and tricuspid regurgitation (TR) (5). It is well known that the resulting venous congestion, as well as the forward failure of the right ventricle, can cause organ dysfunction, which is inextricably linked to disease progression (6, 7).
The liver and kidneys are affected by both venous congestion and reduced cardiac output (8, 9). There are various methods for assessing hepatorenal dysfunction, for example liver and kidney sonography, which we and others have proven to be feasible and highly relevant for patients with pulmonary hypertension (PH) (10, 11). A more simple yet comprehensive method for assessing hepatorenal dysfunction is based on the Model for End-stage Liver Disease Sodium (MELDNa) score (12–14). The MELDNa score is usually used for patients with liver cirrhosis or end-stage liver disease, particularly for organ allocation, but its prognostic and clinical relevance has also been shown in patients with various other diseases, including left ventricular failure, primary TR, and congenital heart disease (6, 15). In addition, the MELDNa score is easily available in clinical routine as it is based on commonly measured laboratory parameters.
It is, however, unclear whether the non-invasive assessment of hepatorenal dysfunction is also associated with the severity of PH and whether the MELDNa score is prognostically relevant for patients with CTEPH. Furthermore, the underlying pathomechanism leading to hepatorenal dysfunction in patients with CTEPH is not fully understood. Therefore, the aim of this study was to determine the association of hepatorenal dysfunction (measured using the MELDNa score) with right heart function and survival in patients with CTEPH.
2. Materials and methods
2.1. Study design and patients
All patients with confirmed CTEPH enrolled between June 2013 and January 2021 in the Giessen PH Registry (16) were included in this retrospective study. The diagnosis was made by a multidisciplinary board of physicians, radiologists, and surgeons according to the contemporary guidelines (17). The date of the diagnostic right heart catheterization was defined as the date of diagnosis. Patients with missing data for calculation of the MELDNa score and patients taking vitamin K antagonists were excluded from the primary analyses. Patients with missing data for calculation of the MELD excluding international normalized ratio (INR) score (defined below) were excluded from the sensitivity analyses. All patients were followed up until November 2022.
The study was approved by the Institutional Review Board of the University of Giessen (No. 266/11), and all patients gave their written informed consent.
2.2. Right heart echocardiography and catheterization
Right heart echocardiography and catheterization were performed as described previously (18, 19). In brief, all echocardiographic parameters were obtained according to the current guidelines (20) via a GE Vivid E9 ultrasound unit (GE Healthcare, Wauwatosa, Wisconsin, United States). Tricuspid annular plane systolic excursion (TAPSE) was measured in M-mode at the lateral tricuspid valve annulus. Right atrial (RA) area was measured at end-systole corresponding to the maximal area. RA pressure (RAP) was estimated from the diameter of the inferior vena cava and its inspiratory collapsibility (17). Pulmonary arterial systolic pressure was estimated by adding estimated RAP to the tricuspid valve gradient (21). The severity of TR was categorized into three levels as previously described (22). Right heart speckle-tracking echocardiography was performed according to the consensus document of the European Association of Cardiovascular Imaging/American Society of Echocardiography/Industry Task Force (23).
All echocardiographic parameters that were not routinely acquired were obtained by an independent investigator who was blinded to the clinical data. Echocardiographic measurements and analyses were performed with EchoPac software (version 201, GE Healthcare).
2.3. Assessment of hepatorenal dysfunction
The surrogate parameter for hepatorenal dysfunction used in this paper was the MELDNa score, which adds sodium levels to the original MELD score (12). The MELDNa score was calculated as MELD score − serum Na − (0.025 * MELD score * (140 − serum Na)) + 140, while the MELD score was calculated as 10*(0.957*ln(creatinine)+0.378*ln(bilirubin)+1.12* ln(International Normalized Ratio))+6.43. Hepatorenal dysfunction was defined as a MELDNa score above the third quartile (> 11).
In sensitivity analyses, hepatorenal dysfunction was defined as a MELD excluding INR (MELD-XI) score (24) above the third quartile (≥ 12 at baseline and ≥ 11 during follow-up). The MELD-XI score, which has been associated with prognosis in patients with acute heart failure (25), was calculated as 5.11 x ln(bilirubin) + 11.76 x ln(creatinine) + 9.44, as described by Heuman et al. (24).
2.4. Statistical analyses
Statistical analyses were performed using R version 4.2.1 (The R Foundation for Statistical Computing) and SPSS version 29 (IBM, Armonk, United States). All parameters were evaluated based on the Shapiro–Wilk normality test and histograms for normal distribution. When normally distributed, data are presented as the mean (standard deviation) and compared using Student’s t-tests. Otherwise, numbers are presented as median [Q1, Q3] and compared using Wilcoxon rank tests. The linear association between variables was assessed using Spearman’s rho. Furthermore, we used univariate and multivariate binary regression analyses to investigate the influence of different parameters on hepatorenal dysfunction in patients with CTEPH. In addition, survival analyses were performed using Kaplan–Meier estimators as well as univariate and multivariate Cox regression.
3. Results
3.1. Study population
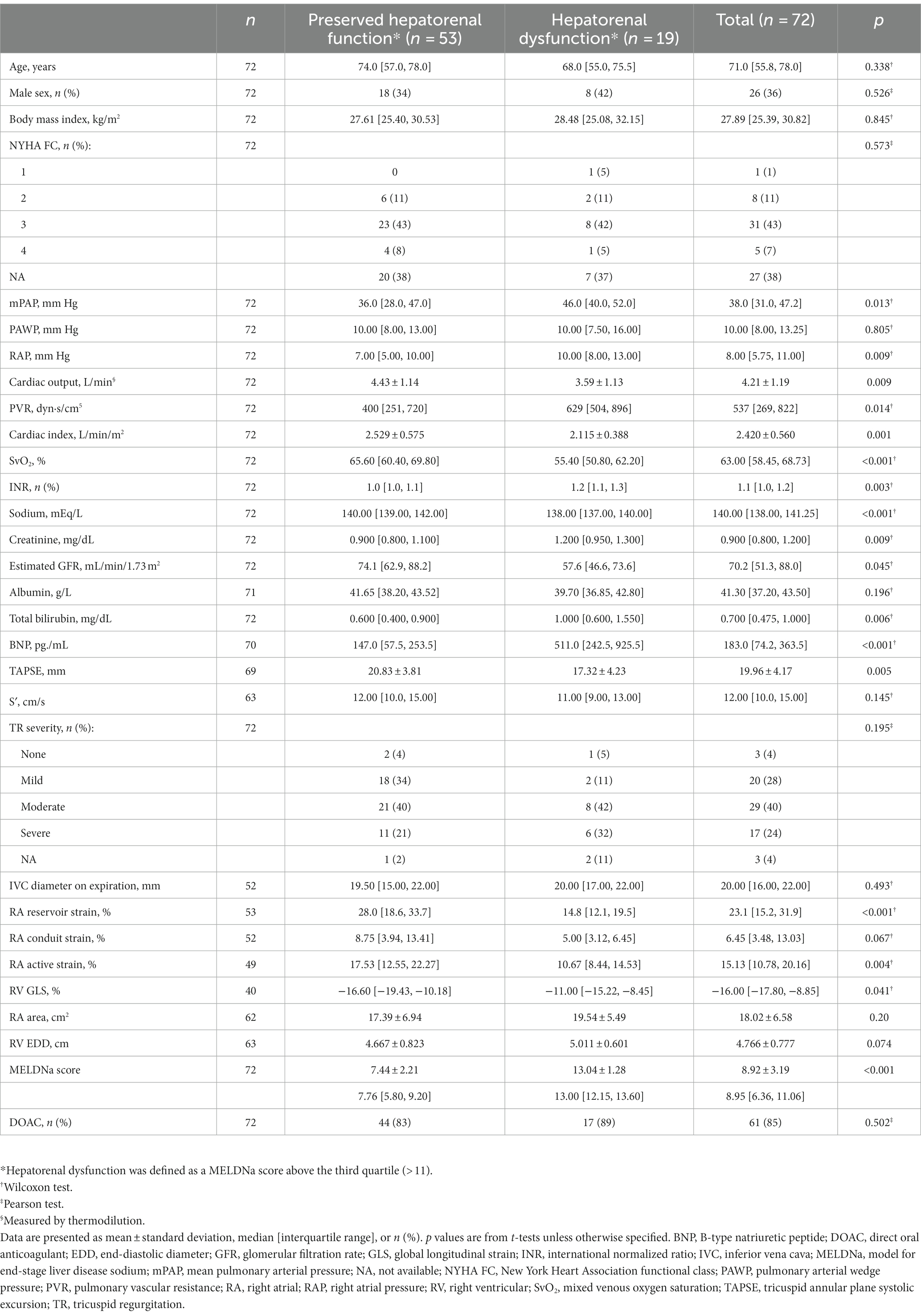
In total, 117 patients with CTEPH had available MELD-XI scores (screening population; Supplementary Table 1), of whom 111 also had available MELDNa scores (Supplementary Table 2). Of the 111 patients, 39 were excluded from the primary analyses because they used vitamin K antagonists. Seventy-two patients remained and were included in the primary analyses; 74% were female and the median age was 71 [56, 78] years. Baseline characteristics of the study cohort indicated a marked impairment of pulmonary hemodynamics, as shown in Table 1. In addition, the majority of the included patients (64%) showed at least moderate TR. Further echocardiographic and laboratory parameters are shown in Table 1. The median MELDNa score was 8.95 [6.36, 11.06].
3.2. Association of hepatorenal dysfunction with right heart parameters in CTEPH
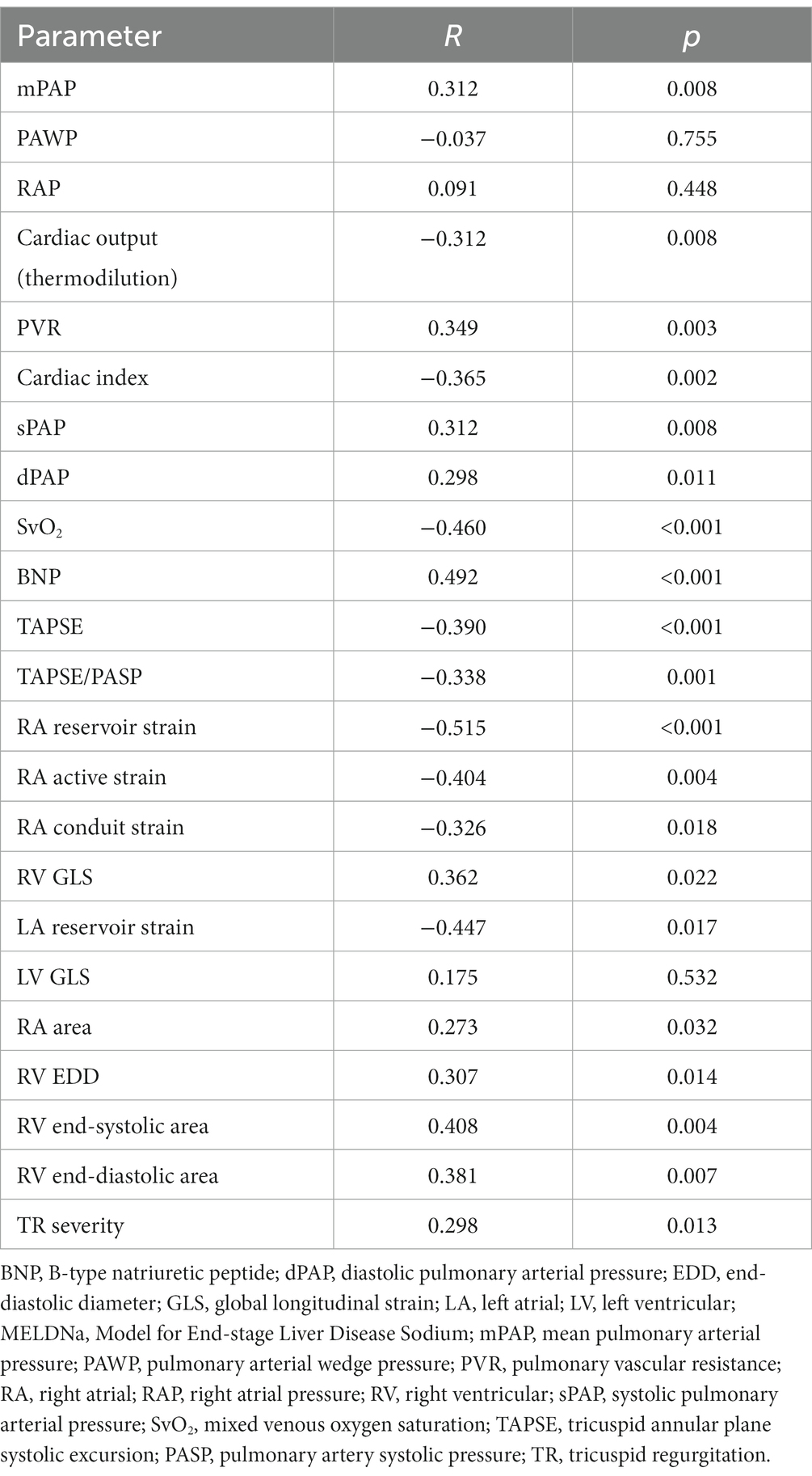
Correlation analyses indicated that the MELDNa score has a linear association with RA and RV function (Table 2). In particular, the MELDNa score was associated with RA reservoir strain (rho: − 0.515, p < 0.001), RV end-systolic area (rho: 0.408, p = 0.004), and TAPSE (rho: − 0.390, p < 0.001). TR severity (rho: 0.298, p = 0.013) and RA area (rho: 0.273, p = 0.032) were also significantly linked to hepatorenal (dys)function. Left ventricular global longitudinal strain (GLS), however, was not associated with the MELDNa score (p = 0.532).
The parameters most stringently related to the MELDNa score were ascertained by multivariate linear regression analysis using the forward selection technique The following parameters were included: systolic, diastolic, and mean pulmonary arterial pressure, pulmonary vascular resistance, cardiac index, sex, TR severity, TAPSE, RA reservoir strain, RV GLS, RA area, RV diameter, RV end-systolic area, and RAP. Interestingly, the model showed that RA reservoir strain (β-coefficient: − 0.365, p = 0.003) and TR severity (β-coefficient: 0.385, p = 0.002), but not RV systolic (dys)function, were strongly related to hepatorenal (dys)function.
The study cohort was then divided into two groups based on the MELDNa score. Patients with a high MELDNa score (above the third quartile [> 11]) were defined as patients with hepatorenal dysfunction. Patients fulfilling this definition of hepatorenal dysfunction had more severely impaired pulmonary hemodynamics (Table 1) and significantly higher B-type natriuretic peptide (BNP) concentrations (511.0 [242.5, 925.5] pg./mL vs. 147.0 [57.5, 253.5] pg./mL, p < 0.001) than those without hepatorenal dysfunction. Furthermore, RV and RA strain were more severely impaired (TAPSE: 17.32 ± 4.23 mm vs. 20.83 ± 3.81 mm, p = 0.005; RV GLS: − 11.00 [− 15.22, − 8.45]% vs. − 16.60 [−19.43, −10.18]%, p = 0.041; RA reservoir strain: 14.8 [12.1, 19.5]% vs. 28.0 [18.6, 33.7]%, p < 0.001). No other significant differences between the two groups were detected (Table 1).
3.3. Prognostic relevance of hepatorenal dysfunction in CTEPH
Next, Kaplan–Meier analysis was performed to investigate the prognostic relevance of hepatorenal dysfunction in patients with CTEPH (Figure 1). Survival at 1, 3, and 5 years was 98, 98, and 95%, respectively, in patients with preserved hepatorenal function but significantly lower in patients with hepatorenal dysfunction (79, 64, and 57%, respectively). Univariate Cox regression showed a significantly increased hazard ratio (HR) per unit increase of MELDNa (HR: 1.6 [95% confidence interval (CI): 1.2, 2.1], p = 0.001). Hepatorenal dysfunction remained as an independent predictor of mortality even after adjusting for age, RV systolic function (TAPSE), and TR severity (HR: 1.6 [95% CI: 1.1, 2.3], p = 0.017). Further adjustment for direct oral anticoagulant (DOAC) intake did not change the results (HR: 1.6 [95% CI: 1.1, 2.4], p = 0.019).
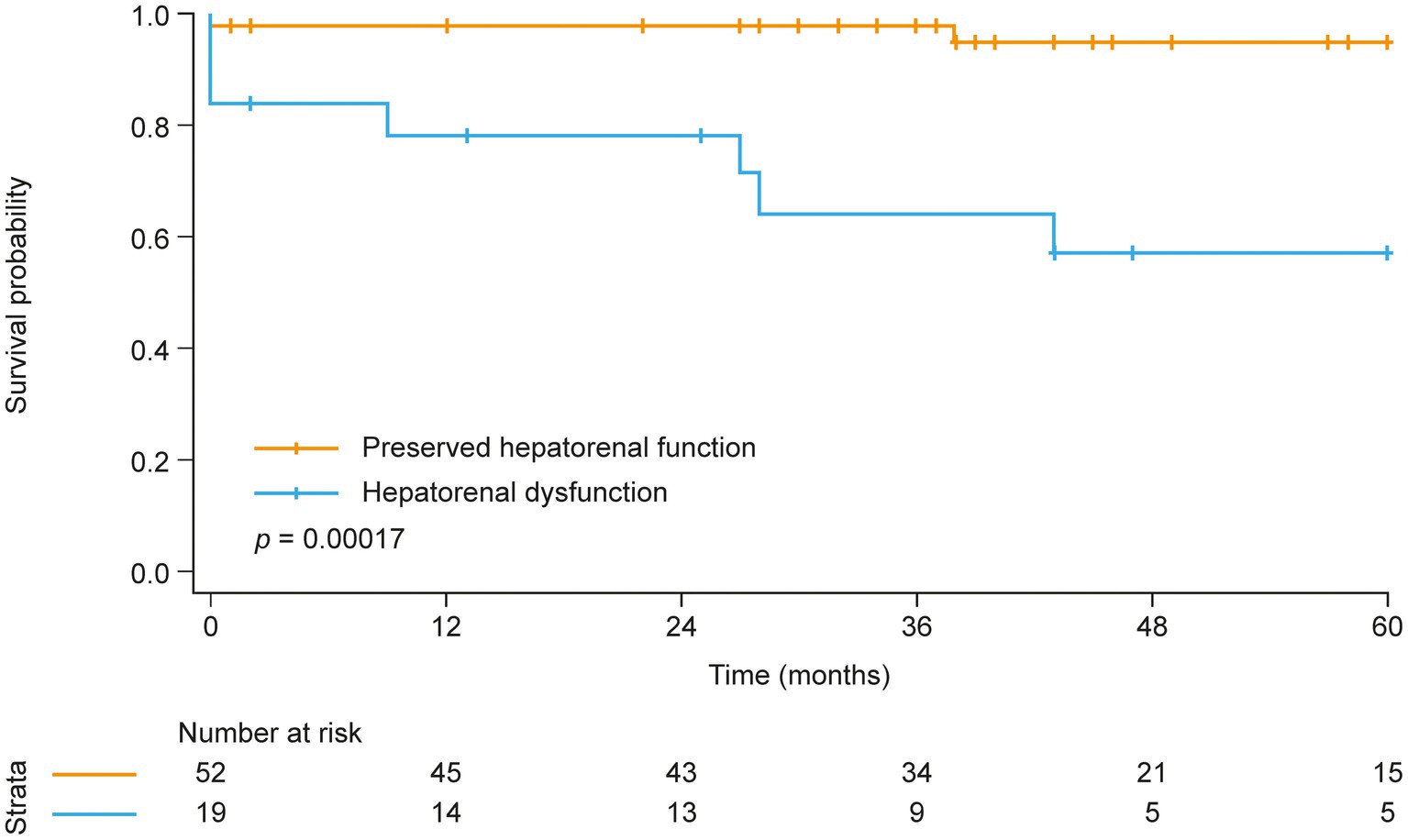
Figure 1. Kaplan–Meier analysis of survival in patients with and without hepatorenal dysfunction at baseline.
A sensitivity analysis of the screening population (n = 117) stratified by the MELD-XI score (24) also showed impaired survival in patients with hepatorenal dysfunction at baseline (Supplementary Figure 1A; HR: 3.67 [95% CI: 1.55, 8.65], p = 0.003).
3.4. Patient characteristics and clinical and prognostic relevance of hepatorenal dysfunction at follow-up
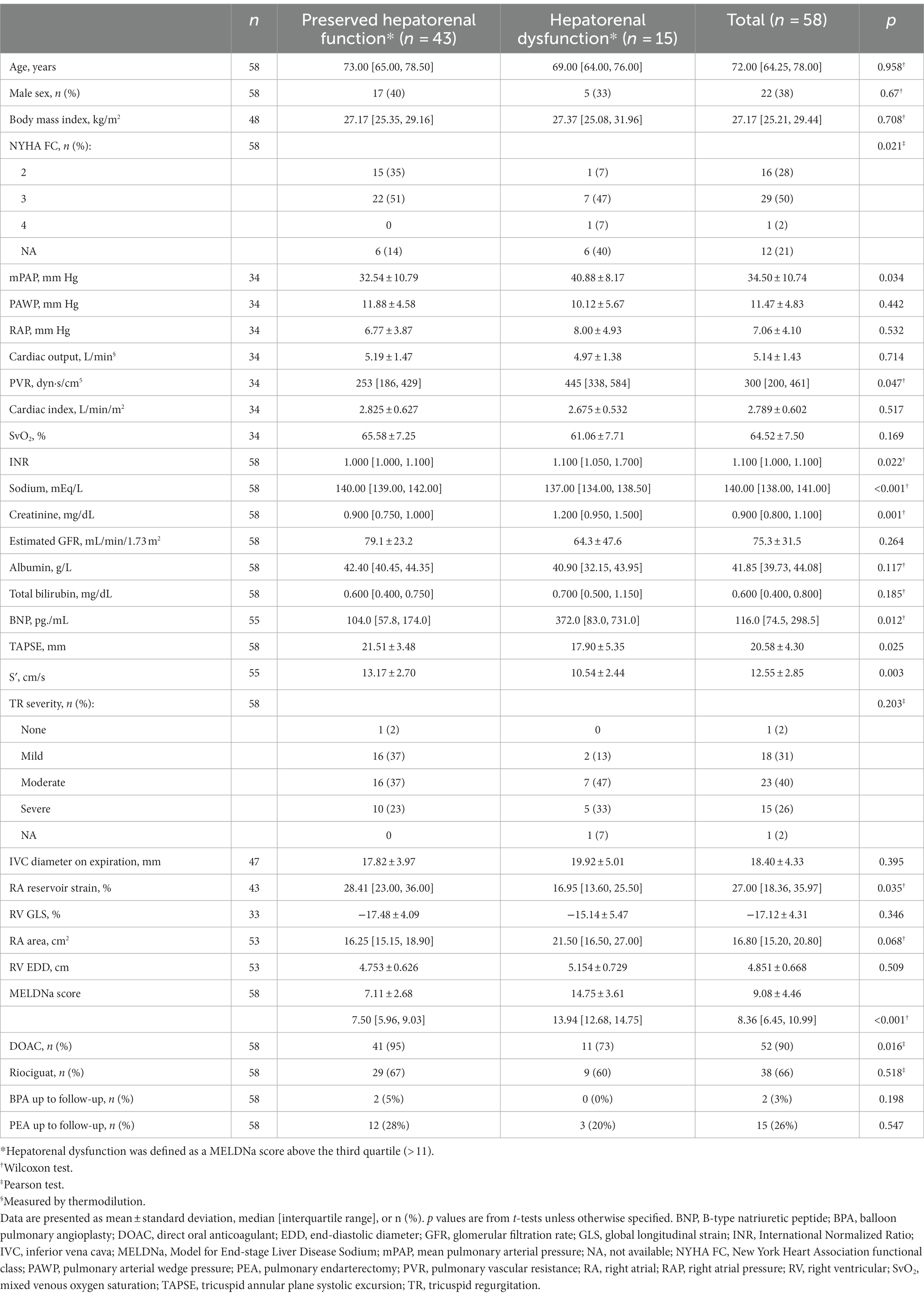
Follow-up data were available in 58 cases in the study cohort. The median time interval between diagnosis and follow-up was 6 [3, 9] months. Characteristics of the patients at follow-up are shown in Table 3. During the follow-up period, 15 (26%) and 2 (3%) patients underwent pulmonary endarterectomy and balloon pulmonary angioplasty, respectively, while PH-targeted medication was initiated in 38 patients (66%).
Dividing the study population into the two groups with and without hepatorenal dysfunction as defined above, the analysis showed a similar interdependency of parameters as observed at baseline: Patients with hepatorenal dysfunction (MELDNa score above the third quartile [> 11]) had more severe impairment of pulmonary hemodynamics, higher BNP concentrations, and impaired RA reservoir strain compared with patients without hepatorenal dysfunction (Table 3). Kaplan–Meier analysis again showed that hepatorenal dysfunction was associated with poor survival (Figure 2A): Survival at 1, 3, and 5 years was 100, 69, and 69%, respectively, in the group with hepatorenal dysfunction, compared with 100, 98, and 98%, respectively, in the rest of the cohort (log-rank p = 0.076). Consistent with this, univariate Cox regression showed a significantly increased HR per unit increase of MELDNa (HR: 1.2 [95% CI: 1.0, 1.5], p = 0.028). Multivariate Cox regression indicated that hepatorenal dysfunction is associated with survival independent of age, RV systolic function (mirrored by TAPSE), and TR severity (HR: 1.8 [95% CI: 1.1, 3.0], p = 0.017). Further adjustment for DOAC intake did not change the results (HR: 1.8 [95% CI: 1.1, 2.9], p = 0.013).
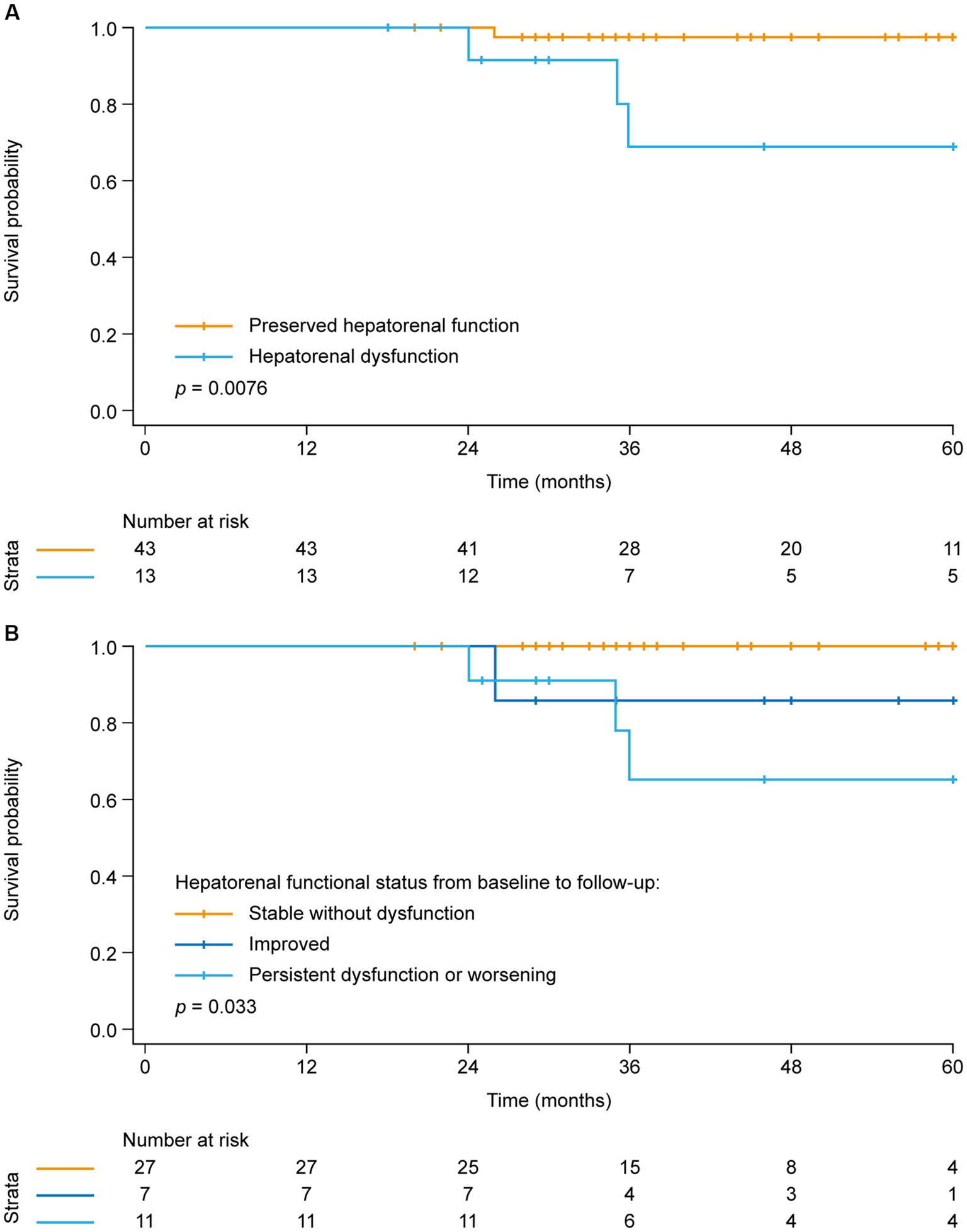
Figure 2. Kaplan–Meier analysis of survival in (A) patients with and without hepatorenal dysfunction at follow-up and (B) patients stratified by hepatorenal functional status change from baseline to follow-up.
In the screening population, follow-up data were available in 91 cases (Supplementary Table 3). A sensitivity analysis of the screening population stratified by the MELD-XI score (24) also showed impaired survival in patients with hepatorenal dysfunction at follow-up (Supplementary Figure 1B; HR: 7.62 [95% CI: 2.23, 26.08], p = 0.001).
3.5. Improvement of the MELDNa score: a prognostically relevant difference
Finally, the cohort was stratified into three groups according to the change in hepatorenal functional status (based on the MELDNa score) from baseline to follow-up (Figure 2B). The first group presented with a normal MELDNa score at baseline as well as during follow-up (“stable without dysfunction,” n = 27), whilst the second group was defined as having hepatorenal dysfunction at baseline, but a low MELDNa score during the follow-up (“improved,” n = 7). The third group included those with a high MELDNa score at baseline and during follow-up as well as patients who developed hepatorenal dysfunction during follow-up (“persistent dysfunction or worsening,” n = 12).
It became evident that the patients with persistent hepatorenal dysfunction or worsening during follow-up had the poorest prognosis (with survival at 1, 3, and 5 years of 100, 65, and 65%, respectively), whereas none of the patients with steadily normal hepatorenal function died within 5 years after follow-up. Notably, patients who presented with hepatorenal dysfunction at baseline but improved during follow-up showed higher survival (100, 86, and 86% at 1, 3, and 5 years, respectively) than those who had persistent dysfunction or worsening.
4. Discussion
To the best of our knowledge, this study is the first to show that hepatorenal dysfunction (assessed via the routine lab data-based MELDNa score) is associated with impairment of pulmonary hemodynamics and survival in patients with CTEPH. Venous congestion due to TR and RA engorgement rather than cardiac forward failure is suggested as major link between hemodynamic abnormalities and loss of hepatorenal function.
Hepatorenal dysfunction has been identified as an important prognostic parameter in acute and chronic left heart failure, as well as in congenital heart diseases (6, 15, 26–28). The pathomechanisms behind this close interplay of organs are known to be multifactorial and complex. While heart failure promotes liver disease by ischemia (due to forward failure) as well as congestion (due to backward failure), hepatic dysfunction itself is known to lead to cardiomyocyte apoptosis and cirrhotic cardiomyopathy. This, in turn, accelerates cardiac remodeling, promotes heart failure, and thus increases mortality (29). In the liver, increased venous congestion can lead to congestive hepatopathy, which can cause liver fibrosis and “cirrhose cardiaque” (30). Hepatic vein flow patterns can be used to assess congestive hepatopathy and have been proposed as bedside hepatic dysfunction parameters (10).
Chronic cardiorenal and renocardiac syndromes are characterized by congestive nephropathy, renal hypoperfusion, activation of the renin-angiotensin-aldosterone system and sympathetic nervous system, pro-inflammatory cytokines, and antidiuretic hormone secretion (31), which can then cause further volume retention and cardiac stress (32). Many of these mechanisms have been shown to decrease survival in multiple cardiovascular diseases (33). In this context, intrarenal venous flow patterns and the renal venous stasis index have emerged as feasible echocardiographic parameters with prognostic relevance (11). To sum up, all these mechanisms can lead to a vicious circle in patients with cardiac dysfunction.
Our results suggest that these associations are also applicable for patients with CTEPH. We observed that the MELDNa score predicts mortality in incident as well as during follow-up. Thus, the MELDNa score is a robust and independent predictor of disease severity and survival in CTEPH. Notably, patients whose MELDNa scores improved over time showed substantially higher survival than those with persistent dysfunction or worsening, highlighting the clinical relevance of hepatorenal dysfunction assessed via the MELDNa score.
Even though hepatorenal dysfunction is known to be prognostic in other forms of heart failure, the underlying pathomechanism is still not fully understood (29, 32, 34). In addition to forward failure (characterized by impairment of cardiac output), venous congestion and backflow into the vena cava due to tricuspid insufficiency or RA contraction/RV diastolic dysfunction are conceivable. Thus, we sought to determine the driving force behind hepatorenal dysfunction in CTEPH. Interestingly, our study provides evidence that impaired RA function (mirrored by decreased RA reservoir strain) and TR, rather than RV systolic function, are the primary causes of hepatorenal dysfunction. This corresponds to the very early observation of Kussmaul et al. (35), who already described the clinical significance of venous reflux in patients with restricted cardiac filling in 1873. Bilirubin levels were independently associated with RAP in patients with acute heart failure in a more recent study by Biegus et al. (36). Taken together, alterations of the veno–atrial interaction and TR appear to be the underlying causes of organ dysfunction from a pathophysiological point of view.
Although specific normal values or defined cutoffs for the MELDNa score in the context of right heart failure are currently unavailable, the MELDNa score has been utilized as a prognostic marker in various diseases, including triple valve surgery patients and heart failure patients listed for transplant (14, 37). Notably, the observed MELDNa threshold consistently hovered around 10 in these populations. This consistent threshold suggests that a MELDNa score above 10 may indicate a higher risk or severity of hepatorenal dysfunction. While our study focused on CTEPH patients, the utilization of a threshold comparable to those established in other disease contexts enhances the credibility of our findings.
From a clinical perspective, the MELDNa score and its change over time offer a simple way to assess hepatorenal dysfunction and identify patients at risk using easily obtainable laboratory parameters. The clinical management of those patients may then be improved by increasing the frequency of follow-up visits as well as starting treatment escalation, interventional treatment (balloon pulmonary angioplasty), or re-evaluation of eligibility for surgery (pulmonary endarterectomy). In contrast to other risk scores [such as the 2015 European Society of Cardiology/European Respiratory Society risk stratification (38), which has also been shown to predict survival in CTEPH (39, 40)], the simplicity of the MELDNa score allows for routine use worldwide in an ambulatory setting. However, further comparative studies are necessary to determine the most appropriate method of risk stratification in patients with CTEPH.
This study is limited by its retrospective design and missing data. In particular, the follow-up analysis is limited owing to missing data, which may have introduced a bias into the results. The use of the MELDNa score (rather than the MELD-XI score) necessitated the exclusion of 39 patients who used vitamin K antagonists (35% of the 111 patients with MELDNa data) from the primary analyses, which may also have been a source of bias. We chose to focus on the MELDNa score for the primary analyses because of the prognostic importance of hyponatremia (particularly severe hyponatremia) in pulmonary arterial hypertension (41, 42). DOAC use may also affect the MELDNa score. However, use of the INR for the MELDNa calculation may still be possible if the INR is determined at the DOAC trough level (43). As shown in the baseline characteristics, the frequency of DOAC use did not differ between the groups with and without hepatorenal dysfunction, and adjustment for DOAC use did not alter the prognostic significance of the MELDNa score, minimizing potential bias. Consistent with this, the MELD-XI score excluding INR also predicted survival in patients with CTEPH. Patients using drugs possibly affecting liver or kidney function were not excluded from the study, which may affect the results. Nevertheless, we can show that the MELDNa score can be used very well for routine follow-up in real-world patients with CTEPH.
In conclusion, our data indicate that hepatorenal dysfunction is associated with RA dysfunction and TR, and is a risk factor for mortality when assessed at baseline and during follow-up in patients with CTEPH (Figure 3). Thus, the MELDNa score can help to identify patients at risk. Further prospective studies are required to confirm whether considering the MELDNa score in treatment and risk stratification improves long-term survival in this specific PH population.
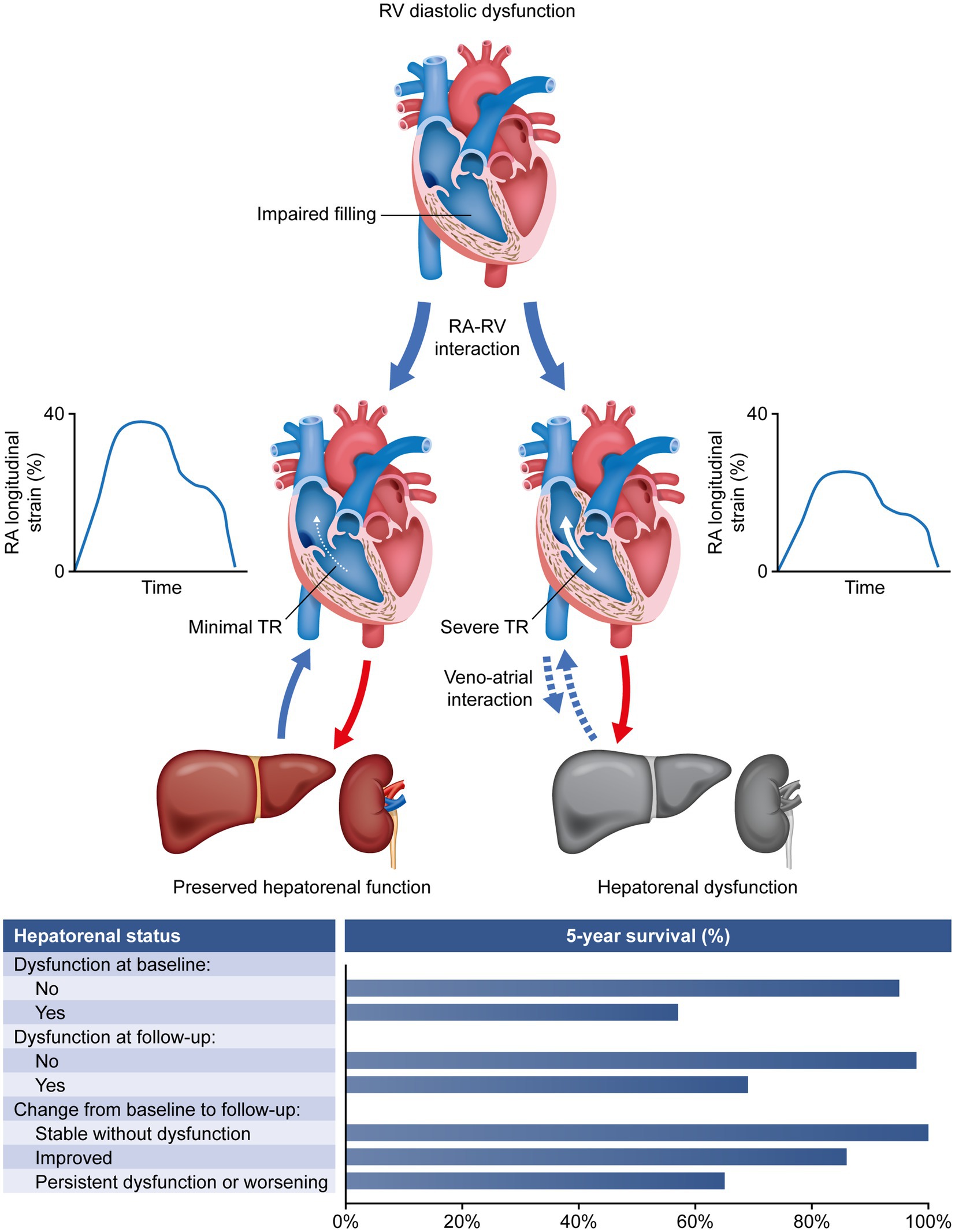
Figure 3. Association of hepatorenal dysfunction with RA dysfunction, TR, and mortality in patients with chronic thromboembolic pulmonary hypertension. RA, right atrial; RV, right ventricular; TR, tricuspid regurgitation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Local Ethics Committee of Giessen. The patients/participants provided their written informed consent to participate in this study.
Author contributions
AY, HG, and KT: study conceptualization. AY, DZ, MR, SS, ZR, NK, FG, WS, HoG, HeG, and KT: study design and data collection, critical revision of the manuscript, approval of manuscript for submission, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AY and HG: data analysis. AY, DZ, HG, and KT: drafting of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Excellence Cluster Cardio-Pulmonary System (ECCPS) and the Collaborative Research Center (SFB) 1213—Pulmonary Hypertension and Cor Pulmonale, grant number SFB1213/1, project B08 (German Research Foundation, Bonn, Germany).
Conflict of interest
AY reports non-financial support from the University of Giessen during the conduct of the study, and personal fees from MSD outside the submitted work. MR reports grants from the German Research Foundation and non-financial support from the University of Giessen during the conduct of the study, and grants from United Therapeutics, grants and personal fees from Bayer, and personal fees from Actelion, Mundipharma, Roche, and OMT outside the submitted work. FG reports no competing interests. WS reports grants from the German Research Foundation and non-financial support from the University of Giessen during the conduct of the study, and personal fees from Pfizer and Bayer Pharma AG outside the submitted work. HAG reports grants from the German Research Foundation and non-financial support from the University of Giessen during the conduct of the study, and personal fees from Bayer, Actelion, Pfizer, Merck, GSK, and Takeda, grants and personal fees from Novartis, Bayer HealthCare, and Encysive/Pfizer, and grants from Aires, the German Research Foundation, Excellence Cluster Cardiopulmonary Research, and the German Ministry for Education and Research outside the submitted work. HG reports grants from the German Research Foundation and non-financial support from the University of Giessen during the conduct of the study, and personal fees from Actelion, AstraZeneca, Bayer, BMS, GSK, Janssen-Cilag, Lilly, MSD, Novartis, OMT, Pfizer, and United Therapeutics outside the submitted work. KT reports grants from the German Research Foundation and non-financial support from the University of Giessen during the conduct of the study, and personal fees from Actelion and Bayer outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1207474/full#supplementary-material
Abbreviations
BNP, B-type natriuretic peptide; CI, confidence interval; CTEPH, chronic thromboembolic pulmonary hypertension; DOAC, direct oral anticoagulant; GLS, global longitudinal strain; HR, hazard ratio; INR, international normalized ratio; MELDNa, Model for End-stage Liver Disease Sodium; MELD-XI, Model for End-stage Liver Disease Excluding International Normalized Ratio; PH, pulmonary hypertension; RA, right atrial; RAP, right atrial pressure; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
References
1. Rako, ZA , Kremer, N , Yogeswaran, A , Richter, MJ , and Tello, K . Adaptive versus maladaptive right ventricular remodelling. ESC Heart Fail. (2022) 10:762–75. doi: 10.1002/ehf2.14233
2. Marston, N , Brown, JP , Olson, N , Auger, WR , Madani, MM , Wong, D, et al. Right ventricular strain before and after pulmonary thromboendarterectomy in patients with chronic thromboembolic pulmonary hypertension. Echocardiography. (2015) 32:1115–21. doi: 10.1111/echo.12812
3. Rehman, MB , Howard, LS , Christiaens, LP , Gill, D , Gibbs, JSR , and Nihoyannopoulos, P . Resting right ventricular function is associated with exercise performance in PAH, but not in CTEPH. Eur Heart J Cardiovasc Imaging. (2018) 19:185–92. doi: 10.1093/ehjci/jex0002
4. Peters, AC , Madhan, AS , Kislitsina, O , Elenbaas, C , Nishtala, A , Freed, B, et al. Temporal trends in right heart strain in patients undergoing pulmonary thromboendarterectomy for chronic thromboembolic pulmonary hypertension. Echocardiography. (2021) 38:1932–40. doi: 10.1111/echo.15246
5. Marcus, JT , Westerhof, BE , Groeneveldt, JA , Bogaard, HJ , de Man, FS , and Vonk, NA . Vena cava backflow and right ventricular stiffness in pulmonary arterial hypertension. Eur Respir J. (2019) 54:1900625. doi: 10.1183/13993003.00625-2019
6. Egbe, AC , Miranda, WR , Dearani, J , Kamath, PS , and Connolly, HM . Prognostic role of hepatorenal function indexes in patients with Ebstein anomaly. J Am Coll Cardiol. (2020) 76:2968–76. doi: 10.1016/j.jacc.2020.10.035
7. Yogeswaran, A , Tello, K , Lund, J , Klose, H , Harbaum, L , Sommer, N, et al. Risk assessment in pulmonary hypertension based on routinely measured laboratory parameters. J Heart Lung Transplant. (2022) 41:400–10. doi: 10.1016/j.healun.2021.10.018
8. Zymlinski, R , Ponikowski, P , and Biegus, J . Looking at the heart failure through the prism of liver dysfunction. Eur J Heart Fail. (2020) 22:1672–4. doi: 10.1002/ejhf.1932
9. Husain-Syed, F , Grone, HJ , Assmus, B , Bauer, P , Gall, H , Seeger, W, et al. Congestive nephropathy: a neglected entity? Proposal for diagnostic criteria and future perspectives. ESC Heart Fail. (2021) 8:183–203. doi: 10.1002/ehf2.13118
10. Fadel, BM , Alkalbani, A , Husain, A , Dahdouh, Z , and Di Salvo, G . Respiratory hemodynamics in the hepatic veins--abnormal patterns. Echocardiography. (2015) 32:705–10. doi: 10.1111/echo.12757
11. Husain-Syed, F , Birk, HW , Ronco, C , Schörmann, T , Tello, K , Richter, MJ, et al. Doppler-derived renal venous stasis index in the prognosis of right heart failure. J Am Heart Assoc. (2019) 8:e013584. doi: 10.1161/JAHA.119.013584
12. Kim, WR , Biggins, SW , Kremers, WK , Wiesner, RH , Kamath, PS , Benson, JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. (2008) 359:1018–26. doi: 10.1056/NEJMoa0801209
13. Stącel, T , Nęcki, M , Latos, M , Urlik, M , Antończyk, R , Kos, A, et al. Model for end-stage liver disease (MELD) score among patients qualified for lung transplantation with end-stage lung diseases with particular consideration of median pulmonary artery pressure. Transplant Proc. (2020) 52:2128–32. doi: 10.1016/j.transproceed.2020.03.043
14. Lim, K , Chow, SCY , Ho, JYK , Wan, S , Underwood, MJ , and Wong, RHL . Hepatorenal dysfunction predicts operative mortality after triple valve surgery: utility of MELD-Na. J Card Surg. (2021) 36:3112–8. doi: 10.1111/jocs.15745
15. Kim, MS , Kato, TS , Farr, M , Wu, C , Givens, RC , Collado, E, et al. Hepatic dysfunction in ambulatory patients with heart failure: application of the MELD scoring system for outcome prediction. J Am Coll Cardiol. (2013) 61:2253–61. doi: 10.1016/j.jacc.2012.12.056
16. Gall, H , Felix, JF , Schneck, FK , Milger, K , Sommer, N , Voswinckel, R, et al. The Giessen pulmonary hypertension registry: survival in pulmonary hypertension subgroups. J Heart Lung Transplant. (2017) 36:957–67. doi: 10.1016/j.healun.2017.02.016
17. Humbert, M , Kovacs, G , Hoeper, MM , Badagliacca, R , Berger, RMF , Brida, M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. (2022) 43:3618–731. doi: 10.1093/eurheartj/ehac237
18. Yogeswaran, A , Richter, MJ , Sommer, N , Ghofrani, HA , Seeger, W , Gall, H, et al. Evaluation of pulmonary hypertension by right heart catheterisation: does timing matter? Eur Respir J. (2020) 56:1901892. doi: 10.1183/13993003.01892-2019
19. Richter, MJ , Zedler, D , Berliner, D , Douschan, P , Gall, H , Ghofrani, HA, et al. Clinical relevance of right atrial functional response to treatment in pulmonary arterial hypertension. Front Cardiovasc Med. (2021) 8:775039. doi: 10.3389/fcvm.2021.775039
20. Rudski, LG , Lai, WW , Afilalo, J , Hua, L , Handschumacher, MD , Chandrasekaran, K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. (2010) 23:685–713. doi: 10.1016/j.echo.2010.05.010
21. Gall, H , Yogeswaran, A , Fuge, J , Sommer, N , Grimminger, F , Seeger, W, et al. Validity of echocardiographic tricuspid regurgitation gradient to screen for new definition of pulmonary hypertension. EClinicalMedicine. (2021) 34:100822. doi: 10.1016/j.eclinm.2021.100822
22. Hahn, RT , Thomas, JD , Khalique, OK , Cavalcante, JL , Praz, F , and Zoghbi, WA . Imaging assessment of tricuspid regurgitation severity. JACC Cardiovasc Imaging. (2019) 12:469–90. doi: 10.1016/j.jcmg.2018.07.033
23. Badano, LP , Kolias, TJ , Muraru, D , Abraham, TP , Aurigemma, G , Edvardsen, T, et al. Industry representatives; reviewers: this document was reviewed by members of the 2016–2018 EACVI scientific documents committee. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. (2018) 19:591–600. doi: 10.1093/ehjci/jey042
24. Heuman, DM , Mihas, AA , Habib, A , Gilles, HS , Stravitz, RT , Sanyal, AJ, et al. MELD-XI: a rational approach to "sickest first" liver transplantation in cirrhotic patients requiring anticoagulant therapy. Liver Transpl. (2007) 13:30–7. doi: 10.1002/lt.20906
25. Biegus, J , Zymlinski, R , Sokolski, M , Siwolowski, P , Gajewski, P , Nawrocka-Millward, S, et al. Impaired hepato-renal function defined by the MELD XI score as prognosticator in acute heart failure. Eur J Heart Fail. (2016) 18:1518–21. doi: 10.1002/ejhf.644
26. Biegus, J , Demissei, B , Postmus, D , Cotter, G , Davison, BA , Felker, GM, et al. Hepatorenal dysfunction identifies high-risk patients with acute heart failure: insights from the RELAX-AHF trial. ESC Heart Fail. (2019) 6:1188–98. doi: 10.1002/ehf2.12477
27. Samsky, MD , Patel, CB , DeWald, TA , Smith, AD , Felker, GM , Rogers, JG, et al. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol. (2013) 61:2397–405. doi: 10.1016/j.jacc.2013.03.042
28. Egbe, AC , Miranda, WR , Anderson, JH , Katta, RR , Goda, AY , Andi, K, et al. Determinants and prognostic implications of hepatorenal dysfunction in adults with congenital heart disease. Can J Cardiol. (2022) 38:1742–50. doi: 10.1016/j.cjca.2022.07.018
29. Xanthopoulos, A , Starling, RC , Kitai, T , and Triposkiadis, F . Heart failure and liver disease: Cardiohepatic interactions. JACC Heart Fail. (2019) 7:87–97. doi: 10.1016/j.jchf.2018.10.007
30. Rosenkranz, S , Howard, LS , Gomberg-Maitland, M , and Hoeper, MM . Systemic consequences of pulmonary hypertension and right-sided heart failure. Circulation. (2020) 141:678–93. doi: 10.1161/CIRCULATIONAHA.116.022362
31. Jönsson, S , Agic, MB , Narfström, F , Melville, JM , and Hultström, M . Renal neurohormonal regulation in heart failure decompensation. Am J Physiol Regul Integr Comp Physiol. (2014) 307:R493–7. doi: 10.1152/ajpregu.00178.2014
32. Rangaswami, J , Bhalla, V , Blair, JEA , Chang, TI , Costa, S , Lentine, KL, et al. McCullough PA; American Heart Association Council on the kidney in cardiovascular disease and council on clinical cardiology. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. (2019) 139:e840–78. doi: 10.1161/CIR.0000000000000664
33. Damman, K , van Deursen, VM , Navis, G , Voors, AA , van Veldhuisen, DJ , and Hillege, HL . Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. (2009) 53:582–8. doi: 10.1016/j.jacc.2008.08.080
34. Deferrari, G , Cipriani, A , and La Porta, E . Renal dysfunction in cardiovascular diseases and its consequences. J Nephrol. (2021) 34:137–53. doi: 10.1007/s40620-020-00842-w
35. Kussmaul, A , and Mt, S . Pericarditis and the paradox pulse. Berl Klin Wochenschr. (1873) 38:1873.
36. Biegus, J , Zymlinski, R , Sokolski, M , Nawrocka, S , Siwolowski, P , Szachniewicz, J, et al. Liver function tests in patients with acute heart failure. Pol Arch Med Wewn. (2012) 122:471–9. doi: 10.20452/pamw.1413
37. Szyguła-Jurkiewicz, B , Nadziakiewicz, P , Zakliczynski, M , Szczurek, W , Chraponski, J , Zembala, M, et al. Predictive value of hepatic and renal dysfunction based on the models for end-stage liver disease in patients with heart failure evaluated for heart transplant. Transplant Proc. (2016) 48:1756–60. doi: 10.1016/j.transproceed.2016.01.079
38. Galie, N , Humbert, M , Vachiery, JL , Gibbs, S , Lang, I , Torbicki, A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. (2015) 46:903–75. doi: 10.1183/13993003.01032-2015
39. Delcroix, M , Staehler, G , Gall, H , Grunig, E , Held, M , Halank, M, et al. Risk assessment in medically treated chronic thromboembolic pulmonary hypertension patients. Eur Respir J. (2018) 52:1800248. doi: 10.1183/13993003.00248-2018
40. Sandqvist, A , Kylhammar, D , Bartfay, SE , Hesselstrand, R , Hjalmarsson, C , Kavianipour, M, et al. Risk stratification in chronic thromboembolic pulmonary hypertension predicts survival. Scand Cardiovasc J. (2021) 55:43–9. doi: 10.1080/14017431.2020.1783456
41. Forfia, PR , Mathai, SC , Fisher, MR , Housten-Harris, T , Hemnes, AR , Champion, HC, et al. Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. (2008) 177:1364–9. doi: 10.1164/rccm.200712-1876OC
42. Rudkovskaia, AA , Tonelli, AR , Rao, Y , Hammel, JP , Buller, GK , Dweik, RA, et al. Is hyponatremia associated with mortality in pulmonary arterial hypertension? Pulm Circ. (2018) 8:2045894018776888. doi: 10.1177/2045894018776888
43. Bundesärztekammer. Neubekanntmachung der Richtlinie gem. § 16 Abs. 1 S. 1 Nrn. 2 und 5 TPG für die Wartelistenführung und Organvermittlung zur Lebertransplantation . (2023). Available at: https://www.bundesaerztekammer.de/fileadmin/user_upload/BAEK/Ueber_uns/Richtlinien_Leitlinien_Empfehlungen/RiliOrgaWlOvLeberTx20230912.pdf. Accessed March 16, 2023.
Keywords: pulmonary hypertension, chronic thromboembolic pulmonary hypertension, hepatorenal function, echocardiography, strain
Citation: Yogeswaran A, Zedler D, Richter MJ, Steinke S, Rako ZA, Kremer NC, Grimminger F, Seeger W, Ghofrani HA, Gall H and Tello K (2023) Hepatorenal dysfunction in patients with chronic thromboembolic pulmonary hypertension. Front. Med. 10:1207474. doi: 10.3389/fmed.2023.1207474
Edited by:
John-David Aubert, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandReviewed by:
Jan Biegus, Wroclaw Medical University, PolandPanagiota Xanthouli, Heidelberg University Hospital, Germany
Andreas Rolf, Kerckhoff Klinik, Germany
Copyright © 2023 Yogeswaran, Zedler, Richter, Steinke, Rako, Kremer, Grimminger, Seeger, Ghofrani, Gall and Tello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Athiththan Yogeswaran, QXRoaXRodGhhbi5Zb2dlc3dhcmFuQGlubmVyZS5tZWQudW5pLWdpZXNzZW4uZGU=
†These authors have contributed equally to this work
 Athiththan Yogeswaran
Athiththan Yogeswaran Daniel Zedler
Daniel Zedler Manuel J. Richter
Manuel J. Richter Sonja Steinke
Sonja Steinke Zvonimir A. Rako
Zvonimir A. Rako Nils C. Kremer
Nils C. Kremer Friedrich Grimminger
Friedrich Grimminger Werner Seeger
Werner Seeger Hossein Ardeschir Ghofrani1
Hossein Ardeschir Ghofrani1 Khodr Tello
Khodr Tello