
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 15 November 2023
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1205446
This article is part of the Research Topic Innovative Tuberculosis Case Finding Interventions: Lessons From the Field View all 15 articles
 Baolin Chen1,2†
Baolin Chen1,2† Yuxiang Bao1,2†
Yuxiang Bao1,2† Jun Chen3
Jun Chen3 Yunpu Zhang3
Yunpu Zhang3 Qifu Wen3
Qifu Wen3 Kai Wang4
Kai Wang4 Xiaoming Cheng1,2*
Xiaoming Cheng1,2* Junyuan Lv1,2,3*
Junyuan Lv1,2,3*Soft tissue tuberculosis is a rare extrapulmonary form of tuberculosis with limited experience in diagnosis and treatment. Soft tissue tuberculosis is an extrapulmonary infection with atypical clinical symptoms that can be easily misdiagnosed. In this article, we report a case of a female patient with isolated soft tissue tuberculosis who presented with a progressively enlarging subcutaneous mass as the primary symptom, and was suspected of having a subcutaneous lipoma after ultrasonography. A review of the literature revealed that soft tissue tuberculosis is insidious and mainly occurs in muscles and subcutaneous tissues. It was indicated by histopathology and qPCR testing for Mycobacterium tuberculosis complex. There is no standard treatment protocol for soft tissue tuberculosis, and a comprehensive regimen of surgical debridement of the lesion combined with chemotherapy can be used following the guidelines for treating extrapulmonary tuberculosis. Early diagnosis and standardized anti-tuberculosis treatment can significantly improve the prognosis of patients.
Tuberculosis is prevalent in underdeveloped and developing nations, including China. Tuberculosis infection occurs primarily in the lung, called pulmonary tuberculosis, but it can also attack other organs or sites outside the lungs causing extrapulmonary tuberculosis (1, 2). A Chinese tuberculosis study reported that extrapulmonary tuberculosis accounts for approximately 24.6% of tuberculosis cases, including the respiratory system (35.5% of extrapulmonary), musculoskeletal system (15.8%), and peripheral lymphatic system (15.8%) (3). Soft tissue tuberculosis is caused by the direct invasion of Mycobacterium tuberculosis or by the spread of tuberculosis lesions from other organs to soft tissues, such as muscles, tendons, and subcutaneous tissues via the bloodstream or lymphatic system. It may occur alone or in association with pulmonary tuberculosis.
Isolated soft tissue tuberculosis is relatively rare, accounting for only 1–2% of all cases of pulmonary and extrapulmonary tuberculosis (4). The clinical presentation of soft tissue tuberculosis is variable and can often be ignored by clinicians (5). Herein, we report a case of soft tissue tuberculosis of the forearm with progressive enlargement of a subcutaneous mass as the first symptom. We also review the relevant literature to discuss its clinical features and prognosis.
The patient was a 40-year-old female admitted to the hospital with a 2 years history of progressive enlargement in the right forearm. The patient had no cough, sputum, hypothermia, night sweats, dyspnea, chest tightness, or fatigue. Physical examination revealed a 6 cm × 3 cm mass was found on the right forearm with a soft texture, no tenderness, and normal skin temperature, mobility, and wrist joint movement. The patient denied any history of local tissue trauma and had no previous history of tuberculosis, immune disorders, tumors, or other systemic diseases.
Superficial ultrasonography of the forearm revealed an irregular mass measuring approximately 56.7 mm × 16.4 mm with unclear borders (Figure 1). CT examination of the chest showed no abnormality (Figure 2). The laboratory blood test revealed a hemoglobin level of 131 g/L, leukocytes of 5.13 × 109/L, neutrophil percentage of 70.6%, lymphocyte percentage of 19.4%, eosinophil percentage of 4.68%, and hematocrit of 20 mm/h. Liver and renal function tests were normal.
The lipoma diagnosis was established according to the examination results, and the mass was resected on August 12, 2022. The mass was observed intraoperatively as a cystic-solid mass with an incomplete covering, and the mass incision revealed grayish-white fluid with small grayish-white particles like fish eggs. Soft tissue tuberculosis of the right forearm was identified by postoperative pathology, which showed fibrocystic tissue with granulation tissue and granuloma formation, inflammatory cell infiltration (Figure 3A), and multinucleated giant cells in some areas (Figure 3B). Staining for acid fast bacilli were positive (Figure 3C) and the IS6110 gene was detected by quantitative real-time PCR (qPCR) using the Mycobacterium tuberculosis Complex kit (Cat.801176, Zeesan Biotech, Xiamen, China), the result showed positive amplification (Figure 4). However, there is no standardized treatment for soft tissue tuberculosis. Considering that the patient lives in a region with a high prevalence of isoniazid resistance (6). The Chinese guideline for diagnosis and treatment of pulmonary tuberculosis suggests that the 2HRZE/4HRE regimen be used in regions where new tuberculosis patients have high prevalence of resistance to isoniazid (7). Finally, we formulated a 2HRZE/4HRE anti-tuberculosis regimen for the patient. It included a 4-drug anti-tuberculosis treatment with isoniazid (H) 300 mg, rifampicin (R) 450 mg, ethambutol (E) 750 mg, and pyrazinamide (Z) 1,500 mg for 2 months, followed by isoniazid (H) 300 mg, rifampicin (R) 450 mg, and ethambutol (E) 750 mg for 4 months. Ten days after the surgery, the patient had good wound healing in the right forearm. After 6 months of anti-tuberculosis treatment follow-up, the patient’s swelling had completely disappeared and the appearance of the right hand was similar to that of the left hand.
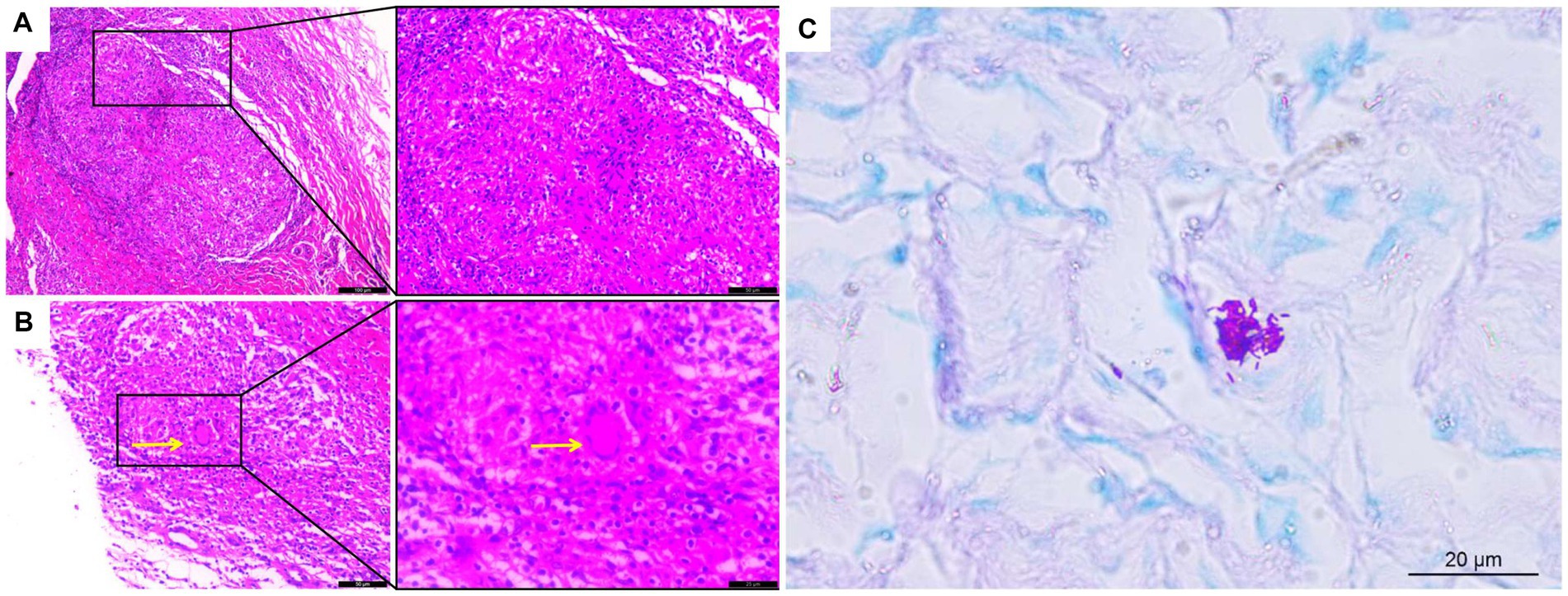
Figure 3. Histological analysis of soft tissue tuberculosis. (A) H&E staining showing granulomas with inflammatory cell infiltration and (B) multinucleated giant cells (×200); (C) Acid-fast bacillus staining was positive. Yellow arrows indicate multinucleated giant cells (×400).
We identified an additional 46 cases (40 articles) in the PubMed database from the construction of the database to the present. The medical subject heading (MESH) terms used were as follows: soft tissue tuberculosis, muscular tuberculosis, muscle tuberculosis, musculoskeletal tuberculosis and tubercular pyomyositis. Inclusion criteria: (1) soft tissue tuberculosis. Exclusion criteria: (1) duplicate publications and cohort study literature; (2) non-English language literature; and (3) undetailed case information. The literature was screened independently by 2 investigators according to the exclusion criteria. In case of disagreement, a senior physician in the relevant field was consulted.
A total of 40 eligible studies involving 46 patients with soft tissue tuberculosis were identified. The details of the pertinent cases described in the literature were compiled, including the patients’ general health status (age, sex, location, and medical history), clinical symptoms, site of onset, pathological detection methods, diagnostic techniques, and treatments (Table 1).
The 46 patients included 25 (54.3%) males and 21 (45.7%) females, with ages ranging from 9 to 85 years, including 41 (89.1%) patients aged >20 years. The cases were distributed as follows: 23 cases in Asian countries, 14 cases in African countries, 4 cases in American countries, 4 cases in European countries, and 1 case in Oceanian countries. The medical background ranged from 2 days to 10 years. Five patients had chronic renal failure, primary dry syndrome, Paget’s disease, SLE, and glucose phosphate dehydrogenase syndrome. Four of these patients received long-term steroids treatment. Eight patients had various degrees of weight loss, two had a history of surgery, two had a history of trauma, and five had a history of tuberculosis.
Of the 46 patients, 18 (39.1%) had local swelling or gradually enlarging masses as their primary clinical symptoms (16), 26 (56.5%) experienced various degrees of pain at the site of onset (19), 1 (2.2%) experienced significant motor dysfunction, and 1 (2.2%) had no apparent clinical manifestations. Weight loss, weakness, anorexia, fever, and night sweats were among the symptoms of tuberculosis toxicity in 10 (21.7%) patients (43). Thirty-eight patients (82.6%) had symptoms at a single site, 8 (17.4%) had symptoms at multiple sites, and 28 (60.8%) had lesions in the muscular areas of the extremity joints, including the thighs, calves, forearms, wrists, and feet. Fifteen patients (32.6%) had infections of the gluteus, thoracodorsal and iliopsoas muscles, with one patient having an abscess in the left psoas muscle involving the iliac crest and the left iliac flank, suggesting osteomyelitis. One patient had an interstitial cystic abscess with necrotic debris extending into the breast tissue. Two patients (4.3%) had a mixed axillary soft tissue lesion and one patient (2.2%) had a temporalis lesion. In overview, none of the individuals with tuberculosis infections in the extremities had combined limb dysfunction.
Clinical diagnosis of soft tissue tuberculosis is challenging and mostly depends on pathological biopsy and culture of Mycobacterium tuberculosis complex (48). In 45 of the 46 patients tuberculosis was diagnosed by histopathological analysis, the Mycobacterium tuberculosis complex culture and qPCR test. One patient received anti-tuberculosis therapy based on clinical features and anti-tuberculosis drug sensitivity data despite being negative on acid-fast bacillus staining and Mycobacterium tuberculosis complex culture (29). HE staining was used to stain the infected tissues, which under the microscope revealed typical caseous necrosis, granuloma development, and occasionally Langhans giant cells. Most patients underwent surgery, including puncture drainage, mass excision biopsy, and abscess dissection. The anti-tuberculosis regimen included a 2–4 months treatment with a combination of the first-line anti-tuberculosis drugs isoniazid (H), rifampicin (R), pyrazinamide (Z), and ethambutol (E), followed by consolidation therapy with isoniazid (H) and rifampicin (R) for 4–10 months. Forty-four patients were reported to have received anti-tuberculosis treatment. Three patients (6.5%) had the most prolonged treatment duration of 12 months, and 17 of the 46 patients (36.9%) were on a 2HRZE/4HR regimen. Early standardized anti-tuberculosis therapy significantly improved patients’ prognosis. After receiving anti-tuberculosis therapy, 43 of the 46 patients experienced improvements, two patients died from spontaneous cerebrovascular accidents, severe shock (9), multi-organ failure (19), and the prognosis for one patient was unclear (20).
The 2019 WHO report estimated a total of 10 million new cases of tuberculosis and 1.5 million tuberculosis-related deaths worldwide (49). Soft tissue tuberculosis is a rare form of extrapulmonary infection caused by Mycobacterium tuberculosis. It is most common in young and middle-aged people and slightly more frequent in males than females (50). According to the literature review, most patients (95.6%) had localized swelling or masses as their primary symptom, and more than half of patients (60.8%) had localized pain or tenderness. Soft tissue tuberculosis had the highest rate of involvement in the extremities (60.8%). However, fewer people (13.0%) developed symptoms of systemic tuberculosis toxicity.
The etiology of soft tissue tuberculosis remains unclear. Nonetheless, two main mechanisms of infection have been described: endogenous and exogenous. Endogenous tuberculosis results from the spread of infection through the lymphatic and blood circulation from tuberculous lesions in other body organs. Indeed, Mycobacterium tuberculosis can easily spread from distant organs via blood or lymph when the body is immunocompromised, such as in HIV infection, diabetes mellitus, renal failure, and long-term use of glucocorticoids (51). On the other hand, exogenous infection is the result of direct invasion of Mycobacterium tuberculosis. About a decade ago, there were case reports in China of tuberculosis bacteria multiplying around the injection site after Bacille Calmette-Guerin (BCG) vaccination, resulting in soft tissue tuberculosis (4). However, no comparable cases have been reported in recent years as a consequence of advances in vaccine preparation techniques in China. Similarly, in an early case report, a patient with tuberculosis suffered a traumatic injury to the buttocks, which resulted in the formation of a cold abscess and a diagnosis of tuberculosis. Thus, the hypothesis was proposed that latent Mycobacterium tuberculosis in the lung spreads to the gluteal muscles via the bloodstream (52). Obviously, the hypothesis has yet to be proven. Only 2 of the 46 patients reported in the literature had a history of trauma.
Of the 46 case studies analyzed in this study, 29 (63%) were diagnosed by surgical debridement or excisional tissue biopsy, and 17 (37%) were diagnosed by ultrasound or CT-guided puncture biopsy. Microscopic examination of pathological sections from 20 patients (43.5%) showed a significant lymphocytic, neutrophilic, or monocytic infiltration with caseous necrosis or granuloma formation and some multinucleated giant cells. All patients had Mycobacterium tuberculosis infection detected by one or more tests, including Gram staining, acid-fast bacilli smear, polymerase chain reaction (PCR), Xpert MTB/RIF test, T-SPOT test, next-generation sequencing (NGS), Mycobacterium tuberculosis tissue or puncture fluid culture, and direct Mycobacterium tuberculosis test (53). The qPCR or gene detection of Mycobacterium tuberculosis is essential for the diagnosis of soft tissue tuberculosis. On the other hand, the acid-fast bacilli smear is less sensitive for detecting Mycobacterium tuberculosis, usually 40% sensitive in extrapulmonary tuberculosis (54). This was also demonstrated in our analysis, where the positive rate of the acid-fast bacilli smear was only 33.3%. Due to the insidious nature of soft tissue tuberculosis symptoms and the low positive predictive value of associated tests, a combination of tests is often used for diagnosis in clinical practice (55). The diagnosis of soft tissue tuberculosis in the analyzed patients was based on pathological examination showing inflammatory cell infiltration, granuloma formation, multinucleated giant cells, positive staining for acid fast bacilli, and positive PCR for Mycobacterium tuberculosis.
Treatment of extrapulmonary tuberculosis consists mainly of lesion debridement and standardized anti-tuberculous drug therapy. Soft tissue tuberculosis with single-site involvement can be surgically excised. However, in patients with multi-site infections and abscess formation, surgical debridement, puncture, and drainage are often used to reduce the lesions and treat them with a sequential standardized anti-tuberculosis regimen (42). Anti-tuberculosis drug therapy is divided into an intensive and a consolidation phase, with emphasis on early treatment, appropriate dosage, regular and complete course, and combination therapy (56). A combination of at least 2 drugs is used to reduce drug resistance. The latest WHO guidelines recommend a 6 months 2HRZE/4HR regimen as the first-line treatment option for pulmonary tuberculosis without arbitrary extension of intensive therapy. Meanwhile, in areas with a high prevalence of isoniazid resistance, the WHO 2022 guideline recommends a 2HRZE/4HR regimen for newly diagnosed patients with extrapulmonary tuberculosis other than central nervous system, bone or joint tuberculosis (57). However, the 2018 edition of the Chinese guideline and the WHO 2010 guideline recommend a 2HRZE/4HRE regimen (7, 58). The literature review shown that 36.9% of patients on the standard 2HRZE/4HR regimen significantly improved. Considering that the patient lives in a region with a high prevalence of isoniazid resistance and the Chinese guideline, we formulated a 2HRZE/4HRE regimen for the patient. Although 2HRZE/4HRE is recommended for new patients with high levels of isoniazid resistance in both the WHO 2010 guideline and the Chinese guideline, the Chinese guideline does not emphasise that 10.3% of previously treated patients are resistant to ethambutol (compared with 2.5% of new patients), which would reduce the effectiveness of this therapy. This is probably why 2HRZE/4HRE remains the usual regimen in China. Regardless of the chosen optimization strategy, it should be noted that long-term use of anti-tuberculosis drugs can cause serious side effects in the internal organs, such as intestinal necrosis (46), hepatic and renal insufficiency (40). The case report and literature review advances our understanding of soft tissue tuberculosis and treatment methods and perform aggressive anti-tuberculosis treatment against drug-resistant Mycobacterium tuberculosis.
In conclusion, comprehensive treatment with surgical excision combined with anti-tuberculosis therapy should be provided for soft tissue tuberculosis. Tuberculosis drug resistance should be taken into account when developing a regimen. Moreover, the regimen should be based on the results of drug susceptibility testing whenever possible.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding authors.
The manuscript is a case report with a literature review. No human studies were carried out in preparing the manuscript. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JL and XC were involved in the conception and design of the work. JL revised the manuscript. JC, YZ, and QW collected the data. KW analyzed the histopathological figures. BC and YB prepared the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by Doctor Foundation of Affiliated Hospital of Zunyi Medical University (No. 201712).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pang, Y, An, J, Shu, W, Huo, F, Chu, N, Gao, M, et al. Epidemiology of extrapulmonary tuberculosis among in patients, China, 2008–2017. Emerg Infect Dis. (2019) 25:457–64. doi: 10.3201/eid2503.180572
2. Cukic, V, and Ustamujic, A. Extrapulmonary tuberculosis in federation of Bosnia and Herzegovina. Mater Sociomed. (2018) 30:153–6. doi: 10.5455/msm.2018.30.153-156
3. Li, T, Yan, X, Du, X, Huang, F, Wang, N, Ni, N, et al. Extrapulmonary tuberculosis in China: a national survey. Int J Infect Dis. (2023) 128:69–77. doi: 10.1016/j.ijid.2022.12.005
4. Gou, LJ, Su, JM, Zhao, Y, and Zhang, FC. Clinical analysis of 20 cases of muscular tuberculosis. Zhonghua Yi Xue Za Zhi. (2012) 92:206–8.
5. Sevgi, DY, Derin, O, Alpay, AS, Gündüz, A, Konuklar, AS, Bayraktar, B, et al. Extrapulmonary tuberculosis: 7 year-experience of a tertiary center in Istanbul. Eur J Intern Med. (2013) 24:864–7. doi: 10.1016/j.ejim.2013.08.704
6. Cao, Z, Lan, Y, Chen, L, Xiang, M, Peng, Z, Zhang, J, et al. Resistance to first-line antituberculosis drugs and prevalence of pnca mutations in clinical isolates of Mycobacterium tuberculosis from Zunyi, Guizhou province of China. Infect Drug Resist. (2019) 12:3093–102. doi: 10.2147/idr.s222943
7. Chinese Medical Association, Medical Journals Publishing House, Chinese Society of General Practice, et al. Guideline for primary care of pulmonary tuberculosis (2018). Chin J Gen Pract. (2019) 18:709–17. doi: 10.3760/cma.j.issn.1671-7368.2019.08.002
8. Franco-Paredes, C, and Blumberg, HM. Psoas muscle abscess caused by Mycobacterium tuberculosis and staphylococcus aureus: case report and review. Am J Med Sci. (2001) 321:415–7. doi: 10.1097/00000441-200106000-00008
9. Ergin, F, Arslan, H, Bilezikçi, B, Ağildere, AM, and Ozdemir, N. Primary tuberculosis in the gluteal muscle of a patient with chronic renal failure. A rare presentation. Nephron. (2001) 89:463–6. doi: 10.1159/000046122
10. Haq, I, Moss, K, and Morris, VH. Myalgia with lymphadenopathy. J R Soc Med. (2001) 94:521–2. doi: 10.1177/014107680109401008
11. Chu, CK, Yang, TL, and Tan, CT. Tuberculous pyomyositis of the temporal muscle in a nonimmunocompromised woman: diagnosis by sonography. J Laryngol Otol. (2004) 118:59–61. doi: 10.1258/002221504322731673
12. Tanomkiat, W, and Buranapanitkit, B. Percutaneous drainage of large tuberculous iliopsoas abscess via a subinguinal approach: a report of two cases. J Orthop Sci. (2004) 9:157–61. doi: 10.1007/s00776-003-0760-5
13. Winzer, KJ, Menenakos, C, Braumann, C, Mueller, JM, and Guski, H. Breast mass due to pectoral muscle tuberculosis mimicking breast cancer in a male patient. Int J Infect Dis. (2005) 9:176–7. doi: 10.1016/j.ijid.2004.07.007
14. Rajapakse, CD, and Shingadia, D. Tuberculous pyomyositis of the left quadratus lumborum. Arch Dis Child. (2006) 91:512. doi: 10.1136/adc.2005.090431
15. Trikha, V, Varshney, MK, and Rastogi, S. Isolated tuberculosis of the vastus lateralis muscle: a case report. Scand J Infect Dis. (2006) 38:304–6. doi: 10.1080/00365540500353267
16. Gottschalk, A, Danz, B, and Volk, M. Multifocal soft tissue tuberculosis in a patient without acute pulmonary involvement. Rofo. (2006) 178:640–2. doi: 10.1055/s-2006-926683
17. Khosrovaneh, A, Camero, LG, Briski, LE, and Khatib, R. Chest wall soft tissue tuberculosis: a protracted course over a 10-year period. Scand J Infect Dis. (2006) 38:129–30. doi: 10.1080/00365540500277243
18. Sabat, D, and Kumar, V. Primary tuberculous abscess of rectus femoris muscle: a case report. J Infect Dev Ctries. (2009) 3:476–8. doi: 10.3855/jidc.421
19. Huang, CC, Liu, MF, Lee, NY, Chang, CM, Lee, HC, Wu, CJ, et al. Fatal tuberculous myositis in an immunocompromised adult with primary Sjögren's syndrome. J Formos Med Assoc. (2010) 109:680–3. doi: 10.1016/s0929-6646(10)60110-6
20. Perez-Alonso, AJ, Husein-Elahmed, H, Duran, CP, Caballero-Marcos, L, and Ramon, JA. Isolated muscle tuberculosis. Med Mal Infect. (2011) 41:559–60. doi: 10.1016/j.medmal.2011.05.002
21. Arora, S, Sabat, D, Sural, S, and Dhal, A. Isolated tuberculous pyomyositis of semimembranosus and adductor magnus: a case report. Orthop Surg. (2012) 4:266–8. doi: 10.1111/os.12011
22. Shields, DW, and Robinson, PG. Iliopsoas abscess masquerading as “sciatica”. BMJ Case Rep. (2012) 2012:bcr2012007419. doi: 10.1136/bcr-2012-007419
23. Elshafie, KT, Al-Hinai, MM, Al-Habsi, HA, Al-Hattali, MS, Hassan, O, and Al-Sukaiti, R. A massive tuberculosis abscess at the erector spinae muscles and subcutaneous tissues in a young man. Sultan Qaboos Univ Med J. (2013) 13:601–5. doi: 10.12816/0003325
24. Lee, HJ, Kim, KW, Kim, KS, Ryu, SH, and Ha, YC. Primary musculoskeletal mycobacterium infection with large cystic masses after total hip arthroplasty. J Arthroplast. (2013) 28:374.e1–3. doi: 10.1016/j.arth.2012.05.009
25. Neogi, DS, Bandekar, SM, and Chawla, L. Skeletal muscle tuberculosis simultaneously involving multiple sites. J Pediatr Orthop B. (2013) 22:167–9. doi: 10.1097/BPB.0b013e328354b04d
26. Sökücü, S, Sökücü, SN, Kabukçuoglu, Y, and Kabukçuoglu, F. Primary skeletal muscle tuberculosis at an unusual site. J Pak Med Assoc. (2013) 63:126–8.
27. Lai, KL, Shi, ZY, Chao, WC, Liu, PY, and Wu, LH. Tuberculous muscle abscess. QJM. (2014) 107:683–4. doi: 10.1093/qjmed/hcu011
28. Dhakal, AK, Shah, SC, Shrestha, D, Banepali, N, and Geetika, KC. Tuberculosis presenting as multiple intramuscular nodules in a child: a case report. J Med Case Rep. (2015) 9:72. doi: 10.1186/s13256-015-0543-6
29. Lombardi, R, Pelusi, S, Airaghi, L, and Fargion, S. Extrapulmonary tuberculosis: an unusual presentation in an immunocompetent patient. BMJ Case Rep. (2015) 2015:bcr2014207146. doi: 10.1136/bcr-2014-207146
30. Meena, M, Dixit, R, Samaria, JK, and Vijayakandeepan Kumaresan, SH. Tuberculosis of the triceps muscle. BMJ Case Rep. (2015) 2015:bcr2014207032. doi: 10.1136/bcr-2014-207032
31. Grigorakos, L, Sgountzos, V, Lazarescu, D, Simopoulou, S, Gkouni, M, Markou, N, et al. Primary thoracic muscle tuberculosis: two case reports. J Med Case Rep. (2016) 10:229. doi: 10.1186/s13256-016-0996-2
32. Kotecha, D, Sardar, M, and Latimer, MD. Tuberculosis presenting as a “swollen calf”. BMJ Case Rep. (2016) 2016:bcr2016216340. doi: 10.1136/bcr-2016-216340
33. Sbai, MA, Benzarti, S, Msek, H, Boussen, M, and Khorbi, A. Pseudotumoral form of soft-tissue tuberculosis of the wrist. Int J Mycobacteriol. (2016) 5:99–101. doi: 10.1016/j.ijmyco.2015.08.001
34. Al-Khazraji, A, Takher, J, Alkhawam, H, and Fabbri, M. Primary tuberculous pyomyositis of the calf muscles. Am J Med Sci. (2017) 353:187–8. doi: 10.1016/j.amjms.2016.05.010
35. Sbai, MA, Benzarti, S, Chalbi, E, Msek, H, and Khorbi, A. Pseudotumoral form of soft tissue tuberculosis of the hand: six cases. Pan Afr Med J. (2016) 25:178. doi: 10.11604/pamj.2016.25.178.8918
36. Alaya, Z, and Osman, W. Isolated muscular tuberculosis: unusual location of the Koch bacillus. Pan Afr Med J. (2017) 26:158. doi: 10.11604/pamj.2017.26.158.11795
37. Fataki, CM, Kasmy, Z, Sahrourdi, S, Raghani, A, Rhars, A, Frikh, M, et al. Primary tuberculous abscess and pyogenic psoas abscess: an uncommon association. Pan Afr Med J. (2017) 28:280. doi: 10.11604/pamj.2017.28.280.13796
38. Hayoun, S, Ouazzani, HE, Habibi, B, Belhabib, S, Souhi, H, Rhorfi, IA, et al. Swelling of the pectoralis muscle revealing isolated muscular tuberculosis. Pan Afr Med J. (2017) 27:44. doi: 10.11604/pamj.2017.27.44.12419
39. Manicketh, I, Panjwani, P, Ravikumar, G, and Prince, ML. Soft tissue tuberculosis – an unusual presentation of a common disease. Indian J Tuberc. (2018) 65:96–7. doi: 10.1016/j.ijtb.2017.04.002
40. Hashimoto, K, Nishimura, S, Oka, N, Kakinoki, R, and Akagi, M. Tuberculoma with phlegmon-like symptoms mimicking soft tissue sarcoma in the wrist: a case report. Mol Clin Oncol. (2018) 9:207–10. doi: 10.3892/mco.2018.1652
41. Moyano-Bueno, D, Blanco, JF, Lopez-Bernus, A, Gutierrez-Zubiaurre, N, Gomez Ruiz, V, Velasco-Tirado, V, et al. Cold abscess of the chest wall: a diagnostic challenge. Int J Infect Dis. (2019) 85:108–10. doi: 10.1016/j.ijid.2019.05.031
42. Zeng, Y, Liu, Y, Xie, Y, Liang, J, Kuang, J, Lu, Z, et al. Muscular tuberculosis: a new case and a review of the literature. Front Neurol. (2019) 10:1031. doi: 10.3389/fneur.2019.01031
43. Murugesh Anand, S, Edwin Fernando, M, Srinivasaprasad, ND, Sujit, S, and Thirumalvalavan, K. Tuberculous myositis and cellulitis in a renal transplant recipient. Indian J Tuberc. (2020) 67:353–6. doi: 10.1016/j.ijtb.2019.04.010
44. Fahad, S, Baloch, N, and Din, NU. Tuberculosis of the flexor carpi radialis muscle – a case report. J Pak Med Assoc. (2020) 70:1645–7. doi: 10.5455/JPMA.40799
45. Othman, SA, Elsharkawy, TM, Alfaifi, D, and Aljehani, Y. The effectiveness of vacuum-assisted closure device in managing intramuscular tuberculosis. Adv Skin Wound Care. (2021) 34:330–3. doi: 10.1097/01.ASW.0000744328.95568.20
46. Mohandes, AF, Karam, B, Alrstom, A, Alasadi, L, Rajab Bek, MW, Daher, N, et al. Primary psoas tuberculosis abscess with an iliac bone lytic lesion: a case report. J Med Case Rep. (2022) 16:209. doi: 10.1186/s13256-022-03417-4
47. He, YG, Huang, YH, Yi, XL, Qian, KL, Wang, Y, Cheng, H, et al. Soft tissue tuberculosis detected by next-generation sequencing: a case report and review of literature. World J Clin Cases. (2023) 11:709–18. doi: 10.12998/wjcc.v11.i3.709
48. Norbis, L, Alagna, R, Tortoli, E, Codecasa, LR, Migliori, GB, and Cirillo, DM. Challenges and perspectives in the diagnosis of extrapulmonary tuberculosis. Expert Rev Anti-Infect Ther. (2014) 12:633–47. doi: 10.1586/14787210.2014.899900
49. Chakaya, J, Khan, M, Ntoumi, F, Aklillu, E, Fatima, R, Mwaba, P, et al. Global tuberculosis report 2020 – reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis. (2021) 113:S7–S12. doi: 10.1016/j.ijid.2021.02.107
50. Kang, W, Yu, J, Du, J, Yang, S, Chen, H, Liu, J, et al. The epidemiology of extrapulmonary tuberculosis in China: a large-scale multi-center observational study. PLoS One. (2020) 15:e0237753. doi: 10.1371/journal.pone.0237753
51. Qian, X, Nguyen, DT, Lyu, J, Albers, AE, Bi, X, and Graviss, EA. Risk factors for extrapulmonary dissemination of tuberculosis and associated mortality during treatment for extrapulmonary tuberculosis. Emerg Microbes Infect. (2018) 7:102–14. doi: 10.1038/s41426-018-0106-1
52. Bai, CH, and Zhang, RM. Skeletal muscle tuberculosis:a case report and a review of literature. J Logist Univ PAPF. (2014) 23:685–7. doi: 10.3969/j.issn.2095-3720.2014.08.017
53. Saktiawati, AMI, Putera, DD, Setyawan, A, Mahendradhata, Y, and van der Werf, TS. Diagnosis of tuberculosis through breath test: a systematic review. EBioMedicine. (2019) 46:202–14. doi: 10.1016/j.ebiom.2019.07.056
54. Njau, AN, Gakinya, SM, Sayed, S, and Moloo, Z. Xpert® MTB/RIF assay on formalin-fixed paraffin-embedded tissues in the diagnosis of extrapulmonary tuberculosis. Afr J Lab Med. (2019) 8:748. doi: 10.4102/ajlm.v8i1.748
55. Plourde, AR, Hall, CR, and Mcelvania, E. The brief case: a real pain in the testicle-a case of extrapulmonary Mycobacterium tuberculosis. J Clin Microbiol. (2022) 60:e0060221. doi: 10.1128/jcm.00602-21
56. Golden, MP, and Vikram, HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. (2005) 72:1761–8.
57. World Health Organization. Consolidated guidelines on tuberculosis: module 4: treatment – drug-susceptible tuberculosis treatment World health Organization (2022). Available at: https://iris.who.inthandle/10665/353829
58. World Health Organization & World Health Organization. Treatment of tuberculosis: guidelines. 4th ed World Health Organization (2010) Available at: https://apps.who.int/iris/handle/10665/44165.
Keywords: soft tissue tuberculosis, extrapulmonary, diagnosis, treatment, case report
Citation: Chen B, Bao Y, Chen J, Zhang Y, Wen Q, Wang K, Cheng X and Lv J (2023) Isolated soft tissue tuberculosis: a case report and literature review. Front. Med. 10:1205446. doi: 10.3389/fmed.2023.1205446
Received: 13 April 2023; Accepted: 31 October 2023;
Published: 15 November 2023.
Edited by:
Daniel Yilma Bogale, Jimma University, EthiopiaReviewed by:
Rizaldy Taslim Pinzon, Duta Wacana Christian University, IndonesiaCopyright © 2023 Chen, Bao, Chen, Zhang, Wen, Wang, Cheng and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoming Cheng, Y3htMTY4OEBzaW5hLmNvbQ==; Junyuan Lv, anVueXVhbmx2QHptdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.