
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med., 31 July 2023
Sec. Translational Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1200947
This article is part of the Research TopicNuclear Hormone Receptors in Cancer and Targeted TherapyView all 6 articles
 Cassandra L. Moyer1
Cassandra L. Moyer1 Powel H. Brown1,2*
Powel H. Brown1,2*Advancements in research have led to the steady decline of breast cancer mortality over the past thirty years. However, breast cancer incidence has continued to rise, resulting in an undue burden on healthcare costs and highlighting a great need for more effective breast cancer prevention strategies, including targeted chemo preventative agents. Efforts to understand the etiology of breast cancer have uncovered important roles for nuclear receptors in the development and progression of breast cancer. Targeted therapies to inhibit estrogen receptor (ER) and progesterone receptor (PR) signaling (selective ER modulators, aromatase inhibitors and selective PR modulators) have shown great promise for the treatment and prevention of hormone receptor (HR)-positive breast cancer. However, these drugs do not prevent HR-negative disease. Therefore, recent efforts have focused on novel targeted therapies with the potential to prevent both HR-positive and HR-negative breast cancer. Among these include drugs that target other nuclear receptors, such as retinoic acid receptor (RAR), retinoid X receptor (RXR) and vitamin D receptor (VDR). In this review we provide an overview of recent preclinical and clinical trials targeting members of the nuclear receptor superfamily for the prevention of breast cancer.
In the last 10 years, the incidence of breast cancer in the United States has steadily increased, with more than 280,000 women expected to be diagnosed in 2023. Despite efficacious early detection measures and significant advances in treatment, breast cancer remains the most common cancer diagnosis and second leading cause of cancer death in women, with over 40,000 patients succumbing to the disease each year (1). Breast cancer is highly heterogenous with varied molecular features and can be classified into subtypes based on the expression of common biomarkers that are known to drive disease progression. These include nuclear estrogen receptor (ER), progesterone receptor (PR) and surface membrane bound human epidermal growth factor receptor 2 (HER2), with 85–90% of breast cancer overexpressing one or more of these receptors (1, 2). It is well established that inhibition of these receptors can halt the progression of BC, leading to the approval of several highly effective breast cancer targeted therapies against ER and HER2.
Endocrine therapies, targeting ER directly or the production of excess estrogen, are commonly used in the adjuvant setting for ER-positive early-stage breast cancer and for the treatment of advanced or metastatic disease in combination with other targeted therapies (3). Due to the development of ER-targeted therapies, women with hormone receptor positive cancer continue to have the best overall survival, even when diagnosed at later stages (4). Similarly, anti-HER2 monoclonal antibodies, tyrosine kinase inhibitors and the recently developed anti-HER2 antibody drug conjugates have shown tremendous success in the treatment of HER2-amplified primary and metastatic breast cancer (5–10). Considering their success, these targeted therapies have also been explored for the prevention of primary breast cancer and recurrence, initially with ER targeted drugs and then aromatase inhibitors. Even more recently, the development of HER2 targeted vaccines have shown promise in preclinical studies to reduce the recurrence of HER2-amplified breast cancer (11, 12).
However, 10–15% of breast cancer patients have tumors that lack ER, PR and HER2 expression, and do not respond to endocrine or anti-HER2 therapies. These triple negative breast cancers (TNBC) appear more frequently in young women (<40 years of age), non-Hispanic black women and women carrying mutations in BRCA1/2 (1, 2). TNBC is more aggressive than hormone receptor positive cancer, and without the same effective targeted therapies available, TNBC patients have poor overall survival (4). Until recently, the standard of care for TNBC has been restricted to chemotherapy, despite the limited benefit particularly in the metastatic setting. However, recent discoveries in breast cancer biology have identified therapeutic molecular targets within TNBC subtypes, including PARP inhibitors for the treatment of women with BRCA mutant breast cancers and immune checkpoint inhibitors for PD-L1-positive advanced disease (13–20). These targeted therapies are currently being tested for the prevention of breast cancer in BRCA mutant and ER-negative preclinical models. However, few patients have BRCA mutant or PD-L1-positive tumors so there maintains an urgent need for novel treatments and preventative agents for high-risk women.
At present, the most effective primary prevention strategy for breast cancer is prophylactic surgery, consisting of both bilateral mastectomy and oophorectomy, which can reduce the risk of breast cancer by 90% (21–23). However, the highly invasive and irreversible nature of these procedures are undesirable, and their use has been limited to only women with hereditary breast cancer syndromes or other high-risk factors. For this reason, preventative agents targeting essential pathways for breast cancer carcinogenesis have been extensively explored. The term chemoprevention was first coined by Michael Sporn, specifically in the context of targeting nuclear receptors with vitamin A or synthetic analogs of vitamin A (retinoids) to prevent chemically induced carcinogenesis (24). He defined chemoprevention more broadly as the ability to inhibit cancer formation using natural or synthetic pharmacological agents, but the idea of targeting nuclear receptors for cancer prevention continues to be greatly considered.
The human nuclear receptor superfamily includes 48 evolutionarily conserved transcription factors that recognize and respond to changes in physiological stimuli, such as steroid hormones, cholesterol metabolites and lipophilic vitamins (25). These receptors can be grouped into hormone receptors (both steroid and non-steroid), with known endogenous ligands, and orphan receptors, without known endogenous ligands, usually requiring heterodimerization with another receptor for transcriptional activation. In the context of breast cancer, several groups have shown that the expression patterns of nuclear receptors can discriminate subtypes, histological grade and even predict treatment response (26, 27). In this review, we will discuss the recent pre-clinical and clinical trials targeting nuclear hormone receptors for the prevention of breast cancer.
Steroid hormone receptors play a critical role in normal breast development as well as the initiation and progression of breast cancer. These receptors include the estrogen receptor (ER), progesterone receptor (PR), glucocorticoid receptor (GR), androgen receptor (AR), and mineralocorticoid receptor (MR) which primarily act as homodimers for transcription regulation. In the classical mode of genomic action, these receptors are inactive in the cytoplasm without ligand, bound to heat shock proteins for stability. Upon exposure to a ligand, the receptors dimerize and translocate to the nucleus where they interact with co-activators and co-repressors (often determined by the type of ligand) and bind specific responsive elements on DNA to activate or repress target gene transcription (28). However, it is also worth noting that hormone receptor signaling can also be activated in a non-classical, ligand-independent manner, such as post-translational phosphorylation by erroneously hyperactive kinases like mitogen-activated protein kinase (MAPK) (29).
During breast cancer carcinogenesis, steroid hormone receptors often become over or under expressed, resulting in the dysregulation of gene expression that can drive tumorigenesis (26, 30). It has long been accepted that the expression of ER and PR are clinically significant as predictors of breast cancer outcome and useful for determining therapeutic strategies (31). Despite the known ligand-independent actions of nuclear receptors, endocrine therapy targeting ligand-dependent ER oncogenic signaling remains the most widely used targeted therapy in breast cancer treatment. More so, many pre-clinical studies and clinical trials have shown that anti-estrogens and anti-progestins can delay or inhibit the formation of breast cancer when used as a preventative therapy.
More than a century ago, it was first noted that advanced, inoperable breast tumors can shrink after the removal of the ovaries, becoming the first reported use of endocrine therapy (32). The mechanism of action was later revealed that estrogen produced by the ovaries can stimulate tumor growth through the overexpression of nuclear ER in breast cancer (33). We now know that an estimated 70–80% of breast cancer is driven by ER signaling (1, 2). When bound to the endogenous ligand estrogen, ER drives tumor proliferation through the activation of direct target genes and upregulation of signaling pathways. It is also known that ER plays a pro-tumorigenic role in the migration and invasion of breast cancer, by stimulating signaling pathways that enhance actin cytoskeleton remodeling and filopodia structure formation (34). Therefore, targeting ER to suppress the hyper-active estrogen signaling pathway has been a highly effective treatment and prevention strategy for ER-positive breast cancer (Figure 1).
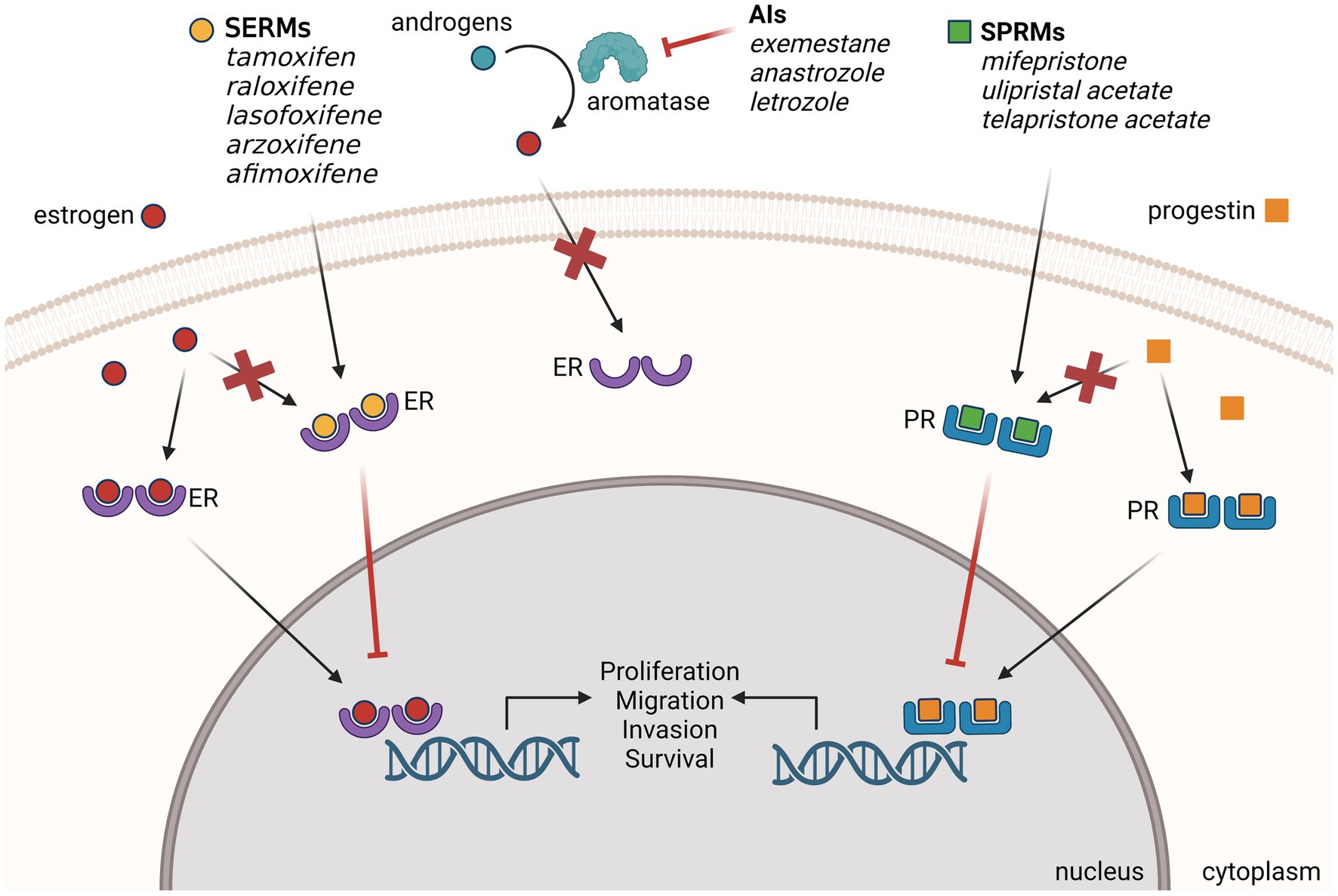
Figure 1. Endocrine therapies used for the prevention of ER-positive BC. Selective estrogen receptor modulators (SERMs), aromatase inhibitors (AIs) and selective progesterone receptor modulators (SPRMs) have demonstrated preclinical and clinical efficacy for the prevention of primary and recurrent breast cancer. SPRMs compete with estrogen to bind ER and block the estrogen signaling that drives breast cancer formation. AIs block estrogen signaling by inhibiting the enzyme aromatase from converting androgen to excess estrogen. SPRMs compete with progestins to bind PR and modulate progesterone signaling. Created with BioRender.com.
Several generations of selective estrogen receptor modulators (SERMs) have been developed as breast specific ER antagonists with varying effects in other tissues, most notably as ER agonists in the bone and uterus (35). Tamoxifen, a first-generation SERM, has been successfully used for several decades for the treatment of ER-positive breast cancer at all stages, in both premenopausal and postmenopausal women. Early clinical trials for the treatment of breast cancer with tamoxifen found a reduction in contralateral breast cancer (36–38), leading to the development of clinical trials with SERMs for the prevention of breast cancer. In a decisive meta-analysis of 20 different clinical trials with 15-years of follow-up, it was found that adjuvant tamoxifen use in women with early-stage breast cancer reduced the risk of ER-positive contralateral breast cancer and recurrence by nearly 50% but had no effect on ER-negative breast cancer recurrence (39).
Ductal carcinoma in situ (DCIS) is considered a non-invasive breast cancer that has been shown to increase the risk for invasive breast cancer (40). DCIS accounts for 20% of all newly diagnosed breast cancers and breast conserving surgery to remove the non-invasive lesions is often the treatment of choice. Due to its common diagnosis among women and higher risk for invasive breast cancer, several clinical trials have explored the ability of tamoxifen to prevent invasive breast cancer in women with DCIS. Initial results and long term follow up of the UK/ANZ DCIS and NASBP B-24 trials both demonstrated that tamoxifen treatment after local excision could reduce the incidence of new breast events and contralateral tumors (41–44). These trials are summarized in Table 1.
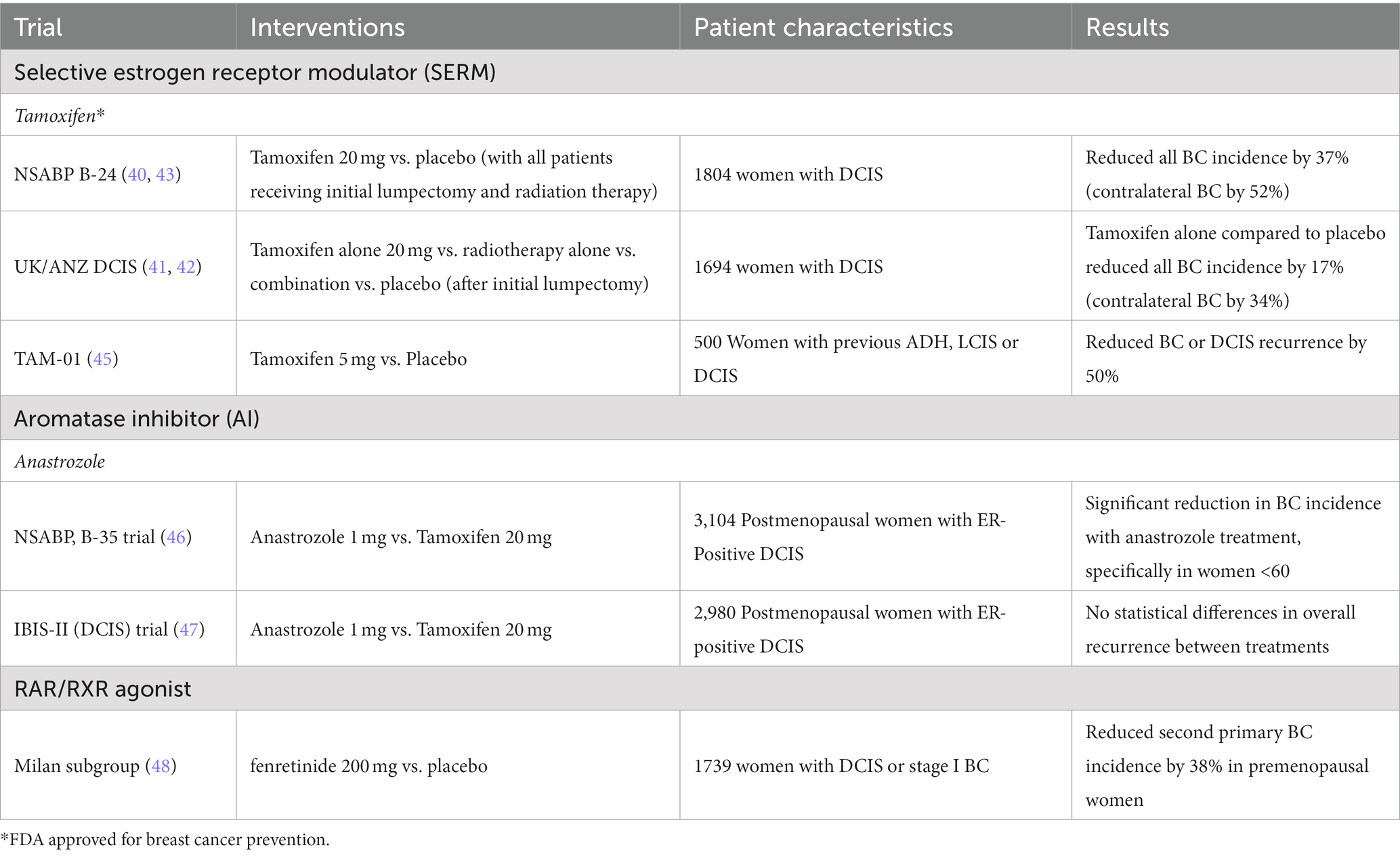
Table 1. Clinical trials targeting ER for DCIS recurrence and invasive breast cancer (BC) prevention.
Four landmark phase III prevention trials, with extensive follow-up of data, have demonstrated that tamoxifen also reduces the incidence of primary ER-positive breast cancer in normal and high-risk women by 35–70% (49–56). These trials have been discussed at length in previous reviews (57, 58) and have been summarized in Table 2. Based on the initial results of the NSABP Breast Cancer Prevention Trial (49) and the other tamoxifen prevention trials (50–52), tamoxifen was FDA-approved for breast cancer risk reduction in pre- and post-menopausal women at increased risk of breast cancer and remains the only preventative agent approved for breast cancer prevention in premenopausal women. Despite the promising success of tamoxifen for the prevention of ER-positive BC, these long-term follow-up studies have documented rare adverse events that warrant caution for use. Most notably, all studies reported that tamoxifen use had common side effects of intensified vasomotor symptoms with increased risk for rare but serious adverse events like thrombosis, pulmonary embolism, cataracts and uterine cancer. Due to concerns about these side effects, many women at high risk of breast cancer often decline to use tamoxifen for breast cancer prevention.
To minimize the adverse events associated with endocrine therapy, second and third generation SERMs have been developed. In several phase III prevention trials for osteoporotic women (MORE, CORE and RUTH trials), the second generation SERM raloxifene was found to reduce the risk of ER-positive breast cancer by 55–84%, without increasing the incidence of endometrial cancer (60–63). However, just as with tamoxifen, these studies reported a significant increased incidence of thrombosis with raloxifene. A subsequent Phase III breast cancer prevention trial, the STAR trial, directly compared the efficacy of tamoxifen and raloxifene for preventing breast cancer in postmenopausal women considered high-risk by the Gail Model for breast cancer risk assessment. This trial initially demonstrated that raloxifene was equally effective as tamoxifen in preventing breast cancer and that it had fewer side effects [fewer hot flushes, thromboses, and no increase in uterine cancer (64, 69)]. These results led raloxifene to also be FDA-approved for breast cancer risk reduction in post-menopausal women. On longer follow-up, raloxifene was found to be slightly less effective than tamoxifen at preventing breast cancer [85% as effective as tamoxifen (65, 70)].
Several phase III prevention trials have explored the use of third generation SERMs for osteoporosis risk reduction in postmenopausal women. In the PEARL trial, lasofoxifene was shown to reduce ER-positive breast cancer by 83% with even fewer reported toxicities than tamoxifen or raloxifene (66). However, this study was limited by low breast cancer incidence and short-term follow-up, lacking sufficient data on long-term benefits or safety, and thus, FDA-approval for breast cancer prevention has not been sought. Similarly, the Generations trial investigating the effects of arzoxifene demonstrated a 70% reduction in ER-positive breast cancer with an increased incidence of thromboembolism and vasomotor symptoms (71). Neither lasofoxifene nor arzoxifene have been FDA approved for the prevention of BC.
More recently, the third generation SERM bazedoxifene in combination with conjugated estrogen (in the drug Duavee) has shown potential for breast cancer prevention. In a pilot study including 28 women at high-risk for breast cancer (non-BRCA1/2 mutation carriers with breast cancer risk of at least twice the average for age group by models of assessment), 6-months of treatment with Duavee significantly reduced mammographic density, proliferation (as assessed by staining for the Ki-67 proliferation marker) and additional breast cancer risk biomarkers while improving menopause-associated symptoms (72). These results supported the development of an ongoing phase IIB trial that will be completed in 2026 (NCT04821141). Another ongoing trial, the PROMISE Study trial is investigating the effects of Duavee on breast cell proliferation in women with ER-positive DCIS, with results expected in 2024 (NCT02694809).
The success of tamoxifen for the prevention of ER-positive breast cancer has prompted additional studies to minimize adverse effects and increase use among high-risk women. A randomized trial in women with ER-positive breast cancer found that low dose tamoxifen (1 or 5 mg) can decrease tumor proliferation, as measured by Ki-67 expression, comparable to that of high dose tamoxifen (20 mg) (59). This finding sparked several clinical trials for the use of low-dose tamoxifen in breast cancer prevention. The HOT study trial in postmenopausal women using HRT first showed a 68% reduction in ER-positive breast cancer incidence among women taking low-dose tamoxifen, with minimal side effects compared to placebo group (45). With 5-years of follow-up, a multicenter phase III trial (TAM-01) in women with previous hormone sensitive breast intraepithelial neoplasia (DCIS, ADH, or ALH), demonstrated that low dose tamoxifen (5 mg) administered for 3-years reduced recurrence and contralateral breast cancer by 50%, without significant differences in thrombosis or uterine cancer compared to placebo (73). This study also revealed that the efficacy of low-dose tamoxifen may be greater in postmenopausal women with lower estradiol levels, and those who never (74). Long-term follow-up of this trial is on-going and will be completed in 2028 (NCT01357772). The KARISMA phase II dose-determination study, including healthy women with higher mammographic density randomized into 0, 1, 2.5, 5, 10 or 20 mg of tamoxifen treatment, revealed that low-dose tamoxifen (2.5 mg) can reduce breast density similarly to the 20 mg high dose with substantially reduced vasomotor symptoms (75). Collectively these studies demonstrate the cancer preventive activity of low dose tamoxifen and suggest that studies of the long-term effects of low-dose tamoxifen are warranted.
Localized treatment of tamoxifen via topical application is also being considered to overcome the adverse effects of systemic tamoxifen therapy. A randomized Phase II trial of 4-hydroxytamoxifen gel (Afimoxifen) versus oral tamoxifen, administered pre-surgery to women with DCIS, demonstrated that the antiproliferative effect of topical tamoxifen is similar to oral tamoxifen, but without the systemic endocrine effects, supporting the rationale for 4-hydroxytamoxifen gel in breast cancer prevention (76). A phase II randomized trial of 4-hydroxytamoxifen gel in healthy women with high breast density was recently completed but results have yet to be published (NCT03199963). Similarly, the phase II Karma CREME-1 trial explored the effects of topical endoxifen, a tamoxifen metabolite, versus placebo on mammographic density of healthy postmenopausal women (NCT04616430). A significant decrease in breast density was observed after 3 months of 20 mg of endoxifen treatment but the development of severe skin rashes led to a high discontinuation rate among participants (77). Although long-term clinical trials in healthy, high-risk women are still needed before topical therapies can be used clinically, these studies support the concept of topical endocrine therapy to prevent the development of ER-positive breast cancer with minimal systemic effects.
To date, clinical trials with SERMs have shown clear efficacy for the prevention of primary and recurrent ER-positive breast cancer, leading to the FDA approval of tamoxifen and raloxifene for high risk women. However, the uptake of SERMs for prevention has been low among high risk women, likely due to concerns about side effects. The field urgently needs a way to effectively prevent ER-positive breast cancer while minimizing adverse events to improve treatment uptake. Ongoing efforts to explore alternative dosing regimens and to develop newer SERMs with reduced toxicity may address these issues but it remains to be seen if patient acceptance will improve. Finally, although SERMs have shown great promise for the prevention of ER-positive breast cancer, they do not prevent ER-negative disease.
An alternative strategy to reduce estrogen signaling in ER-positive breast cancer is through the inhibition of aromatase, an enzyme typically expressed in fat, stromal and muscle tissue but also breast cancer, responsible for converting androgens into estrogen (78). Since their development, aromatase inhibitors (AIs) have been highly effective in reducing circulating estrogen levels, subsequently blocking estrogen signaling in breast tumors. Early clinical trials demonstrated that AIs are more effective than tamoxifen for the treatment of ER-positive BC, without the associated increased risk for thrombosis and uterine cancers (79–84). Additionally, 10-year follow-up of a large clinical trial investigating the efficacy of anastrozole (a third generation AI) versus tamoxifen alone or in combination for the treatment of early-stage breast cancer in postmenopausal women, revealed that treatment with the AI also had a greater reduction in contralateral breast incidence, prompting the exploration of their use in breast cancer prevention [ATAC trial (46)].
As with SERMs, several clinical trials have tested the ability of AIs to reduce invasive breast cancer incidence in women with DCIS (summarized in Table 1). The phase III NSABP B-35 and IBIS-II (DCIS) trials both compared the incidence of invasive breast cancer and toxicities in women with ER-positive DCIS treated with anastrozole or tamoxifen for 5-years. The NSABP B-35 trial found a significant decrease in breast cancer incidence for patients treated with anastrozole compared to tamoxifen at 5-years, but with a similar number of adverse events reported (47). In contrast, the IBIS-II (DCIS) trial found no clear differences in breast cancer prevention efficacy between anastrozole and tamoxifen treatment, concluding that chemoprevention with AI is not superior to SERMs (68). However, the recently published long-term follow-up of the IBIS-II trial in high-risk postmenopausal women (relative risk of breast cancer at least twice that of the general population), comparing anastrozole to placebo, demonstrated a significant reduction in ER-positive breast cancer incidence (54%) without a significant difference in adverse events observed during the 5-year treatment period or 12-years of follow-up (67). These findings indicate that anastrozole is a suitable option for the prevention of ER-positive breast cancer in postmenopausal women, but it has yet to be FDA approved for this purpose.
A true phase III breast cancer prevention trial to explore the efficacy of AIs in high-risk women has also been completed (summarized in Table 2). The MAP.3 trial, comparing the incidence of invasive breast cancer with third generation AI exemestane versus placebo in a cohort of high-risk postmenopausal women (Gail risk score greater than 1.66% or previously diagnosed with atypical ductal hyperplasia, LCIS or DCIS), showed an impressive 74% reduction in ER-positive breast cancer during a short-term follow-up (85). However, significant age-related bone loss, despite calcium and vitamin D supplementation, contributed to significant discontinuation (86, 87). The long-term follow-up of this trial has yet to be published. To alleviate the unwanted side effects, low-dose exemestane trials are currently in progress. A recent phase IIb trial in postmenopausal women with early stage breast cancer demonstrated that exemestane given three days a week is not inferior to daily dosing [NCT02598557 (88)]. Future comparison studies of low-dose exemestane and low-dose tamoxifen in the prevention setting are needed.
The AI letrozole has been effective as a first-line treatment for advanced stage ER-positive breast cancer in postmenopausal women and can also provide benefit as an adjuvant therapy for early-stage hormone responsive breast cancer (81, 89). Early clinical trials for breast cancer prevention demonstrated that 6 months of letrozole treatment in postmenopausal women taking hormone replacement therapies could reduce Ki-67 proliferation markers, prompting further studies (90). Recently, the phase III NRG Oncology/NSABP B-42 trial explored the effects of letrozole versus placebo on disease free survival in postmenopausal women with previously treated ER-positive breast cancer (91). Study investigators found no significant reduction in breast cancer recurrence with 5 years of letrozole therapy but did note a reduction in distant recurrence among letrozole users. Importantly, there were no significant differences in adverse events between treatment groups providing evidence for letrozole as a well-tolerated preventative therapy. An ongoing phase III trial in postmenopausal women carrying BRCA1/2 mutations is underway to investigate breast cancer incidence and recurrence with letrozole therapy (LIBER trial; NCT00673335). The results from this trial are expected in 2023.
Like SERMs, the major problems with the use of AIs for breast cancer prevention are the associated treatment toxicities (namely hot flushes, osteoporosis, bone pain and bone fractures) and the inability to prevent ER-negative breast cancer. Despite the promise of AIs for ER-positive breast cancer prevention, their use is largely restricted to postmenopausal women whom lack estrogen-producing ovaries. This unfortunately excludes premenopausal women from the benefits of AIs, specifically those at high-risk for breast cancer who are already in desperate need for effective preventative agents. To date, no AIs have been approved by the FDA for breast cancer prevention, however, they are often considered for breast cancer prevention in high-risk postmenopausal women as “off label” treatments.
PR and its endogenous ligand progesterone (P4) are essential to normal and pregnancy associated mammary gland development. PR mainly exists as two functionally active isoforms; the full-length receptor PR-B preferentially binds with co-activators of gene transcription while the truncated receptor PR-A shows a greater binding affinity for co-repressors (92–94). In a knockout mouse model, the complete loss of PR-B, resulted in reduced pregnancy associated side branching and lobuloalveolar development (95, 96). Although PR-A was not essential for normal mammary development, there is evidence that PR-A may suppress the function of PR-B (97). As with ER, the expression of PR is tightly regulated under normal conditions and becomes dysregulated in breast cancer. The ratio of PR-A:PR-B is strongly associated with breast cancer progression and endocrine therapy response, with PR-A rich tumors associated to poor disease-free survival (98–101). PGR (NR3C3), the gene encoding the many known isoforms of PR, is a direct target gene of ER and therefore depends on ER expression. For this reason, PR expression by IHC is also prognostic for breast cancer overall and disease-free survival. ER-positive/PR-negative tumors are less responsive to SERMS likely because the loss of PR indicates tumors with nonfunctional ER signaling (102, 103).
Although it is recognized that ER drives PR expression, it is also known that PR-A can inhibit ER transcriptional activity and plays an important role in the formation of breast cancer (104, 105). Recently it was found that postmenopausal women with higher levels of circulating P4 are at increased risk for breast cancer (106). While the exact role of P4-PR in breast cancer development and progression is still unknown, it has been shown that PR activation can contribute to the proliferation and invasion of breast cancer cells via activated EGF signaling and induction of VEGF (107–109). It is also known that P4-PR induces receptor activator of the nuclear factor kappa-B ligand (RANKL) paracrine signaling from luminal cells to promote mammary epithelial proliferation and carcinogenesis (110, 111) suggesting PR as an ideal target for breast cancer prevention. It is worth noting that RANKL targeted therapies are also being explored for the prevention of BC, although a recent phase III trial has demonstrated no significant decrease in contralateral breast cancer incidence among postmenopausal women treated with the RANKL monoclonal antibody, denosumab (112, 113).
Because of the known pro-tumorigenic mechanisms of PR activation, the use of antagonistic selective progesterone receptor modulators (SPRMs) are being investigated for the treatment and prevention of PR-positive breast cancer (Figure 1). These modulators often compete with agonists (like endogenous P4) for higher affinity binding to PR but depend on the ratio of PR-A:PR-B in the tissue, making them highly tissue specific with minimal side effects (114).
Several SPRMs have been investigated for the treatment of PR-positive breast cancer. Mifepristone, ulipristal acetate, and telapristone acetate have been shown to decrease cell proliferation, inhibit cell cycle progression, and increase apoptosis of breast cancer cell lines (115, 116). Additionally, preclinical studies have also revealed that mifepristone and telapristone acetate can inhibit angiogenesis and migration of breast cancer cells in vivo (117, 118). Recently, the phase I MIPRA trial exploring the effects of mifepristone in women with breast cancer pre-selected for high PR-A:PR-B ratios in the tumor demonstrated a significant decrease in Ki-67 proliferation marker with an increase in Cleaved caspase 3 compared to baseline expression after two weeks of treatment (119). Similarly, a phase II trial investigating the effects of telapristone acetate for 2–10 weeks before surgery of early-stage breast cancer patients showed a significant decrease in tumor proliferation in a subset of patients (120).
Using a preclinical prevention model of Brca1 mutant breast cancer, it has been demonstrated that mifepristone, ulipristal acetate and telapristone acetate can reduce proliferation and inhibit the formation of tumors (121–123), highlighting the potential for SPRMs in the prevention of breast cancer for women with BRCA1/2 mutations. In a recent phase II clinical trial investigating the effects of mifepristone (50 mg) on BRCA1/2 carriers, healthy premenopausal women with and without BRCA mutations were treated for 12 weeks and assessed for breast epithelial proliferation and side effects (NCT01898312). Recently published results show that mifepristone, but not the vitamin treatment placebo, reduced both the mitotic age and proportion of luminal progenitor cells in the normal breast tissue of healthy women and BRCA1/2 mutation carriers (124), suggesting mifepristone may be suitable for breast cancer prevention. The same group completed a similar phase II trial investigating the effects of anti-progestin ulipristal acetate (5 mg daily) on surrogate markers of breast cancer risk in high-risk premenopausal women (BRCA1/2 mutation carriers or high lifetime risk by assessment models; NCT02408770). In congruence with the mifepristone results, ulipristal acetate also reduced the normal breast tissue mitotic age (124). Similarly, a phase I trial in very young, healthy women (<40 years), comparing the effects of ulipristal acetate to combined oral contraceptive pill on the proliferation of breast cells (NCT02922127), demonstrated that ulipristal acetate drastically decreases Ki-67 proliferation and reduces the background parenchymal enhancement of normal breast tissue (125).
Of the SPRMs with activity in breast tissue, telapristione acetate has been found to exert the greatest anti-tumorigenic effect in several in vitro breast cancer models (118), warranting consideration for breast cancer prevention. In a pre-clinical model of Sprague–Dawley rats, telapristone acetate was shown to prevent spontaneous mammary hyperplasia and pre-malignant lesions and suppress tumor formation in the N-methyl-N-nitrosourea (MNU) induced mammary carcinogenesis model (126). To explore the feasibility of telapristone acetate in breast cancer prevention, Lee et al studied the bioavailability of telapristone acetate as a topical gel or implant in an athymic nude rat model. Like afimoxifene and endoxifen gel, the investigators found that effective telapristone acetate levels could be achieved in the mammary tissue (127). A recently published phase II trial comparing oral to transdermal delivery of telapristone acetate in women undergoing mastectomies confirmed that local drug distribution patterns were similar between treatment groups, establishing the feasibility of topical telapristone acetate for breast cancer prevention in high-risk women (128). A summary of these trials can be found in Table 3.
Like SERMs, a challenge for SPRMs in breast cancer prevention is toxicity and tolerability in the patient population. For the most part, SPRMs are well-tolerated but adverse events have been associated with treatment, including hot flushes, nausea and vomiting (129, 130). In addition, a few clinical trials with ulipristal acetate and telapristone acetate for the treatment of uterine fibroids were suspended due to liver toxicity concerns (114), bringing into question the potential for long-term use. Of course, SPRMs are known for their utility as emergency contraceptives and must be used with caution in women of child-bearing age. This consideration could limit the use of SPRMs among premenopausal women for breast cancer prevention. Despite these restrictions, preclinical findings and recent phase I/II clinical trials have demonstrated that SPRMs should be tested in Phase III breast cancer prevention trials. Long-term studies on breast cancer incidence and treatment toxicity are needed to assess the safety of these treatments.
Non-steroid hormone receptors typically function as heterodimeric transcription factors with retinoid X receptor (RXR) and are retained in the nucleus even in the inactive state, bound to DNA response elements with transcriptional co-repressors. Upon binding of a specific ligand, these nuclear receptors will dissociate with inhibitory factors and recruit co-regulators to modulate target gene transcription. There are many nuclear receptors known to dimerize with RXR for transcriptional regulation, including RXR itself as a homodimer (Figure 2). Nuclear receptors with known endogenous ligands can regulate gene transcription through heterodimeric binding with RXR in the absence of RXR ligand (known as permissive heterodimers). These partner receptors include retinoic acid receptor (RAR), vitamin D receptor (VDR), peroxisomal proliferator-activated receptor (PPAR), liver X receptor (LXR), thyroid receptor (TR), RAR-related orphan receptor (ROR), pregnane X receptor (PXR), and farnesoid X receptor (FXR). However, RXR can also bind nuclear receptors without known endogenous ligands, referred to as orphan receptors. These nuclear receptors include the chicken ovalbumin upstream promoter transcription factors (COUP-TF1/2), nerve growth factor-induced protein IB (NGF IB/Nur77), nuclear receptor related 1 (Nurr1), neuron-derived orphan receptor 1 (NOR1), and V-erbA-related protein 2 (EAR2). Here we will discuss the known mechanisms of non-steroid hormone receptors RAR, RXR and VDR in breast cancer carcinogenesis as well as recent efforts to target these nuclear receptors for breast cancer prevention (Figure 3).
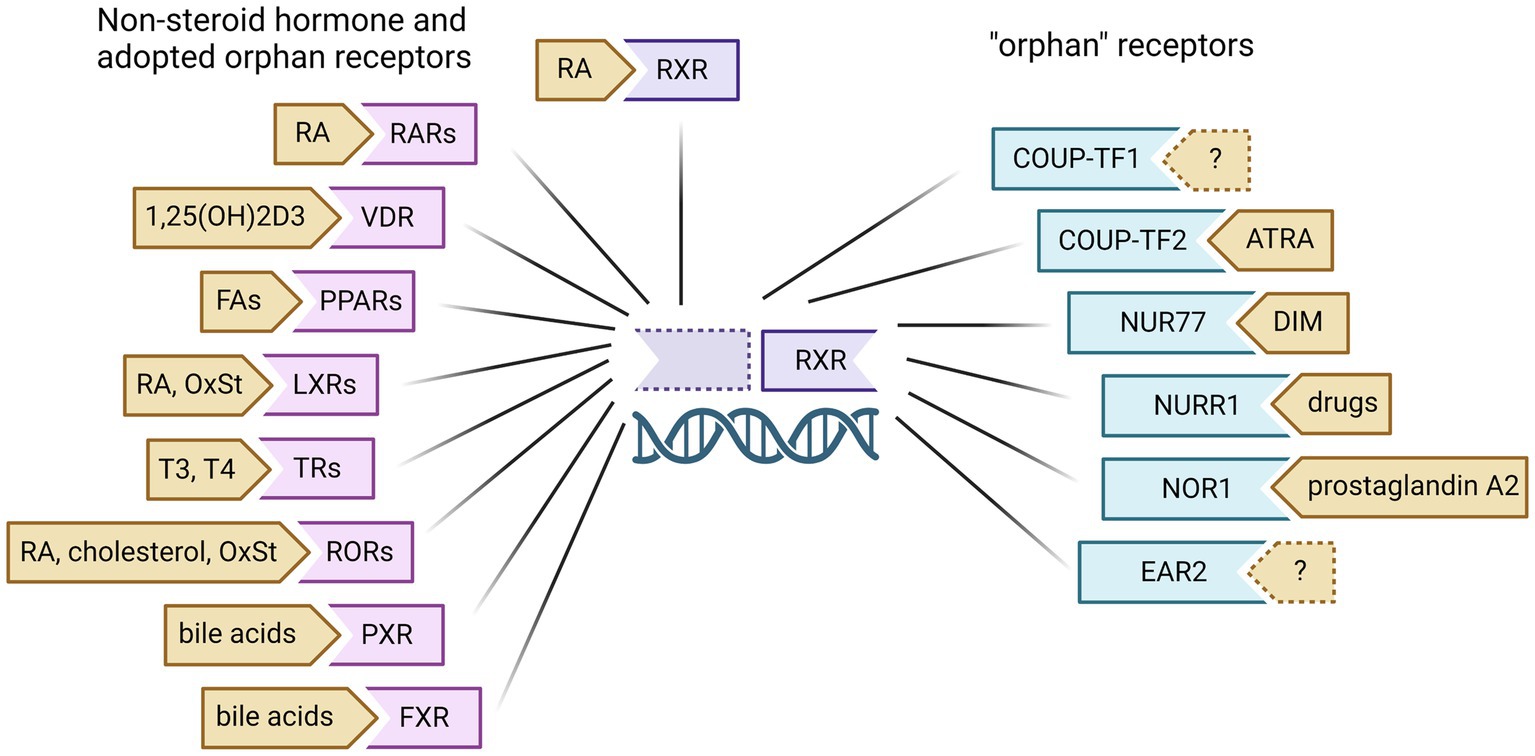
Figure 2. Retinoid X Receptor (RXR)dimeric partners and known ligands. RXR can homodimerize to modulate gene transcription upon binding of ligands like 13-cis-retinoic acid (RA). RXR can also heterodimerize with non-steroid hormone receptors and several orphan receptors to regulate the gene transcription of other nuclear receptors. These partner receptors include retinoic acid receptor (RAR), vitamin D receptor (VDR), peroxisomal proliferator-activated receptor (PPAR), liver X receptor (LXR), thyroid receptor (TR), RAR-related orphan receptor (ROR), pregnane X receptor (PXR), farnesoid X receptor (FXR), chicken ovalbumin upstream promoter transcription factors (COUP-TF1/2), nerve growth factor-induced protein IB (NGF IB/Nur77), nuclear receptor related 1 (Nurr1), neuron-derived orphan receptor 1 (NOR1), and V-erbA-related protein 2 (EAR2). RA, retinoic acid; FA, fatty acids; OxSt, oxysterols; ATRA, all-trans retinoic acid; DIM, 3,3`-diindolylmethane. Created with BioRender.com.

Figure 3. Non-steroid hormone nuclear receptors targeted for the prevention of BC. Retinoids, rexinoids and VDR agonists have been identified, developed and tested to target RAR, RXR and VDR gene transcription for the prevention of primary and recurrent breast cancer. Upon ligand binding, dimeric receptors can modulate the transcription of genes involved in proliferation, apoptosis, lipid metabolism, and cell homeostasis to inhibit carcinogenesis (*denotes treatments that have been tested in clinical trials. Those in red have been tested in breast cancer prevention trials). Created with BioRender.com.
The retinoic acid receptor (RAR) subfamily consists of three members: RARα, RARβ and RARγ. These nuclear receptors are activated by the major bioactive metabolite of vitamin A, retinoic acid (RA), and heterodimerize with the retinoid X receptor (RXR) to regulate the transcription of target genes involved in cell growth, differentiation and death. The first chemo-prevention trial with retinoids was in patients with head and neck cancers at high risk for recurrent and second primary tumors. This study demonstrated that treatment with 13-cis-RA (isotretinoin) could significantly reduce the formation of second primary tumors (131) and showed great promise for the use of retinoids in cancer prevention. Since then, retinoids have been explored for the prevention of retinoblastoma, lung cancer, skin cancer and breast cancer.
For years, it has been known that treatment with RA can inhibit the growth and induce apoptosis of breast cancer cell lines in vitro and in vivo, similar to the effect of tamoxifen (132–134). In preclinical trials, treatment with 9-cis-RA has been shown to suppress the formation of ER-positive mammary tumors in rats exposed to MNU (135) and 9-cis-RA can suppress mammary tumorigenesis in C3(1)-SV40 transgenic mice (136). However, even when given at therapeutic concentrations, RA is associated with adverse effects, such as teratogenicity and skin irritation, that limit its clinical utility. This is in part due to non-genomic, RAR-independent effects of RA on several cell signaling pathways. For this reason, synthetic RAR agonists (retinoids) with fewer toxicities have been developed.
The synthetic retinoid fenretinide was developed in the late 1960s and shown to preferentially accumulate in mammary tissue and inhibit the formation of tumors in a chemically induced rat model of mammary carcinoma (137). Since then, fenretinide has been extensively studied for the prevention of many cancer types, including prostate and oral cancer, due to its favorable toxicity (48, 138). A 15-year follow-up of a phase III clinical trial in women with previous DCIS or stage I breast cancer demonstrated that five years of fenretinide treatment in premenopausal women can significantly reduce the incidence of second primary breast cancer regardless of the initial hormone status (139), suggesting retinoids can prevent both ER-positive and ER-negative breast cancer (summarized in Table 1). The trial also reported a reduced incidence of ovarian carcinoma during the intervention phase, although the protective effects did not last upon discontinuation of fenretinide treatment (140). These findings prompted a phase III prevention trial to investigate the effects of fenretinide on breast cancer incidence in premenopausal high-risk individuals with familial or genetic risk for breast cancer (NCT01479192). Unfortunately, the trial was terminated due to low patient accrual. Recently, a non-aqueous microemulsion for the prolonged release of fenretinide in the mammary tissue was developed for the intended use in breast cancer prevention. In a preclinical study of chemically induced mammary tumors, it was demonstrated that local injection of fenretinide microemulsion significantly reduced the incidence of mammary tumors in Sprague–Dawley rats, without systemic side effects (141). The same group is currently exploring topical administration of fenretinide which may prove more favorable for prevention trials (142).
Like RAR, the retinoid X receptor subfamily is made up of three members: RXRα, RXRβ and RXRγ. In contrast to RAR, RXR can homodimerize or heterodimerize with a variety of other nuclear receptor partners to modulate the signaling of additional gene targets. For some of these heterodimers, a single partner ligand alone can activate gene transcription (permissive heterodimer) while for others, the heterodimer partner ligand must be present for transcription modulation (non-permissive heterodimer). RXR can also form heterodimers with orphan nuclear receptors, lacking known endogenous ligands. Many synthetic agonists with high specificity for RXR (rexinoids), have been developed to mimic the growth inhibitory effects of RAR agonists while avoiding the toxicity associated with natural retinoids.
The third-generation retinoid bexarotene (LGD1069) has been widely studied in the prevention of many cancers. In preclinical models of ER-negative BC, our lab has shown that 9-cis-RA and bexarotene can suppress mammary tumorigenesis and prevent the development of premalignant lesions (143–145). This suppression is mediated in part by a decrease in cyclin D1 and COX2 expression (146, 147) as well as an induction of cellular senescence (148). Together, these preclinical findings supported a clinical trial investigating the effects of bexarotene on breast cell proliferation in women with known or suspected BRCA1/2 mutations (NCT00055991). Pre- and post-menopausal women were treated with bexarotene or placebo for 4 weeks and proliferation associated biomarkers were compared between core needle biopsies at pre and post treatment timepoints. Results from this trial revealed no differences in Ki-67 or cyclin D1 expression between bexarotene and placebo treated groups. Though, in a subgroup analysis of postmenopausal women only, the decrease in cyclin D1 expression was significantly reduced by 65% compared to placebo (149), suggesting a potential benefit for postmenopausal high-risk women (summarized in Table 4). However, this study also found that bexarotene treatment was associated with toxicities including hypertriglyceridemia, subclinical hypothyroidism and skin reactions, likely due to weak RAR-binding. To limit the systemic toxicities, a topical bexarotene gel has been developed. A recent phase I dose escalation study has shown that topical bexarotene can penetrate the breast tissue at 10 mg per every other day but still results in unwanted skin reactions that may limit compliance (156).
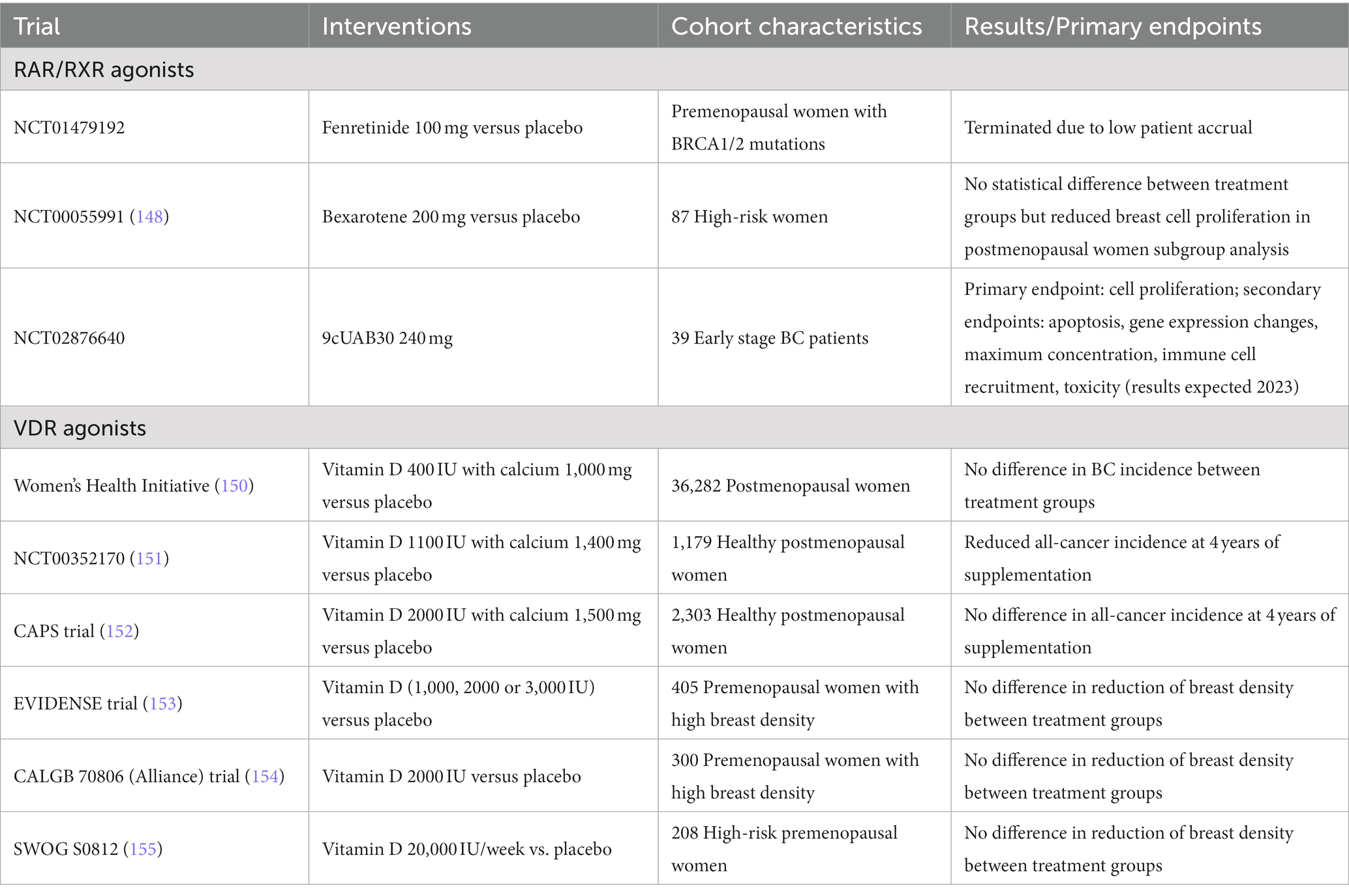
Table 4. Clinical trials targeting non-steroid hormone nuclear receptors for breast cancer prevention.
To avoid the unwanted toxicity associated with weak RAR-binding, even more specific RXR agonists have been developed. Our group has previously shown that the rexinoid LG100268 is more effective for mammary tumor prevention with reduced toxicity compared to bexarotene in preclinical models of ER-negative breast cancer (157). Karen Liby and colleagues further demonstrated that this inhibition may not only be due to a direct effect on breast cell proliferation but also due to the stimulation of immune cells (158). Similarly, the fourth generation rexinoid IRX4204 was shown to prevent mammary carcinogenesis in the ER-negative MMTV-neu mouse model with demonstrated effects on the activity of RAW264.7 macrophage-like cells (159). Neither LG100268 or IRX4204 have been explored in clinical trials for breast cancer treatment or prevention but IRX4204 has been tested for the treatment of taxane-resistant, castration-resistant metastatic prostate cancer with no reported serious adverse events (160).
The highly specific rexinoid, 9cUAB30, has also been evaluated for chemoprevention in preclinical models of BC. Using the MNU induced mammary cancer model in female Sprague–Dawley rats, it was demonstrated that 9cUAB30 can delay the formation of tumors without signs of treatment toxicity (161). Based on these findings, a phase I, placebo-controlled, dose escalation trial was conducted in healthy volunteers to evaluate the safety and pharmacokinetics of 9cUAB30. Results of this study demonstrated that 9cUAB30 is well tolerated, with no dose limiting toxicities and no evidence of elevated triglycerides or cholesterol, a concerning side effect that has been observed with other rexinoids (162). A phase Ib trial to study the biologic effects of 9cUAB30 on presurgical treatment of early stage breast cancer is ongoing and is expected to be completed in 2023 (NCT02876640).
In the inactive state, vitamin D receptor (VDR) exists as a monomer in solution or homodimer bound to VDR response elements on DNA. When activated by its endogenous ligand, 1,25-dihydroxyvitamin D3 (cholecalciferol), VDR preferentially heterodimerizes with RXR to regulate the transcription of VDR target genes (163–165). In normal breast tissue, VDR is essential for the negative growth regulation of the mammary gland during puberty and is known to regulate casein expression during pregnancy as well as post lactation involution (166, 167). As with other nuclear receptors, expression of VDR is often dysregulated in breast cancer. Among human breast tumors, higher VDR expression is associated with decreased Ki-67 staining and better outcomes, with the lowest VDR expression often found in TNBC samples (168). In addition, polymorphisms in the VDR gene have been associated with increased risk for breast cancer (169, 170) and a meta-analysis of 11 studies on circulating vitamin D levels and breast cancer risk demonstrated a 45% reduction in breast cancer risk among women with the highest levels of 25-hydroxyvitamin D (25(OH)D), the major circulating form of vitamin D (171). Similarly, a pooled analysis of 11 case–control studies on circulating D3 and breast cancer risk found that serum 25(OH)D levels of 47 ng/mL or greater was associated with a 50% lower risk of breast cancer (172). In addition, an analysis of data from two independent cohorts (Lappe clinical trial cohort and GrassrootsHealth prospective cohort) found that women with 25(OH)D concentrations above 40 ng/mL had a 67% reduced risk of all invasive cancers (173). Together, these findings suggest vitamin D levels and VDR expression play an important role in breast cancer carcinogenesis.
Previous studies demonstrated that in vitro treatment with vitamin D inhibits the growth of breast cancer cell lines (174, 175). More recently, in a preclinical model of obesity induced BC, investigators found that treatment with dietary vitamin D could delay tumor appearance and inhibit the growth of mammary tumors through repressed estrogen signaling and decreased leptin signaling, associated with a decrease in insulin resistance (176). Although vitamin D supplementation is generally well tolerated, the use in cancer prevention has been hindered due to hypercalcemic toxicity. To overcome this problem, several non-hypercalcemic vitamin D analogues were created and tested for anti-tumor effects. Analogues EM1 and UVB1 have been shown to inhibit the growth of human breast cancer cell lines in vitro and in vivo, without inducing hypercalcemia (177). More recently, it was demonstrated that EM1 and UVB1 can also reduce the viability of HER2-overexpressed and TNBC patient derived xenografts and even inhibit the formation and growth of anti-HER2 resistant organoids (178). Not only do these studies highlight the potential for VDR modulation without side effects, but they also demonstrate that targeting VDR can prevent both ER-positive and ER-negative BC.
Because VDR expression is known to be inversely correlated with breast cancer aggressiveness, several recent preclinical studies have also investigated role of VDR in metastasis prevention. Knockdown of VDR in the TNBC MDA-MD-231 cell line has been shown to significantly increase metastases to the bone of female Balb/c nu/nu mice (179). In the aggressive MMTV-PyMT mouse mammary tumor model, it was demonstrated that a low vitamin D diet accelerates carcinogenesis and lung metastases. When vitamin D was replenished to mice via perfusion, primary tumor formation was delayed and spontaneous lung metastasis was reduced (180). This metastatic prevention with vitamin D supplementation was found to be mediated, in part, through the modulation of cancer associated chemokine interaction of C-X-C Motif Chemokine Ligand 12 (CXCL12) with C-X-C Motif Chemokine Receptor 4 (CXCR4), which is inappropriately elevated with vitamin D deficiency (181).
Due to the plethora of preclinical findings that demonstrate a role for VDR in breast cancer formation and growth, several clinical trials have investigated the preventative effect of vitamin D supplementation on breast cancer risk. In the Women’s Health Study with over 10,000 premenopausal women and 10 years of follow-up, investigators found that higher intake of vitamin D is associated with a 35% reduction in breast cancer risk (150). However, there was no significant reduction of invasive breast cancer incidence among more than 36,000 postmenopausal women from the Women’s Health Initiative whom were randomized to calcium with vitamin D supplementation compared to placebo for 7 years (151), suggesting postmenopausal women may not benefit from the breast cancer preventative effects of vitamin D. In contrast, a randomized trial investigating vitamin D and calcium supplementation (alone or in combination) versus placebo in healthy postmenopausal women of rural Nebraska, Lappe and colleagues found that increasing serum calcium and vitamin D reduced all-cancer risk (152). However, in an expanded randomized trial with healthy postmenopausal women in the same rural Nebraska communities, it was found that supplementation with calcium and vitamin D did not result in a significantly lower risk for all cancer types after 4 years of treatment (153). To date, combined clinical trial results on the effects of vitamin D supplementation and breast cancer risk have been inconclusive.
Because increased breast density is associated with increased risk for BC, and chemo preventative agents such as SERMs have been shown to effectively decrease mammographic density, several recent clinical trials have addressed the consequence of vitamin D supplementation on breast density in premenopausal women. A summary of these trials can be found in Table 4. In the EVIDENSE trial (NCT01747720) investigating the effects of 1,000, 2000 or 3,000 IU vitamin D supplementation on mammographic density of healthy, premenopausal women, it was demonstrated that one year of D3 supplementation did not reduce breast density more than placebo (154). Similarly, the CALGB 70806 (Alliance) trial in healthy, premenopausal women randomized to 2000 IU vitamin D or placebo daily for one year, examined the effect of vitamin D supplementation on breast density (NCT01224678). Although there was a trend towards decreased mammographic density among women with the highest baseline density, there was no significant difference between vitamin D treatment and placebo (155). In the SWOG S0812 (NCT01097278) trial investigating the effects of vitamin D supplementation in high-risk, premenopausal women (Gail score greater than 1.66%, mammographic density greater than 50%, known BRCA1/2 mutation or diagnosed with ADH, LCIS or DCIS), there was no statistically significant difference in mammographic density between 20,000 IU per week and placebo treatment (182). Despite the fact all three trials reported few side effects and high tolerance, the null findings on mammographic density do not support the use of vitamin D supplementation for breast cancer risk reduction.
There are several reasons that may explain the discrepancy between recent clinical trial results and preclinical model findings. Serum levels of 25(OH)D, used in clinical trials to assess vitamin D uptake, may not reflect local concentrations of active metabolite in the breast tissue. It is also possible that the concentration of other nuclear receptor ligands can inhibit the activity of VDR. For example, RXR ligands are known to destabilize the VDR-RXR heterodimer (164) and could explain the discrepancies in response to vitamin D supplementation. It is also known that estrogen, phytoestrogens and retinoids can modulate VDR expression, further highlighting the complexity of targeting single nuclear receptors for prevention (183, 184). Non-hypercalcemic analogues have shown promise in preclinical studies and may provide more specificity for VDR activity, but these have yet to be tested in clinical trials. In addition, very little is known about VDR post-translational modifications and the role of VDR monomers in BC. It was recently found that cytoplasmic VDR can potentiate the growth of breast cancer cell lines in the absence of ligand altogether (185), suggesting a ligand-independent function for VDR that has yet to be fully understood in the context of breast cancer formation and treatment resistance. In summary, more research is needed to elucidate the complexity of vitamin D signaling in both the normal mammary and tumor state to better understand the role of VDR in breast cancer prevention.
Since endocrine targeted therapies can effectively prevent hormone receptor positive breast cancer and other nuclear receptor targets have been shown to prevent hormone receptor negative BC, it is reasonable that combination treatments for the prevention of breast cancer should be explored. Several pre-clinical trials have already investigated the effects of RXR agonists with ER modulators for the prevention of BC. Combination treatment of the rexinoid 9cUAB30 with the SERM tamoxifen was shown to inhibit the formation of mammary tumors in the MNU induced rat model more than single agent treatment (161). In the MMTV-neu ER-negative mammary tumor model, it was demonstrated that the rexinoid LG100268 in combination with the SERMs arzoxifene or acolbifene can synergize to prevent the formation of mammary tumors (186). Similarly, using a p53-null preclinical model, our group has shown that LG100268 with the SERM tamoxifen can reduce Ki-67 and cyclin D1 expression in normal mammary tissue and prevent both ER-positive and ER-negative mammary tumors (187). These studies provide the rationale for exploring the use of rexinoids with SERMs for the prevention of breast cancer in high-risk women.
More recently, it has been demonstrated that retinoids can block PR binding at shared DNA response element regions and inhibit P4 stimulated growth of ER-positive breast cancer xenografts, suggesting a cross-talk between PR and RAR in regulating a subset of hormone responsive breast cancer (188). It has also been shown that the addition of vitamin D analogs could potentiate the antitumor effect of the AI anastrozole in MCF7 tumor bearing mice via regulation of both VDR and ER signaling (189). In addition, VDR activation, with vitamin D or the synthetic analogue EB1089, could re-sensitize an antiestrogen resistant MCF7 breast cancer cell line to tamoxifen treatment and reduce the incidence of ER-positive mammary tumors in a preclinical model (190). These findings suggest that targeting multiple nuclear receptors may be more efficacious for breast cancer prevention than single agent therapies.
Despite promising preclinical results, very few combinations have been explored in clinical trials, likely due to toxicity concerns. In a randomized double-blind phase II trial of low-dose tamoxifen and the retinoid fenretinide alone or in combination in high-risk premenopausal women (Gail risk score greater than 1.3% or diagnosed with LCIS, DCIS or stage I ER-positive breast cancer), most with intraepithelial neoplasia, it was found that both tamoxifen and fenretinide alone could reduce breast density and neoplastic events compared to placebo. However, the combination of low-dose tamoxifen with fenretinide did not show synergistic interaction, despite being well-tolerated by patients (191, 192).
Targeting nuclear receptors for breast cancer prevention has been shown to be possible in multiple phase III trials. At present, only the anti-estrogen SERMs tamoxifen and raloxifene are FDA approved for breast cancer prevention. However, several other drugs have been found to reduce breast cancer risk in women without breast cancer including other SERMs (lasofoxifene) and aromatase inhibitors (anastrozole and exemestane). These drugs are FDA-approved for the treatment of breast cancer, so they can be used off-label, but are generally not used often. The most commonly used off-label drugs for breast cancer prevention are the aromatase inhibitors, anastrozole or exemestane, which can be considered for women who have contraindications for SERM use (such as having a prior deep venous thrombosis).
There is no “predictive biomarker” in the normal breast tissue to predict a response to a preventive drug. Thus, breast cancer prevention drugs are chosen based on their overall efficacy and tolerability to individual women. Mild common toxicities (hot flushes) and rare more serious toxicities (blood clots and uterine cancer) have limited the use of SERMs for prevention among eligible high-risk women. AIs are more effective in treating and preventing breast cancer but are associated with a different spectrum of side effects that also limit the use of these agents for breast cancer prevention. Lower doses, modified treatment schedules, and local administration routes are currently being explored to determine if these efficacious drugs can be given more safely. Advancements in localized treatments are expected to overcome toxicity concerns and improve tolerability among high-risk women. But despite the success and specificity of SERMs and AIs for prevention, these endocrine therapies are only effective for the prevention of ER-positive breast cancer and have no effect on ER-negative disease.
Another concern for the use of anti-estrogen therapy is the intrinsic resistance that can occur among a small subset of patients. In the treatment setting, to overcome de novo and acquired resistance of ER-positive breast cancers to anti-estrogen therapy, it is common practice to add CDK4/6 inhibitors (such as palbociclib, ribociclib, and abemaciclib) to anti-estrogen therapy for the treatment of early and late stage breast cancers. In addition, anti-estrogen selective estrogen degraders (SERDS), such as fulvestrant, are also used to treat metastatic ER-positive breast cancers that have arisen after prior anti-estrogen therapy. These strategies have not yet been used in the prevention setting. However, for ER-positive breast cancers that arise after anti-estrogen preventive therapy, the combination of a different anti-estrogen drug plus a CDK4/6 inhibitor is often used for treatment.
The most common form of intrinsic resistance to anti-estrogen therapy in the prevention setting is the development of an ER-negative breast cancer, not prevented by anti-estrogen therapy. ER-negative breast cancers that arise after anti-estrogen preventive therapy are currently treated with standard chemotherapy. A major focus of the field has been to develop targeted preventive strategies (drug therapy or vaccines) to prevent these ER-negative breast cancers. While several agents reviewed here have shown promise in preclinical models for the prevention of ER-negative breast cancers, none have yet been approved for human use.
Recent efforts to target AR have shown promising results in decreasing the growth of both HR-positive and HR-negative breast cancer. A preclinical treatment study using the antiandrogen enzalutamide demonstrated growth inhibition of AR-positive breast cancer including in a TNBC model (193). In addition, a phase II clinical trial in AR-positive/ER-negative advanced breast cancer patients with the AR antagonist bicalutamide demonstrated a clinical benefit rate of 19% at six months with an improved progression free survival of 12 weeks (194). However, as with other endocrine therapies, associated toxicities with first and second-generation AR antagonists has limited their therapeutic potential. To date, AR targeted therapies have not been explored for breast cancer prevention. Additional studies are needed to improve our understanding of AR and its role in breast cancer development and prevention.
Targeted therapies for non-steroid hormone receptors, like RXR and VDR, are proving to be less toxic with the desired ability to prevent both ER-negative and ER-positive disease in preclinical studies. However, results from clinical trials have yet to demonstrate effective breast cancer prevention in women. The development of novel agonists and analogs with greater specificity may prove to be more efficacious in clinical trials. More recently, several groups have shown that GR, LXR and PPAR may play distinct yet important roles in the development and progression of BRCA mutant breast cancer (195–197), suggesting that other nuclear receptors could be targeted for the prevention of breast cancer. Efforts to combine nuclear receptor targeted therapies may demonstrate greater preventative effects and should be investigated.
The prevention of TNBC remains a major unsolved problem. Just as with treatment options for these aggressive cancers, novel effective preventative therapies need to be developed for high-risk women, especially those with BRCA1/2 mutations. Studies using PARP inhibitors and other signaling transduction inhibitors for prevention in preclinical models are currently ongoing and several groups are exploring the utility of vaccines against breast cancer neoantigens. If these strategies prove to demonstrate moderate efficacy for breast cancer prevention, combination treatments with effective nuclear receptor targeted therapies should also be explored. However, to develop acceptable and effective prevention therapies, it will be necessary to first overcome concerns about the toxicity of these interventions.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
This work was funded by the CFP Foundation (Odyssey Fellowship, CM), the Breast Cancer Research Foundation (PB) and NCI PREVENT Program (75N91019D00021, PB).
We want to thank Michelle Savage for assistance with the editing and submission of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Giaquinto, AN , Sung, H , Miller, KD , Kramer, JL , Newman, LA , Minihan, A, et al. Breast cancer statistics, 2022. CA Cancer J Clin. (2022) 72:524–41. doi: 10.3322/caac.21754
2. Howlader, N , Altekruse, SF , Li, CI , Chen, VW , Clarke, CA , Ries, LAG, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. (2014) 106:dju055. doi: 10.1093/jnci/dju055
3. Harbeck, N , and Gnant, M . Breast cancer. Lancet. (2017) 389:1134–50. doi: 10.1016/S0140-6736(16)31891-8
4. Howlader, N , Cronin, KA , Kurian, AW , and Andridge, R . Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomark Prev. (2018) 27:619–26. doi: 10.1158/1055-9965.EPI-17-0627
5. Cobleigh, MA , Vogel, CL , Tripathy, D , Robert, NJ , Scholl, S , Fehrenbacher, L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. (1999) 17:2639–48. doi: 10.1200/JCO.1999.17.9.2639
6. Slamon, DJ , Leyland-Jones, B , Shak, S , Fuchs, H , Paton, V , Bajamonde, A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. (2001) 344:783–92. doi: 10.1056/NEJM200103153441101
7. Geyer, CE , Forster, J , Lindquist, D , Chan, S , Romieu, CG , Pienkowski, T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. (2006) 355:2733–43. doi: 10.1056/NEJMoa064320
8. Baselga, J , Bradbury, I , Eidtmann, H , di Cosimo, S , de Azambuja, E , Aura, C, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. (2012) 379:633–40. doi: 10.1016/S0140-6736(11)61847-3
9. Verma, S , Miles, D , Gianni, L , Krop, IE , Welslau, M , Baselga, J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. (2012) 367:1783–91. doi: 10.1056/NEJMoa1209124
10. Harbeck, N . Neoadjuvant and adjuvant treatment of patients with HER2-positive early breast cancer. Breast. (2022) 62:S12–6. doi: 10.1016/j.breast.2022.01.006
11. McCarthy, PM , Clifton, GT , Vreeland, TJ , Adams, AM , O’Shea, AE , and Peoples, GE . AE37: a HER2-targeted vaccine for the prevention of breast cancer recurrence. Expert Opin Investig Drugs. (2021) 30:5–11. doi: 10.1080/13543784.2021.1849140
12. Mittendorf, EA , Ardavanis, A , Symanowski, J , Murray, JL , Shumway, NM , Litton, JK, et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence. Ann Oncol. (2016) 27:1241–8. doi: 10.1093/annonc/mdw150
13. Somlo, G , Frankel, PH , Arun, BK , Ma, CX , Garcia, AA , Cigler, T, et al. Efficacy of the PARP inhibitor veliparib with carboplatin or as a single agent in patients with germline BRCA1- or BRCA2-associated metastatic breast cancer: California cancer consortium trial NCT01149083. Clin Cancer Res. (2017) 23:4066–76. doi: 10.1158/1078-0432.CCR-16-2714
14. Robson, M , Im, SA , Senkus, E , Xu, B , Domchek, SM , Masuda, N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. (2017) 377:523–33. doi: 10.1056/NEJMoa1706450
15. Robson, ME , Tung, N , Conte, P , Im, SA , Senkus, E , Xu, B, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. (2019) 30:558–66. doi: 10.1093/annonc/mdz012
16. Tutt, ANJ , Garber, JE , Kaufman, B , Viale, G , Fumagalli, D , Rastogi, P, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. (2021) 384:2394–405. doi: 10.1056/NEJMoa2105215
17. Nanda, R , Liu, MC , Yau, C , Shatsky, R , Pusztai, L , Wallace, A, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. (2020) 6:676–84. doi: 10.1001/jamaoncol.2019.6650
18. Yee, D , DeMichele, AM , Yau, C , Isaacs, C , Symmans, WF , Albain, KS, et al. Association of event-free and distant recurrence-free survival with individual-level pathologic complete response in neoadjuvant treatment of stages 2 and 3 breast cancer: three-year follow-up analysis for the I-SPY2 adaptively randomized clinical trial. JAMA Oncol. (2020) 6:1355–62. doi: 10.1001/jamaoncol.2020.2535
19. Lyons, TG . Targeted therapies for triple-negative breast cancer. Curr Treat Options in Oncol. (2019) 20:82. doi: 10.1007/s11864-019-0682-x
20. Won, KA , and Spruck, C . Triple-negative breast cancer therapy: current and future perspectives (review). Int J Oncol. (2020) 57:1245–61. doi: 10.3892/ijo.2020.5135
21. Hartmann, LC , Sellers, TA , Schaid, DJ , Frank, TS , Soderberg, CL , Sitta, DL, et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst. (2001) 93:1633–7. doi: 10.1093/jnci/93.21.1633
22. Rebbeck, TR , Friebel, T , Lynch, HT , Neuhausen, SL , van ‘t Veer, L , Garber, JE, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. (2004) 22:1055–62. doi: 10.1200/JCO.2004.04.188
23. Domchek, SM , Friebel, TM , Singer, CF , Evans, DG , Lynch, HT , Isaacs, C, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. (2010) 304:967–75. doi: 10.1001/jama.2010.1237
24. Sporn, MB , Dunlop, NM , Newton, DL , and Smith, JM . Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids). Fed Proc. (1976) 35:1332–8.
25. Mangelsdorf, DJ , Thummel, C , Beato, M , Herrlich, P , Schütz, G , Umesono, K, et al. The nuclear receptor superfamily: the second decade. Cells. (1995) 83:835–9. doi: 10.1016/0092-8674(95)90199-X
26. Muscat, GE , Eriksson, NA , Byth, K , Loi, S , Graham, D , Jindal, S, et al. Research resource: nuclear receptors as transcriptome: discriminant and prognostic value in breast cancer. Mol Endocrinol. (2013) 27:350–65. doi: 10.1210/me.2012-1265
27. Lin, ML , Patel, H , Remenyi, J , Banerji, CRS , Lai, CF , Periyasamy, M, et al. Expression profiling of nuclear receptors in breast cancer identifies TLX as a mediator of growth and invasion in triple-negative breast cancer. Oncotarget. (2015) 6:21685–703. doi: 10.18632/oncotarget.3942
28. Griekspoor, A , Zwart, W , Neefjes, J , and Michalides, R . Visualizing the action of steroid hormone receptors in living cells. Nucl Recept Signal. (2007) 5:e003:nrs.05003. doi: 10.1621/nrs.05003
29. Kato, S , Endoh, H , Masuhiro, Y , Kitamoto, T , Uchiyama, S , Sasaki, H, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. (1995) 270:1491–4. doi: 10.1126/science.270.5241.1491
30. Doan, TB , Graham, JD , and Clarke, CL . Emerging functional roles of nuclear receptors in breast cancer. J Mol Endocrinol. (2017) 58:R169–90. doi: 10.1530/JME-16-0082
31. Onitilo, AA , Engel, JM , Greenlee, RT , and Mukesh, BN . Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. (2009) 7:4–13. doi: 10.3121/cmr.2008.825
32. Beatson, GT . On the Treatment of Inoperable Cases of Carcinoma of the Mamma: Suggestions for a New Method of Treatment, with Illustrative Cases. Trans Med Chir Soc Edinb. (1896) 15:153–79.
33. Jensen, EV . On the mechanism of estrogen action. Perspect Biol Med. (1962) 6:47–59. doi: 10.1353/pbm.1963.0005
34. Chakravarty, D , Nair, SS , Santhamma, B , Nair, BC , Wang, L , Bandyopadhyay, A, et al. Extranuclear functions of ER impact invasive migration and metastasis by breast cancer cells. Cancer Res. (2010) 70:4092–101. doi: 10.1158/0008-5472.CAN-09-3834
35. Arnott, J , Martinkovich, S , Planey, SL , and Shah, D . Selective estrogen receptor modulators: tissue specificity and clinical utility. Clin Interv Aging. (2014) 9:1437–52. doi: 10.2147/CIA.S66690
36. Adjuvant tamoxifen in the management of operable breast cancer: the Scottish Trial. Report from the Breast Cancer Trials Committee, Scottish Cancer Trials Office (MRC), Edinburgh. Lancet. (1987) 2:171–5. doi: 10.1016/S0140-6736(87)90762-8
37. Boccardo, F , Rubagotti, A , Bruzzi, P , Cappellini, M , Isola, G , Nenci, I, et al. Chemotherapy versus tamoxifen versus chemotherapy plus tamoxifen in node-positive, estrogen receptor-positive breast cancer patients: results of a multicentric Italian study. Breast Cancer Adjuvant Chemo-Hormone Therapy Cooperative Group. J Clin Oncol. (1990) 8:1310–20. doi: 10.1200/JCO.1990.8.8.1310
38. Fisher, B , Costantino, J , Redmond, C , Poisson, R , Bowman, D , Couture, J, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. (1989) 320:479–84. doi: 10.1056/NEJM198902233200802
39. Davies, C , Godwin, J , Gray, R , Clarke, M , Cutter, D , Darby, S, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. (2011) 378:771–84. doi: 10.1016/S0140-6736(11)60993-8
40. Habel, LA , Moe, RE , Daling, JR , Holte, S , Rossing, MA , and Weiss, NS . Risk of contralateral breast cancer among women with carcinoma in situ of the breast. Ann Surg. (1997) 225:69–75. doi: 10.1097/00000658-199701000-00008
41. Fisher, B , Dignam, J , Wolmark, N , Wickerham, DL , Fisher, ER , Mamounas, E, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. (1999) 353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9
42. Houghton, J , George, WD , Cuzick, J , Duggan, C , Fentiman, IS , Spittle, M, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet. (2003) 362:95–102. doi: 10.1016/S0140-6736(03)13859-7
43. Cuzick, J , Sestak, I , Pinder, SE , Ellis, IO , Forsyth, S , Bundred, NJ, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. (2011) 12:21–9. doi: 10.1016/S1470-2045(10)70266-7
44. Wapnir, IL , Dignam, JJ , Fisher, B , Mamounas, EP , Anderson, SJ , Julian, TB, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. (2011) 103:478–88. doi: 10.1093/jnci/djr027
45. DeCensi, A , Bonanni, B , Maisonneuve, P , Serrano, D , Omodei, U , Varricchio, C, et al. A phase-III prevention trial of low-dose tamoxifen in postmenopausal hormone replacement therapy users: the HOT study. Ann Oncol. (2013) 24:2753–60. doi: 10.1093/annonc/mdt244
46. Cuzick, J , Sestak, I , Baum, M , Buzdar, A , Howell, A , Dowsett, M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. (2010) 11:1135–41. doi: 10.1016/S1470-2045(10)70257-6
47. Margolese, RG , Cecchini, RS , Julian, TB , Ganz, PA , Costantino, JP , Vallow, LA, et al. Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet. (2016) 387:849–56. doi: 10.1016/S0140-6736(15)01168-X
48. Pienta, KJ , Esper, PS , Zwas, F , Krzeminski, R , and Flaherty, LE . Phase II chemoprevention trial of oral fenretinide in patients at risk for adenocarcinoma of the prostate. Am J Clin Oncol. (1997) 20:36–9. doi: 10.1097/00000421-199702000-00008
49. Fisher, B , Costantino, JP , Wickerham, DL , Redmond, CK , Kavanah, M , Cronin, WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. (1998) 90:1371–88. doi: 10.1093/jnci/90.18.1371
50. Powles, T , Eeles, R , Ashley, S , Easton, D , Chang, J , Dowsett, M, et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet. (1998) 352:98–101. doi: 10.1016/S0140-6736(98)85012-5
51. Veronesi, U , Maisonneuve, P , Rotmensz, N , Costa, A , Sacchini, V , Travaglini, R, et al. Italian randomized trial among women with hysterectomy: tamoxifen and hormone-dependent breast cancer in high-risk women. J Natl Cancer Inst. (2003) 95:160–5. doi: 10.1093/jnci/95.2.160
52. Cuzick, J , Forbes, JF , Sestak, I , Cawthorn, S , Hamed, H , Holli, K, et al. Long-term results of tamoxifen prophylaxis for breast cancer--96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. (2007) 99:272–82. doi: 10.1093/jnci/djk049
53. Powles, TJ , Ashley, S , Tidy, A , Smith, IE , and Dowsett, M . Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. (2007) 99:283–90. doi: 10.1093/jnci/djk050
54. Fisher, B , Costantino, JP , Wickerham, DL , Cecchini, RS , Cronin, WM , Robidoux, A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. (2005) 97:1652–62. doi: 10.1093/jnci/dji372
55. Veronesi, U , Maisonneuve, P , Rotmensz, N , Bonanni, B , Boyle, P , Viale, G, et al. Tamoxifen for the prevention of breast cancer: late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J Natl Cancer Inst. (2007) 99:727–37. doi: 10.1093/jnci/djk154
56. Cuzick, J , Sestak, I , Cawthorn, S , Hamed, H , Holli, K , Howell, A, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. (2015) 16:67–75. doi: 10.1016/S1470-2045(14)71171-4
57. den Hollander, P , Savage, MI , and Brown, PH . Targeted therapy for breast cancer prevention. Front Oncol. (2013) 3:250. doi: 10.3389/fonc.2013.00250
58. Jahan, N , Jones, C , and Rahman, RL . Endocrine prevention of breast cancer. Mol Cell Endocrinol. (2021) 530:111284. doi: 10.1016/j.mce.2021.111284
59. Decensi, A , Robertson, C , Viale, G , Pigatto, F , Johansson, H , Kisanga, ER, et al. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst. (2003) 95:779–90. doi: 10.1093/jnci/95.11.779
60. Cummings, SR , Eckert, S , Krueger, KA , Grady, D , Powles, TJ , Cauley, JA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. (1999) 281:2189–97. doi: 10.1001/jama.281.23.2189
61. Cauley, JA , Norton, L , Lippman, ME , Eckert, S , Krueger, KA , Purdie, DW, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res Treat. (2001) 65:125–34. doi: 10.1023/A:1006478317173
62. Martino, S , Cauley, JA , Barrett-Connor, E , Powles, TJ , Mershon, J , Disch, D, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. (2004) 96:1751–61. doi: 10.1093/jnci/djh319
63. Barrett-Connor, E , Mosca, L , Collins, P , Geiger, MJ , Grady, D , Kornitzer, M, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. (2006) 355:125–37. doi: 10.1056/NEJMoa062462
64. Land, SR , Wickerham, DL , Costantino, JP , Ritter, MW , Vogel, VG , Lee, M, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. (2006) 295:2742–51. doi: 10.1001/jama.295.23.joc60075
65. Hortobagyi, GN , and Brown, PH . Two good choices to prevent breast cancer: great taste, less filling. Cancer Prev Res (Phila). (2010) 3:681–5. doi: 10.1158/1940-6207.CAPR-10-0101
66. LaCroix, AZ , Powles, T , Osborne, CK , Wolter, K , Thompson, JR , Thompson, DD, et al. Breast cancer incidence in the randomized PEARL trial of lasofoxifene in postmenopausal osteoporotic women. J Natl Cancer Inst. (2010) 102:1706–15. doi: 10.1093/jnci/djq415
67. Cuzick, J , Sestak, I , Forbes, JF , Dowsett, M , Cawthorn, S , Mansel, RE, et al. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet. (2020) 395:117–22. doi: 10.1016/S0140-6736(19)32955-1
68. Forbes, JF , Sestak, I , Howell, A , Bonanni, B , Bundred, N , Levy, C, et al. Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): a double-blind, randomised controlled trial. Lancet. (2016) 387:866–73. doi: 10.1016/S0140-6736(15)01129-0
69. Vogel, VG , Costantino, JP , Wickerham, DL , Cronin, WM , Cecchini, RS , Atkins, JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. (2006) 295:2727–41. doi: 10.1001/jama.295.23.joc60074
70. Vogel, VG , Costantino, JP , Wickerham, DL , Cronin, WM , Cecchini, RS , Atkins, JN, et al. Update of the national surgical adjuvant breast and bowel project study of tamoxifen and raloxifene (STAR) P-2 trial: preventing breast cancer. Cancer Prev Res (Phila). (2010) 3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076
71. Powles, TJ , Diem, SJ , Fabian, CJ , Neven, P , Wickerham, DL , Cox, DA, et al. Breast cancer incidence in postmenopausal women with osteoporosis or low bone mass using arzoxifene. Breast Cancer Res Treat. (2012) 134:299–306. doi: 10.1007/s10549-012-2041-5
72. Fabian, CJ , Nye, L , Powers, KR , Nydegger, JL , Kreutzjans, AL , Phillips, TA, et al. Effect of bazedoxifene and conjugated estrogen (duavee) on breast cancer risk biomarkers in high-risk women: a pilot study. Cancer Prev Res (Phila). (2019) 12:711–20. doi: 10.1158/1940-6207.CAPR-19-0315
73. DeCensi, A , Puntoni, M , Guerrieri-Gonzaga, A , Caviglia, S , Avino, F , Cortesi, L, et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J Clin Oncol. (2019) 37:1629–37. doi: 10.1200/JCO.18.01779
74. DeCensi, A , Puntoni, M , Johansson, H , Guerrieri-Gonzaga, A , Caviglia, S , Avino, F, et al. Effect modifiers of low-dose tamoxifen in a randomized trial in breast noninvasive disease. Clin Cancer Res. (2021) 27:3576–83. doi: 10.1158/1078-0432.CCR-20-4213
75. Eriksson, M , Eklund, M , Borgquist, S , Hellgren, R , Margolin, S , Thoren, L, et al. Low-dose tamoxifen for mammographic density reduction: a randomized controlled trial. J Clin Oncol. (2021) 39:1899–908. doi: 10.1200/JCO.20.02598
76. Lee, O , Page, K , Ivancic, D , Helenowski, I , Parini, V , Sullivan, ME, et al. A randomized phase II presurgical trial of transdermal 4-hydroxytamoxifen gel versus oral tamoxifen in women with ductal carcinoma in situ of the breast. Clin Cancer Res. (2014) 20:3672–82. doi: 10.1158/1078-0432.CCR-13-3045
77. Bäcklund, M , Eriksson, M , Gabrielson, M , Hammarström, M , Quay, S , Bergqvist, J, et al. Topical endoxifen for mammographic density reduction – a randomized controlled trial. Oncologist. (2022) 27:e597–600. doi: 10.1093/oncolo/oyac102
78. Sasano, H , Miki, Y , Nagasaki, S , and Suzuki, T . In situ estrogen production and its regulation in human breast carcinoma: from endocrinology to intracrinology. Pathol Int. (2009) 59:777–89. doi: 10.1111/j.1440-1827.2009.02444.x
79. Baum, M , Budzar, AU , Cuzick, J , Forbes, J , Houghton, JH , Klijn, JG, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. (2002) 359:2131–9. doi: 10.1016/s0140-6736(02)09088-8
80. Thürlimann, B , Hess, D , Köberle, D , Senn, I , Ballabeni, P , Pagani, O, et al. Anastrozole ('Arimidex') versus tamoxifen as first-line therapy in postmenopausal women with advanced breast cancer: results of the double-blind cross-over SAKK trial 21/95--a sub-study of the TARGET (Tamoxifen or 'Arimidex' Randomized Group Efficacy and Tolerability) trial. Breast Cancer Res Treat. (2004) 85:247–54. doi: 10.1023/B:BREA.0000025420.78346.f9
81. Mouridsen, H , Gershanovich, M , Sun, Y , Pérez-Carrión, R , Boni, C , Monnier, A, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. (2003) 21:2101–9. doi: 10.1200/JCO.2003.04.194
82. Paridaens, R , Dirix, L , Lohrisch, C , Beex, L , Nooij, M , Cameron, D, et al. Mature results of a randomized phase II multicenter study of exemestane versus tamoxifen as first-line hormone therapy for postmenopausal women with metastatic breast cancer. Ann Oncol. (2003) 14:1391–8. doi: 10.1093/annonc/mdg362
83. Coombes, RC , Hall, E , Gibson, LJ , Paridaens, R , Jassem, J , Delozier, T, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. (2004) 350:1081–92. doi: 10.1056/NEJMoa040331
84. Coombes, RC , Kilburn, LS , Snowdon, CF , Paridaens, R , Coleman, RE , Jones, SE, et al. Survival and safety of exemestane versus tamoxifen after 2-3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet. (2007) 369:559–70. doi: 10.1016/S0140-6736(07)60200-1
85. Goss, PE , Ingle, JN , Alés-Martínez, JE , Cheung, AM , Chlebowski, RT , Wactawski-Wende, J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. (2011) 364:2381–91. doi: 10.1056/NEJMoa1103507
86. Cheung, AM , Tile, L , Cardew, S , Pruthi, S , Robbins, J , Tomlinson, G, et al. Bone density and structure in healthy postmenopausal women treated with exemestane for the primary prevention of breast cancer: a nested substudy of the MAP.3 randomised controlled trial. Lancet Oncol. (2012) 13:275–84. doi: 10.1016/S1470-2045(11)70389-8
87. Meggetto, O , Maunsell, E , Chlebowski, R , Goss, P , Tu, D , and Richardson, H . Factors associated with early discontinuation of study treatment in the mammary prevention. 3 breast cancer chemoprevention trial. J Clin Oncol. (2017) 35:629–35. doi: 10.1200/JCO.2016.68.8895
88. de Censi, A , Serrano, D , Gandini, S , Thomas, PS , Crew, KD , Kumar, NB, et al. A randomized presurgical trial of alternative dosing of exemestane in postmenopausal women with early-stage ER-positive breast cancer. J Clin Oncol. (2022) 40:519–9. doi: 10.1200/JCO.2022.40.16_suppl.519
89. Muss, HB , Tu, D , Ingle, JN , Martino, S , Robert, NJ , Pater, JL, et al. Efficacy, toxicity, and quality of life in older women with early-stage breast cancer treated with letrozole or placebo after 5 years of tamoxifen: NCIC CTG intergroup trial MA.17. J Clin Oncol. (2008) 26:1956–64. doi: 10.1200/JCO.2007.12.6334
90. Fabian, CJ , Kimler, BF , Zalles, CM , Khan, QJ , Mayo, MS , Phillips, TA, et al. Reduction in proliferation with six months of letrozole in women on hormone replacement therapy. Breast Cancer Res Treat. (2007) 106:75–84. doi: 10.1007/s10549-006-9476-5
91. Mamounas, EP , Bandos, H , Lembersky, BC , Jeong, JH , Geyer, CE Jr, Rastogi, P, et al. Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2019) 20:88–99. doi: 10.1016/S1470-2045(18)30621-1
92. Wen, DX , Xu, YF , Mais, DE , Goldman, ME , and McDonnell, D . The A and B isoforms of the human progesterone receptor operate through distinct signaling pathways within target cells. Mol Cell Biol. (1994) 14:8356–64. doi: 10.1128/mcb.14.12.8356-8364.1994
93. Giangrande, PH , Pollio, G , and McDonnell, DP . Mapping and characterization of the functional domains responsible for the differential activity of the A and B isoforms of the human progesterone receptor. J Biol Chem. (1997) 272:32889–900. doi: 10.1074/jbc.272.52.32889
94. Giangrande, PH , Kimbrel, E , Edwards, DP , and McDonnell, DP . The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol. (2000) 20:3102–15. doi: 10.1128/MCB.20.9.3102-3115.2000
95. Shyamala, G , Yang, X , Silberstein, G , Barcellos-Hoff, MH , and Dale, E . Transgenic mice carrying an imbalance in the native ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. Proc Natl Acad Sci U S A. (1998) 95:696–701. doi: 10.1073/pnas.95.2.696
96. Mulac-Jericevic, B , Lydon, JP , DeMayo, FJ , and Conneely, OM . Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. (2003) 100:9744–9. doi: 10.1073/pnas.1732707100
97. Vegeto, E , Shahbaz, MM , Wen, DX , Goldman, ME , O'Malley, BW , and McDonnell, DP . Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. (1993) 7:1244–55. doi: 10.1210/mend.7.10.8264658
98. Graham, JD , Yeates, C , Balleine, RL , Harvey, SS , Milliken, JS , Bilous, AM, et al. Characterization of progesterone receptor A and B expression in human breast cancer. Cancer Res. (1995) 55:5063–8.
99. Mote, PA , Bartow, S , Tran, N , and Clarke, CL . Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat. (2002) 72:163–72. doi: 10.1023/A:1014820500738
100. Hopp, TA , Weiss, HL , Hilsenbeck, SG , Cui, Y , Allred, DC , Horwitz, KB, et al. Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin Cancer Res. (2004) 10:2751–60. doi: 10.1158/1078-0432.CCR-03-0141
101. Mote, PA , Gompel, A , Howe, C , Hilton, HN , Sestak, I , Cuzick, J, et al. Progesterone receptor A predominance is a discriminator of benefit from endocrine therapy in the ATAC trial. Breast Cancer Res Treat. (2015) 151:309–18. doi: 10.1007/s10549-015-3397-0
102. Osborne, CK , Schiff, R , Arpino, G , Lee, AS , and Hilsenbeck, VG . Endocrine responsiveness: understanding how progesterone receptor can be used to select endocrine therapy. Breast. (2005) 14:458–65. doi: 10.1016/j.breast.2005.08.024
103. Cui, X , Schiff, R , Arpino, G , Osborne, CK , and Lee, AV . Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol. (2005) 23:7721–35. doi: 10.1200/JCO.2005.09.004
104. Zheng, ZY , Bay, BH , Aw, SE , and Lin, VCL . A novel antiestrogenic mechanism in progesterone receptor-transfected breast cancer cells. J Biol Chem. (2005) 280:17480–7. doi: 10.1074/jbc.M501261200
105. Finlay-Schultz, J , Gillen, AE , Brechbuhl, HM , Ivie, JJ , Matthews, SB , Jacobsen, BM, et al. Breast cancer suppression by progesterone receptors is mediated by their modulation of estrogen receptors and RNA polymerase III. Cancer Res. (2017) 77:4934–46. doi: 10.1158/0008-5472.CAN-16-3541
106. Trabert, B , Bauer, DC , Buist, DSM , Cauley, JA , Falk, RT , Geczik, AM, et al. Association of circulating progesterone with breast cancer risk among postmenopausal women. JAMA Netw Open. (2020) 3:e203645. doi: 10.1001/jamanetworkopen.2020.3645
107. Hyder, SM , Huang, JC , Nawaz, Z , Boettger-Tong, H , Mäkelä, S , Chiappetta, C, et al. Regulation of vascular endothelial growth factor expression by estrogens and progestins. Environ Health Perspect. (2000) 108:785–90. doi: 10.1289/ehp.00108s5785
108. Carvajal, A , Espinoza, N , Kato, S , Pinto, M , Sadarangani, A , Monso, C, et al. Progesterone pre-treatment potentiates EGF pathway signaling in the breast cancer cell line ZR-75. Breast Cancer Res Treat. (2005) 94:171–83. doi: 10.1007/s10549-005-7726-6
109. Moore, MR , Spence, JB , Kiningham, KK , and Dillon, JL . Progestin inhibition of cell death in human breast cancer cell lines. J Steroid Biochem Mol Biol. (2006) 98:218–27. doi: 10.1016/j.jsbmb.2005.09.008
110. Joshi, PA , Jackson, HW , Beristain, AG , di Grappa, MA , Mote, PA , Clarke, CL, et al. Progesterone induces adult mammary stem cell expansion. Nature. (2010) 465:803–7. doi: 10.1038/nature09091
111. Gonzalez-Suarez, E , Jacob, AP , Jones, J , Miller, R , Roudier-Meyer, MP , Erwert, R, et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. (2010) 468:103–7. doi: 10.1038/nature09495
112. Nolan, E , Vaillant, F , Branstetter, D , Pal, B , Giner, G , Whitehead, L, et al. RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nat Med. (2016) 22:933–9. doi: 10.1038/nm.4118
113. Gnant, M , Pfeiler, G , Steger, GG , Egle, D , Greil, R , Fitzal, F, et al. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2019) 20:339–51. doi: 10.1016/S1470-2045(18)30862-3
114. Bouchard, P , Chabbert-Buffet, N , and Fauser, BC . Selective progesterone receptor modulators in reproductive medicine: pharmacology, clinical efficacy and safety. Fertil Steril. (2011) 96:1175–89. doi: 10.1016/j.fertnstert.2011.08.021
115. Gaddy, VT , Barrett, JT , Delk, JN , Kallab, AM , Porter, AG , and Schoenlein, PV . Mifepristone induces growth arrest, caspase activation, and apoptosis of estrogen receptor-expressing, antiestrogen-resistant breast cancer cells. Clin Cancer Res. (2004) 10:5215–25. doi: 10.1158/1078-0432.CCR-03-0637
116. Liang, Y , Hou, M , Kallab, A , Barrett, J , el Etreby, F , and Schoenlein, P . Induction of antiproliferation and apoptosis in estrogen receptor negative MDA-231 human breast cancer cells by mifepristone and 4-hydroxytamoxifen combination therapy: a role for TGFbeta1. Int J Oncol. (2003) 23:369–80. doi: 10.3892/ijo.23.2.369
117. Esber, N , Cherbonnier, C , Resche-Rigon, M , Hamze, A , Alami, M , Fagart, J, et al. Anti-tumoral effects of anti-progestins in a patient-derived breast cancer xenograft model. Horm Cancer. (2016) 7:137–47. doi: 10.1007/s12672-016-0255-4
118. Lee, O , Choi, MR , Christov, K , Ivancic, D , and Khan, SA . Progesterone receptor antagonism inhibits progestogen-related carcinogenesis and suppresses tumor cell proliferation. Cancer Lett. (2016) 376:310–7. doi: 10.1016/j.canlet.2016.04.010
119. Elía, A , Saldain, L , Vanzulli, SI , Helguero, LA , Lamb, CA , Fabris, V, et al. Beneficial effects of mifepristone treatment in patients with breast cancer selected by the progesterone receptor isoform ratio: results from the MIPRA trial. Clin Cancer Res. (2023) 29:866–77. doi: 10.1158/1078-0432.CCR-22-2060
120. Lee, O , Sullivan, ME , Xu, Y , Rogers, C , Muzzio, M , Helenowski, I, et al. Selective progesterone receptor modulators in early-stage breast cancer: a randomized, placebo-controlled phase II window-of-opportunity trial using telapristone acetate. Clin Cancer Res. (2020) 26:25–34. doi: 10.1158/1078-0432.CCR-19-0443
121. Poole, AJ , Li, Y , Kim, Y , Lin, SCJ , Lee, WH , and Lee, EYHP . Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science. (2006) 314:1467–70. doi: 10.1126/science.1130471
122. Communal, L , Vilasco, M , Hugon-Rodin, J , Courtin, A , Mourra, N , Lahlou, N, et al. Proliferation and ovarian hormone signaling are impaired in normal breast tissues from women with BRCA1 mutations: benefit of a progesterone receptor modulator treatment as a breast cancer preventive strategy in women with inherited BRCA1 mutations. Oncotarget. (2016) 7:45317–30. doi: 10.18632/oncotarget.9638
123. Lee, O , Bosland, MC , Wang, M , Shidfar, A , Hosseini, O , Xuei, X, et al. Selective progesterone receptor blockade prevents BRCA1-associated mouse mammary tumors through modulation of epithelial and stromal genes. Cancer Lett. (2021) 520:255–66. doi: 10.1016/j.canlet.2021.07.034
124. Bartlett, TE , Evans, I , Jones, A , Barrett, JE , Haran, S , Reisel, D, et al. Antiprogestins reduce epigenetic field cancerization in breast tissue of young healthy women. Genome Med. (2022) 14:64. doi: 10.1186/s13073-022-01063-5
125. Westhoff, CL , Guo, H , Wang, Z , Hibshoosh, H , Polaneczky, M , Pike, MC, et al. The progesterone-receptor modulator, ulipristal acetate, drastically lowers breast cell proliferation. Breast Cancer Res Treat. (2022) 192:321–9. doi: 10.1007/s10549-021-06503-1
126. Wiehle, R , Lantvit, D , Yamada, T , and Christov, K . CDB-4124, a progesterone receptor modulator, inhibits mammary carcinogenesis by suppressing cell proliferation and inducing apoptosis. Cancer Prev Res (Phila). (2011) 4:414–24. doi: 10.1158/1940-6207.CAPR-10-0244
127. Lee, O , Ivancic, D , Allu, S , Shidfar, A , Kenney, K , Helenowski, I, et al. Local transdermal therapy to the breast for breast cancer prevention and DCIS therapy: preclinical and clinical evaluation. Cancer Chemother Pharmacol. (2015) 76:1235–46. doi: 10.1007/s00280-015-2848-y
128. Lee, O , Pilewskie, M , Karlan, S , Tull, MB , Benante, K , Xu, Y, et al. Local transdermal delivery of telapristone acetate through breast skin, compared with oral treatment: a randomized double-blind, placebo-controlled phase II trial. Clin Pharmacol Ther. (2021) 109:728–38. doi: 10.1002/cpt.2041
129. Perrault, D , Eisenhauer, EA , Pritchard, KI , Panasci, L , Norris, B , Vandenberg, T, et al. Phase II study of the progesterone antagonist mifepristone in patients with untreated metastatic breast carcinoma: a National Cancer Institute of Canada Clinical Trials Group study. J Clin Oncol. (1996) 14:2709–12. doi: 10.1200/JCO.1996.14.10.2709
130. Liu, JH , Soper, D , Lukes, A , Gee, P , Kimble, T , Kroll, R, et al. Ulipristal acetate for treatment of uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. (2018) 132:1241–51. doi: 10.1097/AOG.0000000000002942
131. Hong, WK , Lippman, SM , Itri, LM , Karp, DD , Lee, JS , Byers, RM, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. (1990) 323:795–801. doi: 10.1056/NEJM199009203231205
132. Lacroix, A , and Lippman, ME . Binding of retinoids to human breast cancer cell lines and their effects on cell growth. J Clin Invest. (1980) 65:586–91. doi: 10.1172/JCI109703
133. Darro, F , Cahen, P , Vianna, A , Decaestecker, C , Nogaret, JM , Leblond, B, et al. Growth inhibition of human in vitro and mouse in vitro and in vivo mammary tumor models by retinoids in comparison with tamoxifen and the RU-486 anti-progestagen. Breast Cancer Res Treat. (1998) 51:39–55. doi: 10.1023/A:1006098124087
134. Prakash, P , Russell, RM , and Krinsky, NI . In vitro inhibition of proliferation of estrogen-dependent and estrogen-independent human breast cancer cells treated with carotenoids or retinoids. J Nutr. (2001) 131:1574–80. doi: 10.1093/jn/131.5.1574
135. Anzano, MA , Byers, SW , Smith, JM , Peer, CW , Mullen, LT , Brown, CC, et al. Prevention of breast cancer in the rat with 9-cis-retinoic acid as a single agent and in combination with tamoxifen. Cancer Res. (1994) 54:4614–7.
136. Wu, K , Kim, HT , Rodriquez, JL , Munoz-Medellin, D , Mohsin, SK , Hilsenbeck, SG, et al. 9-cis-Retinoic acid suppresses mammary tumorigenesis in C3(1)-simian virus 40 T antigen-transgenic mice. Clin Cancer Res. (2000) 6:3696–704.
137. Moon, RC , Thompson, HJ , Becci, PJ , Grubbs, CJ , Gander, RJ , Newton, DL, et al. N-(4-Hydroxyphenyl)retinamide, a new retinoid for prevention of breast cancer in the rat. Cancer Res. (1979) 39:1339–46.
138. Chiesa, F , Tradati, N , Marazza, M , Rossi, N , Boracchi, P , Mariani, L, et al. Prevention of local relapses and new localisations of oral leukoplakias with the synthetic retinoid fenretinide (4-HPR). Preliminary results. Eur J Cancer B Oral Oncol. (1992) 28:97–102. doi: 10.1016/0964-1955(92)90035-y
139. Veronesi, U , Mariani, L , Decensi, A , Formelli, F , Camerini, T , Miceli, R, et al. Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann Oncol. (2006) 17:1065–71. doi: 10.1093/annonc/mdl047
140. de Palo, G , Mariani, L , Camerini, T , Marubini, E , Formelli, F , Pasini, B, et al. Effect of fenretinide on ovarian carcinoma occurrence. Gynecol Oncol. (2002) 86:24–7. doi: 10.1006/gyno.2002.6663
141. Salata, GC , Malagó, ID , Carvalho Dartora, VFM , Marçal Pessoa, AF , Fantini, MCA , Costa, SKP, et al. Microemulsion for prolonged release of fenretinide in the mammary tissue and prevention of breast cancer development. Mol Pharm. (2021) 18:3401–17. doi: 10.1021/acs.molpharmaceut.1c00319
142. Apolinário, AC , Salata, GC , de Souza, MM , Chorilli, M , and Lopes, LB . Rethinking breast cancer chemoprevention: technological advantages and enhanced performance of a nanoethosomal-based hydrogel for topical administration of fenretinide. AAPS PharmSciTech. (2022) 23:104. doi: 10.1208/s12249-022-02257-1
143. Wu, K , Zhang, Y , Xu, XC , Hill, J , Celestino, J , Kim, HT, et al. The retinoid X receptor-selective retinoid, LGD1069, prevents the development of estrogen receptor-negative mammary tumors in transgenic mice. Cancer Res. (2002) 62:6376–80.
144. Wu, K , Kim, HT , Rodriquez, JL , Hilsenbeck, SG , Mohsin, SK , Xu, XC, et al. Suppression of mammary tumorigenesis in transgenic mice by the RXR-selective retinoid, LGD1069. Cancer Epidemiol Biomark Prev. (2002) 11:467–74.
145. Li, Y , Zhang, Y , Hill, J , Kim, HT , Shen, Q , Bissonnette, RP, et al. The rexinoid, bexarotene, prevents the development of premalignant lesions in MMTV-erbB2 mice. Br J Cancer. (2008) 98:1380–8. doi: 10.1038/sj.bjc.6604320
146. Kong, G , Kim, HT , Wu, K , DeNardo, D , Hilsenbeck, SG , Xu, XC, et al. The retinoid X receptor-selective retinoid, LGD1069, down-regulates cyclooxygenase-2 expression in human breast cells through transcription factor crosstalk: implications for molecular-based chemoprevention. Cancer Res. (2005) 65:3462–9. doi: 10.1158/0008-5472.CAN-03-2912
147. Wu, K , DuPré, E , Kim, H , Tin-U, CK , Bissonnette, RP , Lamph, WW, et al. Receptor-selective retinoids inhibit the growth of normal and malignant breast cells by inducing G1 cell cycle blockade. Breast Cancer Res Treat. (2006) 96:147–57. doi: 10.1007/s10549-005-9071-1
148. Shilkaitis, A , Bratescu, L , Green, A , Yamada, T , and Christov, K . Bexarotene induces cellular senescence in MMTV-Neu mouse model of mammary carcinogenesis. Cancer Prev Res (Phila). (2013) 6:299–308. doi: 10.1158/1940-6207.CAPR-12-0260
149. Brown, P , Arun, B , Miller, A , Isaacs, C , Gutierrez, C , Huang, J, et al. Abstract CN04-04: phase II trial of bexarotene in women at high risk of breast cancer: comparison of protein and RNA biomarkers. Cancer Prev Res. (2014) 1:CN04-04. doi: 10.1158/1940-6207.PREV-08-CN04-04
150. Lin, J , Manson, JE , Lee, IM , Cook, NR , Buring, JE , and Zhang, SM . Intakes of calcium and Vitamin D and breast cancer risk in women. Arch Intern Med. (2007) 167:1050–9. doi: 10.1001/archinte.167.10.1050
151. Chlebowski, RT , Johnson, KC , Kooperberg, C , Pettinger, M , Wactawski-Wende, J , Rohan, T, et al. Calcium plus Vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. (2008) 100:1581–91. doi: 10.1093/jnci/djn360
152. Lappe, JM , Travers-Gustafson, D , Davies, KM , Recker, RR , and Heaney, RP . Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. (2007) 85:1586–91. doi: 10.1093/ajcn/85.6.1586
153. Lappe, J , Watson, P , Travers-Gustafson, D , Recker, R , Garland, C , Gorham, E, et al. Effect of Vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA. (2017) 317:1234–43. doi: 10.1001/jama.2017.2115
154. Brisson, J , Bérubé, S , Diorio, C , Mâsse, B , Lemieux, J , Duchesne, T, et al. A randomized double-blind placebo-controlled trial of the effect of Vitamin D(3) supplementation on breast density in premenopausal women. Cancer Epidemiol Biomark Prev. (2017) 26:1233–41. doi: 10.1158/1055-9965.EPI-17-0249
155. Wood, ME , Liu, H , Storrick, E , Zahrieh, D , le-Petross, HC , Jung, SH, et al. The influence of vitamin d on mammographic density: results from CALGB 70806 (Alliance) a randomized clinical trial. Cancer Prev Res (Phila). (2021) 14:753–62. doi: 10.1158/1940-6207.CAPR-20-0581
156. Thomas, PS , Patel, AB , Lee, JJ , Liu, DD , Hernandez, M , Muzzio, M, et al. Phase I dose escalation study of topical bexarotene in women at high risk for breast cancer. Cancer Prev Res (Phila). (2023) 16:47–55. doi: 10.1158/1940-6207.CAPR-22-0210
157. Li, Y , Zhang, Y , Hill, J , Shen, Q , Kim, HT , Xu, X, et al. The Rexinoid LG100268 prevents the development of preinvasive and invasive estrogen receptor negative tumors in MMTV-erbB2 mice. Clin Cancer Res. (2007) 13:6224–31. doi: 10.1158/1078-0432.CCR-06-2681
158. Leal, AS , Zydeck, K , Carapellucci, S , Reich, LA , Zhang, D , Moerland, JA, et al. Retinoid X receptor agonist LG100268 modulates the immune microenvironment in preclinical breast cancer models. NPJ Breast Cancer. (2019) 5:39. doi: 10.1038/s41523-019-0135-5
159. Liby, K , Royce, DB , Risingsong, R , Williams, CR , Wood, MD , Chandraratna, RA, et al. A new rexinoid, NRX194204, prevents carcinogenesis in both the lung and mammary gland. Clin Cancer Res. (2007) 13:6237–43. doi: 10.1158/1078-0432.CCR-07-1342
160. Kabbinavar, FF , Zomorodian, N , Rettig, M , Khan, F , Greenwald, DR , DiCarlo, B, et al. An open-label phase II clinical trial of the RXR agonist IRX4204 in taxane-resistant, castration-resistant metastatic prostate cancer (CRPC). J Clin Oncol. (2014) 32:169–9. doi: 10.1200/jco.2014.32.4_suppl.169
161. Grubbs, CJ , Hill, DL , Bland, KI , Beenken, SW , Lin, TH , Eto, I, et al. 9cUAB30, an RXR specific retinoid, and/or tamoxifen in the prevention of methylnitrosourea-induced mammary cancers. Cancer Lett. (2003) 201:17–24. doi: 10.1016/S0304-3835(03)00461-0
162. Kolesar, JM , Andrews, S , Green, H , Havighurst, TC , Wollmer, BW , DeShong, K, et al. A randomized, placebo-controlled, double-blind, dose escalation, single dose, and steady-state pharmacokinetic study of 9cUAB30 in healthy volunteers. Cancer Prev Res (Phila). (2019) 12:903–12. doi: 10.1158/1940-6207.CAPR-19-0310
163. Cheskis, B , and Freedman, LP . Ligand modulates the conversion of DNA-bound vitamin D3 receptor (VDR) homodimers into VDR-retinoid X receptor heterodimers. Mol Cell Biol. (1994) 14:3329–38. doi: 10.1128/mcb.14.5.3329-3338.1994
164. Thompson, PD , Jurutka, PW , Haussler, CA , Whitfield, GK , and Haussler, MR . Heterodimeric DNA binding by the Vitamin D receptor and retinoid X receptors is enhanced by 1,25-dihydroxyvitamin D3 and inhibited by 9-cis-retinoic acid. Evidence for allosteric receptor interactions. J Biol Chem. (1998) 273:8483–91. doi: 10.1074/jbc.273.14.8483
165. Yasmin, R , Williams, RM , Xu, M , and Noy, N . Nuclear import of the retinoid X receptor, the Vitamin D receptor, and their mutual heterodimer. J Biol Chem. (2005) 280:40152–60. doi: 10.1074/jbc.M507708200
166. Zinser, G , Packman, K , and Welsh, J . Vitamin D(3) receptor ablation alters mammary gland morphogenesis. Development. (2002) 129:3067–76. doi: 10.1242/dev.129.13.3067
167. Zinser, GM , and Welsh, J . Accelerated mammary gland development during pregnancy and delayed postlactational involution in Vitamin D3 receptor null mice. Mol Endocrinol. (2004) 18:2208–23. doi: 10.1210/me.2003-0469
168. Santagata, S , Thakkar, A , Ergonul, A , Wang, B , Woo, T , Hu, R, et al. Taxonomy of breast cancer based on normal cell phenotype predicts outcome. J Clin Invest. (2014) 124:859–70. doi: 10.1172/JCI70941
169. KHAN, MI , BIELECKA, ZF , NAJM, MZ , BARTNIK, E , CZARNECKI, JS , CZARNECKA, AM, et al. Vitamin D receptor gene polymorphisms in breast and renal cancer: current state and future approaches (review). Int J Oncol. (2014) 44:349–63. doi: 10.3892/ijo.2013.2204
170. Iqbal, MUN , and Khan, TA . Association between Vitamin D receptor [Cdx2, Fok1, Bsm1, Apa1, Bgl1, Taq1, and Poly (A)] gene polymorphism and breast cancer: a systematic review and meta-analysis. Tumour Biol. (2017) 39:101042831773128. doi: 10.1177/1010428317731280
171. Chen, P , Hu, P , Xie, D , Qin, Y , Wang, F , and Wang, H . Meta-analysis of Vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. (2010) 121:469–77. doi: 10.1007/s10549-009-0593-9
172. Mohr, SB , Gorham, ED , Alcaraz, JE , Kane, CJ , Macera, CA , Parsons, JK, et al. Serum 25-hydroxyvitamin D and prevention of breast cancer: pooled analysis. Anticancer Res. (2011) 31:2939–48.
173. McDonnell, SL , Baggerly, C , French, CB , Baggerly, LL , Garland, CF , Gorham, ED, et al. Serum 25-hydroxyvitamin D concentrations >/=40 ng/ml are associated with >65% lower cancer risk: pooled analysis of randomized trial and prospective cohort study. PLoS One. (2016) 11:e0152441. doi: 10.1371/journal.pone.0152441
174. Frampton, RJ , Omond, SA , and Eisman, JA . Inhibition of human cancer cell growth by 1,25-dihydroxyvitamin D3 metabolites. Cancer Res. (1983) 43:4443–7.
175. Colston, KW , Mackay, AG , James, SY , Binderup, L , Chander, S , and Coombes, RC . EB1089: a new Vitamin D analogue that inhibits the growth of breast cancer cells in vivo and in vitro. Biochem Pharmacol. (1992) 44:2273–80. doi: 10.1016/0006-2952(92)90669-A
176. Swami, S , Krishnan, AV , Williams, J , Aggarwal, A , Albertelli, MA , Horst, RL, et al. Vitamin D mitigates the adverse effects of obesity on breast cancer in mice. Endocr Relat Cancer. (2016) 23:251–64. doi: 10.1530/ERC-15-0557
177. Ferronato, MJ , Salomón, DG , Fermento, ME , Gandini, NA , López Romero, A , Rivadulla, ML, et al. Vitamin D analogue: potent antiproliferative effects on cancer cell lines and lack of hypercalcemic activity. Arch Pharm (Weinheim). (2015) 348:315–29. doi: 10.1002/ardp.201400448
178. Ferronato, MJ , Nadal Serrano, M , Arenas Lahuerta, EJ , Bernadó Morales, C , Paolillo, G , Martinez-Sabadell Aliguer, A, et al. Vitamin D analogues exhibit antineoplastic activity in breast cancer patient-derived xenograft cells. J Steroid Biochem Mol Biol. (2021) 208:105735. doi: 10.1016/j.jsbmb.2020.105735
179. Horas, K , Zheng, Y , Fong-Yee, C , Macfarlane, E , Manibo, J , Chen, Y, et al. Loss of the Vitamin D receptor in human breast cancer cells promotes epithelial to mesenchymal cell transition and skeletal colonization. J Bone Miner Res. (2019) 34:1721–32. doi: 10.1002/jbmr.3744
180. Rossdeutscher, L , Li, J , Luco, AL , Fadhil, I , Ochietti, B , Camirand, A, et al. Chemoprevention activity of 25-hydroxyvitamin D in the MMTV-PyMT mouse model of breast cancer. Cancer Prev Res (Phila). (2015) 8:120–8. doi: 10.1158/1940-6207.CAPR-14-0110
181. Li, J , Luco, AL , Camirand, A , St-Arnaud, R , and Kremer, R . Vitamin D regulates CXCL12/CXCR4 and epithelial-to-mesenchymal transition in a model of breast cancer metastasis to lung. Endocrinology. (2021) 162:bqab049. doi: 10.1210/endocr/bqab049
182. Crew, KD , Anderson, GL , Hershman, DL , Terry, MB , Tehranifar, P , Lew, DL, et al. Randomized double-blind placebo-controlled biomarker modulation study of Vitamin D supplementation in premenopausal women at high risk for breast cancer (SWOG S0812). Cancer Prev Res (Phila). (2019) 12:481–90. doi: 10.1158/1940-6207.CAPR-18-0444
183. Byrne, IM , Flanagan, L , Tenniswood, MPR , and Welsh, JE . Identification of a hormone-responsive promoter immediately upstream of exon 1c in the human Vitamin D receptor gene. Endocrinology. (2000) 141:2829–36. doi: 10.1210/endo.141.8.7618
184. Wietzke, JA , and Welsh, J . Phytoestrogen regulation of a Vitamin D3 receptor promoter and 1,25-dihydroxyvitamin D3 actions in human breast cancer cells. J Steroid Biochem Mol Biol. (2003) 84:149–57. doi: 10.1016/S0960-0760(03)00024-4
185. Trivedi, T , Zheng, Y , Fournier, PGJ , Murthy, S , John, S , Schillo, S, et al. The Vitamin D receptor is involved in the regulation of human breast cancer cell growth via a ligand-independent function in cytoplasm. Oncotarget. (2017) 8:26687–701. doi: 10.18632/oncotarget.15803
186. Liby, K , Rendi, M , Suh, N , Royce, DB , Risingsong, R , Williams, CR, et al. The combination of the rexinoid, LG100268, and a selective estrogen receptor modulator, either arzoxifene or acolbifene, synergizes in the prevention and treatment of mammary tumors in an estrogen receptor-negative model of breast cancer. Clin Cancer Res. (2006) 12:5902–9. doi: 10.1158/1078-0432.CCR-06-1119
187. Mazumdar, A , Medina, D , Kittrell, FS , Zhang, Y , Hill, JL , Edwards, DE, et al. The combination of tamoxifen and the rexinoid LG100268 prevents ER-positive and ER-negative mammary tumors in p53-null mammary gland mice. Cancer Prev Res (Phila). (2012) 5:1195–202. doi: 10.1158/1940-6207.CAPR-11-0524
188. Fettig, LM , McGinn, O , Finlay-Schultz, J , LaBarbera, DV , Nordeen, SK , and Sartorius, CA . Cross talk between progesterone receptors and retinoic acid receptors in regulation of cytokeratin 5-positive breast cancer cells. Oncogene. (2017) 36:6074–84. doi: 10.1038/onc.2017.204
189. Filip-Psurska, B , Psurski, M , Anisiewicz, A , Libako, P , Zbrojewicz, E , Maciejewska, M, et al. Vitamin D compounds PRI-2191 and PRI-2205 enhance anastrozole activity in human breast cancer models. Int J Mol Sci. (2021) 22:2781. doi: 10.3390/ijms22052781
190. Li, Y , Cook, KL , Yu, W , Jin, L , Bouker, KB , Clarke, R, et al. Inhibition of antiestrogen-promoted pro-survival autophagy and tamoxifen resistance in breast cancer through Vitamin D receptor. Nutrients. (2021) 13:1715. doi: 10.3390/nu13051715
191. Decensi, A , Robertson, C , Guerrieri-Gonzaga, A , Serrano, D , Cazzaniga, M , Mora, S, et al. Randomized double-blind 2 x 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women. J Clin Oncol. (2009) 27:3749–56. doi: 10.1200/JCO.2008.19.3797
192. Serrano, D , Gandini, S , Guerrieri-Gonzaga, A , Feroce, I , Johansson, H , Macis, D, et al. Quality of Life in a Randomized Breast Cancer Prevention Trial of Low-Dose Tamoxifen and Fenretinide in Premenopausal Women. Cancer Prev Res (Phila). (2018) 11:811–8. doi: 10.1158/1940-6207.CAPR-18-0073
193. Cochrane, DR , Bernales, S , Jacobsen, BM , Cittelly, DM , Howe, EN , D’Amato, NC, et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. (2014) 16:R7. doi: 10.1186/bcr3599
194. Gucalp, A , Tolaney, S , Isakoff, SJ , Ingle, JN , Liu, MC , Carey, LA, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res. (2013) 19:5505–12. doi: 10.1158/1078-0432.CCR-12-3327
195. Kim, KH , Yoon, JM , Choi, AH , Kim, WS , Lee, GY , and Kim, JB . Liver X receptor ligands suppress ubiquitination and degradation of LXRalpha by displacing BARD1/BRCA1. Mol Endocrinol. (2009) 23:466–74. doi: 10.1210/me.2008-0295
196. BRACAPSVilasco, M , Communal, L , Hugon-Rodin, J , Penault-Llorca, F , Mourra, N, et al. Loss of glucocorticoid receptor activation is a hallmark of BRCA1-mutated breast tissue. Breast Cancer Res Treat. (2013) 142:283–96. doi: 10.1007/s10549-013-2722-8
Keywords: nuclear receptors, hormone receptors, breast cancer, prevention, targeted therapy
Citation: Moyer CL and Brown PH (2023) Targeting nuclear hormone receptors for the prevention of breast cancer. Front. Med. 10:1200947. doi: 10.3389/fmed.2023.1200947
Received: 05 April 2023; Accepted: 30 June 2023;
Published: 31 July 2023.
Edited by:
Anders Strom, University of Houston, United StatesReviewed by:
Xuxu Gou, University of California, San Francisco, United StatesCopyright © 2023 Moyer and Brown. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Powel H. Brown, UEhCcm93bkBtZGFuZGVyc29uLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.