
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Med. , 21 June 2023
Sec. Geriatric Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1198579
This article is part of the Research Topic Therapeutic Neuromodulation for Aging-Related Disorders Associated with the Autonomic Nervous System View all 5 articles
 Qiongnan Bao1,2†
Qiongnan Bao1,2† Yiwei Liu3†
Yiwei Liu3† Xinyue Zhang1,2†
Xinyue Zhang1,2† Yaqin Li1
Yaqin Li1 Ziqi Wang4
Ziqi Wang4 Fang Ye5
Fang Ye5 Xia He6
Xia He6 Manze Xia1,2
Manze Xia1,2 Zhenghong Chen1,2
Zhenghong Chen1,2 Jin Yao1,2
Jin Yao1,2 Wanqi Zhong1,2
Wanqi Zhong1,2 Kexin Wu1,2
Kexin Wu1,2 Ziwen Wang1,2
Ziwen Wang1,2 Mingsheng Sun1,2
Mingsheng Sun1,2 Jiao Chen1,2
Jiao Chen1,2 Xiaojuan Hong1,2
Xiaojuan Hong1,2 Ling Zhao1,2*
Ling Zhao1,2* Zihan Yin1,2*
Zihan Yin1,2* Fanrong Liang1,2*
Fanrong Liang1,2*Background: Amnestic mild cognitive impairment (aMCI) is a pre-dementia condition associated with declined cognitive function dominated by memory impairment. The occurrence of aMCI is associated with the gut-brain axis. Previous studies have shown cognitive improvements in MCI after acupuncture treatment. This study evaluates whether acupuncture can produce a therapeutic effect in patients with aMCI by modulating the gut-brain axis.
Methods and design: This is a prospective, parallel, multicenter randomized controlled trial. A total of 40 patients with aMCI will be randomly assigned to an acupuncture group (AG) or a waiting-list group (WG), participants in both groups will receive health education on improving cognitive function at each visit, and acupuncture will be conducted twice a week for 12 weeks in the AG. Another 20 matched healthy volunteers will be enrolled as normal control. The primary outcome will be the change in Alzheimer’s Disease Assessment Scale-cognitive scale score before and after treatment. Additionally, functional magnetic resonance imaging data, faeces, and blood will be collected from each participant to characterize the brain function, gut microbiota, and inflammatory cytokines, respectively. The differences between patients with aMCI and healthy participants, and the changes in the AG and WG groups before and after treatment will be observed. Ultimately, the correlation among brain function, gut microbiota, inflammatory cytokines, and clinical efficacy evaluation in patients with aMCI will be analyzed.
Discussion: This study will identify the efficacy and provide preliminary data on the possible mechanism of acupuncture in treating aMCI. Furthermore, it will also identify biomarkers of the gut microbiota, inflammatory cytokines, and brain function correlated with therapeutic effects. The results of this study will be published in peer-reviewed journals.
Clinical trial registration:
Mild cognitive impairment (MCI) refers to a transitional phase between cognitive changes of normal ageing and dementia, which is often seen in Alzheimer’s disease (AD) (1). It is characterized by a decline in memory, language and executive function (2). The prevalence of MCI varies between 10% and 20% in adults aged ≥65 years and increases with age (3), and approximately 5 million individuals in the United States aged ≥65 years have MCI (4). The proportion of people with MCI increased from 11.9% in people aged 60–69 years, 19.3% in those aged 70–79 years, and 24.4% in those aged 80–89 years in China (5). The World Health Organization has reported that more than 245 million people in the Western Pacific Region are >65 years old, with this number projected to double by 2050 (6). Thus, the number of patients with MCI tends to increase.
MCI is classified into amnestic MCI (aMCI) and non-amnestic MCI (naMCI) (2). As the most common subtype of MCI, aMCI refers to a syndrome of cognitive decline dominated by memory impairment, with preserved independent functional abilities (7). Individuals with aMCI are more likely to deteriorate to dementia than the age-matched normal population or people with naMCI (8, 9). Because of the progressive decline in cognition, aMCI markedly affects the patient’s quality of life and imposes a substantial financial burden on families and society (10). In the United States, patients with aMCI have significantly less annual household income (average of $29,754) compared to individuals with normal cognition (average of $45,500) (11). This makes aMCI a global concern that needs urgent attention and solutions.
Early recognition and effective intervention for aMCI are critical for slowing down disease progression (4). Based on the updated guideline of the American Academy of Neurology, no pharmacological or dietary agents have been proven to improve the clinical symptoms or delay the progression of aMCI (12). The efficacy of traditional drugs is insufficient (13). For example, cholinesterase inhibitors have limited effects on cognitive function over the short-term but substantially high adverse effects (14). Other management options recommended for aMCI, such as lifestyle changes, aerobic exercise and social engagement, also seem unsatisfactory (3). Therefore, alternative interventions to manage aMCI have become of interest.
As a traditional Chinese medicine (TCM) component, acupuncture has been applied to various neurological diseases for thousands of years. According to TCM theory, acupuncture involves inserting needles into acupoints to adjust Qi and blood of meridians and balance yin and yang throughout the body, thus normalizing the patient’s health status (15). Abundant randomized controlled trials (16, 17) and systematic reviews/meta-analyses (18, 19) have shown that acupuncture can be used to treat MCI. Xu et al. (20) found that the effect of acupuncture on MCI was better than western medicines. Additionally, our previous systematic review (18) indicated that acupuncture is an effective therapy for overall cognitive function improvement in patients with MCI. Acupuncture seems to be a promising complementary therapy for aMCI; however, the underlying mechanisms of acupuncture are still under debate.
Previous studies found that patients with aMCI have changes in brain structure and function, including reduced cortical thickness, and abnormal activity and functional connectivity in specific brain regions (21, 22). In recent years, evidence has established that aMCI occurrence is associated with gut microbiota alteration and neuroinflammatory response (23). Changes in gut microbiota and its metabolites interact with human brain development and cognitive function (24, 25). The dynamic bidirectional connection pathway between the gastrointestinal tract and the nervous system is termed the “gut-brain axis” (26). Gut microbiota taxa could be used as key early indicators of cognitive performance in patients with aMCI (23). Sodium oligomannate has been demonstrated to reverse cognitive impairment by remodelling the gut microbiome and limiting neuroinflammation (27). Based on this, gut microbiome dysbiosis and neuroinflammation may be critical factors contributing to cognitive impairment in patients with aMCI. In recent years, researchers found that acupuncture improved cognition in animal models of AD by modulating the microbiota-gut-brain axis (28–30). Additionally, a review demonstrated that acupuncture exerts an anti-neuroinflammation effect through multiple pathways (31). Thus, we hypothesise that acupuncture may improve the cognitive function of patients with aMCI by regulating the gut-brain axis.
Evidently, the gut-brain axis may play a crucial role in the aMCI pathogenesis. To our knowledge, no study has been conducted to elucidate the underlying mechanisms of acupuncture for aMCI based on the gut-brain axis. Therefore, we designed a parallel-arm, randomised, controlled clinical trial to explore the therapeutic mechanisms of acupuncture for aMCI from the perspective of gut-brain modulation.
1. To observe the efficacy and safety of acupuncture in patients with aMCI;
2. To investigate whether acupuncture can regulate brain function by affecting the gut microbiome and expression of inflammatory cytokines in the gut-brain axis, thus playing a role in improving cognitive function in patients with aMCI.
This is a parallel-designed, prospective, assessor-blinded multicentre (n = 4) randomised controlled study to explore whether acupuncture can improve clinical symptoms of patients with aMCI by regulating the gut-brain axis. Forty patients with aMCI will be randomly and evenly assigned to either the acupuncture group (AG) or the waiting-list group (WG). Various scales will be assessed, functional Magnetic Resonance Imaging (fMRI) scans will be performed, and blood and faeces samples will be collected from both groups of patients before and after the intervention. In addition, 20 healthy participants will be recruited as normal control, and relevant tests and examinations will be conducted at enrollment. Cognitive-related scale, daily-living ability scale, emotion scale, mental behaviour scale and sleep scale will be used to observe the changes in clinical symptoms of patients with aMCI and to identify the therapeutic effect of acupuncture on aMCI. The gut-brain interaction characteristics of patients will be determined, and the regulatory effect of acupuncture on gut microbiota, inflammation cytokines and brain function will be explored. Subsequently, the correlation analysis of clinical efficacy can be related to gut microbiota composition, inflammation cytokines and brain function. The flow chart of trial procedures is displayed in Figure 1, and the study schedule is depicted in Table 1. The trial will be conducted at four sub-centres (West China Hospital of Sichuan University, Sichuan Provincial People’s Hospital, Chengdu Fourth People’s Hospital, and Sichuan Provincial Rehabilitation Hospital). The study protocol reporting follows the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines and checklist (32).
Eligible participants will be recruited from clinical centres, communities and welfare institutes in Sichuan province. Written informed consent will be obtained before participation if the participants meet the inclusion criteria.
Patients who meet the following criteria will be included: (1) diagnosis of aMCI according to the Jak/Bondi 2014 diagnostic criteria (33); (2) right-handedness and age between 50 and 80 years; (3) disease course ≥6 months; (4) a Clinical Dementia Rating (CDR) score of 0.5; (5) a Hachinski Incheinic Score (HIS) ≤ 4; (6) education level ≥ 8 years (including vocational education); (7) volunteering to cooperate and signing an informed consent form; and (8) no contraindications to MRI scanning, such as pacemaker implantation, metal fixed dentures.
Patients who meet any of the following criteria will be excluded: (1) receiving treatment that interferes with cognitive function (e.g., management of acute psychotic episodes, such as memantine, rivasmine, donepezil); (2) a history of neurological conditions affecting cognitive function confirmed by examination, except in patients with suspected early AD, including Parkinson’s disease, vascular dementia, brain tumour, traumatic brain injury, or other diseases which might lead to neurological injury and abnormal brain structure; (3) presence of a systemic diseases that could cause cognitive decline, such as anaemia, Hashimoto’s encephalopathy, metabolic encephalopathy, hepatic encephalopathy, renal encephalopathy; (4) infection, other focal injury, multiple or important brain memory area infarcts, or severe leukodystrophy (Fazekas score ≥ 3) indicated by brain MRI; (5) a history of tumour, psychiatric illness (e.g., bipolar disorder, schizophrenia) or severe depression (Hamilton Depression [HAMD] scale score ≥ 24) and anxiety (Hamilton anxiety [HAMA] scale score ≥ 29); (6) haemorrhagic disease, bleeding tendency or severe skin infection; (7) severe drug dependence, smoking, drug or alcohol abuse; (8) pregnant, potentially pregnant, or lactating females; (9) receipt of any acupuncture therapy or participation in other clinical trials during the 6 months prior to enrolment; and (10) regularly use of probiotics, prebiotics, or antibiotics.
Participants who meet all of the following criteria will be included as normal control: (1) right-handedness and age ≥ 50 years and < 80 years; (2) no cognitive impairment according to Jak/Bondi criteria (non-subjective cognitive decline/MCI/dementia) and normal daily living ability (a Functional Activities Questionnaire [FAQ] score < 9); (3) no contraindications to MRI scanning, such as pacemaker implantation, metal fixed dentures; and (4) volunteering to cooperate and signing an informed consent form.
Participants who meet any of the following criteria will be excluded from the normal control group: (1) claustrophobia; (2) severe cranial anatomical asymmetry or definite lesions found in MRI; (3) regularly use of probiotics, prebiotics, or antibiotics; or (4) participation in other clinical trials.
Patients with aMCI will be randomly divided into AG or WG according to the computer-generated random numbers. The random sequence with an identifying letter will be sealed in a light-tight envelope, which will be opened when eligible participants are enrolled. During the research process, the sequence generation and allocation will be performed by an independent person not participating in the trial. Healthy participants will be enrolled as the normal control without randomization.
Blinded evaluation will be conducted, and patients will be treated separately. The efficacy will be evaluated by a third person unaware of the group assignments; blinded statistical analysis will be used in the data summary stage, and the investigators, clinical operators, efficacy evaluators, and data statisticians will be separated throughout the whole study process.
Previous studies (34, 35) have suggested that 15 participants should be included in each group to ensure a stable statistical effect for brain fMRI data analysis. In most similar studies (36–38) which used fMRI, gut microbiota, or inflammatory cytokines as indicators to explore the mechanisms of acupuncture, the sample size was mostly 15–20 participants per group. Thus, the sample size in our study will be 20 participants per group. Additionally, 20 healthy participants will be included.
Patients in the AG will receive acupuncture treatment. Standardised acupuncture treatment will be based on the Standards for Reporting Interventions in Controlled Trials (STRICTA) (Table 2). The following acupoints will be used: Shenting (GV 24), Baihui (GV 20), Anmian (EX-HN 18), Shenmen (HT 7), and Taixi (KI 3). The orientation and manipulation of the acupoints will be performed based on TCM standards, as described in Figure 2. Licensed acupuncturists will use single-use sterile needles (Hwato, Suzhou, China; 0.25 × 25 mm) to insert acupoints after skin disinfection. Subsequently, twisting, thrusting, and rotation will be conducted by uniform reinforcing-reducing methods to create the experience termed De Qi within the range of patient tolerance. All needles will be manipulated manually every 10 min to maintain the De Qi sensation, the twisting angle will be 90–180 degrees, and the frequency will be 60–90 times/min, with an amplitude of lifting and inserting of 0.3–0.5 cm. The treatment will include 24–30 min sessions over 12 weeks.
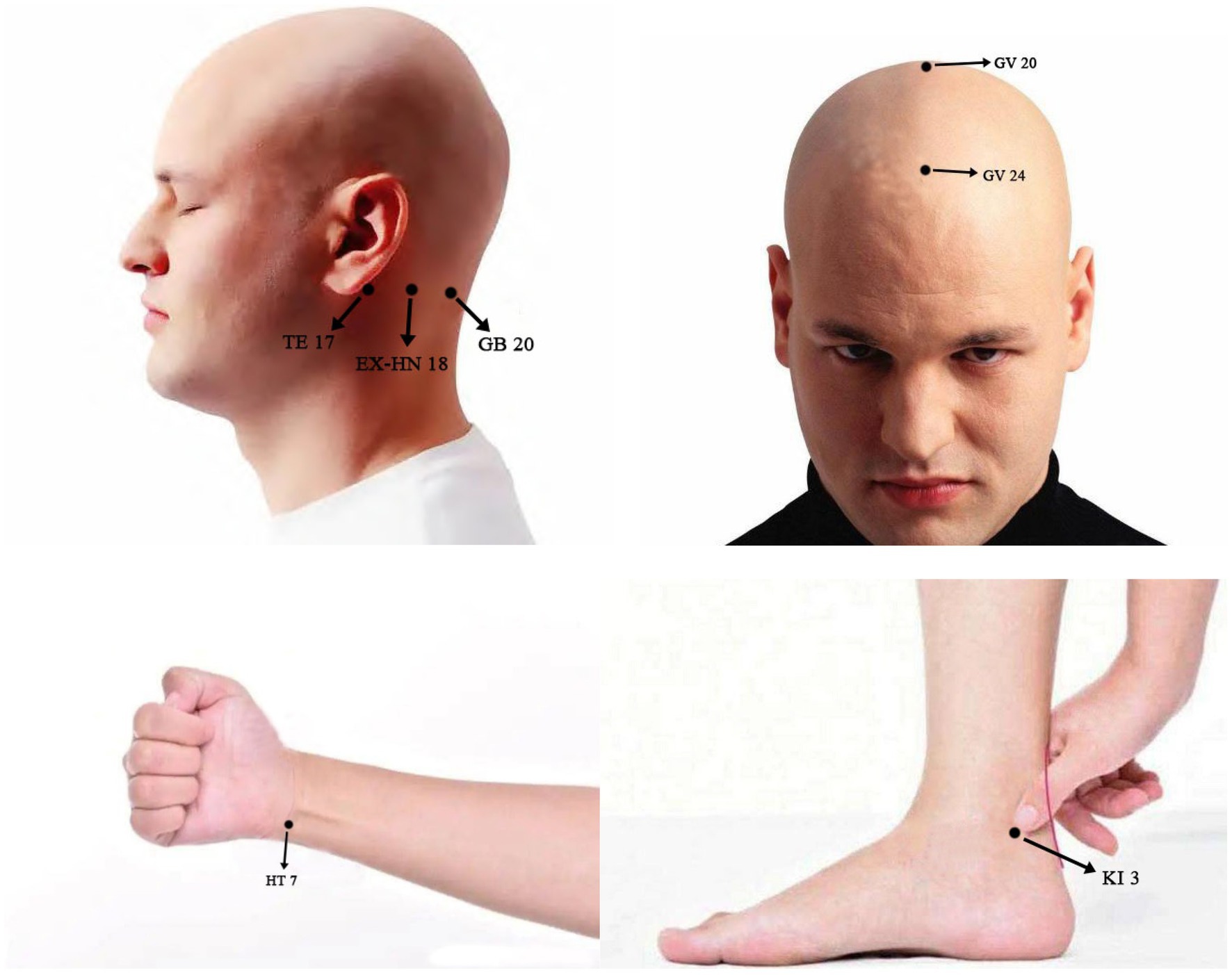
Figure 2. Locations and manipulations of acupoints selected in this study: GV 20 (Baihui), 5 cun directly above the midpoint of the anterior hairline, at the midpoit of the connecting the apexes of the two auricles. Subcutaneous insertion to a depth of 0.5-1 cun with manipulation for the deqi. GV 24 (Shenting), 0.5 cun directly above the midpoint of the anterior hairline. Subcutaneous insertion to a depth of 0.3-0.5 cun with manipulation for the deqi. EX-HN 18 (Anmian), at the neck, the midpoint of the line between GB20 (Fengchi) and TE 17 (Yifeng). Subcutaneous insertion to a depth of 0.5-1 cun with manipulation for the deqi. HT 7 (Shenmen), at the ulnar end of the transverse crease of the wrist, in the depression on the radial side of the tendon of m. flexor carpi ulnaris. Subcutaneous insertion to a depth of 0.3-0.5 cun with manipulation for the deqi. KI 3 (Taixi), posterior to the medial malleolus, in the depression between tip of the medial malleolus and tendo calcaneus. Subcutaneous insertion to depth of 0.5-1 cun with manipulation for the deqi.
Patients in the WG will not undergo acupuncture treatment during the observation period, and they will be informed that 24 free acupuncture sessions can be provided to them after observation.
Both groups will receive 10–15 min of aMCI-related health education at each visit to improve their cognitive function. The health education content is referred to in the Dietary and lifestyle guidelines for the Prevention of Alzheimer’s Disease, published in Neurobiology of Aging in 2014 (39). During the trial, patients will be allowed to receive basic treatment, such as blood pressure control, glucose control, and other supportive care. The time, dose, frequency, and response to treatment will be recorded. Participants will also be advised to avoid using other medications that affect cognitive function or acupuncture, massage, application, etc. The clinician will formulate a corresponding treatment plan and make detailed records if the symptoms are severe.
The healthy participants will not receive any treatment. Matched healthy individuals without cognitive impairment will be determined based on their medical history and cognitive function tests. The general information and cognitive assessment results will be recorded. fMRI images, faecal samples and fasting blood samples of healthy participants will be acquired at enrollment.
Changes in the Alzheimer’s Disease Assessment Scale-cognitive (ADAS-cog) score, assessed from the baseline to end of treatment, will be used to evaluate the cognitive function of patients. The ADAS-cog is designed as a rating scale for the severity of cognitive dysfunction in people with AD and comprises 12 subscales designed to assess several cognitive domains, including word recall and recognition, naming, instruction, language comprehension and expression, orientation, praxis, attention and other cognitive abilities. The total score is the sum of the scores of all subscales, with higher scores indicating greater cognitive impairment.
The following clinical efficacy-related outcomes in patients with aMCI will be used for this study: improvement of cognition, daily living ability, mental behaviour, emotion, and sleep. These outcomes will be measured at baseline and after 12 weeks of the intervention. Each participant will be evaluated by a professional physician unaware of the intervention regimen. Medication combination, compliance, dropout, and elimination cases will be recorded.
The Auditory Verbal Learning Test – Huashan version (AVLT-H) scale will be adopted to evaluate the memory function of patients. It is an objective test proven to be a sensitive measure for diagnosing aMCI (40). The word list consists of 12 two-character Chinese words from three semantic categories (flowers, occupations, and apparel), with four words in each category. The AVLT-H score is allocated as follows: (1) the immediate recall total score representing verbal working memory; (2) the short-term and long-term delayed recall score expressing recall memory; (3) the category-cued recall score; and (4) the recognition score. Considering that delayed recall memory is the first domain to be impaired in individuals with MCI (41), we will measure the changes in long-term delayed recognition scores in this study. The higher the score, the better the memory function.
The Montreal Cognitive Assessment-Basic (MoCA-B) scale will be used to evaluate the cognitive function of patients. The MoCA-B scale is an effective cognitive tool for detecting MCI among older Chinese individuals across all education levels (41). The MoCA-B assesses nine cognitive domains (executive function, language, memory, attention, orientation, calculation, concentration, conceptual thinking, and visual perception) with a total score of 30. The cut-off score for MCI is 16–22 for individuals with mid-level education and 17–24 for those with high-level education. The lower the score, the worse the cognitive function.
The FAQ will be used to evaluate the daily living ability of patients. It is a 10-item measure of difficulties in activities of daily living (42); each item is rated from 0 (no difficulty or independent) to 3 (dependent). The total severity (total sum score from all 10 items, range 0–30) reflects the extent of functional impairment. A total score > 9 indicates impairment of daily living ability; the higher the score, the lower the daily living ability.
The HAMD and HAMA scales will be used to evaluate the emotional status of patients. Anxiety and depression have been identified as risk factors for progression from aMCI to AD dementia (43). Approximately 90% of patients with AD have a variety of psychiatric symptoms, of which depression is the most common type (44, 45). The HAMD scale comprises 17 items that measure somatic and affective symptoms of depression. Each item is scored for severity on a scale of 0–4, with a higher score reflecting higher symptom severity. A total score of 0–7 is generally considered normal; ≥ 7 points indicate probable depression; ≥ 17 points, depression; and ≥ 24 points, severe depression. The HAMA scale comprises 14 items, and each item is rated from 0 (absent) to 4 (severe enough to affect daily life). The total score is the sum of the individual scores of the 14 items. The scoring standards for HAMA are: < 7 points, no anxiety; ≥ 7 points indicate probable anxiety; ≥ 14 points, anxiety; and ≥ 29 points, severe anxiety.
The Mild Behavioral Impairment Checklist (MBI-C) scale will be used to evaluate the mental behaviour of patients. Mild behavioural impairment (MBI) has been proposed as an early manifestation of dementia. The MBI-C is a reliable tool for identifying psychological and behavioural changes in patients with MCI (46). It includes 34 items organised in five domains (decreased motivation, affective dysregulation, impulse dyscontrol, social inappropriateness, and abnormal perception and thought). For each item, a “yes” or “no” question is followed by a severity rating scale of 1 – mild, 2 – moderate, or 3 – severe. MBI-C is especially useful for detecting MBI in people with MCI; the total score is the sum of scores of 34 items, with higher scores indicating the worse MBI.
The Pittsburgh Sleep Quality Index (PSQI) will be used to assess the sleep quality of the participants in the last month before and after the intervention. Sleep disturbances are common in people with MCI and may accelerate MCI progression (47). The PSQI provides a global sleep quality score based on seven components (sleep quality, latency, duration, efficiency, disturbance, use of sleep medication and daytime dysfunction). The sum of scores for these seven components yielded one total score. A global PSQI score > 5 is considered poor sleep quality.
fMRI images of all patients will be acquired using a 3.0 T superconducting magnetic resonance scanner (Siemens Medical, Erlangen, Germany) before and after the intervention. All participants will be instructed to keep their eyes closed and bodies aplanatic, and not to think or fall asleep during scanning. The scanning procedure will include a T1 – weighted imaging (T1WI) and BOLD-fMRI. The scanning parameters will be as follows: (1) T1WI: repetition time (TR)/echo time (TE)/T1 = 2000 ms/30 ms/900 ms, field of view (FOV) = 240 mm × 240 mm, voxel size = 0.9 mm × 0.9 mm × 0.9 mm, matrix = 256 × 256, flip angle (FA): 8°, and slice thickness: 0.9 mm; (2) BOLD-fMRI: TR/TE = 2000 ms/30 ms; matrix = 64 × 64, FOV = 240 mm × 240 mm, voxel size = 3.8 mm × 3.8 mm × 4.4 mm, FA = 90°, and slice thickness = 4.4 mm.
Faecal samples of all patients will be collected at the beginning and end of the intervention. In the morning, participants will be requested to collect mid-medial faecal samples (at least 2 g) using a disposable stool kit. After the samples are obtained, they will be stored at − 20°C freezers, sent to the laboratory, and placed in the refrigerator at −80°C within 24 h for preservation. We will use a metagenomic sequencing technique to determine the gut microbiota of participants. The DNA of gut microbiota from faecal samples will be extracted using the kit. Subsequently, we will establish a DNA library (paired-end; insert length 350 bp of each sample) according to the manufacturer instructions of Illumina, and conduct high-throughput sequencing using paired-end readings of length 2 × 100 bp. Subsequently, cluster analysis, assembly and annotation of metagenomic data will be conducted. Finally, the annotation, composition, differences, and comparison of species in the intestinal microbiota of patients will be analysed, and the similarities and differences in the diversity and abundance of gut flora will be compared between the AG and WG.
Fasting blood samples of all patients will be collected at the beginning and end of the intervention. After obtaining the samples, a serum sample from the participants will be collected using sterile storage tubes; next, we will weigh 500 μL serum sample per tube using a sterilised centrifuge tube, and obtain multiple tubes of each sample for backup. The collected sample will be stored at − 20°C freezers, sent to the laboratory, and placed in the refrigerator at − 80°C within 24 h. Human serum tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-18 (IL-18), cyclooxygenase-1 (COX-1), and cyclooxygenase-2 (COX-2) enzyme-linked immunosorbent assay (ELISA) kit will be used for detection.
Drug use, health education completion, acupuncture expectation and treatment satisfaction will be evaluated for patients at the beginning and end of the intervention.
Physical examinations and vital sign tests are required for all participants. If any adverse events (AEs) occur, a detailed record will be made, the participant will receive appropriate treatment immediately, and the cause of the AEs will be analysed. Most AEs after acupuncture are mild, including the sensation of soreness and distention or localised ecchymoma and bruising that disappear after rest and pressing. Serious AEs will be reported to the ethical committee of the Affiliated Hospital of Chengdu University of TCM in time. When an AE occurs, participants will decide whether or not to quit the trial based on their own will.
Before the implementation of this trial, a clinical study manual will be developed, and all investigators will receive specialised training to familiarise them with the clinical study protocol and ensure consistency in the evaluation of efficacy outcomes. Additionally, acupuncture procedures will be stipulated to ensure the accuracy of the acupuncture technique, and acupuncturists will be supervised irregularly. The case report forms will retain all original data of patients and be stored in a secure and restricted access environment. The data will then be entered into a pre-designed, password-protected electronic database by an investigator unaware of the group assignments. Only designated members of the research team will have access to the database. To ensure privacy, all study research documents will be stored in a cabinet in a locked office in Chengdu. The ethical committee of the Affiliated Hospital of Chengdu University of TCM may inspect the study records and monitor the trial.
Data analysis will be performed using IBM SPSS version 21.0 (IBM Corp, New York). All data of clinical symptom outcomes will be analysed according to the intention to treat population. The Chi-Square test/Fisher’s Exact test will be used to analyse and count data. Continuous variables as means ± standard deviation or median (± interquartile range), and categorical variables as the number and percentage of participants.
The measurement data includes various scale scores, indicators of brain function, DNA library of gut microbiota, and expression levels of inflammatory cytokines. After exploratory analyses, for variables with normal distribution, independent samples t-test will be used for inter-group comparison, and paired samples t-test will be used for intra-group comparison. The Mann–Whitney U test will be used for non-normal distributed variables. The correlation analysis between clinical efficacy, gut microbiota, inflammatory cytokines, and brain function will be conducted using multiple linear regression analysis. p < 0.05 will be considered the threshold for statistical significance.
We proposed a randomised controlled trial to investigate whether acupuncture can exert therapeutic effects in aMCI. The relationship between clinical efficacy and the gut-brain axis will also be explored.
The gut-brain axis, which is composed of the gut microbiota, central nervous system (CNS), blood–brain barrier (BBB), and various cytokines, has a profound impact on the brain and has multiple effects on memory, mood and behaviour (48). The human gastrointestinal tract is home to diverse microbial community genomes known as the “gut microbiome”. Accumulated evidence indicates that gut microbiota dysbiosis plays a crucial role in the onset and progression of MCI (23). Contrarily, MCI can also cause changes in the diversity and abundance of intestinal microbiota via the gut-brain axis (49). Studies have found that the gut microbiota genera in patients with MCI were similar to that in patients with AD but differed from that in healthy controls, and the alteration of microbiomes was correlated with the clinical severity score (50). In gut microbiome dysbiosis, gut microbiota can generate neurotoxic substances like lipopolysaccharide (LPS) and trimethylamine, which trigger the immune cells residing in the brain and activate the immune system, leading to neuroinflammatory responses and causing heightened gut permeability (51, 52), thereby influencing BBB and brain function. Data suggest that patients with MCI express greater levels of LPS and pro-inflammatory cytokines in the blood compared to healthy participants (53, 54).
Additionally, strong evidence supports that neuroinflammation is key in chronic neurodegenerative disease progression (55), and the occurrence of MCI is closely related to the neuroinflammatory response. Neuroinflammation serves as a broad range of immune responses in the CNS with microglia and astrogliosis as its pathological markers (56), presented by elevated cytokines and inflammatory mediators, causing synaptic disturbances and neuritic dystrophy thus contributing to the initial cognitive decline (57). For example, increased basal production of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) and decreased production of anti-inflammatory cytokines (IL-4 and IL-10) were observed in senescence-accelerated mouse models (58). TNF-α is a multi-potent, inflammatory cytokine that can induce apoptosis via receptor activation (55); TNF-α messenger ribonucleic acid levels are related to the degree of apoptosis in hippocampal neurons, which is closely related to cognition (55). IL-1β can upregulate the production of other pro-inflammatory cytokines, prostaglandins, and toxic mediators by initiating a vicious cycle of biochemical pathways (59). Moreover, COX-2 is an inflammatory molecule related to neurodegeneration and associated with synaptic functioning and memory formation, the expression of which is mainly observed in neurons (55). Researchers found that acupuncture reduces LPS, TNF-α and IL-1β concentration and regulates BBB disruption in animal models of AD by adjusting the gut microbiota (49). Therefore, the gut-brain axis may be the target for acupuncture treatment of aMCI.
Recently, increasing number of randomized clinical trial (36, 60, 61) have recruited healthy volunteers and determined the pathological features in the population of patients by comparing them with patients. Patients with MCI were reported to have altered levels of peripheral and cerebrospinal fluid inflammatory markers, abnormal cognitive-related brain network function, and distinct gut microbiota composition compared with healthy controls (62–64). In order to discover potential biomarkers associated with aMCI, in this study, healthy participants will be enrolled to assess fMRI, gut microbiota, and inflammatory cytokines, and the differences between patients with aMCI and healthy controls will be identified. The findings will help us to understand the gut-brain axis characteristics of patients with aMCI.
According to the theory of TCM, aMCI belongs to the “amnesia” category, caused by a deficiency of kidney essence and brain marrow. The brain is the marrow sea and the house of the original spirit. The heart stores the spirit and controls mental activities. When the original spirit is damaged, disease occurs. The GV 20 is an acupoint of the governor vessel which runs through the brain and is the most frequently used acupoint in the treatment of MCI (65). GV 24 is located at the anterior part of the frontal lobe and is closely related to memory and thinking. Studies (66, 67) have found that acupuncture at GV24 or GV20 based on the gut-brain axis can relieve gastrointestinal symptoms and regulate the gut microbiome. EX-HN 18 is an extraordinary acupoint for insomnia, associated with cognitive decline in older adults. Treatment of insomnia can help prevent cognitive decline during ageing (68, 69). HT 7 is a specific acupoint of the heart meridian, which can regulate the mind and emotion, research shows that later-life emotional disorder increase the risk of cognitive impairment (70), and the gut microbes are involved in the onset and development of mood disturbance (71). It has been discovered that the gut-brain axis may be the common target of TCM treatment for insomnia and emotional diseases (72, 73). Besides, mindful awareness practice can ameliorate cognitive impairment and modulate the gut microbiome (74). KI 3, an acupoint of the kidney meridian, has the effect of tonifying the kidney and thus nourishing the brain marrow. A Delphi expert consensus survey (75) recommended selecting acupoints on the heart and kidney meridian for cognitive impairment. Thus, the above acupoints were selected to treat aMCI in this study.
The protocol has some limitations. First, the patients and acupuncturists cannot be blinded due to the nature of the intervention. In order to minimise the performance and detection bias, allocation concealment will be conducted in this study, and outcome measures will be taken by an assessor blinded to group allocation and intervention. Second, a fixed acupuncture regimen is designed and will be used for every participant in the trial without syndrome differentiation, which may fail to show the full efficacy of acupuncture. Third, the sample size is relatively small. The potential limitations of this study may impact the results, which may lead to the need for future studies.
This is the first randomised trial to integrate acupuncture into the gut-brain axis in patients with aMCI. Brain function, gut flora, and neuroinflammatory cytokines are indicators of functioning of the gut-brain axis. The differences in the above indicators between patients with aMCI and healthy individuals will be identified to determine gut-brain interaction characteristics in patients with aMCI. Additionally, changes in these indicators between the AG and WG before and after treatment will also be investigated to illustrate the potential mechanism of acupuncture’s therapeutic effects on patients with aMCI. The main advantage of this study is the collection and evaluation of multifaceted data. We hypothesise that acupuncture therapy can improve clinical symptoms in patients with aMCI by regulating the gut-brain axis. If the hypothesis is proven, acupuncture therapy would not only serve as a novel alternative approach for treating aMCI, but also offer new information to further clarify the mechanism of the effects of acupuncture.
The local ethical committee of the Affiliated Hospital of Chengdu University of TCM has approved the study protocol (ethical approval number: 2022KL – 041).
FRL was the study sponsor. FRL, LZ, and ZHY conceived the study. QNB, YWL, and XYZ wrote the first draft of the current protocol, with FRL, ZHY, and LZ providing input to the final draft. ZHY, YQL, QNB, ZQW, FY, XH, YWL, XYZ, MZX, ZHC, JY, WQZ, and KXW were in charge of recruiting participants. ZHY, YWL, YQL, and XYZ provided treatment. ZWW and MSS carried out statistical analysis in the trial. JC and XJH were responsible for the quality control of the trial. All authors contributed to the article and approved the submitted version.
This study was financially supported by the Central financial transfer payment to local projects in 2022 of the National Administration of Traditional Chinese Medicine.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Petersen, RC, Smith, GE, Waring, SC, Ivnik, RJ, Tangalos, EG, and Kokmen, E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. (1999) 56:303–8. doi: 10.1001/archneur.56.3.303
2. Petersen, RC. Mild cognitive impairment as a diagnostic entity. Alzheimers Dement. (2008) 256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x
3. Langa, KM, and Levine, DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. (2014) 312:2551–61. doi: 10.1001/jama.2014.13806
4. 2022 Alzheimer's disease facts and figures. Alzheimers Dement. (2022) 18:700–89. doi: 10.1002/alz.12638
5. Jia, L, Du, Y, Chu, L, Zhang, Z, Li, F, Lyu, D, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. (2020) 5:e661–71. doi: 10.1016/S2468-2667(20)30185-7
6. WHO. Aging and health. Available at: https://www.who.int/westernpacific/health-topics/aging#tab=tab_1 (Accessed February 20, 2023).
7. Petersen, RC. Clinical practice. Mild cognitive impairment. N Engl J Med. (2011) 364:2227–34. doi: 10.1056/NEJMcp0910237
8. Petersen, RC, Doody, R, Kurz, A, Mohs, RC, Morris, JC, Rabins, PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. (2001) 58:1985–92. doi: 10.1001/archneur.58.12.1985
9. Petersen, RC, Stevens, JC, Ganguli, M, Tangalos, EG, Cummings, JL, and DeKosky, ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the quality standards Subcommittee of the American Academy of neurology. Neurology. (2001) 56:1133–42. doi: 10.1212/wnl.56.9.1133
10. Tahami Monfared, AA, Byrnes, MJ, White, LA, and Zhang, Q. The humanistic and economic burden of Alzheimer's disease. Neurol Ther. (2022) 11:525–51. doi: 10.1007/s40120-022-00335-x
11. Ton, TGN, DeLeire, T, May, SG, Hou, N, Tebeka, MG, Chen, E, et al. The financial burden and health care utilization patterns associated with amnestic mild cognitive impairment. Alzheimers Dement. (2017) 13:217–24. doi: 10.1016/j.jalz.2016.08.009
12. Petersen, RC, Lopez, O, Armstrong, MJ, Getchius, T, Ganguli, M, Gloss, D, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of neurology. Neurology. (2018) 90:126–35. doi: 10.1212/WNL.0000000000004826
13. Winblad, B, Palmer, K, Kivipelto, M, Jelic, V, Fratiglioni, L, Wahlund, L, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the international working group on mild cognitive impairment. J Intern Med. (2004) 256:240–6. doi: 10.1111/j.1365-2796.2004.01380.x
14. Patnode, CD, Perdue, LA, Rossom, RC, Rushkin, MC, Redmond, N, Thomas, RG, et al. Screening for cognitive impairment in older adults: an evidence update for the U.S. preventive services task force [internet] Agency for Healthcare Research and Quality (US) (2020).
15. Zhang, B, Shi, H, Cao, S, Xie, L, Ren, P, Wang, J, et al. Revealing the magic of acupuncture based on biological mechanisms: A literature. Biosci Trends. (2022) 16:73–90. doi: 10.5582/bst.2022.01039
16. Kim, J, Cho, M, Shin, J, Park, G, and Lee, J. Factors contributing to cognitive improvement effects of acupuncture in patients with mild cognitive impairment: a pilot randomized controlled trial. Trials. (2021) 22:341. doi: 10.1186/s13063-021-05296-4
17. Yuan, H, Liu, Y, Zhang, H, Liu, Y, Li, X, and Ni, J. Tongdu Xingshen acupuncture and moxibustion combined with cognitive training in treatment of post-stroke mild cognitive impairment: a randomized controlled trial. Zhongguo Zhen Jiu. (2022) 42:839–43. doi: 10.13703/j.0255-2930.20210811-0005
18. Yin, Z, Li, Y, Jiang, C, Xia, M, Chen, Z, Zhang, X, et al. Acupuncture for mild cognitive impairment: a systematic review with meta-analysis and trial sequential analysis. Front Neurol. (2022) 13:1091125. doi: 10.3389/fneur.2022.1091125
19. Kim, H, Kim, HK, Kim, SY, Kim, YI, Yoo, HR, and Jung, IC. Cognitive improvement effects of electro-acupuncture for the treatment of MCI compared with Western medications: a systematic review and Meta-analysis. BMC Complement Altern Med. (2019) 19:13. doi: 10.1186/s12906-018-2407-2
20. Xu, J, and Peng, C. The clinical study of the electroacupuncture for treatment of amnestic mild cognitive impairment. Chin J Gen Pract. (2017) 15:393–6. doi: 10.16766/j.cnki.issn.1674-4152.2017.03.009
21. Li, K, Qu, H, Ma, M, Xia, C, Cai, M, Han, F, et al. Correlation between brain structure atrophy and plasma amyloid-β and phosphorylated tau in patients with Alzheimer's disease and amnestic mild cognitive impairment explored by surface-based Morphometry. Front Aging Neurosci. (2022) 14:816043. doi: 10.3389/fnagi.2022.816043
22. Min, J, Zhou, X, Zhou, F, Tan, Y, and Wang, W. A study on changes of the resting-state brain function network in patients with amnestic mild cognitive impairment. Braz J Med Biol Res. (2019) 52:e8244. doi: 10.1590/1414-431X20198244
23. Aljumaah, MR, Bhatia, U, Roach, J, Gunstad, J, and Azcarate Peril, MA. The gut microbiome, mild cognitive impairment, and probiotics: A randomized clinical trial in middle-aged and older adults. Clin Nutr. (2022) 41:2565–76. doi: 10.1016/j.clnu.2022.09.012
24. Singh, N, Singh, V, Rai, SN, Mishra, V, Vamanu, E, and Singh, MP. Deciphering the gut microbiome in neurodegenerative diseases and metagenomic approaches for characterization of gut microbes. Biomed Pharmacother. (2022) 156:113958. doi: 10.1016/j.biopha.2022.113958
25. Sharon, G, Sampson, TR, Geschwind, DH, and Mazmanian, SK. The central nervous system and the gut microbiome. Cells. (2016) 167:915–32. doi: 10.1016/j.cell.2016.10.027
26. Westfall, S, Lomis, N, Kahouli, I, Dia, SY, Singh, SP, and Prakash, S. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci. (2017) 74:3769–87. doi: 10.1007/s00018-017-2550-9
27. Wang, X, Sun, G, Feng, T, Zhang, J, Huang, X, Wang, T, et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer's disease progression. Cell Res. (2019) 29:787–803. doi: 10.1038/s41422-019-0216-x
28. He, C, Huang, Z, Yu, C, Wang, X, Jiang, T, Wu, M, et al. Preventive electroacupuncture ameliorates D-galactose-induced Alzheimer's disease-like inflammation and memory deficits, probably via modulating the microbiota-gut-brain axis. Iran J Basic Med Sci. (2021) 24:341–8. doi: 10.22038/ijbms.2021.49147.11256
29. Yang, B, He, M, Chen, X, Sun, M, Pan, T, Xu, X, et al. Acupuncture effect assessment in APP/PS1 transgenic mice: on regulating learning-memory abilities, gut microbiota, and microbial metabolites. Comput Math Methods Med. (2022) 2022:1–20. doi: 10.1155/2022/1527159
30. Cai, M, Lee, J, and Yang, EJ. Electroacupuncture attenuates cognition impairment via anti-neuroinflammation in an Alzheimer's disease animal model. J Neuroinflammation. (2019) 16:264. doi: 10.1186/s12974-019-1665-3
31. Xin, Y, Wang, J, and Xu, A. Electroacupuncture ameliorates neuroinflammation in animal models. Acupunct Med. (2022) 40:474–83. doi: 10.1177/09645284221076515
32. Chan, A, Tetzlaff, JM, Gøtzsche, PC, Altman, DG, Mann, H, Berlin, JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. (2013) 346:e7586. doi: 10.1136/bmj.e7586
33. Bondi, MW, Edmonds, EC, Jak, AJ, Clark, LR, Delano-Wood, L, McDonald, CR, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis. (2014) 42:275–89. doi: 10.3233/JAD-140276
34. Szucs, D, and Ioannidis, JP. Sample size evolution in neuroimaging research: An evaluation of highly-cited studies (1990-2012) and of latest practices (2017-2018) in high-impact journals. NeuroImage. (2020) 221:117164. doi: 10.1016/j.neuroimage.2020.117164
35. Qiu, K, Jing, M, Sun, R, Yang, J, Liu, X, He, Z, et al. The status of the quality control in acupuncture-neuroimaging studies. Evid Based Complement Alternat Med. (2016) 2016:3685785–14. doi: 10.1155/2016/3685785
36. Kang, B, Zhao, C, Ma, J, Wang, H, Gu, X, Xu, H, et al. Electroacupuncture alleviates pain after total knee arthroplasty through regulating neuroplasticity: A resting-state functional magnetic resonance imaging study. Brain Behav. (2023) 13:e2913. doi: 10.1002/brb3.2913
37. Bao, C, Wu, L, Wang, D, Chen, L, Jin, X, Shi, Y, et al. Acupuncture improves the symptoms, intestinal microbiota, and inflammation of patients with mild to moderate Crohn's disease: A randomized controlled trial. Eclinicalmedicine. (2022) 45:101300. doi: 10.1016/j.eclinm.2022.101300
38. Friedman, R, Johnson, AR, Shillue, K, Fleishman, A, Mistretta, C, Magrini, L, et al. Acupuncture treatment for breast Cancer-related lymphedema: A randomized pilot study. Lymphat Res Biol. (2023). doi: 10.1089/lrb.2022.0001
39. Barnard, ND, Bush, AI, Ceccarelli, A, Cooper, J, de Jager, CA, Erickson, KI, et al. Dietary and lifestyle guidelines for the prevention of Alzheimer's disease. Neurobiol Aging. (2014) 35:S74–8. doi: 10.1016/j.neurobiolaging.2014.03.033
40. Zhao, Q, Lv, Y, Zhou, Y, Hong, Z, and Guo, Q. Short-term delayed recall of auditory verbal learning test is equivalent to long-term delayed recall for identifying amnestic mild cognitive impairment. PLoS One. (2012) 7:e51157. doi: 10.1371/journal.pone.0051157
41. Huang, L, Chen, K, Lin, B, Tang, L, Zhao, Q, Lv, Y, et al. Chinese version of Montreal cognitive assessment basic for discrimination among. Neuropsychiatr Dis Treat. (2018) 14:2133–40. doi: 10.2147/NDT.S174293
42. González, DA, Gonzales, MM, Resch, ZJ, Sullivan, AC, and Soble, JR. Comprehensive evaluation of the functional activities questionnaire (FAQ) and its reliability and validity. Assessment. (2022) 29:748–63. doi: 10.1177/1073191121991215
43. Palmer, K, Di Iulio, F, Varsi, AE, Gianni, W, Sancesario, G, Caltagirone, C, et al. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer's disease: the role of depression and apathy. J Alzheimers Dis. (2010) 20:175–83. doi: 10.3233/JAD-2010-1352
44. Mayor, S. Signs of depression and apathy precede memory problems in Alzheimer's disease, study shows. BMJ. (2015) 350:h190. doi: 10.1136/bmj.h190
45. Yang, H, Hong, W, Chen, L, Tao, Y, Peng, Z, and Zhou, H. Analysis of risk factors for depression in Alzheimer's disease patients. Int J Neurosci. (2020) 130:1136–41. doi: 10.1080/00207454.2020.1730369
46. Xu, L, Li, T, Xiong, L, Wang, X, Ismail, Z, Fukuda, M, et al. Reliability and validity of the Chinese version of mild behavioral impairment. J Alzheimers Dis. (2021) 81:1141–9. doi: 10.3233/JAD-210098
47. Ezzati, A, and Pak, VM. The effects of time-restricted eating on sleep, cognitive decline, and Alzheimer's disease. Exp Gerontol. (2022) 171:112033. doi: 10.1016/j.exger.2022.112033
48. Russo, R, Cristiano, C, Avagliano, C, De Caro, C, La Rana, G, Raso, GM, et al. Gut-brain Axis: role of lipids in the regulation of inflammation, pain and CNS diseases. Curr Med Chem. (2018) 25:3930–52. doi: 10.2174/0929867324666170216113756
49. Zhang, Y, Ding, N, Hao, X, Zhao, J, Zhao, Y, Li, Y, et al. Manual acupuncture benignly regulates blood-brain barrier disruption and reduces lipopolysaccharide loading and systemic inflammation, possibly by adjusting the gut microbiota. Front Aging Neurosci. (2022) 14:1018371. doi: 10.3389/fnagi.2022.1018371
50. Li, B, He, Y, Ma, J, Huang, P, Du, J, Cao, L, et al. Mild cognitive impairment has similar alterations as Alzheimer's disease in gut microbiota. Alzheimers Dement. (2019) 15:1357–66. doi: 10.1016/j.jalz.2019.07.002
51. Hang, Z, Lei, T, Zeng, Z, Cai, S, Bi, W, and Du, H. Composition of intestinal flora affects the risk relationship between Alzheimer's disease/Parkinson's disease and cancer. Biomed Pharmacother. (2022) 145:112343. doi: 10.1016/j.biopha.2021.112343
52. Yu, W, Gao, D, Jin, W, Wang, Z, Li, Y, Peng, X, et al. Intestinal Flora Dysbiosis aggravates cognitive dysfunction associated with Neuroinflammation in heart failure. J Card Fail. (2020) 26:885–94. doi: 10.1016/j.cardfail.2020.02.002
53. Escobar, YH, O'Piela, D, Wold, LE, and Mackos, AR. Influence of the microbiota-gut-brain axis on cognition in Alzheimer's disease. J Alzheimers Dis. (2022) 87:17–31. doi: 10.3233/JAD-215290
54. Guzman-Martinez, L, Maccioni, RB, Andrade, V, Navarrete, LP, Pastor, MG, and Ramos-Escobar, N. Neuroinflammation as a common feature of neurodegenerative disorders. Front Pharmacol. (2019) 10:1008. doi: 10.3389/fphar.2019.01008
55. Shabab, T, Khanabdali, R, Moghadamtousi, SZ, Kadir, HA, and Mohan, G. Neuroinflammation pathways: a general review. Int J Neurosci. (2017) 127:624–33. doi: 10.1080/00207454.2016.1212854
56. Yang, Q, and Zhou, J. Neuroinflammation in the central nervous system: symphony of glial cells. Glia. (2019) 67:1017–35. doi: 10.1002/glia.23571
57. Furman, JL, Sama, DM, Gant, JC, Beckett, TL, Murphy, MP, Bachstetter, AD, et al. Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer's disease. J Neurosci. (2012) 32:16129–40. doi: 10.1523/JNEUROSCI.2323-12.2012
58. Yin, F, Sancheti, H, Patil, I, and Cadenas, E. Energy metabolism and inflammation in brain aging and Alzheimer's disease. Free Radic Biol Med. (2016) 100:108–22. doi: 10.1016/j.freeradbiomed.2016.04.200
59. Swaroop, S, Sengupta, N, Suryawanshi, AR, Adlakha, YK, and Basu, A. HSP60 plays a regulatory role in IL-1β-induced microglial inflammation via TLR4-p38 MAPK axis. J Neuroinflammation. (2016) 13:27. doi: 10.1186/s12974-016-0486-x
60. Jang, J, Kim, J, Kwon, O, Jung, SY, Lee, H, Cho, S, et al. Effectiveness and therapeutic mechanism of Pharmacopuncture for pain in Parkinson's disease: A study protocol for a pilot pragmatic randomized, Assessor-blinded, usual care-controlled, three-arm parallel trial. Int J Environ Res Public Health. (2023) 20:20. doi: 10.3390/ijerph20031776
61. Yao, J, Yan, X, Chen, L, Li, Y, Zhang, L, Chen, M, et al. Efficacy and MicroRNA-gut microbiota regulatory mechanisms of acupuncture for severe chronic constipation: study protocol for a randomized controlled trial. Front Med. (2022) 9:906403. doi: 10.3389/fmed.2022.906403
62. Pan, Q, Li, Y, Guo, K, Xue, M, Gan, Y, Wang, K, et al. Elderly patients with mild cognitive impairment exhibit altered gut microbiota. J Immunol Res. (2021) 2021:5578958. doi: 10.1155/2021/5578958
63. Li, H, Hou, X, Liu, H, Yue, C, He, Y, and Zuo, X. Toward systems neuroscience in mild cognitive impairment and Alzheimer's disease: a meta-analysis of 75 fMRI studies. Hum Brain Mapp. (2015) 36:1217–32. doi: 10.1002/hbm.22689
64. Shen, X, Niu, L, Wang, Y, Cao, X, Liu, Q, Tan, L, et al. Inflammatory markers in Alzheimer's disease and mild cognitive impairment: a meta-analysis and systematic review of 170 studies. J Neurol Neurosurg Psychiatry. (2019) 90:590–8. doi: 10.1136/jnnp-2018-319148
65. Li, H, Xue, C, and Chen, X. Rules of Acupoints selection of ancient acupuncture in treating amnestic mild cognitive impairment based on R language data mining technology. J Clin Acupunct Moxibustion. (2022) 38:47–52. doi: 10.19917/j.cnki.1005-0779.022173
66. Chen, L, Xu, W, Pei, L, Wu, X, Geng, H, Guo, J, et al. Effect of Tiaoshen Jianpi acupuncture therapy on gut microbiota and fecal short-chain fatty acids in patients with diarrhea type irritable bowel syndrome. Chinese Acupunct Moxibustion. (2021) 41:137–41. doi: 10.13703/j.0255-2930.20200205-k0002
67. Wang, J, Liu, Y, Huang, H, Wu, J, and Wang, W. Influence of acupuncture on the clinical manifestations and gastrointestinal symptoms of children with autism spectrum disorder. Zhongguo Zhen Jiu. (2022) 42:1373–6. doi: 10.13703/j.0255-2930.20220111-0004
68. Sánchez-García, S, Moreno-Tamayo, K, Ramírez-Aldana, R, García-Peña, C, Medina-Campos, RH, García Dela Torre, P, et al. Insomnia impairs both the pro-BDNF and the BDNF levels similarly to older adults with cognitive decline: An exploratory study. Int J Mol Sci. (2023) 24:24. doi: 10.3390/ijms24087387
69. Sewell, KR, Rainey-Smith, SR, Villemagne, VL, Peiffer, J, Sohrabi, HR, Taddei, K, et al. The interaction between physical activity and sleep on cognitive function and brain beta-amyloid in older adults. Behav Brain Res. (2023) 437:114108. doi: 10.1016/j.bbr.2022.114108
70. Yang, M, Chen, B, Zhou, H, Mai, N, Zhang, M, Wu, Z, et al. Relationships among short self-reported sleep duration, cognitive impairment, and insular functional connectivity in late-life depression. J Alzheimers Dis. (2023):1–11. doi: 10.3233/JAD-220968
71. Yu, S, Wang, L, Jing, X, Wang, Y, and An, C. Features of gut microbiota and short-chain fatty acids in patients with. Front Psychol. (2023) 14:1088268. doi: 10.3389/fpsyg.2023.1088268
72. Feng, W, Yang, Z, Liu, Y, Chen, R, Song, Z, Pan, G, et al. Gut microbiota: A new target of traditional Chinese medicine for insomnia. Biomed Pharmacother. (2023) 160:114344. doi: 10.1016/j.biopha.2023.114344
73. Tsai, S, Nithiyanantham, S, Satyanarayanan, SK, and Su, K. Anti-inflammatory effect of traditional Chinese medicine on the concept of mind-body Interface. Adv Exp Med Biol. (2023) 1411:435–58. doi: 10.1007/978-981-19-7376-5_19
74. Khine, WWT, Voong, ML, Ng, TKS, Feng, L, Rane, GA, Kumar, AP, et al. Mental awareness improved mild cognitive impairment and modulated gut microbiome. Aging (Albany NY). (2020) 12:24371–93. doi: 10.18632/aging.202277
Keywords: acupuncture, amnestic mild cognitive impairment, randomized controlled trial, gut-brain axis, protocol
Citation: Bao Q, Liu Y, Zhang X, Li Y, Wang Z, Ye F, He X, Xia M, Chen Z, Yao J, Zhong W, Wu K, Wang Z, Sun M, Chen J, Hong X, Zhao L, Yin Z and Liang F (2023) Clinical observation and mechanism of acupuncture on amnestic mild cognitive impairment based on the gut-brain axis: study protocol for a randomized controlled trial. Front. Med. 10:1198579. doi: 10.3389/fmed.2023.1198579
Received: 01 April 2023; Accepted: 31 May 2023;
Published: 21 June 2023.
Edited by:
Sae Uchida, Tokyo Metropolitan Institute for Geriatrics and Gerontology, JapanReviewed by:
Mayura Shimura, Tsukuba University of Technology, JapanCopyright © 2023 Bao, Liu, Zhang, Li, Wang, Ye, He, Xia, Chen, Yao, Zhong, Wu, Wang, Sun, Chen, Hong, Zhao, Yin and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fanrong Liang, YWN1cmVzZWFyY2hAMTI2LmNvbQ==; Zihan Yin, eWluemloYW5Ac3R1LmNkdXRjbS5lZHUuY24=; Ling Zhao, emhhb2xpbmdAY2R1dGNtLmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.