- 1Department of Anaesthesiology and Pain Medicine, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland
- 2Unit for Research in Anaesthesia, Department of Paediatric Anaesthesia, IRCCS Istituto Giannina Gaslini, Genova, Italy
- 3Department of Emergency Medicine, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland
- 4Department of Anaesthesiology and Intensive Care Medicine, Cantonal Hospital St. Gallen, St. Gallen, Switzerland
- 5University of Bern, Bern, Switzerland
- 6School of Medicine, Sigmund Freud University Vienna, Vienna, Austria
- 7ERC Research Net, Niel, Belgium
Introduction: Little is known about intraoperative cardiac arrest during anesthesia care. In particular, data on characteristics of cardiac arrest and neurological survival are scarce.
Methods: We conducted a single-center retrospective observational study evaluating anesthetic procedures from January 2015 until December 2021. We included patients with an intraoperative cardiac arrest and excluded cardiac arrest outside of the operating room. The primary outcome was the return of spontaneous circulation (ROSC). Secondary outcomes were sustained ROSC over 20 min, 30-day survival, and favorable neurological outcome according to Clinical Performance Category (CPC) 1 and 2.
Results: We screened 228,712 anesthetic procedures, 195 of which met inclusion criteria and were analyzed. The incidence of intraoperative cardiac arrest was 90 (CI 95% 78–103) in 100,000 procedures. The median age was 70.5 [60.0; 79.4] years, and two-thirds of patients (n = 135; 69.2%) were male. Most of these patients with cardiac arrest had ASA physical status IV (n = 83; 42.6%) or V (n = 47; 24.1%). Cardiac arrest occurred more frequently (n = 104; 53.1%) during emergency procedures than elective ones (n = 92; 46.9%). Initial rhythm was pre-dominantly non-shockable with pulseless electrical activity mostly. Most patients (n = 163/195, 83.6%; CI 95 77.6–88.5%) had at least one instance of ROSC. Sustained ROSC over 20 min was achieved in most patients with ROSC (n = 147/163; 90.2%). Of the 163 patients with ROSC, 111 (68.1%, CI 95 60.4–75.2%) remained alive after 30 days, and most (n = 90/111; 84.9%) had favorable neurological survival (CPC 1 and 2).
Conclusion: Intraoperative cardiac arrest is rare but is more likely in older patients, patients with ASA physical status ≥IV, cardiac and vascular surgery, and emergency procedures. Patients often present with pulseless electrical activity as the initial rhythm. ROSC can be achieved in most patients. Over half of the patients are alive after 30 days, most with favorable neurological outcomes, if treated immediately.
1. Introduction
Cardiac arrest is one of the leading causes of death in Europe (1). Patients with cardiac arrest must be treated immediately with basic life support to minimize no-flow time. Basic life support includes chest compressions with ventilation of the lungs, defined as cardiopulmonary resuscitation (CPR), and early defibrillation (2, 3). Early detection of cardiac arrest, high-quality CPR, and prompt defibrillation are crucial for patients’ survival with favorable neurological outcomes (4, 5). To ensure a favorable outcome, reversible causes of cardiac arrest—referred to by the mnemonic H’s and T’s—must be diagnosed and treated (4). Intraoperative cardiac arrest is a unique form of in-hospital cardiac arrest (IHCA) (6–8) and is feared by patients undergoing anesthesia (9). The limited data that exist suggest that the incidence of perioperative cardiac arrest is between 0.5 and 3 per 10,000 procedures for adult patients (10–13) and between 0.5 and 10 per 10,000 procedures for pediatric patients (14–18).
Patients undergoing anesthesia are considered highly monitored, which may contribute to early detection of cardiac arrest triggering the start of the chain of survival (6, 19). Furthermore, the personnel and equipment needed to provide advanced life support are assumed to be on site already, further accelerating proper resuscitative efforts. On the other hand, there has been a demographic change in patients undergoing anesthesia over the last decades. Nowadays, patients are older and present with a higher BMI and American Society of Anesthesiologists (ASA) physical status score (20), which contributes to a higher baseline risk for cardiac arrest (13).
A registry study with data collected from 2008 until 2012, including more than 1.8 million non-cardiac operations, identified ASA physical status, anesthesia technique, case urgency, type of surgery, and systemic inflammatory response syndrome (SIRS)/sepsis as the strongest predictors of intraoperative cardiac arrest (10). The in-hospital mortality after intraoperative cardiac arrest was reported to be around 35%, and 30-day mortality was up to 71% (21, 22). Some reports suggested that incidence is negatively associated with the higher resource areas (13, 23).
Data on the characteristics of intraoperative cardiac arrest are scarce. Furthermore, most published studies focused only on survival and did not investigate neurological outcome. Therefore, the sequela of intraoperative cardiac arrest for patients regarding neurological outcomes and health-related quality of life is underreported. Thus, we conducted our study to bridge this knowledge gap.
2. Materials and methods
2.1. Ethics committee approval and trial registration
The study protocol was approved by the responsible Cantonal Ethics Committee of Bern (BASEC 2021-02330), and the trial was registered with ClinicalTrials.gov (NCT05316779). For the retrospective part of the study, existing general consent was checked, and for the telephone interview, informed consent was obtained.
2.2. Setting
The Bern University Hospital is one of the largest academic university-affiliated hospitals in Switzerland, with emergency departments for children and adults, and is a certified cardiac arrest center (24). The Department of Anaesthesiology and Pain Medicine oversees around 32,000 procedures annually, including about 23,360 (73%) electives and 8,640 (27%) emergency procedures (25). Patients scheduled for elective surgery have a visit to the pre-anesthetic clinic around 2–8 weeks before the surgery. This aims to assess the individual perioperative risk for morbidity and mortality regarding patient and surgery-related factors. Therefore, the anesthesiologist uses the ASA physical status, a classification system considering the patients’ medical co-morbidities within five categories. In brief, a patient with ASA physical status 1 is healthy and ASA physical status 5 is moribund (26). The higher the category, the higher the peri-operative risk for morbidity and mortality (27). Individual patient-centered optimization can be undertaken during and after the pre-anesthetic visit by following departmental standard operating procedures and current guidelines (28).
Specialized pediatric anesthesiologists provide anesthesia for children <16 years of age in the Bern Children’s Hospital operating rooms. Post-resuscitation care is provided by specialized pediatric critical care physicians in the dedicated pediatric intensive care unit. Adolescents over 16 years are treated in the adults’ hospital areas. All clinical staff undergo a mandatory annual one-day European Resuscitation Council Advanced Life Support refresher course in groups of 6 persons to ensure that CPR competencies conform to current resuscitation guidelines. Furthermore, each staff member has a one-day interprofessional and interdisciplinary in-situ high-fidelity simulation training in the operating room, followed by video debriefing.
2.3. Participants
We included all cardiac arrest patients treated in the 7 years between January 1, 2015 and December 31, 2021 in the operating room under anesthesia care after the departmental anesthesia sign-in (25). We excluded patients with cardiac arrest outside the operating room (i.e., cardiac catheter laboratory, wards, intensive care units, emergency room, or other locations in the hospital), patients admitted to the operating room under ongoing CPR, and procedures with planned extracorporeal circulation or cardiopulmonary bypass after cannulation.
2.4. Procedures and measures
We conducted a two-phased single-center retrospective observational study. In the first phase, between April 14 and May 31, 2022, we screened the departmental anesthesia information system for patients with cardiac arrest. We defined cardiac arrest as at least five chest compressions or one defibrillation having been delivered to the patient, according to the definition of the 7th National Audit Project (29). Data were extracted into the departmental Research Electronic Data Capture (REDCap, Vanderbilt University, United States) using the Utstein-style template for in-hospital cardiac arrest (30). The data included patients’ demographics (sex, age, weight, and height), medical data (ASA physical status, cardiac and vascular surgery vs. non-cardiac surgery), and emergency category (elective vs. emergency procedure). In a second step, between May 1 and June 30, 2022, we invited all survivors to a telephone interview to assess their neurological status. We followed the applicable strengthening the reporting of observational studies in epidemiology (STROBE) guidelines (31).
2.4.1. Primary outcome
The primary endpoint was the return of spontaneous circulation (ROSC) over 1 min at any time during cardiopulmonary resuscitation.
2.4.2. Secondary outcomes
Secondary outcomes were sustained ROSC over 20 min, 30-day survival with favorable neurological outcome, and 3 months of survival. Neurological outcome was assessed with the cerebral performance category (CPC) (32). The CPC has a five-point scale, with lower numbers indicating better neurological outcomes. Favorable neurological survival was defined as CPC 1 and 2. Patients’ health-related quality of life was assessed with the Short Form 12 (SF-12), which has two core dimensions, the Mental component summary and the Physical component summary, covered by 12 questions, each calculated on a scale of 0–100 (33). The score is age-dependent, with higher values corresponding to better health-related quality of life. The telephone interview captured the SF-12 and the CPC for three different time points: (I) at 30 days after cardiac arrest, (II) at three months after cardiac arrest, and (III) at the time point of the telephone interview. If the telephone interview was not possible, the 30-day CPC was retrospectively determined based on documentation from the electronic medical record.
2.5. Statistical analysis
Categorical variables were given in absolute numbers and percentages. Continuous variables were presented as means ± standard deviation (SD) and skewed data with median and interquartile range [IQR]. Student’s t-tests were used to compare continuous, normally distributed data, and Mann–Whitney or Kruskal–Wallis tests for skewed data. Categorical variables were compared with chi-squared tests or Fisher’s exact tests. As exploratory analyses, univariable odds ratios were derived for a pre-selected number of variables employing logistic regression for the two binary outcomes ROSC (yes vs. no) and CPC (categories 1–2 vs. 3–5). A survival analysis using Kaplan–Meier estimates was computed for the entire cohort. We computed univariable, between-group significance tests in terms of survival times using a log-rank test concerning the type of surgery (cardio and vascular vs. non-cardiac), urgency (emergency vs. non-emergency) and the type of the initial cardiac rhythm (shockable vs. non-shockable). The significance level of probability was defined as ≤0.05. All calculations were performed with the R statistical software (34).
3. Results
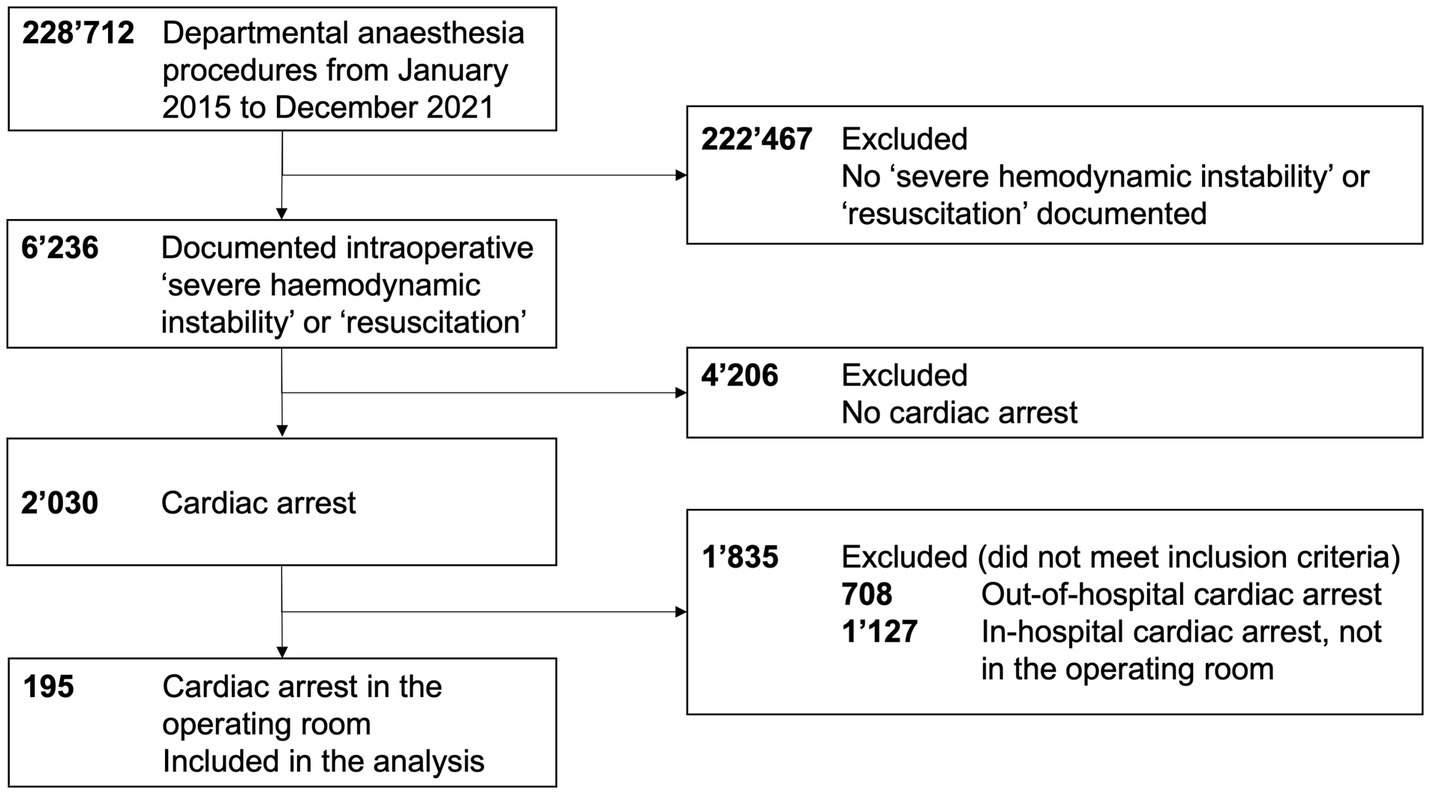
We screened 228,712 departmental anesthesia procedures, 6,245 of which were labeled with the header “severe cardiopulmonary instability” or “resuscitation” in the electronic medical record. Last, we included 195 patients with intraoperative cardiac arrest in this analysis, as displayed in Figure 1.
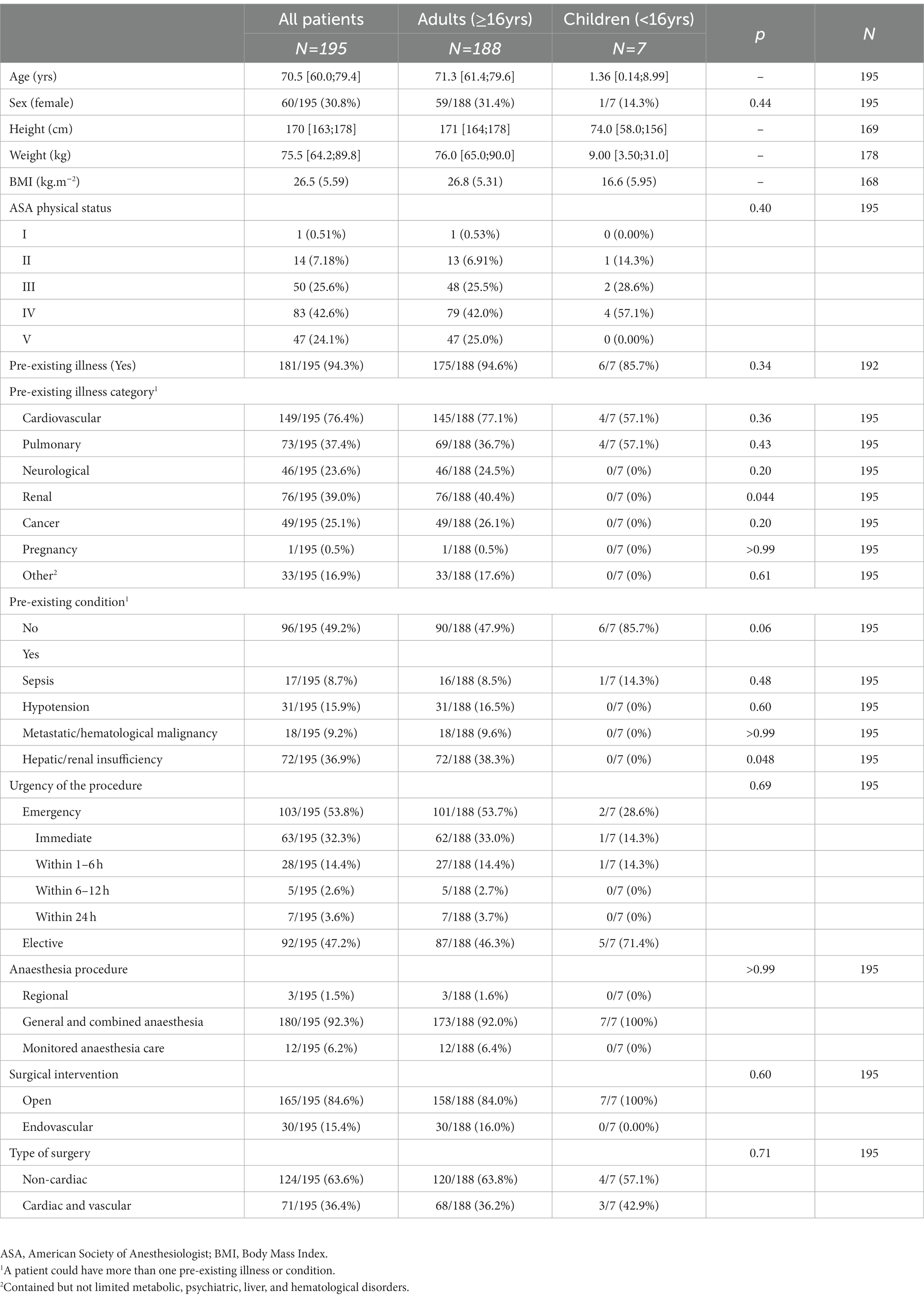
Patients’ baseline characteristics are summarized in Table 1. The median age was 70.5 [60.0; 79.4] years, and two-thirds of patients (n = 135; 69.2%) were male. Most patients had an ASA physical status ≥IV (IV; n = 83; 42.6% and V, n = 47; 24.1%). Cardiac arrest occurred more frequently (n = 103; 53.1%) during emergency procedures than elective ones (n = 92; 46.9%). The responsible surgeon classified almost one-third of the procedures (32.3%; n = 63) as immediate urgent intervention (Supplemental Tables S1, S2). The overall annual incidence of intraoperative cardiac arrest was 90 (CI 95% 78–103) in 100,000 procedures, with 24 (CI 95% 10–50) in children and 100 (CI 95% 86–115) in adults, respectively. The estimated annual incidence of intraoperative cardiac arrest was 58 (CI 95% 47–71) in 100,000 for elective and 175 (CI 95% 143–213) in 100,000 for emergency procedures.
3.1. Intraoperative cardiac arrest
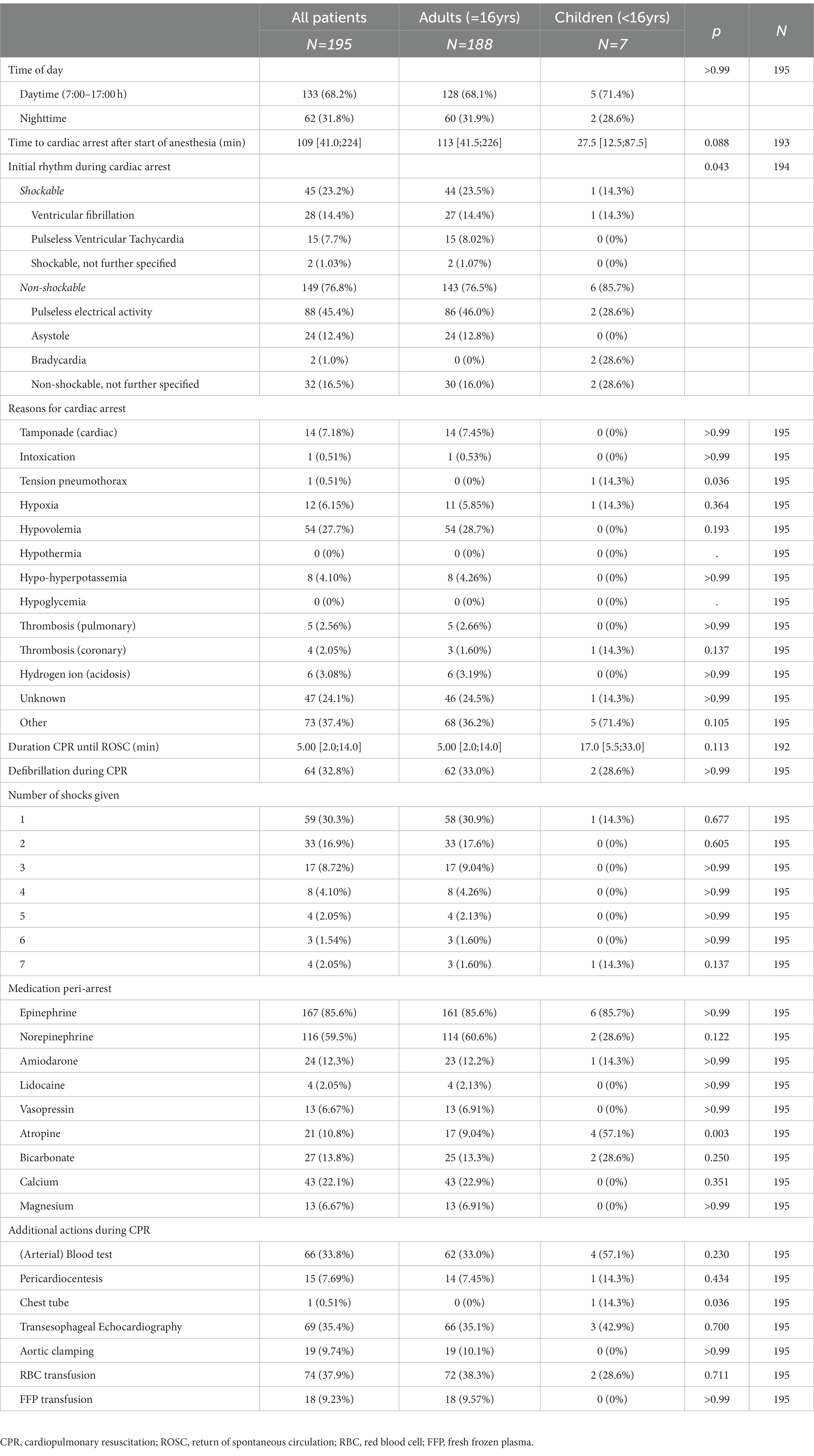
Cardiac arrest-related data are summarized in Table 2. The median time to cardiac arrest after the start of anesthesia was 109 [41.0; 224.0] minutes. Initial rhythm was pre-dominantly non-shockable and mostly pulseless electrical activity (PEA). The median duration of CPR until ROSC was 5.0 [2.0; 14.0] minutes. One-third of the patients (n = 64; 32.8%) received one or more defibrillations during CPR. Univariable (unadjusted) odds ratios for the outcome ROSC were lower for patients with ASA physical status IV-V (OR 0.11; CI 95% 0.02–0.37; p = 0.003) and cardiac and vascular surgery (OR 0.37; CI 95% 0.17–0.81; p = 0.013), as summarized in Table 3. Longer CPR duration in minutes also had a lower odds ratio for ROSC (OR 0.94; CI 95% 0.92–0.97; p < 0.001), but was only an effect modulator.

Table 3. Univariable (unadjusted) odds ratios for the outcomes (A) return of spontaneous circulation (ROSC) and (B) 30-day Clinical Performance Category (CPC) 1 and 2 indicating favorable neurological survival.
3.2. Survival and neurological outcomes
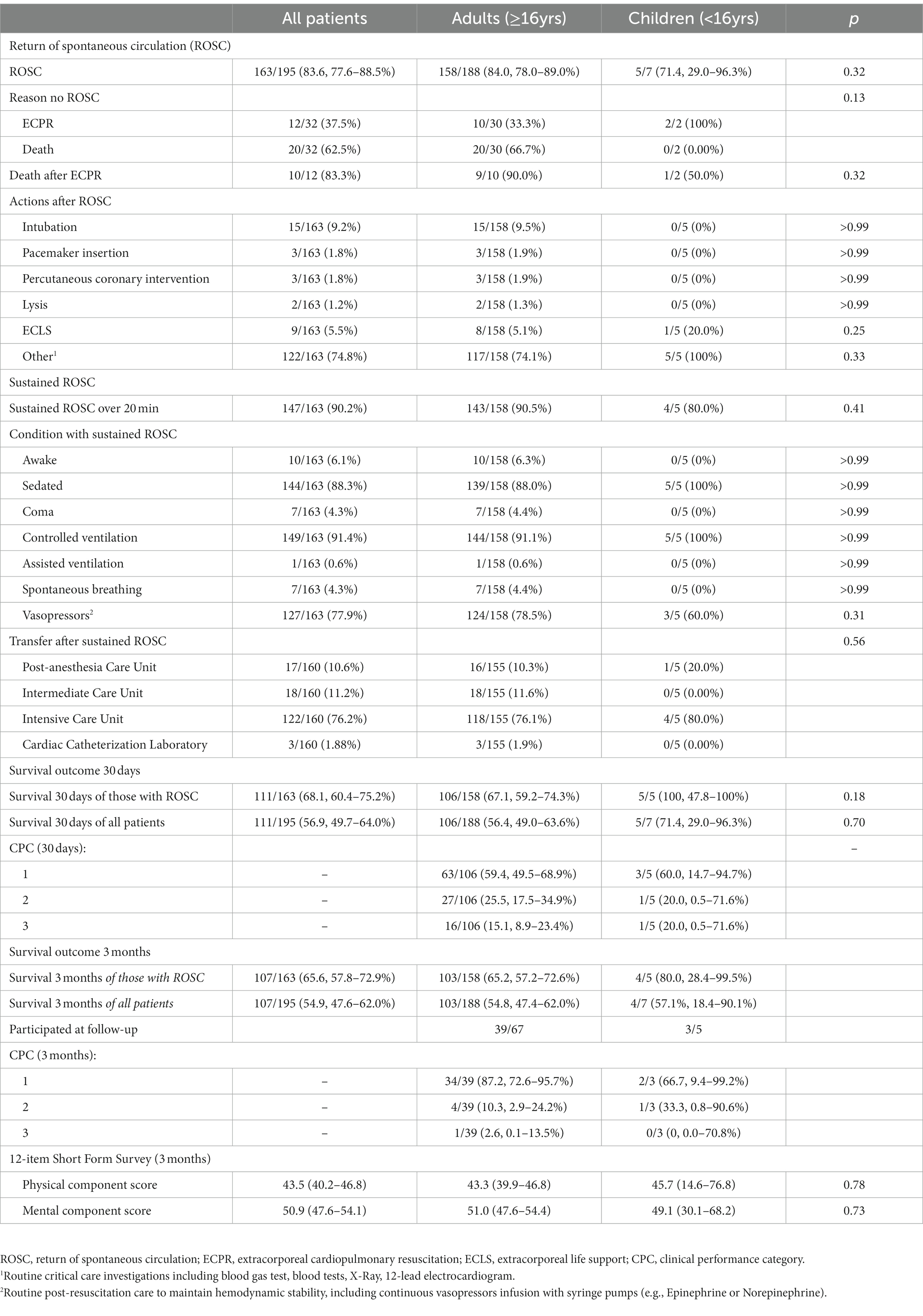
Primary and secondary outcomes are summarized in Table 4. Most patients (n = 163/195, 83.6%; CI 95 77.6–88.5%) had ROSC at least once, while the others died (n = 20/32; 62.5%) or received extracorporeal cardiopulmonary resuscitation (ECPR) (n = 12/32; 37.5%). Sustained ROSC over 20 min was achieved in most patients with ROSC (n = 147/163; 90.2%). Of the 163 patients with ROSC, 111 (68.1, 60.4–75.2%) remained alive after 30 days (Supplemental Tables S3, S4). Most (n = 90; 84.9%) of the patients with ROSC had favorable neurological survival (CPC 1 and 2). Long-term survival was higher for elective procedures than for emergency procedures, as displayed in Figure 2. However, we could not determine which factors produced higher odds ratios for favorable neurological survival, as summarized in Table 3.

Figure 2. Kaplan–Meier survival estimates for (A) all patients, (B) emergency vs. elective procedures, (C) cardiac and vascular vs. non-cardiac surgery, and (D) shockable vs. non-shockable first rhythm during cardiac arrest.
3.3. Follow-up data on health-related quality of life
At the time-point of the telephone interview, 72 members of the cohort were still alive, and 42 (58.3%) participated in the assessment. The majority (n = 41/42; 97.6%) had favorable neurological survival 3 months after the cardiac arrest. The health-related quality of life after 3 months assessed with the SF-12 showed a lower physical component score and a slightly higher mental component score than in the average Swiss population (35).
4. Discussion
This retrospective single-center study assessed characteristics and neurological survival following intraoperative cardiac arrest over 7 years. The overall incidence of intraoperative cardiac arrest was 80 (CI 95% 69–92) in 100,000 procedures. Pulseless electrical activity was most often the first rhythm in this cohort. Most patients had ROSC at least once over a one-minute period and sustained ROSC over 20 min. Over half of the patients had 30-day survival, most with a favorable neurological outcome (CPC 1 and 2). The health-related quality of life assessed 3 months after the cardiac arrest with the SF-12 was lower in the physical component score but higher in the mental component score than the average Swiss population.
Our study’s cardiac arrest incidences were comparable to previously reported pediatric data (10–13) but higher for adult (14–18) patients. The higher incidence in adult patients might be explained by including cardiac and vascular surgery. However, incidences vary in their classification (e.g., cardiac and vascular vs. non-cardiac surgery or elective vs. emergency procedures) and might be a problem of definition (1). Cardiac arrest in our presented cohort occurred more frequently during emergency procedures, similar to earlier reports (10). These patients are likely underdiagnosed regarding their co-morbidities and risks for cardiac arrest compared to elective patients, who can be clinically assessed in more detail in the pre-anesthetic visit and optimized if needed (28). Thus “prevention”—identified as a crucial step to avoid or indicate preparations in case of a cardiac arrest—is often limited in emergency patients (3). Furthermore, several emergency patients, especially those with highly urgent surgery, are considered to have severe trauma (e.g., polytrauma) or non-trauma (e.g., acute aortic dissection or a diagnosis of sepsis) are not expected to survive without surgery, as reflected by the high proportion of ASA physical status V.
Pulseless electrical activity was a leading non-shockable first rhythm in the presented cohort, and might be related to hypovolemic or distributive shock. Intraoperative hypovolemic or distributive shock is characterized by reduced circulating blood volume, and can be diagnosed by severe hypotension as a surrogate. While intraoperative hemorrhage might be the most significant and obvious cause during procedures, as reflected by the high number of red blood cell transfusions in this cohort, blood loss should be carefully monitored. Investigations (e.g., measurement of hemoglobin values and hemostasis) should be undertaken to identify appropriate treatment (36). Pre-existing conditions (e.g., sepsis), pre-existing illness (e.g., renal or liver failure), and pharmacological treatment (e.g., antihypertensive, antithrombotic, or anticoagulation) can contribute to intravascular hypovolemia or vasoplegia and should be anticipated early. Avoiding perioperative hemodynamic imbalance is also a key message underlined in the European Society of Cardiology guidelines to improve patients’ outcomes in non-cardiac surgery (28). Severe anaphylaxis from medication (e.g., antibiotics, neuromuscular blocking agents) or material (e.g., latex, prosthesis) used (37), or a patient’s position during the procedure (e.g., extreme reverse Trendelenburg or beach chair positions) should also be taken into account as a cause for cardiac arrest. The anesthesiologist’s task is to prevent the relative hypovolemia caused by vasodilation due to anesthesia overdose by monitoring anesthetic depth with a patient-centered electroencephalogram, and by being vigilant concerning absolute hypovolemia by treating volume loss and hypotension at an early stage.
An analysis of more than 700 episodes of pulseless electrical activity during CPR for patients with in-hospital cardiac arrest showed that it was the primary rhythm in around 60% (n = 423) of their included episodes (38). ROSC was recorded in over half of these episodes, with initial pulseless electrical activity (n = 230). However, only 9% of these patients survived until hospital discharge. In a recent registry data analysis of over 3,400 in-hospital cardiac arrest patients, an initial shockable rhythm was associated with an increased probability of 30-day survival (RR 2.31; CI 95% 2.02–2.64) compared to a non-shockable rhythm (39). In this registry analysis, only 16% of the patients with a non-shockable rhythm survived until 30 days (39). In contrast, in our analysis, the corresponding risk ratio for 30-day survival was 1.2 (95%-CI: 0.87–1.68) with an initial shockable rhythm. In our cohort, 30-day survival was higher than reported by Stankovic et al. (39) and significantly higher than reported in the registry analysis (22). Unfortunately, all studies (22, 38, 39) did not report the neurological outcome of the patients.
Furthermore, we can add that most survivors had favorable neurological outcomes. We hypothesize that this resulted from early resuscitative interventions based on mandatory high-quality education spaced over time for the entire anesthesia team. The health-related quality of life was in line with the findings of another in-hospital cardiac arrest cohort outside the operating room from our research group (8).
Compared to cardiac arrest registry data, our study showed real-life data on intraoperative cardiac arrests derived from a large Swiss University Hospital, including cardiac and vascular surgery, an adjunct emergency room for all ages, a cardiac arrest center, and intensive care units.
4.1. Limitations
Our study has several limitations. The retrospective design might lead to an underestimation of cases and missing data. Given the overall low number of cases, the statistical analysis, especially for the pediatric cohort, should be seen as descriptive rather than explorative. Unfortunately, the cohort was not assessed by CPC and the SF-12 before hospital admission. The number of assessable survivors for the neurological follow-up might have been higher if they had been contacted directly after 3 months, and the SF-12 could have been more precise. The single-center design might also contribute to difficult generalizability of the data.
5. Conclusion
Intraoperative cardiac arrest is rare but is more likely in older patients, patients with ASA physical status ≥IV, cardiac and vascular surgery, and emergency procedures. Patients often present with pulseless electrical activity as the initial rhythm. ROSC can be achieved in most patients. Over half of the patients are alive after 30 days, most with favorable neurological outcomes, if treated immediately.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the study protocol was approved by the Ethics Commission of the Canton of Bern (BASEC 2021-02330). According to Swiss Law, participants gave general consent for analyzing their health-related data in the retrospective part of the study and written informed consent for the neurological follow-up. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
AF and LF helped with the study design, conduct, patient recruitment, analysis, manuscript writing, and funding. MC-C and MK helped with the study conduct, patient recruitment, and manuscript finalization. ND and UP helped with the study design, analysis, and manuscript finalization. MH helped with the study design, statistical analysis, and manuscript writing. TR and RG helped with the study design, conduct, analysis, manuscript writing, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by a grant from the Burgergemeinde Bern (2022–1029) and the Department of Anaesthesiology and Pain Medicine, Inselspital (FUAD-2-22). The article processing charges were covered by the University of Bern. Open access funding by University of Bern.
Acknowledgments
The authors want to thank all the patients who participated in this study and were willing to report widely on their functional and neurological status to contribute to this trial. The authors thank all the Department of Anaesthesiology and Pain Medicine clinicians. The authors thank Simon Goerge (medical student) for his help with data acquisition and Jeannie Wurz for her careful proofreading of the language.
Conflict of interest
RG is the European Resuscitation Council (ERC) Board Director of Guidelines and ILCOR, and the ILCOR Education, Implementation and Team Task Force Chair.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1198078/full#supplementary-material
Abbreviations
ASA, American Society of Anesthesiologists; CPR, cardiopulmonary resuscitation; CPC, clinical performance category; ECPR, extracorporeal cardiopulmonary resuscitation; IHCA, in-hospital cardiac arrest; OR, odds ratio; ROSC, return of spontaneous circulation; SF-12, 12-Item Short Form Survey.
References
1. Gräsner, JT , Herlitz, J , Tjelmeland, IBM , Wnent, J , Masterson, S , Lilja, G, et al. European resuscitation council guidelines 2021: epidemiology of cardiac arrest in Europe. Resuscitation. (2021) 161:61–79. doi: 10.1016/j.resuscitation.2021.02.007
2. Perkins, GD , Gräsner, JT , Semeraro, F , Olasveengen, T , Soar, J , Lott, C, et al. European resuscitation council guidelines 2021: executive summary. Resuscitation. (2021) 161:1–60. doi: 10.1016/j.resuscitation.2021.02.003
3. Andersen, LW , Holmberg, MJ , Berg, KM , Donnino, MW , and Granfeldt, A . In-hospital cardiac arrest: a review. JAMA. (2019) 321:1200–10. doi: 10.1001/jama.2019.1696
4. Soar, J , Bottiger, BW , Carli, P , Couper, K , Deakin, CD , Djarv, T, et al. European resuscitation council guidelines 2021: adult advanced life support. Resuscitation. (2021) 161:115–51. doi: 10.1016/j.resuscitation.2021.02.010
5. Bircher, NG , Chan, PS , and Xu, Y . American Heart Association's get with the guidelines-resuscitation I. delays in cardiopulmonary resuscitation, defibrillation, and epinephrine administration all decrease survival in in-hospital cardiac arrest. Anesthesiology. (2019) 130:414–22. doi: 10.1097/ALN.0000000000002563
6. Lott, C , Truhlář, A , Alfonzo, A , Barelli, A , González-Salvado, V , Hinkelbein, J, et al. European resuscitation council guidelines 2021: cardiac arrest in special circumstances. Resuscitation. (2021) 161:152–219. doi: 10.1016/j.resuscitation.2021.02.011
7. Hinkelbein, J , Andres, J , Thies, KC , and De Robertis, E . Perioperative cardiac arrest in the operating room environment: a review of the literature. Minerva Anestesiol. (2017) 83:1190–8. doi: 10.23736/S0375-9393.17.11802-X
8. Fuchs, A , Kaser, D , Theiler, L , Greif, R , Knapp, J , and Berger-Estilita, J . Survival and long-term outcomes following in-hospital cardiac arrest in a Swiss University Hospital: a prospective observational study. Scand J Trauma Resusc Emerg Med. (2021) 29:115. doi: 10.1186/s13049-021-00931-0
9. Mavridou, P , Dimitriou, V , Manataki, A , Arnaoutoglou, E , and Papadopoulos, G . Patient's anxiety and fear of anesthesia: effect of gender, age, education, and previous experience of anesthesia. A survey of 400 patients. J Anesth. (2013) 27:104–8. doi: 10.1007/s00540-012-1460-0
10. Kaiser, HA , Saied, NN , Kokoefer, AS , Saffour, L , Zoller, JK , and Helwani, MA . Incidence and prediction of intraoperative and postoperative cardiac arrest requiring cardiopulmonary resuscitation and 30-day mortality in non-cardiac surgical patients. PLoS One. (2020) 15:e0225939. doi: 10.1371/journal.pone.0225939
11. Goswami, S , Brady, JE , Jordan, DA , and Li, G . Intraoperative cardiac arrests in adults undergoing noncardiac surgery: incidence, risk factors, and survival outcome. Anesthesiology. (2012) 117:1018–26. doi: 10.1097/ALN.0b013e31827005e9
12. Sobreira-Fernandes, D , Teixeira, L , Lemos, TS , Costa, L , Pereira, M , Costa, AC, et al. Perioperative cardiac arrests – a subanalysis of the anesthesia-related cardiac arrests and associated mortality. J Clin Anesth. (2018) 50:78–90. doi: 10.1016/j.jclinane.2018.06.005
13. Bainbridge, D , Martin, J , Arango, M , and Cheng, D . Perioperative and anaesthetic-related mortality in developed and developing countries: a systematic review and meta-analysis. Lancet. (2012) 380:1075–81. doi: 10.1016/S0140-6736(12)60990-8
14. Morray, JP , Geiduschek, JM , Ramamoorthy, C , Haberkern, CM , Hackel, A , Caplan, RA, et al. Anesthesia-related cardiac arrest in children: initial findings of the pediatric perioperative cardiac arrest (POCA) registry. Anesthesiology. (2000) 93:6–14. doi: 10.1097/00000542-200007000-00007
15. Habre, W , Disma, N , Virag, K , Becke, K , Hansen, TG , Jöhr, M, et al. Incidence of severe critical events in paediatric anaesthesia (APRICOT): a prospective multicentre observational study in 261 hospitals in Europe. Lancet Respir Med. (2017) 5:412–25. doi: 10.1016/S2213-2600(17)30116-9
16. Disma, N , Veyckemans, F , Virag, K , Hansen, TG , Becke, K , Harlet, P, et al. Morbidity and mortality after anaesthesia in early life: results of the European prospective multicentre observational study, neonate and children audit of anaesthesia practice in Europe (NECTARINE). Br J Anaesth. (2021) 126:1157–72. doi: 10.1016/j.bja.2021.02.016
17. Hauser, ND , Sommerfield, A , Drake-Brockman, TFE , Slevin, L , Chambers, NA , Bergesio, R, et al. Anaesthesia related mortality data at a tertiary pediatric hospital in western Australia. Acta Anaesthesiol Scand. (2022) 67:142–9. doi: 10.1111/aas.14163
18. Meyer, HM , Thomas, J , Wilson, GS , and de Kock, M . Anesthesia-related and perioperative mortality: an audit of 8493 cases at a tertiary pediatric teaching hospital in South Africa. Paediatr Anaesth. (2017) 27:1021–7. doi: 10.1111/pan.13214
19. Klein, AA , Meek, T , Allcock, E , Cook, TM , Mincher, N , Morris, C, et al. Recommendations for standards of monitoring during anaesthesia and recovery 2021: guideline from the Association of Anaesthetists. Anaesthesia. (2021) 76:1212–23. doi: 10.1111/anae.15501
20. Kane, AD , Soar, J , Armstrong, RA , Kursumovic, E , Davies, MT , Oglesby, FC, et al. Patient characteristics, anaesthetic workload and techniques in the UK: an analysis from the 7th National Audit Project (NAP7) activity survey. Anaesthesia. (2023) 78:701–11. doi: 10.1111/anae.15989
21. Fielding-Singh, V , Willingham, MD , Fischer, MA , Grogan, T , Benharash, P , and Neelankavil, JP . A population-based analysis of intraoperative cardiac arrest in the United States. Anesth Analg. (2020) 130:627–34. doi: 10.1213/ANE.0000000000004477
22. Kazaure, HS , Roman, SA , Rosenthal, RA , and Sosa, JA . Cardiac arrest among surgical patients: an analysis of incidence, patient characteristics, and outcomes in ACS-NSQIP. JAMA Surg. (2013) 148:14–21. doi: 10.1001/jamasurg.2013.671
23. Braz, LG , Einav, S , Heesen, MA , Betini, M , Corrente, JE , Pacchioni, M, et al. Association between intra-operative cardiac arrest and country human development index status: a systematic review with meta-regression analysis and meta-analysis of observational studies. Anaesthesia. (2021) 76:1259–73. doi: 10.1111/anae.15374
24. Grübl, T , Nauheimer, D , Wolff, H , Gehret, G , Rott, N , Schmidbauer, W, et al. Zertifizierung von Cardiac-Arrest-Zentren. Notfall + Rettungsmedizin. (2022) 26:23–9. doi: 10.1007/s10049-021-00975-w
25. Fuchs, A , Frick, S , Huber, M , Riva, T , Theiler, L , Kleine-Brueggeney, M, et al. Five-year audit of adherence to an anaesthesia pre-induction checklist. Anaesthesia. (2022) 77:751–62. doi: 10.1111/anae.15704
26. American Society of Anesthesiologists . ASA physical status classification system. Available at: https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system (Accessed May 2, 2023).
27. Hackett, NJ , De Oliveira, GS , Jain, UK , and Kim, JY . ASA class is a reliable independent predictor of medical complications and mortality following surgery. Int J Surg. (2015) 18:184–90. doi: 10.1016/j.ijsu.2015.04.079
28. Halvorsen, S , Mehilli, J , Cassese, S , Hall, TS , Abdelhamid, M , Barbato, E, et al. 2022 ESC guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur Heart J. (2022) 43:3826–924. doi: 10.1093/eurheartj/ehac270
29. Kane, AD , Armstrong, RA , Kursumovic, E , Cook, TM , Oglesby, FC , Cortes, L, et al. Methods of the 7(th) National Audit Project (NAP7) of the Royal College of Anaesthetists: peri-operative cardiac arrest. Anaesthesia. (2022) 77:1376–85. doi: 10.1111/anae.15856
30. Nolan, JP , Berg, RA , Andersen, LW , Bhanji, F , Chan, PS , Donnino, MW, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein resuscitation registry template for in-hospital cardiac arrest: a consensus report from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia). Resuscitation. (2019) 144:166–77. doi: 10.1016/j.resuscitation.2019.08.021
31. von Elm, E , Altman, DG , Egger, M , Pocock, SJ , Gøtzsche, PC , and Vandenbroucke, JP . The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
32. Jennett, B , and Bond, M . Assessment of outcome after severe brain damage. Lancet. (1975) 1:480–4. doi: 10.1016/S0140-6736(75)92830-5
33. Ware, J Jr, Kosinski, M , and Keller, SD . A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. (1996) 34:220–33. doi: 10.1097/00005650-199603000-00003
34. R Development Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2021).
35. Perneger, TV , and Burnand, B . A simple imputation algorithm reduced missing data in SF-12 health surveys. J Clin Epidemiol. (2005) 58:142–9. doi: 10.1016/j.jclinepi.2004.06.005
36. Kietaibl, S , Ahmed, A , Afshari, A , Albaladejo, P , Aldecoa, C , Barauskas, G, et al. Management of severe peri-operative bleeding: guidelines from the European Society of Anaesthesiology and Intensive Care: second update 2022. Eur J Anaesthesiol. (2023) 40:226–304. doi: 10.1097/EJA.0000000000001803
37. Hepner, DL , and Castells, MC . Anaphylaxis during the perioperative period. Anesth Analg. (2003) 97:1381–95. doi: 10.1213/01.ANE.0000082993.84883.7D
38. Norvik, A , Unneland, E , Bergum, D , Buckler, DG , Bhardwaj, A , Eftestøl, T, et al. Pulseless electrical activity in in-hospital cardiac arrest – a crossroad for decisions. Resuscitation. (2022) 176:117–24. doi: 10.1016/j.resuscitation.2022.04.024
Keywords: anesthesia, cardiac arrest, cardiopulonary resuscitation, perioperative care (intraoperative care), ROSC (return of spontaneous circulation), functional outcomes, health-related quality of life
Citation: Fuchs A, Franzmeier L, Cheseaux-Carrupt M, Kaempfer M, Disma N, Pietsch U, Huber M, Riva T and Greif R (2023) Characteristics and neurological survival following intraoperative cardiac arrest in a Swiss University Hospital: a 7-year retrospective observational cohort study. Front. Med. 10:1198078. doi: 10.3389/fmed.2023.1198078
Edited by:
Sebastian Schnaubelt, Medical University of Vienna, AustriaReviewed by:
Jan-Steffen Pooth, University of Freiburg Medical Center, GermanyEnrico Baldi, San Matteo Hospital Foundation (IRCCS), Italy
Copyright © 2023 Fuchs, Franzmeier, Cheseaux-Carrupt, Kaempfer, Disma, Pietsch, Huber, Riva and Greif. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Fuchs, YWxleGFuZGVyLmZ1Y2hzQGluc2VsLmNo
 Alexander Fuchs
Alexander Fuchs Lea Franzmeier1
Lea Franzmeier1 Marie Cheseaux-Carrupt
Marie Cheseaux-Carrupt Markus Huber
Markus Huber Thomas Riva
Thomas Riva Robert Greif
Robert Greif