- Unit of Obstetrics and Gynaecologic Oncology, Azienda USL-IRCCS di Reggio Emilia, Reggio Emilia, Italy
Objectives: The aim of this narrative review is to summarize the available evidence on the use of minimal invasive surgery (MIS) in the management of epithelial ovarian cancer (EOC).
Background: MIS is currently performed to stage and treat EOC at different stage of presentation. We will evaluate risks and benefits of minimally invasive surgery for early stage EOC treatment, then potential advantages provided by staging laparoscopy in identifying patients suitable for primary cytoreductive surgery (PDS) will be discussed. Finally we will investigate the growing role of MIS in the treatment of advanced EOC after neoadjuvant chemotherapy (NACT) and in the treatment of EOC recurrence.
Methods: An electronic database search was performed on PubMed, Medline, and Google Scholar for relevant studies up to December 2022.
Conclusion: LPS represents a feasible surgical procedure for the staging and treatment in early, advanced and EOC relapse in selected patients treated in high-volume oncological centers by surgeons with adequate experience in advanced surgical procedures. Despite the increasing use of MIS over the last few years, randomized clinical trials are still needed to prove its effectiveness.
Introduction
Epithelial ovarian cancer (EOC) affects approximately 225,000 women each year worldwide, and 140,000 die from this disease (1). EOC is usually diagnosed at an advanced stage because it is asymptomatic or has nonspecific symptoms that delay diagnosis (2). EOC usually affects old women, median age at diagnosis is 63 years old (2). The cornerstone of EOC treatment is surgery and adjuvant chemotherapy. EOC surgery requires multivisceral resection and several chemotherapies with a strong impact both on the body and on the emotional sphere of the patients because they upset the routine of EOC patients (3). Carboplatin and paclitaxel is the standard regimen in EOC patients, with a response rate of approximately 65%, median progression-free survival ranging from 16 to 21 months, and median overall survival ranging from 32 to 57 months (4). It is important to schedule surgery with a gynecologic oncologist in a referral centers to diagnose, stage, and treat this ominous disease (5). Many papers from different countries over the last 20 years have demonstrated how treatment at high volume referral centers can ensure cures associated with better oncological outcome (6, 7). High volume hospitals may ensure an interdisciplinary surgical approach and a multidisciplinary team that could manage EOC patients with a significant improvement in survival (8). Unfortunately, despite several evidences supporting centralization, not all EOC patients are treated in high-volume hospitals (9).
Minimally invasive surgery (MIS) in gynecologic oncology has widely expanded in the treatment of endometrial, cervical, and more recently in ovarian cancer (10). The term MIS refers to a wide variety of minimally invasive surgical approaches ranging from standard laparoscopy, robotics, mini-laparoscopy, and single-port laparoscopy. Based on patient characteristics, tumor extent, and type of surgery, surgeons choose the most appropriate method. The laparoscopic approach to adnexal masses started in the late 1970s (11) and it became more popular in gynecologic oncology after Daniel Dargent, Querleu and colleague described the first cases of laparoscopic pelvic lymphadenectomy in patients with cervical cancer and paraaortic lymph node dissection for carcinoma of the ovary or fallopian tube restaging (12–14).
By that time a growing numbers of data collected through many retrospective and prospective studies have been published to prove feasibility of laparoscopic surgery in the treatment of EOC in its different stages of presentation ranging from the treatment of early disease and staging procedures to the treatment of advanced EOC after neoadjuvant chemotherapy and in selected patients with recurrent tumors (15–17).
All those studies showed that in the past decade the indications for MIS in EOC staging and treatment have expanded beyond endometrial cancer staging to include surgical management of ovarian cancers. In this narrative review we will assess the role of laparoscopic surgery in treatment of early stage EOC (ESOC), the role of staging laparoscopy in surgical planning of primary debulking surgery, the role of laparoscopy in interval cytoreductive surgery of advanced ovarian cancer after neo adjuvant chemotherapy (NACT) and in recurrent EOC treatment.
Materials and methods
A search of public databases (PubMed, MEDLINE, Google Scholar) until December 2022 was carried out to identify studies comparing the effectiveness of MIS in EOC treatment.
A string search was generated with the following medical subject: “minimal invasive surgery,” “diagnostic laparoscopy,” “early epithelial ovarian cancer,” “advanced ovarian cancer,” “interval debulking surgery,” “neoadjuvant chemotherapy,” “ovarian cancer recurrence,” “cytoreductive surgery.” All pertinent articles were retrieved, and the relative reference lists were reviewed in order to identify additional studies that could potentially be included. The shared criteria for considering a publication relevant were the design of the study, the sample size and the length of follow-up in studies reporting surgical data, the number of citations in other journals and in the case of publications performed by the same working group an attempt was made to consider the more recent publication. Non English language published literature, duplicates and abstract without full text have been excluded The publications found were equally divided and subjected to reading among the authors (MG, VDM, GA, DP, GD, GC, LA). In case of disagreements in the selection, a final decision was taken upon discussion with 2 authors (MG, VDM).
Role of minimally invasive surgery in early ovarian cancer
ESOC only accounts for 20% to 25% of all EOC cases. Women with stage I have a 5-year survival rate of almost 90% as compared to 46% for women with advanced stages (1, 18). Most EOC are detected at an advanced stage since to date there are no tests or instrumental screening investigations applicable on a large scale to identify patients in the initial stage of the disease. As the development and validation of emerging biomarkers takes time, a renewed focus has been placed on improving clinical trial design in light of the failure of CA125-and Transvaginal ultrasound-based screening trials in order to identify approaches that can reduce EOC mortality efficiently (19). But in a context of daily clinical practice where laparoscopy is universally used for the treatment of ovarian pathology, ESOC is often detected during the removal of suspected benign ovarian tumors so a surgeon with the skills to perform a surgical-staging procedure might not be present.
Complete staging surgery for ESOC in fact includes hysterectomy, bilateral salpingo-oophorectomy, omentectomy, peritoneal biopsy, pelvic and para-aortic lymph node dissection, and peritoneal washings to identify occult advanced-stage disease (20, 21). Achieving accurate cancer staging is critical to predicting prognoses and to decide postsurgical treatment. The presence of microscopic metastases is found in up to 30% of women with apparent ESOC so obtaining prognostic information from a restaging procedure is essential in patients who did not undergo complete staging at the time of the initial surgery (22). Recent advances in technology and an increase in laparoscopic surgical expertise have resulted in the use of MIS to treat or restage ESOC worldwide (23). Laparoscopy guarantees advantages with better clinical outcomes in terms of less postoperative pain, less blood loss, shorter hospital day, and faster onset chemotherapy than laparotomy as previously published (24–26). As a result of the lack of randomized controlled trials (RCTs) and a lack of high-quality evidence, the Cochrane Collaboration concluded there was insufficient evidence to quantify the risks and benefits of laparoscopy when used for the treatment of ESOC (27). But considering the advantages associated with the laparoscopic approach even in the absence of RTC, the NCCN and ESMO-ESGO guidelines recommend a minimally invasive approach in the presence of gynecological oncological surgeons qualified in the staging and restaging of the EOC (5, 28). However main concerns remains about laparoscopic surgery disadvantages such as the potential rupture of the ovarian capsule, the risk of port site metastasis and the inability for lymph node manual assessment and palpation. Laparoscopy is in fact associated with a higher upstaging rate than laparotomy in the case of ovarian tumor rupture during surgery (24, 29–33). A study by Lee et al. revealed that tumor size was larger in the laparotomy group (29), this suggests the surgeon can select a surgical approach tailored to the ovarian neoplasia diameter to avoid undesired ruptures and in selected patients laparotomy and laparoscopy showed a similar incidence of tumor rupture as reported by Park (10.5% vs. 12.1% p = 1.000) (30). In case of intraoperative e tumor rupture, there is an immediate up staging, which may necessitate adjuvant chemotherapy and adversely affect the prognosis (34) but the rupture and not the surgical approach that can occur even during laparotomy seems to worsen the prognosis. The prognostic value needs to be investigated by RTC. Another potential disadvantage of MIS is the risk of port-site metastasis. There are a number of factors that can increase the risk of port-site metastases, including the presence of large masses in the abdomen and especially if concomitant ascites is present as reported in the Cochrane review (27). Some authors reported an incidence of port site metastases of up to 16% but in one of the largest detailed series reported by Zivancovic and by Rutten in 2008 and in 2017 the reported incidence was between 1.96 and 3% in line with other reports suggesting that port-site metastases in patients whose ovarian cancers are staged by laparoscopy may be rare (0%–2%) and only 1 case of the 20 port site metastases reported out of 1,694 patients undergoing laparoscopy did not present additional localization of disease in the abdomen (35–38). The correct staging of retroperitoneal disease may raise some concerns about MIS but meta-analysis has already shown for at least 10 years that no significant difference exists between MIS and traditional approaches when it comes to the size or number of lymph nodes removed during surgery (39). Lymph node metastases incidence in apparent early stage epithelial ovarian cancer is estimated to be around 14%–15% with approximately 37% of patients have only para-aortic positive nodes, 35% have only pelvic positive nodes, and the remaining 28% have both pelvic and para-aortic involvement. As stated by international guidelines a complete pelvic and para-aortic lymphadenectomy is recommended as part of surgical staging for ESOC and data on nodal status appear relevant to guide decisions on adjuvant therapy (40). However, the prognostic importance of the information provided by full nodal dissection must be balanced against the morbidity related to such a radical surgical procedure. Due to a low prevalence of nodal metastases in some histological subtypes (e.g., mucinous carcinoma of expansive subtype or low grade carcinoma), the indication for staging surgery in these cases may be questioned (28). Lymphatic mapping for the assessment of sentinel lymph nodes (SLNs) is a widely accepted part of the surgical treatment of breast and cutaneous melanoma and has been successfully implemented in several gynecological malignancies. The reported experience of SLN in ovarian cancer is restricted to a few studies with a small patient sample. Uccella et al. have recently published the largest prospective study (SELLY) on SLN in ovarian cancer with ICG alone as their laparoscopically tracer (41, 42). There is currently no recommendation from NCCN Clinical Practice Guidelines in Oncology 2022 edition (5) for application of SLN technique in ovarian cancer but there is growing support for its feasibility, and its acceptable negative predictive value. However, further evidence from phase III clinical studies is required to clarify the true negative predictive value, critically regarding patient safety. MIS safety has been examined in one of the largest multicenter studies currently available in the literature that included 300 patients with apparent ESOC referred to seven gynecologic oncology units for laparoscopic staging. In this study, MIS was found to have an advantage in terms of reducing morbidity and improving postoperative recovery. In addition, overall survival (OS), disease-free survival (DFS) and the rates of recurrence were similar to those reported in the literature for open surgical treatment of ESOC (43). Nevertheless, a recent systematic review and meta-analysis on retrospective or prospective data by Knisely et al. on patients with epithelial serous ovarian cancer who were treated with laparoscopic surgery indicates that laparoscopic surgery is a safe and technically feasible procedure (44). Even if data on survival and recurrence in patients staged by MIS show survival rates of approximately 90% at follow-up, similar to that observed in patients staged by laparotomy, these studies do not collect data on long term follow up as seen in Table 1. Studies of this size and duration are unlikely to be able to demonstrate the effectiveness of MIS in the EOC treatment (16, 23, 25, 30, 31, 45–50). In addition, MIS for staging and treatment of ESOC has not been the subject of a randomized controlled trial. For confirmation of these findings, and the development of selection criteria for MIS in ovarian cancer, large prospective randomized trial studies are required.
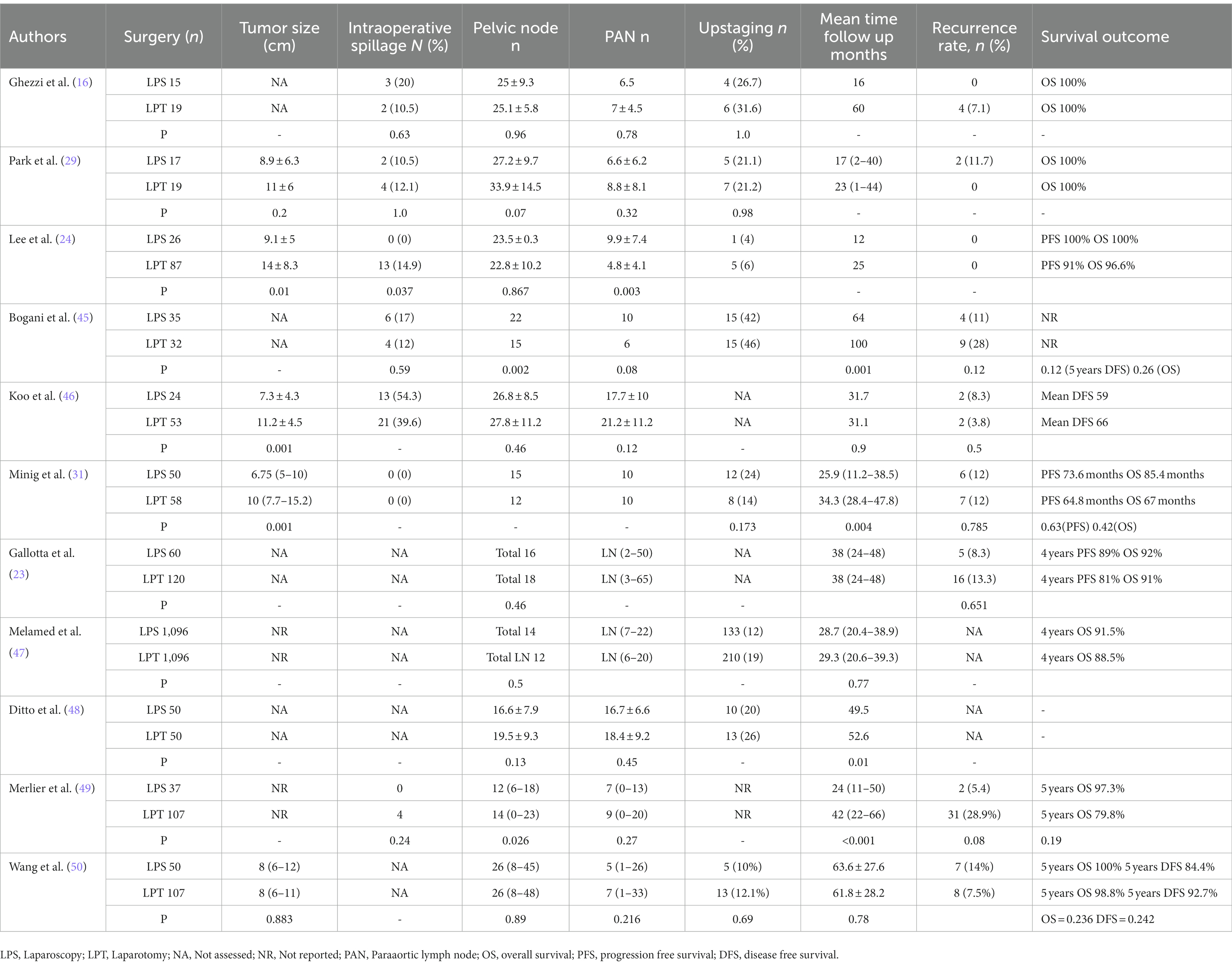
Table 1. Studies of laparoscopic surgical staging vs. open surgery for early ovarian cancer considering number of patients (n) tumor size, % of intraoperative spillage, lymph nodes removed, upstaging %, recurrence rate and survival outcome during follow up period.
Role of laparoscopy in surgical planning of primary debulking surgery for ovarian cancer
Primary debulking surgery (PDS) is recommended as the standard surgical treatment for EOC (28) and optimal debulking surgery with removal of all visible disease remains the most significant prognostic factor for increasing survival in ovarian cancer patients (51, 52). Unfortunately, only about 30% of patients with ovarian cancer benefit from ultra-radical surgery with no visible residual tumor. In this context, it is imperative to identify patients with extensive disease who will likely have residual tumor after surgery despite being treated with radical multi-organ surgery with a moderate-high risk of complications and with no long-term survival benefits. The use of neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS), as an alternative strategy for such patients, has gained popularity over years (53–57). Studies suggest that this approach reduces surgical complexity and postoperative complications, especially in patients with a low performance status and/or high volume of disease (58–62), even if long term benefits remains controversial as a treatment option for EOC (57). While numerous efforts have been made to develop prediction models that integrate imaging techniques (CT, PET-TC), serum tumor markers, and clinical characteristics, they have been unable to accurately predict the effectiveness of optimal debulking (63). This results in the need to perform futile laparotomies, leading to possible intra and postoperative complications, an extended hospital stay, additional financial costs, and could cause a delay in initiating chemotherapy (25, 64, 65).
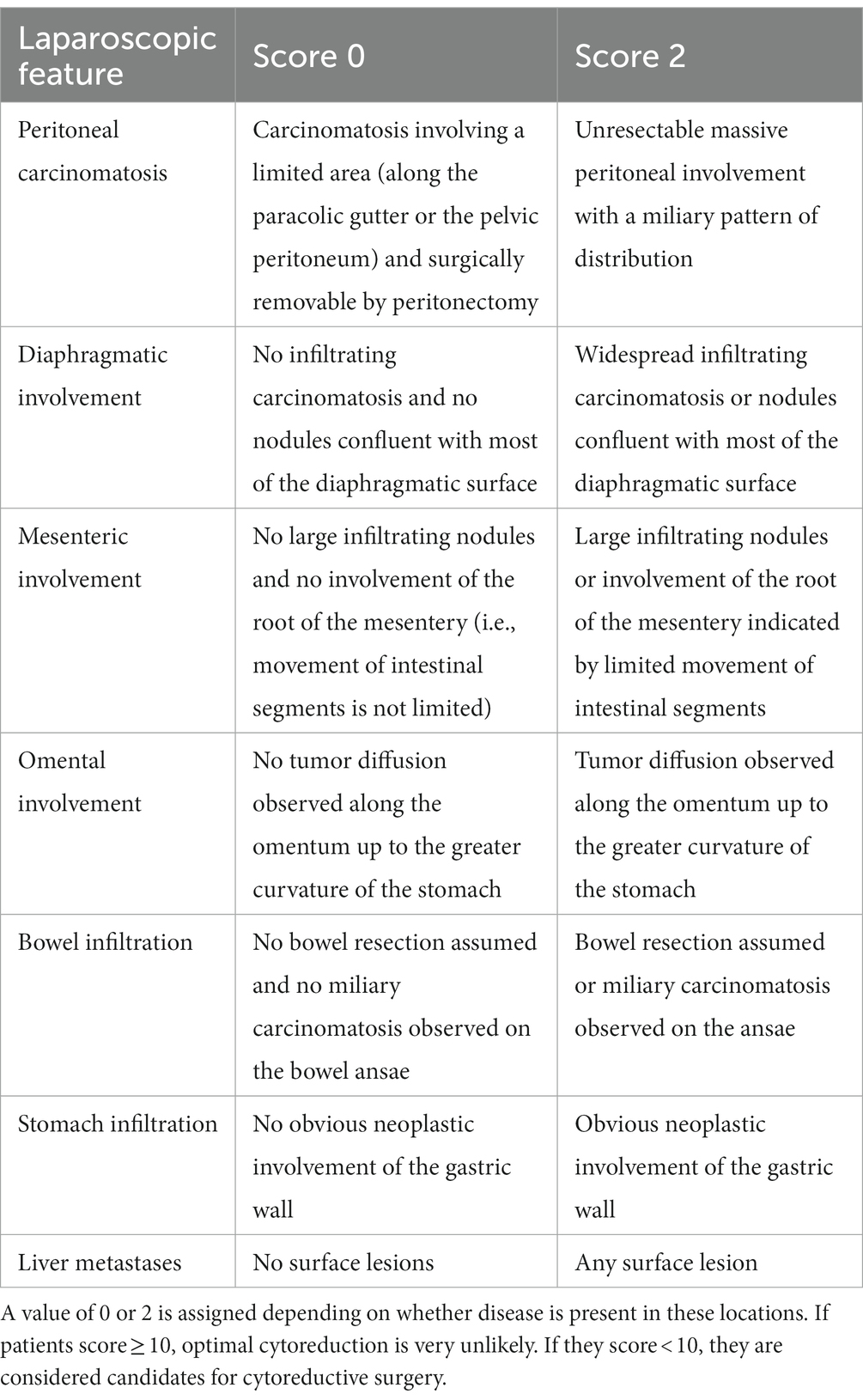
Starting from the experience reported by Vergote (66) on the use of staging laparoscopy (S-LPS) to guide the decision to perform or not perform PDS, the use of laparoscopy for advanced EOC surgery is increasingly being used as a tool to plan ultra-radical surgery and to reduce the number of futile laparotomies. Various retrospective and prospective studies had been published by that time (15, 66–70) and Fagotti et al. (71) developed an S-LPS-based quantitative model, see Table 2. Using an S-LPS predictive index value (PIV), they developed a simple scoring system to estimate the likelihood of optimal cytoreduction based on the presence of (I) omental cake, (II) peritoneal carcinomatosis, (III) diaphragmatic carcinomatosis, (IV) mesenteric retraction, (V) infiltration of the bowel and/or (VI) stomach, and (VII) liver metastases. Two points were assigned to each parameter if it was present. In patients with scores greater than 8, suboptimal surgery was predicted with a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 70%. With a PIV cut-off of 8, complete cytoreduction was 0%, whereas unnecessary laparotomies were 40.5% (15). A prospective multicenter trial (Olympia-MITO 13) conducted on 120 patients in four different centers aimed at evaluating if the PIV assessment was feasible and reproducible in external centers. Results published in 2013 showed that Fagotti index (PIV) reached an accuracy rate of 80% or greater and could be replicated in different centers with different expertise (72). A randomized controlled study published by Rutten et al. (36) showed that suboptimal PDS was estimated to have decreased from 39% to 10% using S-LPS, suggesting that S-LPS should be adopted as a standard clinical procedure.
The possible standardization of the S-LPS in determining disease resectability in patients with suspected advanced EOC and the numerous publications among different working groups allowed to evaluate the applicability of the technique through a Cochrane review recently published in 2019 (73). Analyses were performed on 18 studies involving 14 cohorts of patients. S-LPS had good overall accuracy but some women still had suboptimal resected disease (i.e., >1 cm residual tumor) at PDS. S-LPS, in fact, cannot be used to evaluate a substantial number of women. For example presence of adhesions may restrict access to the abdomen or prevent the exploration of the peritoneum in its entirety and S-LPS is incapable of assessing specific areas associated with suboptimal debulking (retroperitoneal, mesenteric, or retro-hepatic and peripancreatic region). Furthermore S-LPS is considered an easy and low-morbidity method of assessing advanced EOC patients but even if the complication rate for S-LPS is considered low (reported between 1% and 5%) the procedure still requires general anesthesia, and some complications have been reported as severe, thus potentially delaying the primary treatment (surgery or NACT) (70). Additionally, as previously described port-site metastases have been reported in up to 3% of cases following S-LPS, with ascites associated with an increased risk (74, 75). In conclusion, as often happens in the history of medicine regarding novel techniques applications, the absence of entirely univocal data and above all different surgical backgrounds have arisen a situation where S-LPS has motivated some institutions to include it as part of their standard diagnostic procedure, some others only perform S-LPS when there is doubt about resectability, while others do not perform S-LPS at all. Despite the personal affection or not to this technique S-LPS should be performed by oncological gynecologists minimizing the risk of complication and lowering the risk that less experienced gynecologists can send too many patients to NACT, in order to perform a potentially less demanding interval debulking surgery (9, 76). The focus in ovarian cancer referral centers should be on choosing the right surgical approach to treat patients with advanced-stage EOC, and aiming to improve surgical outcomes and the survival rate of patients by implementing systematic quality improvement initiatives.
Minimally invasive interval cytoreductive surgery vs. laparotomy in the treatment of advanced ovarian cancer after NACT
Neoadjuvant chemotherapy has become increasingly popular worldwide as an alternative to primary cytoreductive surgery for advanced ovarian cancer. According to statistics from 2016, 1 in 3 patients with ovarian cancer stage IIIC or IV underwent chemotherapy before surgery (70). Women with high perioperative risk, or poor likelihood of achieving optimal cytoreduction during primary surgery, should receive neoadjuvant chemotherapy and interval surgery, according to guidelines developed by the American Society of Clinical Oncology and the Society of Gynaecologic Oncology (53). Women who have responded to neoadjuvant chemotherapy could benefit from minimally invasive interval cytoreduction as reported by small uncontrolled observational studies that shows better perioperative outcomes a high rate of complete cytoreduction and excellent progression-free survival rates (77–79). The National Cancer Database was utilized by Melamed et al. (80) in analyzing 450 women undergoing minimally invasive cytoreduction vs. 2,621 women undergoing laparotomy. It was found that both groups had similar overall survival rates and surgical outcomes, despite adjusting for several potential confounders. More recently, the INTERNATIONAL MISSION trial, a multi-center retrospective study demonstrated a median progression-free survival of 23 months and a 5-year overall survival rate of 52% in 127 women who underwent MIS after neoadjuvant chemotherapy for EOC with 96.1% patients with no residual tumor and 4.7% complication rate and a 3.9% conversion rate to laparotomy (81). Even though there are no randomized studies, the use of minimally invasive interval cytoreductive surgeries keeps rising (82) and the National Cancer Network Guidelines endorse the MIS as an approach for interval debulking surgery in “select patients” (83). Two meta-analyses have recently been published showing no deleterious survivals or recurrences associated with MIS for ovarian cancer. For women with advanced ovarian cancer who have responded to neoadjuvant chemotherapy, the current limited evidence suggests minimally invasive cytoreductive surgery is equivalent to open interval cytoreductive surgery at this time (44, 84). An ongoing randomized trial comparing these approaches will provide an assessment of the oncologic efficacy of MIS (85).
Minimally invasive surgery for ovarian cancer recurrence
In approximately 75% of cases of EOC, recurrence occurs within 2 years from initial diagnosis (1). Recurrent ovarian cancer is typically treated with systemic chemotherapy and the effects of surgery, is still under debate due to the conflicting data obtained from retrospective reports that strongly support radical secondary cytoreduction effects of surgery (86–90). This data have been questioned by a large randomized trial promoted by the Gynaecologic Oncology Group (GOG-213), where secondary surgery did not improve overall survival, but only disease-free survival (91). Despite this, controversy surrounds the GOG-213 data due to the low rate of complete cytoreduction (63%). No matter what the debate on these topics is, surgical secondary cytoreduction (SSC) remains an important treatment option for ovarian cancer recurrence as suggested by ESMO-ESGO recommendation (28). MIS can have multiple indication in women with ovarian cancer recurrence. Considering that even in recurrence as well in naive patients a minimal invasive approach can be useful to assess the extent of disease and potential cytoreduction in addition to the already well-known benefits previously debated on intraoperative blood loss, transfusion risks, perioperative and postoperative complications, including a shorter time to initiation of adjuvant chemotherapy. The quality of evidence in ovarian cancer recurrence treatment by MIS is low, being based mainly on case reports, retrospective case series and retrospective comparative studies (92–101). In three studies comparing MIS vs. laparotomy the rate of optimal cytoreduction by minimally invasive secondary cytoreductive surgery is consistent across studies, ranging from 70% to 98% and no statistically significant differences on disease free and overall survival (92–94) Table 3. However, MIS requires a high level of expertise and skills, especially in this setting of patients, and should be performed in high-volume oncological centers with adequate experience in advanced surgical procedures. No guidelines or consistent data are currently available to identify patients eligible for MIS for recurrences and no predictors of its feasibility are currently available so the decision to undergo laparoscopic or robotic debulking is left to surgeons’ discretion in different studies. Most MIS candidates had single-site disease or few relapses. In order to confirm or not MIS, a diagnostic laparoscopy, together with preoperative imaging may be useful (102). In conclusion for the treatment of recurrent EOC, MIS may be deemed an alternative to laparotomy in highly selected cases at dedicated oncology centers and in the context of well-conducted research.
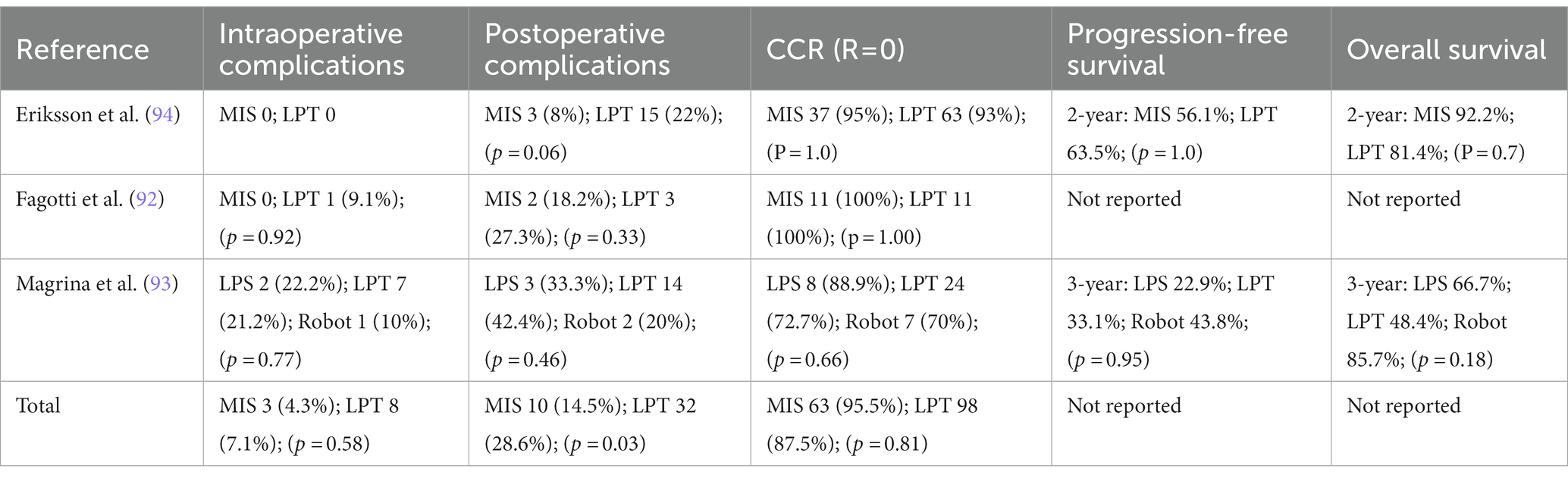
Table 3. Intraoperative and postoperative complication, complete cytoreduction rate (CCR = residual tumor = 0) and survival outcomes in EOC recurrence treated by minimally invasive surgery.
Discussion
The cornerstone of EOC management is surgery and adjuvant chemotherapy. It is important to schedule surgery with a gynecologic oncologist in a referral center to diagnose, stage, and treat this disease (5–9). In the 1970s, MIS started to be used in gynecological malignant pathologies and during years the number of surgeons who thought MIS was appropriate for the treatment of endometrial, cervical an ovarian cancer increased significantly (10). The rate of serious complications associated with MIS is low. The most common complications are vessels and bowel injuries resulting from the initial abdominal access and are usually related to prior abdominal surgery, previous pelvic inflammatory disease or diverticulitis and severe obesity (103). On the other hand any type of MIS offers better outcomes than laparotomy in terms of a shorter hospital stay, decreased perioperative morbidity, less postoperative pain, and faster recovery. When EOC is treated with MIS, several advantages are available and due to smaller incisions that heal more quickly adjuvant chemotherapy can begin sooner. MIS has been nowadays incorporated in EOC treatment to evaluate optimal debulking surgery with staging laparoscopy and to manage ESOC and advanced stage disease at primary diagnosis and after NACT, and recurrent disease. According to an ovarian cancer patient’s stage, the MIS procedure will depend on the goal of surgery. Although existing studies do not demonstrate deleterious survival effects associated with MIS for ovarian cancer, these data must be viewed with caution given the significant methodological shortcomings in the existing literature and we cannot ignore lesson learned from Laparoscopic Approach to Cervical Cancer trial that showed how MIS in early-stage cervical cancer patients had a detrimental effect (104).
We must be aware that the absence of significant differences both positively and negatively on survival between minimally invasive vs. open approach presented in many studies might be due to the small sample size of the studies not able to detect any statistically significant difference. Therefore, to validate the use of MIS larger prospective studies and the development of future randomized interventional studies are needed in order to identify patients affected by ovarian cancer at different stage who can benefit from a successful minimally invasive approach (40).
Conclusion
MIS can be proposed in a wide variety of situations characterizing EOC, from staging laparoscopy to treatment of recurrence. Women treated will experience a lower rate of intra and post-operative complications than those undergoing open surgery. It is of crucial importance that all ovarian cancer patients should be treated in a referral high volume cancer centers with surgeons with an intensive training in MIS. It is in fact important to keep in mind that surgical comparative studies are based on the level of each surgeon, so findings cannot be applied equally to all surgeons and to all centers. If these assumptions are met and patient selection is correct, a minimal invasive approach can be a real advantage in any stage EOC treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MG conceived and wrote the manuscript. GA, DP, GDI, and GC revised the literature and wrote the manuscript. LA revised the manuscript. VDM conceived and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was partially supported by Italian Ministry of Health—Ricerca Corrente Annual Program 2024.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel, RL, Miller, KD, and Jemal, A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
2. American Cancer Society. Available at: https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html (n.d.). (Accessed 3 March 2023).
3. Mangone, L, Mandato, VD, Gandolfi, R, Tromellini, C, and Abrate, M. The impact of epithelial ovarian cancer diagnosis on women's life: a qualitative study. Eur J Gynaecol Oncol. (2014) 35:32–8.
4. Ozols, RF, Bundy, BN, Greer, BE, Fowler, JM, Clarke-Pearson, D, Burger, RA, et al. Gynecologic oncology group. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a gynecologic oncology group study. J Clin Oncol. (2003) 21:3194–200. doi: 10.1200/JCO.2003.02.153
5. National Comprehensive Cancer Network. Ovarian Cancer including Fallopean tube Cancer and primary peritoneal Cancer, (Version 1), (2022). Available at: https://www.nccn.org/professionals/physician_gls/pdf/ovarian
6. Mandato, VD, Abrate, M, de Iaco, P, Pirillo, D, Ciarlini, G, Leoni, M, et al. Clinical governance network for clinical audit to improve quality in epithelial ovarian cancer management. J Ovarian Res. (2013) 6:19. doi: 10.1186/1757-2215-6-19
7. Bristow, RE, Santillan, A, Diaz-Montes, TP, Gardner, GJ, Giuntoli, RL 2nd, Meisner, BC, et al. Centralization of care for patients with advanced-stage ovarian cancer: a cost-effectiveness analysis. Cancer. (2007) 109:1513–22. doi: 10.1002/cncr.22561
8. Mangone, L, Marinelli, F, Bisceglia, I, Braghiroli, MB, Mastrofilippo, V, Cerullo, L, et al. Cancer in a northern Italian Province and the multidisciplinary team. Cancers. (2022) 15:299. doi: 10.3390/cancers15010299
9. Mandato, VD, Torricelli, F, Uccella, S, Pirillo, D, Ciarlini, G, Ruffo, G, et al. An Italian National Survey on ovarian Cancer treatment at first diagnosis. There's none so deaf as those who will not hear. J Cancer. (2021) 12:4443–54. doi: 10.7150/jca.57894
10. Conrad, LB, Ramirez, PT, Burke, W, Naumann, RW, Ring, KL, Munsell, MF, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. (2015) 25:1121–7. doi: 10.1097/IGC.0000000000000450
11. Mangioni, C, Bolis, G, Molteni, P, and Belloni, C. Indications, advantages, and limits of laparoscopy in ovarian cancer. Gynecol Oncol. (1979) 7:47–55. doi: 10.1016/0090-8258(79)90080-5
13. Querleu, D, Leblanc, E, and Castelain, B. Laparoscopic pelvic lymphadenectomy in the staging of early carcinoma of the cervix. Am J Obstet Gynecol. (1991) 164:579–81. doi: 10.1016/S0002-9378(11)80025-6
14. Querleu, D, and LeBlanc, E. Laparoscopic infrarenal paraaortic lymph node dissection for restaging of carcinoma of the ovary or fallopian tube. Cancer. (1994) 73:1467–71. doi: 10.1002/1097-0142(19940301)73:5<1467::AID-CNCR2820730524>3.0.CO;2-B
15. Fagotti, A, Ferrandina, G, Fanfani, F, Ercoli, A, Lorusso, D, Rossi, M, et al. A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: a pilot study. Ann Surg Oncol. (2006) 13:1156–61. doi: 10.1245/ASO.2006.08.021
16. Ghezzi, F, Cromi, A, Uccella, S, Bergamini, V, Tomera, S, Franchi, M, et al. Laparoscopy versus laparotomy for the surgical management of apparent early stage ovarian cancer. Gynecol Oncol. (2007) 105:409–13. doi: 10.1016/j.ygyno.2006.12.025
17. Gueli Alletti, S, Capozzi, VA, Rosati, A, de Blasis, I, Cianci, S, Vizzielli, G, et al. Laparoscopy vs. laparotomy for advanced ovarian cancer: a systematic review of the literature. Minerva Med. (2019) 110:341–57. doi: 10.23736/S0026-4806.19.06132-9
18. Lheureux, S, Gourley, C, Vergote, I, and Oza, AM. Epithelial ovarian cancer. Lancet. (2019) 393:1240–53. doi: 10.1016/S0140-6736(18)32552-2
19. Liberto, JM, Chen, SY, Shih, IM, Wang, TH, Wang, TL, and Pisanic, TR 2nd. Current and emerging methods for ovarian Cancer screening and diagnostics: a comprehensive review. Cancers. (2022) 14:2885. doi: 10.3390/cancers14122885
20. Leblanc, E, Querleu, D, Narducci, F, Occelli, B, Papageorgiou, T, and Sonoda, Y. Laparoscopic restaging of early stage invasive adnexal tumors: a 10-year experience. Gynecol Oncol. (2004) 94:624–9. doi: 10.1016/j.ygyno.2004.05.052
21. Gueli Alletti, S, Restaino, S, Finelli, A, Ronsini, C, Lucidi, A, Scambia, G, et al. Step by step Total laparoscopic hysterectomy with uterine arteries ligation at the origin. J Minim Invasive Gynecol. (2020) 27:22–3. doi: 10.1016/j.jmig.2019.06.001
22. Bae, J, Choi, JS, Lee, WM, Koh, AR, Jung, US, Ko, JH, et al. Feasibility and efficacy of laparoscopic restaging surgery for women with unexpected ovarian malignancy. Eur J Obstet Gynecol Reprod Biol. (2015) 193:46–50. doi: 10.1016/j.ejogrb.2015.06.027
23. Gallotta, V, Petrillo, M, Conte, C, Vizzielli, G, Fagotti, A, Ferrandina, G, et al. Laparoscopic versus laparotomic surgical staging for early-stage ovarian cancer: a case-control study. J Minim Invasive Gynecol. (2016) 23:769–74. doi: 10.1016/j.jmig.2016.03.006
24. Nezhat, FR, Ezzati, M, Chuang, L, Shamshirsaz, AA, Rahaman, J, and Gretz, H. Laparoscopic management of early ovarian and fallopian tube cancers: surgical and survival outcome. Am J Obstet Gynecol. (2009) 200:83.e1–6. doi: 10.1016/j.ajog.2008.08.013
25. Lee, M, Kim, SW, Paek, J, Lee, SH, Yim, GW, Kim, JH, et al. Comparisons of surgical outcomes, complications, and costs between laparotomy and laparoscopy in early-stage ovarian cancer. Int J Gynecol Cancer. (2011) 21:251–6. doi: 10.1097/IGC.0b013e318208c71c
26. Ghezzi, F, Malzoni, M, Vizza, E, Cromi, A, Perone, C, Corrado, G, et al. Laparoscopic staging of early ovarian cancer: results of a multi-institutional cohort study. Ann Surg Oncol. (2012) 19:1589–94. doi: 10.1245/s10434-011-2138-9
27. Falcetta, FS, Lawrie, TA, Medeiros, LRF, da Rosa, MI, Edelweiss, MI, Stein, AT, et al. Laparoscopy versus laparotomy for FIGO stage I ovarian cancer. Cochrane Database Syst Rev. (2016) 2016:CD005344. doi: 10.1002/14651858.CD005344.pub4
28. Colombo, N, Sessa, C, Bois, AD, Ledermann, J, McCluggage, WG, McNeish, I, et al. ESMO–ESGO ovarian Cancer consensus conference working group. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Cancer. (2019) 29:728–60. doi: 10.1136/ijgc-2019-000308
29. Lee, CL, Kay, N, Chen, HL, Yen, CF, and Huang, KG. The roles of laparoscopy in treating ovarian cancer. Taiwan J Obstet Gynecol. (2009) 48:9–14. doi: 10.1016/S1028-4559(09)60029-2
30. Park, JY, Kim, DY, Suh, DS, Kim, JH, Kim, YM, Kim, YT, et al. Comparison of laparoscopy and laparotomy in surgical staging of earlystage ovarian and fallopian tubal cancer. Ann Surg Oncol. (2008) 15:2012–9. doi: 10.1245/s10434-008-9893-2
31. Minig, L, Saadi, J, Patrono, MG, Giavedoni, ME, Cárdenas-Rebollo, JM, Perrotta, M, et al. Laparoscopic surgical staging in women with early stage epithelial ovarian cancer performed by recently certified gynecologic oncologists. Eur J Obstet Gynecol Reprod Biol. (2016) 201:94–100. doi: 10.1016/j.ejogrb.2016.03.029
32. Chereau, E, Lavoue, V, Ballester, M, Coutant, C, Selle, F, Cortez, A, et al. External validation of a laparoscopic-based score to evaluate resectability for patients with advanced ovarian cancer undergoing interval debulking surgery. Anticancer Res. (2011) 31:4469–74.
33. Wu, TI, Lee, CL, Liao, PJ, Huang, KG, Chang, TC, Chou, HH, et al. Survival impact of initial surgical approach in stage I ovarian cancer. Chang Gung Med J. (2010) 33:558–67.
34. Vergote, I. Prognostic factors in stage I ovarian carcinoma. Verh K Acad Geneeskd Belg. (2001) 63:257–71.
35. Zivanovic, O, Sonoda, Y, Diaz, JP, Levine, DA, Brown, CL, Chi, DS, et al. The rate of portsite metastases after 2251 laparoscopic procedures in women with underlying malignant disease. Gynecol Oncol. (2008) 111:431–7. doi: 10.1016/j.ygyno.2008.08.024
36. Rutten, MJ, van Meurs, HS, van de Vrie, R, Gaarenstroom, KN, Naaktgeboren, CA, van Gorp, T, et al. Laparoscopy to predict the result of primary Cytoreductive surgery in patients with advanced ovarian Cancer: a randomized controlled trial. J Clin Oncol. (2017) 35:613–21. doi: 10.1200/JCO.2016.69.2962
37. Ramirez, PT, Wolf, JK, and Levenback, C. Laparoscopic portsite metastases: etiology and prevention. Gynecol Oncol. (2003) 91:179–89. doi: 10.1016/S0090-8258(03)00507-9
38. Panici, PB, Palaia, I, Bellati, F, Pernice, M, Angioli, R, and Muzii, L. Laparoscopy compared with laparoscopically guided minilaparotomy for large adnexal masses: a randomized controlled trial. Obstet Gynecol. (2007) 110:241–8. doi: 10.1097/01.AOG.0000275265.99653.64
39. Park, HJ, Kim, DW, Yim, GW, Nam, EJ, Kim, S, and Kim, YT. Staging laparoscopy for the management of early-stage ovarian cancer: a metaanalysis. Am J Obstet Gynecol. (2013) 209:58.e1–8. doi: 10.1016/j.ajog.2013.04.013
40. Kampan, NC, Teik, CK, and Shafiee, MN. Where are we going with sentinel nodes mapping in ovarian cancer? Front Oncol. (2022) 12:999749. doi: 10.3389/fonc.2022.999749.
41. Scambia, G, Nero, C, Uccella, S, Vizza, E, Ghezzi, F, Cosentino, F, et al. Sentinel-node biopsy in early stage ovarian cancer: a prospective multicentre study (SELLY). Int J Gynecol Cancer. (2019) 29:1437–9. doi: 10.1136/ijgc-2019-000886
42. Uccella, S, Nero, C, Vizza, E, Vargiu, V, Corrado, G, Bizzarri, N, et al. Sentinel-node biopsy in early-stage ovarian cancer: preliminary results of a prospective multicentre study (SELLY). Am J Obstet Gynecol. (2019) 221:324.e1–324.e10. doi: 10.1016/j.ajog.2019.05.005
43. Gallotta, V, Ghezzi, F, Vizza, E, Chiantera, V, Ceccaroni, M, Franchi, M, et al. Laparoscopic staging of apparent early stage ovarian cancer: results of a large, retrospective, multi-institutional series. Gynecol Oncol. (2014) 135:428–34. doi: 10.1016/j.ygyno.2014.09.006
44. Knisely, A, Gamble, CR, St Clair, CM, Hou, JY, Khoury-Collado, F, Gockley, AA, et al. The role of minimally invasive surgery in the care of women with ovarian cancer: a systematic review and Meta-analysis. J Minim Invasive Gynecol. (2021) 28:537–43. doi: 10.1016/j.jmig.2020.11.007
45. Bogani, G, Cromi, A, Serati, M, di Naro, E, Casarin, J, Pinelli, C, et al. Laparoscopic and open abdominal staging for early-stage ovarian cancer: our experience, systematic review, and meta-analysis of comparative studies. Int J Gynecol Cancer. (2014) 24:1241–9. doi: 10.1097/IGC.0000000000000214
46. Koo, YJ, Kim, JE, Kim, YH, Hahn, HS, Lee, IH, Kim, TJ, et al. Comparison of laparoscopy and laparotomy for the management of early-stage ovarian cancer: surgical and oncological outcomes. J Gynecol Oncol. (2014) 25:111–7. doi: 10.3802/jgo.2014.25.2.111
47. Melamed, A, Keating, NL, Clemmer, JT, Bregar, AJ, Wright, JD, Boruta, DM, et al. Laparoscopic staging for apparent stage I epithelial ovarian cancer. Am J Obstet Gynecol. (2017) 216:50.e1–50.e12. doi: 10.1016/j.ajog.2016.08.030
48. Ditto, A, Bogani, G, Martinelli, F, Signorelli, M, Chiappa, V, Scaffa, C, et al. Minimally invasive surgical staging for ovarian carcinoma: a propensity-matched comparison with traditional open surgery. J Minim Invasive Gynecol. (2017) 24:98–102. doi: 10.1016/j.jmig.2016.09.018
49. Merlier, M, Kerbage, Y, Pierache, A, Ramdane, N, Canlorbe, G, Bolze, PA, et al. Impact on prognosis of the surgical route, laparoscopy or laparotomy, for the surgical staging of early stage ovarian Cancer-a study from the FRANCOGYN group. J Clin Med. (2020) 9:3528. doi: 10.3390/jcm9113528
50. Wang, Y, Yin, J, Li, Y, Shan, Y, Gu, Y, and Jin, Y. Laparoscopic and Laparotomic restaging in patients with apparent stage I epithelial ovarian Cancer: a comparison of surgical and oncological outcomes. Front Oncol. (2022) 12:913034. doi: 10.3389/fonc.2022.913034
51. Bristow, RE, Tomacruz, RS, Armstrong, DK, Trimble, EL, and Montz, FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. (2002) 20:1248–59. doi: 10.1200/JCO.2002.20.5.1248
52. Chang, SJ, Hodeib, M, Chang, J, and Bristow, RE. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: a meta-analysis. Gynecol Oncol. (2013) 130:493–8. doi: 10.1016/j.ygyno.2013.05.040
53. Vergote, I, Tropé, CG, Amant, F, Kristensen, GB, Ehlen, T, Johnson, N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. (2010) 363:943–53. doi: 10.1056/NEJMoa0908806
54. Kehoe, S, Hook, J, Nankivell, M, Jayson, GC, Kitchener, H, Lopes, T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. (2015) 386:249–57. doi: 10.1016/S0140-6736(14)62223-6
55. Fagotti, A, Ferrandina, MG, Vizzielli, G, Pasciuto, T, Fanfani, F, Gallotta, V, et al. Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION-NCT01461850). Int J Gynecol Cancer. (2020) 30:1657–64. doi: 10.1136/ijgc-2020-001640
56. Onda, T, Satoh, T, Ogawa, G, Saito, T, Kasamatsu, T, Nakanishi, T, et al. Comparison of survival between primary debulking surgery and neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomised trial. Eur J Cancer. (2020) 130:114–25. doi: 10.1016/j.ejca.2020.02.020
57. Chiofalo, B, Bruni, S, Certelli, C, Sperduti, I, Baiocco, E, and Vizza, E. Primary debulking surgery vs. interval debulking surgery for advanced ovarian cancer: review of the literature and meta-analysis. Minerva Med. (2019) 110:330–40. doi: 10.23736/S0026-4806.19.06078-6
58. Meyer, LA, Cronin, AM, Sun, CC, Bixel, K, Bookman, MA, Cristea, MC, et al. Use and effectiveness of neoadjuvant chemotherapy for treatment of ovarian Cancer. J Clin Oncol. (2016) 34:3854–63. doi: 10.1200/JCO.2016.68.1239
59. Meyer, LA, He, W, Sun, CC, Zhao, H, Wright, AA, Suidan, RS, et al. Neoadjuvant chemotherapy in elderly women with ovarian cancer: rates of use and effectiveness. Gynecol Oncol. (2018) 150:451–9. doi: 10.1016/j.ygyno.2018.06.020
60. Wright, AA, Bohlke, K, Armstrong, DK, Bookman, MA, Cliby, WA, Coleman, RL, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian Cancer: Society of Gynecologic Oncology and American Society of clinical oncology clinical practice guideline. J Clin Oncol. (2016) 34:3460–73. doi: 10.1200/JCO.2016.68.6907
61. Pinelli, C, Morotti, M, Casarin, J, Tozzi, R, Ghezzi, F, Mavroeidis, VK, et al. Interval Debulking surgery for advanced ovarian Cancer in elderly patients (≥70 y): does the age matter? J Investig Surg. (2021) 34:1023–30. doi: 10.1080/08941939.2020.1733146
62. Tozzi, R, Casarin, J, Baysal, A, Pinelli, C, Matak, L, Ghanbarzadeh, N, et al. Morbidity of multiple bowel resection compared to single bowel resection after debulking surgery for ovarian cancer. Eur J Obstet Gynecol Reprod Biol. (2019) 240:215–9. doi: 10.1016/j.ejogrb.2019.07.011
63. Gómez-Hidalgo, NR, Martinez-Cannon, BA, Nick, AM, Lu, KH, Sood, AK, Coleman, RL, et al. Predictors of optimal cytoreduction in patients with newly diagnosed advanced-stage epithelial ovarian cancer: time to incorporate laparoscopic assessment into the standard of care. Gynecol Oncol. (2015) 137:553–8. doi: 10.1016/j.ygyno.2015.03.049
64. Rutten, MJ, van de Vrie, R, Bruining, A, Spijkerboer, AM, Mol, BW, Kenter, GG, et al. Predicting surgical outcome in patients with International Federation of Gynecology and Obstetrics stage III or IV ovarian cancer using computed tomography: a systematic review of prediction models. Int J Gynecol Cancer. (2015) 25:407–15. doi: 10.1097/IGC.0000000000000368
65. Harrison, RF, Cantor, SB, Sun, CC, Villanueva, M, Westin, SN, Fleming, ND, et al. Cost-effectiveness of laparoscopic disease assessment in patients with newly diagnosed advanced ovarian cancer. Gynecol Oncol. (2021) 161:56–62. doi: 10.1016/j.ygyno.2021.01.024
66. Vergote, I, de Wever, I, Tjalma, W, van Gramberen, M, Decloedt, J, and van Dam, P. Neoadjuvant chemotherapy or primary debulking surgery in advanced ovarian carcinoma: a retrospective analysis of 285 patients. Gynecol Oncol. (1998) 71:431–6. doi: 10.1006/gyno.1998.5213
67. Deffieux, X, Castaigne, D, and Pomel, C. Role of laparoscopy to evaluate candidates for complete cytoreduction in advanced stages of epithelial ovarian cancer. Int J Gynecol Cancer. (2006) 16:35–40. doi: 10.1136/ijgc-00009577-200602001-00006
68. Angioli, R, Palaia, I, Zullo, MA, Muzii, L, Manci, N, Calcagno, M, et al. Diagnostic open laparoscopy in the management of advanced ovarian cancer. Gynecol Oncol. (2006) 100:455–61. doi: 10.1016/j.ygyno.2005.09.060
69. Brun, JL, Rouzier, R, Selle, F, Houry, S, Uzan, S, and Daraï, E. Neoadjuvant chemotherapy or primary surgery for stage III/IV ovarian cancer: contribution of diagnostic laparoscopy. BMC Cancer. (2009) 9:171. doi: 10.1186/1471-2407-9-171
70. Nezhat, FR, DeNoble, SM, Liu, CS, Cho, JE, Brown, DN, Chuang, L, et al. The safety and efficacy of laparoscopic surgical staging and debulking of apparent advanced stage ovarian, fallopian tube, and primary peritoneal cancers. JSLS. (2010) 14:155–68. doi: 10.4293/108680810X12785289143990
71. Fagotti, A, Fanfani, F, Ludovisi, M, Lo Voi, R, Bifulco, G, Testa, AC, et al. Role of laparoscopy to assess the chance of optimal cytoreductive surgery in advanced ovarian cancer: a pilot study. Gynecol Oncol. (2005) 96:729–35. doi: 10.1016/j.ygyno.2004.11.031
72. Fagotti, A, Vizzielli, G, de Iaco, P, Surico, D, Buda, A, Mandato, VD, et al. A multicentric trial (Olympia-MITO 13) on the accuracy of laparoscopy to assess peritoneal spread in ovarian cancer. Am J Obstet Gynecol. (2013) 209:462.e1–462.e11. doi: 10.1016/j.ajog.2013.07.016
73. van de Vrie, R, Rutten, MJ, Asseler, JD, Leeflang, MMG, Kenter, GG, Mol, BWJ, et al. Laparoscopy for diagnosing resectability of disease in women with advanced ovarian cancer. Cochrane Database Syst Rev. (2019) 2019:CD009786. doi: 10.1002/14651858.CD009786.pub3
74. Nagarsheth, NP, Rahaman, J, Cohen, CJ, Gretz, H, and Nezhat, F. The incidence of port-site metastases in gynecologic cancers. JSLS. (2004) 8:133–9.
75. van Dam, P, DeCloedt, J, Tjalma, WA, Buytaert, P, Becquart, D, and Vergote, IB. Trocar implantation metastasis after laparoscopy in patients with advanced ovarian cancer: can the risk be reduced? Am J Obstet Gynecol. (1999) 181:536–41. doi: 10.1016/S0002-9378(99)70489-8
76. Melamed, A, Hinchcliff, EM, Clemmer, JT, Bregar, AJ, Uppal, S, Bostock, I, et al. Trends in the use of neoadjuvant chemotherapy for advanced ovarian cancer in the United States. Gynecol Oncol. (2016) 143:236–40. doi: 10.1016/j.ygyno.2016.09.002
77. Nezhat, FR, Pejovic, T, Finger, TN, and Khalil, SS. Role of minimally invasive surgery in ovarian cancer. J Minim Invasive Gynecol. (2013) 20:754–65. doi: 10.1016/j.jmig.2013.04.027
78. Gueli Alletti, S, Bottoni, C, Fanfani, F, Gallotta, V, Chiantera, V, Costantini, B, et al. Minimally invasive interval debulking surgery in ovarian neoplasm (MISSION trial-NCT02324595): a feasibility study. Am J Obstet Gynecol. (2016) 214:503.e1–6. doi: 10.1016/j.ajog.2015.10.922
79. Corrado, G, Mancini, E, Cutillo, G, Baiocco, E, Vici, P, Sergi, D, et al. Laparoscopic Debulking surgery in the Management of Advanced Ovarian Cancer after Neoadjuvant Chemotherapy. Int J Gynecol Cancer. (2015) 25:1253–7. doi: 10.1097/IGC.0000000000000491
80. Melamed, A, Nitecki, R, Boruta, DM, del Carmen, MG, Clark, RM, Growdon, WB, et al. Laparoscopy compared with laparotomy for Debulking ovarian Cancer after neoadjuvant chemotherapy. Obstet Gynecol. (2017) 129:861–9. doi: 10.1097/AOG.0000000000001851
81. Fagotti, A, Gueli Alletti, S, Corrado, G, Cola, E, Vizza, E, Vieira, M, et al. The INTERNATIONAL MISSION study: minimally invasive surgery in ovarian neoplasms after neoadjuvant chemotherapy. Int J Gynecol Cancer. (2019) 29:5–9. doi: 10.1136/ijgc-2018-000012
82. Melamed, A, and Rauh-Hain, JA. Minimally invasive interval cytoreductive surgery: it’s time for a randomized trial. Int J Gynecol Cancer. (2019) 29:1339–40. doi: 10.1136/ijgc-2019-000971
83. Armstrong, DK, Alvarez, RD, and Bakkum-Gamez, JN, et al. NCCN clinical practice guideline in oncology: ovarian cancer including fallopian tube cancer and primary peritoneal cancer. version 1. (2020) Available at: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf.
84. Tang, Q, Liu, W, Jiang, D, Tang, J, Zhou, Q, and Zhang, J. Perioperative and survival outcomes of robotic-assisted surgery, comparison with laparoscopy and laparotomy, for ovarian Cancer: a network Meta-analysis. J Oncol. (2022) 30:2084774. doi: 10.1155/2022/2084774
85. Nitecki, R, Rauh-Hain, JA, Melamed, A, Scambia, G, Pareja, R, Coleman, RL, et al. Laparoscopic cytoreduction after neoadjuvant ChEmotherapy (LANCE). Int J Gynecol Cancer. (2020) 30:1450–4. doi: 10.1136/ijgc-2020-001584
86. Shi, T, Zhu, J, Feng, Y, Tu, D, Zhang, Y, Zhang, P, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. (2021) 22:439–49. doi: 10.1016/S1470-2045(21)00006-1
87. Harter, P, Sehouli, J, Reuss, A, Hasenburg, A, Scambia, G, Cibula, D, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the multicenter intergroup study DESKTOP II. A project of the AGO Kommission annals of oncology special article 703 OVAR, AGO study group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. (2011) 21:289–95. doi: 10.1097/IGC.0b013e31820aaafd
88. Lee, CK, Lord, S, Grunewald, T, Gebski, V, Hardy-Bessard, AC, Sehouli, J, et al. Impact of secondary cytoreductive surgery on survival in patients with platinum sensitive recurrent ovarian cancer: analysis of the CALYPSO trial. Gynecol Oncol. (2015) 136:18–24. doi: 10.1016/j.ygyno.2014.09.017
89. Fotopoulou, C, Zang, R, Gultekin, M, Cibula, D, Ayhan, A, Liu, D, et al. Value of tertiary cytoreductive surgery in epithelial ovarian cancer: an international multicenter evaluation. Ann Surg Oncol. (2013) 20:1348–54. doi: 10.1245/s10434-012-2673-z
90. Cianci, S, Ronsini, C, Vizzielli, G, Tropea, A, Biondi, A, Scambia, G, et al. Cytoreductive surgery followed by HIPEC repetition for secondary ovarian cancer recurrence. Updat Surg. (2019) 71:389–94. doi: 10.1007/s13304-018-0600-y
91. Coleman, RL, Spirtos, NM, Enserro, D, Herzog, TJ, Sabbatini, P, Armstrong, DK, et al. Secondary surgical Cytoreduction for recurrent ovarian Cancer. N Engl J Med. (2019) 381:1929–39. doi: 10.1056/NEJMoa1902626
92. Fagotti, A, Costantini, B, Gallotta, V, Cianci, S, Ronsini, C, Petrillo, M, et al. Minimally invasive secondary cytoreduction plus HIPEC versus open surgery plus HIPEC in isolated relapse from ovarian cancer: a retrospective cohort study on perioperative outcomes. J Minim Invasive Gynecol. (2015) 22:428–32. doi: 10.1016/j.jmig.2014.11.008
93. Magrina, JF, Cetta, RL, Chang, YH, Guevara, G, and Magtibay, PM. Analysis of secondary cytoreduction for recurrent ovarian cancer by robotics, laparoscopy and laparotomy. Gynecol Oncol. (2013) 129:336–40. doi: 10.1016/j.ygyno.2013.01.015
94. Eriksson, AGZ, Graul, A, Yu, MC, Halko, A, Chi, DS, Zivanovic, O, et al. Minimal access surgery compared to laparotomy for secondary surgical cytoreduction in patients with recurrent ovarian carcinoma: perioperative and oncologic outcomes. Gynecol Oncol. (2017) 146:263–7. doi: 10.1016/j.ygyno.2017.05.022
95. Gallotta, V, Conte, C, Giudice, MT, Nero, C, Vizzielli, G, Gueli Alletti, S, et al. Secondary laparoscopic Cytoreduction in recurrent ovarian Cancer: a large, Single-Institution Experience. J Minim Invasive Gynecol. (2018) 25:644–50. doi: 10.1016/j.jmig.2017.10.024
96. Nezhat, FR, Denoble, SM, Cho, JE, Brown, DN, Soto, E, Chuang, L, et al. Safety and efficacy of video laparoscopic surgical debulking of recurrent ovarian, fallopian tube, and primary peritoneal cancers. JSLS. (2012) 16:511–8. doi: 10.4293/108680812X13462882736691
97. Trinh, H, Ott, C, and Fanning, J. Feasibility of laparoscopic debulking with electrosurgical loop excision procedure and argon beam coagulator at recurrence in patients with previous laparotomy debulking. Am J Obstet Gynecol. (2004) 190:1394–7. doi: 10.1016/j.ajog.2004.02.034
98. Nezhat, FR, Finger, TN, Vetere, P, Radjabi, AR, Vega, M, Averbuch, L, et al. Comparison of perioperative outcomes and complication rates between conventional versus robotic-assisted laparoscopy in the evaluation and management of early, advanced, and recurrent stage ovarian, fallopian tube, and primary peritoneal cancer. Int J Gynecol Cancer. (2014) 24:600–7. doi: 10.1097/IGC.0000000000000096
99. Mutlu, L, Khadraoui, W, Khader, T, and Menderes, G. Robotic tumor Debulking off external iliac vessels for the Management of Recurrent Ovarian Cancer. J Minim Invasive Gynecol. (2020) 27:1021–2. doi: 10.1016/j.jmig.2019.10.002
100. Magrina, JF, Guardiola, TC, Magtibay, PM 3rd, Kosiorek, HE, and Magtibay, PM. Minimally invasive surgery for resection of diaphragm metastases in ovarian Cancer. J Minim Invasive Gynecol. (2019) 26:1268–72. doi: 10.1016/j.jmig.2018.12.003
101. Yang, W, Cheng, Z, Dai, H, Long, C, and Liu, H. Laparoscopic-based score assessment combined with a multiple disciplinary teamin management of recurrent ovarian cancer: a single-center prospective study for personalized surgical therapy. Medicine. (2017) 96:e7440. doi: 10.1097/MD.0000000000007440
102. Mascilini, F, Quagliozzi, L, Moro, F, Moruzzi, MC, Gallotta, V, Alletti, SG, et al. Role of intraoperative ultrasound to extend the application of minimally invasive surgery for treatment of recurrent gynecologic Cancer. J Minim Invasive Gynecol. (2018) 25:848–54. doi: 10.1016/j.jmig.2017.12.023
103. Ahmad, G, O’Flynn, H, Duffy, JM, Phillips, K, and Watson, A. Laparoscopic entry techniques. Cochrane Database Syst Rev. (2012) 2:CD006583. doi: 10.1002/14651858.CD006583.pub5
104. Ramirez, PT, Frumovitz, M, Pareja, R, Lopez, A, Vieira, M, Ribeiro, R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical Cancer. N Engl J Med. (2018) 379:1895–904. doi: 10.1056/NEJMoa1806395
Keywords: minimal invasive surgery, diagnostic laparoscopy, early ovarian cancer, advanced ovarian cancer, interval debulking surgery, neoadjuvant chemotherapy, ovarian cancer recurrence, cytoreduction
Citation: Generali M, Annunziata G, Pirillo D, D’Ippolito G, Ciarlini G, Aguzzoli L and Mandato VD (2023) The role of minimally invasive surgery in epithelial ovarian cancer treatment: a narrative review. Front. Med. 10:1196496. doi: 10.3389/fmed.2023.1196496
Edited by:
Antonio Simone Laganà, University of Palermo, ItalyReviewed by:
Pengpeng Qu, Tianjin Central Hospital for Gynecology and Obstetrics, ChinaCarlo Ronsini, Universitàdegli Studi della Campania "Luigi Vanvitelli," Italy
Copyright © 2023 Generali, Annunziata, Pirillo, D’Ippolito, Ciarlini, Aguzzoli and Mandato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vincenzo Dario Mandato, ZGFyaW9tYW5kYXRvQGdtYWlsLmNvbQ==
 Matteo Generali
Matteo Generali Gianluca Annunziata
Gianluca Annunziata Vincenzo Dario Mandato
Vincenzo Dario Mandato