- 1Division of Angiology, Department of Internal Medicine, Medical University of Graz, Graz, Austria
- 2Clinical Institute of Medical and Chemical Laboratory Diagnostics, Medical University of Graz, Graz, Austria
- 3Center of Medical Research (ZMF), Medical University of Graz, Graz, Austria
- 4Division of Restorative Dentistry, Endodontics, Periodontology and Prosthodontics, Department of Dental Medicine and Oral Health, Medical University of Graz, Graz, Austria
- 5Division of Endocrinology and Diabetology, Department of Internal Medicine, Endocrinology Lab Platform, Medical University of Graz, Graz, Austria
- 6Division of Pulmonology, Department of Internal Medicine, Medical University of Graz, Graz, Austria
- 7Ludwig Boltzmann Institute for Lung Vascular Research, Graz, Austria
- 8Division of Nephrology, Department of Internal Medicine, Medical University of Graz, Graz, Austria
- 9Division of Rheumatology, Department of Internal Medicine, Medical University of Graz, Graz, Austria
Objectives: Pathways contributing to endothelial dysfunction in patients with limited cutaneous systemic sclerosis (lcSSc) are largely unknown. The aim of this study was to investigate potential associations of amino acids and parameters of bone metabolism with endothelial dysfunction and vasculopathy-related changes in patients with lcSSc and early-stage vasculopathy.
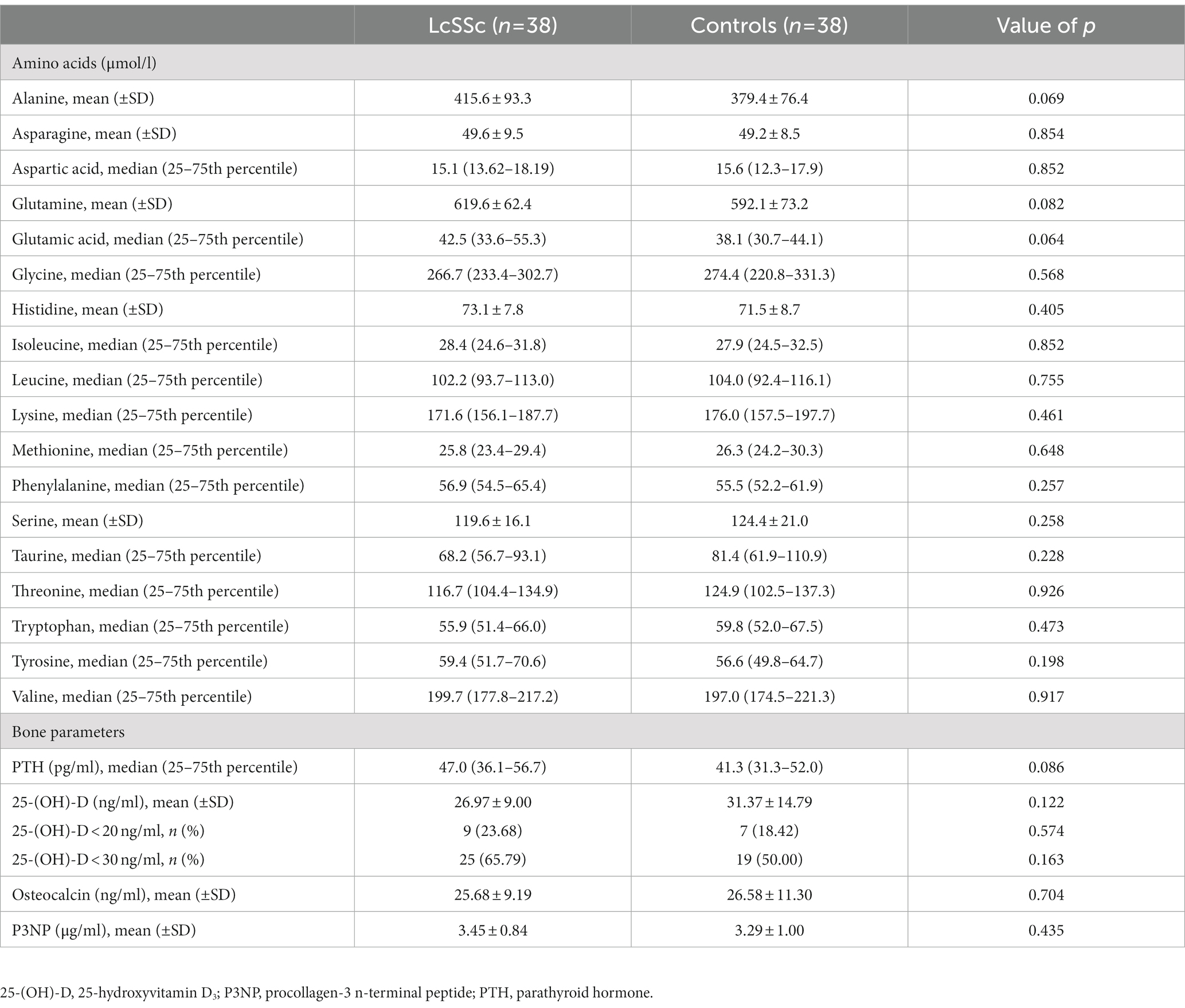
Methods: Amino acids, calciotropic parameters, including 25-hydroxyvitamin D and parathyroid hormone (PTH), and bone turnover parameters, including osteocalcin and N-terminal peptide of procollagen-3 (P3NP), were measured in 38 lcSSc patients and 38 controls. Endothelial dysfunction was assessed by biochemical parameters, pulse-wave analysis, flow-mediated and nitroglycerine-mediated dilation. Additionally, vasculopathy-related and SSc-specific clinical changes including capillaroscopic, skin, renal, pulmonary, gastrointestinal and periodontal parameters were recorded.
Results: No significant differences in amino acids, calciotropic and bone turnover parameters were observed between lcSSc patients and controls. In patients with lcSSc, several significant correlations were found between selected amino acids, parameters of endothelial dysfunction, vasculopathy-related and SSc-specific clinical changes (all with p < 0.05). In addition, significant correlations were observed between PTH and 25-hydroxyvitamin D with homoarginine, and between osteocalcin, PTH and P3NP with modified Rodnan skin score and selected periodontal parameters (all with p < 0.05). Vitamin D deficiency defined as 25-hydroxyvitamin D < 20 ng/ml was associated with the presence of puffy finger (p = 0.046) and early pattern (p = 0.040).
Conclusion: Selected amino acids may affect endothelial function and may be associated to vasculopathy-related and clinical changes in lcSSc patients, while the association with parameters of bone metabolism seems to be minor.
Introduction
Systemic sclerosis (SSc) represents a rare autoimmune connective tissue disease with potentially serious complications due to its effects on skin and internal organs. There are three hallmarks in the pathogenesis of SSc, including autoimmune activation, tissue fibrosis and vasculopathy, while vasculopathy seems to occur predominantly in limited cutaneous SSc (lcSSc) (1–3). Due to interactions between different cells types, extracellular matrix and cytokines, structural and functional changes of micro-and macrovascular vessels occur. They lead to clinical manifestations of SSc, including Raynaud’s phenomenon, capillary changes or pulmonary arterial hypertension (PAH) (3–5). Besides these commonly vasculopathy-driven symptoms and complications, vasculopathy and endothelial dysfunction may also contribute to other manifestations of SSc, like skin, renal and gastrointestinal involvement or periodontal disease (6–9).
Multiple parameters of endothelial dysfunction are known so far, including pulse-wave velocity (PWV), flow-mediated dilation (FMD), nitroglycerine-mediated dilation (NMD), biochemical parameters of the arginine metabolism or endothelial microparticles (EMP), which have also been evaluated in SSc (10–12). However, potential contributors to endothelial dysfunction and vasculopathic changes in SSc are still largely unknown. While both arginine and homoarginine have been investigated as potential contributors to endothelial dysfunction in SSc, the knowledge on potential influences of other amino acids on parameters of endothelial dysfunction and vasculopathic changes in SSc are limited (12–14). Another extensively investigated metabolic parameter is vitamin D and vitamin D deficiency is commonly found in patients with SSc, which may also be associated with SSc-related complications (15). Especially, as vitamin D has immunomodulatory effects by inhibition of T helper-1 lymphocytes and proinflammatory cytokines on the one hand and promotion of anti-inflammatory cytokines on the other hand, these properties may contribute to SSc-related complications (16). Additionally, vitamin D also exhibits antifibrotic properties, with a reported downregulation of several profibrotic biomarkers, and cardioprotective effects by downregulation of prothrombotic factors and exerting anti-hypertrophic effects on cardiomyocytes (16–18). However, vitamin D revealed inconsistent findings in endothelial dysfunction, also in SSc (19–21). Other parameters of bone metabolism, such as parathyroid hormone (PTH) and osteocalcin, may also affect endothelial dysfunction potentially by oxidative stress (22, 23). However, data about the interaction of bone metabolism on endothelial dysfunction in SSc are still lacking.
This study aimed to evaluate associations between proteinogenic amino acids, calciotropic and bone turnover parameters with endothelial dysfunction and vasculopathy-related changes in patients with lcSSc.
Materials and methods
Study design and patient cohort
As this study is a sub-analysis of a prior case–control study of lcSSc patients in order to investigate endothelial dysfunction at early-stage vasculopathy, full details about the study design and parameter measurements have been previously described (12). Briefly, patients with lcSSc and a control group of age-, race-and sex-matched subjects with primary Raynaud’s phenomenon were included. Inclusion criterion for the group of patients with lcSSc was the presence of diagnosed lcSSc according to the EULAR/ACR criteria of 2013 (2). Exclusion criteria for both groups were age < 18 years, presence of diffuse cutaneous SSc or other connective tissues diseases, preexisting or existing PAH, digital ulcers, endoscopic approved reflux, diabetes mellitus or symptomatic atherosclerotic cardiovascular diseases, recent pregnancy or malignancies, acute infections at time of enrolment and current intake (<24 h) of prostanoids, calcium channel blockers, phosphodiesterase-5 inhibitors or endothelin-receptor inhibitors. Primary endpoint was the difference of amino acids and metabolic bone parameters between patients with lcSSc and controls. Secondary endpoints were associations between amino acids and metabolic bone parameters with parameters of endothelial dysfunction and vasculopathy-related as well as SSc-specific clinical changes.
Evaluation of amino acids, calciotropic and bone turnover parameters
Fasting blood samples were obtained by standard phlebotomy during study inclusion. Following amino acids were assessed by high-performance liquid chromatography: alanine, asparagine, aspartic acid, glutamine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, serine, threonine, tryptophan, tyrosine, and valine. Proline and cysteine could not be measured by this method, and taurine was evaluated instead. 25-hydroxyvitamin D [25-(OH)-D] and PTH were measured as calciotropic parameters, N-terminal peptide of procollagen-3 (P3NP) and osteocalcin were measured as bone turnover parameters by routine laboratory work-up. Vitamin D insufficiency was defined as serum 25-(OH)-D levels <30 ng/ml, vitamin D deficiency was defined as serum 25-(OH)-D levels <20 ng/ml.
Evaluation of endothelial dysfunction
Arterial stiffness was evaluated by automated pulse-wave analysis via an oscillometric device (I.E.M. Mobil-O-Graph, I.E.M., Stolberg, Germany) while vascular reactivity was measured by FMD and NMD according to the guidelines by Corretti et al. (24). PWV of >10 m/s, FMD < 7% and NMD < 15.6% were defined as pathologic (25–27). Parameters of arginine metabolism, including asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), homoarginine, arginine, citrulline and ornithine, were measured by high-performance liquid chromatography. Measurement of EMP was performed according to the recommendations published by Cossarizza et al. (28) identifying EMP as CD31+/CD42b-events (29). Detailed information about the measurement methods of the respective parameters of endothelial dysfunction have been previously published by Jud et al. (12).
Vasculopathy-related and SSc-specific clinical changes
Capillaroscopic changes were evaluated by nailfold videocapillaroscopy (NVC) of the second to fifth digit on both hands (Skinview, Optometron Ltd., Oskar-Messterstr., Ismaning, Germany). Morphological changes of the capillaries including SSc-specific capillary patterns, capillaroscopic skin ulcer risk index (CSURI) and microangiopathy evolution score (MES) were recorded (30, 31). Skin involvement, including telangiectasia, puffy finger, sclerodactyly, and modified Rodnan Skin Score (mRSS) were recorded by physical examination. Renal involvement was evaluated by estimated glomerular filtration rate (eGFR) with CKD-EPI equation and urinary total protein/creatinine ratio. Evaluation of subclinical PAH was performed by the DETECT algorithm while evaluation of gastrointestinal involvement was performed by UCLA SCTC GIT 2.0 questionnaire (32, 33). Disease severity was evaluated by the revised European Scleroderma Trials and Research Group (EUSTAR) index (34). Parameters of periodontal disease, including pocket depth, plaque index, bleeding on probing (BOP), bone loss and bone support ratio were evaluated by dental and oral examinations with a dentist’s mirror and a periodontal probe as well as by digital dental panoramic radiograph (Orthophos XG 3D, Dentsply Sirona, Charlotte, USA) (35, 36). Detailed information about the measurement methods of vasculopathy-related and SSc-specific clinical changes have been previously published (9, 12).
Statistical analysis
Categorical variables were represented by frequency and percentages and analyzed by Chi-square test. Continuous variables were given as means and standard deviation or median and interquartile range. The normal distribution was examined with Shapiro–Wilk test and Student’s t-test was used for normally distributed data while Mann–Whitney-U test was used for non-normally distributed data. Pearson’s correlation coefficient was utilized for normally distributed variables and Spearman’s correlation coefficient was used for non-normally distributed variables. Statistical significance was assumed for p values<0.05 and statistical analyses were executed with SPSS version 27.0.
Ethical approval
This study was approved by the local ethics committee (protocol number EK 29–361 ex 16/17) and was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013. All patients gave their written informed consent after being accurately informed about that clinical study.
Results
Patients’ characteristics have been described elsewhere (9, 12). Median uric acid levels were 4.6 mg/dL (3.9–5.4 mg/dl 25th–75th percentile) in patients with lcSSc and 4.3 mg/dL (3.7–5.3 mg/dL 25th–75th percentile) in controls (p = 0.313). Mean disease duration was 7.1 ± 5.8 years in patients with lcSSc and 5.7 ± 3.2 years in controls (p = 0.191). No significant differences regarding amino acids, calciotropic or bone turnover parameters were observed between patients with lcSSc and controls. Only a trend toward higher levels of alanine, glutamic acid, glutamine, and PTH was observed in patients with lcSSc. Neither the rate of vitamin D insufficiency nor vitamin D deficiency differed between both groups (Table 1). At study inclusion, four lcSSc patients (10.5%) and two controls (5.3%) (p = 0.654) had a pre-existing osteoporosis, which was recorded by patient’s history, while 10 lcSSc patients (26.3%) and three controls (7.9%) (p = 0.065) had a pre-existing treatment against osteoporosis. Of those patients with a pre-existing therapy against osteoporosis, all had a peroral vitamin D supplementation, one lcSSc patient had additional denosumab and another lcSSc patient had additional alendronic acid. No patient had any pre-existing hyperparathyroidism.
Correlations between amino acids and parameters of bone metabolism with endothelial dysfunction
Significantly positive correlations were found between NMD, histidine and serine as well as between PWV and tyrosine. Significantly negative correlations between PWV, glycine and serine were also observed. Additionally, threonine revealed significantly negative correlations with PWV, augmentation index and EMP. Of the investigated arginine metabolites, significantly positive correlations were observed between ADMA and leucine, between citrulline, asparagine and glutamine, and between ornithine, asparagine, isoleucine, leucine, methionine, phenylalanine, taurine, and threonine. Significantly negative correlation between citrulline and tryptophan was also found. Neither SDMA nor arginine nor homoarginine correlated with any of the investigated amino acids. Homoarginine correlated significantly positively with PTH and negatively with 25-(OH)-D (Figure 1). Pathologic values of FMD, NMD and PWV were not associated with vitamin D insufficiency or deficiency (Table 2).
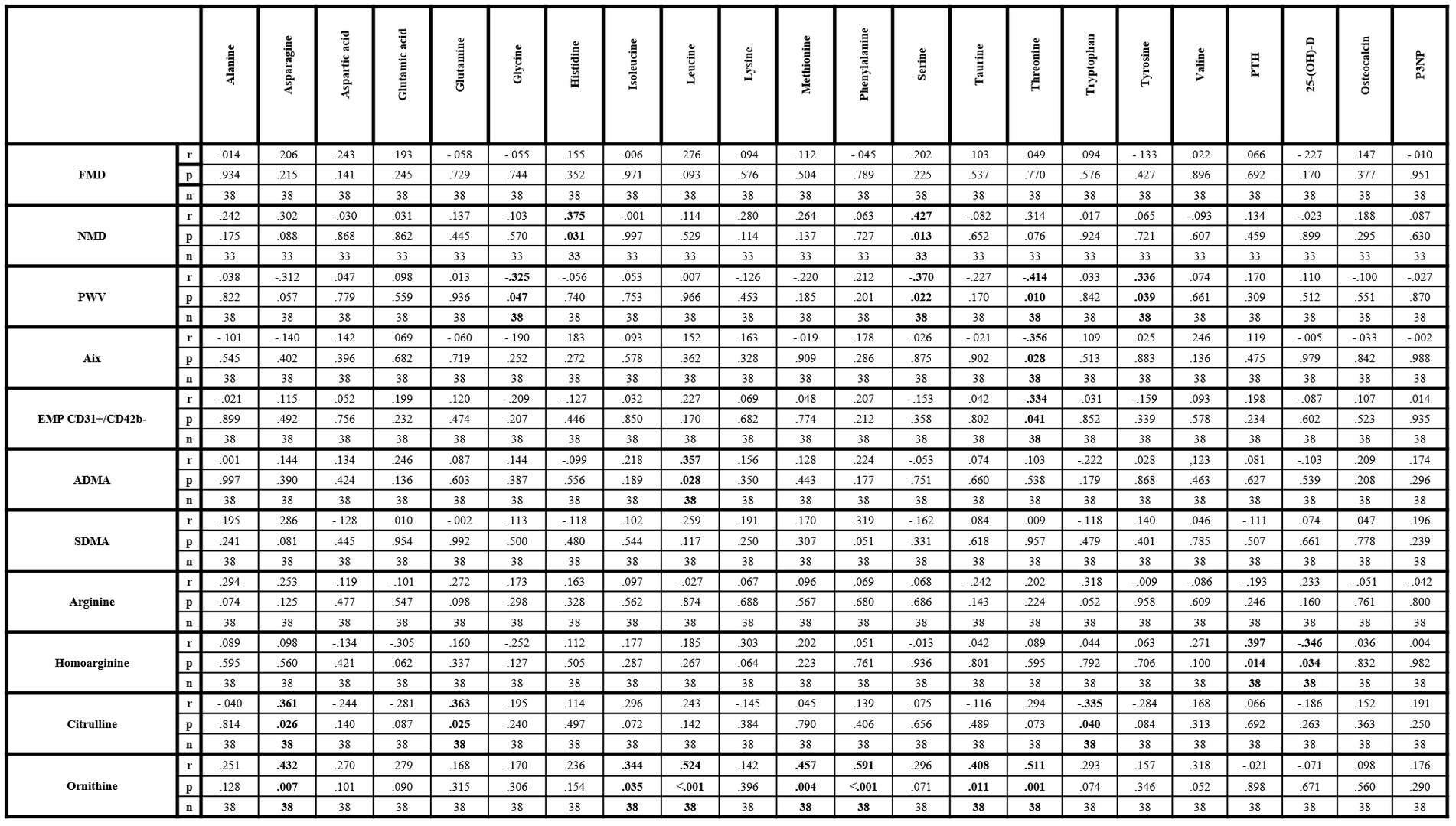
Figure 1. Correlation matrix of amino acids and metabolic bone parameters with parameters of endothelial dysfunction within lcSSc patients. 25-(OH)-D, 25-hydroxyvitamin D3; ADMA, asymmetric dimethylarginine; Aix, augmentation index; EMP, endothelial-derived microparticles; FMD, flow-mediated dilation; NMD, nitroglycerin-mediated dilation; P3NP, procollagen-3 n-terminal peptide; PTH, parathyroid hormone; PWV, pulse-wave velocity; SDMA, symmetric dimethylarginine. Bold values indicate statistical significance.
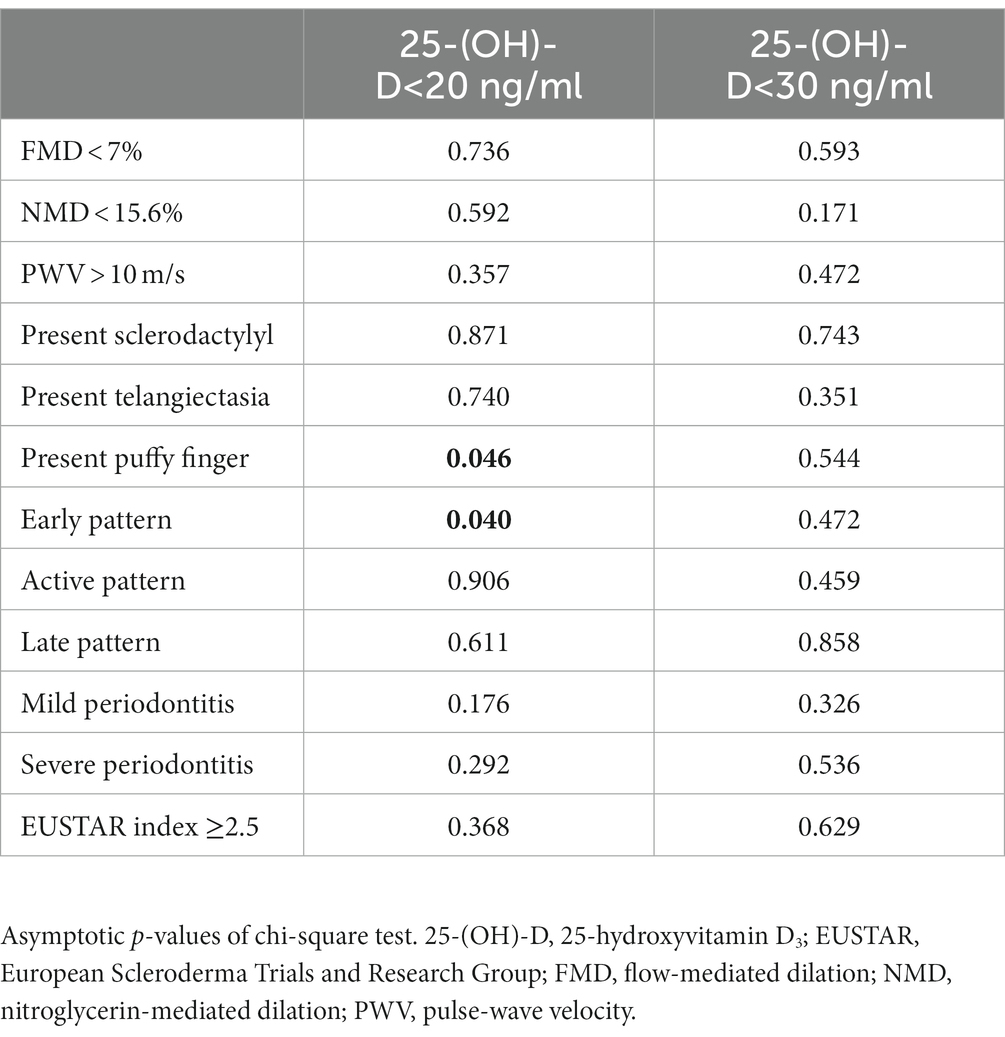
Table 2. Associations of parameters of endothelial dysfunction and SSc-specific clinical changes with vitamin D deficiency and insufficiency.
Correlations between amino acids and parameters of bone metabolism with vasculopathy-related and SSc-specific changes
CSURI revealed a significantly positive correlation with isoleucine and leucine while MES did not show any correlation with amino acids. MRSS was significantly positive correlated with aspartic acid and glutamic acid and negatively with asparagine and methionine. Additional significantly negative correlations were observed between eGFR, alanine and tyrosine while protein/creatinine ratio did not correlate with any amino acid. The DETECT score correlated significantly positively with isoleucine, leucine, phenylalanine and valine, but neither the total score of the UCLA SCTC GIT 2.0 questionnaire nor the EUSTAR index revealed a correlation with any amino acid. Significantly negative correlations were found between threonine, plaque index and BOP as well as between BOP and asparagine.
Of the investigated parameters of bone metabolism, significantly positive correlations were observed between PTH and plaque index as well as between BOP and osteocalcin. P3NP revealed significantly negative correlations with mRSS and alveolar bone loss. Vitamin D revealed no correlation with any vasculopathy-related or SSc-specific parameter (Figure 2). Vitamin D insufficiency was not associated with any of the investigated SSc-specific clinical changes, while significant associations were found between vitamin D deficiency, puffy finger and early pattern (Table 2).
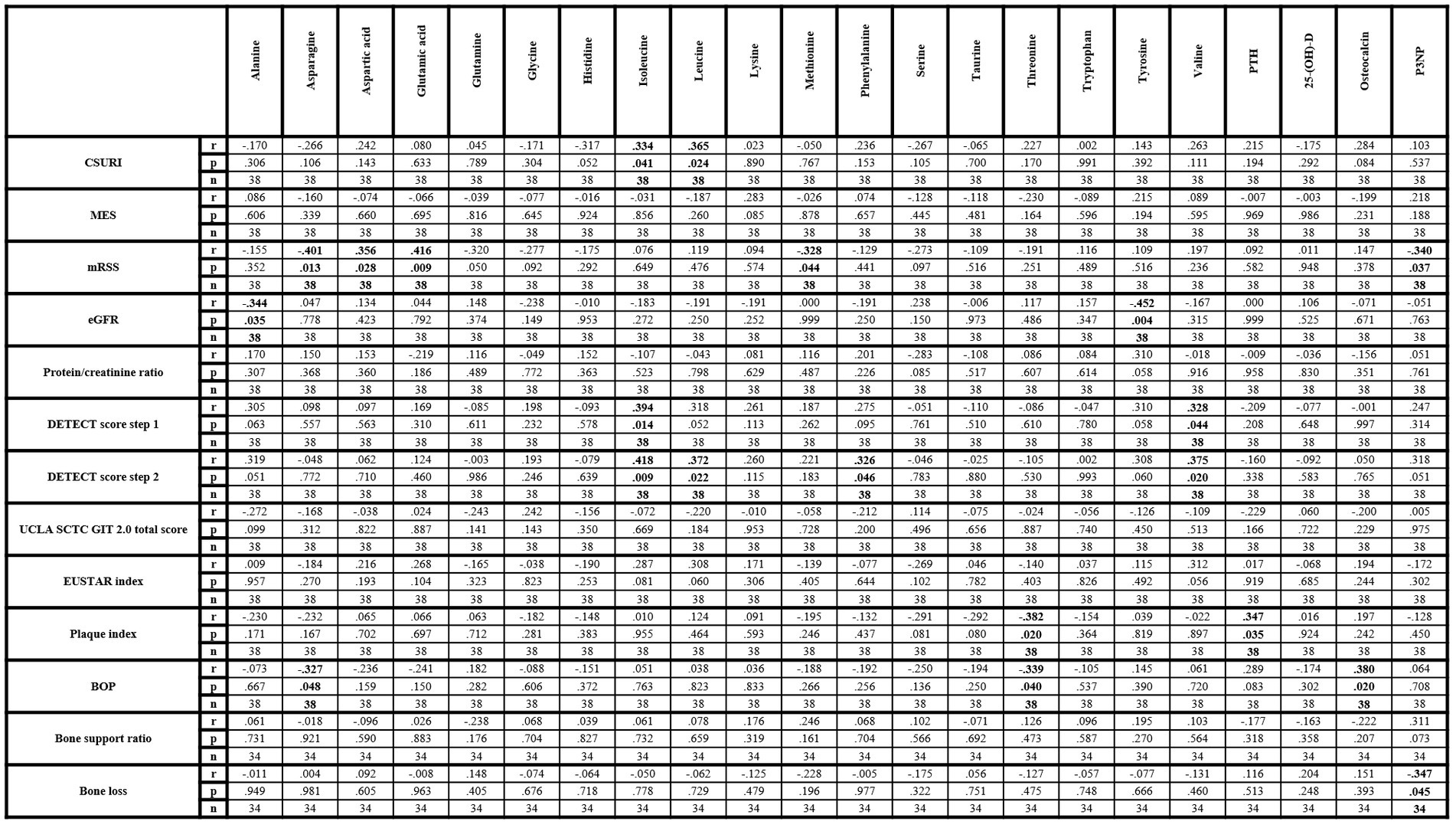
Figure 2. Correlation matrix of amino acids and metabolic bone parameters with clinical parameters within lcSSc patients. 25-(OH)-D, 25-hydroxyvitamin D3; BOP, bleeding on probe; CSURI, capillaroscopic skin ulcer risk index; eGFR, estimated glomerular filtration rate; EUSTAR, European Scleroderma Trials and Research Group; MES, microangiopathy evolution score; mRSS, modified Rodnan Skin Score; P3NP, procollagen-3 n-terminal peptide; PTH, parathyroid hormone. Bold values indicate statistical significance.
Discussion
Endothelial dysfunction plays an important role in the development of SSc-related vasculopathy while the promoting factors of endothelial dysfunction in SSc subtypes and its potential interaction with other pathophysiologic pathways still need to be elucidated. This study was able to demonstrate potential interactions between selected proteinogenic amino acids, calciotropic and bone turnover parameters as well as endothelial dysfunction, vasculopathy-related and clinical changes in lcSSc. However, none of the measured amino acids, calciotropic and bone turnover parameter revealed a significant difference between patients with lcSSc and controls. One possible explanation for the missing relevant difference of the measured parameters may be the early disease stage in the group of lcSSc patients, as none of the investigated subjects had typical SSc-related complications, like PAH, digital ulcers or renal crisis. Only two lcSSc patients (5.3%) had incipient interstitial lung disease. Compared to the study by Smolenska et al. (14), which also investigated amino acids, but in a patient cohort with lcSSc and diffuse cutaneous SSc, the rate of organ involvement was higher than in our study and the serum levels of many amino acids differed substantially compared to our results, suggesting a potential influence of the SSc subtype and activity. Previous studies on parameters of bone metabolism, especially on 25-(OH)-D, included patients with other SSc subtypes and with SSc-related complications, which may also influence the results on those parameters (37–39). In addition, it has to be noted that patients with primary Raynaud’s phenomenon may be not ideal controls for study measurements of parameters of bone metabolism, as even pediatric patients with primary Raynaud’s phenomenon may already have low serum vitamin D levels which could bias our results on parameters of bone metabolism (40).
Certain amino acids revealed significant correlations with parameters of endothelial dysfunction and some of the measured amino acids, including glutamic acid, glutamine, glycine, histidine, leucine, methionine, serine, or tyrosine, have been reported to have potential protective cardiovascular properties (41, 42). Interestingly, leucine may be also an inhibitor of nitric oxide synthase (43). These data are, however, controversial to our findings. On the one hand, positive correlations were found between histidine and NMD and between leucine and ADMA, while negative correlations were observed between glycine, serine and PWV. These results are indicating potential vasoprotective effects for glycine, serine and histidine and potential damaging effects for leucine, which are in accordance with previous studies (41–43). On the other hand, glutamic acid revealed no correlations with any of the measured parameters of endothelial dysfunction. Glutamine and methionine were only positively correlated with citrulline and ornithine, while tyrosine was positively correlated with PWV, although inverse associations have been reported (41). Interestingly, threonine revealed also negative correlations with parameters of arterial stiffness and with EMP suggesting a potential unknown vasoprotective effects. However, the underlying interactions need to be investigated by further studies. Besides ADMA, only citrulline and ornithine correlated with other amino acids, but not arginine, homoarginine or SDMA. The correlations between asparagine, glutamine, citrulline, and ornithine may be expectable due to interactive metabolic processes between these amino acids (44). Conversely, especially the correlations of ornithine with isoleucine, leucine, methionine, phenylalanine, taurine and threonine are of interest as these amino acids have no known metabolic pathway interactions. Potential changes in other metabolic pathways, like polyamine metabolism, which were not investigated in this study, may contribute to these correlations (45, 46). Finally, significant correlations between homoarginine, PTH and 25-(OH)-D only were observed. This might indicate a potential interaction, possibly via osteo−/chondrogenic transformation of vascular smooth muscle cells and subsequent vascular calcification, as previously reported (47). However, this effect may be marginal or undetectable via the systemic detection or the current study design as neither PWV nor augmentation index correlated with PTH or 25-(OH)-D.
CSURI correlated with isoleucine and leucine suggesting a contribution of capillary damage by these chemically similar amino acids, potentially via an interaction of ADMA. At least leucine may act as an inhibitor of nitric oxide synthase and ADMA was shown to correlate with CSURI in a previous study (12, 43). Overall, the association of amino acids with capillary changes seems to be low, as no other amino acid correlated neither with CSURI nor with MES. Skin sclerosis, which was evaluated by mRSS, revealed correlations with asparagine, aspartic acid, glutamic acid and methionine. Elevated glutamate, an anion of glutamic acid, and decreased levels of asparagine have been associated with calcinosis and extensive scleroderma (14). Our data are comparable to these results, as asparagine and glutamine revealed negative correlations while aspartic acid and glutamic acid showed positive correlations with mRSS. Since both, asparagine and glutamine, are reported to be mediators of immune function, dysbalance of their metabolic turn-over may impair immunoregulation with subsequent contribution to skin sclerosis (48, 49). In addition, lower methionine levels may promote skin sclerosis as methionine may affect inflammatory and fibrotic processes (50).
Negative correlations between eGFR, alanine and tyrosine but not with urinary total protein/creatinine ratio were also observed. Alanine was previously described as a potential biomarker in patients with acute kidney injury and showed associations with the prevalence of chronic kidney disease (51, 52). Moreover, impaired kidney function leads to reduced conversion of phenylalanine to tyrosine (53). Thus, these associations seem to be a result of impaired kidney function rather than specific SSc-related changes in the kidney. Nevertheless, whether these alterations may reflect kidney dysfunction associated with vasculopathy in SSc patients need to be elucidated in more detail.
Subclinical PAH, as evaluated by the DETECT score, correlated with isoleucine, leucine, phenylalanine and valine. Again, this might, at least partially, be due to potential interactions via ADMA, since ADMA previously showed associations with the DETECT score (12). On the other hand, associations of higher phenylalanine and valine levels with pulmonary involvement were observed in previous studies, suggesting therefore a potential interaction between selected amino acids with PAH (14, 54). Interestingly, no amino acid was associated with gastrointestinal involvement evaluated by UCLA SCTC GIT 2.0 questionnaire or with disease activity as measured by EUSTAR index. While potential involvement of amino acids in gastrointestinal changes and disease activity cannot be excluded, as only two of many potential parameters were evaluated, further studies are needed to evaluate other parameters of gastrointestinal involvement and disease activity.
Further potential associations between threonine, plaque index and BOP were observed, suggesting anti-inflammatory effects of threonine. Negative correlations of P3NP with mRSS and bone loss indicating that P3NP, as a marker of collagen synthesis in bone and muscles, may have an impact on collagen turn-over in skin and alveolar bone (55). Additionally, positive associations between PTH and plaque index were observed which may be explained by higher serum and salivary phosphorus levels, which were found in patients with hemodialysis and elevated PTH levels (56). The fact that vitamin D did not show any correlation with vasculopathy-related or clinical changes while vitamin D deficiency was associated only with puffy finger and capillaroscopic early pattern, might indicate only marginally influence of 25-(OH)-D in early stage vasculopathy. Similarly, associations between osteocalcin and BOP seem to be by chance without relevant influence on periodontal disease, while no correlation between osteocalcin and bone loss or bone support ratio was observed.
One limitation of this study is the exploratory design with measurements of many parameters without adjustment for multiple testing which allows therefore only some hypothesis generation. Additional limitations are the relatively small sample size and the recruitment of patients with Raynaud’s phenomenon as controls that might affect the results on metabolic parameters (40). However, it has to be noted that this study is a sub-study of a previous investigation on endothelial dysfunction in patients with lcSSc in which the inclusion of patients with Raynaud’s phenomenon as controls was adequate. Strengths are homogenous cohorts with age-, race-and sex-matched controls and absent end-stage vasculopathic changes.
In conclusion, certain amino acids may be associated with endothelial function and may contribute to vasculopathy-related and clinical changes in lcSSc patients with early-stage vasculopathy. However, their clinical relevance in SSc, especially as predictors of SSc-related complications, and potential interaction with other metabolic parameters needs to be further elucidated. The relevance of the investigated calciotropic and bone turnover parameters on endothelial dysfunction and on vasculopathy-related and clinical changes seems to be marginally. Nevertheless, further studies will probably reveal potential interactions of metabolic parameters in SSc.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committee of the Medical University of Graz. The patients/participants provided their written informed consent to participate in this study.
Author contributions
PJ and FH contributed to study design, study measurements, and funding acquisition. PJ contributed to writing of the manuscript and data analysis. AM, HS, BA, GW, BO-P, VF, and GK contributed to study measurements, data interpretation, and reviewing of the manuscript. BO and FM-F contributed to data interpretation and reviewing of the manuscript. MB contributed to reviewing of the manuscript and supervised the project. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Actelion Pharmaceuticals, Vienna, Austria. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
We thank Gudrun Dimsity, Larissa Krempel, Nicole Peischl, Verena Zrim, Cornelia Missbrenner, Dorrit Münzer-Ornik, Beate Weismaier, and Alexandra Kinzelmann for their assistance with the measurements of parameters of endothelial dysfunction, bone and dental parameters.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Denton, CP, and Khanna, D. Systemic sclerosis. Lancet. (2017) 399:1685–99. doi: 10.1016/S0140-6736(17)30933-9
2. van den Hoogen, F, Khanna, D, Fransen, J, Johnson, SR, Baron, M, Tyndall, A, et al. Classification criteria for systemic sclerosis: an ACR-EULAR collaborative initiative. Arthritis Rheum. (2013) 65:2737–47. doi: 10.1002/art.38098
3. Kahaleh, B. Vascular disease in scleroderma: mechanisms of vascular injury. Rheum Dis Clin N Am. (2008) 34:57–71. doi: 10.1016/j.rdc.2007.12.004
4. Szucs, G, Timar, O, Szekanecz, Z, Dér, H, Kerekes, G, Szamosi, S, et al. Endothelial dysfunction precedes atherosclerosis in systemic sclerosis--relevance for prevention of vascular complications. Rheumatology. (2007) 46:759–62. doi: 10.1093/rheumatology/kel426
5. Haustein, UF. Systemic sclerosis–an update. Lab Med. (2011) 42:562–72. doi: 10.1309/LM9OB5BZ4OSNVGVF
6. Manetti, M, Guiducci, S, Romano, E, Bellando-Randone, S, Conforti, ML, Ibba-Manneschi, L, et al. Increased serum levels and tissue expression of matrix metalloproteinase-12 in patients with systemic sclerosis: correlation with severity of skin and pulmonary fibrosis and vascular damage. Ann Rheum Dis. (2012) 71:1064–72. doi: 10.1136/annrheumdis-2011-200837
7. Trostle, DC, Bedetti, CD, Steen, VD, Al-Sabbagh, MR, Zee, B, and Medsger, TA Jr. Renal vascular histology and morphometry in systemic sclerosis. A case-control autopsy study. Arthritis Rheum. (1988) 31:393–400. doi: 10.1002/art.1780310311
8. Bandini, G, Cometi, L, Accogli, E, Domanico, A, Tofani, L, Bruni, C, et al. Ultrasound evaluation of bowel vasculopathy in systemic sclerosis. Eur J Intern Med. (2022) 100:62–8. doi: 10.1016/j.ejim.2022.01.026
9. Jud, P, Wimmer, G, Meinitzer, A, Strohmaier, H, Schwantzer, G, Moazedi-Fürst, F, et al. Periodontal disease and its association to endothelial dysfunction and clinical changes in limited systemic sclerosis: a case-control study. J Periodontal Res. (2023) 58:621–33. doi: 10.1111/jre.13111
10. Cypiene, A, Laucevicius, A, Venalis, A, Dadoniene, J, Ryliskyte, L, Petrulioniene, Z, et al. The impact of systemic sclerosis on arterial wall stiffness parameters and endothelial function. Clin Rheumatol. (2008) 27:1517–22. doi: 10.1007/s10067-008-0958-1
11. Lammi, MR, Saketkoo, LA, Okpechi, SC, Dadoniene, J, Ryliskyte, L, Petrulioniene, Z, et al. Microparticles in systemic sclerosis: potential pro-inflammatory mediators and pulmonary hypertension biomarkers. Respirology. (2019) 24:675–83. doi: 10.1111/resp.13500
12. Jud, P, Meinitzer, A, Strohmaier, H, Schwantzer, G, Foris, V, Kovacs, G, et al. Evaluation of endothelial dysfunction and clinical events in patients with early-stage vasculopathy in limited systemic sclerosis. Clin Exp Rheumatol. (2021) 39:57–65. doi: 10.55563/clinexprheumatol/243mpp
13. Papageorgiou, N, Androulakis, E, Papaioannou, S, Antoniades, C, and Tousoulis, D. Homoarginine in the shadow of asymmetric dimethylarginine: from nitric oxide to cardiovascular disease. Amino Acids. (2015) 47:1741–50. doi: 10.1007/s00726-015-2017-y
14. Smolenska, Z, Zabielska-Kaczorowska, M, Wojteczek, A, Kutryb-Zajac, B, and Zdrojewski, Z. Metabolic pattern of systemic sclerosis: Association of Changes in plasma concentrations of amino acid-related compounds with disease presentation. Front Mol Biosci. (2020) 7:585161. doi: 10.3389/fmolb.2020.585161
15. Diaconu, AD, Ostafie, I, Ceasovschih, A, Șorodoc, V, Lionte, C, and Ancuța, C. Role of vitamin D in systemic sclerosis: a systematic literature review. J Immunol Res. (2021) 2021:9782994. doi: 10.1155/2021/9782994
16. Umar, M, Sastry, KS, and Chouchane, AI. Role of vitamin D beyond the skeletal function: a review of the molecular and clinical studies. Int J Mol Sci. (2018) 19:1618. doi: 10.3390/ijms19061618
17. Wu-Wong, JR, Nakane, M, and Ma, J. Vitamin D analogs modulate the expression of plasminogen activator inhibitor-1, thrombospondin-1 and thrombomodulin in human aortic smooth muscle cells. J Vasc Res. (2007) 44:11–8. doi: 10.1159/000097812
18. Chen, S, Law, CS, Grigsby, CL, Olsen, K, Hong, TT, Zhang, Y, et al. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. (2011) 124:1838–47. doi: 10.1161/CIRCULATIONAHA.111.032680
19. Mazidi, M, Karimi, E, Rezaie, P, and Vatanparast, H. The impact of vitamin D supplement intake on vascular endothelial function; a systematic review and meta-analysis of randomized controlled trials. Food Nutr Res. (2017) 61:1273574. doi: 10.1080/16546628.2016.1273574
20. Park, EK, Park, JH, Kweon, SM, Kim, GT, and Lee, SG. Vitamin D deficiency is associated with digital ulcer but not with atherosclerosis or arterial stiffness in patients with systemic sclerosis: a pilot study. Clin Rheumatol. (2017) 36:1325–33. doi: 10.1007/s10067-017-3622-9
21. González-Martín, JJ, Novella-Navarro, M, Calvo-Aranda, E, Cabrera-Alarcón, JL, Carrión, O, Abdelkader, A, et al. Endothelial dysfunction and subclinical atheromatosis in patients with systemic sclerosis. Clin Exp Rheumatol. (2020) 38:48–52.
22. Gambardella, J, De Rosa, M, Sorriento, D, Prevete, N, Fiordelisi, A, Ciccarelli, M, et al. Parathyroid hormone causes endothelial dysfunction by inducing mitochondrial ROS and Specific oxidative signal transduction modifications. Oxid Med Cell Longev. (2018) 2018:9582319.
23. Tacey, A, Hayes, A, Zulli, A, and Levinger, I. Osteocalcin and vascular function: is there a cross-talk? Mol Metab. (2021) 49:101205. doi: 10.1016/j.molmet.2021.101205
24. Corretti, MC, Anderson, TJ, Benjamin, EJ, Celermajer, D, Charbonneau, F, Creager, MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the international brachial artery reactivity task force. J Am Coll Cardiol. (2002) 39:257–65. doi: 10.1016/s0735-1097(01)01746-6
25. Williams, B, Mancia, G, Spiering, W, Agabiti Rosei, E, Azizi, M, Burnier, M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. (2018) 39:3021–104. doi: 10.1093/eurheartj/ehy339
26. Maruhashi, T, Kajikawa, M, Kishimoto, S, Hashimoto, H, Takaeko, Y, Yamaji, T, et al. Diagnostic criteria of flow-mediated vasodilation for Normal endothelial function and nitroglycerin-induced vasodilation for Normal vascular smooth muscle function of the brachial artery. J Am Heart Assoc. (2020) 9:e013915. doi: 10.1161/JAHA.119.013915
27. Moens, AL, Goovaerts, I, Claeys, MJ, and Vrints, CJ. Flow-mediated vasodilation: a diagnostic instrument, or an experimental tool? Chest. (2005) 127:2254–63. doi: 10.1378/chest.127.6.2254
28. Cossarizza, A, Chang, HD, Radbruch, A, Acs, A, Adam, D, Adam-Klages, S, et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur J Immunol. (2019) 49:1457–973. doi: 10.1002/eji.201970107
29. Deng, F, Whang, S, and Zang, L. Endothelial microparticles act as novel diagnostic and therapeutic biomarkers of circulatory hypoxia-related diseases: a literature review. J Cell Mol Med. (2017) 21:1698–710. doi: 10.1111/jcmm.13125
30. Sebastiani, M, Manfredi, A, Colaci, M, D'amico, R, Malagoli, V, Giuggioli, D, et al. Capillaroscopic skin ulcer risk index: a new prognostic tool for digital skin ulcer development in systemic sclerosis patients. Arthritis Rheum. (2009) 61:688–94. doi: 10.1002/art.24394
31. Sulli, A, Secchi, ME, Pizzorni, C, and Cutolo, M. Scoring the nailfold microvascular changes during capillaroscopic analysis in systemic sclerosis patients. Ann Rheum Dis. (2008) 67:885–7. doi: 10.1136/ard.2007.079756
32. Coghlan, JG, Denton, CP, Grünig, E, Bonderman, D, Distler, O, Khanna, D, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis. (2014) 73:1340–9. doi: 10.1136/annrheumdis-2013-203301
33. Khanna, D, Nagaraja, V, Gladue, H, Bonderman, D, Distler, O, Khanna, D, et al. Measuring response in the gastrointestinal tract in systemic sclerosis. Curr Opin ‑Rheumatol. (2013) 25:700–6. doi: 10.1097/01.bor.0000434668.32150.e5
34. Valentini, G, Iudici, M, Walker, UA, Jaeger, VK, Baron, M, Carreira, P, et al. The European scleroderma trials and research group (EUSTAR) task force for the development of revised activity criteria for systemic sclerosis: derivation and validation of a preliminarily revised EUSTAR activity index. Ann Rheum Dis. (2017) 76:270–6. doi: 10.1136/annrheumdis-2016-209768
35. Mühlemann, HR, and Son, S. Gingival sulcus bleeding--a leading symptom in initial gingivitis. Helv Odontol Acta. (1971) 15:107–13.
36. O'Leary, TJ, Drake, RB, and Naylor, JE. The plaque control record. J Periodontol. (1972) 43:38. doi: 10.1902/jop.1972.43.1.38
37. Sampaio-Barros, MM, Takayama, L, Sampaio-Barros, PD, Bonfá, E, and Pereira, RM. Low vitamin D serum levels in diffuse systemic sclerosis: a correlation with worst quality of life and severe capillaroscopic findings. Rev Bras Reumatol Engl Ed. (2016) 56:337–4. doi: 10.1016/j.rbre.2016.05.006
38. Atteritano, M, Sorbara, S, Bagnato, G, Miceli, G, Sangari, D, Morgante, S, et al. Bone mineral density, bone turnover markers and fractures in patients with systemic sclerosis: a case control study. PLoS One. (2013) 8:e66991. doi: 10.1371/journal.pone.0066991
39. Trombetta, AC, Smith, V, Gotelli, E, Ghio, M, Paolino, S, Pizzorni, C, et al. Vitamin D deficiency and clinical correlations in systemic sclerosis patients: a retrospective analysis for possible future developments. PLoS One. (2017) 12:e0179062. doi: 10.1371/journal.pone.0179062
40. Kisla Ekinci, RM, Taskin Karacay, IE, and Celik, U. Serum vitamin B12 and D levels in children with primary Raynaud phenomenon: a retrospective cohort study. Eur J Clin Nutr. (2022) 76:1615–7. doi: 10.1038/s41430-022-01151-0
41. Jennings, A, MacGregor, A, Welch, A, Chowienczyk, P, Spector, T, and Cassidy, A. Amino acid intakes are inversely associated with arterial stiffness and central blood pressure in women. J Nutr. (2015) 145:2130–8. doi: 10.3945/jn.115.214700
42. Menni, C, Mangino, M, Cecelja, M, Psatha, M, Brosnan, MJ, Trimmer, J, et al. Metabolomic study of carotid-femoral pulse-wave velocity in women. J Hypertens. (2015) 33:791–6. doi: 10.1097/HJH.0000000000000467
43. de Meirelles, LR, Mendes-Ribeiro, AC, Santoro, MM, Mendes, MA, da Silva, MN, Mann, GE, et al. Inhibitory effects of endogenous L-arginine analogues on nitric oxide synthesis in platelets: role in platelet hyperaggregability in hypertension. Clin Exp Pharmacol Physiol. (2007) 34:1267–71. doi: 10.1111/j.1440-1681.2007.04712.x
44. Kuo, MT, Chen, HHW, Feun, LG, and Savaraj, N. Targeting the proline-glutamine-asparagine-arginine metabolic axis in amino acid starvation cancer therapy. Pharmaceuticals (Basel). (2021) 14:72. doi: 10.3390/ph14010072
45. Somani, RR, Rai, PR, and Kandpile, PS. Ornithine decarboxylase inhibition: a strategy to combat various diseases. Mini Rev Med Chem. (2018) 18:1008–21. doi: 10.2174/1389557517666170927130526
46. Tomonaga, S, Kawase, T, Tsukahara, T, Ohta, Y, and Shiraishi, JI. Metabolism of imidazole dipeptides, taurine, branched-chain amino acids, and polyamines of the breast muscle are affected by post-hatch development in chickens. Meta. (2022) 12:86. doi: 10.3390/metabo12010086
47. Alesutan, I, Feger, M, Tuffaha, R, Castor, T, Musculus, K, Buehling, SS, et al. Augmentation of phosphate-induced osteo−/chondrogenic transformation of vascular smooth muscle cells by homoarginine. Cardiovasc Res. (2016) 110:408–18. doi: 10.1093/cvr/cvw062
48. Cruzat, V, Macedo Rogero, M, Noel Keane, K, Curi, R, and Newsholme, P. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. (2018) 10:1564. doi: 10.3390/nu10111564
49. Gao, N, Ding, L, Pang, J, Zheng, Y, Cao, Y, Zhan, H, et al. Metabonomic-Transcriptome integration analysis on osteoarthritis and rheumatoid arthritis. Int J Genomics. (2020) 2020:5925126. doi: 10.1155/2020/5925126
50. Navik, U, Sheth, VG, Khurana, A, Jawalekar, SS, Allawadhi, P, Gaddam, RR, et al. Methionine as a double-edged sword in health and disease: current perspective and future challenges. Ageing Res Rev. (2021) 72:101500. doi: 10.1016/j.arr.2021.101500
51. Iwata, Y, Nakade, Y, Kitajima, S, Yoneda-Nakagawa, S, Oshima, M, Sakai, N, et al. Protective effect of d-alanine against acute kidney injury. Am J Physiol Renal Physiol. (2022) 322:F667–79. doi: 10.1152/ajprenal.00198.2021
52. Lee, H, Jang, HB, Yoo, MG, Park, SI, and Lee, HJ. Amino acid metabolites associated with chronic kidney disease: an eight-year follow-up Korean epidemiology study. Biomedicine. (2020) 8:222. doi: 10.3390/biomedicines8070222
53. Kopple, JD. Phenylalanine and tyrosine metabolism in chronic kidney failure. J Nutr. (2007) 137:1586S–90S. doi: 10.1093/jn/137.6.1586S
54. Tan, R, Li, J, Liu, F, Liao, P, Ruiz, M, Dupuis, J, et al. Phenylalanine induces pulmonary hypertension through calcium-sensing receptor activation. Am J Physiol Lung Cell Mol Physiol. (2020) 319:L1010–20. doi: 10.1152/ajplung.00215.2020
55. Berry, SD, Ramachandran, VS, Cawthon, PM, Gona, P, McLean, RR, Cupples, LA, et al. Procollagen type III N-terminal peptide (P3NP) and lean mass: a cross-sectional study. J Frailty Aging. (2013) 2:129–34.
Keywords: systemic sclerosis, endothelial dysfunction, amino acids, vitamin D, bone metabolism
Citation: Jud P, Meinitzer A, Strohmaier H, Arefnia B, Wimmer G, Obermayer-Pietsch B, Foris V, Kovacs G, Odler B, Moazedi-Fürst F, Brodmann M and Hafner F (2023) Association of amino acids and parameters of bone metabolism with endothelial dysfunction and vasculopathic changes in limited systemic sclerosis. Front. Med. 10:1193121. doi: 10.3389/fmed.2023.1193121
Edited by:
Iain Brownlee, Northumbria University, United KingdomReviewed by:
Antonietta Gigante, Sapienza University of Rome, ItalyBo Shi, Northwestern University, United States
Copyright © 2023 Jud, Meinitzer, Strohmaier, Arefnia, Wimmer, Obermayer-Pietsch, Foris, Kovacs, Odler, Moazedi-Fürst, Brodmann and Hafner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philipp Jud, cGhpbGlwcC5qdWRAbWVkdW5pZ3Jhei5hdA==
 Philipp Jud
Philipp Jud Andreas Meinitzer2
Andreas Meinitzer2 Behrouz Arefnia
Behrouz Arefnia Gernot Wimmer
Gernot Wimmer Barbara Obermayer-Pietsch
Barbara Obermayer-Pietsch Vasile Foris
Vasile Foris Balazs Odler
Balazs Odler Marianne Brodmann
Marianne Brodmann