- 1Department of Orthopedics, The First Affiliated Hospital of Chongqing Medical University, Orthopedic Laboratory of Chongqing Medical University, Chongqing, China
- 2Department of Neurology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
Background: Tranexamic acid (TXA) has previously been shown to be effective in reducing intraoperative blood loss (IBL) and transfusion requirements in spine surgery. A conventional TXA regimen is a simple preoperative or intraoperative administration. However, the hyperfibrinolysis caused by surgical trauma lasts at least 24 h, and a single dose of TXA cannot cover the whole process of hyperfibrinolysis. Moreover, its ability to control postoperative blood loss (PBL) may be insufficient. Therefore, this study aimed to explore the effects and safety of sequential perioperative intravenous TXA for reducing bleeding after posterior lumbar interbody fusion (PLIF).
Methods: Patients requiring PLIF were randomly divided into two groups. All patients were intravenously injected with 1 g of TXA 15 min before skin resection. Every day after the surgery, 200 ml saline was intravenously injected for 1–3 days in Group A, while Group B received 1 g of TXA instead of saline. The total blood loss (TBL), IBL, PBL, HCT, Hb, blood transfusion volume, inflammation-related indicators, and complications were recorded.
Results: TBL, PBL, and hidden blood loss (HBL) in Group B were significantly lower than those in Group A (P < 0.05). The maximum decreases in HCT and Hb in Group B were also significantly lower than those in Group A (P < 0.05), and the drainage removal time (DRT) was sooner in Group B than in Group A (P = 0.003). On the 3rd and 5th days after surgery, the level of CRP in Group B was significantly lower than that in Group A (P < 0.05). Similarly, IL-6 levels were significantly lower in Group B for the first 5 days postoperatively (P < 0.001). Sex, operation time, level of decompression, length of incision, and change in HCT were significant predictors of both TBL and HBL. TBL was also significantly associated with BMI and preoperative fibrinogen, while postoperative TXA was a significant predictor of HBL only.
Conclusion: Intravenous injection of 1 g of TXA 15 min before skin resection combined with continuous intravenous injection of 1 g of TXA 1 to 3 days after PLIF can reduce postoperative bleeding and shorten the time to drainage tube removal. In addition, it can also inhibit the postoperative inflammatory response.
Clinical trial registration: ChiCTR2200056210.
Introduction
Hemorrhage is one of the greatest challenges in posterior lumbar interbody fusion (PLIF) surgery. Total blood loss (TBL) includes both intraoperative blood loss (IBL) and postoperative blood loss (PBL). Hidden blood loss (HBL) is mainly PBL. HBL accounts for 45 to 47% of TBL (1, 2) in spinal surgery. Massive HBL may increase the blood transfusion rate, infection rate, risk of complications, and adverse symptoms such as anemia, which seriously affect the speed of recovery after surgery, extend the length of hospital stay, increase medical costs, and even threaten patients' lives (3, 4). Therefore, how to reduce HBL effectively has become an urgent problem to be solved in PLIF surgery.
As an antifibrinolytic drug, TXA has been widely used in surgical hemostasis. TXA can strongly absorb the lysine-binding site of the fibrinogen (FIB) affinity site on plasminogen and fibrinolytic enzymes in a reversible competitive manner, which hinders the binding between fibrinolytic enzymes and fibrin, inhibits fibrin degradation caused by fibrinolytic enzymes (5), and reduces the formation of D-dimer, thereby achieving hemostasis. At the same time, TXA can modulate the immune response and reduce the postsurgical inflammatory response (6–8). Compared with topical administration after activation of the fibrinolytic system, preoperative intravenous administration of TXA can improve its speed and effectiveness by inhibiting thrombus decomposition (9). Concurrently, intravenous TXA has been shown to be effective in reducing IBL and transfusion requirements (5, 10, 11).
Although the optimal dose of TXA has not yet been determined, most previous studies have used one dose of 1 g of TXA before or during surgery to control wellbeing (9, 11–14), with no additional benefits associated with higher doses (12, 15). The half-life of TXA is 2–11 h, with most of the drugs expelled from the kidney within 24 h (16). However, Xie et al. (17) and Blani et al. (18) reported that fibrinolysis peaks at 6 h postoperatively and persisted for at least 24 h. Therefore, to inhibit fibrinolysis completely, a single dose of TXA is not sufficient. Since increasing the dose increases the risk of epilepsy (12, 19), another strategy that has been investigated and shown to effectively and safely reduce HBL in total knee and total hip arthroplasty is the administration of multiple doses (20, 21). There are only limited studies on multiple TXA use in PLIF. Therefore, this study aimed to investigate whether the strategy of multiple intravenous sequential use of TXA can better inhibit postoperative bleeding after PLIF compared with a single dose. After consideration of the half-life and the large number of studies reporting minimum Hb and HCT on the 3rd postoperative day (14, 22, 23), the effect of three additional doses of TXA administered at 24-h intervals was assessed.
Materials and methods
Sample size
The sample size was calculated using PASS 15 (NCSS, LLC, Kaysville, USA) statistical software. According to the result of our preliminary study, the HBL of Group A was approximately 552 ml and that of Group B was approximately 457 ml. In order to show an HBL difference with a power of 0.80 at a 95% significance level, we needed 57 patients for each treatment group. To compensate for the expected 5% dropout rate, we decided to include at least 60 patients in each group (24).
Study design and patient population
This was a prospective, single-center, double-blind, randomized controlled clinical study. From February 2022 to June 2022, patients who underwent PLIF surgery were randomly divided into Group A and Group B. This study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University Review Board (2021-321) and registered on the Chinese Clinical Trials Registry (ChiCTR2200056210).
Inclusion criteria
The inclusion criteria were as follows: (1) age between 18 and 80 years; (2) patients who underwent complete laminectomy PLIF treatment; and (3) written informed consent.
Exclusion criteria
The exclusion criteria were as follows: (1) Hb: female < 110 g/L, male < 120 g/L; (2) patients with abnormal coagulation function, preoperative platelet count (PLT) < 100 × 109/L, international normalized ratio (INR) > 1.4, prolonged activated partial thromboplastin time (APTT) > 1.4-fold normal; (3) patients who had taken antiplatelet aggregates, such as aspirin or anticoagulants, in the last month; (4) patients with a history of thromboembolisms; (5) presence of thrombosis on lower extremity deep venous ultrasonography; (6) allergic to TXA; (7) patients who are not recommended for surgery under general anesthesia after consultation with relevant experts due to serious heart or respiratory diseases and renal or liver dysfunction; (8) American Society of Anesthesiologists (ASA) physical status >III; (9) history of any psychiatric illness (e.g., depression, mania, and affective disorders.); (10) previous lumbar surgery; and (11) lumbar vertebral tumors, tuberculosis, infection, or lumbar vertebral fracture caused by trauma.
Study design
All patients were randomly assigned into two groups by a sealed envelope method. The study had a double-blind design with products masked and coded so that the participants and treating clinicians were unaware of which product each patient was receiving. Patients in Group A were intravenously injected with 1 g of TXA (200 ml TXA sodium chloride injection) 15 min before skin resection, and 200 ml of normal saline was intravenously injected every day after surgery for 1–3 days (at 24-h intervals from the first infusion). Patients in Group B were intravenously injected with 1 g of TXA 15 min before skin resection and intravenously injected with 1 g of TXA every day after surgery for 1–3 days.
Surgical technique
All operations were performed by a single surgeon with over 10 years of experience in spinal surgery. The patient was in a prone position under general anesthesia. After routine disinfection, the back fascia consistent with the midline skin incision was longitudinally divided, and the paravertebral muscles were dissected from the spinous process and lamina to expose the facet joints. The pedicle screws were implanted successively. After complete laminectomy and discectomy, autologous bone particles and intervertebral fusion cages were tilted into the intervertebral space. Then, the incision was rinsed, hemostasis was accomplished, two drainage tubes were placed, and layer-by-layer suturing was carried out to close the wound. Postoperative drainage of <50 mL in 24 h was considered standard for the removal of drainage tubes (25), and the removal time was recorded. The patient was discharged when the incision was no longer swelling or producing exudate after the drainage tube was removed.
Outcome evaluation
The primary observational indicators of this study were HBL and PBL. The secondary observations include TBL, drainage removal time (DRT), length of hospitalization (LOS), interleukin-6 (IL-6) (the determination method was flow cytometry), C-reactive protein (CRP), a decline of hematocrit and hemoglobin level, TXA complications, liver and kidney function indicators, and coagulation function indicators.
TBL was estimated according to the formula of Nadler and Gross as follows: (1) preoperative blood volume (PBV) was calculated as follows: k1 × height (m)3 + k2 × weight (kg) + k3, where k1 = 0.3669, and k2 = 0.03219, k3 = 0.6041 for men and k1 = 0.3561, k2 = 0.03308, and k3 = 0.1833 for women (26). (2) TBL was calculated as follows: PBV × (HCTpre – HCTpost)/HCTave, where HCTpre is the preoperative hematocrit and HCTpost is the lowest postoperative HCT. By this time, the patient's fluid shifts would have been largely completed and hemodynamically stable. HCTave is the mean of HCTpre and HCTpost (27). ΔHCT was calculated as follows: HCTpre – HCTpost.
Hidden blood loss = TBL – IBL – PBL, of which IBL was estimated by weighing the surgical sponges, measuring blood collected by the suction canisters, and subtracting all irrigation fluids added to the surgical field (1 g of blood = 1 ml of blood). Note: The wound exudates after the operation were ignored because of their small size and difficulty of measurement. If the drainage volume was <50 mL/24 h, extubation was performed. PBL = (WDPOD1 × HctPOD1 + WDPOD2 × HctPOD2)/HCTave. WDPOD1 = the volume of wound drainage on POD1; HctPOD1 = the Hct of wound drainage on POD1; and POD = postoperative days.
Hb, prothrombin time (PT), INR, APTT, and D-dimer (D-D) were measured before the operation and on the first, third, and 5th days after the operation. Venous Doppler ultrasonography examination was performed before the operation and 1 week and 1 month after surgery. If the patient had symptoms such as lower limb pain, swelling, and other symptoms, venous Doppler was performed. The incidence of thromboembolic events was tracked for 1 month after the operation. The maximum Hb drop was defined as the difference between the preoperative Hb and the minimal Hb level obtained postoperatively during hospitalization and before any blood transfusion.
An allogenic transfusion was given if the Hb level was <70 g/L or between 70 and 100 g/L with sustained blood loss or anemic symptoms (such as dizziness, palpitation, and paleness). The standard of transfusing fresh frozen plasma (FFP, 200 ml/time) was INR > 1.5 or APTT > 1.5-fold normal baseline, and the input standard of transfusing platelets (1 U/time) was PLT < 100 × 109/L. If the patient received a blood transfusion, the post-transfusion Hb values were not included in the statistical analysis.
In addition, the general information collected for each patient included sex, age, body mass index (BMI), duration of operation, and hospitalization expenses.
Statistical analysis
The Kolmogorov–Smirnov test was used to test whether the quantitative data obeyed the normal distribution in this study. The quantitative values in this study are expressed as the mean ± standard deviation or median (quartile), and the frequency and percentages of categorical data were calculated. The Mann–Whitney U-test and t-test were used for comparisons of the variables between the two groups. A multiple regression analysis was used to examine the variables that affected TBL and HBL. In cases with a p-value of < 0.05, the results were considered statistically significant. The statistical analysis was performed using SPSS 25.0 software (SPSS Inc, Chicago, Illinois).
Results
Patient information
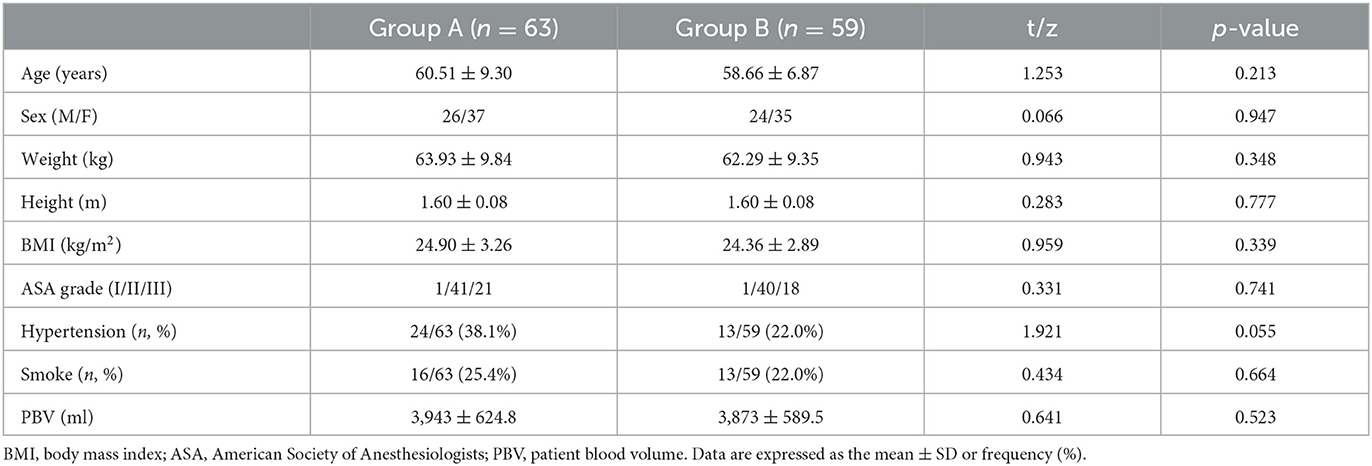
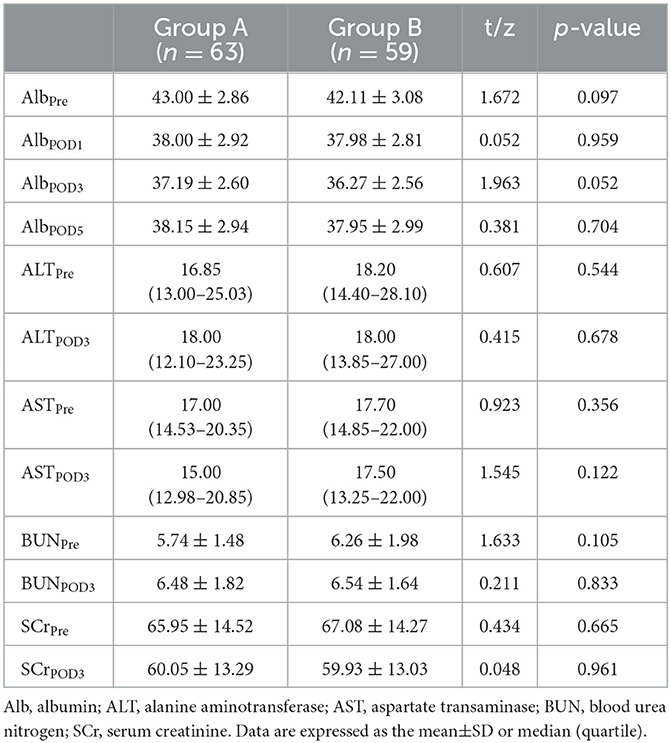
Of 130 eligible consecutive patients, two patients refused to participate, and five patients did not meet the inclusion criteria. Then, 123 patients were enrolled and divided into Group A (63 cases) and Group B (60 cases). During the trial, one patient in Group B developed unbearable vomiting and palpitations after intravenous TXA and was discontinued. Finally, 122 patients were included in the final analysis (Figure 1). All patients underwent surgery successfully. There was no significant difference between the groups in demographic characteristics as shown in Table 1. There was also no significant difference in operation time, level of decompression, or length of incision (Table 2).
Blood loss and blood transfusion-related parameters
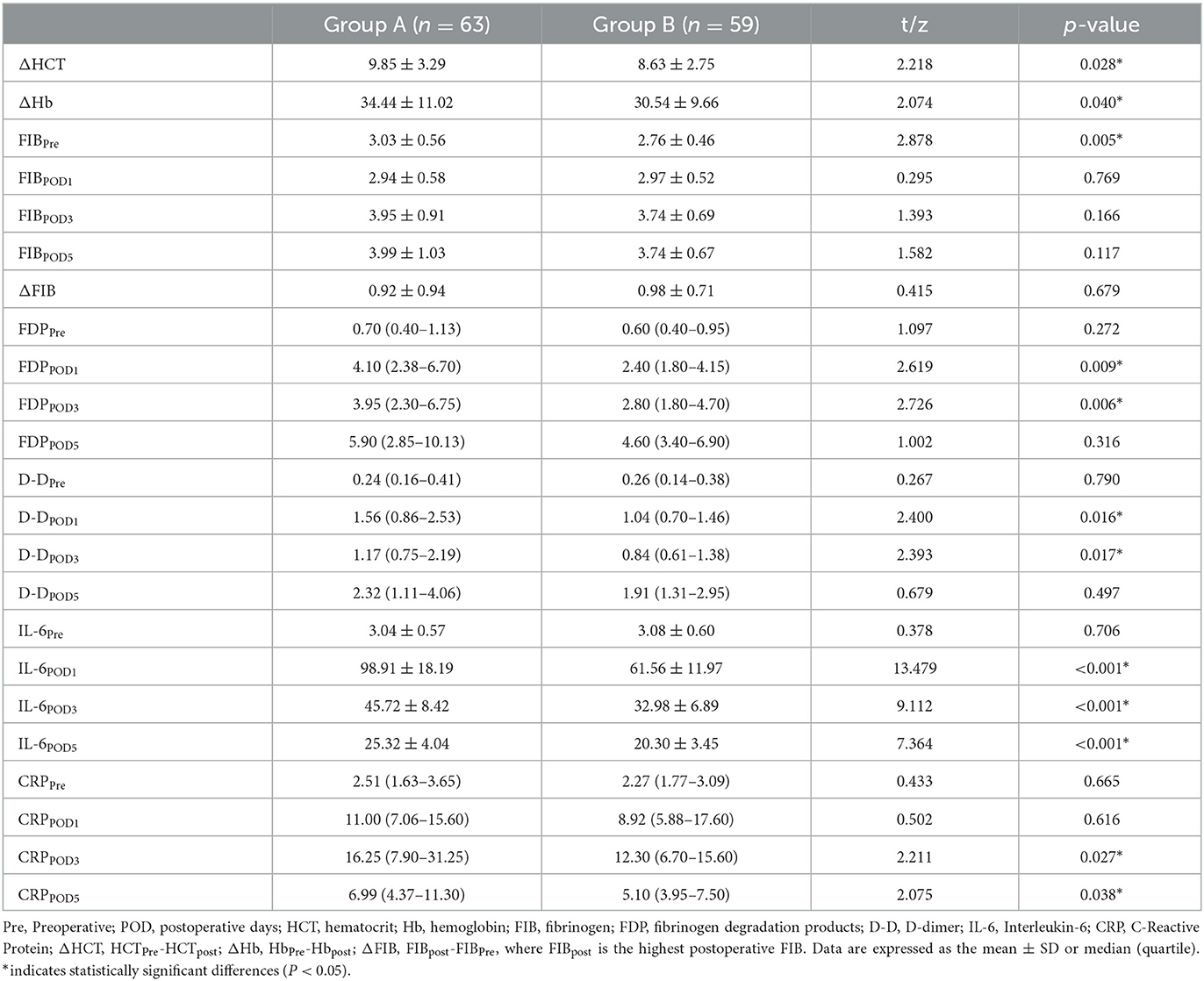
There was no significant difference in IBL between the two groups (304.00 (227.25–360.25) ml and 287.00 (231.50–366.50) ml, P = 0.924). The mean TBL, PBL, and HBL in Group B (933.08 ± 335.80 ml, 178.56 ± 83.34 ml, 434.60 ± 155.87 ml) were significantly lower than those in Group A (1,076.49 ± 410.97 ml, mean difference = 143.41 ml, 95% CI, 8.36–278.47 ml, P = 0.038); (220.71 ± 104.83 ml, mean difference = 42.15 ml, 95% CI, 8.05–76.25 ml, P = 0.016); and (533.73 ± 205.44 mL, mean difference = 99.13 mL, 95% CI, 33.43–164.83 mL, P = 0.003) (Figure 2). There was no significant difference in HCT or Hb between the two groups preoperatively and postoperatively (Figure 3). It is interesting that the mean ΔHCT and ΔHb in Group B (8.63 ± 2.75%, 30.54 ± 9.66 g/L) were significantly lower than those in Group A (9.85 ± 3.29%, mean difference = 1.22%, 95% CI, 0.13–2.31%, P = 0.028; 34.44 ± 11.02 g/L, mean difference = 3.90 g/L, 95% CI, 0.18–7.63 g/L, P = 0.040) (Table 3). There was no significant difference in the blood transfusion rate between the two groups. There were five cases of intraoperative blood transfusion, including three cases in Group B and two cases in Group A (Table 2).
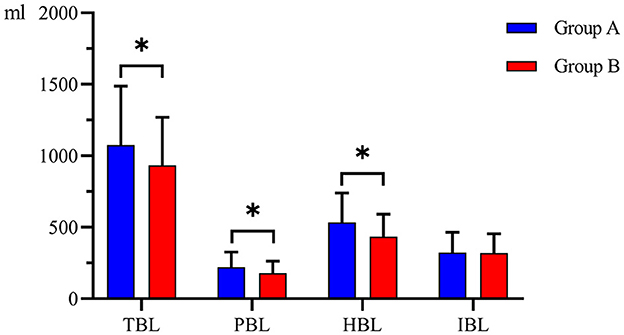
Figure 2. Blood loss between the two groups. Group A was preoperative single-dose intravenous TXA. Group B was sequential multi-dose intravenous TXA. TBL indicates total blood loss. IBL indicates intraoperative blood loss. PBL indicates postoperative blood loss. HBL indicates hidden blood loss. *indicates statistically significant differences (P < 0.05).
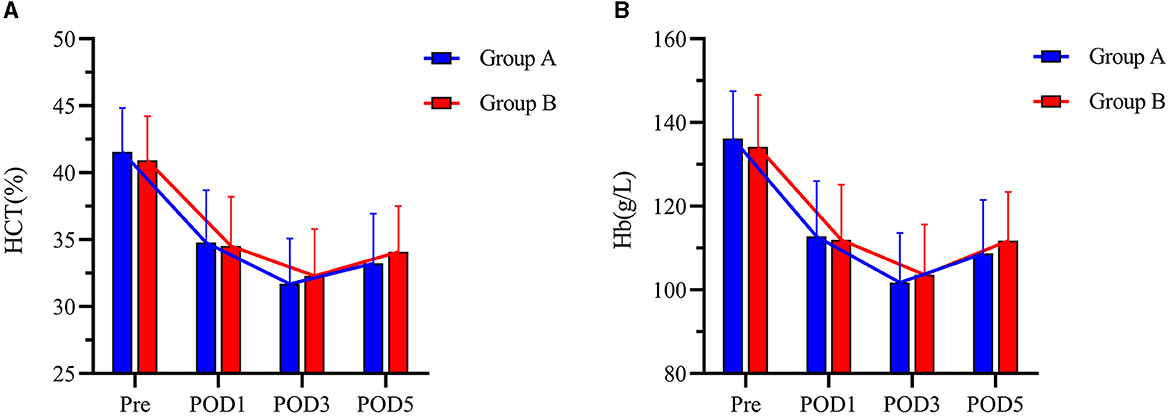
Figure 3. (A, B) HCT and Hb levels at Pre, POD1, POD3, and POD5 between the two groups. Pre represents preoperative. POD1 represents postoperative day 1. POD3 represents postoperative day 3. POD5 represents postoperative day 5. Group A was preoperative single-dose intravenous TXA. Group B was sequential multi-dose intravenous TXA.
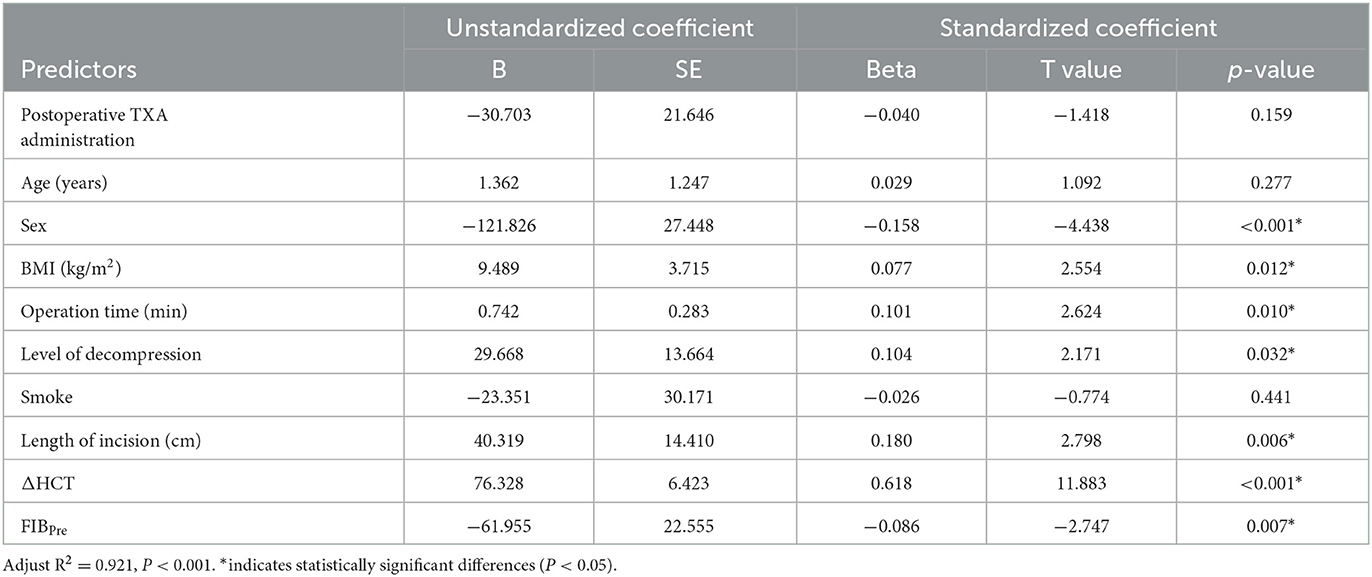
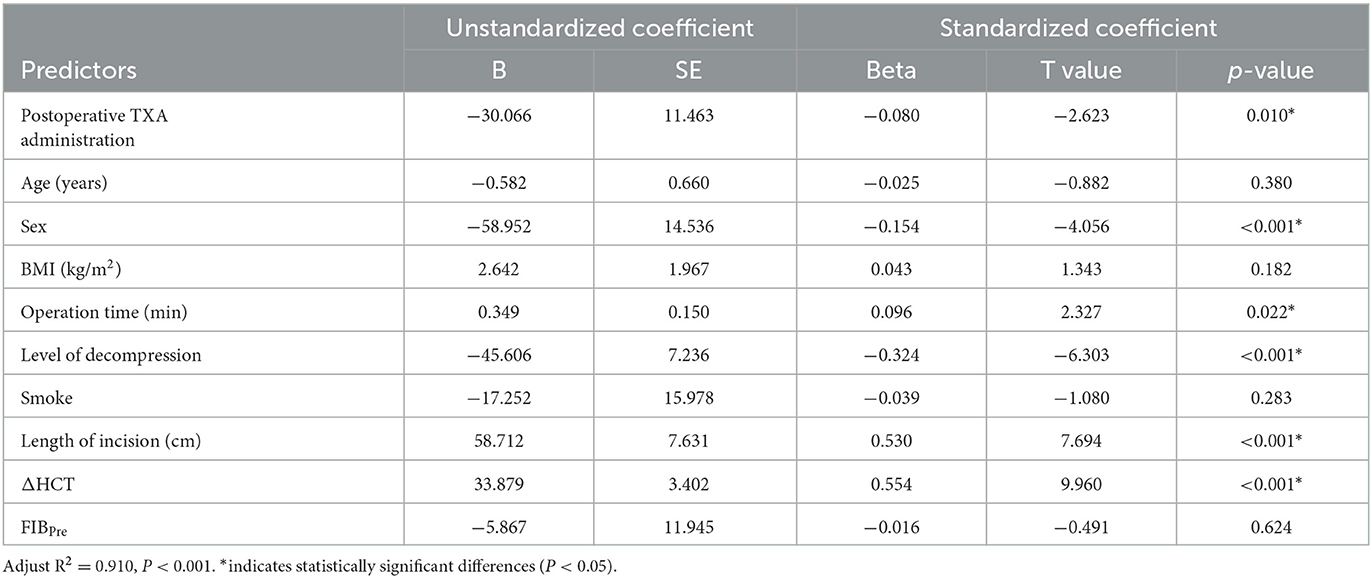
A multiple regression analysis showed that TBL was positively correlated with BMI, operation time, level of decompression, length of incision, and ΔHCT. It was negatively correlated with preoperative levels of FIB (Table 4). HBL was positively correlated with operation time, length of incision, and ΔHCT. It was negatively correlated with the level of decompression and postoperative TXA (Table 5). As compared to male patients, female patients had a reduced risk of both TBL and HBL (Tables 4, 5).
The preoperative FIB and TBL were analyzed by a scatter plot, and an equation was fitted. Figure 4 shows the scatter plot of the preoperative fibrinogen level and total bleeding volume. There was a negative correlation between these two variables. The fitted equation of Group A was Y = −282.3 × X + 1,931 and that of Group B was Y = −85.03 × X + 1,168. This result indicated that postoperative intravenous sequential TXA injection can attenuate the increase in TBL caused by low preoperative FIB.
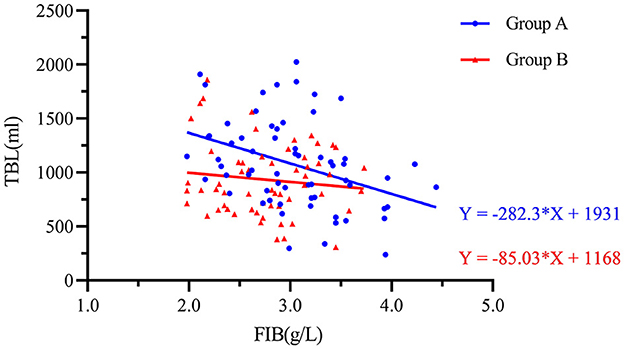
Figure 4. Scatter plot showing the negative logarithmic correlation between the preoperative fibrinogen concentration (g/L) and TBL (mL). Group A was preoperative single-dose intravenous TXA. Group B was sequential multi-dose intravenous TXA.
Inflammation markers
There was no significant difference in the IL-6 levels between the two groups preoperatively; however, IL-6 was significantly reduced in Group B compared to Group A 1–5 days postoperatively (P < 0.001). The CRP levels did not differ between the two groups at Pre and POD1. However, at 3 and 5 days after the operation, the CRP levels in Group B [12.30 (6.70–15.60) mg/L, 5.10 (3.95–7.50) mg/L] were lower than those in Group A [16.25 (7.90–31.25) mg/L, P = 0.027; 6.99 (4.37–11.30) mg/L, P = 0.038] (Table 3). This indicated that postoperative intravenous sequential application of TXA could reduce the inflammatory response.
Coagulation indicators
Coagulation parameters including PLT, PT, INR, APTT, and TT of all patients were changed after surgery but did not differ between the two treatment groups at any timepoint (Figure 5).
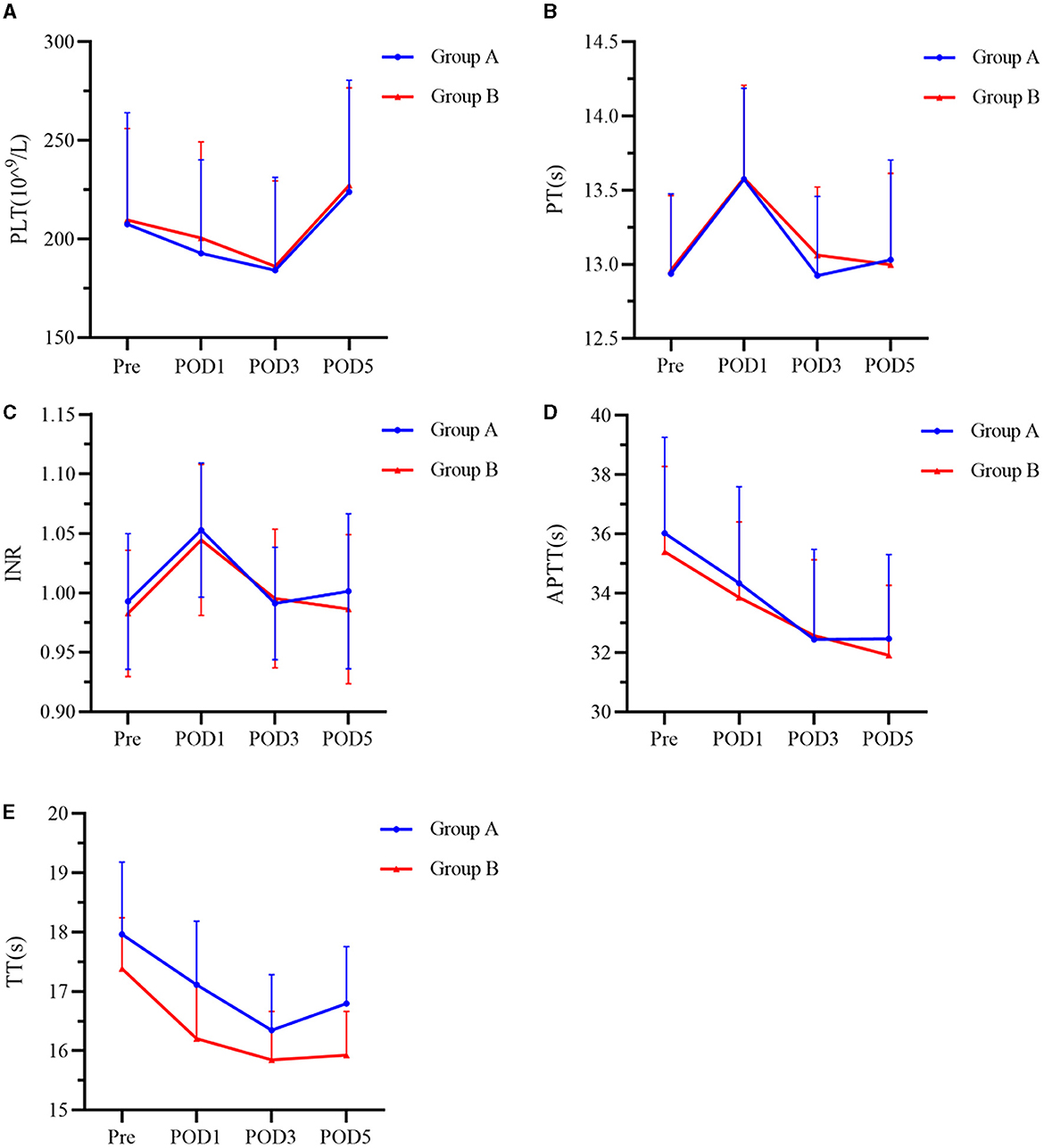
Figure 5. (A–E) Index of coagulation function at Pre, POD1, POD3, and POD5 between the two groups. Group A was preoperative single-dose intravenous TXA. Group B was sequential multi-dose intravenous TXA. PLT represents platelets, PT represents prothrombin time, INR represents international normalized ratio, APTT represents activated partial thromboplastin time, and TT represents thrombin time. Pre represents preoperative. POD1 represents postoperative day 1. POD3 represents postoperative day 3. POD5 represents postoperative day 5.
Surgical outcomes and complications
In Group B, the DRT was earlier (2.92 ± 0.34 days vs. 3.13 ± 0.42 days, P = 0.003), and the LOS was shorter (6.07 ± 0.52 days vs. 6.27 ± 0.52 days, P = 0.033). One case of muscular calf vein thrombosis occurred in both groups (Table 2). No adverse reactions associated with blood transfusion and no drug-related complications, such as DVT, PEs, cardiac infarction, impaired hepatic and renal function, or epilepsy, were found in any patient (Table 6).
Discussion
Although blood conservation strategies, such as hypotensive anesthesia and avoiding abdominal compression, are employed, a spinal fusion operation may still lead to considerable blood loss. Therefore, safe and effective intervention strategies are still needed. In reviewing the scientific database of available literature on this topic, we found that TXA has been widely used in orthopedic surgery and has a positive hemostatic effect (10, 11, 28). At present, the optimal dose of TXA is not determined, however, a large number of studies use a TXA dose of 1 g (12, 15, 29, 30). The specification of TXA sodium chloride injection used in our study was 0.5 g/bottle. If considering weight adaption, it is difficult to achieve accurate dosage in clinical practice. Therefore, 1 g of TXA was selected, regardless of body weight.
The application of TXA in previous research mainly focused on preoperative or intraoperative assessments, but a few studies have focused on the role of HBL in PLIF and its regulatory factors (9, 31). Since Sehat et al. reported that HBL accounted for 49% of the total blood loss after total hip replacement, surgeons have gradually become aware that HBL plays an important role in perioperative TBL (32). This reveals why even if the patient's IBL or PBL seems acceptable, the patient may develop anemia after surgery, resulting in hypoperfusion coagulation dysfunction (33).
In our study, HBL accounted for 46.58% of TBL in Group B and 49.58% of TBL in Group A (Table 2). This result is slightly higher than that of Smorgick et al. and Xu et al. (1, 2). The multiple regression analysis showed that compared with women, male patients had a higher risk of increased TBL and HBL, which was consistent with the results of Cushner et al. (34). We speculate that this may be due to men having more muscle and less fat in their lower back than women (35) or because men have larger lumbar lamina (36), leading to more intraoperative and postoperative bleeding. The proportion of men in our study was 40.98% higher than that in Smorgick et al. (38.6%) and Xu et al. (30.0%) (1, 2). This may partially explain the slightly higher proportion of HBL in our study. At the same time, there were statistical differences between DRT and LOS in our study, but the mean difference between the two groups was not obvious and unlikely to be clinically significant. However, we further analyzed the data based on the level of decompression and found that there were no statistical differences in the number of DRT in Group A (2.96 ± 0.34) and Group B (2.93 ± 0.73, P = 0.822) on the level 1. On level 2, there were significant differences between Group A (3.13 ± 0.35) and Group B (2.96 ± 0.20, P = 0.025). On level 3, the number of DRT in Group A (3.71 ± 0.49) was significantly more than that in Group B (3.14 ± 0.38, P = 0.031). No difference in LOS between the two groups may be due to the small sample size and the single-center study design. Additional multi-center and larger samples are needed for evaluating whether multi-dose TXA can effectively shorten LOS. In this study, sequential perioperative intravenous TXA also did not reduce the rate of blood transfusion. This too may reflect the small number of patients in the study requiring blood transfusion (4.1%).
Many studies have confirmed the safety of using TXA with routine methods (37); however, the safety of postoperative intravenous sequential application of TXA still needs further investigation in consideration of an increased risk of DVT for higher doses of TXA or prolonged use (38–40). In our study, one patient in each group developed intermuscular venous thrombosis, but no other serious thromboembolism events occurred. There were no complications of DVT, PE, impaired hepatic or renal function, or myocardial infarction during the one-month follow-up. This may be because TXA does not increase the risk of thromboembolism (41) but is also likely due to the small sample size of the study, which is insufficient to find all adverse reactions.
The prethrombotic state, also known as the hypercoagulable state, refers to a pathological state characterized by an imbalance in coagulation and anticoagulant factors, which makes patients prone to thrombosis. Molecular marker levels (such as FIB, FDP, and D-D) are reliable indicators of the prethrombotic state (42). D-D is used as a marker for thrombosis and secondary fibrinolysis. Interestingly, the average value of D-D at POD3 in Group B [0.84 (0.61–1.38) mg/L] was significantly lower than that in Group A [1.17(0.75–2.19) mg/L, P = 0.017] (Table 3). This shows that the sequential application of TXA after the operation can effectively reduce the formation of D-D.
Fibrinolytic enzymes can induce monocytes to release proinflammatory cytokines and tissue factor expression, which can be inhibited by TXA in some instances (43). At the same time, TXA can also play an anti-inflammatory role by regulating a complement system (44). Relevant to this study, Wang et al. found that additional doses of TXA could further inhibit the postoperative inflammatory response (45). In the present study, we observed reduced proinflammatory IL-6 for 1–5 days post-operatively in patients treated with sequential TXA. Similarly, CRP levels were lower in this group on postoperative days 3 and 5, suggesting an anti-inflammatory effect associated with sequential multi-dose intravenous TXA, similar to the findings reported in previous studies (8, 45).
Limitations
Our research has several limitations, which deserve further discussion. This was a single-center study, and the sample size was relatively small. Only the effect of 1 g of TXA was discussed in this study. Multi-center studies with larger numbers of patients and different doses are needed to confirm the beneficial effect of TXA to reduce postoperative and hidden blood loss after PILF.
Conclusion
This study showed for the first time that sequential intravenous application of 1 g of TXA at 24-h intervals 1–3 days after the operation could effectively reduce TBL, PBL, and HBL without increasing complications. In addition, this treatment regime could also inhibit the postoperative inflammatory response and shorten the time to drainage tube removal. This study provides new ideas for bleeding management after spinal fusion.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of the First Affiliated Hospital of Chongqing Medical University Review Board (2021-321) and registered on Chinese Clinical Trials Registry (ChiCTR2200056210). The patients/participants provided their written informed consent to participate in this study.
Author contributions
WJ and ZH worked on the design and setting up of the study. WD and YL performed the data analysis and drafted the manuscript. DL, ZM, and MC were responsible for collecting the data. XZ, JS, NZ, and JH performed the PLIF surgery. All authors have critically reviewed and approved the final manuscript.
Funding
This study was supported by the Science Health Joint Medical Scientific Research Project of Chongqing (2021MSXM027).
Acknowledgments
The authors would like to thank all the patients, clinicians, nurses, and technicians who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Smorgick Y, Baker KC, Bachison CC, Herkowitz HN, Montgomery DM, Fischgrund JS. Hidden blood loss during posterior spine fusion surgery. Spine J. (2013) 13:877–81. doi: 10.1016/j.spinee.2013.02.008
2. Xu D, Ren Z, Chen X, Zhuang Q, Hui S, Sheng L, et al. The further exploration of hidden blood loss in posterior lumbar fusion surgery. Orthop Traumatol Surg Res. (2017) 103:527–30. doi: 10.1016/j.otsr.2017.01.011
3. Wu Y-S, Zhang H, Zheng W-H, Feng Z-H, Chen Z-X, Lin Y. Hidden blood loss and the influential factors after percutaneous kyphoplasty surgery. Eur Spine J. (2017) 26:1878–83. doi: 10.1007/s00586-017-4950-9
4. Wang X, Yang R, Sun H, Zhang Y. Different Effects of Intravenous, Topical, and Combined Application of Tranexamic Acid on Patients with Thoracolumbar Fracture. World Neurosurg. (2019) 127:e1185–9. doi: 10.1016/j.wneu.2019.04.095
5. Peters A, Verma K, Slobodyanyuk K, Cheriyan T, Hoelscher C, Schwab F, et al. Antifibrinolytics reduce blood loss in adult spinal deformity surgery: a prospective, randomized controlled trial. Spine. (2015) 40:E443–E449. doi: 10.1097/BRS.0000000000000799
6. Draxler DF, Yep K, Hanafi G, Winton A, Daglas M, Ho H, et al. Tranexamic acid modulates the immune response and reduces postsurgical infection rates. Blood Adv. (2019) 3:1598–609. doi: 10.1182/bloodadvances.2019000092
7. Li Y, Xie H, Deng Z, Wang B, Tang Y, Zhao Z, et al. Tranexamic acid ameliorates rosacea symptoms through regulating immune response and angiogenesis. Int Immunopharmacol. (2019) 67:326–34. doi: 10.1016/j.intimp.2018.12.031
8. Zhang S, Xu H, Xie J, Cao G, Lei Y, Pei F. Tranexamic acid attenuates inflammatory effect and modulates immune response in primary total knee arthroplasty: a randomized, placebo-controlled, pilot trial. Inflammopharmacology. (2020) 28:839–49. doi: 10.1007/s10787-020-00695-6
9. Mu X, Wei J, Wang C, Ou Y, Yin D, Liang B, et al. Intravenous administration of tranexamic acid significantly reduces visible and hidden blood loss compared with its topical administration for double-segment posterior lumbar interbody fusion: a single-center, placebo-controlled, randomized trial. World Neurosurg. (2019) 122:e821–7. doi: 10.1016/j.wneu.2018.10.154
10. Li G, Sun T-W, Luo G, Zhang C. Efficacy of antifibrinolytic agents on surgical bleeding and transfusion requirements in spine surgery: a meta-analysis. Eur Spine J. (2017) 26:140–54. doi: 10.1007/s00586-016-4792-x
11. Zufferey P, Merquiol F, Laporte S, Decousus H, Mismetti P, Auboyer C, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. (2006) 105:1034–46. doi: 10.1097/00000542-200611000-00026
12. Cheriyan T, 2nd SPM, Bianco K, Slobodyanyuk K, Rattenni RN, Lafage V, et al. Efficacy of tranexamic acid on surgical bleeding in spine surgery: a meta-analysis. Spine J. (2015) 15:752–61. doi: 10.1016/j.spinee.2015.01.013
13. Chen X, Zhu X, Yang S, Lin W, Wang L. Tranexamic acid treatment decreases hidden blood loss in total knee arthroplasty. Am J Ther. (2016) 23:e1397–405. doi: 10.1097/MJT.0000000000000230
14. Ren Z, Li S, Sheng L, Zhuang Q, Li Z, Xu D, et al. Topical use of tranexamic acid can effectively decrease hidden blood loss during posterior lumbar spinal fusion surgery: A retrospective study. Medicine (Baltimore). (2017) 96:e8233. doi: 10.1097/MD.0000000000008233
15. Ker K, Prieto-Merino D, Roberts I. Systematic review, meta-analysis and meta-regression of the effect of tranexamic acid on surgical blood loss. Br J Surg. (2013) 100:1271–9. doi: 10.1002/bjs.9193
16. Andersson L, Eriksson O, Hedlund PO, Kjellman H, Lindqvist B. Special considerations with regard to the dosage of tranexamic acid in patients with chronic renal diseases. Urol Res. (1978) 6:83–8. doi: 10.1007/BF00255578
17. Xie J, Ma J, Yao H, Yue C, Pei F. Multiple boluses of intravenous tranexamic acid to reduce hidden blood loss after primary total knee arthroplasty without tourniquet: a randomized clinical trial. J Arthroplasty. (2016) 31:2458–64. doi: 10.1016/j.arth.2016.04.034
18. Blanié A, Bellamy L, Rhayem Y, Flaujac C, Samama CM, Fontenay M, et al. Duration of postoperative fibrinolysis after total hip or knee replacement: a laboratory follow-up study. Thromb Res. (2013) 131:e6–e11. doi: 10.1016/j.thromres.2012.11.006
19. Lecker I, Wang D-S, Whissell PD, Avramescu S, Mazer CD, Orser BA. Tranexamic acid-associated seizures: Causes and treatment. Ann Neurol. (2016) 79:18–26. doi: 10.1002/ana.24558
20. Cao G, Xie J, Huang Z, Huang Q, Chen G, Lei Y, et al. Efficacy and safety of multiple boluses of oral vs. intravenous tranexamic acid at reducing blood loss after primary total knee arthroplasty without a tourniquet: A prospective randomized clinical trial. Thromb Res. (2018) 171:68–73. doi: 10.1016/j.thromres.2018.09.054
21. Cao G, Huang Z, Xie J, Huang Q, Xu B, Zhang S, et al. The effect of oral vs. intravenous tranexamic acid in reducing blood loss after primary total hip arthroplasty: A randomized clinical trial. Thromb Res. (2018) 164:48–53. doi: 10.1016/j.thromres.2018.02.007
22. Borghi B, Pignotti E, Montebugnoli M, Bassi A, Corbascio M, de Simone N, et al. Autotransfusion in major orthopaedic surgery: experience with 1785 patients. Br J Anaesth. (1997) 79:662–4. doi: 10.1093/bja/79.5.662
23. Wu Y-G, Zeng Y, Shen B, Si H-B, Cao F, Yang T-M, et al. Combination of erythropoietin and tranexamic acid in bilateral simultaneous total hip arthroplasty: a randomised, controlled trial. Hip Int. (2016) 26:331–7. doi: 10.5301/hipint.5000356
24. Nirmalan PK, Thomas R. Sample sizes for clinical trials. Ophthalmology. (2007) 114:615. doi: 10.1016/j.ophtha.2006.11.002
25. Jiang W, Fu M, Dong W, Zhou N, Shen J, Zhang X, et al. Use of a multifunctional cocktail for postoperative bleeding and pain control in spinal fusion: a randomized, double-blind, controlled trial. Spine. (2022) 47:1328–1335. doi: 10.1097/BRS.0000000000004249
26. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. (1962) 51:224–32.
27. Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology. (1983) 58:277–80. doi: 10.1097/00000542-198303000-00016
28. Kushioka J, Yamashita T, Okuda S, Maeno T, Matsumoto T, Yamasaki R, et al. High-dose tranexamic acid reduces intraoperative and postoperative blood loss in posterior lumbar interbody fusion. J Neurosurg Spine. (2017) 26:363–7. doi: 10.3171/2016.8.SPINE16528
29. Fujikawa T. Tranexamic Acid in Patients Undergoing Noncardiac Surgery. N Engl J Med. (2022) 386:1986–97.
30. Chakravarthy VB, Yokoi H, Coughlin DJ, Manlapaz MR, Krishnaney AA. Development and implementation of a comprehensive spine surgery enhanced recovery after surgery protocol: the Cleveland Clinic experience. Neurosurg Focus. (2019) 46:E11. doi: 10.3171/2019.1.FOCUS18696
31. Hao S, Li H, Liu S, Meng S, Zhang X, Wang L, et al. The effect of intravenous unit-dose tranexamic acid on visible and hidden blood loss in posterior lumbar interbody fusion: a randomized clinical trial. Sci Rep. (2023) 13:4714. doi: 10.1038/s41598-022-27307-3
32. Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee arthroplasty? Correct blood loss management should take hidden loss into account. Knee. (2000) 7:151–155. doi: 10.1016/S0968-0160(00)00047-8
33. Smith GH, Tsang J, Molyneux SG, White TO. The hidden blood loss after hip fracture. Injury. (2011) 42:133–5. doi: 10.1016/j.injury.2010.02.015
34. Cushner FD, Friedman RJ. Blood loss in total knee arthroplasty. Clin Orthop Relat Res. (1991) 269:98–101. doi: 10.1097/00003086-199108000-00015
35. Urrutia J, Besa P, Lobos D, Campos M, Arrieta C, Andia M, et al. Lumbar paraspinal muscle fat infiltration is independently associated with sex, age, and inter-vertebral disc degeneration in symptomatic patients. Skeletal Radiol. (2018) 47:955–61. doi: 10.1007/s00256-018-2880-1
36. Xu R, Burgar A, Ebraheim NA, Yeasting RA. The quantitative anatomy of the laminas of the spine. Spine. (1999) 24:107–113. doi: 10.1097/00007632-199901150-00002
37. Andersson L, Nilsson IM, Niléhn JE, Hedner U, Granstrand B, Melander B. Experimental and clinical studies on AMCA, the antifibrinolytically active isomer of p-aminomethyl cyclohexane carboxylic acid. Scand J Haematol. (1965) 2:230–47. doi: 10.1111/j.1600-0609.1965.tb01300.x
38. Endo Y, Nishimura S, Miura A. Deep-vein thrombosis induced by tranexamic acid in idiopathic thrombocytopenic purpura. JAMA. (1988) 259:3561–2. doi: 10.1001/jama.1988.03720240023026
39. Kim C, Park SS-H, Davey JR. Tranexamic acid for the prevention and management of orthopedic surgical hemorrhage: current evidence. J Blood Med. (2015) 6:239–44. doi: 10.2147/JBM.S61915
40. Upadhyay SP, Mallick PN, Jagia M, Singh RKA. Acute arterial thrombosis associated with inadvertent high dose of tranexamic acid. Indian J Crit Care Med. (2013) 17:237–9. doi: 10.4103/0972-5229.118443
41. Carabini LM, Moreland NC, Vealey RJ, Bebawy JF, Koski TR, Koht A, et al. A randomized controlled trial of low-dose tranexamic acid vs. placebo to reduce red blood cell transfusion during complex multilevel spine fusion surgery. World Neurosurg. (2018) 110:e572–9. doi: 10.1016/j.wneu.2017.11.070
42. Girolami A, Cosi E, Ferrari S, Lombardi AM, Girolami B. Prethrombotic, prothrombotic, thrombophilic states, hypercoagulable state, thrombophilia etc.: semantics should be respected even in medical papers. J Thromb Thrombolysis. (2017) 43:390–3. doi: 10.1007/s11239-016-1459-8
43. Syrovets T, Jendrach M, Rohwedder A, Schüle A, Simmet T. Plasmin-induced expression of cytokines and tissue factor in human monocytes involves AP-1 and IKKbeta-mediated NF-kappaB activation. Blood. (2001) 97:3941–50. doi: 10.1182/blood.V97.12.3941
44. Barrett CD, Moore HB, Kong Y-W, Chapman MP, Sriram G, Lim D, et al. Tranexamic acid mediates proinflammatory and anti-inflammatory signaling via complement C5a regulation in a plasminogen activator-dependent manner. J Trauma Acute Care Surg. (2019) 86:101–7. doi: 10.1097/TA.0000000000002092
Keywords: spine surgery, posterior lumbar interbody fusion, perioperative blood loss, hidden blood loss, tranexamic acid
Citation: Dong W, Liang Y, Li D, Ma Z, Cheng M, Zhang X, Shen J, Zhou N, Hao J, Jiang W and Hu Z (2023) The effect of sequential perioperative intravenous tranexamic acid in reducing postoperative blood loss and hidden blood loss after posterior lumbar interbody fusion: a randomized controlled trial. Front. Med. 10:1192971. doi: 10.3389/fmed.2023.1192971
Received: 24 March 2023; Accepted: 17 July 2023;
Published: 04 August 2023.
Edited by:
Lei Kuang, Central South University, ChinaReviewed by:
Siegmund Lang, University Medical Center Regensburg, GermanyHayley Louise Letson, James Cook University, Australia
Copyright © 2023 Dong, Liang, Li, Ma, Cheng, Zhang, Shen, Zhou, Hao, Jiang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Jiang, MTM1Mjc1ODA1OTZAMTYzLmNvbQ==; Zhenming Hu, c3BpbmVjZW50ZXJAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Wei Dong1
Wei Dong1 Yi Liang
Yi Liang Jie Hao
Jie Hao Wei Jiang
Wei Jiang Zhenming Hu
Zhenming Hu