
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 29 June 2023
Sec. Dermatology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1187937
This article is part of the Research Topic Updates in Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) View all 6 articles
 Chun-Bing Chen1,2,3,4,5,6,7,8,9†
Chun-Bing Chen1,2,3,4,5,6,7,8,9† Wei-Kai Hung1,2†
Wei-Kai Hung1,2† Chuang-Wei Wang1,2,3,4,5
Chuang-Wei Wang1,2,3,4,5 Chih-Chun Lee10
Chih-Chun Lee10 Shuen-Iu Hung2
Shuen-Iu Hung2 Wen-Hung Chung1,2,3,4,5,7,9,11,12,13,14*
Wen-Hung Chung1,2,3,4,5,7,9,11,12,13,14*Drug reaction with eosinophilia and systemic symptoms or drug-induced hypersensitivity syndrome (DRESS/DIHS) is one type of severe cutaneous adverse reaction (SCAR). It is featured by fever, widespread skin lesions, protracted clinical course, internal organ involvement, and possibly long-term autoimmune sequelae. The presence of high-risk human leukocyte antigen (HLA) alleles, hypersensitivity reaction after culprit drug ingestion, and human herpesvirus reactivation may all contribute to its complex clinical manifestations. Some recent studies focusing on the roles of involved cytokines/chemokines and T cells co-signaling pathways in DRESS/DIHS were conducted. In addition, some predictors of disease severity and prognosis were also reported. In this review, we provided an update on the current understanding of the pathogenesis, potential biomarkers, and the relevant therapeutic rationales of DRESS/DIHS.
Drug hypersensitivity reaction are adverse effects that occur when the immune system reacts inappropriately to a medication. These reactions can range from mild to severe and can involve various organs and tissues, with the skin being the most commonly affected. These reactions can be categorized into two primary types: immediate drug hypersensitivity reactions and delayed drug hypersensitivity reactions (1, 2). Immediate hypersensitivity reactions occur within hours of drug exposure and are typically mediated by Immunoglobulin E (IgE) antibodies. These reactions can manifest as urticaria (hives), angioedema (swelling of the deeper layers of the skin), bronchospasm, or anaphylaxis. Delayed hypersensitivity reactions, on the other hand, occur days to weeks after drug exposure and are usually mediated by T cells. These reactions can present as various skin manifestations, including maculopapular eruptions, or severe cutaneous adverse reactions (SCARs), such as Stevens–Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis, and drug reaction with eosinophilia and systemic symptoms (DRESS). These severe reactions can cause systemic involvements and can be fatal (1, 2). DRESS syndrome is one of the life-threatening drug-induced SCARs. It is characterized by distinctive cutaneous manifestations, internal organ involvement, hematologic abnormalities, and probably long-term autoimmune sequelae (3). Typically, the symptoms develop 2 weeks to 3 months after taking the causative drugs. Patients may present with extensive erythema, facial edema, lymphadenopathy, and high-grade fever (4). Various degrees of internal organ involvement including hepatitis, nephritis, interstitial pneumonia, and myocarditis may occur. Marked elevated levels of blood eosinophils and the presence of atypical lymphocytes are hallmarks of hematologic manifestations.
Historically, similar manifestations were described as a variety of entities according to the causative drugs, like phenytoin hypersensitivity (5), dapsone hypersensitivity (or sulfone syndrome) (6), and allopurinol hypersensitivity syndrome (7). The term “DRESS” was first introduced by Bocquet et al. to make the description of this syndrome more consistent and unambiguous (8). Instead, another term “drug-induced hypersensitivity syndrome (DIHS)” is more widely used by Japanese experts (9). The diagnostic criteria of DRESS defined by the Registry of Severe Cutaneous Adverse Reactions (RegiSCAR) group and the criteria of DIHS are similar except for the inclusion of the status of Human herpesvirus 6 (HHV-6) reactivation in the diagnostic criteria of DIHS (10, 11). Current consensus denotes that these two terms (DRESS and DIHS) are likely within the same disease spectrum, and the diagnosis of DIHS may represent a more severe phenotype (10). In this review, we aimed to summarize the updated understanding of the pathogenesis and biomarkers of DRESS/DIHS as well as the relevant therapeutic implications.
DRESS is considered a T cell-mediated delayed-type hypersensitivity reaction in the Gell and Coombs classification (12). Traditionally, DRESS is classified as a type IVb reaction that corresponds with CD8+ and CD4+ T cells responses underlying the production of interferon-γ (IFN-γ), interleukin (IL)-4, IL-5, and IL-13, resulting in eosinophilia. Currently, there are several proposed pathomechanisms majorly involved in DRESS. DRESS is a drug hypersensitivity reaction due to specific culprit drugs induced immune response in genetically susceptible patients, and HHV reactivation may synergistically contribute to its pathogenesis. The diverse manifestations of DRESS/DIHS may result from the complex interplay between the drug-specific and the antiviral immune responses (Figure 1).
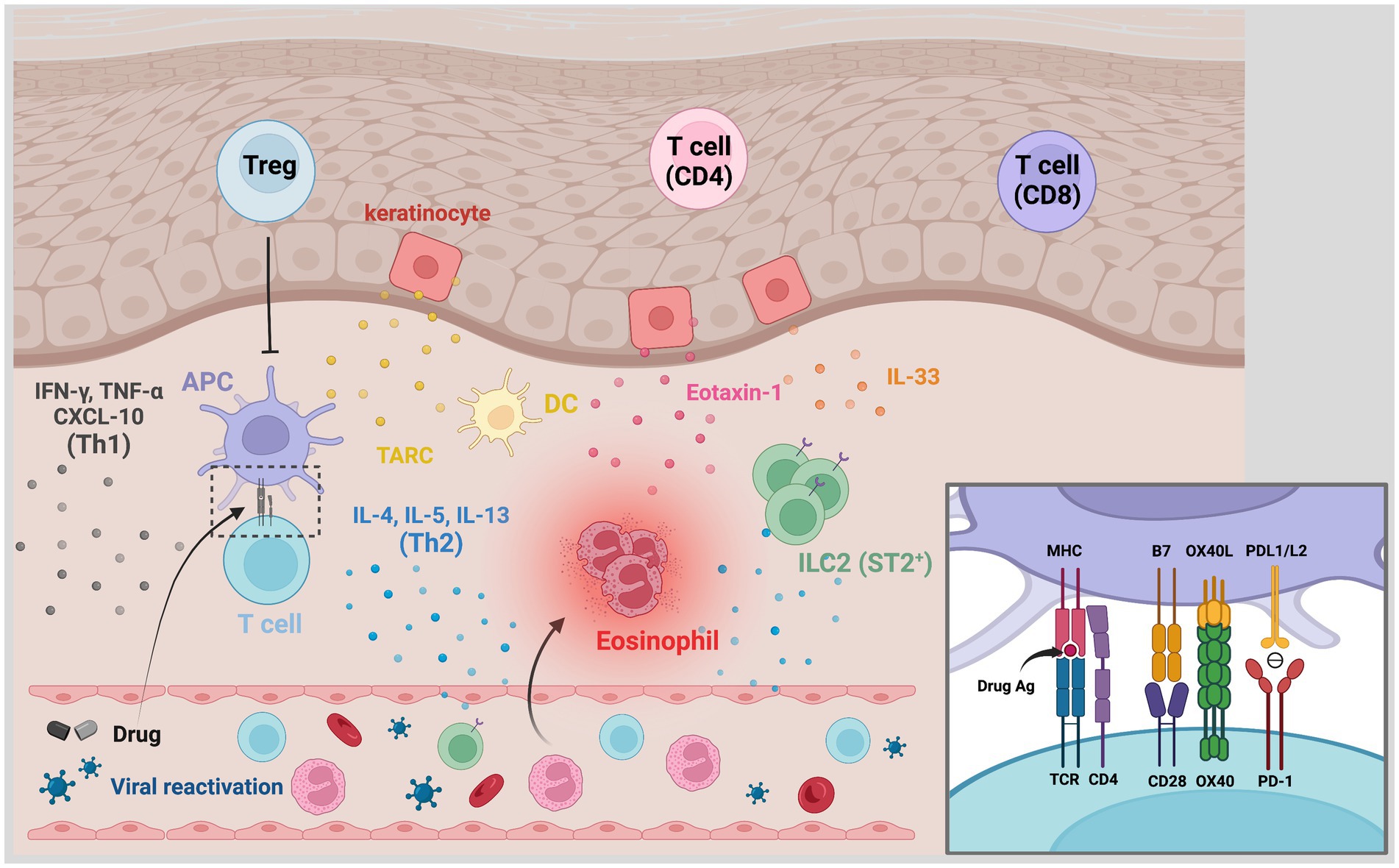
Figure 1. Pathogenesis of DRESS/DIHS. The affected skin is rich in CD4+ and CD8+ T cells, regulatory T cells (Tregs), plasma dendritic cells, and type 2 innate lymphoid cells (ILC2). TARC secreted by keratinocytes and DCs would recruit type 2 T helper (Th2) cells to the skin. Through antigen presentation, drug-specific T cells are activated and produced Th2-associated cytokines including IL-4, IL-5, and IL-13. Increased IL-33 levels, which may be secreted by keratinocytes and macrophages, activate ILC2 via its receptor ST2 and promote the production of Th2-associated cytokines. The IL-5, eotaxin-1, and TARC synergistically promote the local accumulation of harmful eosinophils. Th1-associated cytokines and chemokines like IFN-γ, TNF-α, and C-X-C motif chemokine 10 (CXCL-10) have also overexpressed in some cases. Viral reactivation is another characteristic hallmark in DRESS/DIHS. The inset shows the involved signals of antigen presentation. Traditionally, the specific antigen presented by the MHC molecule is recognized by antigen-specific TCR with the aid of B7-CD28 costimulation. The costimulatory interaction between OX40L and OX40, which is presented by activated T cells, may prevent T cells from being inhibited by Tregs. The binding of PD-1 and PDL1/L2 otherwise transmit signals to inhibit T cell proliferation and cytokines production. A compromised inhibitory mechanism of Tregs and PD-1/PDL1/L2 axes can lead to the promotion of these hypersensitivity reactions. CXCL-10, C-X-C motif chemokine 10; DC, dendritic cell; DRESS/DIHS, drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome; IFN-γ, interferon-γ; ILC2, type 2 innate lymphoid cells; PD-1, programmed cell death protein-1; PD-L1, programmed death ligand 1; TARC, thymus activation-regulated chemokine; TNF-α, tumor necrosis factor-α; Treg, regulatory T cell.
Human leukocyte antigen (HLA) molecules have an essential role in the immune reaction. They specifically present antigens to the T cell receptors (TCRs) (13). Antigen-specific T cells are then selected and the following immune responses are initiated (13). The HLA genes are located at the major histocompatibility complex (MHC) region on chromosome 6p21.3 and are the most polymorphic genes in the human genome (14, 15). Many pharmacogenetic studies have been conducted to investigate the association between HLA alleles and the specific drug-induced DRESS/DIHS. Until now, a variety of risky HLA alleles have been reported. These associated HLA alleles are usually drug- and ethnic-specific (see Table 1). The results suggest these “risky” HLA molecules may preferentially present specific drug antigens to specific TCRs and initiate adverse immune responses. Therefore, people with risky HLA alleles are more susceptible to specific drug hypersensitivity reactions. According to the different prevalence of specific risk alleles in different ethnicities, pre-prescription screening tests were recommended by some organizations for populations at risk (68). The preemptive strategies have demonstrated some success in lowering the incidence of SCARs (69).
Traditionally, it was commonly believed that T cells typically recognized peptides with at least 8–9 amino acids (MHC class I) and 12–15 amino acids (MHC class II) presented by antigen-presenting cells (APCs) (70). In drug hypersensitivity, several models were proposed for recognition of the small drug compounds by T cells with subsequent initiation of immune response. Currently, three main models were widely discussed (Figure 2): the hapten/pro-hapten model, the pharmacological interactions model (p-i concept), and the altered peptide repertoire model (71). In the hapten/pro-hapten model, the drug (hapten) or its reactive metabolites (pro-hapten after processing) would covalently bind to a larger protein or peptide. This formation of a hapten-protein/peptide complex called “haptenation” make this complex recognizable to the T cell receptor and gains the ability to activate the downstream immune response (71). The theory was best demonstrated in cases of penicillin-induced cutaneous adverse reactions (72). In the p-i concept, by contrast, the causative drugs may noncovalently and directly bind to the immune receptors like TCR or specific HLA molecules. The binding of these drugs is sufficient to stimulate the signaling transmission through the TCR without the need for a classic antigen-processing pathway (73). This concept was demonstrated and introduced by delicate studies conducted by Pichler’s group (74–76) and supported by the study conducted by Wei et al. (77) in the field of SCAR. In the study conducted by Wei et al. (77), the carbamazepine can directly interact with the HLA-B*15:02 molecule, be presented to APCs, and initiate immune responses (77). No intracellular antigen processing was involved in the HLA presentation of carbamazepine (77). Similar interaction was also seen in the hypersensitivity reaction induced by dapsone (78). The concept of the altered peptide repertoire model was demonstrated in the case of abacavir hypersensitivity syndrome in HLA-B*57:01-positive individuals (79–81). Abacavir binds noncovalently to the antigen-binding groove of HLA-B*57:01, which causes the conformational changes of the binding groove and alters the repertoire of endogenous peptides presented by HLA-B*57:01. Therefore, previously tolerated self-peptides become recognizable and may elicit hypersensitivity reactions. However, interestingly, these models may not be mutually exclusive in drug hypersensitivity reactions. For example, the hapten/pro-hapten theory and p-i concept had both been proposed in sulfamethoxazole-induced hypersensitivity reactions (82, 83). Different antigen-presenting mechanisms may be involved in different cases, further investigation may be needed to elucidate the actual mechanism in different drugs-induced DRESS/DIHS.
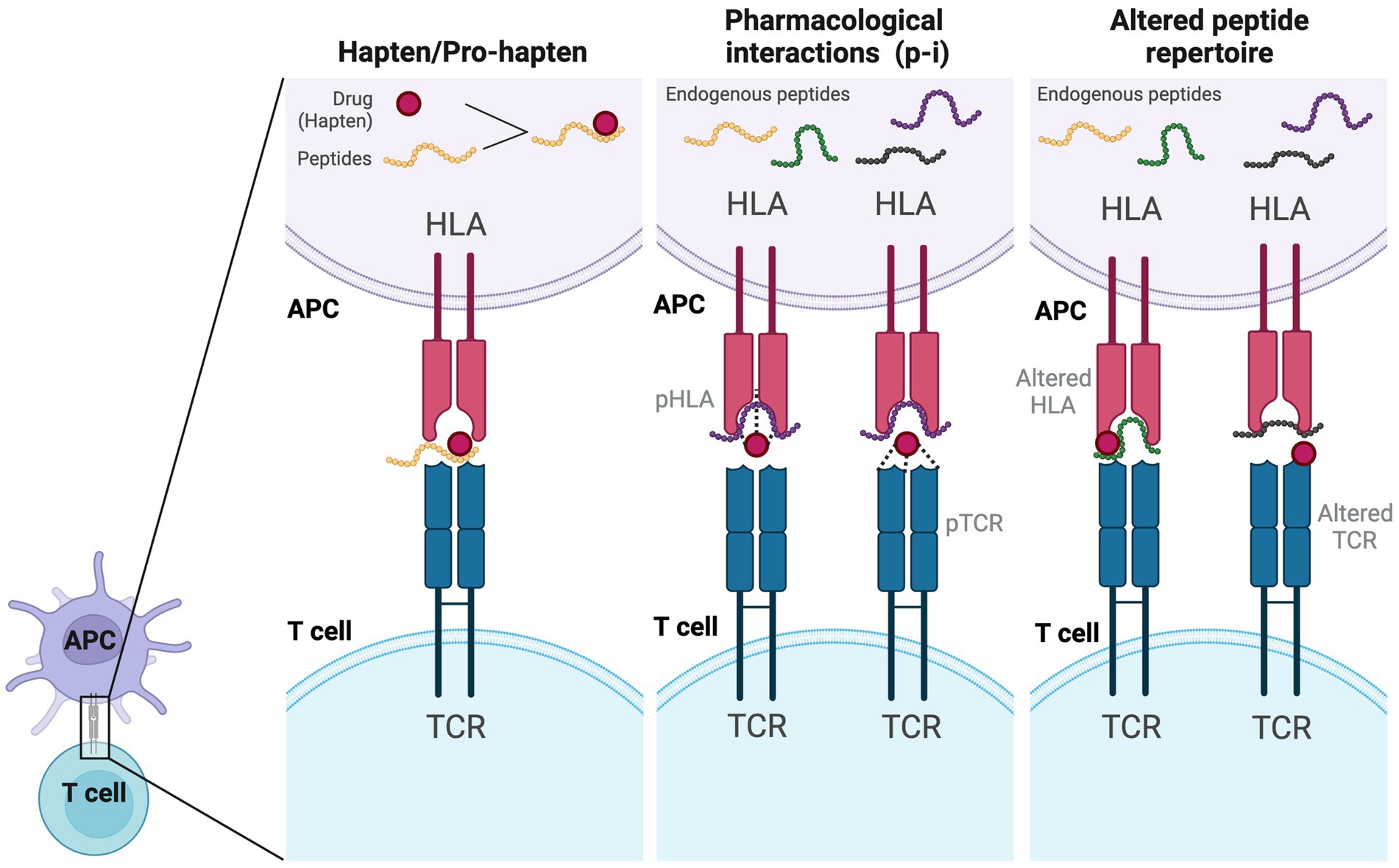
Figure 2. Models of antigen presentation and T-cell activation by drugs. In the hapten/pro-hapten model, the drug (hapten) covalently binds to a larger self-peptide and forms a neoantigen which could be presented by specific HLA alleles and recognized by the specific T cell receptor. In the p-i model, the drug would bind to the HLA or TCR noncovalently, which is a processing-independent interaction and can stimulate T cell activation directly. In the altered self-peptide repertoire model, the drug may bind to either the HLA (altered HLA) or TCR (altered TCR), leading to a conformational change and altering the repertoire of self-peptides presented to T cells. APC, antigen-presenting cell; HLA, human leukocyte antigen; TCR, T cell receptor.
For T cell activation to be initiated, two signals are required: the interaction of TCR MHC/TCR interaction (signal 1), and simultaneous costimulatory signals (signal 2) provided by a set of costimulatory molecules. The interaction between CD28 presented on T cells and the CD80/CD86 expressed on the surface of APCs is the prototype for this crucial signaling pathway. Asides from the CD28-CD80/CD86, there are several costimulatory and coinhibitory signaling molecules playing potentially important roles in the pathogenesis of DRESS/DIHS, such as OX40/OX40L, PD-1/PD-L1, and CTLA-4/CD80/CD86 axis (84).
OX40, also known as CD134, is a member of the tumor necrosis factor (TNF) receptor superfamily. Unlike other molecules in the TNF receptor superfamily, the OX40 was not expressed by naïve T cells but transiently expressed by antigen-activated T cells. The ligand for OX40 (OX40L) is expressed broadly by professional APCs, vascular endothelial cells, activated natural killer (NK) cells, and the responding CD4 T cells (85). At least two mechanisms of OX40-OX40L interaction were suggested including (1) promoting the expansion and survival of effector T cells and the generation of memory T cells, and (2) disrupting T-cell tolerance by antagonizing Treg-mediated suppression (85). The pathogenic role of OX40-OX40L interaction has been demonstrated in various immune diseases and cancers (86, 87).
Beyond the above-mentioned role in T cell activation, recent studies further delineated another role of OX40 in DRESS/DIHS. Miyagawa et al. found both the OX40-expressing CD4+ T cells and the OX40L-positive cells (in peripheral blood mononuclear cells, PBMCs) were upregulated in DRESS/DIHS in the acute stage compared with other drug eruptions (88, 89). HHV-6 reactivation is regarded to be one of the key contributors to the development of DRESS/DIHS. To be noticed, OX40 was previously identified as a specific receptor that helps the entry of HHV-6 to T cells (90). Recent studies demonstrated that the levels of serum soluble OX40 (sOX40), which may be produced by OX40 shedding and/or alternative splicing, were also increased in the serum of DRESS/DIHS patients, and were correlated with the peak level of HHV-6 viral loads (91). Moreover, the increased percentage of OX40 expression and level of sOX40 were both correlated with the serum levels of TARC/CCL17, a Th2-associated chemokine that is associated with disease severity in patients of DRESS/DIHS (92). In another study, Lee et al. demonstrated increased OX40-expressing CD4+ T cells in the lesional skin in DRESS/DIHS, and the frequency of OX40+ CD4 T cells was also correlated with the DRESS/DIHS severity score (93). These advanced investigations suggest that OX40/OX40L axis plays essential roles not only in T cell activation and Treg regulation but also in the HHV-6 replication and initiation of DRESS/DIHS.
PD-1, PD-L1, and CTLA-4 are well-known coinhibitory molecules since the concept of immune checkpoint blockade emerged as a promising strategy to defeat cancers. Many monoclonal antibodies targeting these molecules or their ligands were approved and have had a large success in cancer treatment (94).
PD-1 is a checkpoint receptor primarily found on activated CD4+ T cells, activated CD8+ T cells, and peripheral B cells (95). Its ligand PD-L1 is expressed on T cells, B cells, macrophages, and dendritic cells (DCs) while another ligand PD-L2 is expressed primarily on APCs (95). Ligation of PD-1/PD-L initiates immunosuppressive signals and inhibits the T cells proliferation, cytokine production, and cytotoxicity of T cells (95, 96). CTLA-4, a structural homolog of CD28, is expressed on CD4+ T cells and CD8+ T cells and would competitively bind to the same ligands of CD80/CD86 on APCs (87). The binding of CTLA-4 transmits an inhibitory signal to negatively regulate the T cell’s response (97).
There have been several case reports of immune checkpoint inhibitors induced DRESS/DIHS, showing the potential involvement of these coinhibitory molecules in the pathogenesis of DRESS/DIHS. Two cases developed DRESS/DIHS after using nivolumab, one is for metastatic renal cell carcinoma and another one is for gastric carcinoma (98, 99). For treating metastatic melanoma, two cases developed DRESS/DIHS after using ipilimumab alone or the combined use of ipilimumab plus nivolumab (100, 101). In addition, an increased incidence of hypersensitivity reaction was also observed when patients received sulfasalazine with concurrent immune checkpoint inhibitors (102). This phenomenon suggests that the immune regulatory pathway acts as another factor in determining drug susceptibility (103, 104). Hammond et al. also provided some evidence that checkpoint inhibition may reduce the threshold for drug-specific T-cell priming, which explain the tendency of drug hypersensitivity in these population (105). The exact roles of these coinhibitory molecules in the development of DRESS/DIHS are still under-investigated, and further studies are warranted.
Different types of cytokines and chemokines are involved in the pathogenesis of DRESS/DIHS (summarized in Table 2). DRESS/DIHS is classically considered a Th2-driven reaction with hallmarks of activated lymphocytes and eosinophilia. Th2-associated cytokines such as interleukin (IL)-4, IL-5, and IL-13 were secreted by activated Th2 cells and have a crucial impact on the development of DRESS/DIHS (118, 119). Increased levels of IL-5 were found in the plasma of hypersensitivity syndrome and associated with the generation of eosinophilia in these patients. IL-5 is not only a primary growth factor for eosinophils but also a Th2 chemokine, which plays a key role in promoting the differentiation, survival, and migration of eosinophils (120). Eotaxin-1, a chemokine also known as cysteine cysteine ligand 11 (CCL11), was also identified to be able to regulate eosinophils recruitment and activation synergistically with IL-5 (121). Circulating IL-4- and IL-13-producing CD4 + T cells were significantly higher in patients with DRESS (119). Of note, IL-13-producing T cells were significantly dominant skin-homing CLA+ cells, and the proportions of circulating IL-13-producing cells were correlated with serum thymus activation-regulated chemokine (TARC) levels.
TARC, known as CC chemokine ligand 17 (CCL17), was found to be increased in the serum in DRESS/DIHS patients (92, 116). TARC was produced mainly by DCs, Langerhans cells, and keratinocytes (122). Its main ligand, CC chemokine receptor type 4 (CCR4), is expressed predominantly by Th2-type T cells (122, 123). The TARC/CCL17 can serve as a recruiter for CCR4+ Th2 cells to the inflamed tissue and enhance the type 2 immune responses (122, 123). In DRESS/DIHS, the increased level of serum TARC/CCL17 was found, which is correlated with the blood eosinophil count (124). Moreover, the higher level of TARC/CCL17 in DRESS/DIHS patients at the acute stage was also correlated with the disease activity, and it may predict the presence of HHV-6 reactivation (92, 116).
In addition, IL-33, a member of the IL-1 cytokine superfamily, also activates immune cells and promotes Th2-associated cytokines production via selectively binding to its receptor ST2 (125). Recently, an increased number of type 2 innate lymphoid cells (ILC2s) expressing ST2 were identified in the skin and blood in patients with DRESS/DIHS at the acute stage and these patients presented with increased serum soluble ST2 levels (114). The study suggests the IL-33/ ST2 pathway and ILC2s are involved in the pathogenesis of DRESS/DIHS and more studies may be warranted to confirm this relationship.
Nevertheless, the different types of immune reactions may not be exclusive. In addition to interleukin (IL)-4, 5, and 13, which are predominant cytokines involved in Th2 response, tumor necrosis factor (TNF)-α and interferon (IFN)-γ (typically for Th1 response) are also found to be overexpressed in DRESS/DIHS in some studies (109, 112, 126) and the elevated levels of TNF-α and IL-6 preceded the HHV-6 infection (112). TNF-α and IFN-γ were primarily produced by the expanded population of activated CD8+ T cells (110). Paradoxically, in patients with DRESS/DIHS and HHV-6 reactivation, TNF-α and IFN-γ levels were lower during an early stage (127). Moreover, overexpression of granulysin, granzyme B, and soluble Fas ligand (sFasL) in the skin lesions and increased levels of them in the serum were also noticed in DRESS/DIHS (106–108). The elevated levels of sFasL and granzyme B were also found to be correlated with the elevation of liver enzymes (108). Granulysin and granzyme B are two cytotoxic molecules that were regarded as key mediators in the SJS/TEN (128, 129). sFasL were found elevated in SJS/TEN and could be an indicator for early diagnosis of SJS/TEN (130). These findings suggest that these cytotoxic proteins may also play important roles in the pathogenesis of cutaneous and liver manifestations in DRESS/DIHS. Recently, interferon-γ-induced protein (IP)-10/CXCL10, which is a major chemokine involved in the Th1 cell priming and differentiation, has also been reported to be associated with the development of long-term sequelae in DIHS/DRESS and DRESS with HHV-6 reactivation (127, 131). The detailed role of these Th1 cytokines in DRESS/DIHS is not clear, and further research is warranted for clarifying their function.
In another study including an analysis of 40 DRESS/DIHS patients, overexpression of IL-17 and IL-17E was demonstrated (110). In previous mice models, IL-17E could regulate Th2 immune response and result in an elevated level of eosinophils, IL-4, IL-5, eotaxin, and immunoglobulin E (132). These results suggest IL-17E may also play some role in amplifying the immune response in DRESS/DIHS. Other cytokines such as IL-2 and IL-15 were also found elevated in the PBMC and serum in DRESS/DIHS patients, respectively (109–111, 113). The IL-2 was crucial for the proliferation and activation of T cells, and the elevation of IL-15 seemed to be associated with the development of CMV reactivation in DRESS/DIHS (113, 133).
Association between the reactivation of latent viruses of the HHV family and DRESS/DIHS has been well studied. The most well-known scenario is HHV-6 reactivation, which can be found in 43–100% of patients with DRESS/DIHS and was included in the diagnostic criteria for DIHS (127, 134, 135). The presence of HHV-6 reactivation may represent a more severe phenotype (135, 136). Other members of the HHV family like EBV, CMV, and HHV-7 were also reported to be involved in the pathogenesis of DRESS/DIHS (110, 137).
HHV-6 infects T cells and establishes life-long latency in humans (138). One study demonstrated that the HHV-6-positive monomyeloid precursors (CD11b+CD13+CD14−CD16high) would be recruited to the lesional skin in the acute stage of DRESS/DIHS (139). The responsible chemoattractant may be high-mobility group box (HMGB)-1, which is a member of the damage-associated molecular pattern molecule (DAMP) family (139). The HMGB-1 level has been found to increase in the blood and skin in patients with DRESS/DIHS (139). Subsequently, the recruited monomyeloid precursors may potentiate the transmission of HHV-6 to the skin-resident CD4+ T cells (139). The HHV-6 infection of the CD4+ T cells subset serves as an indispensable step for the replication and activation of the virus (138, 139). In the acute stage of DRESS/DIHS, higher expression of OX40 (CD134), an entry receptor of HHV-6 as aforementioned, by activated CD4+ T cells has also been found (88). Hence, these studies suggested that the skin might be the primary site where HHV-6 reactivation starts (88, 139).
There have been several proposed mechanisms eliciting HHV-6 reactivation during the development of DRESS/DIHS. One possible theory is the direct effect of culprit drugs or their metabolites on viral reactivation. In one in vitro study conducted by Mardivirin et al., HHV-6 replication was increased by amoxicillin in the MT4 cell line (140). Similar effects were observed in other in vitro studies of valproic acid, which enhanced both HHV-6 and CMV replications (141, 142). Another theory proposed is related to the alterations of cellular and cytokine profiles in the early stage of DRESS/DIHS. In the early stage, the numbers of B cells and serum gammaglobulin reduced and Treg cells expanded (143–146). Serum levels of pro-inflammatory cytokines and chemokines such as TNF-α, IFN-γ, IL-1, IL-2, and IL-6 have been found lower in DRESS/DIHS patients with HHV-6 reactivation than the patients without (127). In addition, the plasmacytoid dendritic cells (pDCs), which produced type 1 interferon (IFN-α/β) upon activation to help neighboring cells resist viral infection, have also been found to have decreased levels in the circulation in patients with DRESS/DIHS around the time of viral reactivation (147–150). These relatively immunocompromised conditions were supposed to be related to the viral reactivation in DRESS/DIHS. On the contrary, the number of pDCs increased in the dermis of lesional skin, suggesting that pDCs may be recruited from circulation to skin during HHV-6 reactivation and enhance antiviral response nearby (147). In addition, the levels of TARC/CCL17 are also significantly elevated at an acute stage in DRESS/DIHS, and their levels are even higher in patients with the presence of HHV-6 reactivation (116, 117). As aforementioned, TARC/CCL17 would recruit CCR4+ Th2 cells and promote subsequent Th2 responses (122, 123).
Recently, Pichler et al. postulated another theory called the “virus-release hypothesis” (151). In this hypothesis (Figure 3), DRESS/DIHS caused by intense drug-induced p-i stimulation would activate polyclonal cytotoxic T lymphocytes (151, 152). Within these p-i-activated T cells, there are also herpes virus-specific cytotoxic T cells. The virus-specific T cells kill the herpes virus-infected cells and lead to the release of intracellular herpes viruses. Therefore, blood viremia could be detected with or without symptoms of viral reactivation. This hypothesis can be supported by the previous study results, which denoted the possibility of simultaneously increased serum viral loads of different herpes viruses like HHV-6, CMV, and EBV (153). Most patients with viremia do not present with associated symptoms. However, patients with CMV reactivation are more commonly related to poor prognosis and complications (113, 151). Nevertheless, whether the herpes virus reactivation or viremia is a causative or reactive factor in the pathogenesis of DRESS/DIHS is still in debate. More studies are warranted to uncover this mysterious veil.
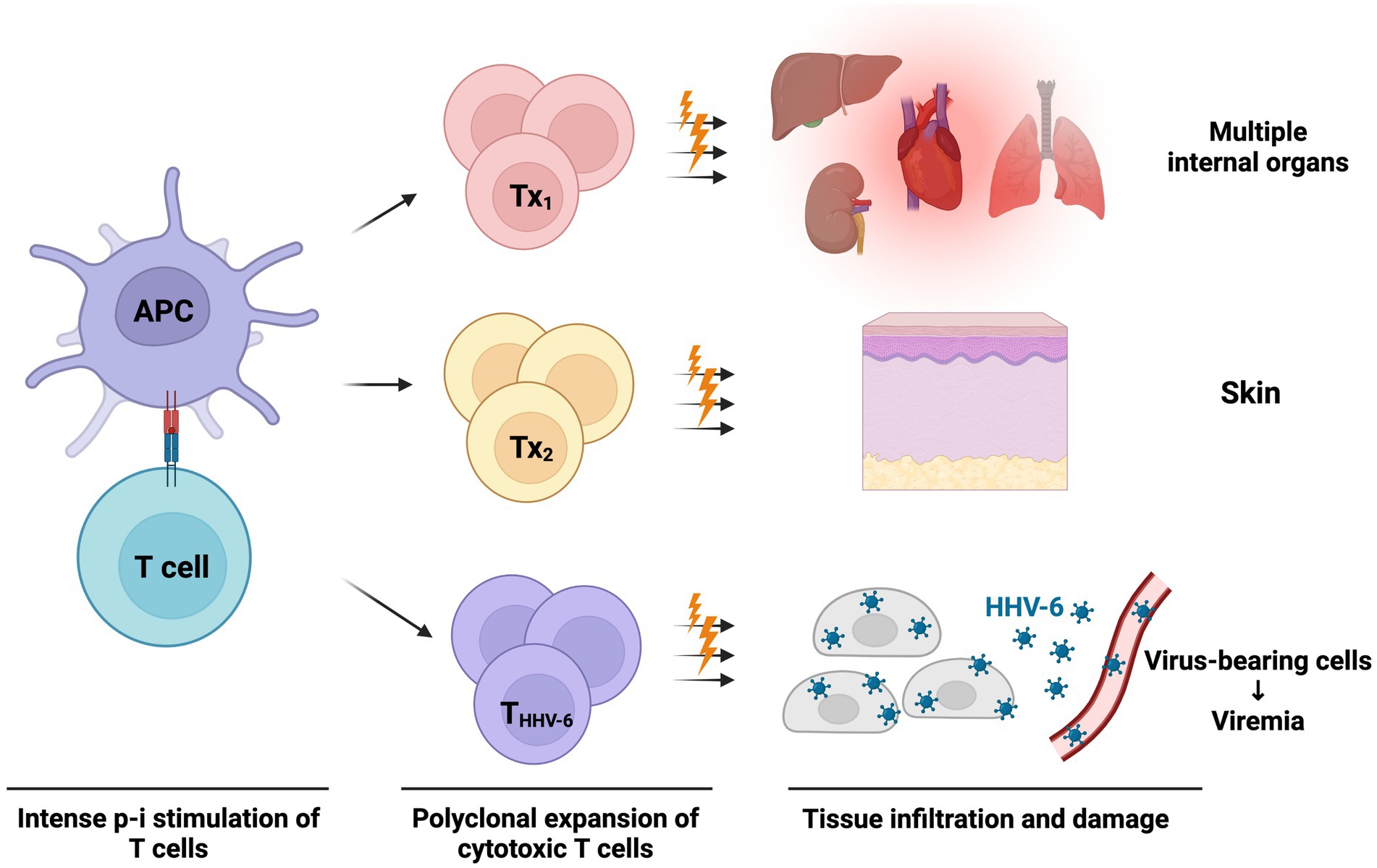
Figure 3. The virus-release hypothesis proposed by Pichler et al. (151) The massive p-i stimulation in DRESS/DIHS can lead to strong polyclonal T-cell expansion and activation. These p-i activated T cells are composed of cytotoxic T cells targeted at various internal organs, skin, and even virus peptide-specific T cells (may be reactive against HHV-6, CMV, EBV, etc.). These cytotoxic T cells attack organs, skin, and herpes peptide-expressing cells, causing acute symptoms of DRESS/DIHS and subsequent release of the prefabricated viruses. Therefore, viremia of various herpes viruses may be detected at a later stage. APC, antigen-presenting cell; CMV, cytomegalovirus; DRESS/DIHS, drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome; EBV, Epstein–Barr virus; HHV-6, human herpesvirus-6; THHV-6, HHV-6 specific cytotoxic T cell; Tx, drug-stimulated T cells against various tissue.
The COVID-19 pandemic has adversely affected our healthcare system across different countries and has a large impact on dermatology practice (154, 155). Different kinds of dermatoses development after the prolonged use of self-protect equipment, COVID-19 infection, and administration of COVID-19 vaccines were frequently reported (155). Drug hypersensitivity reactions like maculopapular drug rashes, have been reported associated with COVID-19 infection (151, 156). There were several supporting evidence for the potential pathomechanism including an increased SARS-CoV-2 spike protein receptor (angiotensin-converting enzyme 2) expressed by keratinocytes, and SARS-CoV-2 RNA isolated from the skin of COVID-19 infected patients (156). The use of systemic treatment for these immune-mediated dermatological disorders could be more complex (157). Moreover, DRESS/DIHS and other SCARs were reported potentially to be induced by the COVID-19 vaccinations (158–160). Schroeder et al. reported a definite case of DRESS/DIHS after the administration of second dose of Pfizer/BioNtech COVID-19 vaccine (160). The authors hypothesized that the reaction of skin and internal organs may be caused by the inefficient detoxification of the vaccine and accumulation of the reactive metabolites (160). However, the exact immune mechanism and causal relationship between COVID-19 vaccines and SCARs are hard to ascertained. More research is warranted to delineate the pathogenesis of COVID-19 vaccines on SCARs.
DRESS/DIHS presents with diverse clinical manifestations and has potential life-threatening risks (3, 68, 161). Risk stratification and biomarker monitoring of these patients are important for guiding management strategy.
Clinically, the outcomes of patients may differ due to the different causative drugs. For example, anticonvulsants and allopurinol were reported to be associated with a poorer prognosis compared with other culprit drugs like antibiotics (162, 163). Dermatological features with the presence of facial edema (164), laboratory examinations with higher lymphocyte count (165), severe liver injury (165), and the presence of HHV reactivation (166) were also linked to a worse prognosis or more severe disease. In addition, tachycardia, leukocytosis, tachypnea, coagulopathy, gastrointestinal bleeding, and systemic inflammatory response syndrome were associated with poor outcomes in DRESS patients (167). Recently, there were two scoring systems proposed to assess the disease severity and predict outcomes. One scoring system proposed by Mizukawa et al. composed of multiple clinical and laboratory data, including age, duration of drug exposure, allopurinol exposure, pulsed steroid use, the extent of skin involvement, fever, appetite loss, renal dysfunction, liver dysfunction and elevated CRP (168). The patients with a total score ≥ 4 represent severe cases with a predilection for later development of CMV diseases and complications (168). The other risk prediction model developed by Sharma et al. included 6 variables (age, sex, rash morphology, facial edema, medication class, and antinuclear antibody positivity) that were associated with the risk of the recurrence of DRESS syndrome (169). However, these models are derived from relatively small numbers of cases and the generalizability to other populations needs further validation.
Aside from these clinical features, some biomarkers were mentioned in studies to provide diagnostic and prognostic clues. Serum TARC/CCL17, soluble ST2, and sOX40 levels were all elevated at the acute stage in patients with DRESS/DIHS (91, 114, 116, 124, 170). The serum TARC/CCL17 levels were correlated with other indicators of systemic inflammation, and the soluble ST2 levels were correlated with IL-33 and alanine aminotransferase levels (114, 170). These are potential biomarkers of early identification and disease severity stratification in DRESS/DIHS. In addition, the elevated levels of serum TNF-α, TARC/CCL17, and sOX40 were also possible indicators of HHV-6 reactivation in DRESS/DIHS (117, 170, 171). Developing a scoring system using the above distinct clinical presentations and potential biomarkers may be a promising strategy for risk stratification and better outcome prediction. However, the availability of measuring these biomarkers may limit the application, especially in resource-poor healthcare facilities.
Since DRESS/DIHS is a relatively rare disease, no prospective randomized control trials have yet been performed to evaluate the efficacy and safety of each treatment modality. The clinical phenotype is heterogenous, and the management approach should be optimized according to the severity and the extent of organ involvement. Currently, there is still no international consensus or guideline on the use of immunomodulant in the treatment of DRESS/DIHS. Recently, there is a guideline for the diagnosis, management, treatment, and prevention of DRESS syndrome conducted by Spanish specialists and experts (68). The Spanish guideline made a comprehensive summary of current management considerations and provided a consensus-based stepwise management algorithm (68).
In general, the gold standard for treatment is causative drug identification and withdrawal with supportive care. Closely monitoring and assessing clinical symptoms, laboratory data, and imaging results are crucial. Multidisciplinary team care and timely consultation with other specialists are also important, especially while severe organ involvement. Systemic corticosteroids usually remain the first line of treatment, though the efficacy and risk profile of this treatment is difficult to be investigated in randomized controlled trials (68). A moderate-to-high dose (0.5–1 mg/kg/day of prednisolone equivalent dose) may be used to achieve improvement of the clinical symptoms and laboratory parameters (68, 172, 173). If the patients presented with more extensive organ involvement, a higher dose of corticosteroids may be applied. Initiation of systemic corticosteroids is recommended as first line therapy in patients with severe organ injury, such as nephritis (174), hepatitis (175), and pneumonitis if there are no contraindications (68). For patients present with more extensive organ involvement, initiating a higher dose of corticosteroids may be warranted (68). However, some disadvantages of systemic corticosteroids were reported including disease flare-ups during tapering (172), increased risk of opportunistic infection (162), and viral reactivations of HHV-6 and CMV (134). Therefore, the benefits and harms should be balanced while applying systemic corticosteroids for patients with DRESS/DIHS. For patients with a mild form of DRESS/DIHS without severe organ involvement, some authors advocate for using topical high-potency corticosteroids alone because of a lower complication rate compared with patients using systemic corticosteroids (162, 176, 177).
Other therapeutic agents have also been reported, though the evidence is mainly from small case series or case reports (see Table 3; Supplementary Table S1). Cyclosporin is another frequently reported potential therapeutic agent. Successful responses in treating patients with DRESS/DIHS have been observed in some case reports (178–181). A recent case–control study involving 26 patients (5 using cyclosporin and 21 using systemic corticosteroids) with DRESS/DIHS demonstrated cyclosporin is an effective treatment with good tolerability in patients contraindicated with systemic corticosteroids usage (183). Another study conducted by our team previously involving 8 DRESS/DIHS patients showed cyclosporin may be an effective and safe alternative treatment as a steroid-sparing agent for recalcitrant corticosteroid-dependent DRESS (182). Therefore, as per the Spanish guideline (68), cyclosporin can be considered as a second-line therapy for patients with poor control with corticosteroids or as a first-line therapy if the systemic corticosteroids are contraindicated. The recommended doses of cyclosporin use are still undetermined, but 3–5 mg/kg/day for a short course (7-day) with subsequent tapering may be considered according to current evidence. An open-labeled randomized clinical trial to compare the efficacy between high-dose cyclosporin (5 mg/kg/day) and pulse systemic corticosteroids in DRESS/DIHS is recently under enrolling (NCT04988256). More evidence of the efficacy and safety of cyclosporin may be established in the future.
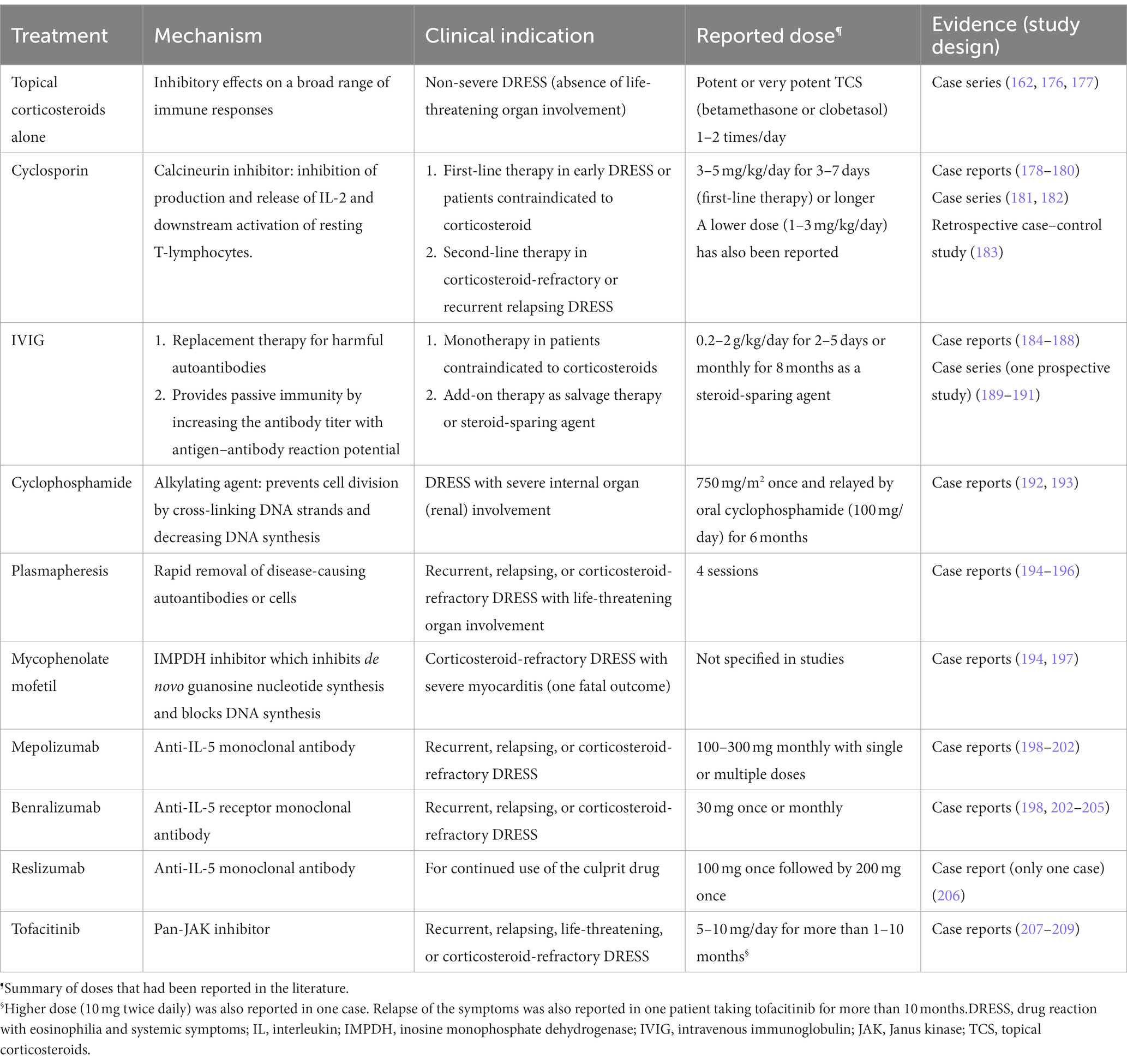
Table 3. Summary of current alternative therapeutic options and evidence other than systemic corticosteroids.
For intravenous immunoglobulin therapy (IVIG), inconsistent treatment results were reported in different studies (184–190). Successfully treated cases were reported from case reports and case series with variable doses and duration (0.2 to 2 g/kg/day for 2 to 5 days) as monotherapy, salvage therapy, or combined therapy with systemic corticosteroids. However, in a relatively large prospective study involving six patients, the author did not support the IVIGs as monotherapy for DRESS/DIHS because of severe adverse events and the absence of beneficial effects (191). Mycophenolate mofetil, cyclophosphamide, rituximab, and plasmapheresis are other potential modalities for management, but the evidence of their effect is primarily from case reports (192–197). These agents may be considered when the patients are refractory to the above therapies. Antiviral agents like ganciclovir and valganciclovir can be considered add-on therapies if viral reactivations with suspected of contributing to severe complications (e.g., encephalitis, hemophagocytosis, or severe erosive colitis) (68).
Target-specific biologic agents have emerged and shown promise in treating a variety of autoimmune and inflammatory diseases. Since IL-5 is one of the key pathogenic cytokines in DRESS/DIHS, IL-5/IL-5 receptor (IL-5R) blockade has been reported to be a potential strategy in the management of recalcitrant DRESS/DIHS (198). There are currently three available humanized monoclonal antibodies for IL-5/IL-5R blockade. Mepolizumab and reslizumab, directly target the IL-5 and inhibit IL-5 signaling; while benralizumab targets the α subunit of the IL-5R. Until now, 15 cases had been reported. Mepolizumab was used in seven cases (198–202), benralizumab in eight cases (198, 202–205), and reslizumab in one case of DRESS/DIHS (206). In cases using mepolizumab or reslizumab, multiple injections for 3 months may be needed to reach complete remission because relapses are common after administration (198–201, 206). Only one case demonstrated obvious clinical improvement after single dose of mepolizumab (202). By contrast, in cases using benralizumab, one subcutaneous injection at a dose of 30 mg may be sufficient in most patients (198, 203–205). However, the effectiveness of these agents for DRESS/DIHS was established based on a small number of cases.
Another signaling pathway-targeted therapy also provides a promising future for treating patients with DRESS/DIHS. By using high-output single-cell transcriptomic analysis, the Janus kinase-signal transducer and activator of transcription (JAK–STAT) signaling pathway showed significantly upregulated in a patient with DRESS/DIHS refractory to high dose corticosteroid, mycophenolate mofetil, and cyclosporine (207). A pan-JAK inhibitor with tofacitinib (up to 10 mg per day) was then administered according to the identified JAK3 and STAT1 signatures, and the disease was getting controlled well after tofacitinib treatment (207). The JAK–STAT pathway is responsible for the upstream signal for many cytokines including IL-4 (JAK1, JAK23, TYK2), IL-5 (JAK1, JAK2), and IL-13 (JAK1, TYK2), which were involved in the pathogenesis of DRESS/DIHS (210). There were another two case reports demonstrating the effectiveness of tofacitinib in treating life-threatening DRESS/DIHS complicated with myocarditis (208, 209). Although disease relapse was noticed when tofacitinib was suddenly stopped in these cases, symptoms would improve rapidly after tofacitinib was reintroduced. Long-term use and slow tapering of tofacitinib may be needed.
Moreover, OX40-OX40L and TARC/CCL17-CCR4 interaction pathways may also be potential targets for treating DRESS/DIHS. The therapeutic application of the available biologics of anti-OX40 and anti-OX40L antibodies, such as rocatinlimab and amlitelimab, has been under investigation for many immune diseases and cancers as well as Th2 dominant atopic dermatitis (87, 211). Mogamulizumab, a humanized anti-CCR4 monoclonal antibody targeting TARC/CCL17-CCR4 axis, was also applied for treating refractory or relapsed adult T cell leukemia/lymphoma and cutaneous T cell lymphoma (212). The potential therapeutic effects of these novel drugs on drug hypersensitivity reactions still need further investigation.
DRESS/DIHS is a rare but severe adverse drug reaction with distinct clinical features and complicated pathomechanisms. Genetic polymorphism, specific signaling pathways in T cell activation, cytokines and chemokines production, and HHV reactivation are involved in the pathogenesis of DRESS/DIHS. Some scoring systems and potential biomarkers are proposed, but not widely applied in clinical practice yet. Systemic corticosteroids are still the first line of DRESS/DIHS management, and other steroid-sparing immunomodulators may be promising treatment modalities, especially for refractory cases and those contraindicated to corticosteroids. More research is required to clarify the pathogenesis and determine the advantages and risks of the newly developed treatment modalities.
C-BC, W-KH, and C-CL: conceptualization. C-BC and W-KH: methodology and investigation. C-BC and W-HC: resources, supervision, and project administration. C-BC, W-KH, C-CL, and C-WW: writing—original draft preparation. C-BC, W-KH, C-WW, C-CL, S-IH, and W-HC: writing—review and editing. C-BC, C-CL, and S-IH: visualization. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the Ministry of Science and Technology, Taiwan (MOST 108-2314-B-182A-104-MY3, 109-2320-B-182A-008-MY3, 110-2320-B-182A-014-MY3, 111-2326-B-182A-003-MY3, 111-2314-B-182A-113-MY3, and NSTC 111-2314-B-182A-113-MY3) and Chang Gung Memorial Hospital (CORPG3L0471, CORPG3L0472, and CORPG3M0361).
Figures 1–3 were created with BioRender.com.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1187937/full#supplementary-material
1. Hausmann, O , Schnyder, B , and Pichler, WJ . Drug hypersensitivity reactions involving skin. Handb Exp Pharmacol. (2010) 196:29–55. doi: 10.1007/978-3-642-00663-0_2
2. Pichler, WJ . Immune pathomechanism and classification of drug hypersensitivity. Allergy. (2019) 74:1457–71. doi: 10.1111/all.13765
3. Hama, N , Abe, R , Gibson, A , and Phillips, EJ . Drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): clinical features and pathogenesis. J Allergy Clin Immunol Pract. (2022) 10:1155–1167 e5. doi: 10.1016/j.jaip.2022.02.004
4. Hung, WK , and Chung, WH . Drug reaction with eosinophilia and systemic symptoms. N Engl J Med. (2022) 387:167. doi: 10.1056/NEJMicm2116076
5. Haruda, F . Phenytoin hypersensitivity: 38 cases. Neurology. (1979) 29:1480–5. doi: 10.1212/WNL.29.11.1480
6. Tomecki, KJ , and Catalano, CJ . Dapsone hypersensitivity. The sulfone syndrome revisited. Arch Dermatol. (1981) 117:38–9. doi: 10.1001/archderm.1981.01650010044023
7. Singer, JZ , and Wallace, SL . The allopurinol hypersensitivity syndrome. Unnecessary morbidity and mortality. Arthritis Rheum. (1986) 29:82–7. doi: 10.1002/art.1780290111
8. Bocquet, H , Bagot, M , and Roujeau, JC . Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin Cutan Med Surg. (1996) 15:250–7. doi: 10.1016/S1085-5629(96)80038-1
9. Shiohara, T , Inaoka, M , and Kano, Y . Drug-induced hypersensitivity syndrome (DIHS): a reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol Int. (2006) 55:1–8. doi: 10.2332/allergolint.55.1
10. Shiohara, T , and Mizukawa, Y . Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): an update in 2019. Allergol Int. (2019) 68:301–8. doi: 10.1016/j.alit.2019.03.006
11. Kardaun, SH , Sekula, P , Valeyrie-Allanore, L , Liss, Y , Chu, CY , Creamer, D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. (2013) 169:1071–80. doi: 10.1111/bjd.12501
12. Pichler, WJ . Delayed drug hypersensitivity reactions. Ann Intern Med. (2003) 139:683–93. doi: 10.7326/0003-4819-139-8-200310210-00012
13. Rudolph, MG , Stanfield, RL , and Wilson, IA . How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. (2006) 24:419–66. doi: 10.1146/annurev.immunol.23.021704.115658
14. Horton, R , Wilming, L , Rand, V , Lovering, RC , Bruford, EA , Khodiyar, VK, et al. Gene map of the extended human MHC. Nat Rev Genet. (2004) 5:889–99. doi: 10.1038/nrg1489
15. Chung, WH , Hung, SI , and Chen, YT . Human leukocyte antigens and drug hypersensitivity. Curr Opin Allergy Clin Immunol. (2007) 7:317–23. doi: 10.1097/ACI.0b013e3282370c5f
16. Sukasem, C , Sririttha, S , Chaichan, C , Nakkrut, T , Satapornpong, P , Jaruthamsophon, K, et al. Spectrum of cutaneous adverse reactions to aromatic antiepileptic drugs and human leukocyte antigen genotypes in Thai patients and meta-analysis. Pharmacogenomics J. (2021) 21:682–90. doi: 10.1038/s41397-021-00247-3
17. McCormack, M , Alfirevic, A , Bourgeois, S , Farrell, JJ , Kasperavičiūtė, D , Carrington, M, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. (2011) 364:1134–43. doi: 10.1056/NEJMoa1013297
18. Ozeki, T , Mushiroda, T , Yowang, A , Takahashi, A , Kubo, M , Shirakata, Y, et al. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum Mol Genet. (2011) 20:1034–41. doi: 10.1093/hmg/ddq537
19. Kim, SH , Lee, KW , Song, WJ , Kim, SH , Jee, YK , Lee, SM, et al. Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res. (2011) 97:190–7. doi: 10.1016/j.eplepsyres.2011.08.010
20. Genin, E , Chen, DP , Hung, SI , Sekula, P , Schumacher, M , Chang, PY, et al. HLA-A*31:01 and different types of carbamazepine-induced severe cutaneous adverse reactions: an international study and meta-analysis. Pharmacogenomics J. (2014) 14:281–8. doi: 10.1038/tpj.2013.40
21. Hung, SI , Chung, WH , Jee, SH , Chen, WC , Chang, YT , Lee, WR, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions [comparative study research support, non-U.S. Gov't]. Pharmacogenet Genomics. (2006) 16:297–306. doi: 10.1097/01.fpc.0000199500.46842.4a
22. Mockenhaupt, M , Wang, CW , Hung, SI , Sekula, P , Schmidt, AH , Pan, RY, et al. HLA-B*57:01 confers genetic susceptibility to carbamazepine-induced SJS/TEN in Europeans. Allergy. (2019) 74:2227–30. doi: 10.1111/all.13821
23. Ramírez, E , Bellón, T , Tong, HY , Borobia, AM , de Abajo, FJ , Lerma, V, et al. Significant HLA class I type associations with aromatic antiepileptic drug (AED)-induced SJS/TEN are different from those found for the same AED-induced DRESS in the Spanish population. Pharmacol Res. (2017) 115:168–78. doi: 10.1016/j.phrs.2016.11.027
24. Mortazavi, H , Rostami, A , Firooz, A , Esmaili, N , Ghiasi, M , Lajevardi, V, et al. Association between human leukocyte antigens and cutaneous adverse drug reactions to antiepileptics and antibiotics in the Iranian population. Dermatol Ther. (2022) 35:e15393. doi: 10.1111/dth.15393
25. Wong, CSM , Yap, DYH , Ip, P , Wong, WHS , Chua, GT , Yeung, CK, et al. HLA-B*15:11 status and carbamazepine-induced severe cutaneous adverse drug reactions in HLA-B*15:02 negative Chinese. Int J Dermatol. (2022) 61:184–90. doi: 10.1111/ijd.15792
26. Yampayon, K , Sukasem, C , Limwongse, C , Chinvarun, Y , Tempark, T , Rerkpattanapipat, T, et al. Influence of genetic and non-genetic factors on phenytoin-induced severe cutaneous adverse drug reactions. Eur J Clin Pharmacol. (2017) 73:855–65. doi: 10.1007/s00228-017-2250-2
27. Tassaneeyakul, W , Prabmeechai, N , Sukasem, C , Kongpan, T , Konyoung, P , Chumworathayi, P, et al. Associations between HLA class I and cytochrome P450 2C9 genetic polymorphisms and phenytoin-related severe cutaneous adverse reactions in a Thai population. Pharmacogenet Genomics. (2016) 26:225–34. doi: 10.1097/FPC.0000000000000211
28. Manuyakorn, W , Likkasittipan, P , Wattanapokayakit, S , Suvichapanich, S , Inunchot, W , Wichukchinda, N, et al. Association of HLA genotypes with phenytoin induced severe cutaneous adverse drug reactions in Thai children. Epilepsy Res. (2020) 162:106321. doi: 10.1016/j.eplepsyres.2020.106321
29. Chang, CC , Ng, CC , Too, CL , Choon, SE , Lee, CK , Chung, WH, et al. Association of HLA-B*15:13 and HLA-B*15:02 with phenytoin-induced severe cutaneous adverse reactions in a Malay population. Pharmacogenomics J. (2017) 17:170–3. doi: 10.1038/tpj.2016.10
30. Chung, WH , Chang, WC , Lee, YS , Wu, YY , Yang, CH , Ho, HC, et al. Genetic variants associated with phenytoin-related severe cutaneous adverse reactions. JAMA. (2014) 312:525–34. doi: 10.1001/jama.2014.7859
31. Kim, BK , Jung, JW , Kim, TB , Chang, YS , Park, HS , Moon, J, et al. HLA-A*31:01 and lamotrigine-induced severe cutaneous adverse drug reactions in a Korean population. Ann Allergy Asthma Immunol. (2017) 118:629–30. doi: 10.1016/j.anai.2017.02.011
32. Kazeem, GR , Cox, C , Aponte, J , Messenheimer, J , Brazell, C , Nelsen, AC, et al. High-resolution HLA genotyping and severe cutaneous adverse reactions in lamotrigine-treated patients. Pharmacogenet Genomics. (2009) 19:661–5. doi: 10.1097/FPC.0b013e32832c347d
33. Hung, SI , Chung, WH , Liou, LB , Chu, CC , Lin, M , Huang, HP, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. (2005) 102:4134–9. doi: 10.1073/pnas.0409500102
34. Tassaneeyakul, W , Jantararoungtong, T , Chen, P , Lin, PY , Tiamkao, S , Khunarkornsiri, U, et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genomics. (2009) 19:704–9. doi: 10.1097/FPC.0b013e328330a3b8
35. Kaniwa, N , Saito, Y , Aihara, M , Matsunaga, K , Tohkin, M , Kurose, K, et al. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics. (2008) 9:1617–22. doi: 10.2217/14622416.9.11.1617
36. Kang, HR , Jee, YK , Kim, YS , Lee, CH , Jung, JW , Kim, SH, et al. Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics. (2011) 21:303–7. doi: 10.1097/FPC.0b013e32834282b8
37. Kim, MY , Yun, J , Kang, DY , Kim, TH , Oh, MK , Lee, S, et al. HLA-A*24:02 increase the risk of allopurinol-induced drug reaction with eosinophilia and systemic symptoms in HLA-B*58:01 carriers in a Korean population; a multicenter cross-sectional case-control study. Clin Transl Allergy. (2022) 12:e12193. doi: 10.1002/clt2.12193
38. Saag, M , Balu, R , Phillips, E , Brachman, P , Martorell, C , Burman, W, et al. High sensitivity of human leukocyte antigen-b*5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clin Infect Dis. (2008) 46:1111–8. doi: 10.1086/529382
39. Hetherington, S , Hughes, AR , Mosteller, M , Shortino, D , Baker, KL , Spreen, W, et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. (2002) 359:1121–2. doi: 10.1016/S0140-6736(02)08158-8
40. Mallal, S , Nolan, D , Witt, C , Masel, G , Martin, AM , Moore, C, et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. (2002) 359:727–32. doi: 10.1016/S0140-6736(02)07873-X
41. Hughes, AR , Mosteller, M , Bansal, AT , Davies, K , Haneline, SA , Lai, EH, et al. Association of genetic variations in HLA-B region with hypersensitivity to abacavir in some, but not all, populations. Pharmacogenomics. (2004) 5:203–11. doi: 10.1517/phgs.5.2.203.27481
42. Manglani, MV , Gabhale, YR , Lala, MM , Sekhar, R , and More, D . HLA-B*5701 allele in HIV-infected Indian children and its association with Abacavir hypersensitivity. Indian Pediatr. (2018) 55:140–1. doi: 10.1007/s13312-018-1248-x
43. Martin, AM , Nolan, D , James, I , Cameron, P , Keller, J , Moore, C, et al. Predisposition to nevirapine hypersensitivity associated with HLA-DRB1*0101 and abrogated by low CD4 T-cell counts. AIDS. (2005) 19:97–9. doi: 10.1097/00002030-200501030-00014
44. Chantarangsu, S , Mushiroda, T , Mahasirimongkol, S , Kiertiburanakul, S , Sungkanuparph, S , Manosuthi, W, et al. HLA-B*3505 allele is a strong predictor for nevirapine-induced skin adverse drug reactions in HIV-infected Thai patients. Pharmacogenet Genomics. (2009) 19:139–46. doi: 10.1097/FPC.0b013e32831d0faf
45. Littera, R , Carcassi, C , Masala, A , Piano, P , Serra, P , Ortu, F, et al. HLA-dependent hypersensitivity to nevirapine in Sardinian HIV patients. AIDS. (2006) 20:1621–6. doi: 10.1097/01.aids.0000238408.82947.09
46. Gatanaga, H , Yazaki, H , Tanuma, J , Honda, M , Genka, I , Teruya, K, et al. HLA-Cw8 primarily associated with hypersensitivity to nevirapine. AIDS. (2007) 21:264–5. doi: 10.1097/QAD.0b013e32801199d9
47. Carr, DF , Chaponda, M , Jorgensen, AL , Castro, EC , van Oosterhout, JJ , Khoo, SH, et al. Association of human leukocyte antigen alleles and nevirapine hypersensitivity in a Malawian HIV-infected population. Clin Infect Dis. (2013) 56:1330–9. doi: 10.1093/cid/cit021
48. Gao, S , Gui, XE , Liang, K , Liu, Z , Hu, J , and Dong, B . HLA-dependent hypersensitivity reaction to nevirapine in Chinese Han HIV-infected patients. AIDS Res Hum Retrovir. (2012) 28:540–3. doi: 10.1089/aid.2011.0107
49. Lefebvre, M , Walencik, A , Allavena, C , Billaud, E , Kassi, A , Cesbron, A, et al. Rate of DRESS syndrome with Raltegravir and role of the HLA-B*53: 01 allele. J Acquir Immune Defic Syndr. (2020) 85:e77–80. doi: 10.1097/QAI.0000000000002474
50. Thomas, M , Hopkins, C , Duffy, E , Lee, D , Loulergue, P , Ripamonti, D, et al. Association of the HLA-B*53:01 allele with drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome during treatment of HIV infection with Raltegravir. Clin Infect Dis. (2017) 64:1198–203. doi: 10.1093/cid/cix096
51. Zhang, FR , Liu, H , Irwanto, A , Fu, XA , Li, Y , Yu, GQ, et al. HLA-B*13:01 and the dapsone hypersensitivity syndrome. N Engl J Med. (2013) 369:1620–8. doi: 10.1056/NEJMoa1213096
52. Krismawati, H , Irwanto, A , Pongtiku, A , Irwan, ID , Maladan, Y , Sitanggang, YA, et al. Validation study of HLA-B*13:01 as a biomarker of dapsone hypersensitivity syndrome in leprosy patients in Indonesia. PLoS Negl Trop Dis. (2020) 14:e0008746. doi: 10.1371/journal.pntd.0008746
53. Park, HJ , Park, JW , Kim, SH , Choi, SY , Kim, HK , Jung, CG, et al. The HLA-B*13:01 and the dapsone hypersensitivity syndrome in Korean and Asian populations: genotype- and meta-analyses. Expert Opin Drug Saf. (2020) 19:1349–56. doi: 10.1080/14740338.2020.1796965
54. Satapornpong, P , Pratoomwun, J , Rerknimitr, P , Klaewsongkram, J , Nakkam, N , Rungrotmongkol, T, et al. HLA-B*13:01 is a predictive marker of Dapsone-induced severe cutaneous adverse reactions in Thai patients. Front Immunol. (2021) 12:661135. doi: 10.3389/fimmu.2021.661135
55. Chen, WT , Wang, CW , Lu, CW , Chen, CB , Lee, HE , Hung, SI, et al. The function of HLA-B*13:01 involved in the Pathomechanism of Dapsone-induced severe cutaneous adverse reactions. J Invest Dermatol. (2018) 138:1546–54. doi: 10.1016/j.jid.2018.02.004
56. Wang, CW , Tassaneeyakul, W , Chen, CB , Chen, WT , Teng, YC , Huang, CY, et al. Taiwan/Asian severe cutaneous adverse reaction C. whole genome sequencing identifies genetic variants associated with co-trimoxazole hypersensitivity in Asians. J Allergy Clin Immunol. (2021) 147:1402–12. doi: 10.1016/j.jaci.2020.08.003
57. Sukasem, C , Pratoomwun, J , Satapornpong, P , Klaewsongkram, J , Rerkpattanapipat, T , Rerknimitr, P, et al. Genetic Association of co-Trimoxazole-Induced Severe Cutaneous Adverse Reactions is Phenotype-Specific: HLA class I genotypes and haplotypes. Clin Pharmacol Ther. (2020) 108:1078–89. doi: 10.1002/cpt.1915
58. Nakkam, N , Saksit, N , Konyoung, P , Amornpinyo, W , Khunarkornsiri, U , Purimart, D, et al. Associations of HLA and drug-metabolizing enzyme genes in co-trimoxazole-induced severe cutaneous adverse reactions. Drug Metab Pharmacokinet. (2022) 47:100480. doi: 10.1016/j.dmpk.2022.100480
59. Yang, F , Gu, B , Zhang, L , Xuan, J , Luo, H , Zhou, P, et al. HLA-B*13:01 is associated with salazosulfapyridine-induced drug rash with eosinophilia and systemic symptoms in Chinese Han population. Pharmacogenomics. (2014) 15:1461–9. doi: 10.2217/pgs.14.69
60. Nakamura, R , Ozeki, T , Hirayama, N , Sekine, A , Yamashita, T , Mashimo, Y, et al. Association of HLA-A*11:01 with sulfonamide-related severe cutaneous adverse reactions in Japanese patients. J Invest Dermatol. (2020) 140:1659–1662.e6. doi: 10.1016/j.jid.2019.12.025
61. Rutkowski, K , Taylor, C , and Wagner, A . HLA B62 as a possible risk factor for drug reaction with eosinophilia and systemic symptoms to piperacillin/tazobactam. J Allergy Clin Immunol Pract. (2017) 5:829–30. doi: 10.1016/j.jaip.2016.10.008
62. Konvinse, KC , Trubiano, JA , Pavlos, R , James, I , Shaffer, CM , Bejan, CA, et al. HLA-A*32:01 is strongly associated with vancomycin-induced drug reaction with eosinophilia and systemic symptoms. J Allergy Clin Immunol. (2019) 144:183–92. doi: 10.1016/j.jaci.2019.01.045
63. Bellón, T , Lerma, V , Guijarro, J , Ramírez, E , Martínez, C , Escudero, C, et al. LTT and HLA testing as diagnostic tools in Spanish vancomycin-induced DRESS cases: A case-control study. Front Pharmacol. (2022) 13:959321. doi: 10.3389/fphar.2022.959321
64. Wang, CW , Lin, WC , Chen, WT , Chen, CB , Lu, CW , Hou, HH, et al. Associations of HLA-A and HLA-B with vancomycin-induced drug reaction with eosinophilia and systemic symptoms in the Han-Chinese population. Front Pharmacol. (2022) 13:954596. doi: 10.3389/fphar.2022.954596
65. Balas, A , Ramírez, E , Trigo, E , Cabañas, R , Fiandor, A , Arsuaga, M, et al. HLA-A∗68, −A∗11:01, and -A∗29:02 alleles are strongly associated with benznidazole-induced maculopapular exanthema (MPE)/DRESS. J Allergy Clin Immunol Pract. (2020) 8:3198–3200.e3. doi: 10.1016/j.jaip.2020.05.004
66. Salehi, T , Fleet, AP , Hissaria, P , Carroll, RP , and Au, PC . Human leukocyte antigen association with azathioprine-induced drug hypersensitivity reactions in patients with anti-neutrophil cytoplasmic antibody associated vasculitis. Hum Immunol. (2023) 84:196–8. doi: 10.1016/j.humimm.2022.12.006
67. Saper, VE , Ombrello, MJ , Tremoulet, AH , Montero-Martin, G , Prahalad, S , Canna, S, et al. Severe delayed hypersensitivity reactions to IL-1 and IL-6 inhibitors link to common HLA-DRB1*15 alleles. Ann Rheum Dis. (2022) 81:406–15. doi: 10.1136/annrheumdis-2021-220578
68. Cabanas, R , Ramirez, E , Sendagorta, E , Alamar, R , Barranco, R , Blanca-Lopez, N, et al. Spanish guidelines for diagnosis, management, treatment, and prevention of DRESS syndrome. J Investig Allergol Clin Immunol. (2020) 30:229–53. doi: 10.18176/jiaci.0480
69. Ko, TM , Tsai, CY , Chen, SY , Chen, KS , Yu, KH , Chu, CS, et al. Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. BMJ. (2015) 351:h4848. doi: 10.1136/bmj.h4848
70. Hemmer, B , Kondo, T , Gran, B , Pinilla, C , Cortese, I , Pascal, J, et al. Minimal peptide length requirements for CD4(+) T cell clones--implications for molecular mimicry and T cell survival. Int Immunol. (2000) 12:375–83. doi: 10.1093/intimm/12.3.375
71. Pavlos, R , Mallal, S , Ostrov, D , Buus, S , Metushi, I , Peters, B, et al. T cell-mediated hypersensitivity reactions to drugs. Annu Rev Med. (2015) 66:439–54. doi: 10.1146/annurev-med-050913-022745
72. Padovan, E , Mauri-Hellweg, D , Pichler, WJ , and Weltzien, HU . T cell recognition of penicillin G: structural features determining antigenic specificity. Eur J Immunol. (1996) 26:42–8. doi: 10.1002/eji.1830260107
73. Pichler, WJ . Pharmacological interaction of drugs with antigen-specific immune receptors: the p-i concept. Curr Opin Allergy Clin Immunol. (2002) 2:301–5. doi: 10.1097/00130832-200208000-00003
74. Schnyder, B , Mauri-Hellweg, D , Zanni, M , Bettens, F , and Pichler, WJ . Direct, MHC-dependent presentation of the drug sulfamethoxazole to human alphabeta T cell clones. J Clin Invest. (1997) 100:136–41. doi: 10.1172/JCI119505
75. Zanni, MP , von Greyerz, S , Schnyder, B , Brander, KA , Frutig, K , Hari, Y, et al. HLA-restricted, processing- and metabolism-independent pathway of drug recognition by human alpha beta T lymphocytes. J Clin Invest. (1998) 102:1591–8. doi: 10.1172/JCI3544
76. Zanni, MP , von Greyerz, S , Schnyder, B , Wendland, T , and Pichler, WJ . Allele-unrestricted presentation of lidocaine by HLA-DR molecules to specific alphabeta+ T cell clones. Int Immunol. (1998) 10:507–15. doi: 10.1093/intimm/10.4.507
77. Wei, CY , Chung, WH , Huang, HW , Chen, YT , and Hung, SI . Direct interaction between HLA-B and carbamazepine activates T cells in patients with Stevens-Johnson syndrome. J Allergy Clin Immunol. (2012) 129:1562–9.e5. doi: 10.1016/j.jaci.2011.12.990
78. Jiang, H , Wang, CW , Wang, Z , Dai, Y , Zhu, Y , Lee, YS, et al. Functional and structural characteristics of HLA-B*13:01-mediated specific T cells reaction in dapsone-induced drug hypersensitivity. J Biomed Sci. (2022) 29:58. The authors declare that they have no competing interests. doi: 10.1186/s12929-022-00845-8
79. Ostrov, DA , Grant, BJ , Pompeu, YA , Sidney, J , Harndahl, M , Southwood, S, et al. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc Natl Acad Sci. (2012) 109:9959–64. doi: 10.1073/pnas.1207934109
80. Illing, PT , Vivian, JP , Dudek, NL , Kostenko, L , Chen, Z , Bharadwaj, M, et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire [research support, non-U.S. Gov't]. Nature. (2012) 486:554–8. doi: 10.1038/nature11147
81. Norcross, MA , Luo, S , Lu, L , Boyne, MT , Gomarteli, M , Rennels, AD, et al. Abacavir induces loading of novel self-peptides into HLA-B*57: 01: an autoimmune model for HLA-associated drug hypersensitivity. AIDS. (2012) 26:F21–9. doi: 10.1097/QAD.0b013e328355fe8f
82. Callan, HE , Jenkins, RE , Maggs, JL , Lavergne, SN , Clarke, SE , Naisbitt, DJ, et al. Multiple adduction reactions of nitroso sulfamethoxazole with cysteinyl residues of peptides and proteins: implications for hapten formation. Chem Res Toxicol. (2009) 22:937–48. doi: 10.1021/tx900034r
83. Watkins, S , and Pichler, WJ . Activating interactions of sulfanilamides with T cell receptors. Open J Immunol. (2013) 3:139–57. doi: 10.4236/oji.2013.33019
84. Hsu, YO , Lu, KL , Fu, Y , Wang, CW , Lu, CW , Lin, YF, et al. The roles of Immunoregulatory networks in severe drug hypersensitivity. Front Immunol. (2021) 12:597761. doi: 10.3389/fimmu.2021.597761
85. Ishii, N , Takahashi, T , Soroosh, P , and Sugamura, K . OX40–OX40 ligand interaction in T-cell-mediated immunity and immunopathology. Adv Immunol. (2010) 105:63–98. doi: 10.1016/S0065-2776(10)05003-0
86. Willoughby, J , Griffiths, J , Tews, I , and Cragg, MS . OX40: structure and function—what questions remain? Mol Immunol. (2017) 83:13–22. doi: 10.1016/j.molimm.2017.01.006
87. Fu, Y , Lin, Q , Zhang, Z , and Zhang, L . Therapeutic strategies for the costimulatory molecule OX40 in T-cell-mediated immunity. Acta Pharm Sin B. (2020) 10:414–33. doi: 10.1016/j.apsb.2019.08.010
88. Miyagawa, F , Nakamura, Y , Miyashita, K , Iioka, H , Himuro, Y , Ogawa, K, et al. Preferential expression of CD134, an HHV-6 cellular receptor, on CD4T cells in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic symptoms (DRESS). J Dermatol Sci. (2016) 83:151–4. doi: 10.1016/j.jdermsci.2016.05.001
89. Miyagawa, F , Nakamura-Nishimura, Y , Kanatani, Y , and Asada, H . Correlation between expression of CD134, a human herpesvirus 6 cellular receptor, on CD4+ T cells and Th2-type immune responses in drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms. Acta Derm Venereol. (2020) 100:adv00102. doi: 10.2340/00015555-3465
90. Tang, H , Serada, S , Kawabata, A , Ota, M , Hayashi, E , Naka, T, et al. CD134 is a cellular receptor specific for human herpesvirus-6B entry. Proc Natl Acad Sci U S A. (2013) 110:9096–9. doi: 10.1073/pnas.1305187110
91. Mitsui, Y , Shinkuma, S , Nakamura-Nishimura, Y , Ommori, R , Ogawa, K , Miyagawa, F, et al. Serum soluble OX40 as a diagnostic and prognostic biomarker for drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms. J Allergy Clin Immunol Pract. (2022) 10:558–565 e4. doi: 10.1016/j.jaip.2021.10.042
92. Nakamura-Nishimura, Y , Miyagawa, F , Miyashita, K , Ommori, R , Azukizawa, H , and Asada, H . Serum thymus and activation-regulated chemokine is associated with the severity of drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome. Br J Dermatol. (2018) 178:1430–2. doi: 10.1111/bjd.16132
93. Lee, ES , Kiuchi, Y , Inomata, N , and Sueki, H . Increased expression of human herpes virus 6 receptor CD134/OX40 in skin lesions of patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms. J Dermatol. (2022) 49:e221–3. doi: 10.1111/1346-8138.16341
94. Postow, MA , Sidlow, R , and Hellmann, MD . Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481
95. Keir, ME , Butte, MJ , Freeman, GJ , and Sharpe, AH . PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. (2008) 26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331
96. Zamani, MR , Aslani, S , Salmaninejad, A , Javan, MR , and Rezaei, N . PD-1/PD-L and autoimmunity: A growing relationship. Cell Immunol. (2016) 310:27–41. doi: 10.1016/j.cellimm.2016.09.009
97. Hosseini, A , Gharibi, T , Marofi, F , Babaloo, Z , and Baradaran, B . CTLA-4: from mechanism to autoimmune therapy. Int Immunopharmacol. (2020) 80:106221. doi: 10.1016/j.intimp.2020.106221
98. Lu, J , Thuraisingam, T , Chergui, M , and Nguyen, K . Nivolumab-associated DRESS syndrome: A case report. JAAD Case Rep. (2019) 5:216–8. doi: 10.1016/j.jdcr.2018.11.017
99. Ai, L , Gao, J , Zhao, S , Li, Q , Cui, YH , Liu, Q, et al. Nivolumab-associated DRESS in a genetic susceptible individual. J Immunother Cancer. (2021) 9:e002879. doi: 10.1136/jitc-2021-002879
100. Mirza, S , Hill, E , Ludlow, SP , and Nanjappa, S . Checkpoint inhibitor-associated drug reaction with eosinophilia and systemic symptom syndrome. Melanoma Res. (2017) 27:271–3. doi: 10.1097/CMR.0000000000000326
101. Voskens, CJ , Goldinger, SM , Loquai, C , Robert, C , Kaehler, KC , Berking, C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. (2013) 8:e53745. doi: 10.1371/journal.pone.0053745
102. Ford, M , Sahbudin, I , Filer, A , Steven, N , and Fisher, BA . High proportion of drug hypersensitivity reactions to sulfasalazine following its use in anti-PD-1-associated inflammatory arthritis. Rheumatology. (2018) 57:2244–6. doi: 10.1093/rheumatology/key234
103. Naisbitt, DJ , Olsson-Brown, A , Gibson, A , Meng, X , Ogese, MO , Tailor, A, et al. Immune dysregulation increases the incidence of delayed-type drug hypersensitivity reactions. Allergy. (2020) 75:781–97. doi: 10.1111/all.14127
104. Hammond, S , Thomson, P , Meng, X , and Naisbitt, D . In-vitro approaches to predict and study T-cell mediated hypersensitivity to drugs. Front Immunol. (2021) 12:630530. doi: 10.3389/fimmu.2021.630530
105. Hammond, S , Olsson-Brown, A , Grice, S , Gibson, A , Gardner, J , Castrejon-Flores, JL, et al. Checkpoint inhibition reduces the threshold for drug-specific T-cell priming and increases the incidence of sulfasalazine hypersensitivity. Toxicol Sci. (2022) 186:58–69. doi: 10.1093/toxsci/kfab144
106. Saito, N , Abe, R , Yoshioka, N , Murata, J , Fujita, Y , and Shimizu, H . Prolonged elevation of serum granulysin in drug-induced hypersensitivity syndrome. Br J Dermatol. (2012) 167:452–3. doi: 10.1111/j.1365-2133.2012.10921.x
107. Weinborn, M , Barbaud, A , Truchetet, F , Beurey, P , Germain, L , and Cribier, B . Histopathological study of six types of adverse cutaneous drug reactions using granulysin expression. Int J Dermatol. (2016) 55:1225–33. doi: 10.1111/ijd.13350
108. Yang, F , Chen, SA , Wu, X , Zhu, Q , and Luo, X . Overexpression of cytotoxic proteins correlates with liver function impairment in patients with drug reaction with eosinophilia and systemic symptoms (DRESS). Eur J Dermatol. (2018) 28:13–25. doi: 10.1684/ejd.2017.3211
109. Beeler, A , Engler, O , Gerber, BO , and Pichler, WJ . Long-lasting reactivity and high frequency of drug-specific T cells after severe systemic drug hypersensitivity reactions. J Allergy Clin Immunol. (2006) 117:455–62. doi: 10.1016/j.jaci.2005.10.030
110. Picard, D , Janela, B , Descamps, V , D'Incan, M , Courville, P , Jacquot, S, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a multiorgan antiviral T cell response. Sci Transl Med. (2010) 2:116. doi: 10.1126/scitranslmed.3001116
111. Srinoulprasert, Y , Kumkamthornkul, P , Tuchinda, P , Wongwiangjunt, S , Sathornsumetee, S , Jongjaroenprasert, K, et al. Differential cytokine profiles produced by anti-epileptic drug re-exposure of peripheral blood mononuclear cells derived from severe anti-epileptic drug patients and non-allergic controls. Cytokine. (2022) 157:155951. doi: 10.1016/j.cyto.2022.155951
112. Yoshikawa, T , Fujita, A , Yagami, A , Suzuki, K , Matsunaga, K , Ihira, M, et al. Human herpesvirus 6 reactivation and inflammatory cytokine production in patients with drug-induced hypersensitivity syndrome. J Clin Virol. (2006) 37:S92–6. doi: 10.1016/S1386-6532(06)70019-1
113. Mizukawa, Y , Kimishima, M , Aoyama, Y , and Shiohara, T . Predictive biomarkers for cytomegalovirus reactivation before and after immunosuppressive therapy: A single-institution retrospective long-term analysis of patients with drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic syndrome (DRESS). Int J Infect Dis. (2020) 100:239–46. doi: 10.1016/j.ijid.2020.08.078
114. Tsai, YG , Liou, JH , Hung, SI , Chen, CB , Chiu, TM , Wang, CW, et al. Increased type 2 innate lymphoid cells in patients with drug reaction with eosinophilia and systemic symptoms syndrome. J Invest Dermatol. (2019) 139:1722–31. doi: 10.1016/j.jid.2018.10.048
115. Yang, CW , Cho, YT , Hsieh, YC , Hsu, SH , Chen, KL , and Chu, CY . The interferon-γ-induced protein 10/CXCR3 axis is associated with human herpesvirus-6 reactivation and the development of sequelae in drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. (2020) 183:909–19. doi: 10.1111/bjd.18942
116. Ogawa, K , Morito, H , Hasegawa, A , Daikoku, N , Miyagawa, F , Okazaki, A, et al. Identification of thymus and activation-regulated chemokine (TARC/CCL17) as a potential marker for early indication of disease and prediction of disease activity in drug-induced hypersensitivity syndrome (DIHS)/drug rash with eosinophilia and systemic symptoms (DRESS). J Dermatol Sci. (2013) 69:38–43. doi: 10.1016/j.jdermsci.2012.10.002
117. Ogawa, K , Morito, H , Hasegawa, A , Miyagawa, F , Kobayashi, N , Watanabe, H, et al. Elevated serum thymus and activation-regulated chemokine (TARC/CCL17) relates to reactivation of human herpesvirus 6 in drug reaction with eosinophilia and systemic symptoms (DRESS)/drug-induced hypersensitivity syndrome (DIHS). Br J Dermatol. (2014) 171:425–7. doi: 10.1111/bjd.12948
118. Choquet-Kastylevsky, G , Intrator, L , Chenal, C , Bocquet, H , Revuz, J , and Roujeau, JC . Increased levels of interleukin 5 are associated with the generation of eosinophilia in drug-induced hypersensitivity syndrome. Br J Dermatol. (1998) 139:1026–32. doi: 10.1046/j.1365-2133.1998.02559.x
119. Teraki, Y , and Fukuda, T . Skin-homing IL-13-producing T cells expand in the circulation of patients with drug rash with eosinophilia and systemic symptoms. Dermatology. (2017) 233:242–9. doi: 10.1159/000475546
120. Greenfeder, S , Umland, SP , Cuss, FM , Chapman, RW , and Egan, RW . Th2 cytokines and asthma. The role of interleukin-5 in allergic eosinophilic disease. Respir Res. (2001) 2:71–9. doi: 10.1186/rr41
121. Yawalkar, N , Shrikhande, M , Hari, Y , Nievergelt, H , Braathen, LR , and Pichler, WJ . Evidence for a role for IL-5 and eotaxin in activating and recruiting eosinophils in drug-induced cutaneous eruptions. J Allergy Clin Immunol. (2000) 106:1171–6. doi: 10.1067/mai.2000.110922
122. Catherine, J , and Roufosse, F . What does elevated TARC/CCL17 expression tell us about eosinophilic disorders? Semin Immunopathol. (2021) 43:439–58. doi: 10.1007/s00281-021-00857-w
123. Imai, T , Nagira, M , Takagi, S , Kakizaki, M , Nishimura, M , Wang, J, et al. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol. (1999) 11:81–8. doi: 10.1093/intimm/11.1.81
124. Komatsu-Fujii, T , Kaneko, S , Chinuki, Y , Suyama, Y , Ohta, M , Niihara, H, et al. Serum TARC levels are strongly correlated with blood eosinophil count in patients with drug eruptions. Allergol Int. (2017) 66:116–22. doi: 10.1016/j.alit.2016.06.003
125. Takatori, H , Makita, S , Ito, T , Matsuki, A , and Nakajima, H . Regulatory mechanisms of IL-33-ST2-mediated allergic inflammation. Front Immunol. (2018) 9:2004. doi: 10.3389/fimmu.2018.02004
126. Nishio, D , Izu, K , Kabashima, K , and Tokura, Y . T cell populations propagating in the peripheral blood of patients with drug eruptions. J Dermatol Sci. (2007) 48:25–33. doi: 10.1016/j.jdermsci.2007.05.013
127. Chen, YC , Chiang, HH , Cho, YT , Chang, CY , Chen, KL , Yang, CW, et al. Human herpes virus reactivations and dynamic cytokine profiles in patients with cutaneous adverse drug reactions --a prospective comparative study. Allergy. (2015) 70:568–75. doi: 10.1111/all.12602
128. Chung, WH , Hung, SI , Yang, JY , Su, SC , Huang, SP , Wei, CY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. (2008) 14:1343–50. doi: 10.1038/nm.1884
129. Gibson, A , Deshpande, P , Campbell, CN , Krantz, MS , Mukherjee, E , Mockenhaupt, M, et al. Updates on the immunopathology and genomics of severe cutaneous adverse drug reactions. J Allergy Clin Immunol. (2023) 151:289–300.e4. doi: 10.1016/j.jaci.2022.12.005
130. Abe, R , Shimizu, T , Shibaki, A , Nakamura, H , Watanabe, H , and Shimizu, H . Toxic epidermal necrolysis and Stevens-Johnson syndrome are induced by soluble Fas ligand. Am J Pathol. (2003) 162:1515–20. doi: 10.1016/S0002-9440(10)64284-8
131. Yang, CW , Cho, YT , Hsieh, YC , Hsu, SH , Chen, KL , and Chu, CY . The interferon-gamma-induced protein 10/CXCR3 axis is associated with human herpesvirus-6 reactivation and the development of sequelae in drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. (2020) 183:909–19. doi: 10.1111/bjd.18942
132. Kim, MR , Manoukian, R , Yeh, R , Silbiger, SM , Danilenko, DM , Scully, S, et al. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood. (2002) 100:2330–40. doi: 10.1182/blood-2002-01-0012
133. Abbas, AK , Trotta, E , Marson, A , and Bluestone, JA . Revisiting IL-2: biology and therapeutic prospects. Sci Immunol. (2018) 3:482. doi: 10.1126/sciimmunol.aat1482
134. Ishida, T , Kano, Y , Mizukawa, Y , and Shiohara, T . The dynamics of herpesvirus reactivations during and after severe drug eruptions: their relation to the clinical phenotype and therapeutic outcome. Allergy. (2014) 69:798–805. doi: 10.1111/all.12410
135. Shiohara, T , Iijima, M , Ikezawa, Z , and Hashimoto, K . The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol. (2007) 156:1083–4. doi: 10.1111/j.1365-2133.2007.07807.x
136. Ushigome, Y , Kano, Y , Hirahara, K , and Shiohara, T . Human herpesvirus 6 reactivation in drug-induced hypersensitivity syndrome and DRESS validation score. Am J Med. (2012) 125:e9–e10. doi: 10.1016/j.amjmed.2011.10.027
137. Ang, CC , Wang, YS , Yoosuff, EL , and Tay, YK . Retrospective analysis of drug-induced hypersensitivity syndrome: a study of 27 patients. J Am Acad Dermatol. (2010) 63:219–27. doi: 10.1016/j.jaad.2009.08.050
138. Otani, N , and Okuno, T . Human herpesvirus 6 infection of CD4+ T-cell subsets. Microbiol Immunol. (2007) 51:993–1001. doi: 10.1111/j.1348-0421.2007.tb03996.x
139. Hashizume, H , Fujiyama, T , Kanebayashi, J , Kito, Y , Hata, M , and Yagi, H . Skin recruitment of monomyeloid precursors involves human herpesvirus-6 reactivation in drug allergy. Allergy. (2013) 68:681–9. doi: 10.1111/all.12138
140. Mardivirin, L , Valeyrie-Allanore, L , Branlant-Redon, E , Beneton, N , Jidar, K , Barbaud, A, et al. Amoxicillin-induced flare in patients with DRESS (drug reaction with eosinophilia and systemic symptoms): report of seven cases and demonstration of a direct effect of amoxicillin on human herpesvirus 6 replication in vitro. Eur J Dermatol. (2010) 20:68–73. doi: 10.1684/ejd.2010.0821
141. Mardivirin, L , Lacroix, A , Delebassée, S , Descamps, V , and Ranger-Rogez, S . Enhancement of human herpesvirus 6 replication using sodium valproate [article]. Virologie. (2007) 11:151–3. doi: 10.1684/vir.2011.8928
142. Kuntz-Simon, G , and Obert, G . Sodium valproate, an anticonvulsant drug, stimulates human cytomegalovirus replication. J Gen Virol. (1995) 76:1409–15. doi: 10.1099/0022-1317-76-6-1409
143. Takahashi, R , Kano, Y , Yamazaki, Y , Kimishima, M , Mizukawa, Y , and Shiohara, T . Defective regulatory T cells in patients with severe drug eruptions: timing of the dysfunction is associated with the pathological phenotype and outcome. J Immunol. (2009) 182:8071–9. doi: 10.4049/jimmunol.0804002
144. Kano, Y , Inaoka, M , and Shiohara, T . Association between anticonvulsant hypersensitivity syndrome and human herpesvirus 6 reactivation and hypogammaglobulinemia. Arch Dermatol. (2004) 140:183–8. doi: 10.1001/archderm.140.2.183
145. Kano, Y , Seishima, M , and Shiohara, T . Hypogammaglobulinemia as an early sign of drug-induced hypersensitivity syndrome. J Am Acad Dermatol. (2006) 55:727–8. doi: 10.1016/j.jaad.2006.02.050
146. Yazicioglu, M , Elmas, R , Turgut, B , and Genchallac, T . The association between DRESS and the diminished numbers of peripheral B lymphocytes and natural killer cells. Pediatr Allergy Immunol. (2012) 23:289–96. doi: 10.1111/j.1399-3038.2012.01268.x
147. Sugita, K , Tohyama, M , Watanabe, H , Otsuka, A , Nakajima, S , Iijima, M, et al. Fluctuation of blood and skin plasmacytoid dendritic cells in drug-induced hypersensitivity syndrome. J Allergy Clin Immunol. (2010) 126:408–10. doi: 10.1016/j.jaci.2010.06.004
148. Siegal, FP , Kadowaki, N , Shodell, M , Fitzgerald-Bocarsly, PA , Shah, K , Ho, S, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. (1999) 284:1835–7. doi: 10.1126/science.284.5421.1835
149. Colonna, M , Trinchieri, G , and Liu, YJ . Plasmacytoid dendritic cells in immunity. Nat Immunol. (2004) 5:1219–26. doi: 10.1038/ni1141
150. Hsu, SH , Yang, CW , Hsieh, YC , Chen, KL , Cho, YT , Liau, JY, et al. Plasmacytoid dendritic cells diminution in peripheral blood is prevalent in drug reaction with eosinophilia and systemic symptoms and may precede human herpesvirus 6 reactivation [article]. Dermatol Sin. (2021) 39:175–81. doi: 10.4103/ds.ds_37_21
151. Pichler, WJ , and Bruggen, MC . Viral infections and drug hypersensitivity. Allergy. (2022) 78:60–70. doi: 10.1111/all.15558
152. Pichler, WJ . The important role of non-covalent drug-protein interactions in drug hypersensitivity reactions. Allergy. (2022) 77:404–15. doi: 10.1111/all.14962
153. Kano, Y , Hiraharas, K , Sakuma, K , and Shiohara, T . Several herpesviruses can reactivate in a severe drug-induced multiorgan reaction in the same sequential order as in graft-versus-host disease. Br J Dermatol. (2006) 155:301–6. doi: 10.1111/j.1365-2133.2006.07238.x
154. Lee, C-H , Huang, Y-H , and Chu, C-Y . Taiwan dermatological association recommendations for coronavirus disease of 2019 vaccination in patients treated with immunotherapeutics [review article]. Dermatol Sin. (2021) 39:169–74. doi: 10.4103/ds.ds_50_21
155. Luo, C , Geng, C-Z , Tung, Y-H , Wang, B-L , and Tung, T-H . Coronavirus disease 2019 in dermatology practice: perspective of three levels of prevention on public health [review article]. Dermatol Sin. (2022) 40:143–7. doi: 10.4103/ds.ds_33_22
156. Mitamura, Y , Schulz, D , Oro, S , Li, N , Kolm, I , Lang, C, et al. Cutaneous and systemic hyperinflammation drives maculopapular drug exanthema in severely ill COVID-19 patients. Allergy. (2022) 77:595–608. doi: 10.1111/all.14983
157. Yu, C-L , Lin, Y-T , and Chi, C-C . Recommendations on use of systemic treatments for immune-mediated dermatologic disorders in patients with confirmed COVID-19 infection: A rapid review [review article]. Dermatol Sin. (2022) 40:67–70. doi: 10.4103/1027-8117.349030
158. Wu, PC , Huang, IH , Wang, CW , Chung, WH , and Chen, CB . Severe cutaneous adverse reactions after COVID-19 vaccination: A systematic review. Allergy. (2023) 78:1383–6. doi: 10.1111/all.15642
159. O'Connor, T , O'Callaghan-Maher, M , Ryan, P , and Gibson, G . Drug reaction with eosinophilia and systemic symptoms syndrome following vaccination with the AstraZeneca COVID-19 vaccine. JAAD Case Rep. 20:14–6. doi: 10.1016/j.jdcr.2021.11.028
160. Schroeder, JW , Gamba, C , Toniato, A , and Rongioletti, F . A definite case of drug reaction with eosinophilia and systemic symptoms (DRESS) induced by administration of the Pfizer/BioNTech BNT162b2 vaccine for SARS-CoV2. Clin Dermatol. (2022) 40:591–4. doi: 10.1016/j.clindermatol.2022.02.018
161. Saber, M , Faghihi, G , Seyedghafouri, SA , and Hosseini, SM . Mortality and cause of death in patients with dermatologic diseases: an 11-year record-based observational study. Dermatol Sin. (2023) 41:18. doi: 10.4103/ds.DS-D-22-00134
162. Um, SJ , Lee, SK , Kim, YH , Kim, KH , Son, CH , Roh, MS, et al. Clinical features of drug-induced hypersensitivity syndrome in 38 patients. J Investig Allergol Clin Immunol. (2010) 20:556–62.
163. Cabañas, R , Calderon, O , Ramirez, E , Fiandor, A , Prior, N , Caballero, T, et al. Piperacillin-induced DRESS: distinguishing features observed in a clinical and allergy study of 8 patients. J Investig Allergol Clin Immunol. (2014) 24:425–30.
164. Momen, SE , Diaz-Cano, S , Walsh, S , and Creamer, D . Discriminating minor and major forms of drug reaction with eosinophilia and systemic symptoms: facial edema aligns to the severe phenotype. J Am Acad Dermatol. (2021) 85:645–52. Conflicts of interest None disclosed. doi: 10.1016/j.jaad.2021.04.020
165. Tetart, F , Picard, D , Janela, B , Joly, P , and Musette, P . Prolonged evolution of drug reaction with eosinophilia and systemic symptoms: clinical, Virologic, and biological features. JAMA Dermatol. (2014) 150:206–7. doi: 10.1001/jamadermatol.2013.6698
166. Tohyama, M , Hashimoto, K , Yasukawa, M , Kimura, H , Horikawa, T , Nakajima, K, et al. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol. (2007) 157:934–40. doi: 10.1111/j.1365-2133.2007.08167.x
167. Wei, CH , Chung-Yee Hui, R , Chang, CJ , Ho, HC , Yang, CH , Lin, YJ, et al. Identifying prognostic factors for drug rash with eosinophilia and systemic symptoms (DRESS). Eur J Dermatol. (2011) 21:930–7. doi: 10.1684/ejd.2011.1550
168. Mizukawa, Y , Hirahara, K , Kano, Y , and Shiohara, T . Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms severity score: A useful tool for assessing disease severity and predicting fatal cytomegalovirus disease. J Am Acad Dermatol. (2019) 80:670–678.e2. doi: 10.1016/j.jaad.2018.08.052
169. Sharma, AN , Murphy, K , Shwe, S , Miller, M , Mesinkovska, NA , Rojek, NW, et al. Predicting DRESS syndrome recurrence—the ReDRESS score. JAMA Dermatol. (2022) 158:1445–7. doi: 10.1001/jamadermatol.2022.3491
170. Komatsu-Fujii, T , Chinuki, Y , Niihara, H , Hayashida, K , Ohta, M , Okazaki, R, et al. The thymus and activation-regulated chemokine (TARC) level in serum at an early stage of a drug eruption is a prognostic biomarker of severity of systemic inflammation. Allergol Int. (2018) 67:90–5. doi: 10.1016/j.alit.2017.06.001
171. Taylor, L , and Schwarz, H . Identification of a soluble OX40 isoform: development of a specific and quantitative immunoassay. J Immunol Methods. (2001) 255:67–72. doi: 10.1016/S0022-1759(01)00424-0
172. Wongkitisophon, P , Chanprapaph, K , Rattanakaemakorn, P , and Vachiramon, V . Six-year retrospective review of drug reaction with eosinophilia and systemic symptoms. Acta Derm Venereol. (2012) 92:200–5. doi: 10.2340/00015555-1222
173. Lee, HY . Drug reaction with eosinophilia and systemic symptoms (DRESS). Uptodate. (2023). Retrieved June 17, 2023, from https://www.uptodate.com/contents/drug-reaction-with-eosinophilia-and-systemic-symptoms-dress
174. Fernandez-Juarez, G , Perez, JV , Caravaca-Fontán, F , Quintana, L , Shabaka, A , Rodriguez, E, et al. Duration of treatment with corticosteroids and recovery of kidney function in acute interstitial nephritis. Clin J Am Soc Nephrol. (2018) 13:1851–8. doi: 10.2215/CJN.01390118
175. Hu, PF , Wang, PQ , Chen, H , Hu, XF , Xie, QP , Shi, J, et al. Beneficial effect of corticosteroids for patients with severe drug-induced liver injury. J Dig Dis. (2016) 17:618–27. doi: 10.1111/1751-2980.12383
176. Funck-Brentano, E , Duong, TA , Bouvresse, S , Bagot, M , Wolkenstein, P , Roujeau, JC, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. (2015) 72:246–52. doi: 10.1016/j.jaad.2014.10.032
177. Uhara, H , Saiki, M , Kawachi, S , Ashida, A , Oguchi, S , and Okuyama, R . Clinical course of drug-induced hypersensitivity syndrome treated without systemic corticosteroids. J Eur Acad Dermatol Venereol. (2013) 27:722–6. doi: 10.1111/j.1468-3083.2012.04547.x
178. Zhang, ZX , Yang, BQ , Yang, Q , Wu, M , and Wang, GJ . Treatment of drug-induced hypersensitivity syndrome with cyclosporine. Indian J Dermatol Venereol Leprol. (2017) 83:713–7. doi: 10.4103/ijdvl.IJDVL_1084_16
179. Harman, KE , Morris, SD , and Higgins, EM . Persistent anticonvulsant hypersensitivity syndrome responding to ciclosporin. Clin Exp Dermatol. (2003) 28:364–5. doi: 10.1046/j.1365-2230.2003.01267.x
180. Kuschel, SL , and Reedy, MS . Cyclosporine treatment of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a case report and brief review of the literature. Pract Dermatol. (2018) 2018:41–3.
181. Kirchhof, MG , Wong, A , and Dutz, JP . Cyclosporine treatment of drug-induced hypersensitivity syndrome. JAMA Dermatol. (2016) 152:1254–7. doi: 10.1001/jamadermatol.2016.2220
182. Su, HJ , Chen, CB , Yeh, TY , and Chung, WH . Successful treatment of corticosteroid-dependent drug reaction with eosinophilia and systemic symptoms with cyclosporine. Ann Allergy Asthma Immunol. (2021) 127:674–81. doi: 10.1016/j.anai.2021.08.012
183. Nguyen, E , Yanes, D , Imadojemu, S , and Kroshinsky, D . Evaluation of cyclosporine for the treatment of DRESS syndrome. JAMA Dermatol. (2020) 156:704–6. doi: 10.1001/jamadermatol.2020.0048
184. Kito, Y , Ito, T , Tokura, Y , and Hashizume, H . High-dose intravenous immunoglobulin monotherapy for drug-induced hypersensitivity syndrome. Acta Derm Venereol. (2012) 92:100–1. doi: 10.2340/00015555-1168
185. Scheuerman, O , Nofech-Moses, Y , Rachmel, A , and Ashkenazi, S . Successful treatment of antiepileptic drug hypersensitivity syndrome with intravenous immune globulin. Pediatrics. (2001) 107:E14. doi: 10.1542/peds.107.1.e14
186. Santos, RP , Ramilo, O , and Barton, T . Nevirapine-associated rash with eosinophilia and systemic symptoms in a child with human immunodeficiency virus infection. Pediatr Infect Dis J. (2007) 26:1053–6. doi: 10.1097/INF.0b013e318125655d
187. Cumbo-Nacheli, G , Weinberger, J , Alkhalil, M , Thati, N , and Baptist, AP . Anticonvulsant hypersensitivity syndrome: is there a role for immunomodulation? Epilepsia. (2008) 49:2108–12. doi: 10.1111/j.1528-1167.2008.01720.x
188. Singer, EM , Wanat, KA , and Rosenbach, MA . A case of recalcitrant DRESS syndrome with multiple autoimmune sequelae treated with intravenous immunoglobulins. JAMA Dermatol. (2013) 149:494–5. doi: 10.1001/jamadermatol.2013.1949
189. Eshki, M , Allanore, L , Musette, P , Milpied, B , Grange, A , Guillaume, JC, et al. Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms: a cause of unpredictable multiorgan failure. Arch Dermatol. (2009) 145:67–72. doi: 10.1001/archderm.145.1.67
190. Marcus, N , Smuel, K , Almog, M , Prais, D , Straussberg, R , Landau, D, et al. Successful intravenous immunoglobulin treatment in pediatric severe DRESS syndrome. J Allergy Clin Immunol Pract. (2018) 6:1238–42. doi: 10.1016/j.jaip.2017.10.016
191. Joly, P , Janela, B , Tetart, F , Rogez, S , Picard, D , D'Incan, M, et al. Poor benefit/risk balance of intravenous immunoglobulins in DRESS. Arch Dermatol. (2012) 148:543–4. doi: 10.1001/archderm.148.4.dlt120002-c
192. Laban, E , Hainaut-Wierzbicka, E , Pourreau, F , Yacoub, M , Sztermer, E , Guillet, G, et al. Cyclophosphamide therapy for corticoresistant drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome in a patient with severe kidney and eye involvement and Epstein-Barr virus reactivation. Am J Kidney Dis. (2010) 55:e11–4. doi: 10.1053/j.ajkd.2009.10.054
193. Esposito, AJ , Murphy, RC , Toukatly, MN , Amro, OW , Kestenbaum, BR , and Najafian, B . Acute kidney injury in allopurinol-induced DRESS syndrome: a case report of concurrent tubulointerstitial nephritis and kidney-limited necrotizing vasculitis. Clin Nephrol. (2017) 87:316–9. doi: 10.5414/CN108966
194. Shaughnessy, KK , Bouchard, SM , Mohr, MR , Herre, JM , and Salkey, KS . Minocycline-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome with persistent myocarditis. J Am Acad Dermatol. (2010) 62:315–8. doi: 10.1016/j.jaad.2009.05.046
195. Higuchi, M , Agatsuma, T , Iizima, M , Yamazaki, Y , Saita, T , Ichikawa, T, et al. A case of drug-induced hypersensitivity syndrome with multiple organ involvement treated with plasma exchange. Ther Apher Dial. (2005) 9:412–6. doi: 10.1111/j.1744-9987.2005.00320.x
196. Lo, MH , Huang, CF , Chang, LS , Kuo, HC , Chien, SJ , Lin, IC, et al. Drug reaction with eosinophilia and systemic symptoms syndrome associated myocarditis: a survival experience after extracorporeal membrane oxygenation support. J Clin Pharm Ther. (2013) 38:172–4. doi: 10.1111/jcpt.12025
197. Hagiwara, H , Fukushima, A , Iwano, H , and Anzai, T . Refractory cardiac myocarditis associated with drug rash with eosinophilia and systemic symptoms syndrome due to anti-bipolar disorder drugs: a case report. Eur Heart J Case Rep. (2018) 2:yty100. doi: 10.1093/ehjcr/yty100
198. Gschwend, A , Helbling, A , Feldmeyer, L , Mani-Weber, U , Meincke, C , Heidemeyer, K, et al. Treatment with IL5-/IL-5 receptor antagonists in drug reaction with eosinophilia and systemic symptoms (DRESS). Allergo J Int. (2022):1–8. doi: 10.1007/s40629-022-00224-7
199. Ange, N , Alley, S , Fernando, SL , Coyle, L , and Yun, J . Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome successfully treated with mepolizumab. J Allergy Clin Immunol Pract. (2018) 6:1059–60. doi: 10.1016/j.jaip.2017.10.020
200. Thein, OS , Sutton, B , Thickett, DR , and Parekh, D . Mepolizumab rescue therapy for acute pneumonitis secondary to DRESS. BMJ Case Rep. (2019) 12:231355. doi: 10.1136/bcr-2019-231355
201. Kowtoniuk, R , Pinninti, M , Tyler, W , and Doddamani, S . DRESS syndrome-associated acute necrotizing eosinophilic myocarditis with giant cells. BMJ Case Rep. (2018) 2018. doi: 10.1136/bcr-2018-226461 [Epub ahead of print].
202. Rubin, L , Talmon, A , Ribak, Y , Kessler, A , Martin, Y , Haran, TK, et al. Novel targeted inhibition of the IL-5 axis for drug reaction with eosinophilia and systemic symptoms syndrome [original research]. Front Immunol. (2023) 14:1134178. doi: 10.3389/fimmu.2023.1134178
203. Schmid-Grendelmeier, P , Steiger, P , Naegeli, MC , Kolm, I , Claudia Cécile Valérie, L , Maverakis, E, et al. Benralizumab for severe DRESS in two COVID-19 patients. J Allergy Clin Immunol Pract. (2021) 9:481–483.e2. doi: 10.1016/j.jaip.2020.09.039
204. Lang, CCV , Schmid-Grendelmeier, P , Maverakis, E , and Brüggen, MC . Reply to "Benralizumab: A potential tailored treatment for life-threatening DRESS in the COVID-19 era". J Allergy Clin Immunol Pract. (2021) 9:3531–2. doi: 10.1016/j.jaip.2021.06.048
205. Mesli, F , Dumont, M , Soria, A , Groh, M , Turpin, M , Voiriot, G, et al. Benralizumab: A potential tailored treatment for life-threatening DRESS in the COVID-19 era. J Allergy Clin Immunol Pract. (2021) 9:3529–3531.e1. doi: 10.1016/j.jaip.2021.06.047
206. Park, H , Choi, GS , and Lee, EM . Successful treatment of Imatinib-induced DRESS syndrome using Reslizumab without cessation of Imatinib: A case report. Case Rep Oncol. (2021) 14:1548–54. doi: 10.1159/000519471
207. Kim, D , Kobayashi, T , Voisin, B , Jo, JH , Sakamoto, K , Jin, SP, et al. Targeted therapy guided by single-cell transcriptomic analysis in drug-induced hypersensitivity syndrome: a case report. Nat Med. (2020) 26:236–43. doi: 10.1038/s41591-019-0733-7
208. Damsky, WE , Vesely, MD , Lee, AI , Choi, J , Meyer, AC , Chen, M, et al. Drug-induced hypersensitivity syndrome with myocardial involvement treated with tofacitinib. JAAD Case Rep. (2019) 5:1018–26. doi: 10.1016/j.jdcr.2019.07.004
209. Chowdhury, M , Azari, BM , Desai, NR , and Ahmad, T . A novel treatment for a rare cause of cardiogenic shock. JACC Case Rep. (2020) 2:1461–5. doi: 10.1016/j.jaccas.2020.02.004
210. Morris, R , Kershaw, NJ , and Babon, JJ . The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. (2018) 27:1984–2009. doi: 10.1002/pro.3519
211. Lé, AM , and Torres, T . OX40-OX40L inhibition for the treatment of atopic dermatitis-focus on Rocatinlimab and Amlitelimab. Pharmaceutics. (2022) 14:2753. doi: 10.3390/pharmaceutics14122753
Keywords: drug reaction with eosinophilia and systemic symptoms, DIHS, hypersensitivity, eosinophilia, HHV-6
Citation: Chen C-B, Hung W-K, Wang C-W, Lee C-C, Hung S-I and Chung W-H (2023) Advances in understanding of the pathogenesis and therapeutic implications of drug reaction with eosinophilia and systemic symptoms: an updated review. Front. Med. 10:1187937. doi: 10.3389/fmed.2023.1187937
Received: 16 March 2023; Accepted: 05 June 2023;
Published: 29 June 2023.
Edited by:
Yen Loo Lim, National Skin Centre, SingaporeReviewed by:
Irina Khamaganova, Pirogov Russian National Research Medical University, RussiaCopyright © 2023 Chen, Hung, Wang, Lee, Hung and Chung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Hung Chung, d2VuaHVuZ2NodW5nQHlhaG9vLmNvbQ==; Y2h1bmcxQGNnbWgub3JnLnR3
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.