- 1Division of Hematology and Oncology, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan
- 2Division of Neurology, China Medical University Hospital, Taichung, Taiwan
- 3Eye Center, China Medical University Hospital, Taichung, Taiwan
- 4China Medical University, Taichung, Taiwan
Hematopoietic stem cell transplantation (HSCT) recipients affected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have a high mortality rate. The American Society of Transplantation and Cellular Therapy (ASTCT) and the European Society for Blood and Marrow Transplantation (EBMT) recommend vaccination for these vulnerable populations. However, emerging data suggested that vaccination might elicit immunological adverse events, including an exacerbation of graft-vs.-host disease (GVHD). Herein, we report a case of severe optic neuritis developed shortly after AstraZeneca COVID-19 vaccination in an allogeneic HSCT recipient with underlying chronic GVHD. The patient had a headache 5 days after vaccination, and the disease progressed rapidly to complete blindness 17 days after the vaccination. The diagnosis of optic neuritis was well-confirmed by the presence of an anti-myelin oligodendrocyte glycoprotein antibody and the typical features of MRI image and Ophthalmoscopy. Other differential diagnoses, such as infection or leukemia relapse in the central nervous system (CNS), were carefully excluded. A timely high-dose corticosteroid was administered, and her visual acuity improved rapidly. She returned to her baseline status 1 month later. With more than 1 year of follow-up, no optic neuritis or leukemia relapse was observed. In summary, allogeneic transplant recipients can develop severe optic neuritis after vaccination. Optic neuritis can be an exacerbation of GVHD or rarely a sporadic adverse event of vaccination. Furthermore, our experience indicates that a prompt diagnosis and early steroid treatment are vital for a good recovery.
Introduction
Hematopoietic stem cell transplantation (HSCT) recipients have a higher mortality rate after getting COVID-19. According to a meta-analysis of 14 studies, COVID-19-related mortality was around 4% in the general population before the introduction of vaccination (1). However, the overall survival at 30 days after diagnosis of COVID-19 was 68 and 67% for recipients of allogeneic and autologous HSCT, respectively (2). Vaccination against COVID-19 is thus recommended by major societies such as the American Society of Hematology—American Society of Transplantation and Cellular Therapy (ASH-ASTCT) and the European Society for Blood and Marrow Transplantation (EBMT). Nevertheless, three studies have reported a risk of eliciting or worsening graft-vs.-host disease (GVHD) after the administration of mRNA vaccines (3–5). The safety data of AstraZeneca (AZ) adenoviral vaccine on the HSCT recipients are sparse. Herein, we introduce a rare case of anti-myelin oligodendrocyte glycoprotein (anti-MOG) antibody-positive optic neuritis developed shortly after AZ vaccination in a recipient of allogeneic HSCT with underlying chronic GVHD (cGVHD).
Case description
A 60-year-old female presented to the clinic with a history of acute onset of visual loss 17 days after receiving the first dose of the AstraZeneca (AZ) COVID-19 vaccine. She underwent a matched unrelated donor peripheral blood stem cell transplant for AML in 2009. Mild cGVHD involving only the lacrimal gland (sicca syndrome) developed after the transplant, and she needs a regular artificial tear for symptom control. Neither topical steroids nor systemic immunosuppressive agents were used. The patient received the first dose of the AZ vaccine during the COVID-19 pandemic (the Day of vaccination is defined as Day 0). She suffered from intermittent headaches since Day 5, followed by bilateral periorbital pain on Day 14. Blurred vision of left eye developed on Day 15 and rapidly progressed to right eye on Day 17 with total blindness of left eye. Outside the ocular problem, the patient did not have other neurological symptoms, such as focal weakness or sensory deficit. There were also no fever, joint pain, and other symptoms suggesting infectious or rheumatological diseases.
The nasopharyngeal swab PCR for COVID-19 was negative. Ophthalmoscopic examination on Day 18 disclosed left optic disk papilledema with retinal hemorrhage (Figure 1A). Contrast brain MRI revealed swelling of the left optic nerve with increased enhancement, compatible with optic neuritis (Figure 1B). A cerebrospinal fluid (CSF) study showed no pleocytosis (WBC 5 cells/μL). The IgG index was 0.64 (normal range 0.3–0.7), and the micro-albumin was 27.0 mg/dL (normal range 10–30 mg/dL). Microbiological studies of CSF using FilmArray Meningitis/Encephalitis panel (BioFire Diagnostics, Salt Lake City, UT), which detects Escherichia coli, Haemophilus influenzae, Listeria monocytogenes, Neisseria meningitidis, Streptococcus agalactia and streptococcus pneumoniae, Cytomegalovirus, Enterovirus, Herpes simplex virus (HSV)-1, HSV-2, Human herpesvirus-6, Human parechovirus, Varicella zoster virus, and Cryptococcus, were all negative. Bone marrow biopsy showed no leukemia relapse. Whole-body positron emission tomography/computed tomography scan revealed a negative finding. Anti-aquaporin-4 antibody was negative, and the anti-MOG antibody (cell-based immunofluorescence test) was positive.
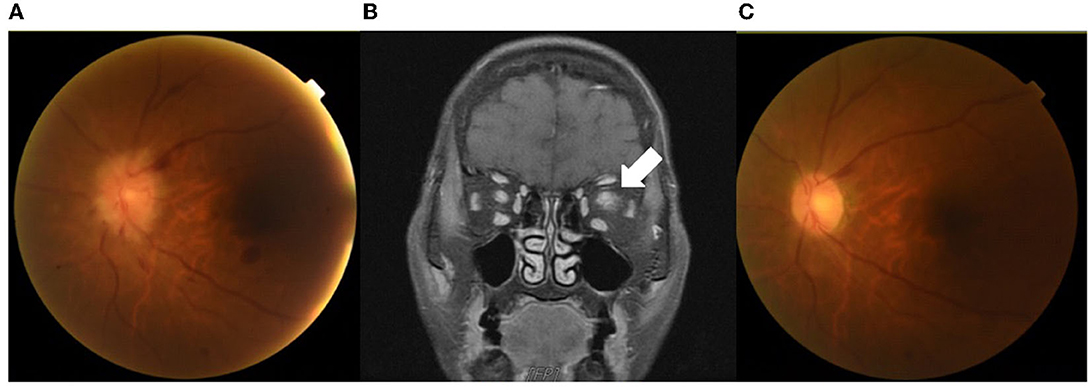
Figure 1. Images of contrast MRI and ophthalmoscopy. An ophthalmoscopic examination on Day 18 showed left optic disk papilledema with retinal hemorrhage (A). Brain MRI after gadolinium administration on the same day showed swelling of the left optic nerve with increased enhancement (arrow), compatible with optic neuritis (B). An ophthalmoscopic examination on Day 45 showed the resolution of papilledema and retinal hemorrhage (C).
The patient received pulse therapy with methylprednisolone 250 mg daily from Day 19 to 21, then switched to oral prednisolone 1 mg/kg from Day 22. Her visual acuity improved rapidly and has almost returned to her baseline status on Day 28. Repeated ophthalmoscopic examination on Day 42 shows no papillary or retinal edema. The prednisolone was tapered off gradually over the next 4 months. We have followed the patient for over a year, and no optic neuritis or leukemia relapse has occurred. The patient reported that the ocular condition is now the same as before the vaccination. However, using artificial tears of roughly the same frequency remains needed to control dry eyes.
Discussion
Optic neuritis is a rare immune-mediated neurological disturbance. While ocular involvement is commonly seen in patients with cGVHD, the process is most often limited to the anterior eye segment. Few cases of cGVHD with the presentation of optic neuritis have been reported, but none were associated with vaccination (Table 1) (6–9). Besides, our case is the first report of documented anti-MOG positivity in this setting, which further supports the attacks of immune-mediated demyelination targeting optic nerves.
The safety issue of vaccination for patients with active GVHD is unclear because most clinical trials likely exclude them. According to a retrospective cohort study that enrolled 34 patients diagnosed with cGVHD and received two doses of mRNA SARS-CoV-2 vaccine during the COVID-19 pandemic, nine (26.5%) experienced worsening of their cGVHD symptoms after vaccination (10). All these nine patients received the Pfizer mRNA vaccine, and the most common organs involved are the skin, mouth, and eyes. Ali et al. also reported a worsening of cGVHD in 3.5% and new GVHD in 9.7% of allogeneic HSCT recipients who received Pfizer (BNT162b2) or Moderna (mRNA-1273) vaccines (10). Our case is unique in its provoked by the vector-based AZ vaccine and the unusual site of immune attack. The exact mechanism of vaccination-associated cGVHD remains unknown, although significantly higher cytokine (Interleukin-18, Angiopoietin-2, Interferon-gamma) were observed in patients with suspected cGVHD aggravation after vaccination (11). On the other hand, sporadic cases of anti-MOG positive optic neuritis after the AZ COVID-19 vaccine had been reported in non-transplant patients. However, most of these cases showed multiple site involvements or combined with myelitis or extensive encephalomyelitis rather than isolated optic neuritis (12). Whether the optic neuritis seen in this patient is a GVHD aggravation or a new, vaccination-associated event is not very critical because the treatment for both conditions is likely the same. A prompt diagnosis and early corticosteroid treatment are even more important. In the current case, we used a high-dose steroid as the initial treatment, which aligns with the recommendation for treating idiopathic optic neuritis with significant visual loss (13). The patient got a robust and sustained response, and there was no recurrence of neuritis after discontinuation of steroid.
In conclusion, although SARS-CoV-2 vaccination is generally considered safe and recommended for allogeneic HSCT patients, severe optic neuritis could develop due to GVHD exacerbation or vaccination itself. Careful monitoring after vaccination remains warranted, especially in patients with active GVHD. A quick diagnosis and early administration of high dose steroid can rapidly restore visual acuity.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
S-KJ and Y-CS conducted the clinical consultation and diagnostics. CC was involved in patient care and wrote the first draft. S-PY was involved in patient care and revised the manuscript. All authors have read and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Parohan M, Yaghoubi S, Seraji A, Javanbakht MH, Sarraf P, Djalali M. Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Aging Male. (2020) 23:1416–24. doi: 10.1080/13685538.2020.1774748
2. Sharma A, Bhatt NS, Martin AS, Abid MB, Bloomquist J, Chemaly RF, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. (2021) 8:e185–93. doi: 10.1016/S2352-3026(20)30429-4
3. Ali H, Ngo D, Aribi A, Arslan S, Dadwal S, Marcucci G, et al. Safety and tolerability of SARS-CoV2 emergency-use authorized vaccines for allogeneic hematopoietic stem cell transplant recipients. Transplant Cell Ther. (2021) 27:938.e1–e6. doi: 10.1016/j.jtct.2021.07.008
4. Ram R, Hagin D, Kikozashvilli N, Freund T, Amit O, Bar-On Bar-On Y. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy-a single center prospective cohort study. Transplant Cell Ther. (2021) 27:788–94. doi: 10.1016/j.jtct.2021.06.024
5. Bergman P, Blennow O, Hansson L, Mielke S, Nowak P, Chen P, et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. EBioMedicine. (2021) 74:103705. doi: 10.1016/j.ebiom.2021.103705
6. Diamanti L, Franciotta D, Berzero G, Bini P, Farina LM, Colombo AA, et al. Late post-transplant anti-aquaporin-4 Ab-positive optic neuritis in a patient with AML. Bone Marrow Transplant. (2015) 50:1125–6. doi: 10.1038/bmt.2015.84
7. Moesen I, Kidd DP. Bilateral inflammatory optic neuropathy related to graft versus host disease following allogeneic bone marrow transplantation for Hodgkin disease. Neuroophthalmology. (2014) 38:224–9. doi: 10.3109/01658107.2014.907321
8. Matsuo Y, Kamezaki K, Takeishi S, Takenaka K, Eto T, Nonami A, et al. Encephalomyelitis mimicking multiple sclerosis associated with chronic graft-versus-host disease after allogeneic bone marrow transplantation. Int Med. (2009) 48:1453–6. doi: 10.2169/internalmedicine.48.2003
9. Ooi J, Takahashi S, Tajika K, Tojo A, Tani K, Asano S. Immune-mediated optic neuritis after unrelated allogeneic bone marrow transplantation. Blood. (1998) 91:2619–20. doi: 10.1182/blood.V91.7.2619.2619_2619_2620
10. Trunk AD, Shewan SK, Lee CJ, Parker CJ, Couriel DR. Chronic graft-versus-host disease exacerbation after SARS-CoV-2 vaccination. Bone Marrow Transplant. (2022) 57:502–3. doi: 10.1038/s41409-021-01543-z
11. Pabst C, Benning L, Liebers N, Janssen M, Caille L, Speer C, et al. Humoral responses and chronic GVHD exacerbation after COVID-19 vaccination post allogeneic stem cell transplantation. Vaccines. (2022) 10:330. doi: 10.3390/vaccines10020330
12. Jarius S, Bieber N, Haas J, Wildemann B. MOG encephalomyelitis after vaccination against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2): case report and comprehensive review of the literature. J Neurol. (2022) 269:5198–212. doi: 10.1007/s00415-022-11194-9
Keywords: vaccine, graft-vs.-host disease, hematopoietic stem cell transplant, optic neuritis, COVID-19
Citation: Chu C, Jiang S-K, Shao Y-C and Yeh S-P (2023) Case report: Sudden onset optic neuritis shortly after SARS-CoV-2 vaccination in an allogeneic hematopoietic stem cell transplant recipient with chronic graft-vs.-host disease. Front. Med. 10:1177610. doi: 10.3389/fmed.2023.1177610
Received: 01 March 2023; Accepted: 08 May 2023;
Published: 20 June 2023.
Edited by:
Kris Michael Mahadeo, Duke University, United StatesReviewed by:
Natalie Dailey Garnes, University of Texas MD Anderson Cancer Center, United StatesSabah Kalyoussef, Saint Peter's University Hospital, United States
Mira Kohorst, Mayo Clinic, United States
Copyright © 2023 Chu, Jiang, Shao and Yeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Su-Peng Yeh, c3VwZW5neWVoQGdtYWlsLmNvbQ==
 Chiang Chu
Chiang Chu Shin-Kuang Jiang2
Shin-Kuang Jiang2 Su-Peng Yeh
Su-Peng Yeh