
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med., 17 July 2023
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1175827
Objective: This study aimed to assess the efficacy and safety of Chinese herbal medicine (CHM) plus conventional western medicine (CWM) in comparison with CWM against COVID-19.
Methods: We searched eight electronic databases and three trial registers spanning from January 1, 2020 to May 18, 2023. We included randomized controlled trials (RCTs) comparing the effectiveness and safety of CHM plus CWM and CWM against COVID-19 in our study. The Cochrane Risk of Bias tool 2.0 (RoB2) was applied to evaluate the methodological quality of the included RCTs. The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system was employed to assess the certainty of evidence. Statistical analysis was implemented in R version 4.1.2.
Results: Our study included 50 RCTs involving 11,624 patients. In comparison with sole CWM, CHM plus CWM against COVID-19 significantly enhanced clinical effective rate (RR = 1.18, 95% CI [1.13, 1.22]), improved chest image (RR = 1.19, 95% CI [1.11, 1.28]), inhibited clinical deterioration (RR = 0.45, 95% CI [0.33, 0.60]), lowered mortality (RR = 0.53, 95% CI [0.40, 0.70]), and reduced the total score of TCM syndrome (SMD = −1.24, 95% CI [−1.82, −0.66]). SARS-CoV-2 nucleic acid conversion time (MD = −2.66, 95% CI [−3.88, −1.44]), duration of hospitalization (MD = −2.36, 95% CI [−3.89, −0.82]), and clinical symptom (fever, cough, fatigue, and shortness of breath) recovery times were shorter in CHM plus CWM groups than in CWM groups. Further, CHM plus CWM treatment was more conducive for some laboratory indicators returning to normal levels. No statistical difference was found in the incidence of total adverse reactions between the two groups (RR = 0.97, 95% CI [0.88, 1.07]). We assessed the risk of bias for 246 outcomes, and categorized 55 into “low risk”, 151 into “some concerns”, and 40 into “high risk”. Overall, the certainty of the evidence ranged from moderate to very low.
Conclusions: Potentially, CHM listed in this study, as an adjunctive therapy, combining with CWM is an effective and safe therapy mode for COVID-19. However, more high-quality RCTs are needed to draw more accurate conclusions.
Clinical trial registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=293963.
The coronavirus disease 2019 (COVID-19) is a respiratory infectious disease that poses a severe threat to human health, caused by a novel coronavirus (SARS-CoV-2) (1). Since December 2019, when a large number of COVID-19 cases were detected in Wuhan, China, COVID-19 has evolved and spread rapidly worldwide (2). On March 11, 2020, due to the rapid transmission of the virus and the continued increase in confirmed cases, the World Health Organization (WHO) classified the current 2019 coronavirus disease outbreak as a global pandemic (3). At present, the global situation is still quite grim, and COVID-19 remains a threat to human health.
As the SARS-CoV-2 transmits from person to person, the virus is still in the process of evolution. Based on the impact of variants on transmission, disease severity, and capacity for immune escape, WHO has designated five variants as SARS-CoV-2 Variants of Concern (VOC). In addition, the “Omicron” variant, rampant across the world, is unlikely to be the final VOC (4, 5). The original SARS-CoV-2 and the new variants have posed enormous challenges and threats to global epidemic prevention and control. Scientific research has led to the development of vaccines (6), monoclonal antibodies, biologically active natural products (7–9), and small molecule formulations (10), to the extent that significant progress has been made in mitigating the threat of COVID-19. However, there is still no specific and effective drug to eliminate the virus, and conventional therapy for COVID-19 is mainly symptomatic and supportive treatment by Western medicine (11, 12). In addition, COVID-19 vaccines cannot stop the pandemic completely, as some people will still be infected after vaccination (13). From previous clinical experience, CHM is an option to combat various infectious diseases, such as SARS (14), influenza (15), and Ebola (16). During the early stage of COVID-19 outbreak in China, when the disease was not well-understood and no vaccine was available, Chinese doctors employed CHM to treat COVID-19 and achieved remarkable clinical effects (17). CHM was still widely applied in China for the treatment of COVID-19 patients. The combination of CHM and conventional western medicine (CWM) has been used in 92% of diagnosed COVID-19 cases, and more than 90% of patients received significant therapeutic effects (18). For patients with mild and moderate disease, early CHM treatment could be effective in preventing the progression to severe or critical cases. A lot of clinical practices have shown that early CHM intervention in patients with COVID-19 improved clinical cure rate, delayed disease progression, and lowered the risk of death (19). In the face of this epidemic, a range of Chinese herbal medicines have been recognized as very promising anti-SARS-CoV-2 agents, including active ingredients, monomer preparations, crude extracts, and formulas. All these agents have potential activity against SARS-CoV-2 and have attracted significant attention due to their activities both in vitro and in clinical practice (20). Therefore, CHM therapy has been included in the diagnosis and treatment guidelines for COVID-19 in China. Many CHMs, such as Qingfei Paidu Decoction (Granules), Jinhua Qinggan Granules, Lianhua Qingwen Capsules, and Xuanfei Baidu Granules, were proposed as adjunctive medicines for the treatment of COVID-19 (21).
Relevant systematic reviews concerning CHM efficacy assessment have been published, but with limitations (22–29). Some articles incorporated non-RCTs (e.g., observational studies), or pooled the data from non-RCTs and RCTs together (22, 23). Some articles only centered on a certain type of CHM, while others only focused on mild and moderate participants (18–20). Many articles didn't assess the certainty of evidence (23–25, 27, 28). Some failed to address the risk of bias for each outcome due to the inappropriate use of Cochrane Risk of Bias Tool 2.0 (RoB2) (22, 26, 29). A previous systematic review has only pooled the outcomes with low risk of bias, which could lead to omission of evidence (30). Additionally, some clinical trials employing new CHMs for the treatment of COVID-19 have been published, and the best evidence are in the process of constant change. Currently, no systematic review including data on patients infected with SRAS-CoV-2 Omicron variant has been published. Therefore, building upon published clinical studies, we conducted an assessment on the clinical indicators and the certainty of clinical evidence on the effectiveness and safety of CHM in the treatment of COVID-19 in this systematic review and meta-analysis. In the present study, we summarized the available evidence for CHM as an adjunctive treatment for COVID-19.
Our systematic review was reported in compliance the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement (31). Our study was registered on International Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42021293963.
Inclusion criteria: (a) participants included confirmed COVID-19 patients (including asymptomatic patients), regardless of age, gender, ethnicity or severity of the disease; (b) participants in the intervention group received CHM plus CWM treatments, regardless of dosage, dosage forms, components, administration frequency and administration method; (c) participants in the control group received CWM treatments alone or CWM plus placebo treatments; (d) the study reported our outcome of interest; and (e) only randomized controlled trials (RCTs) were included in our systematic review.
Exclusion criteria: Studies including acupuncture, moxibustion, cupping therapy, massage, qigong therapy and music therapy as well as cohort studies, case-control studies, cross-sectional studies, case reports, clinical experiences, interviews, comments, letters, abstracts, and animal experiments were excluded.
Researchers Z.M. and Y.Z. searched eight databases, including PubMed, Embase, the Cochrane Library, Web of Science, Chinese National Knowledge Infrastructure Database (CNKI), Chinese Science and Technology Journals Database (VIP), Chinese Biomedical Literature Database (CBM) and Wanfang Database. Chinese Clinical Trial Registration Center (ChiCTR), WHO International Clinical Trials Registry Platform (ICTRP), and ClinicalTrials.gov were also searched, ranging from January 1, 2020 to May 18, 2023, without any language and nationality limitations. Taking “PubMed” for example, literature search was conducted by means of a combination of MeSH terms and free-text terms. Specific search strategies were modified based on the characters of different databases and registers. All of the search strategies were listed in Supplementary Table S1.
Four reviewers (S.Y., Y.Y., Y.Z., and J.L.) were divided into two groups. All literature was divided into two parts and screened by two groups respectively. Then each of the researchers within the two groups made study selection by reading the title, abstract and full text independently in NoteExpress version 3.5. Any disagreements in results collation were settled by discussion or consulting to a third reviewer (J.H.).
Data were extracted independently by two reviewers (SY and YY) from the included studies by means of a pre-designed data collection form. Any discrepancies between the two reviewers were settled by discussion or consulting to a third reviewer (FW). Moreover, all data collected were re-checked to ensure their accuracy. The extracted data included: (a) basic information (title, first author's name, and publication year); (b) study details (study design, original places of participants, sample size, severity condition of participants, age, and gender of participants); (c) interventions and controls (CHM, CWM, administration frequency, administration methods, dosage, components, and treatment duration); and (d) outcome measures and adverse reactions. If continuous data were reported as medians and interquartile ranges (IQRs), they would be converted into mean and SD values by mathematical methods, or obtained from other published meta-analyses (32, 33). In case of missing or incomplete information in outcome data, we contacted the corresponding authors via email. If no response was made, the data were re-calculated through the plots digitizer software; otherwise, the data would be excluded.
We selected the outcomes in line with a core outcome set for COVID-19 based on traditional Chinese and Western medicine, as well as advice from clinicians (34). Due to the discrepancy between outcomes reported in the included original studies and our pre-determined outcomes, we adjusted and updated our registration on PROSPERO (on April 4, 2022). The primary outcomes were clinical effective rate and SARS-CoV-2 nucleic acid conversion time, while the secondary outcomes included chest image improvement, duration of hospitalization, condition of disease conversion, death, clinical symptoms recovery time (fever, cough, fatigue, and shortness of breath), total score of traditional Chinese medicine (TCM) syndrome, laboratory indicators, and adverse reactions. The conditions of disease were classified into mild, moderate, severe, or critical. The clinical classification was based on the protocol issued by the National Health Commission of the People's Republic of China, as follows, (1) mild cases: the clinical symptoms were mild and there were no signs of pneumonia on images; (2) moderate cases: COVID-19-related clinical manifestations such as fever and/or respiratory symptoms, and there were radiological findings of pneumonia; (3) severe cases: respiratory distress (respiratory rate ≥ 30 breaths/min), oxygen saturation ≤ 93% on air intake at rest, arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤ 300 mmHg (1 mmHg = 0.133 kPa), or chest imaging showing significant lesion progression within 24 to 48 h > 50%; and (4) critical cases: respiratory failure requiring mechanical ventilation, shock, or other organ failures requiring intensive care unit (ICU) care (21).
RoB2 was employed to assess the risk of bias of each outcome included in the meta-analysis in 5 domains: randomization process, deviation from intended intervention, missing outcome data, outcome measurement, and selection of the reported result (35). Two Signaling questions in the five domains were answered by two reviewers (ZM and YZ) separately, and the discrepancies were resolved by discussion or consulting to a third reviewer (LT). Each domain was categorized as “low risk”, “some concerns” or “high risk”. When all five domains were assessed as “low risk”, the overall bias of the outcome was considered as “low risk”.
The R (version 4.1.2) was used to implement the statistical analysis (36). Considering the heterogeneity in different CHM interventions, we selected the random-effects model for the meta-analysis. The risk ratio (RR) with 95% confidence interval (CI) was evaluated for dichotomous outcomes (e.g., clinical effective rate). The mean difference (MD) with 95% CI was evaluated for continuous outcomes (e.g., SARS-CoV-2 nucleic acid conversion time). Whereas, due to the different rating scales, the total score of TCM syndrome was pooled by using the standardized mean difference (SMD). A significant difference was considered when P < 0.05. The I2 statistic was used to evaluate statistical heterogeneity between studies, and the values of 25, 50, and 75% signified the level of low, moderate, and high heterogeneity, respectively (37). Subgroup and sensitivity analysis were performed to search for sources of heterogeneity and test the robustness of the synthesized results. Egger's test and contour-enhanced funnel plots were accepted to evaluate the publication bias (38, 39). If a quantitative meta-analysis was not feasible, we conducted a qualitative analysis to show differences.
The certainty of evidence was assessed through the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) system (40), which is downgraded for study limitations, inconsistency of results, indirectness of evidence, imprecision, and reporting bias. Optimal information size (OIS) is the number of patients required for an adequately powered trial. OIS was estimated assuming a type I error (α) of 0.05 and power of test (1 – β) of 0.8. If the number of observations did not satisfy the OIS principle, the certainty would potentially be downgraded due to imprecision. If the 95% CI overlaps the no-effect value (RR = 1.0), the certainty of evidence may be downgraded regardless of the OIS (41). The certainty of evidence was classified as high, moderate, low, and very low.
A total of 1,800 studies were selected from eight databases and three registers. A total 773 of which were deleted because of duplication, and 933 were excluded for their titles and abstracts. In 94 studies were assessed for eligibility by reading the full texts, and 44 of which were excluded for the following reasons: (a) duplicated (n = 6); (b) non-RCT (n = 8); (c) non-CHM (n = 7); (d) non-COVID-19 patients (n = 12); (e) inconsistent interventions (n = 5); (f) animal trails (n = 1); (g) CHM in two arms (n = 5). Our systematic review included 50 studies, 49 of which were included in the meta-analysis (Figure 1), 21 were in English, while the rest were in Chinese. Publication times (online) ranged from 2020 to 2023. The funding sources for the included studies were summarized in Supplementary Table S2.
The details of the study design and participants of included studies are shown in Table 1. A total of 11,624 participants were randomized, 11,377 of whom were included in the final analysis, the others were rejected for drop-out, refusing medication, violation of the protocol, or for other reasons. The sample size of included studies ranged from 12 to 3,243, and all of them were from China. The severity of disease in participants was categorized into the level of low, moderate, severe, or critical. One of the studies enrolled asymptomatic COVID-19 patients (42). Among the 50 included studies, 32 were single-center studies, 17 were multi-center studies, and 1 did not report the study design. CHM plus CWM treatment was used in intervention groups and the same CWM treatment in control groups for all of the 50 studies. Information of the name, detailed dosage, use frequency, and administration method of medicine was shown in Table 2. Duration of treatment ranged from 5 to 21 days. In the treatment, 29 RCTs employed Chinese patent medicine, 19 used CHM formulas, and 2 used a combination of both. Totally, CHMs in the 50 included studies have 7 dosage forms, including powder, pill/tablet, decoction, oral liquid, granule, capsule, and injection. The following CHM prescriptions were repeated in the included studies: Lianhua Qingwen capsule (granule) (LHQW), Lianhua Qingke capsule (tablet) (LHQK), Jinhua Qinggan granule (JHQG), Xuebijing injection (XBJ), Huashi Baidu granule (HSBD), Qingfei Paidu decoction (QFPD), Jinyinhua oral liquid (JYH), Maxing Shigan decoction (MXSG), Buzhong Yiqi decoction (BZYQ), and so on. Names of Chinese botanical drugs were consulted in the Chinese pharmacopoeia version 2020 (https://db.ouryao.com) and https://mpns.science.kew.org, and the detailed information of the specific components and contents of CHM prescriptions was shown in Supplementary Table S3. A total of 130 Chinese botanical drugs were used in all CHMs, with Gancao (Glycyrrhiza uralensis Fisch. ex DC.) being the most frequently used option. Botanical drugs with use frequency over ten times were shown in Figure 2.
The assessment results of the risk of bias are shown in Figure 3 and Supplementary Table S4. Totally, 246 outcomes were assessed in 50 studies, a majority of which was at a moderate level of risk of bias: 55 outcomes (22.4%) were categorized into “low risk”, 151 (61.4%) into “some concerns” and 40 (16.3%) into “high risk”.
• Bias arising from the randomization process
As 29 studies failed to mention their allocation concealment implementation, 144 outcomes were determined to be “some concerns” in this domain. Additionally, three studies (43–45) grouped the participants using inappropriate randomization methods, as a result, 9 outcomes were assessed as “high risk” in the three studies.
• Bias due to deviations from intended interventions
Three studies reported deviations from intended interventions, or estimated the effects of assignment to intervention by inappropriate analysis (46–48); therefore, 17 outcomes were assessed as being of “some concerns”. Four studies deviated from the intended intervention on a considerable extent (49–52), leading to imbalances between the two arms, consequently, 14 outcomes were determined to be “high risk”. As failure to appropriately analyze participants in the groups may impose substantial impacts on the results, the results “death” in one study (48) and “conversion to severe cases” in another (46) were evaluated as “high risk” in this domain.
• Bias due to missing outcome data
In total of 19 outcomes were determined to be “some concerns” in the domain of missing outcome data, due to 4 studies failed to report the complete or near complete outcome data from all participants (46, 47, 53, 54). Seventeen outcomes were classified as “high risk” in 8 studies, as missing data in the outcomes might depend on their true values (50, 53–59).
• Bias in measurement of the outcome
As no blind methods were adopted or no information about blind methods were mentioned in 31 studies, 81 outcomes were identified to be “some concerns” in the domain of outcome measurement. Despite of the fact that the participants were aware of the interventions they received, some objective outcomes data was free from influence. Thus, these outcomes were evaluated as “low risk” in this domain.
• Bias in selection of the reported result
The majority of the outcomes were evaluated as “low risk” in the domain of selection of the reported result. A total of 43 outcomes in 9 studies were assessed as being of “some concerns” in this domain (46, 47, 50, 55, 56, 60–63).
The clinical effective rate is defined as [(the number of cured participants in each group + the number of improved participants in each group)/the total number of participants in each group] × 100%. Among the 26 studies that reported the clinical effective rate, 4 were assessed as “low risk” (58–67), 20 as “some concerns” (56, 68–81) and 2 as “high risk” (44, 49). CHM formulas were given in 13 studies (58, 60–63, 68, 70, 71, 73, 74, 79–81), Chinese patent medicine in 12 studies (44, 49, 56, 64–67, 69, 75–78), and a combination of both in 1 study (72). A total of 1,675 participants involved in CHM plus CWM groups and 1,495 in CWM groups. The meta-analysis results indicated that the clinical effective rate of CHM plus CWM treatment groups was better than that of CWM treatment groups (RR = 1.18, 95% CI [1.13, 1.22], I2 = 28%, P < 0.0001, low certainty) (Figure 4A).
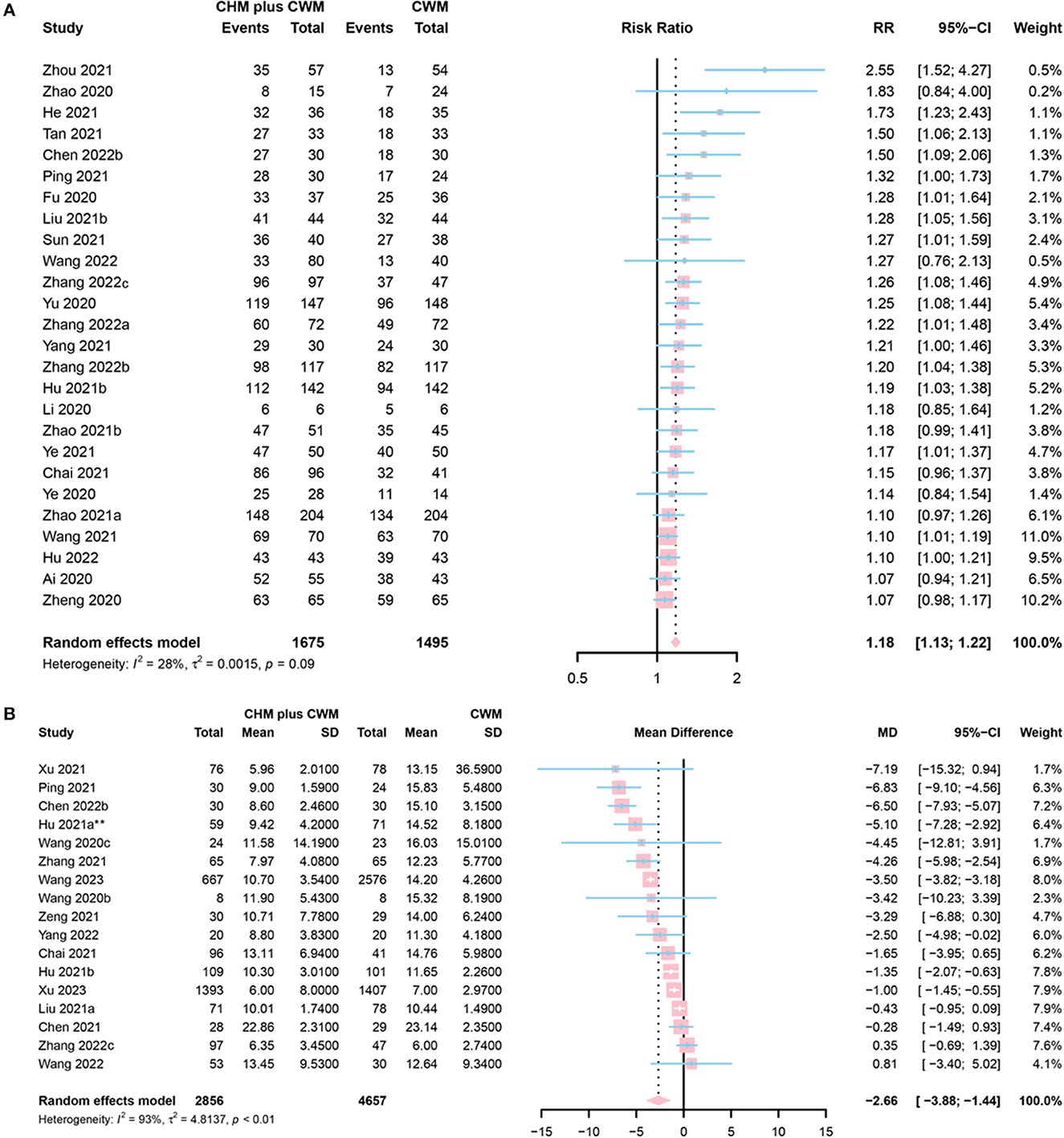
Figure 4. Forest plot of (A) clinical efficacy, and (B) SARS-CoV-2 nucleic acid conversion time. *Data from the 60 mL CHM group were included in the meta-analysis.
A total of 17 studies evaluated SARS-CoV-2 nucleic acid conversion time. Five of them were assessed as “low risk” (59, 65, 82–84), 8 as “some concerns” (41, 42, 46, 48, 60, 61, 63, 85), and 4 as “high risk” (43, 45, 56, 65). Among them, 16 studies enrolled symptomatic cases (41, 43, 45, 46, 48, 56, 59–61, 63, 65, 82–85) and the rest one (42) evaluated nucleic acid conversion time in asymptomatic patients with COVID-19. A total of 2,856 participants involved in CHM plus CWM groups, and 4,657 involved in CWM groups. The results of the meta-analysis showed that CHM plus CWM groups had a shorter SARS-CoV-2 nucleic acid conversion time than those in CWM group (MD = −2.66, 95% CI [−3.88, −1.44], I2 = 93%, P < 0.0001, low certainty) (Figure 4B).
A total of 11 studies mentioned chest image improvement. Two studies were assessed as “low risk” (50, 82), 7 as “some concerns” (42, 48, 76, 77, 80, 86, 97) and 2 as “high risk” (52, 58). A total of 618 participants involved in CHM plus CWM groups, and 594 in CWM groups. The meta-analysis revealed a significantly increasing chest image improvement in CHM plus CWM groups (RR = 1.19, 95% CI [1.11, 1.28], I2 = 0%, P < 0.0001, moderate certainty) (Figure 5A).
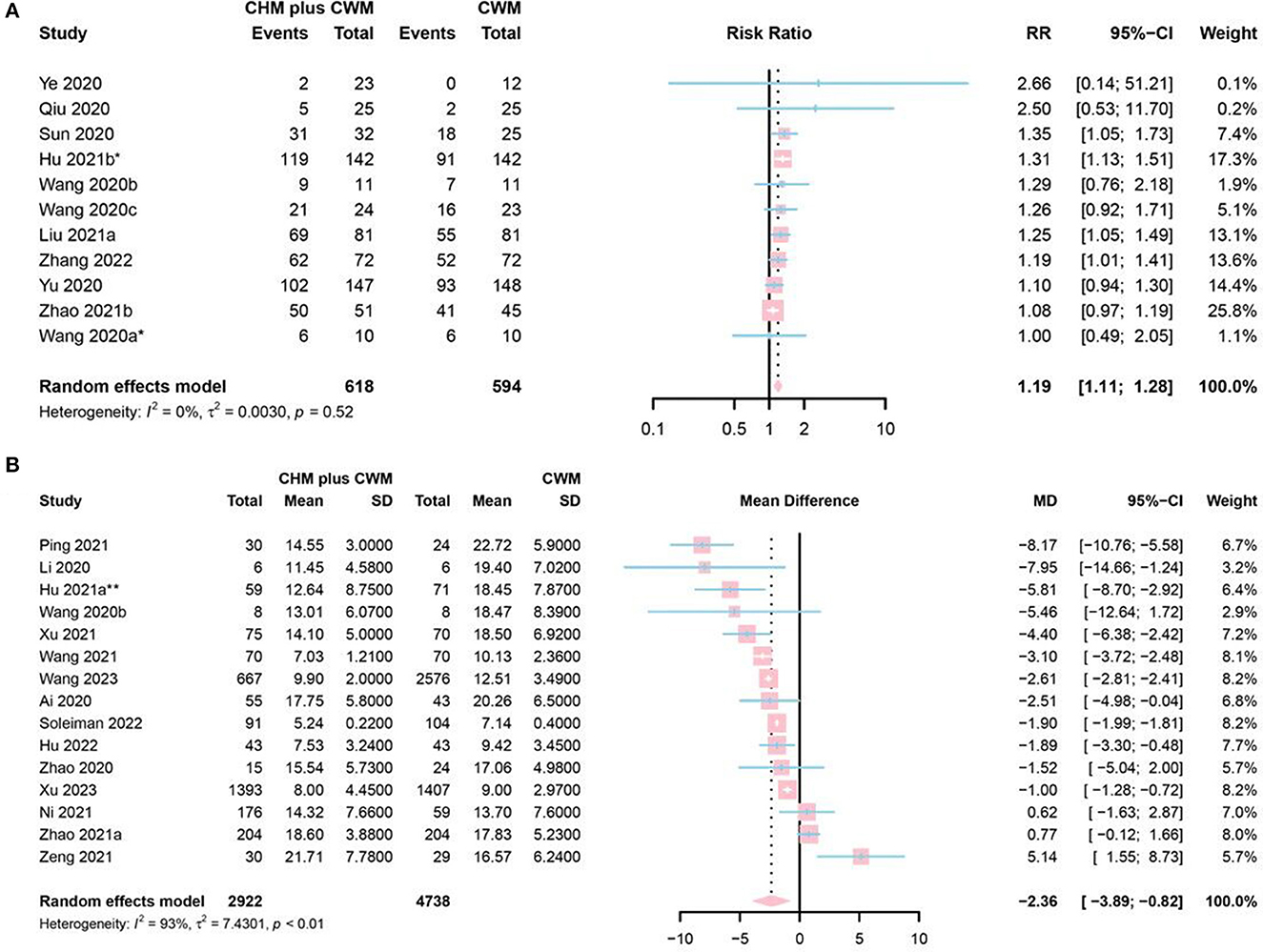
Figure 5. Forest plot of (A) chest image improvement, and (B) duration of hospitalization. *Data from the oral CHM plus inhalation treatment group were included in the meta-analysis; **Data from the 60 mL CHM group were included in the meta-analysis.
Fifteen studies mentioned duration of hospitalization, 1 study identified as “low risk” (83), 8 as “some concerns” (42, 60–62, 71, 73, 78, 79), and 5 as “high risk” (43, 49, 51, 56, 65). A total of 2,922 participants involved in CHM plus CWM groups and 4,738 in CWM groups. The results of the meta-analysis showed that CHM plus CWM groups had a shorter duration of hospitalization than CWM groups (MD = −2.36, 95% CI [−3.89, −0.82], I2 = 93%, P = 0.0026, very low certainty) (Figure 5B).
Conversion to severe cases was defined as (a) changing from mild to moderate, severe or critical condition; (b) changing from moderate to severe or critical condition; or (c) changing from severe to critical condition. A total of 23 studies reported conversion to severe cases. Six of them were assessed as “low risk” (50, 58, 59, 67, 83, 84), 11 as “some concerns” (47, 54, 62, 64, 69, 76, 78, 82, 86, 88, 89), and 6 as “high risk” (46, 52, 53, 55, 57, 65). A total of 2,862 participants involved in CHM plus CWM groups, and 2,791 in CWM group. The disease severity in 4 studies changed from severe to critical (53, 58, 67, 88), and in 16 studies from mild or moderate to severe or critical (46, 47, 52, 54, 55, 59, 62, 64, 65, 69, 76, 82–84, 86, 89). Three studies provided no detailed definition of “rate of conversion to severe cases” (50, 57, 78). The meta-analysis results showed the rate of conversion to severe cases in CHM plus CWM groups was lower than in CWM groups (RR = 0.45, 95% CI [0.33, 0.60], I2= 0%, P < 0.0001, low certainty) (Figure 6A).
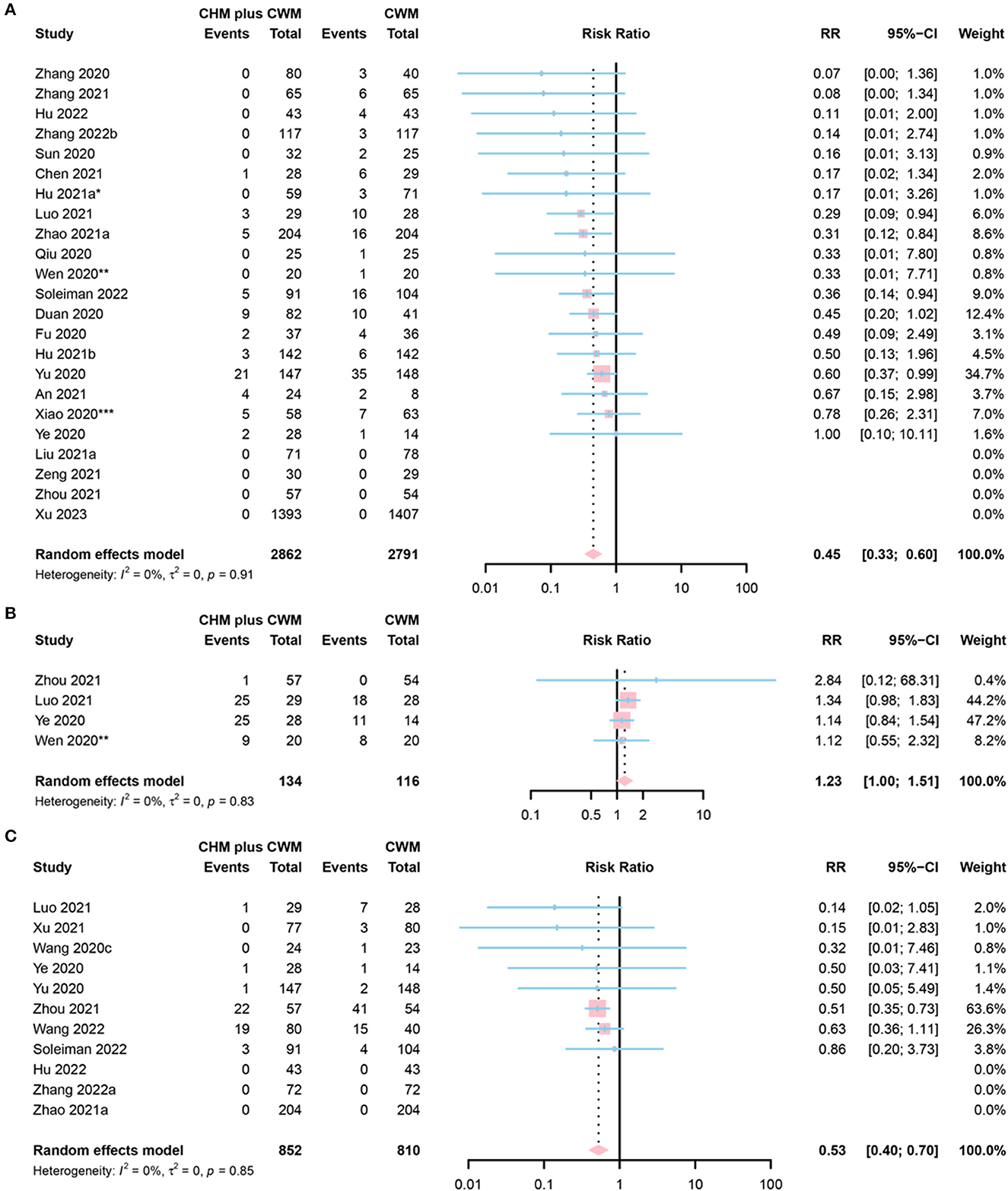
Figure 6. Forest plot of (A) conversion to severe cases, (B) conversion to mild cases, and (C) death. *Data from the 60 mL CHM group were included in the meta-analysis; **Data from the 50 mL CHM group were included in the meta-analysis; ***Data from the Lianhua Qingwen group were included in the meta-analysis.
Conversion to mild cases was defined as: (a) changing from critical to severe, moderate or mild condition; (b) changing from severe to moderate or mild condition; or (c) changing from moderate to mild condition. Of the 4 studies that reported conversion to mild cases, 3 were assessed as “low risk” (43, 47, 50), and 1 as “some concerns” (71). A total of 134 participants involved in CHM plus CWM groups and 116 in CWM groups. The disease severity in 3 studies changed from severe to mild or moderate (43, 47, 71), and in 1 study from critical to severe (50). The results of the meta-analysis indicated that the rate of conversion to mild cases in CHM plus CWM groups was equivalent to that in CWM groups (RR = 1.23, 95% CI [1.00, 1.51], I2 = 0%, P = 0.0539, low certainty) (Figure 6B).
The results of the meta-analysis on 11 studies reporting mortality indicated that the death rate in CHM plus CWM groups was lower than that in CWM groups (RR = 0.53, 95% CI [0.40, 0.70], I2 = 0%, P < 0.0001, moderate certainty). Among 11 studies, 5 were classified into “low risk” (54, 58, 67, 77, 78), 3 into “some concerns” (61, 62, 76), and 3 into “high risk” (43, 48, 53). A total of 852 participants in CHM plus CWM groups and 810 in CWM groups. A forest plot of death was shown in Figure 6C.
Sixteen studies provided fever recovery time. Six studies were assessed as “low risk” (50, 66, 77, 82–84), 9 as “some concerns” (42, 46, 48, 53, 62, 63, 68, 79, 86), and 1 as “high risk” (43). However, a study (77), only reporting the median time to fever symptom recovery of two arms (CHM plus CWM vs. CWM = 2 vs. 3 days, P = 0.0007), was excluded from the meta-analysis. The remaining 15 studies were included in meta-analyses. A total of 559 participants involved in CHM plus CWM groups and 513 in CWM groups. The results of meta-analysis demonstrated that fever recovery time was shorter in CHM plus CWM groups than in CWM groups (MD = −1.28, 95% CI [−1.85, −0.72], I2 = 91%, P < 0.0001, very low certainty) (Figure 7A).
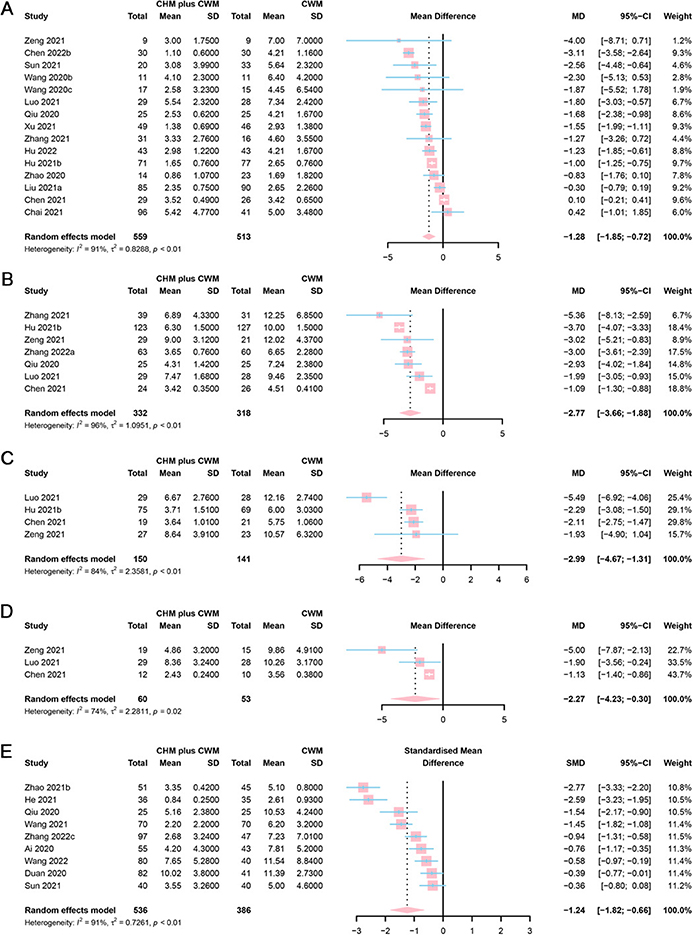
Figure 7. Forest plot of (A) fever, (B) cough, (C) fatigue, (D) shortness of breath recovery time, and (E) total score of TCM syndrome.
Among the 8 studies focused on cough recovery time, 3 were assessed as “low risk” (50, 83, 84), 4 as “some concerns” (46, 53, 77, 86), and 1 as “high risk” (52). However, a study (52), only reporting the median time to cough symptom recovery of two arms (CHM plus CWM vs. CWM = 4 vs. 7 days, P < 0.05), was excluded from the meta-analysis. A total of 332 participants involved in CHM plus CWM groups and 318 in CWM groups. The results of the meta-analysis showed that cough recovery time was shorter in CHM plus CWM groups than in CWM groups (MD = −2.77, 95% CI [−3.66, −1.88], I2 = 96%, P < 0.0001, low certainty) (Figure 7B).
Four studies addressed fatigue recovery time. Two of them were assessed as “low risk” (50, 83), while the other 2 as “some concerns” (46, 53). As the target data in 2 studies were absent, they were excluded from the meta-analysis (50, 83). A total of 150 participants involved in CHM plus CWM groups and 141 in CWM groups. The results demonstrated that the fatigue recovery time was shorter in CHM plus CWM groups than in CWM groups (MD = −2.99, 95% CI [−4.67, −1.31], I2 = 84%, P = 0.0005, low certainty) (Figure 7C).
Three studies reported shortness of breath recovery time. One study was assessed as “low risk” (83), while the other 2 as “some concerns” (46, 53). However, 1 study was excluded for the unavailability of mean and SD values (83). A total of 60 participants involved in CHM plus CWM groups and 53 in CWM groups. The results of the meta-analysis showed that shortness of breath recovery time was shorter in CHM plus CWM groups than in CWM groups (MD = −2.27, 95% CI [−4.23, −0.30], I2 = 74%, P = 0.0238, low certainty) (Figure 7D).
Ten studies included the total score of TCM syndrome. One study was assessed as “low risk” (83), 8 as “some concerns” (47, 60, 61, 66, 70, 73, 80, 86), and 1 as “high risk” (49). Different scoring systems were applied. For example, the scoring system used by Ai et al. study in the measurement of 8 primary symptoms, 3 secondary symptoms, and TCM tongue pulse, with total score ranging from 0 to 66 (49). Higher scores indicated more severe disease conditions. Meanwhile, He et al. adopted a TCM syndrome rating scale with 7 symptoms and a total score ranging from 0 to 21 (70), and Wang et al. used a rating scale with 10 symptoms and a total score ranging from 0 to 60 (73). Thus, the scores were pooled using SMD. However, 1 study was excluded as the absence of necessary data (83). A total of 536 participants involved in CHM plus CWM groups and 386 in CWM groups. The results of the meta-analysis showed that the total score of TCM syndrome of CHM plus CWM groups was lower than CWM groups (SMD = −1.24, 95% CI [−1.82, −0.66], I2 = 91%, P < 0.0001, very low certainty). A forest plot of total score of TCM syndrome was showed in Figure 7E.
In 20 studies, laboratory indicators C-reactive protein (CRP), lymphocyte (LYM), tumor necrosis factor-α (TNF-α), white blood cell (WBC), neutrophil (NEU), high sensitive C-reactive protein (hsCRP), erythrocyte sedimentation rate (ESR), and procalcitonin (PCT) were tested after treatments. Two studies were assessed as “low risk” (58, 83), 15 as “some concerns” (46, 48, 53, 61, 63, 69–71, 73, 75, 76, 79, 80, 85, 88), and 3 as “high risk” (49, 51, 56). As the required data were not obtained from articles and authors, some studies were excluded. Moreover, 1 study was excluded from the meta-analysis of CRP due to the discrepancy in data reported in figures and in texts (53). One study was excluded from the meta-analysis of PCT level, as there was a problem with the units of measurement used in it (46). The results of the meta-analysis were shown in Table 3.
Of the 36 studies reporting adverse reactions, 5 were assessed as “low risk” (50, 53, 67, 83, 84), 22 as “some concerns” (46–48, 61, 62, 64, 66, 68, 69, 71–73, 82, 85, 88, 90–92), and 9 as “high risk” (43, 45, 49, 51, 52, 54, 56, 59, 65). The main adverse reactions contained diarrhea (45–48, 50, 51, 56, 59, 62, 64, 65, 67, 72, 75, 78, 82, 84, 89, 91), vomiting (46, 48, 50, 51, 56, 64, 67, 72, 75, 82, 89, 91), nausea (46, 48, 50, 51, 56, 64, 67, 72, 75, 89, 90), loss of appetite (43, 50, 51, 64, 82), abnormal liver function (43, 46, 50, 53, 62, 77, 83), itchy skin (52, 71, 75, 82, 89, 90), and rash (51, 53, 59, 67, 75, 89–91). We analyzed the differences in the incidence of adverse reactions between the two groups, involving a total of 26 types of CHM preparations (Supplementary Table S5). Commonly, the incidence of adverse reactions in CHM plus CWM treatment is equivalent to CWM group. In comparison to CWM treatment, severe/critical patients after administration of Shenhuang granule had lower risk of some adverse reactions (e.g., hypoalbuminemia, increased blood glucose, thrombocytopenia, increased total bilirubin, increased white blood cell count, abnormal serum sodium, respiratory failure or acute respiratory distress syndrome, cardiopulmonary failure, multiple organ dysfunction syndrome, total serious adverse reactions, and so on), and patients undergoing LHQW had lower incidence of diarrhea (5.6 vs. 13.4%, P = 0.0431); however, mild patients in JHQG group had a significantly increased risk of diarrhea (32.9 vs. 0.0%, P < 0.0001). Four out of 36 studies reported no adverse reactions in CHM plus CWM groups and provided no information about adverse reactions in CWM groups (49, 52, 88, 91); furthermore, the incidence of total adverse reactions is unclear in Zhou et al.'s study (50). Thus, the remaining 31 studies of adverse reactions were included in the meta-analysis. A total of 4,209 participants involved in CHM plus CWM groups and 5,792 in CWM groups. The results of the meta-analysis demonstrated no significant difference in adverse reactions rate between CHM plus CWM groups and CWM groups (RR = 0.97, 95% CI [0.88, 1.07], I2 = 52%, P = 0.5111, very low certainty) (Figure 8).
We further conducted subgroup analysis concerning two primary outcomes. There was no change in the pooled results in any of the subgroups with regard to clinical effective rate (all P < 0.05). For SARS-CoV-2 nucleic acid conversion time, the results showed no statistical difference between CHM plus CWM groups and CWM groups in the subgroup where treatment duration was not reported. The detailed results of the subgroup analysis were shown in Supplementary Figures S1–S8. We implemented sensitivity analysis by means of the leave-one-out method for the primary outcomes (Supplementary Figures S9, S10). In the meta-analysis of clinical effective rate, the I2 was 7% after removing 1 study (67), indicating this study might be the chief reason for the heterogeneity (Supplementary Figure S9).
We assessed publication bias for 10 outcomes indicators (i.e., clinical effective rate, SARS-CoV-2 nucleic acid conversion time, chest image improvement, duration of hospitalization, conversion to severe cases, fever recovery time, LYM count, WBC count, CRP, and adverse reactions) which exceeded 10 studies. For clinical effective rate, an obviously asymmetric funnel plot illustrated considerable publication bias (Figure 9A). Moreover, Egger's test also supported the existence of publication bias, indicating the small-study effects (P < 0.0001). Eleven smaller studies were identified and trimmed with the trim-and-fill method, and the RR value after adjusting for publication bias was 1.13 (95% CI [1.10, 1.17], I2= 47%, P < 0.0001) (Supplementary Figure S11). For conversion to severe cases, a potential publication bias was supported by the mild asymmetry based on visual inspection of Figure 9E. Egger's test (P < 0.0001) also found evidence of publication bias. The results of the trim-and-fill analysis showed an insignificant change in the RR value (RR = 0.49, 95% CI [0.37, 0.64], I2 = 0%, P < 0.0001) (Supplementary Figure S12). Symmetric funnel plots and the Egger's test results suggested no publication bias with respect to other 8 outcomes (Figure 9).
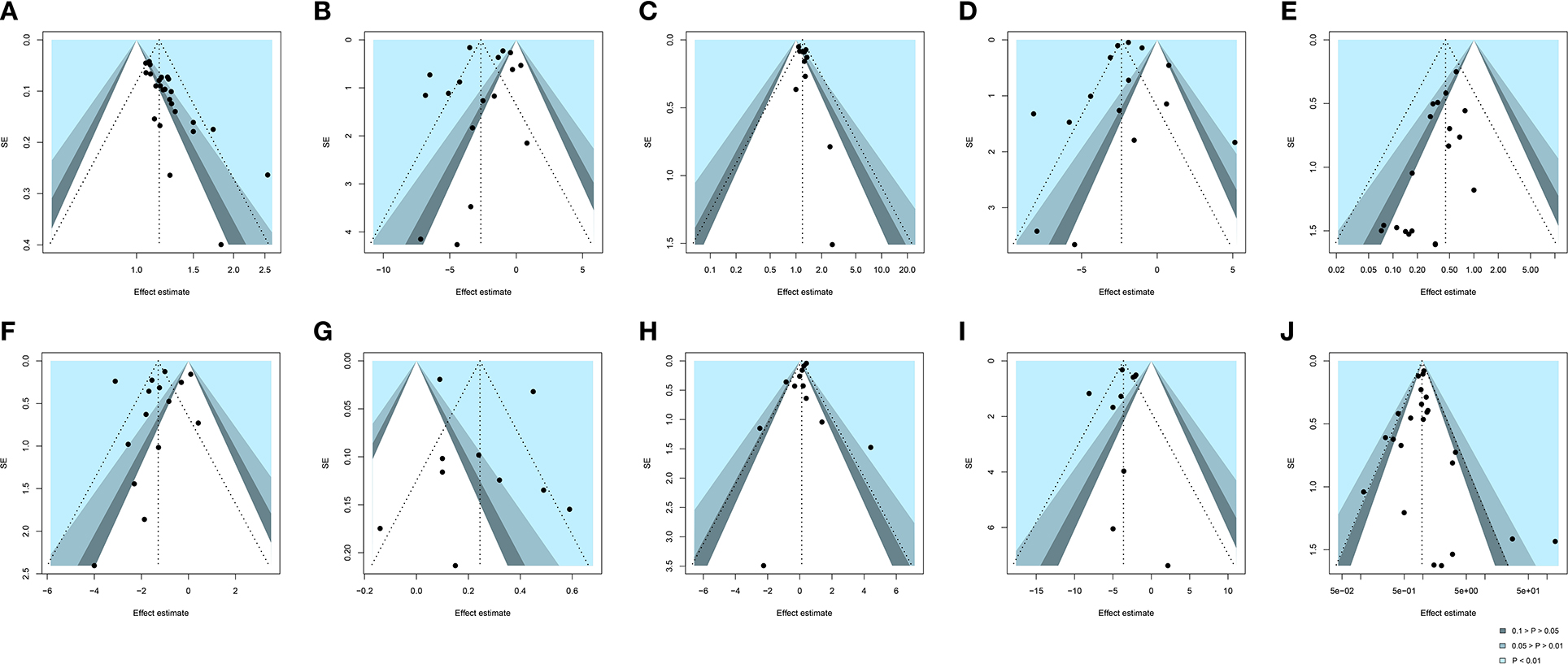
Figure 9. Funnel plot of (A) clinical effective rate, (B) SARS-CoV-2 nucleic acid conversion time, (C) chest image improvement, (D) duration of hospitalization, (E) conversion to severe cases, (F) fever recovery time, (G) LYM count, (H) WBC count, (I) CRP, and (J) adverse reactions. SE, standard error. Black dots mean different studies and contours mean the levels of statistical significance.
CHM has been employed to combat sundry epidemic and endemic diseases for thousands of years. In the 17th century, the world's first medical work on the systematic study of acute infectious diseases was published in China, in which etiology, pathogenesis, symptoms, and treatment of plague were elaborated. Likewise, Chinese traditional therapy has established many representative theories of infectious diseases (92–94). In 2003, CHM therapy was used to prevent and treat severe acute respiratory syndrome (SARS) in China (95). CHM therapy has been reported effective in improving pneumonia symptoms, quality of life, and absorption of pulmonary infiltration, while reducing clinical deterioration and decreasing corticosteroid dosage in SARS patients (96, 97). Similar to SARS-CoV, SARS-CoV-2 infection leads to an over-reaction of the immune system, causing a cytokine storm, which is closely related to the severity of COVID-19 (98). Some components of Chinese botanical drugs were reported beneficial for the suppression of excessive inflammatory responses (99). Furthermore, in 2009, CHM was nominated to treat influenza in the Guidelines for Management of Pandemic (H1N1) 2009 Influenza, published by the Ministry of Health of China (100). Thus, it is widely believed that CHM therapy could potentially provide an effective therapy against COVID-19. Our systematic review, including 50 RCTs, ranging from 2020 to 2023, evaluated and discussed the efficacy and safety of CHM for COVID-19.
Many CHM prescriptions were used repeatedly in these 50 RCTs, including LHQW, JYH, QFPD, JHQG, HSBD, MXSG, and so on. LHQW was the most frequently used CHM prescription, a classical prescription made from Yinqiao powder and MXSG, and contained over a dozen Chinese botanical drugs including Lianqiao [Forsythia suspensa (Thunb.) Vahl], Jinyinhua (Lonicera japonica Thunb.), Mahuang (Ephedra sinica Stapf.), Kuxingren (Prunus armeniaca L.), etc. Researches indicated LHQW can significantly inhibit SARS-CoV-2 replication, alter virus morphology, and exert anti-inflammatory activity in vitro (101). Wang et al. found that LHQW contains 22 key compounds which may have targets with SARS-CoV-2 by Network Pharmacology (102). We listed the chemical structures of 6 main compounds (i.e., quercetin, luteolin, kaempferol, sitosterin, naringenin, and acacetin) of LHQW in Supplementary Figure S13. Quercetin has the largest number of targets. Furthermore, it is also the chief ingredient of Lianqiao and Jinyinhua used in LHQW (102). Meanwhile, systematic reviews of clinical evidence have revealed LHQW could obviously alleviate clinical symptoms, inhibit clinical deterioration, and shorten the course of COVID-19 (25, 103, 104). We have summarized the Chinese botanical drugs that were commonly used in these 50 RCTs. Gancao (Glycyrrhiza uralensis Fisch. ex DC.) was mentioned most frequently. This Chinese botanical drug can be used to improve the symptoms of the respiratory tract, i.e., cough, sore throat (105). In animal models of acute pneumonia, the flavonoids in Gancao reduced the infiltration of neutrophils and inhibited the expression of pro-inflammatory mediators, thus exerting anti-inflammatory activity (106). Besides, glycyrrhetinic acid (Supplementary Figure S14A) in Gancao suppress viral replication and release in host cells (107). Mahuang (Ephedra sinica Stapf.) is a commonly used specie in CHM preparations. The main bioactive components contained in Mahuang are ephedrine and pseudoephedrine (Supplementary Figures S14B, C). Kuxingren (Prunus armeniaca L.) with the main active ingredient of amygdalin (Supplementary Figure S14D), also a very frequently used drug, paired with Mahuang is widely employed to combat respiratory diseases (108). This herb pair (Mahuang-Kuxingren) is popularly used for the treatment of bronchitis and asthma in accordance with the principle of mutual reinforcement and assistance. Kuxingren is defined as a poison based on TCM theory, since amygdalin is a major component of Mahuang but the main source of its toxicity, which can be converted to cyanide in the body and lead to fatal cyanide poisoning (109, 110); whereas the combination with Mahuang can prevented and antagonized the toxicity of Kuxingren and allow the safe use of Kuxingren in the clinic with few associated adverse effects (108). Lianqiao (Forsythia suspensa (Thunb.) Vahl) is widely used as a CHM in Asia, its main function is to clear heat and detoxify (111). Modern pharmacological studies have found that it has anti-inflammatory, antibacterial, antiviral, antioxidant, anti-tumor, neuroprotective and liver protective pharmacological effects (112). However, it is worth noting that for the treatment of COVID-19, combination formulations of multiple herbs are often used, and the possible efficacy and safety risks are often more than just the accumulation of individual herbs, due to the interaction of different herbs. The efficacy and safety of CHM are to some extent related to the dosage, proportioning, processing methods, and quality control of the herbs. In addition, standardized assessment and quality control on many CHM is indeed lacking and further research and standards are needed in the future. Furthermore, WHO reviewed three reports on Chinese herbal medicine and COVID-19 provided by Chinese experts and 12 registered and published RCTs, reached a consensus and recommended a combination of CHM and CWM therapies for the treatment of COVID-19, however, research on the specific dual mechanisms of combining CHM and CWM is lacking and this still needs to be further explored in depth in the future (113).
We found that CHM preparations were effective in treating COVID-19 without increasing the probability of adverse reactions by meta-analysis. As for the clinical effective rate, the results of subgroup and sensitivity analyses were similar to those of the meta-analysis. When a study was removed from the meta-analysis, the I2 was 7%. Thus, we thought this study was the main source of heterogeneity (67). Although the Egger's test suggested the existence of publication bias, the trim-and-fill analysis indicated that the publication bias did not affect the results of the meta-analysis. Therefore, the conclusion from the meta-analysis was considered robust. From meta-analysis results, SARS-CoV-2 nucleic acid conversion time of patients in CHM plus CWM group was 2.66 days shorter than that in CWM group. Sensitivity analysis also did not reveal a significant change when omitting any one study at a time by the leave-one-out method. However, we found no statistical difference between CHM plus CWM groups and CWM groups in the subgroup where treatment duration was not reported. Perhaps the effect depended on the duration of treatment. Additionally, the meta-analysis results indicated that CHM plus CWM could promote the improvement of chest imaging, shorten the duration of hospitalization, suppress clinical deterioration, reduce mortality, shorten the clinical symptoms (i.e., fever, cough, fatigue and shortness of breath) recovery time, reduce the TCM syndrome score, and contribute to the return of some laboratory indicators to the normal levels. Although evidence of publication bias was found for conversion to severe cases by asymmetric funnel plot and Egger's test, trim-and-fill analysis demonstrated the robustness of the pooled results. Overall, no serious adverse reactions have been identified. There was no statistically significant difference in the incidence of adverse reactions between two arms.
There are several noteworthy advantages in our systematic review. We conducted the research under the guidance of the latest international statement for systematic review and meta-analysis (31). We systematically screened the literature that assess the efficacy and safety of CHM in the context of COVID-19. The process of literature selection, data extraction, and evidence quality evaluation was conducted by following the back-to-back principle rigorously. We employed RoB2 to evaluate all the 210 outcomes included in our study, whereas most other systematic reviews only evaluated the overall quality of the original researches. We also measured the certainty of evidence through the GRADE system to determine the confidence of the effect estimates. Additionally, to ensure the high quality of the original evidence, our study only incorporated RCTs and excluded observational studies. Based on an overview that summarized the published systematic reviews regarding CHM and COVID-19, we found that the number of RCTs (50 RCTs) included in our study was more than that in other published systematic reviews (114). Compared with the previous systematic reviews, the sample size of this work is large and the data are updated, making the evidence more reliable. Importantly, another strength of our study is the thorough analysis of the possible adverse reaction produced by use of different CHM preparations. To our knowledge, this article is the first systematic review to include data on patients infected with SARS-CoV-2 Omicron variant to evaluate CHM treatment for COVID-19.
However, some limitations also exist in this study. There were inconsistent treatments with CHM in intervention groups and inconsistency in CWM treatment methods of control groups, however, we did not perform subgroup analyses according to the treatment difference. The heterogeneity among some pooled analysis studies for secondary outcomes was significant, but no subgroup and sensitivity analysis were implemented. Only electronic databases and clinical trial registers were searched. Therefore, some literature from other source that meet the inclusion criteria may have been left out. Ongoing trials and unpublished studies were not included in this work either. As COVID-19 epidemic is a sudden health event, a lack of double-blinded RCTs is widespread, and few RCTs used placebos. Allocation concealment and blind methods were not conducted in many RCTs, so the number of enrolled RCTs with low risk of bias was inadequate. The overall quality of evidence was low. The evidence certainty was mostly low or very low, which meant that differences might exist between the actual and estimated effect. To some extent, perhaps the benefits of CHM have been overstated. Additionally, a multi-center study design was scarce in this study, which could lead to some selection bias. The vast majority of RCTs are conducted in China, leading to limited representativeness of this study, which constituted a major disadvantage. We searched the ongoing RCTs concerning CHM for treating COVID-19 from trial registers. A total of 22 RCTs is being investigated in mainland China, 2 in United States, and 1 in Taiwan. These studies will be included in the next update. Further, the evidence for asymptomatic patients was absent, and we were unable to interpret the effects of CHM on such cases in a full extent.
Based on current evidence, we concluded that CHM therapy was potentially an effective and safe adjunctive treatment for COVID-19. This study provided a list of CHM preparations for the treatment of COVID-19. Further, more double-blinded, multi-center, and large-sample size RCTs of high-quality are needed to perform meta-analysis yielding more accurate results.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
LT conceived the study and registered the protocol. ZM and YZ searched the literature. YZ, SY, YY, JL, and JH screened and included the articles. ZM and YZ assessed the quality of included studies. SY, YY, and FW collected the data. JL and JH re-checked the data. ZM performed data-analysis, interpreted the results, and drafted the manuscript. LT, SY, FW, and ZM participated in manuscript revision. All authors re-read and re-checked the final manuscript.
This study was supported by the Natural Science Foundation of Fujian Province (2022J01311 to LT), the National Natural Science Foundation of China (81860354 to FW), and the Scientific Research Funds of Huaqiao University (21BS105 to LT and 605-50Y18058 to FW). Funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1175827/full#supplementary-material
1. Kumar A, Singh R, Kaur J, Pandey S, Sharma V, Thakur L, et al. Wuhan to World: The COVID-19 Pandemic. Front Cell Infect Microbiol. (2021) 11:596201. doi: 10.3389/fcimb.2021.596201
2. Xu TL, Ao MY, Zhou X, Zhu WF, Nie HY, Fang JH, et al. China's practice to prevent and control COVID-19 in the context of large population movement. Infect Dis Poverty. (2020) 9:115. doi: 10.1186/s40249-020-00716-0
3. World Health Organization (WHO). WHO Director-General's opening remarks at the media briefing on COVID-19–11 March 2020. (2020). Available online at: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-−11-march-2020 (accessed 7 June 2022).
4. World Health Organization (WHO). Coronavirus disease (COVID-19): Variants of SARS-COV-2. (2021). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-(covid-19)-variants-of-sars-cov-2 (accessed 7 June 2022).
5. World Health Organization (WHO). Interim Statement on COVID-19 vaccines in the context of the circulation of the Omicron SARS-CoV-2 Variant from the WHO Technical Advisory Group on COVID-19 Vaccine Composition (TAG-CO-VAC). (2022). Available online at: https://www.who.int/news/item/11-01-2022-interim-statement-on-covid-19-vaccines-in-the-context-of-the-circulation-of-the-omicron-sars-cov-2-variant-from-the-who-technical-advisory-group-on-covid-19-vaccine-composition (accessed 7 June 2022).
6. Tuekprakhon A, Nutalai R, Dijokaite-Guraliuc A, Zhou D, Ginn HM, Selvaraj M, et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. (2022) 185:2422–2433.e13. doi: 10.1016/j.cell.2022.06.005
7. Wang Z, Yang L. Turning the Tide: Natural Products and Natural-Product-Inspired Chemicals as Potential Counters to SARS-CoV-2 Infection. Front Pharmacol. (2020) 11:1013. doi: 10.3389/fphar.2020.01013
8. Yang L, Wang Z. Natural Products, Alone or in Combination with FDA-Approved Drugs, to Treat COVID-19 and Lung Cancer. Biomedicines. (2021) 9:689. doi: 10.3390/biomedicines9060689
9. Wang Z, Wang N, Yang L, Song XQ. Bioactive natural products in COVID-19 therapy. Front Pharmacol. (2022) 13:926507. doi: 10.3389/fphar.2022.926507
10. Wang Z, Yang L, Song XQ. Oral GS-441524 derivatives: Next-generation inhibitors of SARS-CoV-2 RNA-dependent RNA polymerase. Front Immunol. (2022) 13:1015355. doi: 10.3389/fimmu.2022.1015355
11. Dos Santos WG. Natural history of COVID-19 and current knowledge on treatment therapeutic options. Biomed Pharmacother. (2020) 129:110493. doi: 10.1016/j.biopha.2020.110493
12. World Health Organization (WHO). Coronavirus disease (COVID-19).(2021). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19 (accessed 7 June 2022).
13. World Health Organization (WHO). Coronavirus disease (COVID-19): Vaccines. (2022). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-(covid-19)-vaccines (accessed 7 June 2022).
14. Chen Z, Nakamura T. Statistical evidence for the usefulness of Chinese medicine in the treatment of SARS. Phytother Res. (2004) 18:592–4. doi: 10.1002/ptr.1485
15. Wang L, Zhang RM, Liu GY, Wei BL, Wang Y, Cai HY, et al. Chinese herbs in treatment of influenza: a randomized, double-blind, placebo-controlled trial. Respir Med. (2010) 104:1362–9. doi: 10.1016/j.rmed.2010.05.015
16. Cui Q, Du R, Anantpadma M, Schafer A, Hou L, Tian J, et al. Identification of Ellagic acid from plant Rhodiola rosea L. as an anti-ebola virus entry inhibitor. Viruses. (2018) 10:152. doi: 10.3390/v10040152
17. Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. (2020) 14:64–8. doi: 10.5582/bst.2020.01030
18. Huang K, Zhang P, Zhang Z, Youn JY, Wang C, Zhang H, et al. Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: Efficacies and mechanisms. Pharmacol Ther. (2021) 225:107843. doi: 10.1016/j.pharmthera.2021.107843
19. Ren JL, Zhang AH, Wang XJ. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res. (2020) 155:104743. doi: 10.1016/j.phrs.2020.104743
20. Wang Z, Yang L. Chinese herbal medicine: Fighting SARS-CoV-2 infection on all fronts. J Ethnopharmacol. (2021) 270:113869. doi: 10.1016/j.jep.2021.113869
21. National Health Commission of the People's Republic of China. Diagnosis and Treatment Protocol for COVID-19 (Trial 9th Edition). (2022). Available online at: http://www.gov.cn/zhengce/zhengceku/2022-03/15/content_5679257.htm (accessed 7 June 2022).
22. Jiang F, Xu N, Zhou Y, Song J, Liu J, Zhu H, et al. Contribution of traditional Chinese medicine combined with conventional western medicine treatment for the novel coronavirus disease (COVID-19), current evidence with systematic review and meta-analysis. Phytother Res. (2021) 35:5992–6009. doi: 10.1002/ptr.7209
23. Liu M, Gao Y, Yuan Y, Yang K, Shi S, Zhang J, et al. Efficacy and safety of integrated traditional Chinese and western medicine for corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. Pharmacol Res. (2020) 158:104896. doi: 10.1016/j.phrs.2020.104896
24. Du X, Shi L, Cao W, Zuo B, Zhou A. Add-on effect of Chinese herbal medicine in the treatment of mild to moderate COVID-19: A systematic review and meta-analysis. PLoS ONE. (2021) 16:e0256429. doi: 10.1371/journal.pone.0256429
25. Fan Z, Guo G, Che X, Yang Y, Liu Y, Li L, et al. Efficacy and safety of Lianhuaqingwen for mild or moderate coronavirus disease 2019: A meta-analysis of randomized controlled trials. Medicine (Baltimore). (2021) 100:e26059. doi: 10.1097/MD.0000000000026059
26. Liang SB, Fang M, Liang CH, Lan HD, Shen C, Yan LJ, et al. Therapeutic effects and safety of oral Chinese patent medicine for COVID-19: A rapid systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. (2021) 60:102744. doi: 10.1016/j.ctim.2021.102744
27. Fan AY, Gu S, Alemi SF. Research Group for Evidence-based Chinese, M Chinese herbal medicine for COVID-19: Current evidence with systematic review and meta-analysis. J Integr Med. (2020) 18:385–94. doi: 10.1016/j.joim.2020.07.008
28. Xiong X, Wang P, Su K, Cho WC, Xing Y. Chinese herbal medicine for coronavirus disease 2019: A systematic review and meta-analysis. Pharmacol Res. (2020) 160:105056. doi: 10.1016/j.phrs.2020.105056
29. Li L, Xie H, Wang L, Zhang A, Mou X, Lin Y, et al. The efficacy and safety of combined chinese herbal medicine and western medicine therapy for COVID-19: a systematic review and meta-analysis. Chin Med. (2022) 17:77. doi: 10.1186/s13020-022-00600-z
30. Wang H, Xu B, Zhang Y, Duan Y, Gao R, He H, et al. Efficacy and Safety of Traditional Chinese Medicine in Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis. Front Pharmacol. (2021) 12:609213. doi: 10.3389/fphar.2021.609213
31. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. (2021) 18:e1003583. doi: 10.1371/journal.pmed.1003583
32. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
33. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
34. Qiu R, Zhao C, Liang T, Hao X, Huang Y, Zhang X, et al. Core Outcome Set for Clinical Trials of COVID-19 based on traditional Chinese and western medicine. Front Pharmacol. (2020) 11:781. doi: 10.3389/fphar.2020.00781
35. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
36. Shim SR, Kim SJ. Intervention meta-analysis: application and practice using R software. Epidemiol Health. (2019) 41:e2019008. doi: 10.4178/epih.e2019008
37. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
38. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
39. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. (2008) 61:991–6. doi: 10.1016/j.jclinepi.2007.11.010
40. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
41. Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. (2011) 64:1283–93. doi: 10.1016/j.jclinepi.2011.01.012
42. Wang YL, Xue J, Dai EH, Xu ZG, Feng CX, Liu HD, et al. Clinical study on the treatment of patients with novel coronavirus pneumonia and asymptomatic infection with integrated traditional Chinese and western medicine. Hebei J Trad Chin Med. (2020) 42:645–649. doi: 10.3969/j.issn.1002-2619.2020.05.001
43. Xu X, Zhang J, Zheng W, Yang Z, Zhao X, Wang C, et al. Efficacy and safety of Reduning injection in the treatment of COVID-19: a randomized, multicenter clinical study. Ann Palliat Med. (2021) 10:5146–55. doi: 10.21037/apm-20-2121
44. Tan DX, Shi WL, Liu N, Wang HY, Liang ZJ, Li CH, et al. Observation on antiviral and anti-inflammatory effects of Lianhua Qingwen capsule in early stage of COVID-19. J China Prescript Drug. (2021) 19:92–3.
45. Wang M, Shi J, Zhang K, Hong J, Peng X, Tian Y, et al. Efficacy and safety of longyizhengqi granule in treatment of mild COVID-19 patients caused by SARS-CoV-2 omicron variant: a prospective study. Infect Drug Resist. (2023) 16:1611–8. doi: 10.2147/IDR.S389883
46. Chen CW Li XL, Liu YF, Chen S. Clinical Study of Lianhua Qingwen Capsule in the Treatment of Corona Virus Disease 2019. Res Integr Tradit Chin Western Med. (2021) 13:1–4.
47. Duan C, Xia WG, Zheng CJ, Sun GB, Li ZL, Li QL, et al. Clinical observation on jinhua qinggan granule combined with conventional western medicine therapy in treating mild cases of coronavirus disease 2019. J Tradit Chin Med. (2020) 61:1473–7.
48. Wang JB, Wang ZX, Jing J, Zhao P, Dong JH, Zhou YF, et al. Exploring an Integrative Therapy for Treating COVID-19: A Randomized Controlled Trial. Chin J Integr Med. (2020) 26:648–55. doi: 10.1007/s11655-020-3426-7
49. Ai XY, Luo C, Lin LP, Xie M, Fan HM, Tan XH. Therapeutic effect of integrated traditional Chinese and western medicine on COVID-19 in Guangzhou. China Trop Med. (2020) 20:746–50.
50. Hu K, Guan WJ, Bi Y, Zhang W, Li L, Zhang B, et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine. (2021) 85:153242. doi: 10.1016/j.phymed.2020.153242
51. Ni L, Wen Z, Hu X, Tang W, Wang H, Zhou L, et al. Effects of Shuanghuanglian oral liquids on patients with COVID-19: a randomized, open-label, parallel-controlled, multicenter clinical trial. Front Med. (2021) 15:704–17. doi: 10.1007/s11684-021-0853-6
52. Sun HM, Xu F, Zhang L, Wei C, Chen JY, Wang QX, et al. Study on Clinical Efficacy of Lianhua Qingke Granule in Treatment of Mild and Ordinary COVID-19. Chin J Exper Tradit Med Formulae. (2020) 26:29–34.
53. Luo Z, Chen W, Xiang M, Wang H, Xiao W, Xu C, et al. The preventive effect of Xuebijing injection against cytokine storm for severe patients with COVID-19: A prospective randomized controlled trial. Eur J Integr Med. (2021) 42:101305. doi: 10.1016/j.eujim.2021.101305
54. Soleiman-Meigooni S, Hoseini Yekta N, Sheikhan HR, Aminianfar M, Hamidi-Farahani R, Ahmadi M, et al. Efficacy of a standardized herbal formulation from Glycyrrhiza glabra L. as an adjuvant treatment in hospitalized patients with COVID-19: A Randomized Controlled trial. J Ayurveda Integr Med. (2022) 13:100670. doi: 10.1016/j.jaim.2022.100670
55. An X, Xu X, Xiao M, Min X, Lyu Y, Tian, J. Efficacy of Jinhua Qinggan granules combined with western medicine in the treatment of confirmed and suspected COVID-19: a randomized controlled trial. Front Med (Lausanne). (2021) 8:728055. doi: 10.3389/fmed.2021.728055
56. Ping XH, Xu HL, Fu DF, Zhou YF, Liu L, Xu HX. Clinical observation of Jiawei Yupingfeng powder combined with western medicine in the treatment of the novel coronavirus pneumonia. Med Forum. (2021) 25:149–51.
57. Xiao M, Tian J, Zhou Y, Xu X, Min X, Lv Y, et al. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: A randomized controlled trial. Pharmacol Res. (2020) 161:105126. doi: 10.1016/j.phrs.2020.105126
58. Ye YA, Group GCC. Guideline-Based Chinese Herbal Medicine Treatment Plus Standard Care for Severe Coronavirus Disease 2019 (G-CHAMPS): Evidence From China. Front Med (Lausanne). (2020) 7:256. doi: 10.3389/fmed.2020.00256
59. Xu X, Zhou S, Chen C, Li J, Wu H, Jin G, et al. Efficacy and safety of Reyanning mixture in patients infected with SARS-CoV-2 Omicron variant: A prospective, open-label, randomized controlled trial. Phytomedicine. (2023) 108:154514. doi: 10.1016/j.phymed.2022.154514
60. Zhang J, Liu L, Zhang G, Li M, Ma B, Yang W, et al. Treating patients infected with the SARS-CoV-2 Omicron variant with a traditional Chinese medicine, Shufeng Jiedu capsule. Biosci Trends. (2022) 16:238–41. doi: 10.5582/bst.2022.01220
61. Wang Y, Huang D, Wang H, Feng C, Zhong Z, Cai J, et al. Curative effect of integrated Chinese and Western medicine on 80 severe and critical COVID-19 patients. J Beijing Univ Tradit Chin Med. (2022) 45:555–62.
62. Hu H, Wu Z, Ji C, Chen J, Yang K. Clinical effect of integrated traditional Chinese and Western medicine therapy in treatment of the initial pyrexia stage of coronavirus disease 2019: An analysis of 43 cases. Hunan J Tradit Chin Med. (2022) 38:1–4.
63. Chen F, Fu B, Ba Y, Liu X, Zhou Y, Zou Y, et al. Clinical Curative Effect Observation of Combining Traditional Chinese and Western Medicine on Common New Coronavirus Pneumonia in Xiaogan Area. J Sichuan Tradit Chin Med. (2022) 40:23–6.
64. Zhang C, Yao Y, Zhou D, Ouyang Y, Hao W, Yan H, et al. Clinical observation on Ganjiang Xiaochaihu Decoction in treating patients with mild infection of COVID-19 Omicron variant. Shanghai J Tradit Chin Med. (2022) 56:1–4.
65. Hu F, Guo AH, Huang L, Yu WX, Liu GF, Gao XS, et al. Jinyinhua Liquid at Different Dosage Combined with Western Medicine Conventional Treatment for Common Type COVID-19: A Multicenter Trial of 187 Cases. J Tradit Chin Med. (2021) 62:510–5.
66. Sun SQ, Chen FF, Yin CW, Wang J, Cai WR, Guo J, et al. Clinical efficacy of Liushen pills combined with routine treatment for COVID-19 patients. Chin Tradit Patent Med. (2021) 43:2277–80. doi: 10.13422/j.cnki.syfjx.20201438
67. Zhou S, Feng J, Xie Q, Huang T, Xu X, Zhou D, et al. Traditional Chinese medicine shenhuang granule in patients with severe/critical COVID-19: A randomized controlled multicenter trial. Phytomedicine. (2021) 89:153612. doi: 10.1016/j.phymed.2021.153612
68. Chai KQ, Huang FH, Li YP, Zhou Y, Chen YB, Hu X, et al. Clinical study of traditional Chinese medicine against Corona Virus Disease 2019. China J Tradit Chin Med Phar. (2021) 36:7458–62.
69. Fu XX, Lin LP, Tan XH. Clinical study on 37 case of COVID-19 treated with integrated traditional chinese and western medicine. Tradit Chin Drug Res Clin. Pharmacol. (2020) 31:600–4.
70. He Q, Zhang QJ, Gan XW, Li XG. Clinical effect analysis of Buzhong Yiqi Decoction on mild COVID-19. J Emergency in Tradit Chin Med. (2021) 30:385–7.
71. Li YD, Zhang WJ. Evaluation on the Clinical Effect of Traditional Chinese Medicine and Western Medicine Regimens on COVID-19. Guangming J Chin Med. (2020) 35:1273–5.
72. Liu W, Su XY, Liao XL, Pan H, Mei D, Zhang YD. Efficacy analysis of antiviral drugs combined with traditional Chinese medicine in the treatment of mild COVID-19. Contemp Med Symposium. (2021) 19:159–60.
73. Wang Y, Chen L, Zheng L, Ku BQ Yu R, Zhang XF. Clinical effects of Qingfei Paidu Decoction combined with conventional treatment on patients with coronavirus disease 2019. Chinese Tradit Patent Med. (2021) 43:656–9.
74. Yang S, Bu XH, Liu XJ, Huang JL, Wang JM. Analysis of the Clinical Effect of “Preventive Treatment” Theory in the Prevention and Treatment of New Coronavirus Pneumonia in Southwest Shandong Province. Smart Healthcare. (2021) 7:150–2.
75. Ye L, Zhao HJ, Xu SG, Chen WZ. Clinical Study of Modified Shengjiang Powder in the Treatment of Ordinary COVID-19. Chinese Foreign Med Res. (2021) 19:9–13.
76. Yu P, Li YZ, Wan SB, Wang Y. Effects of Lianhua Qingwen granules plus arbidol on treatment of mild corona virus disease-19. Chin Pharmac J. (2020) 55:1042–5.
77. Zhang L, Wu L, Xu X, Yuan Y, Jiang R, Yan X, et al. Efficacy and Safety of Lianhua Qingke tablets in the treatment of mild and common-type COVID-19: a randomized, controlled, multicenter clinical study. Evid Based Complement Alternat Med. (2022) 2022:8733598. doi: 10.1155/2022/8733598
78. Zhao C, Li L, Yang W, Lv W, Wang J, Guo J, et al. Chinese medicine formula huashibaidu granule early treatment for mild COVID-19 patients: an unblinded, cluster-randomized clinical trial. Front Med (Lausanne). (2021) 8:696976. doi: 10.3389/fmed.2021.696976
79. Zhao J, Yang X, Wang C, Song S, Cao K, Wei T, et al. Yidu-toxicity blocking lung decoction ameliorates inflammation in severe pneumonia of SARS-COV-2 patients with Yidu-toxicity blocking lung syndrome by eliminating IL-6 and TNF-a. Biomed Pharmacother. (2020) 129:110436. doi: 10.1016/j.biopha.2020.110436
80. Zhao JL, Yang SL, Ke DF, Qiu L, Jiang L. The efficacy of Anti-viral Formula No. 1 in treating early and middle stage of cold-damp-stagnant lung type COVID-19. Forum Tradit Chin Med. (2021) 36:20–2.
81. Zheng ZZ, Bai ZG Li CJ, Ge SP, Luo Y, He GD. Clinical observation on novel coronavirus pneumonia treated by TCM syndrome differentiation treatment. Med J Commun. (2020) 34:117–8.
82. Liu J, Yang W, Liu Y, Lu C, Ruan L, Zhao C, et al. Combination of Hua Shi Bai Du granule (Q-14) and standard care in the treatment of patients with coronavirus disease 2019 (COVID-19): A single-center, open-label, randomized controlled trial. Phytomedicine. (2021) 91:153671. doi: 10.1016/j.phymed.2021.153671
83. Zeng C, Yuan Z, Zhu J, Wang Y, Xie Y, Ye R, et al. Therapeutic effects of traditional Chinese medicine (Maxingshigan-Weijing Decoction) on COVID-19: An open-label randomized controlled trial. Integr Med Res. (2021) 10:100782. doi: 10.1016/j.imr.2021.100782
84. Zhang XY, Lv L, Zhou YL, Xie LD, Xu Q, Zou XF, et al. Efficacy and safety of Xiyanping injection in the treatment of COVID-19: A multicenter, prospective, open-label and randomized controlled trial. Phytother Res. (2021) 35:4401–10. doi: 10.1002/ptr.7141
85. Yang X, Wang Y, Liu Y, Shang L, Cheng Z, Fang L, et al. Traditional Chinese medicine together with high-dose vitamin C improves the therapeutic effect of western medicine against COVID-19. Am J Transl Res. (2022) 14:501–10.
86. Qiu M, Li QT, Zhu DP, Wang CH, Sun QZ, Qian CF, et al. Efficacy observation of maxing xuanfei jiedu decoction on moderate COVID-19 patients. J Emergency Tradit Chin Med. (2020) 29:1129–1130.
87. Wang YL, Yang XD, Liu YP, Zhang J, Feng YF, Shang L, et al. Clinical effect of the treatment of novel coronavirus pneumonia by internal administration of Traditional Chinese medicine plus fumigation and absorption combined with super dose of vitamin C in treating COVID-19. J Xi'an Jiaotong Univ (Medical Sciences). (2020) 41:931–5.
88. Wen L, Zhou ZG, Jiang DX, Huang K. Effect of Xuebijing injection on inflammatory makers and disease outcome of coronavirus disease 2019. Chin Crit Care Med. (2020) 32:426–9.
89. Zhang YL, Lei L, Xu Y, Wei DR, Hu F. Clinical Efficacy of Jinyinhua Oral Liquid in the Treatment of 80 Patients with Coronavirus Disease 2019. China Pharmaceutical. (2020) 29:23–6.
90. Liao GR. The efficacy and safety of self-made Chinese herbal decoction in patients with COVID-19. Int Infect Dis. (2020) 9:353.
91. Xiong WZ, Wang G, Du J, Ai W. Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19: A pilot randomized clinical trial. Integr Med Res. (2020) 9:100489. doi: 10.1016/j.imr.2020.100489
92. Li LC, Zhang ZH, Zhou WC, Chen J, Jin HQ, Fang HM, et al. Lianhua Qingwen prescription for Coronavirus disease 2019 (COVID-19) treatment: Advances and prospects. Biomed Pharmacother. (2020) 130:110641. doi: 10.1016/j.biopha.2020.110641
93. Du HZ, Hou XY, Miao YH, Huang BS, Liu DH. Traditional Chinese Medicine: an effective treatment for 2019 novel coronavirus pneumonia (NCP). Chin J Nat Med. (2020) 18:206–10. doi: 10.1016/S1875-5364(20)30022-4
94. Yang Y, Islam MS, Wang J, Li Y, Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci. (2020) 16:1708–17. doi: 10.7150/ijbs.45538
95. Luo H, Tang QL, Shang YX, Liang SB, Yang M, Robinson N, et al. Can Chinese Medicine Be Used for Prevention of Corona Virus Disease 2019 (COVID-19)? A Review of Historical Classics, Research Evidence and Current Prevention Programs. Chin J Integr Med. (2020) 26:243–50. doi: 10.1007/s11655-020-3192-6
96. Liu X, Zhang M, He L, Li Y. Chinese herbs combined with Western medicine for severe acute respiratory syndrome (SARS). Cochrane Database Syst Rev. (2012) 10:CD004882. doi: 10.1002/14651858.CD004882.pub3
97. Tong X, Li A, Zhang Z, Duan J, Chen X, Hua C, et al. TCM treatment of infectious atypical pneumonia–a report of 16 cases. J Tradit Chin Med. (2004) 24:266–9.
98. Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. (2021) 93:250–6. doi: 10.1002/jmv.26232
99. Brendler T, Al-Harrasi A, Bauer R, Gafner S, Hardy ML, Heinrich M, et al. Botanical drugs and supplements affecting the immune response in the time of COVID-19: Implications for research and clinical practice. Phytother Res. (2021) 35:3013–31. doi: 10.1002/ptr.7008
100. Chen W, Lim CE, Kang HJ, Liu J. Chinese herbal medicines for the treatment of type A H1N1 influenza: a systematic review of randomized controlled trials. PLoS ONE. (2011) 6:e28093. doi: 10.1371/journal.pone.0028093
101. Runfeng L, Yunlong H, Jicheng H, Weiqi P, Qinhai M, Yongxia S, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res. (2020) 156:104761. doi: 10.1016/j.phrs.2020.104761
102. Wang L, Yang ZH, Zhang HR, Yu HX, Yang K, Fu BH, et al. Lianhua Qingwen for the treatment of new coronavirus (2019-nCoV) pneumonia network pharmacology research and preliminary evidence. Chinese Herbal Med. (2020) 3:772–8.
103. Zeng M, Li L, Wu Z. Traditional Chinese medicine Lianhua Qingwen treating corona virus disease 2019(COVID-19): Meta-analysis of randomized controlled trials. PLoS ONE. (2020) 15:e0238828. doi: 10.1371/journal.pone.0238828
104. Zhuang J, Dai X, Wu Q, Cai H, Fu X, Zhang W, et al. A meta-analysis for Lianhua Qingwen on the treatment of Coronavirus disease 2019 (COVID-19). Complement Ther Med. (2021) 60:102754. doi: 10.1016/j.ctim.2021.102754
105. Silveira D, Prieto-Garcia JM, Boylan F, Estrada O, Fonseca-Bazzo YM, Jamal CM, et al. COVID-19: Is there evidence for the use of herbal medicines as adjuvant symptomatic therapy? Front Pharmacol. (2020) 11:581840. doi: 10.3389/fphar.2020.581840
106. Xie YC, Dong XW, Wu XM, Yan XF, Xie QM. Inhibitory effects of flavonoids extracted from licorice on lipopolysaccharide-induced acute pulmonary inflammation in mice. Int Immunopharmacol. (2009) 9:194–200. doi: 10.1016/j.intimp.2008.11.004
107. Yang R, Wang LQ, Yuan BC, Liu Y. The pharmacological activities of licorice. Planta Med. (2015) 81:1654–69. doi: 10.1055/s-0035-1557893
108. Song S, Ma Q, Tang Q, Chen F, Xing X, Guo Y, et al. Stereoselective metabolism of amygdalin-based study of detoxification of Semen Armeniacae Amarum in the Herba Ephedrae-Semen Armeniacae Amarum herb pair. J Ethnopharmacol. (2016) 179:356–66. doi: 10.1016/j.jep.2015.12.019
109. Li Y, Wang MY, Fan XS, Qi X, Chen Y, Zhang H, et al. Effect of San-ao Decoction, a traditional Chinese prescription, on IL-4 treated normal human bronchial epithelium. J Ethnopharmacol. (2010) 131:104–9. doi: 10.1016/j.jep.2010.06.006
110. Park JH, Seo BI, Cho SY, Park KR, Choi SH, Han CK, et al. Single oral dose toxicity study of prebrewed armeniacae semen in rats. Toxicol Res. (2013) 29:91–8. doi: 10.5487/TR.2013.29.2.091
111. Chen HY, Lin YH, Huang JW, Chen YC. Chinese herbal medicine network and core treatments for allergic skin diseases: Implications from a nationwide database. J Ethnopharmacol. (2015) 168:260–7. doi: 10.1016/j.jep.2015.04.002
112. Dong Z, Lu X, Tong X, Dong Y, Tang L, Liu M. Forsythiae Fructus: A Review on its Phytochemistry, Quality Control, Pharmacology and Pharmacokinetics. Molecules. (2017) 22:1466. doi: 10.3390/molecules22091466
113. World Health Organization (WHO). WHO Expert Meeting on Evaluation of Traditional Chinese Medicine in the Treatment of COVID-19. (2022). Available online at: https://www.who.int/publications/m/item/who-expert-meeting-on-evaluation-of-traditional-chinese-medicine-in-the-treatment-of-covid-19 (accessed 23 May 2023).
Keywords: Chinese herbal medicine, COVID-19, systematic review, meta-analysis, randomized controlled trials
Citation: Tong L, Ma Z, Zhou Y, Yang S, Yang Y, Luo J, Huang J and Wang F (2023) Combination of Chinese herbal medicine and conventional western medicine for coronavirus disease 2019: a systematic review and meta-analysis. Front. Med. 10:1175827. doi: 10.3389/fmed.2023.1175827
Received: 28 February 2023; Accepted: 29 June 2023;
Published: 17 July 2023.
Edited by:
Shisan Bao, The University of Sydney, AustraliaReviewed by:
Liyan Yang, Qufu Normal University, ChinaCopyright © 2023 Tong, Ma, Zhou, Yang, Yang, Luo, Huang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Tong, bGVpdG9uZzAwN0AxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.