- 12nd Pulmonary Medicine Department, Medical School, General University Hospital Attikon, National and Kapodistrian University of Athens, Athens, Greece
- 27th Pulmonary Department, Athens Chest Hospital “Sotiria”, Athens, Greece
- 3Respiratory Medicine Division, Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden
- 4Cardiovascular Research Center, Simches 3 Massachusetts General Hospital, Boston, MA, United States
- 52nd Department of Radiology, Nuclear Medicine Section, Medical School, General University Hospital “Attikon”, National and Kapodistrian University of Athens, Athens, Greece
- 61st Respiratory Medicine Department, Athens Medical School, Sotiria Chest Hospital of Athens, National and Kapodistrian University of Athens, Athens, Greece
- 74th Pulmonary Department, Athens Chest Hospital “Sotiria”, Athens, Greece
- 8Department of Cardiology, Medical School, General University Hospital “Attikon”, National and Kapodistrian University of Athens, Athens, Greece
- 9First Department of Neurology, Medical School, Aeginition Hospital, National and Kapodistrian University of Athens, Athens, Greece
- 10Division of Nuclear Medicine, Biomedical Research Foundation of the Academy of Athens, Athens, Greece
Sarcoidosis is an inflammatory granulomatous disease of unknown etiology involving any organ or tissue along with any combination of active sites, even the most silent ones clinically. The unpredictable nature of the sites involved in sarcoidosis dictates the highly variable natural history of the disease and the necessity to cluster cases at diagnosis based on clinical and/or imaging common characteristics in an attempt to classify patients based on their more homogeneous phenotypes, possibly with similar clinical behavior, prognosis, outcome, and therefore with therapeutic requirements. In the course of the disease's history, this attempt relates to the availability of a means of detection of the sites involved, from the Karl Wurm and Guy Scadding's chest x-ray staging through the ACCESS, the WASOG Sarcoidosis Organ Assessment Instruments, and the GenPhenReSa study to the 18F-FDG PET/CT scan phenotyping and far beyond to new technologies and/or the current “omics.” The hybrid molecular imaging of the 18F-FDG PET/CT scan, by unveiling the glucose metabolism of inflammatory cells, can identify high sensitivity inflammatory active granulomas, the hallmark of sarcoidosis—even in clinically and physiologically silent sites—and, as recently shown, is successful in identifying an unexpected ordered stratification into four phenotypes: (I) hilar–mediastinal nodal, (II) lungs and hilar–mediastinal nodal, (III) an extended nodal supraclavicular, thoracic, abdominal, inguinal, and (IV) all the above in addition to systemic organs and tissues, which is therefore the ideal phenotyping instrument. During the “omics era,” studies could provide significant, distinct, and exclusive insights into sarcoidosis phenotypes linking clinical, laboratory, imaging, and histologic characteristics with molecular signatures. In this context, the personalization of treatment for sarcoidosis patients might have reached its goal.
Introduction
Sarcoidosis is a systemic inflammatory disease of unknown etiology, which occurs in populations worldwide and involves the lungs and the intrathoracic lymph nodes (1–3). The etiologically implicated antigen “still eludes us,” and the disease is considered a dysregulated, immune-mediated response due to its presence, persistence, and failure to clear, leading to tissue granulomas formation (4, 5). The histological hallmark of the disease indeed constitutes well-formed, non-caseating granulomas that may localize in any organ or tissue without boundaries and with any combination patterns of active sites of involvement in most of the cases, notably, even in the clinically silent (6, 7) cases. The detection of granulomas with the abovementioned characteristics is never pathognomonic for the disease (8). Therefore, it is necessary to ensure diagnosis in most cases is associated with the combination of compatible clinical, laboratory, and imaging characteristic patterns with histologic findings, as well as the exclusion of any other etiology of granulomatous inflammation (1, 9).
However, clinical compatibility for sarcoidosis in most cases—beyond the well-known and quite pathognomonic clinical syndromes (phenotypes), such as Löfgren's syndrome and Heerfordt's syndrome—constitutes primarily the unpredictable and “anarchic” tissue distribution of the disease in combination with some characteristic imaging features from the lungs, in addition to the asymptomatic or oligosymptomatic disease's first appearance (1, 10–12). Due to the unpredictability of the sites involved in sarcoidosis, the disease has a highly variable natural history, and since every sarcoidosis patient represents a distinctive case of theirown, individual management strategies may be imperative (13–20).
Clustering or better phenotyping patients with the disease at diagnosis on the basis of clinical and/or imaging common characteristics is an old attempt to assign patients with an unpredictable disease to more or less homogeneous groups possibly with similar clinical behavior, prognosis, and outcome and therefore with similar therapeutics requirements (21–30). In the course of the history of the disease, this attempt relates to the availability of the current means of detection of any site of involvement in a systemic disease, from the chest x-ray of Karl Wurm and Guy Scadding's staging and through the ACCESS study, the WASOG Sarcoidosis Organ Assessment Instrument, and the GenPhenReSa study to the 18F-fluoro-2-deoxyglucose (18F-FDG) positron emission tomography (PET) computed tomography (CT) scan phenotyping and far beyond to new technologies and/or the current “omics” availability (31–41). Stratification of sarcoidosis patients based on T-cell count in Bronchoalveolar Lavage (BAL) and gallium scan, the presence of impaired physiology, fibrosis, and pulmonary hypertension, as well as clinical activity, as reflected by acute or non-acute disease in onset, treatment, and long-term treatment requirements, and the clinical outcome status of the disease have also been described (24, 42–48). Successful phenotyping, to be clinically useful by the physicians in everyday clinical practice, should be easy, simple, reliable in unraveling most, if not all, sites of disease involvement, less expensive, reproducible worldwide in different populations with the disease, and able to offer information concerning the clinical behavior of the individual phenotypes, thus providing valuable information for the decision-making process (49). The corpus of this research article has been focused on the initial evaluation of the sarcoidosis patient.
Ordering the unpredictable
Sometimes sarcoidosis creates order within itself. Sven Löfgren was the first to associate erythema nodosum and bilateral hilar lymphadenopathy with sarcoidosis—an early or acute manifestation of the disease (fever and polyarthritis commonly coexist) that is fairly distinct from tuberculosis, with good prognosis—an assumption that maintains its value till present (50–52). Cristian Heerfordt was the first to describe that, in sarcoidosis, the “febris uveoparotidea subchronica” (fever, parotid enlargement, and uveitis) was occasionally associated with the seventh nerve palsy and other rare manifestations of the disease (53). Both the above two phenotypes though fairly uncommon maintain a significant diagnostic value not necessitating histologic confirmation in most if not in all patients. Prognosis is excellent in Löfgren's, and indeterminate in the Heerfordt's syndrome (1, 8). However, despite the fact that the abovementioned phenotypes are narrow in terms of clinical manifestations and are relatively homogeneous, nothing is known about other silent systemic sites of active disease; better detection and definitive identification of these silent systemic sites are possible with the application of new technologies (7). As disease evolved, technology and research followed its lead.
In the beginning it was Wilhelm Conrad Röntgen
In the beginning, it was Wilhelm Conrad Röntgen (recipient of Nobel Prize in Physics in 1901) who was unaware of sarcoidosis, and by discovering the electromagnetic radiation known as Röntgen rays (X-rays), he offered the availability of chest roentgenogram to the pioneers of the disease (Ernest Besnier, Caesar Boeck, and Jörgen Schaumann, who were the men behind the sarcoidosis disease and the disease was named after them in early days) rendering them aware that the skin, eyes and joints disease in front of them was part of an internal organ, systemic disease involving almost always the lungs and the intrathoracic lymph nodes: the indispensable first encounter of every physician with any sarcoidosis patient to this day (54–56).
Karl Wurm and Guy Scadding's staging first step through chest radiology
The first chest röentgenogram (x-rays) classification of sarcoidosis, which was designed by Karl Wrum, was divided into three stages (stage I bilateral hilar–mediastinal lymphadenopathy, stage II lungs reversible involvement and stage III lungs fibrosis) and was the first attempt to draw prognostic considerations of the disease, and accordingly arriving at therapeutic decisions for sarcoidosis patients (31, 32). It belongs to Guy Scadding the current chest radiological staging of sarcoidosis in “four groups” as firstly reported, [group 1) enlarged hilar lymph-nodes, group 2) hilar nodes and lung shadowing, group 3) lung shadowing and group 4) fibrosis, though not always “sharply demarcated”] and belong to him the seminal conclusions regarding the prognostic significance of the above radiologic grouping as well as the fundamental clinical advice that corticosteroids are necessary only in a minority of patients (33) (Figure 1A), all of which were determined by Guy Scadding. Moreover, Guy Scadding also confirmed previous observations reporting the benign clinical course and spontaneous resolution of the Löfgren's syndrome phenotype. Several years later, Johan Grunewald and Anders Eklund provided additional significant information regarding the influence of the genetic background, human leukocytes antigen (HLA) polymorphisms, particularly the HLA-DRB1*03 allele on the outcome of this phenotype—a new step in the effort to draw information about phenotypes from the science advancement adding to the clinical information the results of the “bench” (11, 23, 57–61). During the early Scadding's times, the awareness of extrathoracic manifestations of sarcoidosis was mostly limited to the skin, eyes, parotids, superficial lymph nodes, joints, and few others, and there were no reliable conclusions that could be added regarding the clinicians' influence on disease's clinical behavior, prognosis, and outcome. In modern times, the necessity to evolve from Karl Wurm and Guy Scadding's staging was unavoidable and possible because of the advancement of technology and scientific thinking (49).
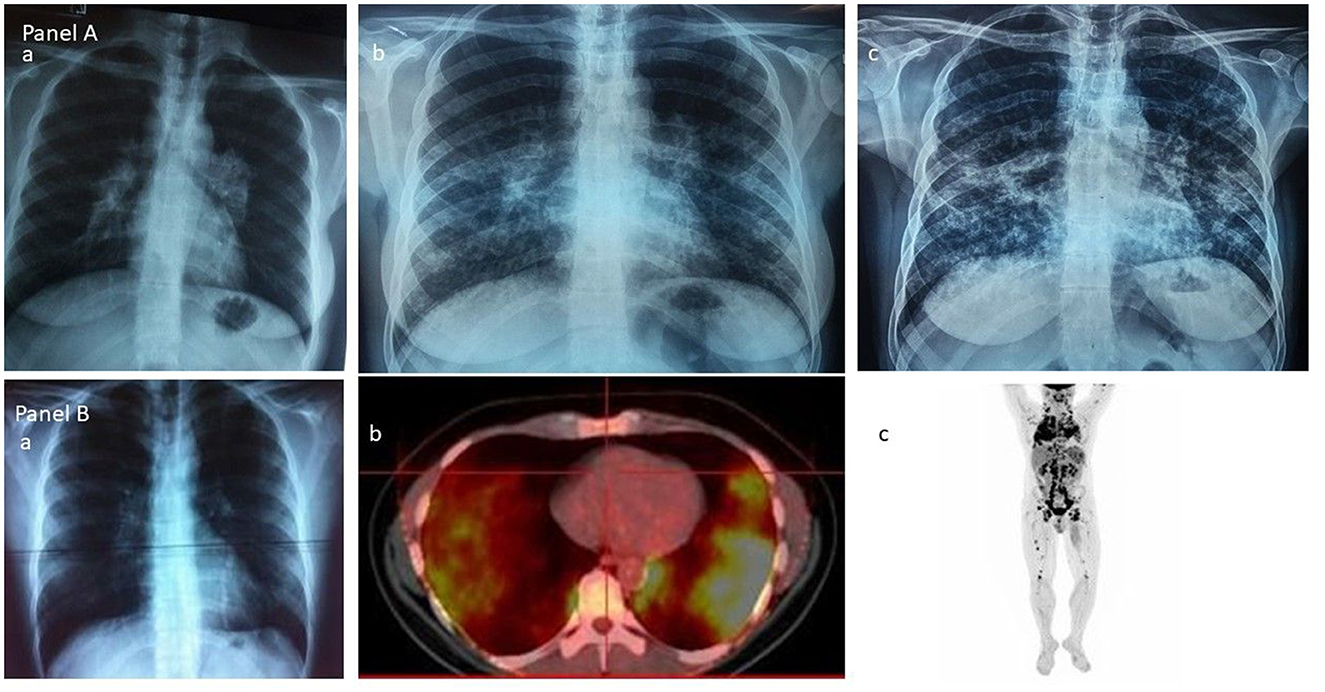
Figure 1. (A) The Scadding's vision of sarcoidosis through the chest roentgenogram: group 1—enlarged hilar lymph-nodes; group 2—hilar nodes and lung shadowing; and group 3—lung shadowing. (B) The current vision of sarcoidosis through the 18F-FDG PET/CT scan: (a) and (b) the lungs; (c) the whole body.
The ACCESS study for the definition of organ involvement in sarcoidosis
To solve the problem of the elusive identification of the etiologically implicated antigen in sarcoidosis, the National Heart, Lung, and Blood Institute (NHLBI) set up the A Case Control Etiologic Study of Sarcoidosis (ACCESS) multicenter study (34–36). In present times, the ACCESS study proposed an instrument to assess and define organ involvement in sarcoidosis, a clear step that has been taken ahead to evolve from Karl Wurm and Guy Scadding's staging. It was successful in identifying differences in HLA gene associations with sarcoidosis among European and African Americans; however, for several reasons, the instrument failed to address all possible sites of the disease's activity, which is indispensable to identifying phenotypes (62). Furthermore, the development of new technologies made the ACCESS instrument outdated, and the necessity for organ assessment in sarcoidosis resulted in developing new instruments.
The WASOG Sarcoidosis Organ Assessment Instrument
The WASOG Sarcoidosis Organ Assessment Instrument was developed as an update of the previous ACCESS tool to establish reliable criteria for the probability of any organ being involved in the disease in a sarcoidosis patient, in the new technology era. The probability of organ involvement from sarcoidosis was based on two criteria: granulomatous inflammation and compatible clinical manifestation, excluding in both cases alternative etiologies. Based on the above findings, clinical manifestations were graded as (1) highly probable, 90% likelihood, (2) probable, 50–90% likelihood, and (3) possible, <50% likelihood. For each manifestation, an agreement of 70% was needed for consensus (Delphi study methodology). Indeterminate probability was defined when consensus was not reached (37).
GenPhenReSa consortium
The GenPhenReSa consortium is the first attempt, entirely utilizing the WASOG Sarcoidosis Organ Assessment Instrument to identify almost all sites of involvement by the disease, in order firstly to identify reliable and homogeneous phenotypes of sarcoidosis appearance, useful cohorts for further biomedical studies and secondly to attempt possible genotype–phenotype associations were detected. The results of the phenotype module of the GenPhenReSa as evidenced by the cluster analysis of 2,163 Caucasian patients phenotyped in 31 different sarcoidosis expert study centers regard the identification of five “distinct” subgroups identified from organ involvement: (1) abdominal organ involvement, (2) ocular-cardiac-cutaneous-central nervous system disease involvement, (3) musculoskeletal-cutaneous involvement, (4) pulmonary and intrathoracic lymph node involvement, and (5) extrapulmonary involvement, defined by the authors as “homogeneous cohorts useful for further biomedical studies” (38). However, some concerns arise at first glance regarding distinctness and homogeneity since the same authors acknowledge that “new technologies will enable better detection of organ involvement”; therefore, frequencies and clusters of organ involvement are likely to change over time. Indeed, using the abovementioned system to define organ involvement appears laborious in terms of the necessity for several tests and examinations as well as somewhat controversial in terms of the homogeneity of clusters since phenotyping appears to overlap and is somewhat ambiguous.
18F-FDG PET/CT scan phenotyping
The hybrid molecular imaging of the 18F-FDG PET/CT scan by unveiling glucose metabolism of inflammatory cells is able to identify, with high sensitivity, inflammatory active granulomas, the hallmark of sarcoidosis, even in clinically and physiologically silent sites, providing simultaneously whole-body PET and CT images (63, 64). Therefore, the 18F-FDG PET/CT scan appears to be the ideal instrument for the assessment of organs involved in sarcoidosis in detecting their intrinsic inflammatory activity (65–67) (Figure 1B) in the most beneficial fashion. Through the 18F-FDG PET/CT scan, investigators may attain a far better understanding of sarcoidosis physical history, behavior, unveiling patterns of disease expression, subdividing patients by clusters, and identifying phenotypes. Recently, Papiris et al. by implementing an 18F-FDG PET/CT scan in newly diagnosed, especially in the treatment-naïve patients with sarcoidosis, identified, despite the random distribution of the disease, by a statistical method called hierarchical cluster analysis, an unexpected ordered stratification into four phenotypes: (I) hilar–mediastinal nodal, (II) lungs and hilar–mediastinal nodal, (III) an extended nodal supraclavicular, thoracic, abdominal, inguinal, and (IV) all the above in addition to systemic organs and tissues such as muscles–bones–spleen and skin (39) (Figure 2). Although this approach represents a simplified assessment of sarcoidosis where the use of only one investigative tool and in “one shot” depicts the universal expression of the disease by its active presence, the approach indisputably offered a much logical picture of the disease much closer to the perception of caring clinicians. Furthermore, this “one-shop-stop” in the acquisition of data offers “an open book” approach to the clinicians in getting themselves exposed to every single “page written by the disease” by scientists and experts who have in-depth knowledge and to decide on therapeutic requirements. The organ clusters (phenotypes) identified in this analysis remain far better than anyone else's analysis carried out before and are in accordance with the existing knowledge of sarcoidosis. The phenotypes I and II appeared to coincide with the familial Scadding's first two stages; however, by applying 18F-FDG PET/CT scan, all other sites of disease involvement were surely excluded, a sort of “pure organs Scadding's stages I and II,” while in Scadding's staging the other coexisting sites of disease involvement slowly got away from the disease scenario and went unidentified. The phenotype III disclosed was rather suspected in sarcoidosis patients and occasionally observed clinically and by other means such as computerized tomography (CT) scans and echocardiogram (ECHO). However, for the first time, the disease involvement was clearly identified in all possible extensions of the actual site, and a lot of work must be done to identify its prognostic significance and therapeutic requirements (apparently necessitating none). Finally, the phenotype IV appears familial to the clinician-caring sarcoidosis patients since it appears to be satisfying to the current knowledge of the systemic nature of the disease, probably relating to the systemic spreading, from the lungs and hilar–mediastinal lymph nodes to the whole body, of the etiologically implicated antigen. Therefore, by clustering investigations of sarcoidosis through the 18F-FDG PET/CT scan, clinically logical phenotypes were identified. However, like all other clustering investigations, some patients may not respect the boundaries but also Scadding's staging by the simplest of the means of investigation, the chest roentgenogram, which clarifies that stages may not be “sharply demarcated,” and we feel right to confirm his elaboration concerning clustering. Based on hierarchical cluster analysis of the study population and implementing adequate preparation with low carbohydrate diet for 24 h followed by 18-h fasting to suppress radiotracer's uptake by normal myocardial cell, no significant difference was detected between the clusters regarding myocardial involvement, suggesting that heart disease could be detected and should be evaluated in any cluster. Given the important prognostic and treatment implications of cardiac involvement, the role of 18F-FDG PET/CT scan has been examined extensively. Although this modality requires a specific protocol including diet restriction and being more prone than cardiac magnetic resonance (CMR) to provide false positive results, it is a technique much more sensitive than transthoracic echocardiogram (70 vs. 25%) that should be used as complementary to CMR for the detection of myocardial inflammation and fibrosis, respectively (16, 39, 68, 69). Certainly, the low specificity of 18F-FDG PET/CT scan poses several concerns regarding the differentiation with any neoplasm or other inflammatory etiology, but in the patient diagnosed with sarcoidosis, further approach in unusual or suspected sites investigated by additional investigative tools including biopsy should be warranted (70). The 18F-FDG PET/CT scan molecular imaging by its high sensibility in detecting organs' intrinsic inflammatory activity, its worldwide availability, the “relatively low” radiation impact, the reasonable cost, and its reliability to evaluate treatment response appears the ideal instrument for phenotyping in sarcoidosis. However, the 18F-FDG PET/CT is unable to “see” the eye; therefore, to obtain a complete phenotype, an ophthalmologic examination is mandatory (71–77). Neurological and endocrinology evaluations are also indispensable for thoroughness to detect neurosarcoidosis, small-fiber neuropathy, and abnormal calcium metabolism (78–81). Until further well-designed multicenter studies are performed to confirm the abovementioned findings as universal patterns of disease behavior, we do not consider this study an ideal destination for sarcoidosis and its assessment but rather treat it as just another journey ahead. 18F-FDG PET/CT should be used rationally and wisely, its radiation exposure that is comparable to whole-body diagnostic CT should also be taken into consideration and should be balanced by its higher sensitivity for both thoracic and extrathoracic disease. The unveiling of the abovementioned phenotypes, especially in treatment naïve patients at the first glance on diagnosis, appears useful in designing future studies with more homogeneous cohorts, toward the acquisition in sarcoidosis patients of a more personalized medicine approach (39). Currently, relevant studies are lacking; whether 18F-FDG PET/CT should be recommended to all patients and whether 18F-FDG PET/CT phenotyping could provide additional information on outcome, treatment indications, silent lung disease, such as asymptomatic radiographic stage I included, should be further validated.
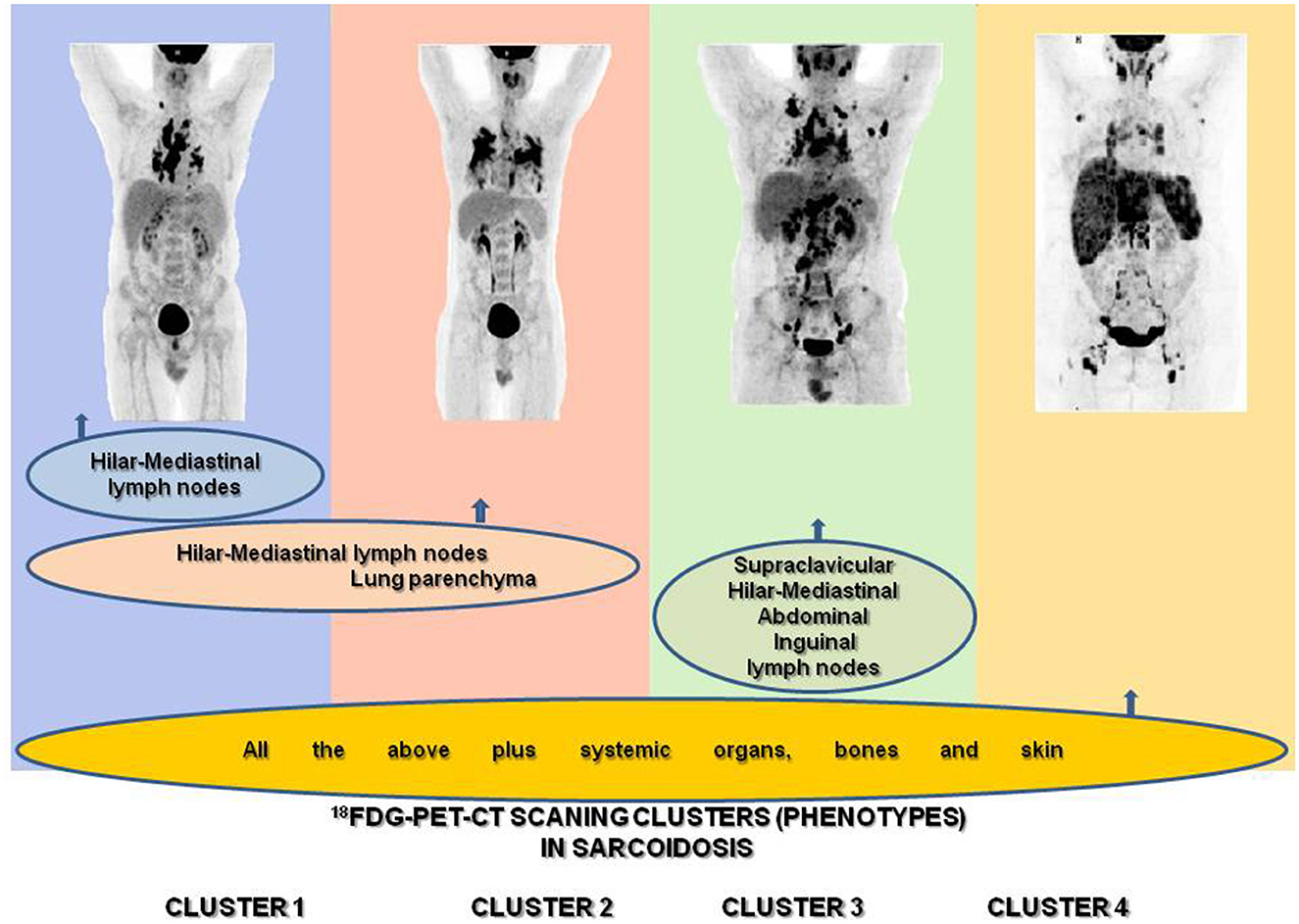
Figure 2. Identification of sarcoidosis 18F-FDG PET/CT scan phenotypes according to organ involvement using two-step cluster analysis: (1) hilar–mediastinal nodal, (2) lungs and hilar–mediastinal nodal, (3) an extended nodal supraclavicular, thoracic, abdominal, inguinal, and (4) all the above in addition to systemic organs and tissues such as muscles–bones–spleen and skin.
The “omics era”
“Omics” is a high-throughput technology that allows for the comprehensive profiling of various biochemical molecules in a context-dependent manner in multiple organisms. “Omics” studies include genetics, epigenetics, transcriptomics, proteomics, lipidomics, metabolomics, and microbiomics. Given that sarcoidosis is a complex, polygenic disease of unknown cause with diverse clinical phenotypes, the “omics” approach adds another layer of information by delving into the molecular signatures that could provide important insights on the pathogenesis of this disease (4, 5, 41, 82–88). Genetic predisposition and immune dysregulation, inherent to sarcoidosis, are investigated by characterizing both previously known and newly discovered immune-cell-specific pathways, gene expression, and epigenetic modifications that may differ between specific tissues and compartments as well as between progressive and non-progressive disease manifestations (41, 89–94). Through “omics,” we have currently gained access to a very advanced group of techniques that can provide conceptual, mechanistic, and molecular interpretations of phenotypes irrespective of treatment. For instance, genome-wide association studies (GWAS) examined the genetic composition of many genomes to identify variants associated with specific traits or diseases. GWASs provide insight into phenotypic biology, predict clinical outcomes, and reveal causal relationships between risk factors and health outcomes. Recent GWAS in European, African American, and Asian populations identified a non-synonymous single-nucleotide polymorphism (SNP), rs1049550, within the annexin A11 (ANXA11) gene as being associated with susceptibility to sarcoidosis (95). The RNA-sequencing (RNA-seq) technologies, such as bulk and single-cell RNA-sequencing (scRNA-seq), permit unbiased interrogation of the whole transcriptome and deep immunophenotyping of heterogeneous single cells suspensions, respectively. The RNA-seq data analysis for differential gene expression has become a standard method for comparing gene expression among healthy and diseased individuals, tissues, and cell types and can provide valuable information about dysregulated pathways and inciting disease mechanisms. ScRNA-seq can then be combined with spatial transcriptomics and cellular proteomics to provide a “transcriptometabolomic” map of the cell's active status in healthy and diseased conditions. Using this approach, a recent study examined the immunostructural stoichiometry of sarcoidosis patients' granulomas, revealing that granulomas hijack the transcriptional programs that regulate normal lymphoid organ development and alter cytokine and chemokine pathways (96). These findings may explain the increased inflammatory activity and 18F-FDG uptake observed in those tissues and aid in identifying potentially targetable molecules for therapeutic development. Another method that combines single-cell transcriptomics with proteomics, which is called cellular indexing of transcriptomes and epitopes (CITE-Seq), is a sequencing-based method that allows simultaneous quantification of cell surface protein and single-cell sequencing data, enabling both surface marker and transcriptomic phenotyping of immune cells that are dysregulated in various diseases (97). Since cell surface proteins are indicators of cell phenotype and functional status, CITE-seq may provide a superior method for profiling immune cells in sarcoidosis when compared with scRNA-seq or proteomic analysis alone (98). While “omics” cannot be directly applied to everyday clinical practice, they could provide significant, distinct, and exclusive insights into the described phenotypes of sarcoidosis by combining clinical, laboratory, imaging, and histologic characteristics with molecular signatures in a sort of “reverse phenotyping” approach. Eventually, the future may see an algorithm combining all the aforementioned methods to efficiently diagnose, phenotype, and eventually lead to efficient sarcoidosis treatment. However, larger studies need to be conducted to arrive to robust conclusions that can be developed further into clinical applications.
Perspective in the evading history of phenotyping in sarcoidosis
Discussion
As we critically reviewed the history of phenotyping in sarcoidosis, we realized that we have constantly been making progress from what could initially be observed by the inquiring minds of scientists only through the naked eye to what we are currently able to observe using the recent great advances in technology, and the progress seems enormous (99–101). For example, the contribution of 18F-FDG PET/CT scan in enriching our ability to screen patients with sarcoidosis for all organs and sites involved, even the most silent ones, in “one shot” is indisputable as well as the ability of -omics studies to provide unbiased insights about pathophysiological and molecular signatures that could be representative of specific sarcoidosis phenotypes, is still unperceivable to clinical observation (39, 41). However, to this day, clinical observation and judgment are the first means of capturing and validating the existence of the phenotypes associated with sarcoidosis. Defining and evolving disease phenotypes is perpetually motivated by the desire to better understand the disease and its many obscure aspects (4). The utility of phenotypes becomes increasingly relevant as studies that intend to elucidate disease mechanisms underlying sarcoidosis pathogenesis and to identify biomarkers that can be used for diagnosis, prediction of outcomes, and optimization of disease management can only be carried out in well-phenotyped populations of multiple ethnic origins; therefore, an initiative requiring international collaboration efforts, such as the Multi-Ethnic Sarcoidosis Genomics Consortium (MESARGEN), may bring together many scientists and clinicians from around the world toward this goal. 1
Due to its high sensitivity in detecting intrinsic inflammatory activity, 18F-FDG PET/CT scanning appears to be the most appropriate tool for guiding and orchestrating our efforts in phenotyping in sarcoidosis in the future. In the era of “omics”, the research could provide unique insights into sarcoidosis phenotypes through the association of clinical, laboratory, imaging, and histologic characteristics with molecular signatures. In this context, the personalization of treatment for sarcoidosis patients might have reached its goal.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SP: concept and design of the study (text and figures), analysis and interpretation of all data, and wrote the manuscript. LK, NR, MS, GL, PG, and EC: interpretation of the data, wrote parts of the manuscript, and revised the work critically for very important intellectual content. AG and MK: produced the figures and revised the work critically for important intellectual content. AP, TR, VA, E-MA, EG, SC, and JG: major contribution in the interpretation of the data and revised the work critically for important intellectual content. EM: major contribution to the concept of the study, to the acquisition, analysis and interpretation of data, had access to all data, supervised the accuracy and integrity of any part of the work, and wrote the final version of the manuscript with SP. All authors read and approved the final version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^Available online at: https://mesargen.org/.
References
1. Grunewald J, Grutters JC, Arkema EV, Saketkoo LA, Moller DR, Müller-Quernheim J. Sarcoidosis. Nat Rev Dis Primers. (2019) 5:45. doi: 10.1038/s41572-019-0096-x
2. Arkema EV, Cozier YC. Sarcoidosis epidemiology: recent estimates of incidence, prevalence and risk factors. Curr Opin Pulm Med. (2020) 26:527–34. doi: 10.1097/MCP.0000000000000715
3. Ma X, Zhu L, Kurche JS, Xiao H, Dai H, Wang C. Global and regional burden of interstitial lung disease and pulmonary sarcoidosis from 1990 to 2019: results from the global burden of disease study 2019. Thorax. (2022) 77:596–605. doi: 10.1136/thoraxjnl-2020-216732
4. Judson MA. A primer on the clinical aspects of sarcoidosis for the basic and translational scientist. J Clin Med. (2021) 10:2857. doi: 10.3390/jcm10132857
5. Grunewald J, Spagnolo P, Wahlström J, Eklund A. Immunogenetics of disease-causing inflammation in sarcoidosis. Clin Rev Allergy Immunol. (2015) 49:19–35. doi: 10.1007/s12016-015-8477-8
6. Tana C, Donatiello I, Caputo A, Tana M, Naccarelli T, Mantini C, et al. Clinical features, histopathology and differential diagnosis of sarcoidosis. Cells. (2021) 11:59. doi: 10.3390/cells11010059
7. Judson MA. Screening sarcoidosis patients for occult disease. Semin Respir Crit Care Med. (2020) 41:741–57. doi: 10.1055/s-0040-1709496
8. Judson MA. The diagnosis of sarcoidosis. Curr Opin Pulm Med. (2019) 25:484–96. doi: 10.1097/MCP.0000000000000596
9. Judson MA. Granulomatous sarcoidosis mimics. Front Med. (2021) 8:680989. doi: 10.3389/fmed.2021.680989
10. Palmucci S, Torrisi SE, Caltabiano DC, Puglisi S, Lentini V, Grassedonio E, et al. Clinical and radiological features of extra-pulmonary sarcoidosis: a pictorial essay. Insights Imaging. (2016) 7:571–87. doi: 10.1007/s13244-016-0495-4
11. Karakaya B, Kaiser Y, van Moorsel CHM, Grunewald J. Löfgren's syndrome: diagnosis, management, and disease pathogenesis. Semin Respir Crit Care Med. (2017) 38:463–76. doi: 10.1055/s-0037-1602380
12. Dua A, Manadan A. Images in clinical medicine. Heerfordt's syndrome, or uveoparotid fever. N Engl J Med. (2013) 369:458. doi: 10.1056/NEJMicm1303454
13. Kolilekas L, Triantafillidou C, Manali E, Rontogianni D, Chatziioannou S, Papiris S. The many faces of sarcoidosis: asymptomatic muscle mass mimicking giant-cell tumor. Rheumatol Int. (2009) 29:1389–90. doi: 10.1007/s00296-009-0989-1
14. Froudarakis ME, Bouros D, Voloudaki A, Papiris S, Kottakis Y, Constantopoulos SH, et al. Pneumothorax as a first manifestation of sarcoidosis. Chest. (1997) 112:278–80. doi: 10.1378/chest.112.1.278
15. Papadavid E, Dalamaga M, Stavrianeas N, Papiris SA. Subcutaneous sarcoidosis masquerading as cellulitis. Dermatology. (2008) 217:212–4. doi: 10.1159/000142945
16. Crouser ED, Maier LA, Wilson KC, Bonham CA, Morgenthau AS, Patterson KC, et al. Diagnosis and detection of sarcoidosis. an official American thoracic society clinical practice guideline. Am J Respir Crit Care Med. (2020) 201:e26–51. doi: 10.1164/rccm.202002-0251ST
17. Baughman RP, Valeyre D, Korsten P, Mathioudakis AG, Wuyts WA, Wells A, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. (2021) 58:2004079. doi: 10.1183/13993003.04079-2020
18. Gupta R, Judson MA, Baughman RP. Management of advanced pulmonary sarcoidosis. Am J Respir Crit Care Med. (2022) 205:495–506. doi: 10.1164/rccm.202106-1366CI
19. Trivieri MG, Spagnolo P, Birnie D, Liu P, Drake W, Kovacic JC, et al. Challenges in cardiac and pulmonary sarcoidosis: Jacc State-of-the-Art review. J Am Coll Cardiol. (2020) 76:1878–901. doi: 10.1016/j.jacc.2020.08.042
20. Calandriello L, D'Abronzo R, Pasciuto G, Cicchetti G, Del Ciello A, Farchione A, et al. Novelties in imaging of thoracic sarcoidosis. J Clin Med. (2021) 10:2222. doi: 10.3390/jcm10112222
21. Lin NW, Arbet J, Mroz MM, Liao SY, Restrepo CI, Mayer AS, et al. Clinical phenotyping in sarcoidosis using cluster analysis. Respir Res. (2022) 23:88. doi: 10.1186/s12931-022-01993-z
22. Loddenkemper R, Kloppenborg A, Schoenfeld N, Grosser H, Costabel U. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis–results of a cooperative study in former West Germany and Switzerland. Watl Study Group. Wissenschaftliche Arbeitsgemeinschaft Für Die Therapie Von Lungenkrankheitan. Sarcoidosis Vasc Diffuse Lung Dis. (1998) 15:178–82.
23. Scadding JG. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years' observation. Br Med J. (1961) 2:1165–72. doi: 10.1136/bmj.2.5261.1165
24. Prasse A, Katic C, Germann M, Buchwald A, Zissel G, Müller-Quernheim J. Phenotyping sarcoidosis from a pulmonary perspective. Am J Respir Crit Care Med. (2008) 177:330–6. doi: 10.1164/rccm.200705-742OC
25. Lundkvist A, Kullberg S, Arkema EV, Cedelund K, Eklund A, Grunewald J, et al. Differences in disease presentation between men and women with sarcoidosis: a cohort study. Respir Med. (2022) 191:106688. doi: 10.1016/j.rmed.2021.106688
26. Abo Al Hayja M, Wahlström J, Kullberg S, Darlington P, Eklund A, Grunewald J. Bronchoalveolar lavage fluid cell subsets associate with the disease course in Löfgren's and Non-Löfgren's sarcoidosis patients. Respir Med. (2021) 186:106521. doi: 10.1016/j.rmed.2021.106521
27. Rossides M, Grunewald J, Eklund A, Kullberg S, Di Giuseppe D, Askling J, et al. Familial aggregation and heritability of sarcoidosis: a Swedish nested case-control study. Eur Respir J. (2018) 52:1800385. doi: 10.1183/13993003.00385-2018
28. Lhote R, Annesi-Maesano I, Nunes H, Launay D, Borie R, Sacré K, et al. Clinical phenotypes of extrapulmonary sarcoidosis: an analysis of a French, multi-ethnic, multicentre cohort. Eur Respir J. (2021) 57:2001160. doi: 10.1183/13993003.01160-2020
29. Spyropoulos G, Domvri K, Manika K, Fouka E, Kontakiotis T, Papakosta D. Clinical, imaging and functional determinants of sarcoidosis phenotypes in a greek population. J Thorac Dis. (2022) 14:1941–9. doi: 10.21037/jtd-21-1760
30. Crouser ED, Amin EN. Severe sarcoidosis phenotypes: an occupational hazard? Chest. (2016) 150:263–5. doi: 10.1016/j.chest.2016.02.663
31. Meier G, Wurm K. On the prognosis of sarcoidosis (Boeck's disease). Beitr Klin Tuberk Spezif Tuberkuloseforsch. (1960) 123:90–7. doi: 10.1007/BF02142474
32. Wurm K. The significance of stage classification of sarcoidosis (Boeck's disease). Dtsch Med Wochenschr. (1960) 85:1541–8. doi: 10.1055/s-0028-1112616
33. Scadding JG. The late stages of pulmonary sarcoidosis. Postgrad Med J. (1970) 46:530–6. doi: 10.1136/pgmj.46.538.530
34. Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H Jr, Bresnitz EA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. (2001) 164:1885–9. doi: 10.1164/ajrccm.164.10.2104046
35. Rossman MD, Kreider ME. Lesson learned from access (a case controlled etiologic study of sarcoidosis). Proc Am Thorac Soc. (2007) 4:453–6. doi: 10.1513/pats.200607-138MS
36. Judson MA, Baughman RP, Teirstein AS, Terrin ML, Yeager H Jr. Defining organ involvement in sarcoidosis: the access proposed instrument. access research group. a case control etiologic study of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. (1999) 16:75–86.
37. Judson MA, Costabel U, Drent M, Wells A, Maier L, Koth L, et al. The WASOG sarcoidosis organ assessment instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. (2014) 31:19–27.
38. Schupp JC, Freitag-Wolf S, Bargagli E, Mihailović-Vučinić V, Rottoli P, Grubanovic A, et al. Phenotypes of organ involvement in sarcoidosis. Eur Respir J. (2018) 51:1700991. doi: 10.1183/13993003.00991-2017
39. Papiris SA, Georgakopoulos A, Papaioannou AI, Pianou N, Kallergi M, Kelekis NL, et al. Emerging phenotypes of sarcoidosis based on 18F-FDG PET/CT: a hierarchical cluster analysis. Expert Rev Respir Med. (2020) 14:229–38. doi: 10.1080/17476348.2020.1684902
40. Garman L, Montgomery CG, Rivera NV. Recent advances in sarcoidosis genomics: epigenetics, gene expression, and gene by environment (G × E) interaction studies. Curr Opin Pulm Med. (2020) 26:544–53. doi: 10.1097/MCP.0000000000000719
41. Bhargava M, Liao SY, Crouser ED, Maier LA, Leach SM. The landscape of transcriptomic and proteomic studies in sarcoidosis. ERJ Open Res. (2022) 8:00621–2021. doi: 10.1183/23120541.00621-2021
42. Pinkston P, Bitterman PB, Crystal RG. Spontaneous release of interleukin-2 by lung T lymphocytes in active pulmonary sarcoidosis. N Engl J Med. (1983) 308:793–800. doi: 10.1056/NEJM198304073081401
43. Schoenberger CI, Line BR, Keogh BA, Hunninghake GW, Crystal RG. Lung inflammation in sarcoidosis: comparison of serum angiotensin-converting enzyme levels with bronchoalveolar lavage and gallium-67 scanning assessment of the T lymphocyte alveolitis. Thorax. (1982) 37:19–25. doi: 10.1136/thx.37.1.19
44. Balbi B, Moller DR, Kirby M, Holroyd KJ, Crystal RG. Increased numbers of T lymphocytes with gamma delta-positive antigen receptors in a subgroup of individuals with pulmonary sarcoidosis. J Clin Invest. (1990) 85:1353–61. doi: 10.1172/JCI114579
45. Walsh SL, Wells AU, Sverzellati N, Keir GJ, Calandriello L, Antoniou KM, et al. An integrated clinicoradiological staging system for pulmonary sarcoidosis: a case-cohort study. Lancet Respir Med. (2014) 2:123–30. doi: 10.1016/S2213-2600(13)70276-5
46. Shlobin OA, Kouranos V, Barnett SD, Alhamad EH, Culver DA, Barney J, et al. physiological predictors of survival in patients with sarcoidosis-associated pulmonary hypertension: results from an international registry. Eur Respir J. (2020) 55:1901747. doi: 10.1183/13993003.01747-2019
47. Kirkil G, Lower EE, Baughman RP. Predictors of mortality in pulmonary sarcoidosis. Chest. (2018) 153:105–13. doi: 10.1016/j.chest.2017.07.008
48. Baughman RP, Nagai S, Balter M, Costabel U, Drent M, du Bois R, et al. Defining the clinical outcome status (COS) in sarcoidosis: results of WASOG task force. Sarcoidosis Vasc Diffuse Lung Dis. (2011) 28:56–64.
49. Culver DA, Baughman RP. It's time to evolve from scadding: phenotyping sarcoidosis. Eur Respir J. (2018) 51:1800050. doi: 10.1183/13993003.00050-2018
50. Lofgren S, Lundback H. The bilateral hilar lymphoma syndrome; a study of the relation to tuberculosis and sarcoidosis in 212 cases. Acta Med Scand. (1952) 142:265–73. doi: 10.1111/j.0954-6820.1952.tb13865.x
51. Lofgren S. Primary pulmonary sarcoidosis. II. Clinical course and prognosis. Acta Med Scand. (1953) 145:465–74. doi: 10.1111/j.0954-6820.1953.tb07044.x
53. Theobald GD, Wilder HL. Heerfordt's syndrome. Trans Am Acad Ophthalmol Otolaryngol. (1953) 57:332–3.
55. Young RC Jr, Rachal RE, Cowan CL Jr. Sarcoidosis–the beginning: historical highlights of personalities and their accomplishments during the early years. J Natl Med Assoc. (1984) 76:887–96.
56. Spagnolo P. Sarcoidosis: a critical review of history and milestones. Clin Rev Allergy Immunol. (2015) 49:1–5. doi: 10.1007/s12016-015-8480-0
57. Wolin A, Lahtela EL, Anttila V, Petrek M, Grunewald J, van Moorsel CHM, et al. SNP variants in major histocompatibility complex are associated with sarcoidosis susceptibility-a joint analysis in four european populations. Front Immunol. (2017) 8:422. doi: 10.3389/fimmu.2017.00422
58. Kaiser Y, Lepzien R, Kullberg S, Eklund A, Smed-Sörensen A, Grunewald J. Expanded lung T-bet+RORγT+ CD4+ T-cells in sarcoidosis patients with a favourable disease phenotype. Eur Respir J. (2016) 48:484–94. doi: 10.1183/13993003.00092-2016
59. Darlington P, Gabrielsen A, Sörensson P, Tallstedt L, Padyukov L, Eklund A, et al. Hla-Alleles associated with increased risk for extra-pulmonary involvement in sarcoidosis. Tissue Antigens. (2014) 83:267–72. doi: 10.1111/tan.12326
60. Grunewald J, Eklund A. Löfgren's syndrome: human leukocyte antigen strongly influences the disease course. Am J Respir Crit Care Med. (2009) 179:307–12. doi: 10.1164/rccm.200807-1082OC
61. Grunewald J, Brynedal B, Darlington P, Nisell M, Cederlund K, Hillert J, et al. Different HLA-DRB1 Allele distributions in distinct clinical subgroups of sarcoidosis patients. Respir Res. (2010) 11:25. doi: 10.1186/1465-9921-11-25
62. Rossman MD, Thompson B, Frederick M, Maliarik M, Iannuzzi MC, Rybicki BA, et al. HLA-DRB1*1101: a significant risk factor for sarcoidosis in blacks and whites. Am J Hum Genet. (2003) 73:720–35. doi: 10.1086/378097
63. Adams H, van Rooij R, van Moorsel CHM, Spee-Dropkova M, Grutters JC, Keijsers RG. Volumetric FDG pet analysis of global lung inflammation: new tool for precision medicine in pulmonary sarcoidosis? Sarcoidosis Vasc Diffuse Lung Dis. (2018) 35:44–54. doi: 10.36141/svdld.v35i1.5807
64. Keijsers RG, van den Heuvel DA, Grutters JC. Imaging the inflammatory activity of sarcoidosis. Eur Respir J. (2013) 41:743–51. doi: 10.1183/09031936.00088612
65. Vagts C, Ascoli C, Fraidenburg DR, Baughman RP, Huang Y, Edafetanure-Ibeh R, et al. Unsupervised clustering reveals sarcoidosis phenotypes marked by a reduction in lymphocytes relate to increased inflammatory activity on 18FDG-PET/CT. Front Med. (2021) 8:595077. doi: 10.3389/fmed.2021.595077
66. Papiris SA, Manali ED, Papaioannou AI, Georgakopoulos A, Kolilekas L, Pianou NK, et al. Prevalence, distribution and clinical significance of joints, muscles and bones in sarcoidosis: an F-FDG-PET/CT study. Expert Rev Respir Med. (2020) 14:957–64. doi: 10.1080/17476348.2020.1775587
67. Papiris SA, Manali ED, Pianou NK, Kallergi M, Papaioannou AI, Georgakopoulos A, et al. 18F-FDG PET/CT in pulmonary sarcoidosis:quantifying inflammation by the TLG index. Expert Rev Respir Med. (2020) 14:103–10. doi: 10.1080/17476348.2020.1682997
68. Vita T, Okada DR, Veillet-Chowdhury M, Bravo PE, Mullins E, Hulten E, et al. Complementary value of cardiac magnetic resonance imaging and positron emission tomography/computed tomography in the assessment of cardiac sarcoidosis. Circ Cardiovasc Imaging. (2018) 11:e007030. doi: 10.1161/CIRCIMAGING.117.007030
69. Alvi RM, Young BD, Shahab Z, Pan H, Winkler J, Herzog E, et al. Repeatability and optimization of FDG positron emission tomography for evaluation of cardiac sarcoidosis. JACC Cardiovasc Imaging. (2019) 12:1284–7. doi: 10.1016/j.jcmg.2019.01.011
70. Chen X, Xu X, Chrysikos S, Zhao M, Zhou Y. Value of 18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in the differential diagnosis of sarcoidosis and lung cancer with lymph node metastasis: a retrospective study. Transl Lung Cancer Res. (2022) 11:1926–35. doi: 10.21037/tlcr-22-611
71. Jung RS, Mittal BR, Maturu NV, Kumar R, Bhattacharya A, Gupta D. Ocular sarcoidosis: does F-FDG PET/CT have any role? Clin Nucl Med. (2014) 39:464–6. doi: 10.1097/RLU.0000000000000419
72. Quinn B, Dauer Z, Pandit-Taskar N, Schoder H, Dauer LT. Radiation dosimetry of 18F-FDG PET/CT: incorporating exam-specific parameters in dose estimates. BMC Med Imaging. (2016) 16:41. doi: 10.1186/s12880-016-0143-y
73. Nishiyama Y, Yamamoto Y, Fukunaga K, Takinami H, Iwado Y, Satoh K, et al. Comparative evaluation of 18F-FDG PET and 67GA scintigraphy in patients with sarcoidosis. J Nucl Med. (2006) 47:1571–6.
74. Braun JJ, Kessler R, Constantinesco A, Imperiale A. 18F-FDG PET/CT in sarcoidosis management: review and report of 20 cases. Eur J Nucl Med Mol Imaging. (2008) 35:1537–43. doi: 10.1007/s00259-008-0770-9
75. Bakker ALM, Mathijssen H, Azzahhafi J, Swaans MJ, Veltkamp M, Keijsers RGM, et al. Effectiveness and safety of infliximab in cardiac sarcoidosis. Int J Cardiol. (2021) 330:179–85. doi: 10.1016/j.ijcard.2021.02.022
76. Sgard B, Brillet PY, Bouvry D, Djelbani S, Nunes H, Meune C, et al. Evaluation of FDG PET combined with cardiac MRI for the diagnosis and therapeutic monitoring of cardiac sarcoidosis. Clin Radiol. (2019) 74:81.e9–18. doi: 10.1016/j.crad.2018.09.015
77. Maturu VN, Rayamajhi SJ, Agarwal R, Aggarwal AN, Gupta D, Mittal BR. Role of serial F-18 FDG PET/CT scans in assessing treatment response and predicting relapses in patients with symptomatic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. (2016) 33:372–80.
78. Raasing L, Vogels OJM, Veltkamp M, Grutters JC. Infliximab decreases inflammatory activity but has no effect on small fiber neuropathy related symptoms in dutch patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. (2022) 39:e2022033. doi: 10.36141/svdld.v39i4.12031
79. Gosselin J, Roy-Hewitson C, Bullis SSM, DeWitt JC, Soares BP, Dasari S, et al. Neurosarcoidosis: phenotypes, approach to diagnosis and treatment. Curr Rheumatol Rep. (2022) 24:371–82. doi: 10.1007/s11926-022-01089-z
80. Walker MD, Shane E. Hypercalcemia: a review. JAMA. (2022) 328:1624–36. doi: 10.1001/jama.2022.18331
81. Gwadera Ł, Białas AJ, Iwański MA, Górski P, Piotrowski WJ. Sarcoidosis and calcium homeostasis disturbances-do we know where we stand? Chron Respir Dis. (2019) 16:1479973119878713. doi: 10.1177/1479973119878713
82. Kaiser Y, Eklund A, Grunewald J. Moving target: shifting the focus to pulmonary sarcoidosis as an autoimmune spectrum disorder. Eur Respir J. (2019) 54:1802153. doi: 10.1183/13993003.021532018
83. Barna BP, Judson MA, Thomassen MJ. Inflammatory pathways in sarcoidosis. Adv Exp Med Biol. (2021) 1304:39–52. doi: 10.1007/978-3-030-68748-9_3
84. Lin NW, Maier LA, Mroz MM, Jacobson S, MacPhail K, Liu S, et al. Genomic biomarkers in chronic beryllium disease and sarcoidosis. Respir Med. (2021) 187:106390. doi: 10.1016/j.rmed.2021.106390
85. Spagnolo P, Maier LA. Genetics in sarcoidosis. Curr Opin Pulm Med. (2021) 27:423–9. doi: 10.1097/MCP.0000000000000798
86. Rivera NV, Patasova K, Kullberg S, Diaz-Gallo LM, Iseda T, Bengtsson C, et al. A gene-environment interaction between smoking and gene polymorphisms provides a high risk of two subgroups of sarcoidosis. Sci Rep. (2019) 9:18633. doi: 10.1038/s41598-019-54612-1
87. Rivera NV, Ronninger M, Shchetynsky K, Franke A, Nöthen MM, Müller-Quernheim J, et al. High-density genetic mapping identifies new susceptibility variants in sarcoidosis phenotypes and shows genomic-driven phenotypic differences. Am J Respir Crit Care Med. (2016) 193:1008–22. doi: 10.1164/rccm.201507-1372OC
88. Gialafos E, Triposkiadis F, Kouranos V, Rapti A, Kosmas I, Manali E, et al. Relationship between tumor necrosis factor-? (TNFA) gene polymorphisms and cardiac sarcoidosis. In Vivo. (2014) 28:1125–9.
89. Konigsberg IR, Lin NW, Liao SY, Liu C, MacPhail K, Mroz MM, et al. Multi-omic signatures of sarcoidosis and progression in bronchoalveolar lavage cells. bioRxiv [Preprint]. (2023). doi: 10.1101/2023.01.26.525601
90. Jiang Y, Jiang D, Costabel U, Dai H, Wang C. A transcriptomics-based meta-analysis identifies a cross-tissue signature for sarcoidosis. Front Med. (2022) 9:960266. doi: 10.3389/fmed.2022.960266
91. Liu J, Ma P, Lai L, Villanueva A, Koenig A, Bean GR, et al. Transcriptional and immune landscape of cardiac sarcoidosis. Circ Res. (2022) 131:654–69. doi: 10.1161/CIRCRESAHA.121.320449
92. Vukmirovic M, Yan X, Gibson KF, Gulati M, Schupp JC, DeIuliis G, et al. Transcriptomics of bronchoalveolar lavage cells identifies new molecular endotypes of sarcoidosis. Eur Respir J. (2021) 58:2002950. doi: 10.1183/13993003.02950-2020
93. Häggmark A, Hamsten C, Wiklundh E, Lindskog C, Mattsson C, Andersson E, et al. Proteomic profiling reveals autoimmune targets in sarcoidosis. Am J Respir Crit Care Med. (2015) 191:574–83. doi: 10.1164/rccm.201407-1341OC
94. Rybicki BA, Sinha R, Iyengar S, Gray-McGuire C, Elston RC, Iannuzzi MC. Genetic linkage analysis of sarcoidosis phenotypes: the sarcoidosis genetic analysis (SAGA) study. Genes Immun. (2007) 8:379–86. doi: 10.1038/sj.gene.6364396
95. Karakaya B, van der Vis JJ, Veltkamp M, Biesma DH, Grutters JC, van Moorsel CHM. ANXA11 rs1049550 associates with Löfgren's syndrome and chronic sarcoidosis patients. Cells. (2022) 11:1557. doi: 10.3390/cells11091557
96. Krausgruber T, Redl A, Barreca D, Doberer K, Romanovskaia D, Dobnikar L, et al. Single-cell and spatial transcriptomics reveal aberrant lymphoid developmental programs driving granuloma formation. Immunity. (2023) 56:289–306.e7. doi: 10.1016/j.immuni.2023.01.014
97. Su Y, Chen D, Yuan D, Lausted C, Choi J, Dai CL, et al. Multi-omics resolves a sharp disease-state shift between mild and moderate COVID-19. Cell. (2020) 183:1479–95.e20. doi: 10.1016/j.cell.2020.10.037
98. Magallon RE, Knapp JR, Harmacek LD, Tu T-HC, Vestal B, Gillespie M, et al. Comparative profiling of the immune system in sarcoidosis via CITE-Seq and flow cytometry. J Immunol. (2020) 204:224.24-.24. doi: 10.4049/jimmunol.204.Supp.224.24
99. Liu A, Sharma L, Yan X, Dela Cruz CS, Herzog EL, Ryu C. Emerging insights in sarcoidosis: moving forward through reverse translational research. Am J Physiol Lung Cell Mol Physiol. (2022) 322:L518–l25. doi: 10.1152/ajplung.00266.2021
100. Miedema JR, Bonella F, Grunewald J, Spagnolo P. Looking into the future of sarcoidosis: what is next for treatment? Curr Opin Pulm Med. (2020) 26:598–607. doi: 10.1097/MCP.0000000000000709
Keywords: sarcoidosis, chest roentgenogram staging, 18F-FDG PET/CT scan phenotyping, omics, personalized treatment
Citation: Papiris SA, Kolilekas L, Rivera N, Spanos M, Li G, Gokulnath P, Chatterjee E, Georgakopoulos A, Kallieri M, Papaioannou AI, Raptakis T, Apollonatou V, Antonogiannaki E-M, Gialafos E, Chatziioannou S, Grunewald J and Manali ED (2023) From Karl Wurm and Guy Scadding's staging to 18F-FDG PET/CT scan phenotyping and far beyond: perspective in the evading history of phenotyping in sarcoidosis. Front. Med. 10:1174518. doi: 10.3389/fmed.2023.1174518
Received: 26 February 2023; Accepted: 05 April 2023;
Published: 10 May 2023.
Edited by:
Theodoros Karampitsakos, University of South Florida, United StatesReviewed by:
Ourania Papaioannou, General University Hospital of Patras, GreeceCarole Y. Perrot, University of South Florida, United States
Dominique Valeyre, Université Sorbonne Paris Nord, France
Shu-Yi Liao, National Jewish Health, United States
Copyright © 2023 Papiris, Kolilekas, Rivera, Spanos, Li, Gokulnath, Chatterjee, Georgakopoulos, Kallieri, Papaioannou, Raptakis, Apollonatou, Antonogiannaki, Gialafos, Chatziioannou, Grunewald and Manali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Spyros A. Papiris, cGFwaXJpc0BvdGVuZXQuZ3I=
 Spyros A. Papiris
Spyros A. Papiris Lykourgos Kolilekas2
Lykourgos Kolilekas2 Natalia Rivera
Natalia Rivera Michail Spanos
Michail Spanos Guoping Li
Guoping Li Andriana I. Papaioannou
Andriana I. Papaioannou Elias Gialafos
Elias Gialafos Effrosyni D. Manali
Effrosyni D. Manali