- 1The Department of Organ Transplantation, Renmin Hospital of Wuhan University, Wuhan, Hubei, China
- 2Department of Urology, Renmin Hospital of Wuhan University, Wuhan, Hubei, China
Post-transplant anemia is one of the most common complications in kidney transplant recipients, severely affecting patient prognosis and quality of life, and is an independent predictor of graft kidney loss and patient mortality. However, our clinical understanding and the attention given to post-transplant anemia are currently insufficient. This paper reviews the current status, risk factors, and therapeutic progress in anemia after transplantation in kidney transplant recipients. We recommend that clinical staff pay attention to anemia and its complications in kidney transplant recipients and intervene early for anemia.
1 Introduction
Kidney transplantation is the most effective treatment for end-stage kidney disease (ESKD), and a successful kidney transplant can restore the patient’s kidney function to almost normal levels, including endocrine functions (1, 2). Post-transplantation anemia (PTA) is a common complication after kidney transplantation, and studies have shown that the incidence of PTA is 20–50% at different stages after transplantation (3–7). Although the vast majority of cases of PTA can be corrected in the early stages following successful kidney transplantation, there are still patients who progress to anemia or secondary anemia, which can seriously affect the recipients’ prognosis. PTA has been shown to reduce patient quality of life (8). Anemia has been linked with significant cardiovascular morbidity and mortality in renal transplant recipients (9, 10). Although anemia is a serious complication of transplantation, it has not attracted the attention of researchers. This paper therefore reviews the current status, risk factors, and available interventions for anemia after transplantation in kidney transplant recipients in order to prompt clinical workers to pay early attention to anemia after kidney transplantation.
2 Classification and diagnosis
Anemia describes a state in which the level of hemoglobin (Hb), the number of red blood cells, and/or the specific capacity of red blood cells within peripheral blood units is below the low normal limit (11). There is currently no exact staging or grading of the degree of anemia after transplantation (11). It is still generally graded using the normal human anemia scale now. In order to identify the effects of anemia on the post-transplant recipient and the transplanted kidney at different times, some centers have differentiated between PTA occurring within 6 months and PTA occurring after 6 months (12), as early and late anemia, respectively.
At present, according to the World Health Organization and the American Transplant Society, anemia is diagnosed in adults living at sea level with Hb ≤130 g/L for men, Hb ≤120 g/L for women, or Hb ≤110 g/L for pregnant women (13). According to the Kidney Disease: Improving Global Outcomes (KDIGO) initiative and the European Kidney Best Practice group, anemia is defined as Hb ≤120 g/L in men and menopausal women and Hb ≤110 g/L in non-menopausal women (14, 15). The reference range for hemoglobin concentrations in the blood may vary depending on the population analyzed, age, sex, environmental conditions, and dietary habits (16).
3 Prevalence and risks of anemia in kidney transplant recipients
3.1 Prevalence of anemia in kidney transplant recipients
Anemia is one of the most common complications in patients with CKD, with both the incidence and degree of anemia gradually increasing as renal function decreases. A study has found that more than 50% of cases of CKD are combined with anemia, while the prevalence of anemia in the uremia phase reaches 90.2% (17). A retrospective study of 649 samples taken in Mexico between 2013 and 2017 found an anemia prevalence of 73.1% in patients prior to kidney transplantation (18). Similarly, another retrospective study in Turkey found that before kidney transplantation, the prevalence of anemia and severe anemia reached 86.7 and 58.8%, respectively (19). Fortunately, kidney transplantation can improve the symptoms of anemia in patients with CKD to some extent. But due to intraoperative blood loss, repeated postoperative blood tests, infection, rejection, delayed recovery of transplanted kidneys, drugs and other factors (6, 20, 21), the incidence of anemia remains high in patients after kidney transplantation. Indeed, the incidence of PTA decreased significantly as kidney function improves after transplantation. The prevalence of PTA at 1, 3, 6, and 12 months after kidney transplantation has been reported as 84.3, 39.5, 26.2, and 21.6%, respectively (22). Since then, the incidence of PTA has remained at high level and even increased. In further studies, the prevalence of post-transplant anemia after kidney transplantation ranged from 25 to 41.4% (12, 23), with a 2-year PTA prevalence of 36.6%, while the incidence of anemia at 3, 5, and 10 years after transplantation was reported to be 41.5, 35.3, and 93.2%, respectively (24).
3.2 Risks of anemia in kidney transplant recipients
3.2.1 Influence of anemia on cardiovascular function in kidney transplant recipients
Lower hemoglobin levels are associated with higher cardiovascular events (10). The annual incidence of cardiovascular disease (CVD) after kidney transplantation is 3.5–5%, which is 50-times that in the general population (25). Among the causes of death among kidney transplant recipients, CVD ranks first (40.9%) (26). Rates of cardiac death in renal transplant recipients (RTRs) are higher than in the general population, with the rate of cardiac death 10-times higher and the annual rate of fatal or non-fatal CV events 50-times that of the general population (27, 28). Anemia is an independent risk factor for clinical and echocardiographic cardiac disease, as well as mortality in end-stage renal disease patients (29). Kidney transplant recipients are considered to be a specific category of patients with CKD and are at risk for CKD-related complications (30). Long-term anemia causes hyperdynamic changes in the circulatory system and long-term overload of the heart and myocardial ischemia. This leads to anemic heart disease, as well as changes in heart rate, arrhythmias, changes in the structure of the heart, and congestive heart failure in severe cases (31). A retrospective cohort study of patients with no clinical heart disease who survived 1 year after kidney transplantation by a Canadian team showed that anemia was an important and major risk factor for left ventricular hypertrophy, 1 to 5 years after transplantation (10). However, most of these studies were observational or small intervention trials, which also focused on patients with CKD and did not take into account the poorer renal function and higher proteinuria in patients with PTA. Therefore, it is important to study whether positive treatment of anemia can improve CVD in PTA patients. However, studies have shown no benefit in correcting anemia or of high hemoglobin levels on cardiovascular disease or survival in CKD patients (32–35).
3.2.2 Influence of anemia on kidney function in kidney transplant recipients
Previous studies have shown an association between anemia and adverse transplant outcomes, including graft failure, rejection and patient survival (36). During a specific physical examination of 18,383 healthy elderly people in Tokyo, Japan, it was found that patients with Hb <12 g/dL (male) or Hb <11 g/dL (female) had a 2.215- or 2.2-fold risk, respectively, of new CKD compared with individuals with normal Hb levels (37). In addition, these patients had 2.618-times the risk of deteriorating kidney function, respectively (37). A study in 385 kidney transplant recipients showed that both persistent anemia and late-onset anemia were associated with an increased risk of graft loss (36). Furthermore, studies such as that conducted by de Andrade have shown that patients with anemia have a 3.8-fold higher risk of losing a transplanted kidney than patients without anemia (38). It’s worth nothing that each increase in the degree of anemia increases the risk of graft loss by 2.77-times (hazard ratio [HR], 2.77; 95% confidence interval [CI], 1.50–5.13) (22). Studies such as that conducted by Jones have shown that patients with anemia have a 5.25-fold risk of transplant kidney failure compared with non-anemic patients (39). PTA also increases the incidence of post-transplant rejection, which is 1.8-times greater than in non-anemic patients (40). Previous studies have suggested that residual renal function is the most important predictor of PTA and that impaired renal function in renal transplant recipients is proportional to the severity of anemia (41). However, the role of correcting anemia on graft kidney function is not clear. A meta-analysis showed no difference between the ESA and no ESA groups (42). Similarly, in CKD patients, no nephroprotective effect of anemia correction has been observed (32). In contrast, the prospective study by Tsujita et al. showed that correcting anemia to target levels (12.5–13.5 g/dL) slowed the time to deterioration of renal function (43). These studies suggest that the occurrence of PTA after kidney transplantation is detrimental to the recipient’s transplant kidney function. We need to pay attention to the occurrence of PTA in the recipient at an early stage. The effectiveness and safety of anemia correction in improving graft outcomes in renal transplant patients remains to be further validated.
3.2.3 Influence of anemia on survival and quality of life in kidney transplant recipients
Previous studies have demonstrated that anemia is associated with increased mortality and morbidity in patients with various diseases. For example, anemia is associated with shortened survival in patients with lung and cervical cancers (44), while severe anemia (Hb <11 g/dL) is consistently associated with high mortality (HR, 4.36; 95% CI, 3.04–6.27) (5). The development of anemia after kidney transplantation has a very significant impact on the recipient’s survival and is usually indirectly caused by the effect of anemia on other functions. However, due to the observational design of studies, causality cannot be affirmed. A retrospective study of 4,217 kidney transplant recipients in France found that all-cause mortality was as high as 6.8% in PTA recipients and 4.55% in the no-PTA group (23). The main reasons for this may be related to the type of the patient’s primary disease, the deterioration of the transplanted kidney function and the long-term use of immunosuppressive drugs. Anemia can also seriously affect the quality of life of kidney transplant recipients. For example, patients with anemia have been shown to experience chronic fatigue, decreased activity endurance, and cognitive decline, as well as increased length of hospital stay and costs associated with anemia (8, 45). Treatment of anemia may have some benefits on the quality of life of patients after kidney transplantation, but whether it will reduce the complications and mortality of patient needs further study.
4 Risk factors for the occurrence of PTA
4.1 Causes of anemia before kidney transplantation
Patients with ESKD often have varying degrees of anemia before surgery. The main reason for this is bone marrow suppression due to decreased erythropoietin (EPO) secreted by the renal interstitial cells and the accumulation of uremic toxins in the blood that inhibit the activity of EPO (46), resulting in decreased erythrocyte production. Additionally, the blood loss caused by hemodialysis or the abnormal iron metabolism caused by blood loss and microvascular inflammation can also lead to the occurrence of anemia (47). Patients with ESKD often use daily diet to control the production of toxins, and may also suffer from a loss of appetite because of the disease, while patients with end-stage kidney disease tend to have uremia toxins exceeding the level that causes catabolism, which may lead to maladaptation of nutrients and a lack of folic acid, vitamin B12, iron, and other hematopoietic substrate. This eventually triggers anemia (11). Anemia may occur in the context of bone metastases in kidney cancer, lupus nephropathy, and hemolytic uremic syndrome, as well as multiple myeloma and diabetic nephropathy (48–50). A retrospective analysis of 410 patients (Hb <10 g/dL) in South Korea found that those with 25-hydroxyvitamin D < 10 ng/dL before kidney transplantation had a higher risk of developing anemia than those with 25-hydroxyvitamin D ≥ 10 ng/dL, while vitamin D deficiency may also be a risk factor for anemia in patients with ESKD (21). A deficiency of levonidin has also been shown to be associated with anemia (51). The degree of anemia in pre-transplant patients affects the development of anemia in post-transplant patients to varying degrees (52). Therefore, the correction of anemia of patient needs to be considered before kidney transplantation and receive early post-transplant work-up.
4.2 Early causes of anemia after transplantation
Early anemia after kidney transplantation is often attributed to iron deficiency, blood loss, immunosuppression, and viral infections. Inadequate iron storage during transplantation, blood loss during surgery, increased iron utilization for compensatory production of red blood cells due to blood loss, and malnutrition can all contribute to iron deficiency (53). Moreover, frequent blood tests in the early postoperative period resulting in frequent small blood loss in patients can exacerbate the incidence and extent of anemia (54). Immunosuppressive drugs are usually used after kidney transplantation to prevent the occurrence of graft and receptor rejection; however, immunosuppression causes the patient’s entire immune system to be suppressed, inhibiting the bone marrow hematopoietic system and leading to the possibility of anemia. A study in Israel evaluating the incidence of anemia in pediatric patients with kidney transplantation and CKD showed that renal function recovered and glomerular filtration rates improved after kidney transplantation, but recipients had a higher incidence of anemia, possibly due to immunosuppressive therapy and EPO resistance (47). In a large cohort study enrolling 864 adult subjects, the prevalence of severe anemia immediately after kidney transplantation was 62.7%, significantly associated with anti-thymocyte globulin (ATG) /anti-lymphocytic globulin (ALG) administration (55). Simultaneously, the use of immunosuppressants decreases the body’s resistance to infection, resulting in various infections (56); cytomegalovirus (57, 58), and parvovirus B19 (59) typically occur early after transplantation, with a very low proportion and count of reticulocytes (60). Infection with parvovirus B19 must be strongly suspected when refractory and severe anemia with reticulocytopenia develops after transplantation (61). Studies have also shown that avoiding the use of steroids in the first 6 months after kidney transplantation also increases the incidence of PTA (62). Acute kidney injury and nutritional deficiencies are also important influencing factors for early PTA (5). Therefore, it is important to monitor the iron stores and immunosuppression status of patients in the early post-transplant period and make timely adjustments to avoid the development of PTA.
4.3 Cause for late PTA
The onset of late PTA is associated with impaired kidney graft function and the development of renal insufficiency (63). Delayed graft function, impaired kidney graft function, and acute rejection are risk factors for PTA (62). Serum creatinine and glomerular filtration rates have been shown to affect Hb in patients (20). For example, when eGFR >60 mL/min/1.73 m2, 18.7% of kidney transplant recipients experienced anemia, compared with only 2.4% of the general population. Further, when eGFR was 30–60 mL/min/1.73 m2, 34.8% of recipients developed anemia after kidney transplantation and 3.8% of the general population suffered from anemia (64). Therefore, renal transplant recipients are more susceptible to the influences of inadequate glomerular filtration rate, which leads to the development of PTA. We need to pay early attention to recipients with impaired kidney graft function and delayed graft function. In kidney transplant patients, kidney graft function deteriorates over time due to various factors. A multicenter study in France confirmed that deterioration of kidney graft function is a significant risk factor for PTA, and that renal transplant recipients have increased proteinuria early in the deterioration of kidney function (23). An increase in urine protein has been shown to be significantly associated with anemia (65). In addition, multiple studies have reported that a longer transplant time is an important influencing factor PTA (9, 23, 66).
4.4 Other causes of anemia after transplantation
Sex is a contributing factor in PTA, and previous studies have suggested a higher incidence of PTA in female kidney transplant recipients (19, 67), which may be associated with menstrual blood loss and resulting iron deficiency in women. Pre-menopausal women recipients may need more attention. In terms of age, pediatric transplant recipients may be more likely to develop PTA than adults (68), and in a multicenter study in Argentina, Hb levels were significantly correlated with age, as lower Hb levels in children (19, 69). At the same time, donor age has been suggested to be a risk factor for PTA (70). This may be because the donor kidney in older donors may have poorer endocrine function. At the same time, studies have pointed out that the non-use of EPO before transplantation is also a major predictor of PTA (66). There are other causes, such as a meta-analysis of 29,061 kidney transplant recipients that showed a significantly increased risk of anemia in patients receiving renin-angiotensin system (RAS) (71).
5 Status of interventions for anemia in kidney transplant recipients
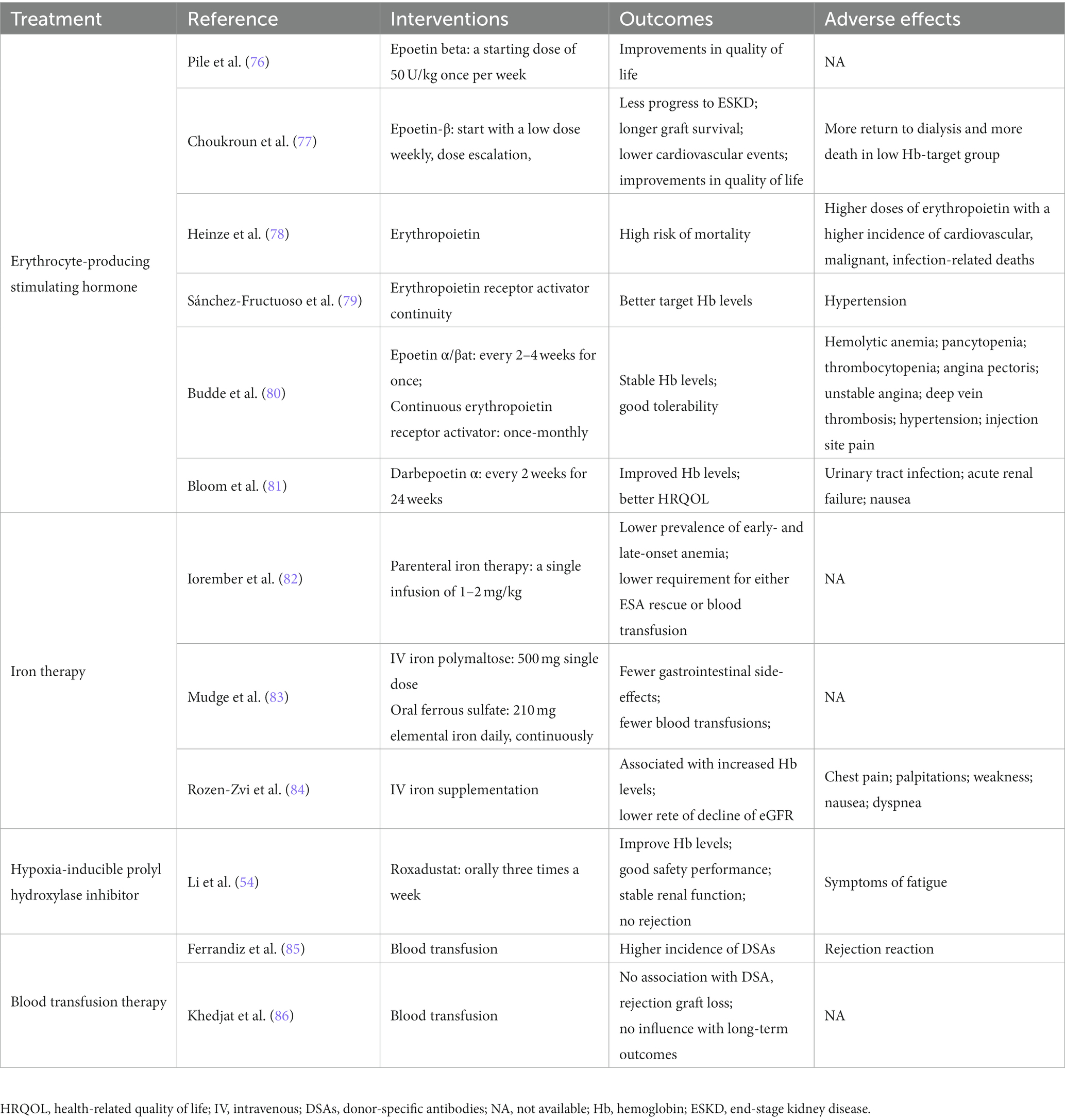
Anemia is a syndrome, not a disease. Therefore, the cause must always be investigated, and treatment must be primarily for causal disorders (72). Based on published evidence, comprehensive anemia testing approximately 3 months after transplantation allows for prompt correction of anemia and treatment and improved prognosis (73). However, there are no specific recommendations for PTA treatment in kidney transplant recipients in KDIGO guidelines, and the current treatment of anemia after kidney transplantation is mainly based on CKD rational for the use of anemia-associated erythropoiesis stimulating agent (ESA), iron therapy, hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHI), and other (74, 75) (Table 1).
5.1 Erythrocyte-producing stimulating hormone (ESA)
According to the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative, hemoglobin (Hb) levels below 11 g/dL are currently proposed treatment targets for anemia (87). KDIGO guidelines recommend initiating ESA therapy in patients with CKD only when Hb concentrations <10.0 g/dL and target Hb levels of 11.5 g/dL (14). Multiple evidence-based medical data show that ESA treatment significantly improves postoperative quality of life and reduces the need for blood transfusions (88). Recombinant human erythropoietin (rhEPO) is the first generation of ESA applied to the clinic, rhEPO is a short-acting preparation, 2 to 3 per week sub-administration; Daepotin α (DPO) belongs to the second generation of ESA. The mechanism of improving anemia is the same as that of rhEPO, but it is more biologically active and only needs to be administered once every 1 to 2 weeks; Persistent Erythropoietin Receptor Activator (CERA) is a third-generation ESA with advantages such as long half-life and low frequency of administration, but studies have confirmed its long-term efficacy and safety are inferior to other ESA (89). ESA is effective in correcting anemia and maintaining hemoglobin concentrations in the target range in most patients with CKD (90). Many post-transplant patients are anemic but only few receive adequate anemia treatment. Even transplant recipients with severe anemia received epoetin in only 17.8% of cases (12). Study showed that target hemoglobin level of 11.5 to 13.5 g/dL improves the vitality and mental health domains in quality of life Short Form (SF) - 36 scores and not associated with adverse changes in cardiovascular outcomes or increased cardiovascular morbidity or thrombotic events. However, treatment did not reduce the rate of decline in graft function (76). The main reason for this may be that some physicians believe that even without treatment, most patients will have symptoms of anemia improving on their own in the short term after surgery. Other studies have shown no effect of ESA on kidney function (91). It has also been reported that the treatment of EPO can cause complications such as hypertension and stroke, resulting in a poor prognosis for patients (92, 93). The laboratory evaluation of anemia including iron status and other substrates and replacement should be performed prior to treatment with ESA with a view to rational use of ESA for desired efficacy. The specific efficacy and influence of the use of ESA requires further study in the future.
5.2 Iron therapy
Iron is the basic ingredient for the synthesis of Hb, and studies have shown that early anemia after kidney transplantation is not due to the incomplete recovery of kidney function, but rather iron deficiency (94). Therefore, kidney transplant recipients with anemia after transplantation should routinely undergo measurement of serum ferritin, transferrin saturation, and other indicators, supplemented by serum hypersensitivity C-reactive protein and other deficit indicators if judged necessary. In addition, the causes of the iron deficiency should be investigated, and timely iron treatment administered according to the needs of patients, iron supplements before or after the kidney transplant for prevention might also be considered.
Iron therapy is typically divided into two categories: oral iron and intravenous iron. Commonly used forms of oral iron include ferrous sulfate, ferrous gluconate, and the newly emerged iron citrate, heme iron polypeptide, for example. The impact of oral iron on iron metabolism is close to the physiological state (95). However, the main disadvantages of oral iron are gastrointestinal adverse effects and the slow absorption of oral iron, which is not conducive to maximum iron utilization and rapid supplementation for patients in urgent need of iron supplementation. Intravenous iron agents include low-molecular weight dextran iron, iron sucrose, iron isomalt sucrose and iron carboxymaltose. The effectiveness and safety of intravenous iron in correcting renal anemia have been confirmed by a of evidence-based medical guidelines (96). However, the irregular application of intravenous iron can cause iron overload and damage to vital organs such as the liver and heart (97). Iron carboxymaltose, commonly used in intravenous iron preparations, was found to induce severe fibroblast growth factor 23-induced hypophosphatemia (98). Hence, we need to be vigilant about the amount of intravenous iron we use and avoid overloading. Gafter-Gvili et al. recommend that the use of an appropriate combination of erythropoietin stimulators and iron agents may be more beneficial in maintaining hemoglobin targeting at 12.5–13 g/dL in kidney transplant recipients (73). The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines for the care of kidney transplant recipients recommend that anemia in kidney transplant patients should be monitored and treated in the same way as patients with CKD (99), while, CKD populations have indicated that an increased risk of stroke and venous thromboembolism when ESA therapy is used to target high Hb levels (32, 35, 100). CAPRIT (77) as the only clinical randomized cntrolled study evaluating the therapeutic target of PTA, divided Hb into normal group (130–150 g/L) and partially corrected group (105–115 g/L). The preservation of renal function and cardiovascular events in the normal group were better than those in the partially corrected group. Therefore, it is suggested that the therapeutic target of PTA is higher than that of other CKD patients.
5.3 Hypoxia-inducible prolyl hydroxylase inhibitor (HIF-PHI)
The safety of ESA in the treatment of anemia has been questioned after an increased risk of death, cardiovascular events, and stroke was observed in the ESA intervention trial (35). Therefore, compared with external supplementation of EPO, stimulating the production of endogenous EPO by other drugs might have higher safety and applicability. HIF-PHI is a novel oral small molecule drug class that stimulates endogenous EPO production and improves iron utilization (101). Four HIF-PHI agents, roxadustat, daprodustat, vadadustat and molidustat, have been investigated in clinical trials. Roxadustat was the first HIF-PHI to enter a Phase 3 clinical trial, and was recently approved for the oral treatment of anemia in China (102). Roxadustat has been shown to correct anemia and maintain hemoglobin levels in the presence of low ferritin saturation and a gradual decline in ferritin levels (103). Roxadustat has also been shown to significantly increase hemoglobin levels and has shown good safety, renal stability, and no rejection in patients with PTA (54). HIF-PHIs were not inferior to ESAs in correcting anemia when using the Hb increase from baseline to the evaluation period as the primary endpoint in most trials (104, 105). However, its high price limits its large application by most patients, while several side effects of roxadustat need to be noted, including gastrointestinal diseases, nasopharyngitis, and back pain (102). A recent meta-analysis showed a 31% higher risk of thrombosis events versus ESAs (105). At present, daprodustat and vadadustat are also approved for listing in Japan, but their effects and safety need to be verified by further clinical studies. In the absence of conclusive data on the reduction of cardiovascular risk with the use of HIF-PHI, their use to correct hemoglobin levels in transplant recipients should be treated with more caution. It is noted that no randomized controlled trial has been designed for kidney transplant recipients so far, given their metabolization by CYP enzymes, possible drug interactions in kidney transplant recipients should also be carefully evaluated.
5.4 Anti-infective and antiviral therapy
Kidney transplant recipients require long-term immunosuppressants after surgery, which primarily work by suppressing the body’s immune system, making patients more susceptible to bacterial and viral infection (106). Anemia due to chronic infection is often improved with anti-infective therapy. To prevent opportunistic infection in kidney transplant recipients, anti-infective and antiviral prophylaxis is recommended after kidney transplantation. In patients with microvirus B19 infection, intravenous immunoglobulin (IVIG) is required in addition to reduced exposure to immunosuppressive drugs, and high doses of IVIG do not aggravate anemia in patients (61, 107).
5.5 Adaptation of immunosuppressants
Many immunosuppressive drugs used after transplantation have potential myelosuppressive effects, and immunosuppressants such as tacrolimus and mycophenolate mofetil (MMF) have been reported to be a cause of PTA after kidney transplantation (108). Anemia has been reported to be more common in patients taking MMF and sirolimus (12). Some studies have shown no relationship between anemia and immunosuppression, which may be due to the already poorer function of the transplanted kidney in such patients (30). Current regimens for adjusting immunosuppressive therapy include switching to low-intensity immunosuppressants or reducing the dose of immunosuppressants (109). However, it is important to note that in the process of reducing immunosuppression intensity, the risk of rejection may be increased.
5.6 Blood transfusion
In non-emergency situations, blood transfusion therapy is generally not recommended for kidney transplant patients. Study has shown that donor-specific antibodies and antibody-mediated rejection (AMR) occurs in patients undergoing transfusion therapy after kidney transplantation (85). The probability of AMR is significantly higher than in non-transfusion patients. In addition, this might interfere with the patients’ opportunity to be re-transplanted. Therefore, a strategy of blood transfusion in the clinic should always be treated with caution. However, in cases where it is necessary, we still do not hesitate to carry out antibody clearance in conjunction with blood transfusions.
6 Discussion
PTA is a common complication after kidney transplantation and yet, despite its high prevalence, low treatment rate, and serious consequences, the condition has not currently attracted sufficient attention. Due to differences in the definition of anemia, ethnicity, follow-up time, and intervention factors, for example, the reported incidence of PTA varies greatly among research centers. Therefore, we need to explore and reach consensus on the assessment criteria applicable to anemia in renal transplant patients in the future.
The main risk factors associated with the development of PTA include transplanted kidney function, polypharmacy, and infection. Risk factors can either aggravate PTA or worsen the disease caused by PTA. There are no national or international detailed systematic reviews or guidelines for the treatment of PTA. There is also insufficient guidance for the diagnosis and treatment of PTA in kidney transplant recipients, while the target level of Hb after PTA treatment also remains controversial. Optimal treatment of PTA may be ambivalent, depending on the underlying cause; for example, infection caused by PTA requires reduced or even discontinued immunosuppressant therapy, while PTA caused by kidney rejection requires immunosuppression to be strengthened. The short-term effects of PTA on kidney transplant patients are unclear and reversible, but their long-term negative effects are known.
This paper reviews the current epidemiology status, risk factors and available interventions for PTA in patients with kidney transplantation. We hope that clinicians will pay attention to PTA after renal transplantation and that systematic guidelines for the prevention and management of PTA after renal transplantation will be available in the near future.
Author contributions
YT and JG drafted the manuscript, undertook the systematic literature search. ZW and JL are conceived and designed the study. JZ and TQ obtained funding and supervised the study. TQ, JL, and JZ had important intellectual input. All authors have approved the final version of manuscript before submission.
Funding
This project is supported by the National Natural Science Foundation of China (Nos. 81870067 and 82170664, JZ) and Wuhan Science and Technology Bureau (No. 2020020601012213, JZ).
Acknowledgments
The authors thank the organ transplant staff at the Renmin Hospital of Wuhan University for their help in preparation of this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ALG, anti-lymphocytic globulin; AMR, antibody-mediated rejection; ATG, anti-thymocyte globulin; CERA, continuous erythropoietin receptor activator; CKD, chronic kidney disease; CVD, cardiovascular disease; EPO, erythropoietin; ESA, erythropoiesis stimulating agent; ESKD, end-stage kidney disease; IVIG, intravenous immunoglobulin; KDIGO, Kidney Disease: Improving Global Outcomes; MMF, mycophenolate mofetil; PTA, post-transplantation anemia.
References
1. Tanrisev, M, Hoscoskun, C, Asci, G, Sozbilen, M, Firat, O, Ertilav, M, et al. (2015). Long-term outcome of kidney transplantation from elderly living and expanded criteria deceased donors. Ren Fail 37:249–53. doi: 10.3109/0886022X.2014.982488
2. Winkelmayer, WC, Chandraker, A, Alan, BM, Kramar, R, and Sunder-Plassmann, G (2006). A prospective study of anaemia and long-term outcomes in kidney transplant recipients. Nephrol Dial Transplant 21:3559–66. doi: 10.1093/ndt/gfl457
3. Hsu, HC, Norton, GR, Peters, F, Robinson, C, Dlongolo, N, Solomon, A, et al. (2021). Association of Post Transplantation Anaemia and Persistent Secondary Hyperparathyroidism with diastolic function in stable kidney transplant recipients. Int J Nephrol Renovasc Dis 14:211–23. doi: 10.2147/IJNRD.S314313
4. Okumi, M, Okabe, Y, Unagami, K, Kakuta, Y, Iizuka, J, Takagi, T, et al. (2019). The interaction between post-transplant anemia and allograft function in kidney transplantation: the Japan academic consortium of kidney transplantation-II study. Clin Exp Nephrol 23:1066–75. doi: 10.1007/s10157-019-01737-2
5. Schechter, A, Gafter-Gvili, A, Shepshelovich, D, Rahamimov, R, Gafter, U, Mor, E, et al. (2019). Post renal transplant anemia: severity, causes and their association with graft and patient survival. BMC Nephrol 20:51. doi: 10.1186/s12882-019-1244-y
6. Gafter-Gvili, A, Ayalon-Dangur, I, Cooper, L, Shochat, T, Rahamimov, R, Gafter, U, et al. (2017). Posttransplantation anemia in kidney transplant recipients: a retrospective cohort study. Medicine 96:e7735. doi: 10.1097/MD.0000000000007735
7. Krischock, LA, van Stralen, KJ, Verrina, E, Tizard, EJ, Bonthuis, M, Reusz, G, et al. (2016). Anemia in children following renal transplantation-results from the ESPN/ERA-EDTA registry. Pediatr Nephrol 31:325–33. doi: 10.1007/s00467-015-3201-8
8. Abaci, SH, Alagoz, S, Salihoglu, A, Yalin, SF, Gulcicek, S, Altiparmak, MR, et al. (2015). Assessment of Anemia and quality of life in patients with renal transplantation. Transplant Proc 47:2875–80. doi: 10.1016/j.transproceed.2015.10.043
9. Rigatto, C, Parfrey, P, Foley, R, Negrijn, C, Tribula, C, and Jeffery, J (2002). Congestive heart failure in renal transplant recipients: risk factors, outcomes, and relationship with ischemic heart disease. J Am Soc Nephrol 13:1084–90. doi: 10.1681/ASN.V1341084
10. Rigatto, C, Foley, R, Jeffery, J, Negrijn, C, Tribula, C, and Parfrey, P (2003). Electrocardiographic left ventricular hypertrophy in renal transplant recipients: prognostic value and impact of blood pressure and anemia. J Am Soc Nephrol 14:462–8. doi: 10.1097/01.ASN.0000043141.67989.39
11. KDOQI and National Kidney Foundation (2006). KDOQI clinical practice guidelines and clinical practice recommendations for Anemia in chronic kidney disease. Am J Kidney Dis 47:S11–S145. doi: 10.1053/j.ajkd.2006.03.011
12. Vanrenterghem, Y, Ponticelli, C, Morales, JM, Abramowicz, D, Baboolal, K, Eklund, B, et al. (2003). Prevalence and management of anemia in renal transplant recipients: a European survey. Am J Transplant 3:835–45. doi: 10.1034/j.1600-6143.2003.00133.x
13. Kasiske, BL, Vazquez, MA, Harmon, WE, Brown, RS, Danovitch, GM, Gaston, RS, et al. (2000). Recommendations for the outpatient surveillance of renal transplant recipients. American Society of Transplantation. J Am Soc Nephrol 11:S1–S86. doi: 10.1681/ASN.V11suppl_1s1
14. Locatelli, F, Nissenson, AR, Barrett, BJ, Walker, RG, Wheeler, DC, Eckardt, KU, et al. (2008). Clinical practice guidelines for anemia in chronic kidney disease: problems and solutions. A position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int 74:1237–40. doi: 10.1038/ki.2008.299
15. Locatelli, F, Barany, P, Covic, A, De Francisco, A, Del, VL, Goldsmith, D, et al. (2013). Kidney disease: improving global outcomes guidelines on anaemia management in chronic kidney disease: a European renal best practice position statement. Nephrol Dial Transplant 28:1346–59. doi: 10.1093/ndt/gft033
16. Beutler, E, and Waalen, J (2006). The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood 107:1747–50. doi: 10.1182/blood-2005-07-3046
17. Stauffer, ME, and Fan, T (2014). Prevalence of anemia in chronic kidney disease in the United States. PLoS One 9:e84943. doi: 10.1371/journal.pone.0084943
18. Rosales, MK, Perez, RE, Cancino, LJ, Diaz, ER, Chacon, PM, Zavalza, CP, et al. (2020). Anemia and erythrocytes: behavior and prevalence 1 year after kidney transplant. Transplant Proc 52:1169–72. doi: 10.1016/j.transproceed.2020.01.053
19. Sert, I, Colak, H, Tugmen, C, Dogan, SM, and Karaca, C (2013). Anemia in living donor kidney transplantation. Transplant Proc 45:2238–43. doi: 10.1016/j.transproceed.2012.12.008
20. Carta, P, Bigazzi, B, Buti, E, Antognoli, G, Di Maria, L, Caroti, L, et al. (2016). Anemia and immunosuppressive regimen in renal transplanted patients: single-center retrospective study. Transplant Proc 48:337–9. doi: 10.1016/j.transproceed.2015.12.054
21. Kim, YL, Kim, H, Kwon, YE, Ryu, DR, Lee, MJ, Park, KS, et al. (2016). Association between vitamin D deficiency and Anemia in patients with end-stage renal disease: a Cross-sectional study. Yonsei Med J 57:1159–64. doi: 10.3349/ymj.2016.57.5.1159
22. Huang, Z, Song, T, Fu, L, Rao, Z, Zeng, D, Qiu, Y, et al. (2015). Post-renal transplantation anemia at 12 months: prevalence, risk factors, and impact on clinical outcomes. Int Urol Nephrol 47:1577–85. doi: 10.1007/s11255-015-1069-y
23. Garrigue, V, Szwarc, I, Giral, M, Soulillou, JP, Legendre, C, Kreis, H, et al. (2014). Influence of anemia on patient and graft survival after renal transplantation: results from the French DIVAT cohort. Transplantation 97:168–75. doi: 10.1097/TP.0b013e3182a94a4d
24. Qw, W (2014). Attention should be paid to the understanding of anemia after kidney transplantation. J Nephrol Dial Kidney Transplant 23:51–2.
25. Longenecker, JC, Coresh, J, Powe, NR, Levey, AS, Fink, NE, Martin, A, et al. (2002). Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE study. J Am Soc Nephrol 13:1918–27. doi: 10.1097/01.ASN.0000019641.41496.1E
26. Gouva, C, Nikolopoulos, P, Ioannidis, JP, and Siamopoulos, KC (2004). Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int 66:753–60. doi: 10.1111/j.1523-1755.2004.00797.x
27. Foley, RN, Parfrey, PS, and Sarnak, MJ (1998). Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32:S112–9. doi: 10.1053/ajkd.1998.v32.pm9820470
28. Liefeldt, L, and Budde, K (2010). Risk factors for cardiovascular disease in renal transplant recipients and strategies to minimize risk. Transpl Int 23:1191–204. doi: 10.1111/j.1432-2277.2010.01159.x
29. Foley, RN, Parfrey, PS, Harnett, JD, Kent, GM, Murray, DC, and Barre, PE (1996). The impact of anemia on cardiomyopathy, morbidity, and and mortality in end-stage renal disease. Am J Kidney Dis 28:53–61. doi: 10.1016/S0272-6386(96)90130-4
30. Sinnamon, KT, Courtney, AE, Maxwell, AP, McNamee, PT, Savage, G, and Fogarty, DG (2007). Level of renal function and serum erythropoietin levels independently predict anaemia post-renal transplantation. Nephrol Dial Transplant 22:1969–73. doi: 10.1093/ndt/gfm100
31. Kajimoto, K, Sato, N, and Takano, T (2016). Association of anemia and renal dysfunction with in-hospital mortality among patients hospitalized for acute heart failure syndromes with preserved or reduced ejection fraction. Eur Heart J Acute Cardiovasc Care 5:89–99. doi: 10.1177/2048872615593387
32. Drueke, TB, Locatelli, F, Clyne, N, Eckardt, KU, Macdougall, IC, Tsakiris, D, et al. (2006). Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355:2071–84. doi: 10.1056/NEJMoa062276
33. O'Riordan, E, and Foley, RN (2000). Effects of anaemia on cardiovascular status. Nephrol Dial Transplant 15:19–22. doi: 10.1093/oxfordjournals.ndt.a027971
34. Parfrey, PS, Foley, RN, Wittreich, BH, Sullivan, DJ, Zagari, MJ, and Frei, D (2005). Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J Am Soc Nephrol 16:2180–9. doi: 10.1681/ASN.2004121039
35. Singh, AK, Szczech, L, Tang, KL, Barnhart, H, Sapp, S, Wolfson, M, et al. (2006). Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355:2085–98. doi: 10.1056/NEJMoa065485
36. Kolonko, A, Pinocy-Mandok, J, Kocierz, M, Kujawa-Szewieczek, A, Chudek, J, Malyszko, J, et al. (2009). Anemia and erythrocytosis after kidney transplantation: a 5-year graft function and survival analysis. Transplant Proc 41:3046–51. doi: 10.1016/j.transproceed.2009.07.090
37. Kaneko, T, Kodani, E, Fujii, H, Asai, R, Seki, M, Nakazato, R, et al. (2021). Anemia and atrial fibrillation as independent risk factors for new-onset chronic kidney disease: the TAMA-MED project-CKD and AF. Clin Kidney J 14:2221–6. doi: 10.1093/ckj/sfab014
38. de Andrade, LG, Abrao, JM, and Carvalho, MF (2012). Anemia at one year is an independent risk factor of graft survival. Int Urol Nephrol 44:263–8. doi: 10.1007/s11255-010-9854-0
39. Jones, H, Talwar, M, Nogueira, JM, Ugarte, R, Cangro, C, Rasheed, H, et al. (2012). Anemia after kidney transplantation; its prevalence, risk factors, and independent association with graft and patient survival: a time-varying analysis. Transplantation 93:923–8. doi: 10.1097/TP.0b013e31824b36fa
40. Chhabra, D, Grafals, M, Skaro, AI, Parker, M, and Gallon, L (2008). Impact of anemia after renal transplantation on patient and graft survival and on rate of acute rejection. Clin J Am Soc Nephrol 3:1168–74. doi: 10.2215/CJN.04641007
41. Choukroun, G, Deray, G, Glotz, D, Lebranchu, Y, Dussol, B, Bourbigot, B, et al. (2008). Incidence and management of anemia in renal transplantation: an observational-French study. Nephrol Ther 4:575–83. doi: 10.1016/j.nephro.2008.04.009
42. Elliott, S, Tomita, D, and Endre, Z (2017). Erythropoiesis stimulating agents and Reno-protection: a meta-analysis. BMC Nephrol 18:14. doi: 10.1186/s12882-017-0438-4
43. Tsujita, M, Kosugi, T, Goto, N, Futamura, K, Nishihira, M, Okada, M, et al. (2019). The effect of maintaining high hemoglobin levels on long-term kidney function in kidney transplant recipients: a randomized controlled trial. Nephrol Dial Transplant 34:1409–16. doi: 10.1093/ndt/gfy365
44. Caro, JJ, Salas, M, Ward, A, and Goss, G (2001). Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer 91:2214–21. doi: 10.1002/1097-0142(20010615)91
45. Kawada, N, Moriyama, T, Ichimaru, N, Imamura, R, Matsui, I, Takabatake, Y, et al. (2009). Negative effects of anemia on quality of life and its improvement by complete correction of anemia by administration of recombinant human erythropoietin in posttransplant patients. Clin Exp Nephrol 13:355–60. doi: 10.1007/s10157-009-0170-x
46. Brigandi, RA, Johnson, B, Oei, C, Westerman, M, Olbina, G, de Zoysa, J, et al. (2016). A novel hypoxia-inducible factor-prolyl hydroxylase inhibitor (GSK1278863) for Anemia in CKD: a 28-day, phase 2A randomized trial. Am J Kidney Dis 67:861–71. doi: 10.1053/j.ajkd.2015.11.021
47. Krause, I, Davidovits, M, Tamary, H, Yutcis, M, and Dagan, A (2016). Anemia and markers of erythropoiesis in pediatric kidney transplant recipients compared to children with chronic renal failure. Pediatr Transplant 20:958–62. doi: 10.1111/petr.12792
48. Aladjidi, N, Leverger, G, Leblanc, T, Picat, MQ, Michel, G, Bertrand, Y, et al. (2011). New insights into childhood autoimmune hemolytic anemia: a French national observational study of 265 children. Haematologica 96:655–63. doi: 10.3324/haematol.2010.036053
49. Mohyuddin, GR, Koehn, K, Shune, L, Aziz, M, Abdallah, AO, McClune, B, et al. (2021). Renal insufficiency in multiple myeloma: a systematic review and meta-analysis of all randomized trials from 2005-2019. Leuk Lymphoma 62:1386–95. doi: 10.1080/10428194.2020.1867725
50. Ito, K, Yokota, S, Watanabe, M, Inoue, Y, Takahashi, K, Himuro, N, et al. (2021). Anemia in diabetic patients reflects severe Tubulointerstitial injury and aids in clinically predicting a diagnosis of diabetic nephropathy. Intern Med 60:1349–57. doi: 10.2169/internalmedicine.5455-20
51. Yang, XL, Ma, YL, Xu, N, and Tian, XK (2017). Effect of levocarnitine on hypotension and muscle spasm in maintenance hemodialysis patients. Chin J Integ Trad West Nephrol 18:254. doi: 10.3969/j.issn.1009-587X.2017.03.023
52. Taegtmeyer, AB, Rogers, P, Breen, JB, Barton, PJ, Banner, NR, and Yacoub, MH (2008). The effects of pre- and post-transplant anemia on 1-year survival after cardiac transplantation. J Heart Lung Transplant 27:394–9. doi: 10.1016/j.healun.2008.01.014
53. Zheng, S, Coyne, DW, Joist, H, Schuessler, R, Godboldo-Brooks, A, Ercole, P, et al. (2009). Iron deficiency anemia and iron losses after renal transplantation. Transpl Int 22:434–40. doi: 10.1111/j.1432-2277.2008.00814.x
54. Li, J, Ma, K, Wang, L, Qi, H, Lv, J, Rao, Y, et al. (2021). Efficacy and safety of roxadustat in the treatment of renal allograft anemia patients: a case series. Ann Palliat Med 10:11859–67. doi: 10.21037/apm-21-2916
55. Rostami, Z, Shafighi, N, Baghersad, MM, and Einollahi, B (2011). Risk factors for immediate anemia in renal transplant recipients: a single-center experience. Transplant Proc 43:581–3. doi: 10.1016/j.transproceed.2011.01.072
56. Kim, HC, Park, SB, Han, SY, and Whang, EA (2003). Anemia following renal transplantation. Transplant Proc 35:302–3. doi: 10.1016/S0041-1345(02)03958-1
57. Hernandez-Gallego, R, Cerezo, I, Robles, NR, Barroso, S, Romanciuc, A, and Cubero, JJ (2018). Anemia as very late-onset cytomegalovirus disease after kidney transplantation. Transpl Infect Dis 20:12797. doi: 10.1111/tid.12797
58. Kumar, V, Gupta, S, Singh, S, Goyal, VK, and Yadav, M (2010). Pure red cell aplasia associated with cytomegalovirus infection. J Pediatr Hematol Oncol 32:315–6. doi: 10.1097/MPH.0b013e3181cb4383
59. Thongprayoon, C, Khoury, NJ, Bathini, T, Aeddula, NR, Boonpheng, B, Lertjitbanjong, P, et al. (2020). Epidemiology of parvovirus B19 and anemia among kidney transplant recipients: a meta-analysis. Urol Ann 12:241–7. doi: 10.4103/UA.UA_89_19
60. Hai, AH, Diem, HT, and Cuong, NT (2019). Parvovirus B19-associated Anemia in kidney transplant recipients: a single-center experience. Transplant Proc 51:2693–6. doi: 10.1016/j.transproceed.2019.03.076
61. Ahmed, W, Dogar, R, and Acharya, S (2017). Parvovirus B19: a rare cause of post-renal transplant Anemia. J Coll Physicians Surg Pak 27:785–7.
62. Oliveira, CM, Timbo, PS, Pinheiro, SR, Leite, JG, Timbo, LS, and Esmeraldo, RM (2013). Post-transplant anemia and associated risk factors: the impact of steroid-free therapy. Sao Paulo Med J 131:369–76. doi: 10.1590/1516-3180.2013.1316523
63. Lorenz, M, Kletzmayr, J, Perschl, A, Furrer, A, Horl, WH, and Sunder-Plassmann, G (2002). Anemia and iron deficiencies among long-term renal transplant recipients. J Am Soc Nephrol 13:794–7. doi: 10.1681/ASN.V133794
64. Chadban, SJ, Baines, L, Polkinghorne, K, Jefferys, A, Dogra, S, Kanganas, C, et al. (2007). Anemia after kidney transplantation is not completely explained by reduced kidney function. Am J Kidney Dis 49:301–9. doi: 10.1053/j.ajkd.2006.11.034
65. Lim, A, Kansal, A, and Kanellis, J (2018). Factors associated with anaemia in kidney transplant recipients in the first year after transplantation: a cross-sectional study. BMC Nephrol 19:252. doi: 10.1186/s12882-018-1054-7
66. Banaga, AS, Yousif, ME, and Elmusharaf, K (2011). Risk factors of post renal transplant anaemia among Sudanese patients, a study in three renal transplant centres. BMC Nephrol 12:37. doi: 10.1186/1471-2369-12-37
67. Banjeglav, J, and Zibar, L (2012). Posttransplantation anemia 6 months after kidney transplantation. Acta Med Croatica 66:4–11.
68. Galutira, PJ, and Del, RM (2012). Understanding renal posttransplantation anemia in the pediatric population. Pediatr Nephrol 27:1079–85. doi: 10.1007/s00467-011-2036-1
69. Petrone, H, Arriola, M, Re, L, Taylor, F, Bruzzone, M, Chiurchu, C, et al. (2010). National survey of anemia prevalence after kidney transplantation in Argentina. Transplant Proc 42:288–90. doi: 10.1016/j.transproceed.2009.12.053
70. Marcen, R, Galeano, C, Fernandez-Rodriguez, A, Jimenez, S, Teruel, JL, Burgos, FJ, et al. (2012). Anemia at 1 year after kidney transplantation has a negative long-term impact on graft and patient outcomes. Transplant Proc 44:2593–5. doi: 10.1016/j.transproceed.2012.09.033
71. Cheungpasitporn, W, Thongprayoon, C, Chiasakul, T, Korpaisarn, S, and Erickson, SB (2015). Renin-angiotensin system inhibitors linked to anemia: a systematic review and meta-analysis. QJM 108:879–84. doi: 10.1093/qjmed/hcv049
72. Moreno, CJ, Romero, CM, and Gutierrez, MM (2009). Classification of anemia for gastroenterologists. World J Gastroenterol 15:4627–37. doi: 10.3748/wjg.15.4627
73. Gafter-Gvili, A, and Gafter, U (2019). Posttransplantation Anemia in kidney transplant recipients. Acta Haematol 142:37–43. doi: 10.1159/000496140
74. Lankhorst, CE, and Wish, JB (2010). Anemia in renal disease: diagnosis and management. Blood Rev 24:39–47. doi: 10.1016/j.blre.2009.09.001
75. Besarab, A, Provenzano, R, Hertel, J, Zabaneh, R, Klaus, SJ, Lee, T, et al. (2015). Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant 30:1665–73. doi: 10.1093/ndt/gfv302
76. Pile, T, Raftery, M, Thuraisingham, R, Kirwan, CJ, Harwood, S, and Yaqoob, MM (2020). Treating Posttransplant Anemia with erythropoietin improves quality of life but does not affect progression of chronic kidney disease. Exp Clin Transplant 18:27–33. doi: 10.6002/ect.2018.0283
77. Choukroun, G, Kamar, N, Dussol, B, Etienne, I, Cassuto-Viguier, E, Toupance, O, et al. (2012). Correction of postkidney transplant anemia reduces progression of allograft nephropathy. J Am Soc Nephrol 23:360–8. doi: 10.1681/ASN.2011060546
78. Heinze, G, Kainz, A, Hörl, WH, and Oberbauer, R (2009). Mortality in renal transplant recipients given erythropoietins to increase haemoglobin concentration: cohort study. BMJ 339:b4018. doi: 10.1136/bmj.b4018
79. Sánchez-Fructuoso, AI, Ruiz, JC, Torregrosa, JV, González, E, Gómez, E, Gallego, RJ, et al. (2012). Anemia control in renal transplant recipients receiving continuous erythropoietin receptor activator (C.E.R.A.) treatment: the AnemiaTrans study. Adv Ther 29:979–91. doi: 10.1007/s12325-012-0063-3
80. Budde, K, Rath, T, and Kliem, V (2014). Anemia control in kidney transplant recipients using once-monthly continuous erythropoietin receptor activator: a prospective, observational study. J Transplant 2014:179705:1–10. doi: 10.1155/2014/179705
81. Bloom, RD, Bolin, P, Gandra, SR, Scarlata, D, and Petersen, J (2011). Impact on health-related quality of life in kidney transplant recipients with late posttransplant anemia administered darbepoetin alfa: results from the STRATA study. Transplant Proc 43:1593–600. doi: 10.1016/j.transproceed.2011.02.009
82. Iorember, F, Aviles, D, and Bamgbola, O (2020). Impact of immediate post-transplant parenteral iron therapy on the prevalence of anemia and short-term allograft function in a cohort of pediatric and adolescent renal transplant recipients. Pediatr Transplant 24:e13787. doi: 10.1111/petr.13787
83. Mudge, DW, Tan, KS, Miles, R, Johnson, DW, Badve, SV, Campbell, SB, et al. (2012). A randomized controlled trial of intravenous or oral iron for posttransplant anemia in kidney transplantation. Transplantation 93:822–6. doi: 10.1097/TP.0b013e318248375a
84. Rozen-Zvi, B, Gafter-Gvili, A, Zingerman, B, Levy-Drummer, RS, Levy, L, Mor, E, et al. (2012). Intravenous iron supplementation after kidney transplantation. Clin Transpl 26:608–14. doi: 10.1111/j.1399-0012.2012.01602.x
85. Ferrandiz, I, Congy-Jolivet, N, Del, BA, Debiol, B, Trébern-Launay, K, Esposito, L, et al. (2016). Impact of early blood transfusion after kidney transplantation on the incidence of donor-specific anti-HLA antibodies. Am J Transplant 16:2661–9. doi: 10.1111/ajt.13795
86. Khedjat, K, Lenain, R, Hamroun, A, Baes, D, Top, I, Labalette, M, et al. (2022). Post-transplantation early blood transfusion and kidney allograft outcomes: a single-center observational study. Transpl Int 35:10279. doi: 10.3389/ti.2022.10279
87. KDOQI (2007). KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis 50:471–530. doi: 10.1053/j.ajkd.2007.06.008
88. Penny, H, Breen, C, Hall, S, Frame, S, and Goldsmith, D (2012). Modern Management of Post-Transplant Anaemia - focus on Mircera (Methoxy polyethylene glycol-Epoetin Beta) and IV Iron therapies: 1276. Transplantation 94:244. doi: 10.1097/00007890-201211271-00454
89. Wilhelm-Leen, ER, and Winkelmayer, WC (2015). Mortality risk of darbepoetin alfa versus epoetin alfa in patients with CKD: systematic review and meta-analysis. Am J Kidney Dis 66:69–74. doi: 10.1053/j.ajkd.2014.12.012
90. Koury, MJ, and Haase, VH (2015). Anaemia in kidney disease: harnessing hypoxia responses for therapy. Nat Rev Nephrol 11:394–410. doi: 10.1038/nrneph.2015.82
91. Martinez, F, Kamar, N, Pallet, N, Lang, P, Durrbach, A, Lebranchu, Y, et al. (2010). High dose epoetin beta in the first weeks following renal transplantation and delayed graft function: results of the neo-PDGF study. Am J Transplant 10:1695–700. doi: 10.1111/j.1600-6143.2010.03142.x
92. Krapf, R, and Hulter, HN (2009). Arterial hypertension induced by erythropoietin and erythropoiesis-stimulating agents (ESA). Clin J Am Soc Nephrol 4:470–80. doi: 10.2215/CJN.05040908
93. McCullough, PA, Barnhart, HX, Inrig, JK, Reddan, D, Sapp, S, Patel, UD, et al. (2013). Cardiovascular toxicity of epoetin-alfa in patients with chronic kidney disease. Am J Nephrol 37:549–58. doi: 10.1159/000351175
94. Yabu, JM, and Winkelmayer, WC (2011). Posttransplantation anemia: mechanisms and management. Clin J Am Soc Nephrol 6:1794–801. doi: 10.2215/CJN.01190211
95. Ratcliffe, LE, Thomas, W, Glen, J, Padhi, S, Pordes, BA, Wonderling, D, et al. (2016). Diagnosis and Management of Iron Deficiency in CKD: a summary of the NICE guideline recommendations and their rationale. Am J Kidney Dis 67:548–58. doi: 10.1053/j.ajkd.2015.11.012
96. Drueke, TB, and Parfrey, PS (2012). Summary of the KDIGO guideline on anemia and comment: reading between the (guide)line(s). Kidney Int 82:952–60. doi: 10.1038/ki.2012.270
97. Kuragano, T, Matsumura, O, Matsuda, A, Hara, T, Kiyomoto, H, Murata, T, et al. (2014). Association between hemoglobin variability, serum ferritin levels, and adverse events/mortality in maintenance hemodialysis patients. Kidney Int 86:845–54. doi: 10.1038/ki.2014.114
98. Mani, LY, Nseir, G, Venetz, JP, and Pascual, M (2010). Severe hypophosphatemia after intravenous administration of iron carboxymaltose in a stable renal transplant recipient. Transplantation 90:804–5. doi: 10.1097/TP.0b013e3181f00a18
99. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group (2009). KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9:S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x
100. Pfeffer, MA, Burdmann, EA, Chen, CY, Cooper, ME, de Zeeuw, D, Eckardt, KU, et al. (2009). A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361:2019–32. doi: 10.1056/NEJMoa0907845
101. Xu, MM, Wang, J, and Xie, JX (2017). Regulation of iron metabolism by hypoxia-inducible factors. Sheng Li Xue Bao 69:598–610. doi: 10.13294/j.aps.2017.0054
102. Roxadustat, DS (2019). Roxadustat: First Global Approval. Drugs 79:563–72. doi: 10.1007/s40265-019-01077-1
103. Chen, N, Hao, C, Peng, X, Lin, H, Yin, A, Hao, L, et al. (2019). Roxadustat for Anemia in patients with kidney disease not receiving dialysis. N Engl J Med 381:1001–10. doi: 10.1056/NEJMoa1813599
104. Locatelli, F, Minutolo, R, De Nicola, L, and Del, VL (2022). Evolving strategies in the treatment of Anaemia in chronic kidney disease: the HIF-prolyl hydroxylase inhibitors. Drugs 82:1565–89. doi: 10.1007/s40265-022-01783-3
105. Charytan, C, Manllo-Karim, R, Martin, ER, Steer, D, Bernardo, M, Dua, SL, et al. (2021). A randomized trial of Roxadustat in Anemia of kidney failure: SIERRAS study. Kidney Int Rep 6:1829–39. doi: 10.1016/j.ekir.2021.04.007
106. Vanichanan, J, Udomkarnjananun, S, Avihingsanon, Y, and Jutivorakool, K (2018). Common viral infections in kidney transplant recipients. Kidney Res Clin Pract 37:323–37. doi: 10.23876/j.krcp.18.0063
107. Malbora, B, Saritas, S, Ataseven, E, Belen, B, Alparslan, C, Yavascan, O, et al. (2017). Pure red cell aplasia due to parvovirus B19: erythropoietin-resistant Anemia in a pediatric kidney recipient. Exp Clin Transplant 15:369–71. doi: 10.6002/ect.2016.0263
108. Lobbes, H, Mahevas, M, Alviset, S, Galicier, L, Costedoat-Chalumeau, N, Amoura, Z, et al. (2021). Pure red cell aplasia in systemic lupus erythematosus, a nationwide retrospective cohort and review of the literature. Rheumatology 61:355–66. doi: 10.1093/rheumatology/keab363
Keywords: kidney transplantation, anemia after transplantation, risk factors, intervention, research progress
Citation: Tang Y, Guo J, Zhou J, Wan Z, Li J and Qiu T (2024) Risk factors and current state of therapy for anemia after kidney transplantation. Front. Med. 10:1170100. doi: 10.3389/fmed.2023.1170100
Edited by:
Laila-Yasmin Mani, University Hospital of Bern, SwitzerlandReviewed by:
François Gaillard, Hospices Civils de Lyon, FranceKarl Martin Wissing, University Hospital Brussels, Belgium
Copyright © 2024 Tang, Guo, Zhou, Wan, Li and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Qiu, cWl1dGFvQHdodS5lZHUuY24=; Jinke Li, MTU0MzQ1NjY2QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yan Tang1,2†
Yan Tang1,2† Jiayu Guo
Jiayu Guo Jiangqiao Zhou
Jiangqiao Zhou Tao Qiu
Tao Qiu