
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Med., 14 June 2023
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1169096
This article is part of the Research TopicThe Current Challenges Underlying Hepatitis D Virus InfectionView all 11 articles
 Lennart Hermanussen1†
Lennart Hermanussen1† Sibylle Lampalzer1†
Sibylle Lampalzer1† Jan-Hendrik Bockmann1,2
Jan-Hendrik Bockmann1,2 Annerose E. Ziegler1
Annerose E. Ziegler1 Felix Piecha1,2
Felix Piecha1,2 Maura Dandri1,2
Maura Dandri1,2 Sven Pischke1,2
Sven Pischke1,2 Friedrich Haag3
Friedrich Haag3 Ansgar W. Lohse1,2
Ansgar W. Lohse1,2 Marc Lütgehetmann2,4
Marc Lütgehetmann2,4 Christina Weiler-Normann1,5†
Christina Weiler-Normann1,5† Julian Schulze zur Wiesch1,2,3*†
Julian Schulze zur Wiesch1,2,3*†Infections with hepatotropic viruses are associated with various immune phenomena. Hepatitis D virus (HDV) causes the most severe form of viral hepatitis. However, few recent data are available on non-disease-specific and non-organ-specific antibody (NOSA) titers and immunoglobulin G (IgG) levels in chronic hepatitis D (CHD) patients. Here, we examined the NOSA titers and IgG levels of 40 patients with CHD and different disease courses and compared them to 70 patients with chronic hepatitis B (CHB) infection. 43% of CHD patients had previously undergone treatment with pegylated interferon-α (IFN-α). The antibody display of 46 untreated patients diagnosed with autoimmune hepatitis (AIH) was used as a reference. The frequency of elevated NOSA titers (CHD 69% vs. CHB 43%, p < 0.01), and the median IgG levels (CHD 16.9 g/L vs. CHB 12.7 g/L, p < 0.01) were significantly higher in CHD patients than in patients with CHB, and highest in patients with AIH (96%, 19.5 g/L). Also, the antinuclear antibody pattern was homogeneous in many patients with AIH and unspecific in patients with viral hepatitis. Additionally, f-actin autoantibodies were only detectable in patients with AIH (39% of SMA). In CHD patients, IgG levels correlated with higher HDV viral loads, transaminases, and liver stiffness values. IgG levels and NOSA were similar in CHD patients irrespective of a previous IFN-α treatment. In summary, autoantibodies with an unspecific pattern are frequently detected in CHD patients with unclear clinical relevance.
Hepatitis D virus (HDV) infection is the most severe form of viral hepatitis. HDV is a defective RNA virus and requires the hepatitis B surface antigen (HBsAg) of the hepatitis B virus (HBV) to complete its lifecycle. Worldwide, 15–20 million people suffer from a chronic HDV infection, which amounts to about 6–8% of the patients with a chronic HBV infection (CHB) (1). Chronic HDV/HBV co- or super-infection (CHD) is associated with higher rates of liver cirrhosis, hepatocellular carcinoma, and higher liver-related mortality compared to patients with HBV mono-infection (2–5). Chronic HDV infection has been a challenge in clinical hepatology in terms of diagnostics, monitoring, and therapy. For example, anti-HDV Immunoglobulin M (IgM) testing was used to diagnose ongoing hepatitis delta replication for a long time before HDV-RNA assays became available, and there have been incremental advances in the standardization of these molecular assays (6, 7). Antiviral treatment with pegylated Interferon-α (IFN-α) has been introduced around 10 years ago with low treatment response rates (8), and only lately therapy with bulevirtide received conditional approval in the EU (9).
Autoimmune hepatitis (AIH) is an immune-mediated chronic liver disease leading to (necro)inflammation, liver cirrhosis with all of its complications. AIH is characterized by elevated serum transaminases and immunoglobulin G (IgG) levels, detectable autoantibodies, and characteristic histopathological findings. Based on different autoantibody profiles, AIH can be classified into two subtypes, autoimmune hepatitis type 1 (AIH-1) and type 2 (AIH-2). (10, 11) Over the years there have been attempts to standardize the assessment of autoantibody profiles (12).
Notably, patients with chronic viral hepatitis may also develop high autoantibody titers (13, 14). Furthermore, overt extra-hepatic manifestations, such as vasculitis, polyarthritis nodosa, glomerulonephritis, dermatitis, polyarthralgia, arthritis, lung disease, and aplastic anemia may also occur (13, 15). Molecular mimicry and bystander activation have been proposed as possible mechanisms to explain the breakdown of self-tolerance caused by viral infection (16). Differentiating autoimmune phenomena in viral hepatitis from true autoimmune (liver) disease is of utmost clinical relevance: a concomitantly existing autoimmune liver disease would necessitate immunosuppressive treatment, which bears the risk of dampening control of the virus-induced liver disease.
Most of the antibodies in viral hepatitis are non-disease-specific and non-organ-specific antibodies (NOSA). Antinuclear antibodies (ANAs) were the first autoantibodies to be associated with AIH (17). Since NOSA have also been detected in patients with viral hepatitis, drug-induced liver injury, Wilson’s disease, alcohol-induced liver disease, non-alcoholic fatty liver disease, and a variety of extrahepatic autoimmune diseases, the specificity of ANAs for a specific (liver) disease is generally low (18).
Anti-smooth muscle antibodies (SMAs) are detected in up to 85% of patients with AIH-1 (19). The SMA titer is also of clinical significance since higher titers have higher AIH-specificity (11). Moreover, sub-specificity toward f-actin has been clearly associated with AIH (11). However, SMA can also be observed in up to 25% of patients with CHB, and chronic hepatitis C virus (HCV) infection (19–22).
The anti-liver-kidney microsomal type 1 antibodies (LKM1), which are targeted against cytochrome P450 CYP2D6 (23) are a hallmark in the diagnosis of AIH-2. In 1983, LKM autoantibodies were also described in CHD patients and later termed LKM-3 (24). These autoantibodies were present in 13–14% of Italian HDV carriers. The major LKM-3 autoantigen was identified as an epitope on family 1 UGTs (UGTl) (25).
Anti-mitochondrial antibodies (AMA) are typically present in patients suffering from primary biliary cholangitis (18) but have also been detected in a small number (3%) of patients with HCV (26). Anti-soluble liver antigen/liver-pancreas antibodies (SLA/LP) are the most specific for AIH-1 among all AIH-related antibodies. However, only a small proportion of patients with AIH show these antibodies that are associated with persistent disease (18, 27, 28).
In patients with CHD, basal cell layer (BCLA) and antithymic antibodies were frequently found, too (29).
The first evidence for autoantibodies in CHD was established as early as 1980 (30). However, in comparison to HBV and HCV infection (31, 32), data for autoimmune phenomena in chronic HDV infection is scarce (31). At the time when the last results on this topic were published nearly 30 years ago, antibody assessment was less standardized, and neither HDV PCR diagnostics, transient liver elastography, nor HDV interferon treatment was readily available (6, 7, 9).
A better knowledge of the autoantibody profiles of CHD might contribute to the understanding of immune-mediated disease progression and extrahepatic manifestations. Autoantibodies could also serve as suitable biomarkers to support the assessment of disease activity, to predict the clinical course, and treatment outcome.
In this study, we cross-sectionally evaluated the autoantibody titers and IgG levels in a single-center cohort of patients with CHD and different disease statuses and compared those to patients with CHB mono-infection and AIH. NOSA titers and IgG levels were correlated to HDV viremia, transaminases, and liver stiffness values from transient elastography as possible markers for the clinical status.
We retrospectively identified and analyzed three different cohorts consisting of 46 patients with AIH, 42 patients with CHD, and 70 patients with CHB. Autoantibody titers were available in 46/46 patients of the AIH cohort, in 40/42 patients of the CHD cohort, and 69/70 patients of the HBV cohort. IgG levels were available in 44/46 patients of the AIH cohort, in 36/42 patients of the CHD cohort, and 61/70 patients of the HBV cohort.
All patients were treated in specialized outpatient clinics at the University Medical Center Hamburg-Eppendorf. The data were collected as part of the clinical routine visits between 2010 and 2019 and retrospectively analyzed. The diagnosis of AIH was secured by serological and histopathological findings as well as treatment response according to the EASL clinical practice guidelines (33). Viral hepatitis was excluded in patients of the AIH group. Inclusion criteria for participants in the CHD cohort were the confirmation of positive HBsAg status as well as proof of anti-HDV, not necessarily an existing viremia. In this present study, 19/41 (46%) of patients with HDV infection had a negative HDV PCR at the time of inclusion.
Patients receiving INF-α therapy within the last 6 months before inclusion, with a history of malignancy, HCV infection, or HIV were excluded. The study was approved by the local ethics committee (WF-035/17).
Testing for autoantibodies was performed by trained personnel at the Institute of Immunology at the University Medical Center Hamburg-Eppendorf. ANA, SMA, LKM-1, and AMA were assessed by immunofluorescence testing. HEp-2 Cells (human epithelioma cells) and tissue slides (Euroimmun, Lübeck, Germany) were incubated in accordance with the manufacturer’s instructions, and interpretation was performed manually using the Eurostar microscope (Euroimmun, Lübeck Germany). SLA was tested by ELISA using enzyme immunoassays from Euroimmun, Germany (11, 34). According to our internal laboratory criteria, we regarded ANA, SMA, and LKM-1 as positive at a titer of >1:80, AMA as positive at titers of ≥1:40, and SLA/LP as positive if ≥20 U/mL. IgG levels were deemed elevated above the cut-off 16 g/L.
All data were collected in a Microsoft® Excel® (Version 12.3.6 for Mac) file and imported into IBM SPSS (Version 24.0 for Mac, Armonk, NY, United States, 2016) for statistical analysis.
Data are presented as absolute numbers and percentages or as the median and interquartile range (IQR). Nominal variables were compared using the Chi-Square test. Metric variables were analyzed using the Mann–Whitney-U-test because there was no normal distribution. Spearman’s rho was used for correlation analysis: the statistical significance is indicated following the correlation coefficient (r) (*p < 0.05, **p < 0.01).
The baseline characteristics of the three patient cohorts are summarized in Table 1. The three cohorts differed in terms of age: patients in the CHB cohort had a median age of 37 years, in the CHD cohort the median age was 45 years, and 54 years in the AIH cohort. Not surprisingly, there were significantly more men in the HDV cohort than in the AIH cohort since AIH is known to be a disease with predominance in women (60% versus 33%) (10).
In the CHB cohort, 16% were under treatment with nucleoside/nucleotide analogs (NA) and had a median viral load under the limit of detection (0–456 IU/mL); 1% had previously undergone treatment with IFN-α and had an HBV DNA load of 130 IU/mL; 83% were therapy naïve and had a median viral load of 2,400 (422–20,250) IU/ml.
In the CHD cohort, 17% of patients were under treatment with nucleoside/nucleotide analogs (NA) and had median HDV and HBV viremia underneath the detection limit (HDV RNA: 0–12,200 IU/mL; HBV DNA: 0–200 IU/mL); 43% of HDV patients had previously undergone treatment with IFN-α and had a median HDV viremia of 32,000 IU/mL (870–220,000) and a median HBV viremia of 70 (6–790) IU/ml; 40% were therapy naïve and had a median HDV viremia under the limit of detection (0–300 IU/mL) and a median HBV DNA load of 378 (35–3,490) IU/ml.
82% of AIH patients were treatment naïve at the time of study inclusion. 7% of patients had received steroids (prednisolone or budesonide) before, and another 11% had previously been treated with steroids and azathioprine.
Patients with CHD had statistically higher IgG levels than patients with CHB (p < 0.01), but lower IgG levels than patients with AIH (p < 0.05). In the AIH cohort, the median IgG level was 19.5 g/L (15.5–27.4) and was elevated in 73% of cases. In the CHD cohort, IgG levels were measured with a median value of 16.9 g/L (12.3–22.4). Elevated IgG levels were detected in 54% of CHD patients. In the CHB cohort, the median IgG level was 12.7 g/L (10.2–14.3) and was elevated in 12% (Figure 1). In addition, patients with CHD showed a weak correlation between IgG levels and HDV viral load levels (r = 0.39*), whereas there was no correlation between IgG levels and HBV viral loads. Thus, patients with detectable HDV RNA (46%) had significantly higher IgG levels compared to patients with undetectable HDV RNA (p > 0.01). Patients with undetectable HDV RNA had similar IgG levels to patients of the CHB cohort. IgG levels were similar in viremic CHD patients regardless of the IFN-α treatment status. IgG levels also correlated weakly significantly with ANA titers in patients with CHD (r = 0.42*). In patients with AIH, IgG levels correlated weakly with SMA titers (r = 0.34*; Table 2).
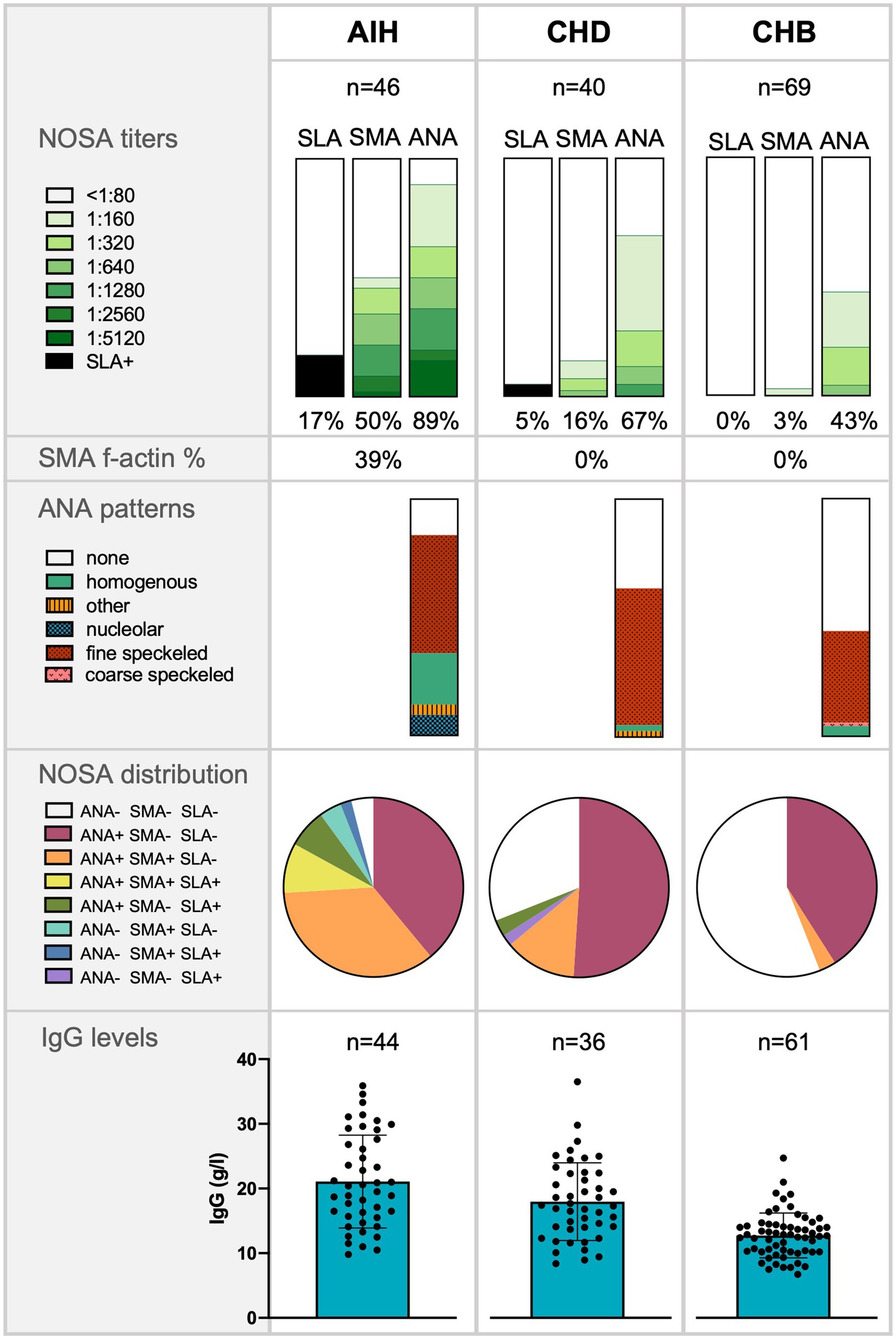
Figure 1. Frequency of non-disease-specific and non-organ-specific antibodies (NOSA), antinuclear antibodies (ANA) patterns, NOSA distribution, and Immunoglobulin G (IgG) levels in patients with autoimmune hepatitis (AIH), chronic hepatitis D (CHD), and chronic hepatitis B (CHB). The significance of IgG levels was tested with a Mann–Whitney-U test: AIH compared to CHD (p < 0.05*); CHD compared to CHB (p < 0.01**).
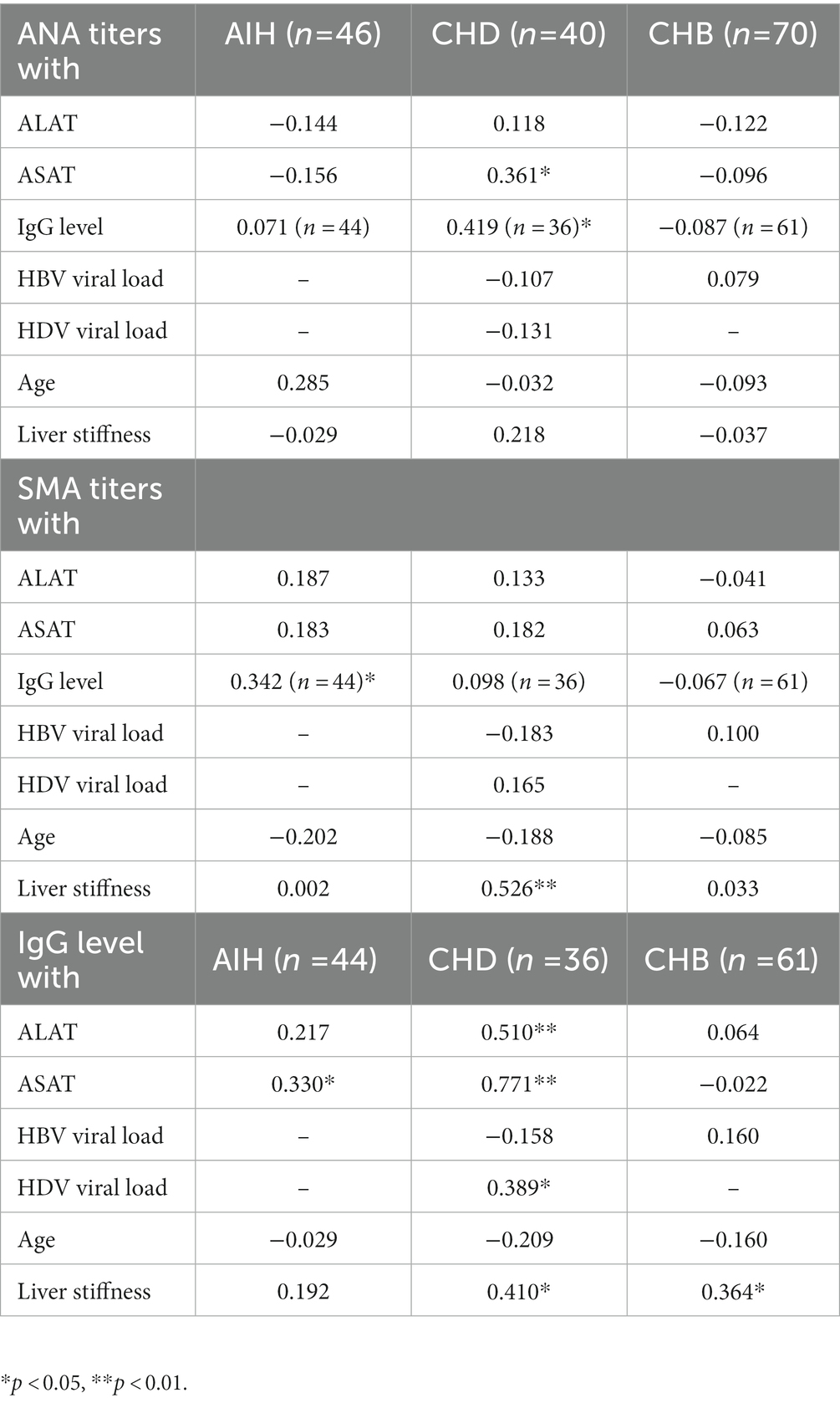
Table 2. Spearman correlation analysis of ANA and SMA titers as well of IgG levels with laboratory parameters in all cohorts.
Positive NOSA titers were found more frequently in the CHD cohort than in the CHB cohort (CHD 69% vs. CHB 43%, p < 0.01). However, positive NOSA titers were significantly less frequent in patients with HDV infection than in patients diagnosed with AIH (CHD 69% vs. AIH 96%, p < 0.01). Notably, the patterns differed between the cohorts: AIH patients displayed significantly more homogenous ANA autoantibodies than the other cohorts. In addition, AIH patients displayed significantly more f-actin autoantibodies than the other cohorts. Details of the ANA staining patterns are shown in Figure 1.
Positive ANA titers were less frequently detected in patients with CHD than in patients with AIH (CHD 67% vs. AIH 89%, p < 0.05). In addition, significantly fewer patients with HDV had high ANA titers of ≥1:320 than patients with AIH (CHD 28% vs. AIH 63%, p < 0.01). However, patients with HDV infection were more likely to have positive ANA titers than patients with CHB (CHD 67% vs. CHB 43%, p < 0.05). CHD patients with detectable HDV RNA were not more likely to have positive ANA titers or ANA titers of ≥1:320 compared to CHD patients without detectable HDV RNA.
In general, positive SMA titers were most frequently observed in patients with AIH (AIH 50% vs. CHD 16% vs. CHB 3%). Specifically, 91% of patients with positive SMA titers in the AIH cohort had high SMA titers of ≥1:320. Patients with CHD tended to have positive SMA titers more frequently than patients with CHB (CHD 16% vs. CHB 3%, p = 0.055). In addition, detectable HDV RNA tended to be associated with positive and high SMA titers ≥1:320 compared to patients with undetectable HDV RNA (>1:80: p = 0.08; ≥1:320: p = 0.08).
Elevated SLA titers were detected in one patient (3%) with CHD and eight patients (17%) diagnosed with AIH, but not in any patient with CHB.
Neither patients with viral hepatitis nor patients with AIH had positive AMA or LKM-1 titers.
Among CHD patients, ANA titers were higher in IFN-α naïve patients than in those who were treated with IFN-α at some time before (median 1:1280 vs. 1:160). SMA titers were detected independently of previous IFN-α treatment. Among the CHD patients who received IFN-α therapy 3/18 (17%) showed sustained response during follow-up. For these patients, NOSA titers were determined before and after treatment and remained unchanged.
The distribution of NOSA was analyzed for each cohort and visualized in Figure 1: Patients with CHB almost exclusively had elevated ANA titers with negative SMA and negative SLA titers. In patients with CHD and patients with AIH, positive ANA titers were also combined with positive SMA titers. However, this pattern occurred significantly less frequently in patients with CHD than in patients with AIH (CHD 13% vs. AIH 35%, p < 0.05). The CHD patient with positive SLA titer was negative for SMA and ANA.
NOSA titers did not correlate with HDV viral load levels.
Transaminases, alanine-aminotransferase (ALAT) and aspartate- aminotransferase (ASAT) were significantly higher in the AIH cohort compared to the two viral hepatitis cohorts (p < 0.01). However, transaminases were significantly higher in patients with HDV infection compared to patients with CHB (p < 0.01). A moderate or weak correlation between transaminases (ALAT; ASAT) and IgG levels were observed in patients with CHD (r = 0.51**; r = 0.77**) and AIH (r = 0.22; r = 0.33*). Transaminase levels were significantly higher in patients with viremic HDV infection than in patients with undetectable HDV RNA, in whom transaminase levels did not exceed those of patients of the CHB cohort. Moreover, a weakly significant correlation between ANA titers and ASAT was seen in patients with HDV infection (r = 0.36*).
There was no statistical difference in liver stiffness values determined by transient elastography among the three different patient cohorts (in kPa: CHB 5.3 (4.3–6.5); CHD 7.7 (6.2–14.0); AIH 8.2 (6.1–17.9)). In CHD patients, liver stiffness values did not correlate with ANA titers (r = 0.22), but showed a moderate correlation with SMA titers (r = 0.53**). In the CHD cohort, liver stiffness values also correlated with HDV viral load (r = 0.52**) and IgG levels (r = 0.41*). CHD patients with liver cirrhosis (liver stiffness values >14 kPa) had significantly higher IgG levels compared to CHD patients with low liver stiffness values (< 6.5 kPa) (p < 0.05). Also, in CHB patients, IgG levels weakly correlated with liver stiffness values (r = 0.36*). In the AIH cohort, no correlation was found between liver stiffness values and ANA titers, SMA titers, or IgG levels.
Biopsies were available for 34 AIH patients, 12 patients with CHD, and 3 patients with CHB (Supplementary Tables 1–3). The median Desmet score for liver fibrosis/cirrhosis was similar for AIH and CHD patients (2/4). The biopsies of patients with CHB did not show fibrosis/cirrhosis (0/4). The median modified Ishak score (mHAI) was higher in AIH patients (9/18, IQR 4–16) compared to patients with CHD (6.5/18, IQR 2–10) and CHB (4,5/18, IQR 3–6), without reaching statistical significance.
Infections with hepatotropic viruses have been associated with various immunopathological manifestations. An association between infection and autoimmunity is well documented, particularly for chronic infections with hepatitis B and hepatitis C viruses (31). In comparison, less data was available on the frequency and pattern of NOSA for CHD and in relation to the disease or treatment status (30, 35).
In chronically HDV-infected individuals, perforin-positive cytotoxic CD4+ T cells accumulate and have been implicated in contributing to the severity of HDV related liver infection (36–38). Also, HDV infection was shown to augment the antiviral state of the hepatocytes, chemokine production, and antigen presentation (39, 40). Though the liver damage that results from chronic HDV infection is considered to be primarily mediated by the immune system (37, 41), little is known about the role of autoantibodies.
In this current study, we cross-sectionally examined available NOSA titers and IgG levels in 42 patients with CHD and 116 controls consisting of 70 patients with CHB, and 46 patients diagnosed with AIH. Because patients with CHD were compared to patients with HBV mono-infection, differences in autoimmune phenomena, represented by autoantibody titers and IgG levels, could be associated with the addition of HDV infection.
This study is limited by its retrospective design, the absence of multicenter validation, and the small sample size. Also, the three cohorts were not matched for age, sex, or degree of liver damage. Only very selected autoantibodies were analyzed as used in the standard workup of elevated liver enzymes. This panel is primarily matched to the autoantibody profile of AIH, PSC, and PBC.
Here, almost exclusively patients with HDV genotype 1 infection were included, as this is the most common genotype circulating in Europe (42). However, recent studies have shown differences in spreading kinetics, treatment outcome, and disease courses between variant HDV genotypes (43, 44). Therefore, it would be of interest whether the autoantibody profile differs, too.
Elevated serum IgG levels are considered a characteristic feature of AIH, as they are detected in up to 85% of patients and are part of the diagnostic score in AIH (45). We found elevated IgG levels in 73% of patients with AIH, and only in 12% of patients with CHB, but also in 54% of patients with CHD. Hartl et al. previously suggested a correlation between ANA/SMA titers and IgG levels in patients with AIH, but IgG levels were not related to the degree of liver fibrosis or intrahepatic inflammatory activity (45). In this current study, there was also a significant correlation between IgG and ANA titers in CHD patients and SMA and IgG in AIH patients. However, there was also a significant correlation between IgG and transaminases, suggesting a relationship between IgG and intrahepatic inflammatory activity in CHD and AIH patients. Also, in the CHD cohort IgG levels and HDV viral loads correlated with higher liver stiffness values. Patients with HDV viremia had significantly higher IgG levels than patients without detectable viremia.
Most data available on the frequency of autoantibodies in CHD patients is historic, when the methodologies applied differend, were less standardized, and IFN-α treatment was not available. In this study, patients with CHD were significantly more likely to have positive/high NOSA titers than patients with CHB (CHD 69% vs. CHB 43%; p < 0.01), but less likely to have positive NOSA titers than patients with AIH (CHD 69% vs. AIH 96%; p < 0.01). The prevalence of positive NOSA titers in AIH patients determined in our study was consistent with the literature (46). In a study by McFarlane et al. from 1995, about 20% of CHD patients (n = 27) had positive NOSA titers, similar to the included patients with HBV mono-infection (n = 22) (35). In a study with 325 Chinese patients diagnosed with CHB, 58.2% showed positive NOSA titers at the same cut-off value as in our analysis, which was significantly higher than in healthy controls (6.7%) (47).
In line with our results, ANA were among the most frequent NOSA in the CHB patients of the Chinese cohort (47). The data base for ANA in CHD is scarce. In contrast to our results, in 1986, Zauli et al. found the prevalence of ANA to be significantly lower in CHD patients (9%) than in CHB patients (48).
Patients with AIH were significantly more likely to have positive SMA titers and, in particular, to have high titers (≥ 1:320) than patients with CHD or CHB. However, 8% of patients with CHD also showed SMA titers, while these antibodies were not present in CHB patients. While patients with HBV mainly had isolated elevated ANA titers, patients with HDV and AIH also had combined elevated ANA and SMA titers. In a previous study from 1986, the prevalence of SMA in CHD did not significantly differ from chronic hepatitis HBV mono-infection (48). Here, SMA titers correlated with liver stiffness values in CHD patients but not in the other cohorts. Larger studies should be conducted to validate whether high SMA titers are indeed associated with activity or prognosis of the HDV liver disease.
SLA is considered the most specific marker for AIH among all AIH-related antibodies. It is still controversial whether the detection of SLA is associated with severe courses of AIH (18, 27).
Rarely, do HCV/LKM-1 positive patients progress to LKM-1 positive autoimmune hepatitis (49). Positive LKM-1 was not detected in any patients of this current study. Whereas the presence of LKM-1 is a typical feature of AIH-2, only patients with AIH-1 were included in this study. In this study, some CHD patients had high mHAI scores. Whether HDV infection – as has been described for chronic HCV infection – can indeed trigger an AIH, is not fully elucidated.
On IFN-α therapy, HCV/LKM-1 positive patients experience increases in aminotransferase levels, occasionally of such magnitude to warrant suspension of treatment (50, 51). Subgroup analysis of CHD patients showed no differences in NOSA titers irrespective of a previous IFN-α-therapy. In the current study, CHD patients with elevated NOSA titers did not experience relevant increases in aminotransferase levels upon IFN-α treatment. In our study, 43% of the CHD patients underwent treatment with pegylated IFN-α, although only 17% of IFN-α treated HDV patients showed a sustained response (HH-CHD8, HH-CHD26, HH-CHD42, Supplementary Table 3). These patients showed similar NOSA titers before and after treatment. Interestingly, in another recently published case of CHD with clinical and histological stigmata of AIH, therapy with the HBV/HDV entry inhibitor bulevirtide led to rapid normalization of immunoglobulin levels (52). Further studies are needed to assess whether such a therapeutic approach may lead to a substantial reduction of immunoglobulin levels. The literature does not indicate benefits in treating CHD patients displaying high NOSA titers with immunomodulating agents such as steroids (53, 54).
The median mHAI score was the highest in the AIH cohort, though not significantly compared to the CHD and CHB cohorts. Interestingly, the CHD patient with the highest mHAI score (10/18) also had highly elevated ANA titers and IgG levels. However, by histology, it is not possible to distinguish between viral hepatitis and AIH with certainty, and elevated NOSA as well as IgG levels were detected in many patients of the CHD cohort. Of note, NOSA patterns differed significantly in patients with AIH and viral hepatitis: the pattern of ANA autoantibodies in AIH patients was significantly more often homogenous than in the other two patient groups. Also, AIH patients displayed f-actin autoantibodies significantly more often than patients with viral hepatitis. If validated in larger studies, such differences in the NOSA titers and pattern may even be used to establish novel biomarkers to delimit the few patients with covert AIH from the majority of patients that demonstrate NOSA rather as an unspecific para-infectious immune phenomenon.
Notably, high NOSA titers might predict the development of autoimmune disease even after sustained HDV control (55). Arbuckle et al. found that ANAs with a dilution titer of 1:120 were present in 78% of lupus erythematosus patients studied 3–9 years before clinical manifestation (56). One patient of the CHD cohort also had cryoglobulins and was diagnosed with membranoproliferative glomerulonephritis (HH-CHD41, Supplementary Table 3). Additionally, prospective studies examining the presence of high autoantibody titers in an HDV cohort are needed to assess their significance as early predictors of autoimmune disease or as a singular para-infectious phenomenon. In this context, it seems important to investigate the prevalence of HDV infection in other autoimmune diseases. Further studies should assess the prevalence of additional clinical symptoms (arthralgias, sicca symptom) and autoantibodies typical of other autoimmune diseases. For example, anti-SSA and anti-SSB in Sjögren’s syndrome, and cryoglobulins have been repeatedly found in patients with chronic hepatitis B, C, and E infection (34).
Possible mechanisms responsible for the immunopathology seen in chronic viral infections, in general, include molecular mimicry, impairment of regulatory T-cell activity, and polyclonal activation of B lymphocytes (31, 57, 58). Polyclonal activation of B cells seems to be a likely explanation of the correlation of IgG levels with HDV disease activity and severity. The persistence of elevated NOSA titers in non-viremic HDV patients could be due to the formation of persisting dysfunctional atypical memory B cells (61, 62). The role of B cells in CHD needs to be clarified in future studies.
In summary, autoantibodies are frequently detected as a para-infectious feature of CHD patients with unclear clinical significance.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Hamburger Ethikkammer. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JSZW, ML, and CW-N initiated and supervised the study. LH, SL, and JSZW wrote the first draft of the manuscript. SL, LH, and CW-N performed analyses and generated data. FH, MD, AWL, AZ, FP, and J-HB discussed the data and corrected the manuscript. All authors contributed to the manuscript and approved the final submitted version.
JSZW, AWL, MD, and ML are funded by the DZIF. JSZW is funded by DFG SFB1328, and the EU (Thervac B).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1169096/full#supplementary-material
1. Dandri, M, Volmari, A, and Lutgehetmann, M. The Hepatitis Delta virus and chronic hepatitis D. J Hepatol. (2022) 77:1448–50. doi: 10.1016/j.jhep.2022.05.022
2. Buti, M, Homs, M, Rodriguez-Frias, F, Funalleras, G, Jardí, R, Sauleda, S, et al. Clinical outcome of acute and chronic Hepatitis Delta over time: a long-term follow-up study. J Viral Hepat. (2011) 18:434–2. doi: 10.1111/j.1365-2893.2010.01324.x
3. Niro, GA, Smedile, A, Ippolito, AM, Ciancio, A, Fontana, R, Olivero, A, et al. Outcome of Chronic Delta hepatitis in Italy: a long-term cohort study. J Hepatol. (2010) 53:834–09. doi: 10.1016/j.jhep.2010.06.008
4. Su, CW, Huang, YH, Huo, TI, Shih, HH, Sheen, IJ, Chen, SW, et al. Genotypes and viremia of hepatitis B and D viruses are associated with outcomes of chronic hepatitis D patients. Gastroenterology. (2006) 130:1625–35. doi: 10.1053/j.gastro.2006.01.035
5. Bockmann, JH, Grube, M, Hamed, V, von Felden, J, Landahl, J, Wehmeyer, M, et al. High rates of cirrhosis and severe clinical events in patients with Hbv/Hdv co-infection: longitudinal analysis of a German cohort. BMC Gastroenterol. (2020) 20:24. doi: 10.1186/s12876-020-1168-9
6. Pfluger, LS, Norz, D, Volz, T, Giersch, K, Giese, A, Goldmann, N, et al. Clinical establishment of a laboratory developed quantitative Hdv Pcr assay on the Cobas6800 high-throughput system. JHEP Rep. (2021) 3:100356. doi: 10.1016/j.jhepr.2021.100356
7. Wranke, A, Heidrich, B, Ernst, S, Calle Serrano, B, Caruntu, FA, Curescu, MG, et al. Anti-Hdv Igm as a marker of disease activity in Hepatitis Delta. PLoS One. (2014) 9:e101002. doi: 10.1371/journal.pone.0101002
8. Wedemeyer, H, Yurdaydin, C, Dalekos, GN, Erhardt, A, Cakaloglu, Y, Degertekin, H, et al. Peginterferon plus Adefovir versus either drug alone for Hepatitis Delta. N Engl J Med. (2011) 364:322–1. doi: 10.1056/NEJMoa0912696
9. Wedemeyer, H, Schoneweis, K, Bogomolov, P, Blank, A, Voronkova, N, Stepanova, T, et al. Safety and efficacy of Bulevirtide in combination with Tenofovir Disoproxil fumarate in patients with hepatitis B virus and hepatitis D virus coinfection (Myr202): a multicentre, randomised, parallel-group, open-label, phase 2 trial. Lancet Infect Dis. (2023) 23:117–9. doi: 10.1016/S1473-3099(22)00318-8
10. Sebode, M, Hartl, J, Vergani, D, and Lohse, AW. Autoimmune hepatitis: from current knowledge and clinical practice to future research agenda. Liver Int. (2018) 38:15–22. doi: 10.1111/liv.13458
11. Galaski, J, Weiler-Normann, C, Schakat, M, Zachou, K, Muratori, P, Lampalzer, S, et al. Update of the simplified criteria for autoimmune hepatitis: evaluation of the methodology for Immunoserological testing. J Hepatol. (2021) 74:312–09. doi: 10.1016/j.jhep.2020.07.032
12. Vergani, D, Alvarez, F, Bianchi, FB, Cancado, EL, Mackay, IR, Manns, MP, et al. Liver autoimmune serology: a consensus statement from the Committee for Autoimmune Serology of the international autoimmune hepatitis group. J Hepatol. (2004) 41:677–3. doi: 10.1016/j.jhep.2004.08.002
13. Easl. Clinical practice guidelines on the Management of Hepatitis B Virus Infection. J Hepatol. (2017) 67:370–98. doi: 10.1016/j.jhep.2017.03.021
14. Pyrsopoulos, NT, and Reddy, KR. Extrahepatic manifestations of chronic viral hepatitis. Curr Gastroenterol Rep. (2001) 3:71–8. doi: 10.1007/s11894-001-0044-1
15. Mazzaro, C, Dal Maso, L, Visentini, M, Gitto, S, Andreone, P, Toffolutti, F, et al. Hepatitis B virus-related Cryogobulinemic Vasculitis. The role of antiviral Nucleot(S)ide analogues: a review. J Intern Med. (2019) 286:290–8. doi: 10.1111/joim.12913
16. Smatti, MK, Cyprian, FS, Nasrallah, GK, Al Thani, AA, Almishal, RO, and Yassine, HM. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. (2019) 11:762. doi: 10.3390/v11080762
17. Mackay, IR, Weiden, S, and Hasker, J. Autoimmune Hepatitis. Ann N Y Acad Sci. (1965) 124:767–09. doi: 10.1111/j.1749-6632.1965.tb19000.x
18. Sebode, M, Weiler-Normann, C, Liwinski, T, and Schramm, C. Autoantibodies in autoimmune liver disease-clinical and diagnostic relevance. Front Immunol. (2018) 9:609. doi: 10.3389/fimmu.2018.00609
19. Johanet, C, and Ballot, E. Auto-antibodies in autoimmune hepatitis: anti-smooth muscle antibodies (Asma). Clin Res Hepatol Gastroenterol. (2012) 36:189–1. doi: 10.1016/j.clinre.2011.10.012
20. Terziroli Beretta-Piccoli, B, Mieli-Vergani, G, and Vergani, D. Autoimmune hepatitis: serum autoantibodies in clinical practice. Clin Rev Allergy Immunol. (2021) 63:124–7. doi: 10.1007/s12016-021-08888-9
21. Deshpande, P, Bundell, C, McKinnon, E, Hellard, M, Ffrench, R, Wilkinson, AL, et al. Frequent occurrence of low-level positive autoantibodies in chronic hepatitis C. Pathology. (2020) 52:576–3. doi: 10.1016/j.pathol.2020.05.001
22. Cassani, F, Cataleta, M, Valentini, P, Muratori, P, Giostra, F, Francesconi, R, et al. Serum autoantibodies in chronic hepatitis C: comparison with autoimmune hepatitis and impact on the disease profile. Hepatology. (1997) 26:561–6. doi: 10.1002/hep.510260305
23. Girardin, F, Daali, Y, Gex-Fabry, M, Rebsamen, M, Roux-Lombard, P, Cerny, A, et al. Liver kidney microsomal type 1 antibodies reduce the Cyp2d6 activity in patients with chronic hepatitis C virus infection. J Viral Hepat. (2012) 19:568–3. doi: 10.1111/j.1365-2893.2011.01578.x
24. Crivelli, O, Lavarini, C, Chiaberge, E, Amoroso, A, Farci, P, Negro, F, et al. Microsomal autoantibodies in chronic infection with the Hbsag Associated Delta (Delta) agent. Clin Exp Immunol. (1983) 54:232–8.
25. Philipp, T, Durazzo, M, Trautwein, C, Alex, B, Straub, P, Lamb, JG, et al. Recognition of uridine diphosphate Glucuronosyl transferases by Lkm-3 antibodies in chronic hepatitis D. Lancet. (1994) 344:578–1. doi: 10.1016/s0140-6736(94)91966-6
26. Colapietro, F, Lleo, A, and Generali, E. Antimitochondrial antibodies: from bench to bedside. Clin Rev Allergy Immunol. (2021) 63:166–7. doi: 10.1007/s12016-021-08904-y
27. Liberal, R, Mieli-Vergani, G, and Vergani, D. Clinical significance of autoantibodies in autoimmune hepatitis. J Autoimmun. (2013) 46:17–24. doi: 10.1016/j.jaut.2013.08.001
28. Hartl, J, Ehlken, H, Weiler-Normann, C, Sebode, M, Kreuels, B, Pannicke, N, et al. Patient selection based on treatment duration and liver biochemistry increases success rates after treatment withdrawal in autoimmune hepatitis. J Hepatol. (2015) 62:642–6. doi: 10.1016/j.jhep.2014.10.018
29. Buti, M, Amengual, MJ, Esteban, R, Pujol, A, Jardi, R, Allende, H, et al. Serological profile of tissue autoantibodies during acute and Chronic Delta hepatitis. J Hepatol. (1989) 9:345–09. doi: 10.1016/0168-8278(89)90144-x
30. Philipp, T, Obermayer-Straub, P, and Manns, MP. Autoantibodies in Hepatitis Delta. Biomed Pharmacother. (1995) 49:344–9. doi: 10.1016/0753-3322(96)82663-1
31. Vergani, D, and Mieli-Vergani, G. Autoimmune manifestations in viral hepatitis. Semin Immunopathol. (2013) 35:73–85. doi: 10.1007/s00281-012-0328-6
32. Shahini, E, Iannone, A, Romagno, D, Armandi, A, Carparelli, S, Principi, M, et al. Clinical relevance of serum non-organ-specific antibodies in patients with Hcv infection receiving direct-acting antiviral therapy. Aliment Pharmacol Ther. (2018) 48:1138–45. doi: 10.1111/apt.14999
33. European Association for the study of the Liver. EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol. (2015) 63:971–1004. doi: 10.1016/j.jhep.2015.06.030
34. Horvatits, T, Schulze Zur Wiesch, J, Polywka, S, Buescher, G, Lütgehetmann, M, Hussey, E, et al. Significance of anti-nuclear antibodies and Cryoglobulins in patients with acute and chronic Hev infection. Pathogens. (2020) 9. doi: 10.3390/pathogens9090755
35. McFarlane, BM, Bridger, CB, Smith, HM, Antonov, KA, Naoumov, N, Williams, R, et al. Autoimmune mechanisms in chronic hepatitis B and Delta virus infections. Eur J Gastroenterol Hepatol. (1995) 7:615–1.
36. Aslan, N, Yurdaydin, C, Wiegand, J, Greten, T, Ciner, A, Meyer, MF, et al. Cytotoxic Cd4 T cells in viral hepatitis. J Viral Hepat. (2006) 13:505–14. doi: 10.1111/j.1365-2893.2006.00723.x
37. Kohsar, M, Landahl, J, Neumann-Haefelin, C, and Schulze Zur Wiesch, J. Human hepatitis D virus-specific T cell epitopes. JHEP Rep. (2021) 3:100294. doi: 10.1016/j.jhepr.2021.100294
38. Landahl, J, Bockmann, JH, Scheurich, C, Ackermann, C, Matzat, V, Heide, J, et al. Detection of a broad range of low-level major histocompatibility complex class ii-restricted, Hepatitis Delta virus (Hdv)-specific T-cell responses regardless of clinical status. J Infect Dis. (2019) 219:568–7. doi: 10.1093/infdis/jiy549
39. Giersch, K, Allweiss, L, Volz, T, Helbig, M, Bierwolf, J, Lohse, AW, et al. Hepatitis Delta co-infection in humanized mice leads to pronounced induction of innate immune responses in comparison to Hbv mono-infection. J Hepatol. (2015) 63:346–3. doi: 10.1016/j.jhep.2015.03.011
40. Dandri, M, Bertoletti, A, and Lutgehetmann, M. Innate immunity in hepatitis B and D virus infection: consequences for viral persistence, inflammation, and T cell recognition. Semin Immunopathol. (2021) 43:535–8. doi: 10.1007/s00281-021-00864-x
41. Abbas, Z, and Afzal, R. Life cycle and pathogenesis of hepatitis D virus: a review. World J Hepatol. (2013) 5:666–5. doi: 10.4254/wjh.v5.i12.666
42. Le Gal, F, Gault, E, Ripault, MP, Serpaggi, J, Trinchet, JC, Gordien, E, et al. Eighth major clade for Hepatitis Delta virus. Emerg Infect Dis. (2006) 12:1447–50. doi: 10.3201/eid1209.060112
43. Giersch, K, Hermanussen, L, Volz, T, Volmari, A, Allweiss, L, Sureau, C, et al. Strong replication interference between Hepatitis Delta viruses in human liver chimeric mice. Front Microbiol. (2021) 12:671466. doi: 10.3389/fmicb.2021.671466
44. Borzacov, LM, de Figueiredo Nicolete, LD, Souza, LF, Dos Santos, AO, Vieira, DS, and Salcedo, JM. Treatment of Hepatitis Delta virus genotype 3 infection with peg-interferon and Entecavir. Int J Infect Dis. (2016) 46:82–8. doi: 10.1016/j.ijid.2016.03.017
45. Hartl, J, Miquel, R, Zachou, K, Wong, GW, Asghar, A, Pape, S, et al. Features and outcome of Aih patients without elevation of igg. JHEP Rep. (2020) 2:100094. doi: 10.1016/j.jhepr.2020.100094
46. Mieli-Vergani, G, Vergani, D, Czaja, AJ, Manns, MP, Krawitt, EL, Vierling, JM, et al. Autoimmune Hepatitis. Nat Rev Dis Primers. (2018) 4:18017. doi: 10.1038/nrdp.2018.17
47. Li, BA, Liu, J, Hou, J, Tang, J, Zhang, J, Xu, J, et al. Autoantibodies in Chinese patients with chronic hepatitis B: prevalence and clinical associations. World J Gastroenterol. (2015) 21:283–1. doi: 10.3748/wjg.v21.i1.283
48. Zauli, D, Crespi, C, Bianchi, FB, Craxi, A, and Pisi, E. Autoimmunity in chronic liver disease caused by Hepatitis Delta virus. J Clin Pathol. (1986) 39:897–9. doi: 10.1136/jcp.39.8.897
49. Gregorio, GV, Pensati, P, Iorio, R, Vegnente, A, Mieli-Vergani, G, and Vergani, D. Autoantibody prevalence in children with liver disease due to chronic hepatitis C virus (Hcv) infection. Clin Exp Immunol. (1998) 112:471–6. doi: 10.1046/j.1365-2249.1998.00574.x
50. Dalekos, GN, Wedemeyer, H, Obermayer-Straub, P, Kayser, A, Barut, A, Frank, H, et al. Epitope mapping of cytochrome P4502d6 autoantigen in patients with chronic hepatitis C during alpha-interferon treatment. J Hepatol. (1999) 30:366–5. doi: 10.1016/s0168-8278(99)80092-0
51. Muratori, L, Zauli, D, Giostra, F, Ballardini, G, Lenzi, M, Cassani, F, et al. Lkm1 appearance in a Hla-Dr3+ patient with chronic hepatitis C during interferon treatment. J Hepatol. (1993) 18:259–09. doi: 10.1016/s0168-8278(05)80258-2
52. Loglio, A, Ferenci, P, Uceda Renteria, SC, Tham, CYL, van Bommel, F, Borghi, M, et al. Excellent safety and effectiveness of high-dose Myrcludex-B monotherapy administered for 48 weeks in Hdv-related compensated cirrhosis: a case report of 3 patients. J Hepatol. (2019) 71:834–9. doi: 10.1016/j.jhep.2019.07.003
53. Niro, GA, Rosina, F, and Rizzetto, M. Treatment of hepatitis D. J Viral Hepat. (2005) 12:2–9. doi: 10.1111/j.1365-2893.2005.00601.x
54. Farci, P. Treatment of chronic hepatitis D: new advances old challenges. Hepatology. (2006) 44:536–9. doi: 10.1002/hep.21351
55. Akmatov, MK, Röber, N, Ahrens, W, Flesch-Janys, D, Fricke, J, Greiser, H, et al. Anti-nuclear autoantibodies in the general German population: prevalence and lack of association with selected cardiovascular and metabolic disorders-findings of a multicenter population-based study. Arthritis Res Ther. (2017) 19:127. doi: 10.1186/s13075-017-1338-5
56. Arbuckle, MR, McClain, MT, Rubertone, MV, Scofield, RH, Dennis, GJ, James, JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. (2003) 349:1526–33. doi: 10.1056/NEJMoa021933
57. Wildner, NH, Ahmadi, P, Schulte, S, Brauneck, F, Kohsar, M, Lutgehetmann, M, et al. B cell analysis in Sars-Cov-2 versus malaria: increased frequencies of Plasmablasts and atypical memory B cells in Covid-19. J Leukoc Biol. (2021) 109:77–90. doi: 10.1002/JLB.5COVA0620-370RR
Keywords: hepatitis D virus, hepatitis B virus, autoimmune hepatitis, autoantibodies, viral hepatitis
Citation: Hermanussen L, Lampalzer S, Bockmann J-H, Ziegler AE, Piecha F, Dandri M, Pischke S, Haag F, Lohse AW, Lütgehetmann M, Weiler-Normann C and Wiesch JSz (2023) Non-organ-specific autoantibodies with unspecific patterns are a frequent para-infectious feature of chronic hepatitis D. Front. Med. 10:1169096. doi: 10.3389/fmed.2023.1169096
Received: 18 February 2023; Accepted: 18 May 2023;
Published: 14 June 2023.
Edited by:
Valentina Svicher, University of Rome Tor Vergata, ItalyReviewed by:
Antonella Olivero, University of Turin, ItalyCopyright © 2023 Hermanussen, Lampalzer, Bockmann, Ziegler, Piecha, Dandri, Pischke, Haag, Lohse, Lütgehetmann, Weiler-Normann and Wiesch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julian Schulze zur Wiesch, anVsaWFuc3p3QGdvb2dsZW1haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.