
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Med. , 11 April 2023
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1167812
This article is part of the Research Topic Soil-Transmitted Helminth Infections from a One Health Perspective View all 5 articles
Editorial on the Research Topic
Soil-transmitted helminth infections from a One Health perspective
One Health is a multidisciplinary approach aimed at protecting and promoting the health of humans, animals, and ecosystems (1). In a public health context, One Health is particularly applied to investigate, understand, control, and prevent infectious and parasitic diseases, with particular attention to zoonoses. One Health highlights that the health of (I) human societies, (II) domestic and wild animals, and (III) the environment is strongly connected and, therefore, public health issues must be addressed by considering such connections (1). Several examples highlight these aspects in the context of infectious and parasitic diseases. Anthropogenic activities that are detrimental to the environment and animals facilitate the transmission of several zoonotic parasites (2). Pervasive human interaction with wild species (e.g., in wet markets) contributed to the emergence of SARS-CoV-2 in the human population and the COVID-19 pandemic (3). Deforestation of tropical forests and habitat fragmentation facilitate the dissemination of several viral, bacterial, and parasitic diseases. By contrast, the preservation of biodiversity and the environment is a protective factor against emerging infectious diseases (4). Currently, there is a growing acceptance of the One Health need to address parasitic diseases in the next decades in a changing, conflicted, and resource-limited world (5, 6).
Soil-transmitted helminths (STHs) are a group of parasites that mainly affect populations in places with sanitation problems as STH transmission particularly occurs through contact with soil contaminated with parasitic eggs or larvae (7, 8). It is estimated that 1.5 billion individuals are infected with STHs worldwide and they are included in the list of neglected tropical diseases by the WHO (8). STH infections cause several health problems, such as malnutrition, iron deficiency, and child developmental deficits (7, 8). Among STHs, roundworms (Ascaris lumbricoides), whipworms (Trichuris trichiura), hookworms (Necator americanus and Ancylostoma duodenale), and Strongyloides stercoralis are of great public health importance for some populations. Zoonotic STHs (parasites transmitted from animals to humans), including Strongyloides fuelleborni, Toxocara canis, Toxocara cati, Ancylostoma ceylanicum, and Ancylostoma brazilienese, also cause concern in several countries (7).
Animal, environmental, and human factors may affect multiple aspects associated with STH infections (Figure 1). Vertebrate animals, such as dogs and cats, influence the risk of zoonotic STH infections (by T. canis, T. cati, etc.) (9). Invertebrate animals can act as mechanical carriers of parasite eggs between different places (10). The lack of environmental sanitation and access to clean water facilitate the contamination of soil and foods with eggs and larvae of STHs, increasing infection risk (8). Human immunological, nutritional, and genetic factors may influence both susceptibility to and consequences of STH infection in the host (e.g., parasite load, affecting the number of eggs excreted in the feces) (7, 11, 12). Socio-economic and cultural factors (e.g., open defecation, walking barefoot, and food habits) also modify the STH infection transmission risk (12, 13). Further discussion about the importance of addressing parasitic diseases under the One Health perspective can be found elsewhere (14, 15).
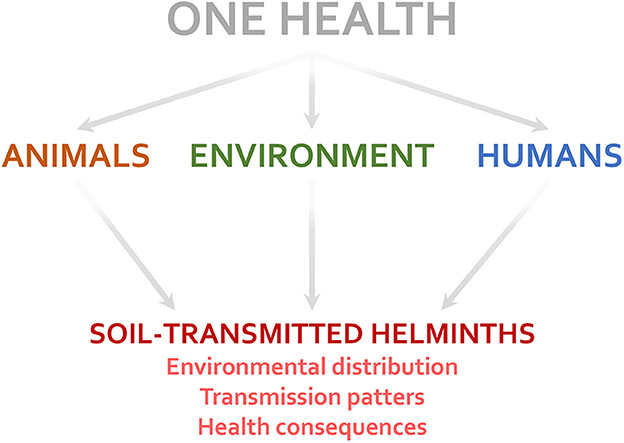
Figure 1. Factors related to the One Health triad (humans, animals, and the environment) influence several aspects of soil-transmitted helminth infections.
The articles published in this Research Topic advanced the knowledge of STHs, with contributions from different perspectives. In a study performed in Slovakia, Ihnacik et al. investigated STHs from the One Health perspective, evaluating human, animal, and environmental (soil) samples. The study focused on the Roma community, a socially vulnerable group of people. In human samples, A. lumbricoides and T. trichiura were the most prevalent parasites. Of note, people belonging to the Roma minority had a higher infection rate (odds ratio: 37.12) than non-Roma inhabitants, highlighting the importance of social issues as determinants of STH infections. Considering the parasitological evaluation of the environment, 26.26% of soil samples were positive for STH eggs. Regarding domestic animals, the authors observed STH eggs in 43.55% of the analyzed dog fecal samples. Finally, Ihnacik et al. reported that climatic factors, ethnicity, and water sanitation and hygiene (WASH) issues are crucial factors influencing the distribution and transmission of STHs in the studied population, suggesting the need for a One Health approach to control STH infection in Slovakia. Of note, other studies performed in different countries supported the role of climatic factors (16, 17), ethnicity (18, 19), and WASH (20, 21) as determinants of STH distribution and transmission.
Helminth infections alter immune responses in different ways, often influencing the susceptibility and outcome of non-parasitic diseases. In this context, Bazargan et al. performed a case-control study and scoping review of the literature to investigate the influence of Toxocara infection on allergic asthma manifestations in humans. For the case-control study, data from 124 healthy individuals and 124 patients with asthma from Iran were evaluated. The authors observed a significant association between asthma severity and age in Toxocara-seropositive individuals. However, no significant association between asthma and Toxocara seropositivity was found. Bazargan et al. suggest the need for further studies investigating the potential influence of Toxocara infection on asthma susceptibility in humans. Case-control, cross-sectional, and cohort studies are a few methodological approaches that can be used for such an investigation.
Malaria and helminth co-infection is a major issue in some African countries. Afolabi et al. performed two surveys among children living in urban and rural settings in Senegal to determine the prevalence of malaria-helminth co-infection. There was a 2.2% prevalence of polyparasitism with Plasmodium falciparum, STHs, Schistosoma haematobium, and Schistosoma mansoni among children in the two study sites. The prevalence of P. falciparum-S. haematobium and P. falciparum-S. mansoni co-infections was 1.1% and 0.7%, respectively. These co-infection rates were considered low, a result potentially linked to the sustained effective control measures for parasitic infections.
Finally, Walker et al. evaluated the effectiveness of One Health interventions against the zoonotic hookworm Ancylostoma ceylanicum, a parasite of dogs but also commonly reported in people from Southeast Asia and the Pacific. The authors modeled the effect of mass drug administration (MDA) on infection control, showing that targeting both dogs and humans could suppress prevalence in humans to ≤1% by the end of 2030, with a 25–50% coverage of the animal reservoir. The study also suggested that the disease transmission cycle could be completely interrupted with an increase in treatment coverage. In brief, this study evidenced the importance of controlling zoonotic STH infections from a One Health perspective.
STH infections should be studied and managed with the One Health perspective in mind, as strongly suggested by the studies included in this Research Topic. This approach is increasingly important in a world with huge environmental and social challenges.
JE wrote the first draft of the manuscript and prepared Figure 1. SC complemented and edited the text. All authors contributed to the article and approved the submitted version.
JE receives a postdoctoral fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Programa Nacional de Pós-Doutorado–PNPD/CAPES, Brazil).
The editors are grateful to all the authors who participated in this Research Topic. We also thank the reviewers who revised the manuscripts submitted to this Research Topic.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. WHO-World Health Organization. Tripartite and UNEP support OHHLEP's definition of “One Health”-Joint Tripartite (FAO, OIE, WHO) and UNEP Statement. (2021). Available online at: https://www.who.int/news/item/01-12-2021-tripartite-and-unep-support-ohhlep-s-definition-of-one-health (accessed on February 9, 2023).
2. Thompson RC. Parasite zoonoses and wildlife: One Health, spillover and human activity. Int J Parasitol. (2013) 43:1079–88. doi: 10.1016/j.ijpara.2013.06.007
3. Holmes EC, Goldstein SA, Rasmussen AL, Robertson DL, Crits-Christoph A, Wertheim JO, et al. The origins of SARS-CoV-2: a critical review. Cell. (2021) 184:4848–56. doi: 10.1016/j.cell.2021.08.017
4. Ellwanger JH, Fearnside PM, Ziliotto M, Valverde-Villegas JM, Veiga ABG, Vieira GF, et al. Synthesizing the connections between environmental disturbances and zoonotic spillover. An Acad Bras Cienc. (2022) 94:e20211530. doi: 10.1590/0001-3765202220211530
5. Jenkins EJ, Simon A, Bachand N, Stephen C. Wildlife parasites in a One Health world. Trends Parasitol. (2015) 31:174–80. doi: 10.1016/j.pt.2015.01.002
6. Hotez PJ. Human parasitology and parasitic diseases: heading towards 2050. Adv Parasitol. (2018) 100:29–38. doi: 10.1016/bs.apar.2018.03.002
7. Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. (2006) 367:1521–32. doi: 10.1016/S0140-6736(06)68653-4
8. WHO-World Health Organization. Soil-Transmitted Helminth Infections. (2022). Available online at: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on January 6, 2023).
9. Dantas-Torres F, Otranto D. Dogs, cats, parasites, and humans in Brazil: opening the black box. Parasit Vectors. (2014) 7:22. doi: 10.1186/1756-3305-7-22
10. Benelli G, Wassermann M, Brattig NW. Insects dispersing taeniid eggs: who and how? Vet Parasitol. (2021) 295:109450. doi: 10.1016/j.vetpar.2021.109450
11. Ellis MK, Raso G, Li YS, Rong Z, Chen HG, McManus DP. Familial aggregation of human susceptibility to co- and multiple helminth infections in a population from the Poyang Lake region, China. Int J Parasitol. (2007) 37:1153–61. doi: 10.1016/j.ijpara.2007.02.008
12. Ellwanger JH, Ziliotto M, Kulmann-Leal B, Chies JAB. Iron deficiency and soil-transmitted helminth infection: classic and neglected connections. Parasitol Res. (2022) 121:3381–92. doi: 10.1007/s00436-022-07697-z
13. Muslim A, Mohd Sofian S, Shaari SA, Hoh BP, Lim YA. Prevalence, intensity and associated risk factors of soil transmitted helminth infections: a comparison between Negritos (indigenous) in inland jungle and those in resettlement at town peripheries. PLoS Negl Trop Dis. (2019) 13:e0007331. doi: 10.1371/journal.pntd.0007331
14. Krecek RC, Rabinowitz PM, Conrad PA. Demystifying and demonstrating the value of a one health approach to parasitological challenges. Vet Parasitol. (2020) 287:109202. doi: 10.1016/j.vetpar.2020.109202
15. Heukelbach J. One health approach to control human and zoonotic hookworm infections. In: Mehlhorn H, Heukelbach J., editors. Infectious Tropical Diseases and One Health in Latin America. Parasitology Research Monographs, vol 16. Cham: Springer (2022).
16. Pullan RL, Brooker SJ. The global limits and population at risk of soil-transmitted helminth infections in 2010. Parasit Vectors. (2012) 5:81. doi: 10.1186/1756-3305-5-81
17. Chammartin F, Scholte RGC, Guimarães LH, Tanner M, Utzinger J, Vounatsou P. Soil-transmitted helminth infection in South America: a systematic review and geostatistical meta-analysis. Lancet Infect Dis. (2013) 13:507–18. doi: 10.1016/S1473-3099(13)70071-9
18. Menzies SK, Rodriguez A, Chico M, Sandoval C, Broncano N, Guadalupe I, et al. Risk factors for soil-transmitted helminth infections during the first 3 years of life in the tropics; findings from a birth cohort. PLoS Negl Trop Dis. (2014) 8:e2718. doi: 10.1371/journal.pntd.0002718
19. Ster IC, Niaz HF, Chico ME, Oviedo Y, Vaca M, Cooper PJ. The epidemiology of soil-transmitted helminth infections in children up to 8 years of age: findings from an Ecuadorian birth cohort. PLoS Negl Trop Dis. (2021) 15:e0009972. doi: 10.1371/journal.pntd.0009972
20. Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, Freeman MC. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med. (2014) 11:e1001620. doi: 10.1371/journal.pmed.1001620
21. Worrell CM, Wiegand RE, Davis SM, Odero KO, Blackstock A, Cuéllar VM, et al. A cross-sectional study of water, sanitation, and hygiene-related risk factors for soil-transmitted helminth infection in urban school- and preschool-aged children in Kibera, Nairobi. PLoS ONE. (2016) 11:e0150744. doi: 10.1371/journal.pone.0150744
Keywords: infection, hookworm, One Health, parasites, public health, roundworm, soil-transmitted helminths, whipworm
Citation: Ellwanger JH and Cavallero S (2023) Editorial: Soil-transmitted helminth infections from a One Health perspective. Front. Med. 10:1167812. doi: 10.3389/fmed.2023.1167812
Received: 16 February 2023; Accepted: 28 March 2023;
Published: 11 April 2023.
Edited and reviewed by: Shisan Bao, The University of Sydney, Australia
Copyright © 2023 Ellwanger and Cavallero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joel Henrique Ellwanger, am9lbC5lbGx3YW5nZXJAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.