
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 17 May 2023
Sec. Intensive Care Medicine and Anesthesiology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1164466
 Christian Bayerl1*†
Christian Bayerl1*† Ann-Kathrin Berg2†
Ann-Kathrin Berg2† Stefan Angermair3
Stefan Angermair3 Damon Kim1
Damon Kim1 Bernd Hamm1
Bernd Hamm1 Katharina Beyer2
Katharina Beyer2 Christian Schineis2
Christian Schineis2Emphysematous diseases of the abdomen are rare with an often inconspicuous presentation of symptoms and rapid lethal outcome if untreated. We report the first successfully treated case of Clostridium perfringens-associated emphysematous hepatitis. In the emergency room, a 79-year-old man presented with shortness of breath and deteriorated general condition since the morning of admission. Initial CT scans showed a small but rapidly expanding gas collection in liver segment 6. Emergency surgery with atypical liver resection was performed immediately. With early resection and prolonged administration of antibiotics in the presence of sepsis, the patient recovered successfully and was discharged 37 days after admission. As in our case, prompt diagnosis with early surgical treatment is crucial for the management of emphysematous hepatitis.
Emphysematous hepatitis is a very rare and rapidly progressive infectious condition characterized by hepatic gas accumulation without fluid components or mass effect and very high mortality (1–10). Only a few cases of this disease have been reported globally (1–14). To the best of our knowledge, this is the first successfully treated patient with Clostridia-associated emphysematous hepatitis who has survived.
A 79-year-old male patient presented to our emergency department with shortness of breath. According to his wife, his general condition had deteriorated since the morning of admission.
He had a medical history of hypertension, hyperlipidemia, non-insulin-dependent diabetes mellitus (NIDDM), benign prostate hyperplasia, atrial fibrillation, and chronic heart failure with an ejection fraction of 30–35% as a result of myocardial infarctions 33 and 7 years before admission with percutaneous coronary intervention, implantation of two drug-eluting stents in the right coronary artery and implantation of a CRT-D. His daily medication included 25 mg of spironolactone, 5 mg of bisoprolol, 100 mg of acetylsalicylic acid, 20.7 mg of atorvastatin, 0.4 mg of tamsulosin, 12.3 mg of dapagliflozin and 3 mg of phenprocoumon.
On examination, the tympanic temperature was 38.5°C, the blood pressure was 137/117 mm Hg, and the pulse was 109 beats per minute. He had tachypnoea of 24 breaths per minute and oxygen saturation of 94 % on ambient air. In the initial physical examination, the abdomen was tender without abdominal guarding. Laboratory tests showed an elevated white blood count of 14,500 per cubic millimeter (reference range: 3,900 to 10,500), a CRP level of 39.7 milligrams per liter (reference: < 5.0), abnormal kidney function (creatinine 2.19 milligrams per decilitre, reference range: 0.70–1.20), and liver function with elevated liver enzymes (alanine aminotransferase of 70 U per liter, reference: < 41; aspartate aminotransferase of 64 U per liter, reference: < 50; alkaline phosphatase of 145 U per liter, reference range: 40–130; gamma-glutamyl transferase of 283 U per liter, reference range: 8–61) and bilirubin (total b. of 2.26 milligrams per decilitre, reference: < 1.20; conjugated b. of 1.25 milligrams per decilitre, reference: < 0.30). His blood glucose level was 225 milligrams per deciliter (reference range: 82–115).
Due to increasing tachypnoea, tachycardia, and progressive hypotension, pulmonary artery embolism was the most likely diagnosis at that point. Therefore, a pulmonary CT angiogram was performed, which showed no evidence of pulmonary artery embolism or relevant abnormalities in the lungs. Purely by chance, the scan found a small, circumscribed gas collection in liver segment 6 with a diameter of 34 millimeters (Figure 1). The patient had no known history of liver or biliary tract disease.
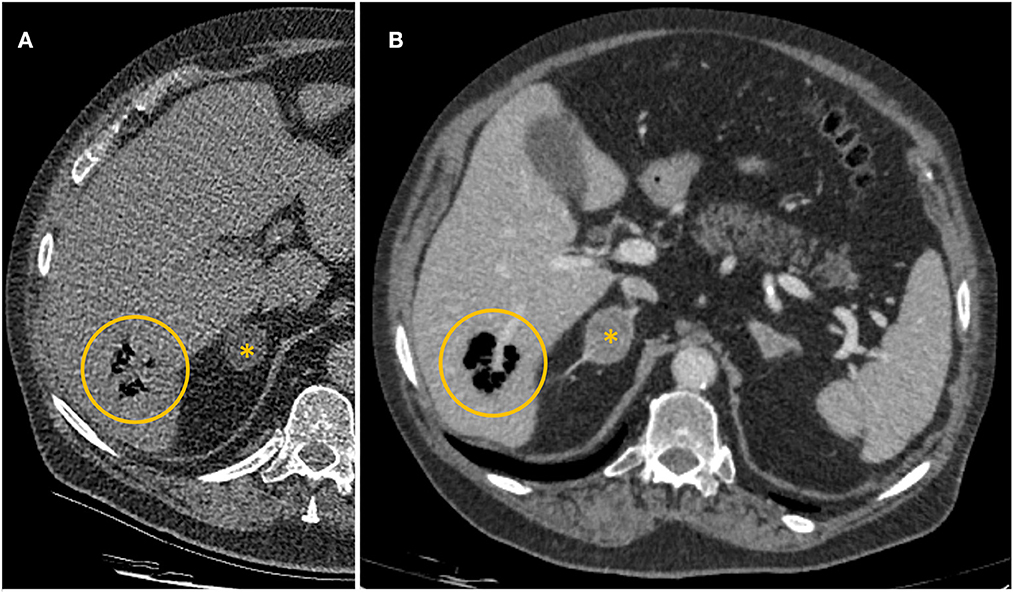
Figure 1. (A) Axial computed tomography pulmonary angiogram on admission. Collection of gas in liver segment 6 (yellow circle). (B) Contrast-enhanced computed tomography of the abdomen 154 min after the first CT scan. Collection of gas in liver segment 6 (yellow circle). Incidental finding of a mass of the right adrenal gland (*).
After the scan, the patient's condition deteriorated rapidly, and he subsequently developed a distributive shock, requiring vasopressor support. After stabilization, a contrast-enhanced CT scan of the abdomen was performed 154 min after the initial thoracic scan. It showed marked enlargement of the periportal gas collection in liver segment 6 from 34 to 40 mm in diameter with well-described margins, no fluid collections, and no evidence of abscess formation (Figure 1). Volumetric analysis of the gas collection showed a 4 fold increase between the two scans. No relevant hepatic vascular or perfusion disorder, especially no gas in the portal vein and its branches could be detected in the acquired delayed phase. Nevertheless, intrahepatic and extrahepatic bile ducts were dilated due to a suspected stricture of the ampulla of Vater. This led to the diagnosis of emphysematous hepatitis with acute liver failure, most likely due to altered drainage of the common bile duct, resulting in changes in clinical presentation and laboratory studies.
Immediately after the second CT scan, the patient was admitted to the interdisciplinary intensive care unit of our hospital and prepared for exploration and non-anatomic liver resection.
Intraoperatively, access was obtained via a costal margin incision. First, cholecystectomy and partial mobilization of the right liver were performed to gain better exposure. The affected liver tissue presented deliquescent underneath an intact liver capsule (Figure 2). The capsule was incised and surgical debridement and atypical liver resection with adequate safety margins were performed by monopolar and bipolar dissection and situational ligation of prominent bile ducts or hepatic vessels. A swab was taken for microbiological testing. During liver debridement and atypical resection, a hemorrhage occurred from a distal branch of the portal vein that ran right through the affected tissue. A 5-min pringle maneuver was applied to control the bleeding by placing a suture around the portal branch. Due to intrahepatic and extrahepatic cholestasis, bile duct revision with insertion of a T-drain was performed to drain bile extra-corporally. Choledocholithiasis could not be detected intraoperatively. After the procedure, the patient was readmitted to the intensive care unit for further stabilization.
The following day, the patient presented with significantly elevated total bilirubin levels of 16.38 milligrams per decilitre due to an insufficiently secreting T-drain. Immediate surgical revision was performed, during which the T-drainage was found to be obstructed and was therefore removed. The site of resection and debridement appeared to be in stable condition, and no infectious progress was noted, so no further debridement was required. A larger T-drain was inserted, and bilirubin levels decreased rapidly over the next couple of hours. An additional ERCP revealed stenosis of the common bile duct due to a parapapillary diverticulum (Figure 3). Therefore, papillotomy and stent placement was performed.
Microbiological examination revealed the presence of Clostridium perfringens and Enterococcus faecium in both blood culture and intraoperative swab.
Postoperatively, as the initial calculated anti-infective therapy, a combination of piperacillin/tazobactam (4.5 g, three times a day for 17 days), vancomycin (sensitive for E. faecium, 2 g continuously over 24 h for 12 days) and Amphotericin B (375 mg, once a day for 5 days) was administered. Following the identification of Clostridium perfringens that were susceptible to clindamycin, the antibiotic regimen was updated to include clindamycin (900 mg, three times a day). After further testing indicated that Clostridium perfringens were also responsive to piperacillin/tazobactam, clindamycin was discontinued after being administered for 6 days. In the further course, vancomycin was replaced by linezolid (600 mg, twice a day for 8 days).
In the further clinical course, despite the T-drain functioning correctly, an increase of CRP and cholestasis enzymes (ALP and gamma-GT) raised concerns for cholangitis. To address this, we intensified the anti-infective therapy by replacing piperacillin/tazobactam with meropenem (1 g, three times a day for 9 days) and caspofungin (70 mg once on the first day, followed by 50 mg once a day for the subsequent 2 days) while also blocking the T-drain. Following a reduction in cholestatic enzymes and infection values, the T-drain was extracted, while the biliary stent was left in position.
Moreover, aside from the antibiotic therapy, differentiated volume and vasopressor therapy in the setting of sepsis was a major challenge, especially due to severe chronic heart failure, developed from 3-vessel coronary heart disease and a history of cardiac infarction.
After 28 days of intensive care therapy, the patient could be transferred to the surgical ward. However, due to respiratory insufficiency caused by pleural effusion, the patient was readmitted to the intensive care unit for 2 days, where he was stabilized. He did not present any further increase in the white blood cell count, liver parameters, or renal retentions markers. Therefore, the patient could be further mobilized and was transferred to a geriatric rehabilitation site 37 days after admission, fully mobilized and in very good general condition. Figure 4 provides an overview of the clinical course with relevant laboratory tests.
Blachar et al. reported the first case of emphysematous hepatitis in 2002 with a fatal outcome 3 days after admission (1). Other reports followed (2–10), with a maximum survival of 8 days after hospitalization (9). Ghosn et al. reported the successful treatment of a 38-year-old patient with radiological features of emphysematous hepatitis (11). Yet, surgical exploration showed the presence of pus, better fitting the diagnosis of a liver abscess rather than emphysematous hepatitis (11). Three other reports of successfully treated patients with emphysematous hepatitis followed by Estébanez-Ferrero et al. (12), Francois et al. (13), and Pan et al. (14), as summarized in Table 1.
Most reported cases of emphysematous hepatitis, including our patient, show an association with diabetes (1, 3, 7, 9, 11, 14), which also appears to be a risk factor for other abdominal emphysematous diseases such as emphysematous cholecystitis or pyelonephritis (15). Recent abdominal interventions or surgeries also appear to be risk factors (1, 2, 4, 6, 10), which were not known for our patient. It is noteworthy that three cases had a history of hilar bile duct carcinoma, which was treated either by bile duct intervention, radiotherapy, or surgery—with the clinical picture of emphysematous hepatitis, all associated with Clostridium perfringens.
Reported pathogens associated with emphysematous hepatitis are Klebsiella pneumoniae (1, 5, 9, 10), Enterobacter cloacae (2), Streptococcus mutans (6), Enterococcus faecalis (6, 10), Enterococcus faecium (11), Aeromonas ichtiosmia (10), Clostridium perfringens (2, 4, 7, 10), Escherichia coli (4, 8, 10–13), Streptococcus anginosus (13) and Klebsiella oxytoca (13, 14). Successfully treated cases revealed Escherichia coli (11–13), Enterococcus faecium (11), Streptococcus anginosus (13), and Klebsiella oxytoca (13, 14) as associated pathogens. In our case, Clostridium perfringens and Enterococcus faecium were detected in the intraoperative swab as well as in the blood culture.
Clostridium perfringens is a Gram-positive, anaerobic bacterium with the ability to produce more than 20 toxins and a remarkably short generation time of 12–17 min at 37°C (16–18). Associated bacteremia shows mortality rates from 26.9% (19) to 74% in septic patients with a rare but well-known complication of massive hemolysis (20). Reported cases of emphysematous hepatitis with Clostridium perfringens in blood culture showed a maximal survival time of 3 days (4).
On this basis, the prognosis of our patient seemed to be poor mainly due to the presence of Clostridium perfringens. Regarding the bacterial spectrum, all successfully treated cases (11–14), which were nevertheless challenging to treat due to their microbiological diversity, appeared to have a more favorable prognosis due to their bacterial spectrum and the lower age of the patients. Not to mention that one case (11) resembled a liver abscess rather than emphysematous hepatitis.
The eradication of the gas-forming Clostridium perfringens requires early surgical resection in combination with prolonged administration of antibiotics, whereas a non-surgical approach, as performed by Francois et al. (13) and Pan et al. (14), would very likely have resulted in a rapid fatal outcome in our patient, especially considering the presence of septic, toxin-induced shock.
As in our case, the initial clinical presentation of emphysematous hepatitis is often inconspicuous and progresses rapidly, as demonstrated clinically and radiologically by the rapid volume expansion of focal gas collections. Considering the size of the affected liver parenchyma, we successfully intervened at a very early stage. Therefore, early abdominal imaging, preferably contrast-enhanced CT with its high sensitivity and specificity in the detection of abnormal gas (15), is crucial for the outcome of patients with emphysematous hepatitis. As therapy, we consider that a “hit hard and early” approach is essential.
Emphysematous hepatitis remains a challenging disease to diagnose and treat. Despite early and radical surgical therapy with early systemic antibiosis, the disease is often lethal.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the patient's wife for the publication of this case report.
CB wrote the original draft and performed the diagnosis in the CT scan. A-KB wrote the original draft and assisted with the first surgery. CS was responsible for conceptualization, edited the manuscript, and indicated and performed the surgery. SA was a supervisor responsible for the ICU patient and edited and reviewed the manuscript. DK, BH, and KB edited and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
We would like to express our sincere gratitude to the clinic staff who cared for the patient during his hospital stay. Their dedication, expertise, and compassion were crucial to the patient's recovery. We thank Dr. Juliane Buchkremer for providing valuable endoscopic images. We acknowledge financial support from the Open Access Publication Fund of Charité – Universitätsmedizin Berlin and the German Research Foundation (DFG).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Blachar A, Federle MP, Brancatelli G. Acute fulminant hepatic infection causing fatal “emphysematous hepatitis”: case report. Abdom Imaging. (2002) 27:188–90. doi: 10.1007/s00261-001-0067-y
2. Letourneau-Guillon L, Audet P, Plasse M, Lepanto L. Answer to case of the month #162. Emphysematous infection of the liver parenchyma. Can Assoc Radiol J. (2010) 61:117–9. doi: 10.1016/j.carj.2009.10.003
3. Chauhan U, Prabhu SM, Shetty GS, Solanki RS, Udiya AK, Singh A. Emphysematous hepatitis–a fatal infection in diabetic patients: case report. Clin Res Hepatol Gastroenterol. (2012) 36:e114–6. doi: 10.1016/j.clinre.2012.05.018
4. Kim JH, Jung ES, Jeong SH, Kim JS, Ku YS, Hahm KB, et al. A case of emphysematous hepatitis with spontaneous pneumoperitoneum in a patient with hilar cholangiocarcinoma. Korean J Hepatol. (2012) 18:94–7. doi: 10.3350/kjhep.2012.18.1.94
5. Lin YS, Wang WS, Chen MJ. Emphysematous changes of the liver. Gastroenterology. (2012) 142:213, 413–4. doi: 10.1053/j.gastro.2011.04.064
6. Nada KM, El Husseini I, Abu Hishmeh ME, Shah NS, Ibragimova N, Basir R. A rare case of septic shock secondary to emphysematous hepatitis. Case Rep Crit Care. (2017) 2017:3020845. doi: 10.1155/2017/3020845
7. Calderon H, Serfin J. 13-Hour progression of emphysematous hepatitis as depicted on repeat computerized tomography. J Surg Case Rep. (2020) 2020:rjaa089. doi: 10.1093/jscr/rjaa089
8. Miranda G, Dionisio AC, Azevedo C, Carvalho E, Semiao M, Branco V, et al. Fulminant emphysematous hepatitis - a rare cause of septic shock. Eur J Case Rep Intern Med. (2020) 7:001539. doi: 10.12890/2020_001539
9. Bofill A, Marco F. Emphysematous hepatitis. N Engl J Med. (2021) 385:e58. doi: 10.1056/NEJMicm2108779
10. Azri A, Pichon J, Fartoukh M, Djibre M. Fatal emphysematous hepatitis with spontaneous pneumoperitoneum. Liver Int. (2020) 40: 1224. doi: 10.1111/liv.14450
11. Ghosn Y, Abdallah A, Hussein Kamareddine M, Geahchan A, Baghdadi A, El-Rassi Z, et al. Gas-forming liver abscess versus emphysematous hepatitis: a radiologic diagnostic dilemma-a case report and review of the literature. Case Rep Hepatol. (2019) 2019:5274525. doi: 10.1155/2019/5274525
12. Estebanez-Ferrero B, Fuentes-Porcel O, Lorenzo-Linan MA, Rico-Morales MDM. Emphysematous hepatitis: a very rare entity with a poor prognosis. Rev Esp Enferm Dig. (2021) 113:612–4. doi: 10.17235/reed.2021.7795/2021
13. Francois S, Aerts M, Reynaert H, Van Lancker R, Van Laethem J, Kunda R, et al. Step-up approach in emphysematous hepatitis: a case report. World J Hepatol. (2022) 14:464–70. doi: 10.4254/wjh.v14.i2.464
14. Pan N, Wang S, Miao Z. Emphysematous hepatitis with successful treatments: a rare case report. Medicine. (2023) 102:e32530. doi: 10.1097/MD.0000000000032530
15. Grayson DE, Abbott RM, Levy AD, Sherman PM. Emphysematous infections of the abdomen and pelvis: a pictorial review. Radiographics. (2002) 22:543–61. doi: 10.1148/radiographics.22.3.g02ma06543
16. Petit L, Gibert M, Popoff MR. Clostridium perfringens: toxinotype and genotype. Trends Microbiol. (1999) 7:104–10. doi: 10.1016/S0966-842X(98)01430-9
17. Kiu R, Hall LJ. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg Microbes Infect. (2018) 7:141. doi: 10.1038/s41426-018-0144-8
18. Li J, McClane BA. Further comparison of temperature effects on growth and survival of Clostridium perfringens type A isolates carrying a chromosomal or plasmid-borne enterotoxin gene. Appl Environ Microbiol. (2006) 72:4561–8. doi: 10.1128/AEM.00177-06
19. Yang CC, Hsu PC, Chang HJ, Cheng CW, Lee MH. Clinical significance and outcomes of Clostridium perfringens bacteremia–a 10-year experience at a tertiary care hospital. Int J Infect Dis. (2013) 17:e955–60. doi: 10.1016/j.ijid.2013.03.001
Keywords: case report, emphysematous hepatitis, computed tomography, liver resection, septic shock, Clostridium perfringens
Citation: Bayerl C, Berg A-K, Angermair S, Kim D, Hamm B, Beyer K and Schineis C (2023) First successful treatment of Clostridium perfringens-associated emphysematous hepatitis: a case report. Front. Med. 10:1164466. doi: 10.3389/fmed.2023.1164466
Received: 12 February 2023; Accepted: 18 April 2023;
Published: 17 May 2023.
Edited by:
Rahul Kashyap, WellSpan Health, United StatesReviewed by:
Gagandeep Dhillon, University of Maryland, Baltimore, United StatesCopyright © 2023 Bayerl, Berg, Angermair, Kim, Hamm, Beyer and Schineis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian Bayerl, Y2hyaXN0aWFuLmJheWVybEBjaGFyaXRlLmRl
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.