
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med., 28 July 2023
Sec. Ophthalmology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1159316
This article is part of the Research TopicUpdate on Glaucoma Research: From Basic Science to Clinical PracticeView all 12 articles
 Mohammad Mohammadi1,2
Mohammad Mohammadi1,2 Adeleh Yarmohammadi3
Adeleh Yarmohammadi3 Amin Salehi-Abargouei4,5,6
Amin Salehi-Abargouei4,5,6 Hamidreza Ghasemirad1
Hamidreza Ghasemirad1 Mohammad Shirvani7,8
Mohammad Shirvani7,8 Hamed Ghoshouni1*†
Hamed Ghoshouni1*†Background: Glaucoma, the leading cause of irreversible blindness, is a common disorder that contributes to gradual optic nerve degeneration. The beneficial impacts of uric acid (UA) have been reported in some neurodegenerative conditions such as Parkinson's disease, Alzheimer's disease, and amyotrophic lateral sclerosis. But the results of current studies about the association between serum UA level and glaucoma are conflicting. The present meta-analysis was conducted to provide a better understanding of the association between serum UA level and glaucoma.
Methods: We searched the databases of PubMed, Scopus, Web of Science, and Google Scholar systematically until November 20, 2022 to identify case-control studies, comparing the serum UA concentrations of the patients with glaucoma and controls. The mean ± standard division difference was used to assess the difference in serum UA concentrations between the glaucoma patients and controls.
Results: Six studies involving 1,221 glaucoma patients and 1,342 control group were included in the present meta-analysis. This meta-analysis using a random effect model indicated that the mean UA level in glaucoma patients was 0.13 (I2 = 91.92%, 95% CI = −0.42 to 0.68) higher than the controls; however, it was not statistically significant.
Conclusions: Our findings provide evidence that glaucoma patients have a higher serum UA level compared to the controls, but this difference is not statistically significant. Prospective studies are needed to determine the possible association between increased UA and glaucoma pathogenesis.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022364055, identifier: CRD42022364055.
Glaucoma is the leading cause of irreversible blindness in the world (1). The prevalence of this disorder is rising and varies globally (2, 3), and it is predicted that the number of glaucoma patients will exceed 110 million people by 2040 (4).
In the early stages, glaucoma might be asymptomatic, or patients may experience blurred or missing areas in their vision field (5). However, the late stages of the condition can result in irreversible blindness, especially if untreated (6).
Although the harmful effects of glaucoma on vision are irreversible, early diagnosis and treatment of this condition can decrease the risk of permanent blindness (7).
Due to the asymptomatic nature of glaucoma, early detection of the disease is challenging, and the number of diagnosed patients with glaucoma is lower than undiagnosed patients (8, 9).
Intraocular pressure (IOP) measurement is one of the main diagnostic tests for the diagnosis and progress monitoring of glaucoma (10). Evaluated IOP is the leading risk factor for glaucoma (11). IOP reflects the balance between the aqueous humor generation and its drainage from the eye through the trabecular meshwork and the Schlemm canal outflow pathway (12). Dysfunction of this outflow pathway elevates IOP, which results in glaucomatous optic neuropathy, but it has been shown that normal IOP also may be found in some glaucoma patients (13). This suggests that other factors may involve in the underlying mechanism of glaucoma and underline the need to prioritize research in this area to promote the clinicians' insight into the development of glaucoma.
Glaucoma has traditionally been considered an eye disease, but recent studies have linked it to central nervous system degeneration (14–16). The neurodegeneration associated with glaucoma contributes to gradual optic nerve degeneration with progressive retinal ganglion cell (RGC) loss, which is the main cause of progressive vision loss (17, 18).
The underlying pathogenesis of glaucomatous optic neuropathy is still unknown. It has been suggested that glaucoma destroys neurons through neuroinflammation and oxidative stress (18). Antioxidants can be protective against glaucoma through different mechanisms such as IOP reduction, promoting vascular health, and prevention of RGC loss (19).
Uric acid (UA) is a purine metabolite that detects intracellularly and in all body fluids (20) and that shown to have both pro-oxidant and antioxidant features in-vitro by production and scavenging of reactive oxygen species (21, 22). The beneficial impacts of UA have been shown in other neurodegenerative conditions, such as Parkinson's disease (23), Huntington's disease (24), Alzheimer's disease (25), and amyotrophic lateral sclerosis (26). However, the role of UA in the underlying mechanism of glaucoma is still unclear.
By exploring the association between uric acid and glaucoma, we can identify potential abnormal metabolic processes in glaucoma patients, thereby considering UA as a biomarker. Data from several case-control studies suggest a significant inverse association between serum concentrations of UA and glaucoma risk (27–29). However, this was not confirmed in all studies, and some studies even reported a significant association between high serum UA concentrations and the risk of glaucoma (30–32). Hence, the current meta-analysis aimed to evaluate the relationship between serum UA concentration and glaucoma in case-control studies.
The present study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (33). The protocol has been registered in the PROSPERO database (registration number: CRD42022364055).
The present systematic review focused on case-control studies that reported the serum UA concentrations in patients with glaucoma and compared them to controls. The investigations should be conducted in vivo in humans, and the subjects could be controls or glaucoma patients.
We included only studies in the English language. We excluded conference meetings and abstracts that were not published in peer-reviewed journals. If original data or exact numbers were unavailable in both groups of patients with glaucoma and controls, they were not included in our quantitative analysis.
To identify studies to be included in this review, a systematic search was performed via PubMed, Scopus, Google Scholar, and Web Of Science databases from inception through November 20, 2022. We used the following search strategy: (“uric acid”[Mesh]) AND (“glaucoma”[Mesh] OR “Intraocular Pressure”[Mesh] OR “Ocular Hypertension” [Mesh]). Moreover, the reference lists of studies obtained in the initial search were manually searched for more relevant articles.
After removing duplicate records, two reviewers (MM and HRG) independently analyzed all titles and abstracts obtained from the searches to identify relevant papers.
The full text of studies that appeared to meet the inclusion criteria were obtained and independently analyzed by two reviewers (MM and HG). The authors resolved the disagreement through a discussion with a third author (AS). Eventually, studies that did not meet the inclusion criteria were discarded.
We applied the Newcastle-Ottawa Quality Assessment Scale (NOS) for case-control studies to evaluate the methodological quality of the studies (34). The NOS contains a star system in which a study is assessed on three domains (Supplementary Table S1); representativeness of study group selection (four items), comparability of groups (two items), and ascertainment of the exposure (three items). Studies scored one star for each area addressed, with a maximum score of 9, of which 7–9, 4–6, 0–3, and scores considered high, fair, low, and quality, respectively. Disagreements were resolved by discussion, and a third author arbitrated unresolved discrepancies.
Using standardized data extraction forms, two reviewers (MM and AS) extracted data independently. In cases of disagreement between the reviewers, a third reviewer (HG) was consulted. The following data were extracted from selected studies: authors' name, publication year, country of study, glaucoma type, number of subjects, age range of subjects, definition, and the mean and standard deviation of serum UA levels. Data were extracted separately for each entity groups (glaucoma patients or controls).
The mean difference (MD) and its corresponding standard error (SE) were calculated by using the mean values and their standard deviations reported/calculated for case and control groups. Then MDs extracted from each study were used as effect size for meta-analysis. The meta-analyses were performed using DerSimonian-Laird random-effects model, which takes the between-study heterogeneity into account. Stata software, version 17.0 (Stata Corp, College Station, TX), was used to analyze the data. Both the Q statistic and I2 statistic measures were used for the evaluation of heterogeneity between studies. P-values < 0.05 for Cochran's Q test and an I2 higher than 25% will be considered as significant heterogeneity (35).
P-values < 0.05 were considered statistically significant. To conduct a sensitivity analysis, each article was removed from the final analysis. Begg's funnel plots and Egger's and Begg's asymmetry tests were used to assess the presence of publication bias (36).
After the systematic search in databases, 289 records were retrieved. By removing 83 duplicate records, 206 were screened, and finally, the six studies met the inclusion criteria and were included in this meta-analysis. The Prisma flowchart shows this process in detail in Figure 1.
Among six studies evaluating the level of UA in serum, 1,221 glaucoma patients were compared to the 1,342 control group. One study recruited patients with normal-tension glaucoma (NTG), another study with primary angle closure glaucoma (PACG), while the patients in the other four studies all were primary open-angle glaucoma (POAG). The largest sample size between these studies included 886 primary angle closure glaucoma patients, with 994 participants as a control group. In comparison, the smallest contained 23 primary open-angle glaucoma patients with 15 participants as a control group. These studies were performed in China (n = 2), Tunis (n = 1), Greece (n = 1), Italy (n = 1), and Japan (n = 1).
The mean UA level across all glaucoma patients ranged between 4.00 ± 0.66 mg/dl In Serra et al. to 6.2 ± 1.9 mg/dl in Elisaf et al. The characteristics of each included study are shown in Table 1.
For quality assessment of included studies, the Newcastle-Ottawa Scale score was used. Almost all studies have good-quality scores, and Supplementary Table S1 shows these scores in detail.
The pooled analysis included all six studies, showing that serum UA level was higher in glaucoma patients than in other patients without glaucoma. In detail, A meta-analysis using a random effect model indicates that the mean UA level in glaucoma patients was 0.13 (I2 = 91.92%, 95% CI = −0.42 to 0.68) higher than the controls; however, it was not statistically significant (Figure 2).
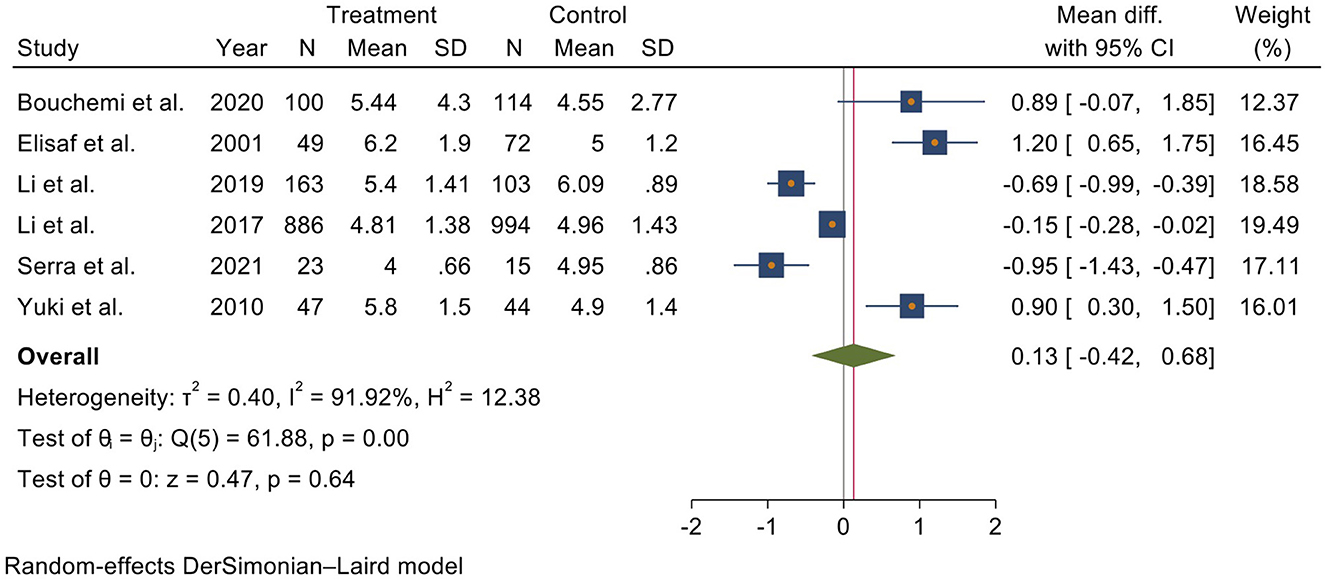
Figure 2. Forest plot of meta-analysis for serum uric acid level in glaucoma patients and control groups.
Each article was removed from the analysis to perform a sensitivity analysis, and no significant effect of a single study was found. The funnel plot and Begg and Egger test showed no evidence of publication bias (P > 0.05) (Supplementary Figure S1).
So far, the association between serum UA concentrations and glaucoma is under debate. To the best of our knowledge, this is the first meta-analysis to examine the relationship between serum UA level and glaucoma.
In the present meta-analysis of 1,221 glaucoma patients compared to 1,342 controls included in 6 case-control studies, serum UA concentration in patients with glaucoma was higher than in the controls, but this association was not statistically significant.
Three out of six case-control studies (27–29) within this meta-analysis found a significant inverse association. In comparison, three other studies (30–32) have reported a positive association between high UA levels and glaucoma (Table 1). These various results may be reflected by the heterogeneity found in the present meta-analysis. The inclusion of various glaucoma types in this meta-analysis and the differences in disease etiology could contribute to this heterogeneity (Table 1).
Some authors have recently found decreased total antioxidant capacity levels in the blood and aqueous humor samples of glaucoma patients (37, 38). Oxidative stress is also suggested to play a role in the physiologic changes in aqueous humor outflow, leading to increased IOP and RGC degeneration in glaucoma (39, 40).
UA is one of the main antioxidants of plasma (21, 41). Wayner et al. (42) reported that urate contributes up to 65% of the overall antioxidant capacity of the plasma. Meanwhile, experimental animal studies and human clinical trials have suggested that higher serum UA concentrations can prevent neuronal degeneration (43, 44).
Li et al. investigated the association between the progression of recently diagnosed PACG and pretreatment UA levels of serum. In this prospective observational study, there was a correlation between a lower baseline serum UA concentration and a higher risk of PACG progression. These findings suggested that higher serum UA values may protect against PACG and suppress the disease progression (45).
On the other hand, some studies have suggested that systemic inflammation is related to glaucomatous damage (46). A recent study by Astafurov et al. (46) showed that glaucoma patients had greater bacterial oral counts in compression to controls and low-dose lipopolysaccharide administration in glaucoma animal models led to neuronal loss and axonal degeneration. In addition, recent studies have reported a significant association between Heliobacter pylori infection and glaucoma (47, 48).
As mentioned above, it seems that glaucoma patients are in a low antioxidative and high oxidative state in the body. UA may be consumed in glaucoma by preferentially reacting with oxidizing agents in the body. These findings are consistent with former studies reporting that subjects with higher UA levels have a decreased risk of glaucoma (27–29) and that the level of UA was negatively related to the glaucoma severity (28, 29).
However, another previous study compared the serum UA levels between pseudoexfoliation patients (the leading cause of secondary glaucoma) and controls and reported that serum UA levels of subjects with and without pseudoexfoliation were similar (49).
The other three studies included in our meta-analysis (30–32) suggested higher serum UA concentrations were found in glaucoma patients in comparison with controls.
Regarding this subject interestingly, elevated levels of UA have been reported in the aqueous humor of some patients with glaucoma (50). Additionally, it has been suggested that oxidative stress can accelerate the apoptosis of trabecular meshwork cells and extracellular matrix accumulation in the trabecular meshwork, leading to increased resistance of the aqueous humor outflow pathway and an increase in IOP (51). It is possible that elevated serum UA may reduce the outflow facility of aqueous humor by impairing the trabecular meshwork physiology, ultimately leading to an increase in IOP and glaucomatous optic neuropathy.
Nevertheless, IOP elevation is insufficient to explain the underlying pathophysiology of glaucoma (52). Therefore, other involving risk factors, particularly the impairment of the vasculature supplying the optic nerve and the tissues around it, have also been suggested (53).
According to the growing body of clinical and experimental research, UA-induced inflammatory response and oxidative stress contribute to microvascular impairments (54, 55). Some in vitro and in vivo findings suggested that UA may contribute to endothelial dysfunction by causing antiproliferative impacts on the endothelium (56, 57), which has been shown to have an important association with open-angle glaucoma (58).
Moreover, it has been reported that an elevated serum UA and its fluctuations were independently related to impaired choroidal and retinal microcirculation (59).
In a recent study, Yang et al. reported that higher serum UA concentrations were noticeably associated with decreased retinal capillary plexus vessel density. These results may support the damaging impact of high serum UA concentrations on the retinal microvasculature and suggest the necessity of regulating serum UA to prevent microvascular alteration (60). In addition, interestingly, it has been reported that a history of chronic renal disease is significantly associated with the higher risk of development of subsequent glaucoma (61).
Serum UA is known as a potential risk factor for the development and progression of chronic renal disease. It has been reported that elevated serum UA levels can cause an increase in glomerular blood pressure leading to renal diseases. Additionally, pilot studies have suggested that lowering serum UA therapies may slow the progression of chronic renal disease (62). In this regard, it is suggested that both the choroid plexus in the human eye and the renal glomerulus have extensive vascular networks with similar structures (63). The underlying mechanism of glaucoma development may be similar to the chronic renal disease. According to these findings, it is possible that higher levels of UA may be contributing to glaucomatous optic neuropathy.
With all these interpretations, this study was a meta-analysis of the case-control studies, and we cannot consider a precise causal role for UA in the pathogenesis of glaucoma.
Due to the limited number of primary studies available, we were unable to perform a separate analysis for UA levels in each glaucoma subtype. Therefore, it is important to interpret the results cautiously, considering the potential variations in UA levels among different glaucoma types.
In addition, systemic diseases and some medications administration may affect the serum UA level (64–66). Except for Bouchemi et al. (32), all the studies analyzed in this meta-analysis excluded individuals with systemic diseases or those taking medications that could affect serum UA levels. Bouchemi et al. (32) did not clearly state the criteria for excluding patients with systemic diseases or those using medications that could impact serum UA levels.
The observational nature of the included studies does not allow us to determine whether UA-lowering interventions can influence the development of glaucoma. Further randomized clinical trials are required to assess whether the UA-lowering medications may be beneficial in managing glaucoma.
The primary objective of our study was to compare serum UA levels between patients with glaucoma and the control group. We did not analyze and compare the concentration of UA levels in the vitreous and/or aqueous humor. Prior studies have shown that UA levels in aqueous humor of patients with glaucoma were higher than controls (32, 50). The exact mechanism by which UA is transferred into the aqueous humor remains unclear. However, several urate transporters that are involved in UA homeostasis, such as the ATP-binding cassette transporters, organic anion transporters, and solute carrier transporters have been identified in the retina and/or ciliary body of human eyes (67–70). These transporters may be involved in regulating of UA levels in human eyes. Future studies should consider comparing the UA levels in the vitreous and/or aqueous humor to gain a more comprehensive insight into its role in the development of glaucoma.
Furthermore, the study population of included studies in our meta-analysis was limited, and it is necessary to conduct more extensive prospective cohort studies to determine the potential link between serum UA levels and glaucoma.
This meta-analysis summarized a large body of evidence from case-control studies on the association between serum UA level and glaucoma. These findings provided evidence that serum UA concentrations are higher in glaucoma patients in comparison with controls, but this association is not statistically significant. However, prospective studies are needed to confirm the exact effect of serum UA concentrations on the risk of glaucoma.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
MM and HGho designed the study. HGha, MM, and AY performed a systematic search and extracted the data. AS-A and HGho conducted statistical analysis. MM, HGho, and MS drafted the paper. All authors read, revised the manuscript, contributed to the article, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1159316/full#supplementary-material
1. Steinmetz JD, Bourne RR, Briant PS, Flaxman SR, Taylor HR, Jonas JB, et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Global Health. (2021) 9:e144–e60. doi: 10.1016/S2214-109X(20)30489-7
2. Pant AB, Wang Y, Mielcarz DW, Kasper EJ, Telesford KM, Mishra M, et al. Alteration of CD39+Foxp3+ CD4 T cell and cytokine levels in EAE/MS following anti-CD52 treatment. J Neuroimmunol. (2017) 303:22–30. doi: 10.1016/j.jneuroim.2016.12.010
3. Wang W, Gawlik K, Lopez J, Wen C, Zhu J, Wu F, et al. Erratum: genetic and environmental factors strongly influence risk, severity and progression of age-related macular degeneration. Signal Transduct Target Ther. (2016) 1:16023. doi: 10.1038/sigtrans.2016.23
4. Tham YC Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. (2014) 121:2081–90. doi: 10.1016/j.ophtha.2014.05.013
5. Crabb DP, Smith ND, Glen FC, Burton R, Garway-Heath DF. How does glaucoma look?: Patient perception of visual field loss. Ophthalmology. (2013) 120:1120–6. doi: 10.1016/j.ophtha.2012.11.043
6. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. (2000) 130:429–40. doi: 10.1016/S0002-9394(00)00538-9
7. Tatham AJ, Weinreb RN, Medeiros FA. Strategies for improving early detection of glaucoma: the combined structure-function index. Clin Ophthalmol. (2014) 8:611–21. doi: 10.2147/OPTH.S44586
8. Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. (2005) 24:39–73. doi: 10.1016/j.preteyeres.2004.06.001
9. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. (2014) 311:1901–11. doi: 10.1001/jama.2014.3192
10. Stein JD, Khawaja AP, Weizer JS. Glaucoma in adults—screening, diagnosis, and management: a review. JAMA. (2021) 325:164–74. doi: 10.1001/jama.2020.21899
11. Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Archives of ophthalmology. (2002) 120:701–13. doi: 10.1001/archopht.120.6.701
12. To CH, Kong CW, Chan CY, Shahidullah M, Do CW. The mechanism of aqueous humour formation. Clin Exp Optom. (2002) 85:335–49. doi: 10.1111/j.1444-0938.2002.tb02384.x
13. Fechtner RD, Weinreb RN. Mechanisms of optic nerve damage in primary open angle glaucoma. Surv Ophthalmol. (1994) 39:23–42. doi: 10.1016/S0039-6257(05)80042-6
14. Yücel YH, Zhang Q, Weinreb RN, Kaufman PL, Gupta N. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res. (2003) 22:465–81. doi: 10.1016/S1350-9462(03)00026-0
15. Weber AJ, Chen H, Hubbard WC, Kaufman PL. Experimental glaucoma and cell size, density, and number in the primate lateral geniculate nucleus. Invest Ophthalmol Vis Sci. (2000) 41:1370–9.
16. Gupta N, Ang LC, Noël de Tilly L, Bidaisee L, Yücel YH. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol. (2006) 90:674–8. doi: 10.1136/bjo.2005.086769
17. Usategui-Martin R, Fernandez-Bueno I. Neuroprotective therapy for retinal neurodegenerative diseases by stem cell secretome. Neural Regen Res. (2021) 16:117–8. doi: 10.4103/1673-5374.283498
18. Gauthier AC, Liu J. Neurodegeneration and neuroprotection in glaucoma. Yale J Biol Med. (2016) 89:73–9.
19. Jabbehdari S, Chen JL, Vajaranant TS. Effect of dietary modification and antioxidant supplementation on intraocular pressure and open-angle glaucoma. Eur J Ophthalmol. (2021) 31:1588–605. doi: 10.1177/1120672120960337
20. Squadrito GL, Cueto R, Splenser AE, Valavanidis A, Zhang H, Uppu RM, et al. Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch Biochem Biophys. (2000) 376:333–7. doi: 10.1006/abbi.2000.1721
21. Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant-and radical-caused aging and cancer: a hypothesis. Proc Nat Acad Sci USA. (1981) 78:6858–62. doi: 10.1073/pnas.78.11.6858
22. Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. (2007) 293:C584–C96. doi: 10.1152/ajpcell.00600.2006
23. Tana C, Ticinesi A, Prati B, Nouvenne A, Meschi T. Uric acid and cognitive function in older individuals. Nutrients. (2018) 10. doi: 10.3390/nu10080975
24. Auinger P, Kieburtz K, McDermott MP. The relationship between uric acid levels and Huntington's disease progression. Mov Disord. (2010) 25:224–8. doi: 10.1002/mds.22907
25. Scheepers L, Jacobsson LTH, Kern S, Johansson L, Dehlin M, Skoog I. Urate and risk of Alzheimer's disease and vascular dementia: a population-based study. Alzheimers Dement. (2019) 15:754–63. doi: 10.1016/j.jalz.2019.01.014
26. Bakshi R, Xu Y, Mueller KA, Chen X, Granucci E, Paganoni S, et al. Urate mitigates oxidative stress and motor neuron toxicity of astrocytes derived from ALS-linked SOD1(G93A) mutant mice. Mol Cell Neurosci. (2018) 92:12–6. doi: 10.1016/j.mcn.2018.06.002
27. Serra R, Coscas F, Pinna A, Peri M, Zucca I, Sellam A, et al. Detection of serum uric acid in primary open angle glaucoma: a pilot study. Eur J Ophthalmol. (2021) 31:1857–61. doi: 10.1177/1120672120944012
28. Li S, Shao M, Tang B, Zhang A, Cao W, Sun X. The association between serum uric acid and glaucoma severity in primary angle closure glaucoma: a retrospective case-control study. Oncotarget. (2017) 8:2816. doi: 10.18632/oncotarget.13745
29. Li S, Shao M, Li D, Tang B, Cao W, Sun X. Association of serum uric acid levels with primary open-angle glaucoma: a 5-year case–control study. Acta Ophthalmologica. (2019) 97:e356–63. doi: 10.1111/aos.13789
30. Elisaf M, Kitsos G, Bairaktari E, Kalaitzidis R, Kalogeropoulos C, Psilas K. Metabolic abnormalities in patients with primary open-angle glaucoma. Acta Ophthalmol Scand. (2001) 79:129–32. doi: 10.1034/j.1600-0420.2001.079002129.x
31. Yuki K, Murat D, Kimura I, Ohtake Y, Tsubota K. Reduced-serum vitamin C and increased uric acid levels in normal-tension glaucoma. Graefes Arch Clin Exp Ophthalmol. (2010) 248:243–8. doi: 10.1007/s00417-009-1183-6
32. Bouchemi M, Soualmia H, Midani F, El Afrit MA, El Asmi M, Feki M. impaired nitric oxide production in patients with primary open-angle glaucoma nitric oxide levels in patients with glaucoma Diminution de la production du monoxyde d'azote chez des patients atteints de glaucome primitif à angle ouvert. La Tunisie Méd. (2020) 98:144–9.
33. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006
34. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
35. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
36. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane (2022). Available online at: www.training.cochrane.org/handbook
37. Ergan E, Ozturk F, Beyazyildiz E, Elgin U, Sen E, Cankaya AB, et al. Oxidant/antioxidant balance in the aqueous humor of patients with glaucoma. Int J Opthalmol. (2016) 9:249. doi: 10.18240/ijo.2016.02.12
38. Nucci C, Di Pierro D, Varesi C, Ciuffoletti E, Russo R, Gentile R, et al. Increased malondialdehyde concentration and reduced total antioxidant capacity in aqueous humor and blood samples from patients with glaucoma. Mol Vis. (2013) 19:1841.
39. Lei Y, Zhang X, Song M, Wu J, Sun X. Aqueous humor outflow physiology in NOS3 knockout mice. Invest Ophthalmol Vis Sci. (2015) 56:4891–8. doi: 10.1167/iovs.15-16564
40. Green K. Free radicals and aging of anterior segment tissues of the eye: a hypothesis. Ophthal Res. (1995) 27(Suppl. 1):143–9. doi: 10.1159/000267860
41. Ghiselli A, Serafini M, Natella F, Scaccini CJ. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med. (2000) 29:1106–14. doi: 10.1016/S0891-5849(00)00394-4
42. Wayner D, Burton G, Ingold K, Barclay L, Locke S. The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim Biophys Acta. (1987) 924:408–19. doi: 10.1016/0304-4165(87)90155-3
43. Aoyama K, Matsumura N, Watabe M, Wang F, Kikuchi-Utsumi K, Nakaki T. Caffeine and uric acid mediate glutathione synthesis for neuroprotection. Neuroscience. (2011) 181:206–15. doi: 10.1016/j.neuroscience.2011.02.047
44. Du Y, Chen CP, Tseng CY, Eisenberg Y, Firestein BL. Astroglia-mediated effects of uric acid to protect spinal cord neurons from glutamate toxicity. Glia. (2007) 55:463–72. doi: 10.1002/glia.20472
45. Li S, Shao M, Cao W, Sun X. Association between pretreatment serum uric acid levels and progression of newly diagnosed primary angle-closure glaucoma: a prospective cohort study. Oxid Med Cell Longev. (2019). doi: 10.1155/2019/7919836
46. Astafurov K, Elhawy E, Ren L, Dong CQ, Igboin C, Hyman L, et al. Oral microbiome link to neurodegeneration in glaucoma. PLoS ONE. (2014) 9:e104416. doi: 10.1371/journal.pone.0104416
47. Zeng J, Liu H, Liu X, Ding C. The relationship between Helicobacter pylori infection and open-angle glaucoma: a meta-analysis. Invest Ophthalmol Vis Sci. (2015) 56:5238–45. doi: 10.1167/iovs.15-17059
48. Kim JM, Kim SH, Park KH, Han SY, Shim HS. Investigation of the association between Helicobacter pylori infection and normal tension glaucoma. Invest Ophthalmol Vis Sci. (2011) 52:665–8. doi: 10.1167/iovs.10-6096
49. Simavli H, Bucak YY, Tosun M, Erdurmuş M. Serum uric acid, alanine aminotransferase, hemoglobin and red blood cell count levels in pseudoexfoliation syndrome. J Ophthalmol. (2015). doi: 10.1155/2015/914098
50. Jampel HD, Moon JI, Quigley HA, Barron Y, Lam K-W. Aqueous humor uric acid and ascorbic acid concentrations and outcome of trabeculectomy. Arch Ophthalmol. (1998) 116:281–5. doi: 10.1001/archopht.116.3.281
51. Saccà SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Archi Ophthalmol. (2005) 123:458–63. doi: 10.1001/archopht.123.4.458
52. Huck A, Harris A, Siesky B, Kim N, Muchnik M, Kanakamedala P, et al. Vascular considerations in glaucoma patients of A frican and E uropean descent. Acta ophthalmologica. (2014) 92:e336–e40. doi: 10.1111/aos.12354
53. Abegão Pinto L, Willekens K, Van Keer K, Shibesh A, Molenberghs G, Vandewalle E, et al. Ocular blood flow in glaucoma–the Leuven Eye Study. Acta Ophthalmol. (2016) 94:592–8. doi: 10.1111/aos.12962
54. Xiong Q, Liu J, Xu Y. Effects of uric acid on diabetes mellitus and its chronic complications. Int J Endocrinol. (2019). doi: 10.1155/2019/9691345
55. Zhu D-D, Wang Y-Z, Zou C, She X-P, Zheng Z. The role of uric acid in the pathogenesis of diabetic retinopathy based on Notch pathway. Biochem Biophys Res Commun. (2018) 503:921–9. doi: 10.1016/j.bbrc.2018.06.097
56. Choi YJ, Yoon Y, Lee KY, Hien TT, Kang KW, Kim KC, et al. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J. (2014) 28:3197–204. doi: 10.1096/fj.13-247148
57. Kanellis J, Kang D-H, editors. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol. (2005) 39–42. doi: 10.1016/j.semnephrol.2004.09.007
58. Cellini M, Strobbe E, Gizzi C, Balducci N, Toschi PG, Campos EC. Endothelin-1 plasma levels and vascular endothelial dysfunction in primary open angle glaucoma. Life Sci. (2012) 91:699–702. doi: 10.1016/j.lfs.2012.02.013
59. Lu Y, Yue J, Chen J, Li X, Wang L, Huang W, et al. Retinal microvasculature and choriocapillaris flow deficit in relation to serum uric acid using swept-source optical coherence tomography angiography. Transl Vis Sci Technol. (2022) 11:9. doi: 10.1167/tvst.11.8.9
60. Yang K, Li C, Shi K, Zhu X, Xiao Y, Su B, et al. Association of serum uric acid with retinal capillary plexus. Front Endocrinol. (2022) 13:855430. doi: 10.3389/fendo.2022.855430
61. Cho HK, Han JC, Choi JA, Chae JE, Kim RB. association between chronic renal disease and the risk of glaucoma development: a 12-year nationwide cohort study. Invest Ophthalmol Vis Sci. (2021) 62:27. doi: 10.1167/iovs.62.6.27
62. Johnson RJ, Nakagawa T, Jalal D, Sánchez-Lozada LG, Kang DH, Ritz E. Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplant. (2013) 28:2221–8. doi: 10.1093/ndt/gft029
63. Wong CW, Wong TY, Cheng C-Y, Sabanayagam C. Kidney and eye diseases: common risk factors, etiological mechanisms, and pathways. Kidney Int. (2014) 85:1290–302. doi: 10.1038/ki.2013.491
64. Jin M, Yang F, Yang I, Yin Y, Luo JJ, Wang H, et al. Uric acid, hyperuricemia and vascular diseases. Front Biosci. (2012) 17:656–69. doi: 10.2741/3950
65. Kim G-H, Jun J-B. Altered serum uric acid levels in kidney disorders. Life. (2022) 12:1891. doi: 10.3390/life12111891
66. Daskalopoulou S, Tzovaras V, Mikhailidis D, Elisaf M. Effect on serum uric acid levels of drugs prescribed for indications other than treating hyperuricaemia. Curr Pharm Des. (2005) 11:4161–75. doi: 10.2174/138161205774913309
67. Nigam SK, Bhatnagar V. The systems biology of uric acid transporters: the role of remote sensing and signaling. Curr Opin Nephrol Hypertens. (2018) 27:305. doi: 10.1097/MNH.0000000000000427
68. Sun H-L, Wu Y-W, Bian H-G, Yang H, Wang H, Meng X-M, et al. Function of uric acid transporters and their inhibitors in hyperuricaemia. Front Pharmacol. (2021) 12:667753. doi: 10.3389/fphar.2021.667753
69. Liu L, Liu X. Roles of drug transporters in blood-retinal barrier. Adv Exp Med Biol. (2019) 1141:467–504. doi: 10.1007/978-981-13-7647-4_10
Keywords: glaucoma, intraocular pressure, uric acid, oxidative stress, systematic review, meta-analysis
Citation: Mohammadi M, Yarmohammadi A, Salehi-Abargouei A, Ghasemirad H, Shirvani M and Ghoshouni H (2023) Uric acid and glaucoma: a systematic review and meta-analysis. Front. Med. 10:1159316. doi: 10.3389/fmed.2023.1159316
Received: 05 February 2023; Accepted: 07 July 2023;
Published: 28 July 2023.
Edited by:
Fabrizio Giansanti, University of Florence, ItalyReviewed by:
Jan Lešták, Eye Clinic JL, CzechiaCopyright © 2023 Mohammadi, Yarmohammadi, Salehi-Abargouei, Ghasemirad, Shirvani and Ghoshouni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hamed Ghoshouni, aGFtZWRnaG9zaG91bmlAZ21haWwuY29t
†ORCID: Hamed Ghoshouni orcid.org/0000-0001-8949-2256
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.