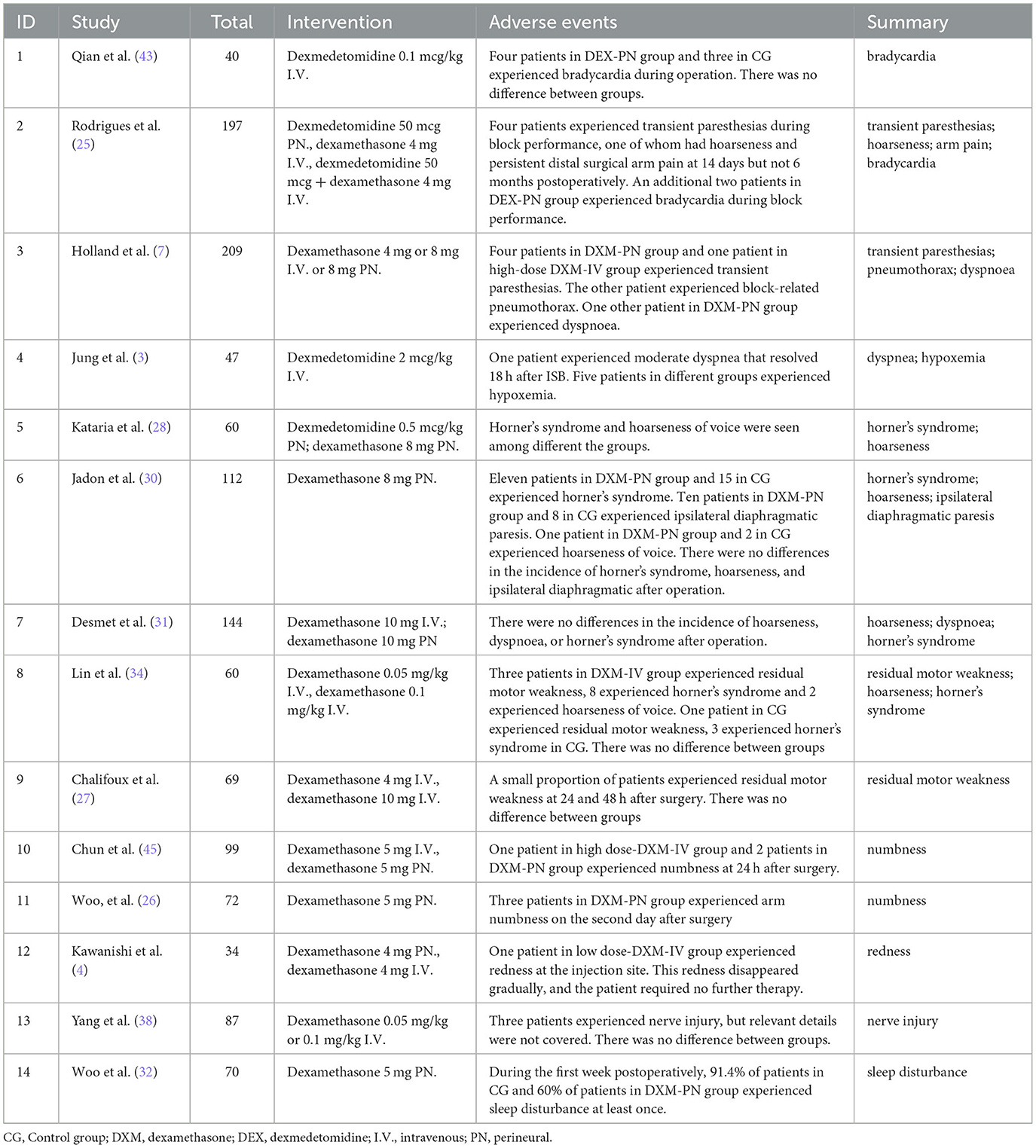
- 1Department of Anesthesiology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Shandong Institute of Anesthesia and Respiratory Critical Medicine, Jinan, Shandong, China
- 2School of Anesthesiology, Weifang Medical University, Weifang, Shandong, China
- 3Department of Anesthesiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
Introduction: Interscalene block (ISB) is widely regarded as the gold standard treatment for acute pain following arthroscopic shoulder surgery. However, a single injection of a local anesthetic for ISB may not offer sufficient analgesia. Various adjuvants have been demonstrated to prolong the analgesic duration of the block. Hence, this study aimed to assess the relative efficacy of dexamethasone and dexmedetomidine as adjuncts to prolong the analgesic duration for a single- shot ISB.
Methods: The efficacy of adjuvants was compared using a network meta-analysis. The methodological quality of the included studies was evaluated using the Cochrane bias risk assessment tool. A comprehensive search of the PubMed, Cochrane, Web of Science, and Embase databases was conducted with a search deadline of March 1, 2023. Various adjuvant prevention randomized controlled trials have been conducted in patients undergoing interscalene brachial plexus block for shoulder arthroscopic surgery.
Results: Twenty-five studies enrolling a total of 2,194 patients reported duration of analgesia. Combined dexmedetomidine and dexamethasone (MD = 22.13, 95% CI 16.67, 27.58), dexamethasone administered perineurally (MD = 9.94, 95% CI 7.71, 12.17), high-dose intravenous dexamethasone (MD = 7.47, 95% CI 4.41, 10.53), dexmedetomidine administered perineurally (MD = 6.82, 95% CI 3.43, 10.20), and low-dose intravenous dexamethasone (MD = 6.72, 95% CI 3.74, 9.70) provided significantly longer analgesic effects compared with the control group.
Discussion: The combination of intravenous dexamethasone and dexmedetomidine provided the greatest effect in terms of prolonged analgesia, reduced opioid doses, and lower pain scores. Furthermore, peripheral dexamethasone in prolonging the analgesic duration and lowering opioid usage was better than the other adjuvants when used a single medication. All therapies significantly prolonged the analgesic duration and reduced the opioid dose of a single-shot ISB in shoulder arthroscopy compared with the placebo.
1. Introduction
Interscalene block (ISB) is widely regarded as the gold standard for the treatment of acute pain following arthroscopic shoulder surgery as it provides great analgesia in the early postoperative period while reducing opioid consumption and adverse effects (e.g., respiratory depression, nausea, and vomiting) (1, 2). However, a single injection of local anesthetic for ISB may not offer sufficient analgesia if used for longer than 14 h. The short duration of analgesia and analgesic effect of a single shot of ISB restrict its use (3). Continuous infusion analgesia using a patient-controlled interscalene catheter might prolong the duration of analgesia; unfortunately, it cannot circumvent the inherent practical problems and complications of plexus catheter infusion maintenance (4).
Various adjuvants have been demonstrated to prolong the analgesic duration of the block, including epinephrine, clonidine, dexmedetomidine, and intravenous and perineural injection of dexamethasone (3, 5). As a highly selective α2 adrenergic receptor agonist, dexmedetomidine is anticipated to have a longer analgesic duration than other adjuvants, without neurotoxicity (6). Dexamethasone, a potent glucocorticoid, is effective at both low (4 mg) and high (8 mg) concentrations. Several animal experiments have proven that these adjuvants prolong the impact of a nerve block, and clinical trials have also verified the beneficial effects on peripheral nerve and brachial plexus block (7). However, different doses and modes of administration of adjuvant therapies affect analgesic duration extension, and a quantitative evaluation of their efficacy is still required.
The objective of this network meta-analysis was to determine the relative efficacy of dexamethasone and dexmedetomidine as adjuncts to prolong the analgesic duration of a single-shot ISB.
2. Method
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline and the Cochrane Handbook for the Systematic Review of Interventions (8, 9). The research reviewed the existing data; thus, neither ethical approval nor patient agreement was necessary.
2.1. Search strategy
Two authors independently designed and conducted a systematic literature search to identify the parallel group and cross-over randomized controlled trials (RCTs) on PubMed, Embase, Web of Science, the Cochrane Central Register of Controlled Trials, the China National Knowledge Infrastructure database, the Chinese Scientific Journal database, and the Wan Fang Database with a search deadline of March 1, 2023. Without any restrictions on the publication year, region, or language, our search method included Medical Topic Headings (MeSH), Emtree phrases, subject headings, and free-text terms, mainly including the following terms: “arthroscopic shoulder surgery,” “dexamethasone,” “dexmedetomidine,” and “adjuncts.” We conducted further analysis to determine whether the material was provided in a non-English language.
In addition, we searched the bibliography lists of relevant previous studies to perform a battery of recursive searches and manual retrieval for potential studies, where only abstracts meeting our eligibility criteria were presented. EndNote X9 was used to manage all the above screening records (Thomson ISI Research Soft, Philadelphia, PA, USA). A comprehensive list of search phrases for each database is available in the “Search Strategies” supplement.
2.2. Eligibility criteria and data abstraction
The inclusion and exclusion criteria were prioritized according to the PICO criteria. RCTs published in peer-reviewed scientific journals compared the efficacy of adjuvants for ISB to control postoperative pain in arthroscopic shoulder surgery. The PICO criteria were classified as follows: Participants: Patients scheduled for elective shoulder arthroscopic surgery were enrolled in this network meta-analysis (NMA). Interventions: Intravenous or perineural injection was administered as adjuvants for ISB in patients undergoing shoulder arthroscopic surgery. Comparators: Interventions themselves or patients who received an ISB alone. Outcomes: The primary outcome was analgesia duration. The postoperative analgesic duration was defined as the time interval between ISB and the request for the first rescue analgesic, the time of the first-time shoulder pain was experienced, and the sensory block duration. The secondary outcomes were opioid consumption and pain score. Consumption of opioids is defined as the use of oral morphine equivalents, according to the general monograph for opioids in the Canadian Pharmacists' Association Compendium of Pharmaceuticals and Specialties. Opioid consumption was converted to morphine equivalents using standard conversions (10). The determination of pain is mainly based on the Visual Analog Scale (VAS) or Numeric Rating Score (NRS), which defines the presence and degree of pain. Different pain levels can be measured on a scale of 0–10, with 0 representing no pain and 10 representing the worst pain. Study design: This review included both parallel-group and crossover RCTs. The studies were divided into six groups: low-dose intravenous dexamethasone (4 mg) (low-dose DXM-IV), high-dose intravenous dexamethasone (8 mg) (high-dose DXM-IV), perineural dexamethasone (DXM-PN), perineural dexmedetomidine (DEX-PN), combined intravenous dexamethasone and dexmedetomidine (DEX-DXM), and control group. Two authors (X-MW and ZL) separately identified the relevant articles. Initial searches were conducted on both the titles and abstracts using the defined eligibility criteria. In this phase, duplicate articles were removed from the retrieved articles simultaneously.
All articles selected from the initial research were retrieved and assessed based on their full text. If no data were available for abstract-only research, they were disregarded. Disagreements were settled by discussions between reviewers and consultation with an independent expert referee (P-CS) to ensure that a consensus was reached on all items.
2.3. Outcome measurement and quality appraisal
According to a pre-tested, nine-item, standardized data extraction form, two independent authors extracted data from each article under the following headings: first author(s), year of publication, patient characteristics, sample size, duration of analgesia, pain scores, opioid consumption, and incidence of complications. The mean and standard deviation (SD) of the duration of analgesia, pain scores, and opioid consumption were extracted as continuous outcomes. If the duration of analgesia and VAS was expressed as median with interquartile range (IQR), it was transformed and expressed as mean ± SD before statistical analysis (8). We presumed that the width of the IQR was equal to 1.35 times the SD and that the median was equal to the mean. The formulas used to get the mean and standard deviation (SD) were all based on the recommendations provided in the Cochrane Handbook for Systematic Reviews (11).
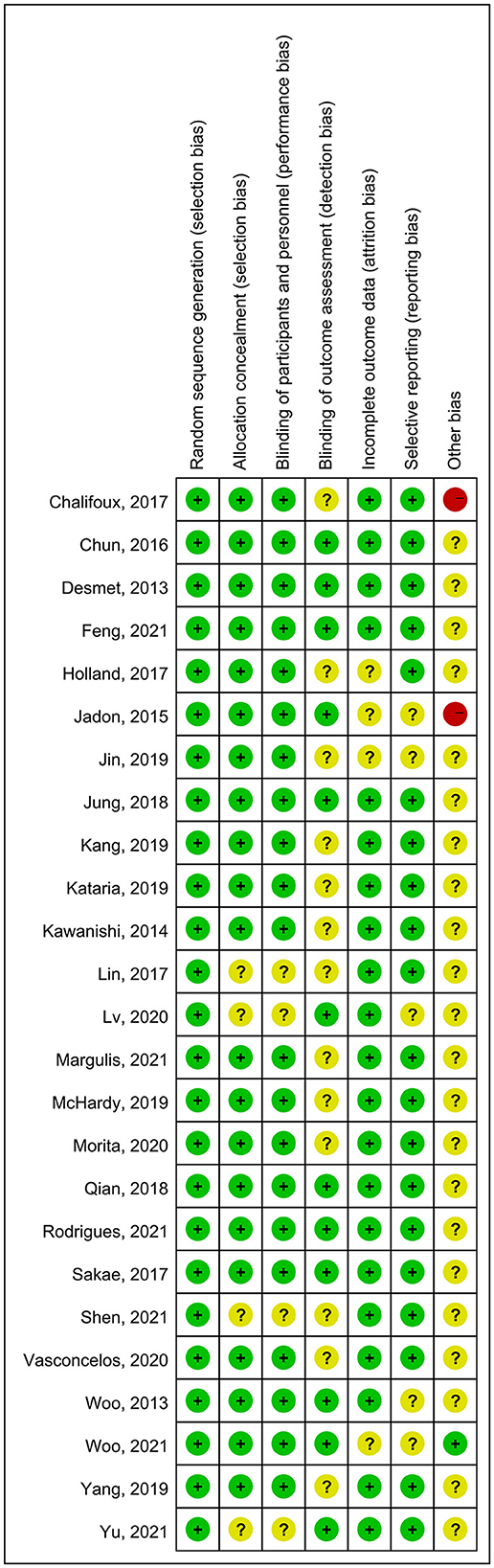
Two independent authors (ZL and X-MW) evaluated and categorized the risk of bias (ROB) using the Cochrane Handbook version 5.1.0 tool in Review Manager (version 5.3) (8). For each trial, we categorized the risk as low, high, or unclear, according to the seven assessment items. For selection bias, we evaluated whether the studies clearly defined the random sequence generation and allocation concealment method. Regarding detection bias, we evaluated studies primarily based on whether the participants, personnel, and outcome evaluators were blinded. We classified patients as high-risk for attrition bias in studies in which essential data were missing, particularly primary outcome data. We assessed selection bias based on whether the research excluded secondary outcomes or provided inadequate data. Other biases were categorized based on a full-text search for evidence that may have contributed to potential inconsistencies among the included studies. In addition, the GRADE method was used to evaluate the quality of evidence for each connection (12).
2.4. Statistical analysis
A network plot was generated to simulate a fully connected network, as an overview of the available evidence for each adjuvant. A comparison-adjusted network funnel plot was used to visually assess publication bias. Both analyses were conducted using the Stata software (version 14.0; Stata Corp, College Station, TX, USA).
Transitivity is the key underlying assumption of the NMA and relates to the validity of making indirect comparisons and the homogeneous distribution of effect modifiers across the included studies. Before performing data analysis, the baseline characteristics of the participants were presented for each intervention group (13–15). We assigned a non-informative prior distribution to the parameters based on a Bayesian framework (16). The Markov chain Monte Carlo method was used to examine all the results, which established three distinct chains with a total number of 50,000 iterations (17–19). For continuous variables, we used the mean difference (MD) to pool the effect size and 95% confidence intervals (CIs) (20, 21). The proportion of the best ranking in all simulated activities was used to calculate the probability of which adjuvant intervention would be the best. For each treatment, the surface under the cumulative ranking curve (SUCRA) was used to estimate cumulative ranking (22). The SUCRA value is presented as a percentage, ranging from 0 to 100%. Higher SUCRA values indicate a better ranking of treatment effectiveness, whereas lower SUCRA values indicate a worse trend (21). By evaluating the trace “history” feature, both the tract plot and the Brooks-Gelman-Rubin diagnostic statistics were considered to ensure convergence. Sensitive analysis by omitting one study in each turn was performed. The above analyses were performed using STATA (ver. 14.2; StataCorp, Lakeway Drive College Station, TX 77845, USA) and OpenBUGS (ver. 3.2.3 rev 1012, Members of OpenBUGS Project Management Group) software. Details of the OpenBUGS code are presented in the “OpenBUGS code” supplement. The node-splitting method was used to assess model inconsistency, where the probability of significant inconsistency was indicated if node-splitting analysis-derived P-values were < 0.05 (8, 18, 23, 24). I2 statistic was tested for assessing substantial heterogeneity, of which the values 25, 50, 75% indicated mild, moderate and high heterogeneity respectively (9). The analysis was performed using “Gemtc” package (version 0.8–2) and “rjags” (version 4–6) in R language (X64 4.12 version).
3. Results
3.1. Baseline characteristics and quality of the included studies
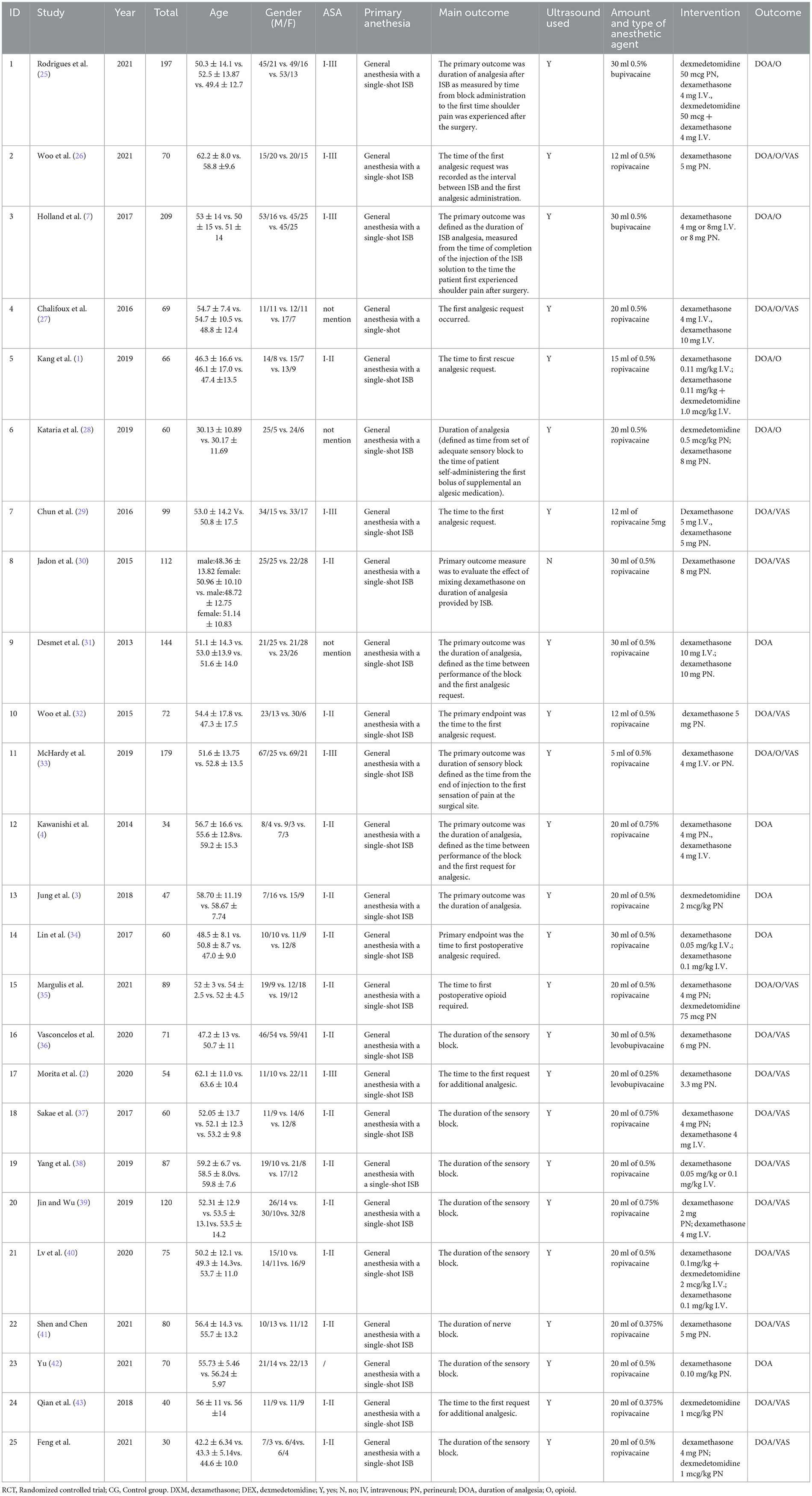
Initially, a total of 48 studies were identified by searching electronic databases and manually, of which 9 articles were removed due to duplication. Furthermore, 3 were excluded owing to irrelevant topics after screening based on the titles and abstracts. Following the full-text screening, 25 articles remained, and 23 articles were excluded for the following reasons: 4 did not report relevant data, 1 did not contain relevant outcomes, 1 was not an RCT, and 1 did not report relevant outcomes. Eventually, a total of 25 RCTs were deemed eligible for review and inclusion in this NMA, and a unanimous agreement was achieved on all articles among the reviewer authors. The literature search and study selection procedures are presented in Figure 1. EndNote X9 software (Clarivate Analytics, London, United Kingdom) was used to import and maintain all reference lists retrieved using a search engine. Table 1 summarizes the essential characteristics of the included studies.
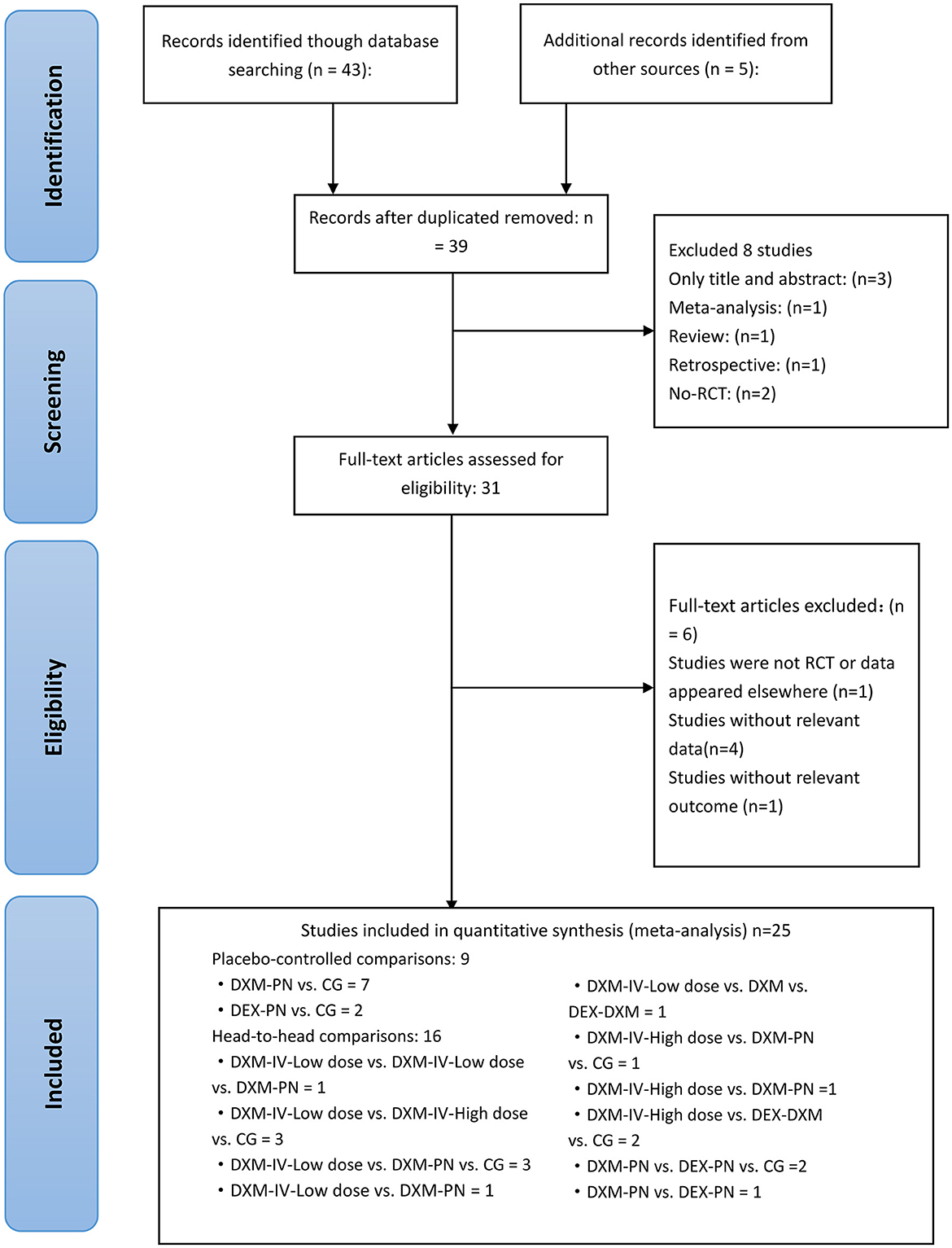
Figure 1. Literature review flowchart; RCT, Randomized controlled trial; CG, Control group. DXM, dexamethasone; DEX, dexmedetomidine; IV, intravenous; PN, perineural.
A total of 2,194 patients who underwent arthroscopic shoulder surgery for arthroscopic rotator cuff, subacromial decompression, and various forms of shoulder surgery were enrolled in 25 studies published between 2013 and 2021 and included in the review. Five therapies were tested in parallel (n = 9) or crossover (n = 16) RCTs (1–4, 7, 25–44). The sample size was largest for the DXM-PN group (n = 665; 17 studies), followed by the control (n = 584; 20 studies), low-dose DXM-IV (n = 369; 9 studies), high-dose DXM-IV (n = 285; 8 studies), DEX-PN (n = 178; 6 studies), and DEX-DXM groups (n = 113; 3 studies). A network map was created to allow direct comparison between the interventions (Figure 2).
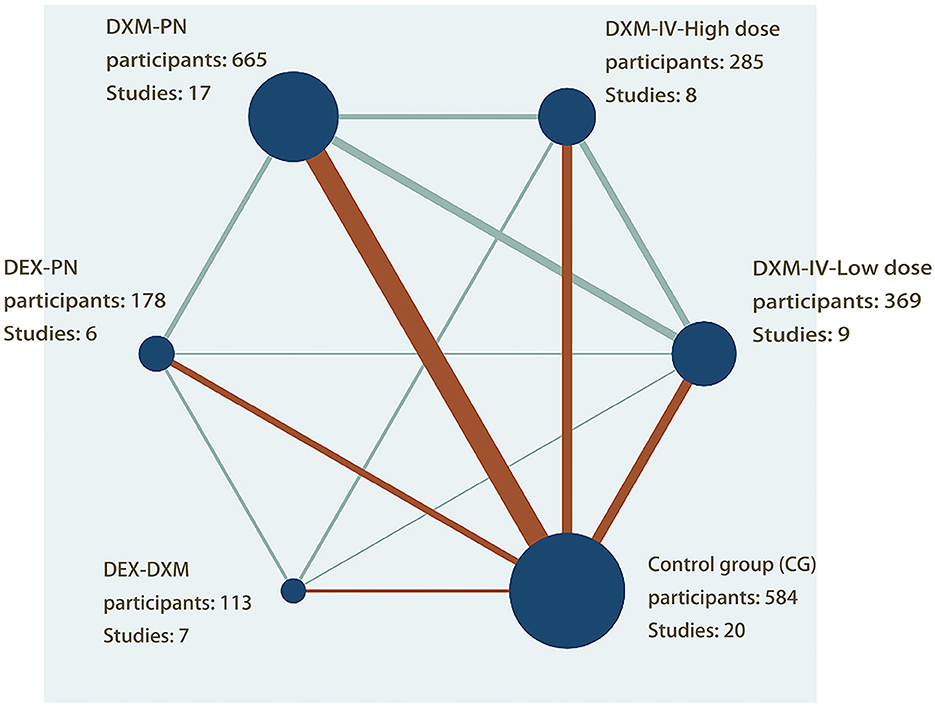
Figure 2. Network plot of evidence of all the trials. The network plot of the intervention network shows the comparion of the sample size to provide anesthesia for patients undergoing arthroscopic shoulder surgery. Each node represented a different method of prevention with the size of the node depending on the number of patients who received the intervention directly, he nodes were connected by lines indicating direct relationships between interventions, with the thickness of the line depending on the amount of direct evidence supporting the intervention.
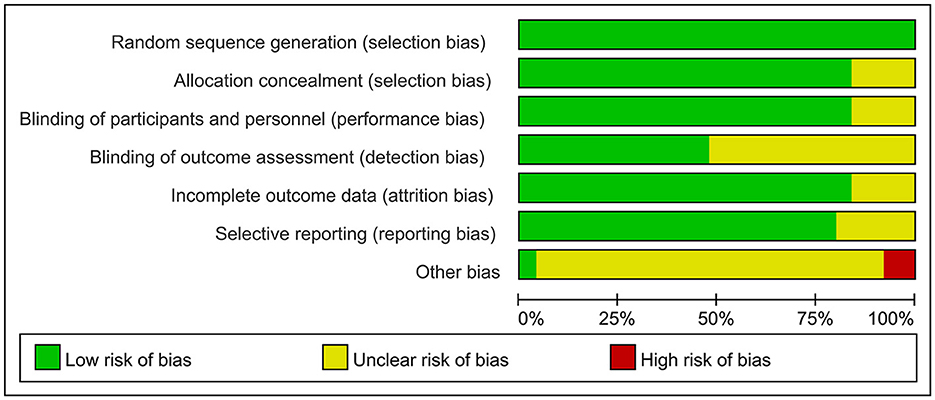
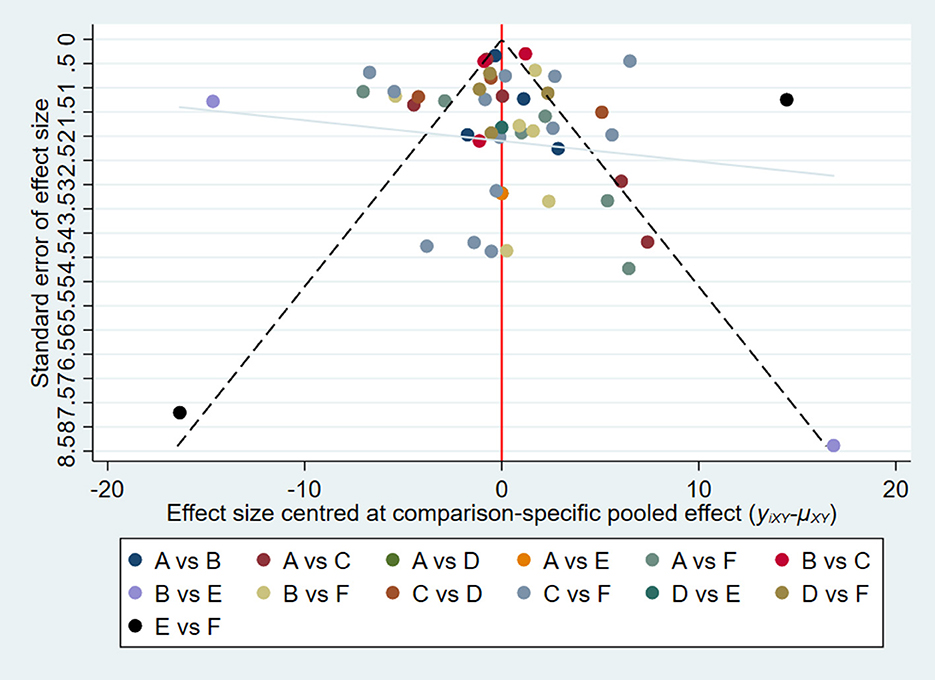
The overall quality of the included studies revealed modest variance. All 25 included trials were randomly allocated and showed a low risk of bias in “random sequence generation.” Twenty studies had a low ROB with selective outcome reporting. Four studies had a high or unclear risk for attrition bias. Twenty-one studies used allocation concealment, whereas 21 fully detailed the blinding of the outcome evaluation. Evaluation of the quality of the included studies is shown in Figures 3, 4. Publication bias was not observed in the funnel plot based on its symmetrical distribution (inverted funnel plot) (Figure 5). When consistency and inconsistency between studies were assessed, all P-values were > 0.05, showing that the effect of consistency between studies was acceptable. According to the I2 value, there was low to moderate heterogeneity among the included studies. Sensitive analysis by omitting one study in each turn indicated the results were unaffected. No single study notably affected the overall summary estimate and P-value. The details are shown in the Supplementary material.
3.2. Duration of analgesia
Twenty-five studies enrolling 2,194 patients reported the duration of analgesia. The placebo group included 584 patients, and the intervention group included 1610 (low-dose DXM-IV = 369, high-dose DXM-IV = 285, DXM-PN = 665, DEX-PN = 178, DEX-DXM = 113). Combined DEX-DXM (MD = 22.13, 95% CI 16.67, 27.58), DXM-PN (MD = 9.94, 95% CI 7.71, 12.17), high-dose DXM-IV (MD = 7.47, 95% CI 4.41, 10.53), DEX-PN (MD = 6.82, 95% CI 3.43, 10.20), and low-dose DXM-IV (MD = 6.72, 95% CI 3.74, 9.70) provided significantly longer analgesic effects compared with the control group.
According to the SUCRA data (Supplementary Figure 1), the combination of DEX-DXM (SUCRA = 98.5%) and DXM-PN (77.6%) had the highest efficacy, followed by high-dose DXM-IV (47.0%), DEX-PN (38.7%), low-dose DXM-IV (36.6%), and control groups (0.3%).
3.3. Opioids consumption
Eight studies enrolling a total of 939 patients reported opioid consumption after surgery. The placebo group included 110 patients, and the intervention group included 829 (low-dose DXM-IV = 248, high-dose DXM-IV = 116, DXM-PN = 252, DEX-PN = 125, DEX-DXM = 88). The DEX-DXM (MD = −4.50, 95% CI −5.25, −3.75), DXM-PN (MD = −4.70, 95% CI −5.53, −3.87), low-dose DXM-IV (MD = −30.03, 95% CI −46.35, −13.71), high-dose DXM-IV (MD =-4.50, 95% CI −5.28, −3.72), and DEX-PN (MD =-4.40, 95% CI −5.31, −3.49) groups had significantly better outcomes than the control group (Supplementary Figure 2).
The SUCRA data showed that the DEX-DXM group (SUCRA = 99.4%) had the highest efficacy, followed by the DXM-PN (SUCRA = 66.7%), low-dose DXM-IV (SUCRA = 47.7%), high-dose DXM-IV (SUCRA = 45.2%), DEX-PN (SUCRA = 41.0%), and control groups (0.5%).
3.4. Pain score
Sixteen studies enrolling a total of 1,307 patients reported pain scores (VAS or NRS) 24 h after surgery. The placebo group included 422 patients, and the intervention group included 885 (low-dose DXM-IV = 202, high-dose DXM-IV = 127, DXM-PN = 471, DEX-PN = 60, DEX-DXM = 25). The combined DEX-DXM (MD = −2.56, 95% CI −4.53, −0.59), high-dose DXM-IV (MD = −1.79, 95% CI −2.93, −0.66), DXM-PN (MD = −1.46, 95% CI −2.17, −0.75), and low-dose DXM-IV (MD = −1.06, 95% CI −2.08, −0.05) groups provided significantly longer analgesic effects than the control group (Supplementary Figure 3).
The SUCRA data showed that the DEX-DXM group (SUCRA = 89.3%) had the highest efficacy, followed by the high-dose DXM-IV (SUCRA = 72.9%), DXM-PN (SUCRA = 60.1%), low-dose DXM-IV (SUCRA = 39.8%), DEX-PN (SUCRA = 36.0%), and control groups (1.9%).
3.5. Adverse events
Two studies referred to transient paresthesias during block performance, 2 studies mentioned bradycardia, 4 studies have described hoarseness of voice, 4 studies pointed Horner' s syndrome, 3 studies mentioned dyspnea, 2 studies referred residual motor weakness, and 2 studies pointed numbness. Redness at the injection site, nerve injury, sleep disturbance and persistent distal surgical arm pain only mentioned in one study. Specific details of adverse events are summarized in Table 2.
4. Discussion
In this study we assessed the relative efficacy of dexamethasone and dexmedetomidine as adjuncts to prolong the analgesic duration for a single- shot ISB. A total of 25 studies were included, including 2194 patients undergoing shoulder arthroscopy. Our study showed that the combination of intravenous dexamethasone and dexmedetomidine provided the greatest effect in terms of prolonged analgesia, reduced opioid doses, and lower pain scores. Furthermore, peripheral dexamethasone in prolonging the analgesic duration and lowering opioid usage was better than the other adjuvants when used a single medication. All therapies significantly prolonged the analgesic duration and reduced the opioid dose of a single-shot ISB in shoulder arthroscopy compared with the placebo.
Shoulder arthroscopy may cause considerable discomfort, particularly during the first 24 h following surgery. Several adjuvants, including intravenous dexamethasone, peripheral dexamethasone, peripheral dexmedetomidine, and the combined application of dexamethasone and dexmedetomidine, have been shown to extend the duration of nerve block (1). Our analysis quantitatively compared the effects of these adjuvants.
The exact mechanism by which dexamethasone prolongs the duration of the sensory blockade is unclear. Although glucocorticoids have been claimed to have direct effects on nerves, other investigations have indicated that dexamethasone may cause peripheral vasoconstriction and impede local anesthetic absorption (5). In a recent retrospective cohort analysis of upper and lower limb surgery under different forms of peripheral nerve block, intravenous dexamethasone was shown to extend the duration of the block when added to ropivacaine (46). Dexamethasone is also recognized as an auxiliary function in regional analgesia according to many single studies and a meta-analysis of 29 studies (47, 48). An RCT demonstrated that in patients who received ultrasound-guided sciatic nerve blocks, there was no significant difference between peripheral and intravenous dexamethasone in terms of the duration of analgesia (49). In major and small orthopedic surgeries, dexamethasone and other glucocorticoids have considerable analgesic effects in the equivalent dosage range of 9 to 40 mg dexamethasone (45, 50–52). In shoulder surgery, relatively little data are available, although dexamethasone (4–8 mg) has been used as an adjuvant for an ISB with a prolonged analgesic effect (53, 54). In addition, corticosteroid injections around the nerve have been utilized for a long time to treat radiculopathy. To date, no clinical studies have identified the neurological problems induced by dexamethasone (5).
Individual studies have shown that dexamethasone may extend the analgesic effect of ropivacaine when administered as an adjuvant; however, there are few direct head-to-head comparisons, and the findings are uncertain or even contradictory. A meta-analysis by Choi et al. involving 393 patients who received dexamethasone demonstrated that dexamethasone as a local anesthetic adjuvant lengthened the analgesic time of brachial plexus block (5). According to our results, peripheral dexamethasone is more efficient than intravenous dexamethasone. High-dose and low-dose intravenous dexamethasone were equivalent.
Dexmedetomidine is currently one of the most commonly used adjuvants for nerve blocking because it has no significant neurotoxicity risk. It is hypothesized that α2 receptor binding in the central nervous system mediates the analgesic effects of dexmedetomidine by decreasing the release of nociceptive transmitters (55). Brummett et al. first reported in 2008 that dexmedetomidine enhanced the duration of sciatic nerve block in rats without causing neurotoxicity (56). Several clinical trials have studied the beneficial effect of a single dose of peripheral dexmedetomidine on prolonging the analgesic time of nerve blocks (6, 57, 58). In a study conducted by Abdallah et al., intravenous or perineural administration of 0.5 mcg/kg dexmedetomidine was compared with the placebo. A total of 24h use of morphine after surgery was decreased in the dexmedetomidine group, but there was no significant difference in resting pain levels between the three groups after 24h (59). Our results are consistent with those of previous studies. Compared with the control group, dexmedetomidine prolonged the analgesic time of the brachial plexus block.
Our NMA demonstrates that the combination of dexamethasone and dexmedetomidine has the greatest analgesic effects in terms of prolonging analgesia. The mechanisms why dexamethasone could prolong analgesia are corticosteroid induced vasoconstriction reducing local anesthetic absorption and the inhibition of potassium channels on nociceptive C-fibers or inhibits inflammatory responses through peripheral and central (60–62). The synergistic mechanism why dexmedetomidine could prolong analgesia may be mediated by the binding of a2 receptors in the central nervous system, which inhibits the release of nociceptive transmitters (55, 63). The combined effects of the two drugs are usually antagonistic, additive, or synergistic (64). This mechanism can potentially be explained using the effect-addition model (65). However, the exact mechanism underlying the interaction between dexamethasone and dexmedetomidine need more studies to confirm.
In all of the studies we included, there was no significant difference in the incidence of adverse reactions between the groups using adjuvants or systemic medications compared to the control group. In addition, no matter what kind of adjuvant is used, there was no significant improvement in the incidence of complications related to nerve block treatments, such as dyspnea, hoarseness, and Horner's syndrome. As for peripheral dexamethasone or dexmedetomidine, studies have revealed that it is typically harmless (48, 66). In a recent study, data from 1026 individuals who received perineural dexmedetomidine were included, and it was shown that none of them experienced any neurotoxicity symptoms and neurologic sequalae (66). However, in patients with pre-existing heart disease, a systemic impact on the cardiovascular system remains a potential concern at high doses. Dexmedetomidine can cause bradycardia which may be the result of decreased central sympathetic output and increased parasympathetic output from cardiac vagal neurons in the brainstem (67). Hussain et al. (68) reported peripheral dexamethasone does not appear to lead to long-term neurologic complications and no persistent neurological deficits were reported in all included RCTs (68). Ma et al. (69) showed during in-vitro studies that dexamethasone may have a protective effect against local anesthetic-induced cell injury (69) and for the treatment of post-traumatic visual disturbance, a series of 2,000 intrathecal injections had no neurological sequelae (70). Some other evidences also suggest that dexamethasone may be neuroprotective, and it has been demonstrated that corticosteroids have no long-term electrophysiological, behavioral, or histological effects on the sciatic nerve tissue of rats (71). In general, the safety profile of perineural dexamethasone is promising.
This study had several limitations. First, it is difficult to evaluate the sensory blocks after surgery. Most studies use the time before the first pain relief as a sign of cessation of the sensory block. Other studies have only described the duration of analgesia or sensory blockade. Furthermore, the “off-label” use of adjuvants surrounding the nerve poses safety concerns. Without human clinical trials, we can only claim that there is no increase in neuronal cell death following exposure to low-dose dexamethasone plus ropivacaine for 2 h compared with ropivacaine alone based on laboratory investigations (72) and there is no neurological damage in perineural dexmedetomidine studies, as previously reported.
Our study has several advantages. When evaluating the prolonged analgesic effects of different adjuvants in the intermuscular sulcus brachial plexus, we restricted the surgery to the same type. Only RCT studies were included in our analysis, and the quality assessment results of all publications were similar.
In conclusion, the combination of intravenous dexamethasone and dexmedetomidine provided the greatest effect in terms of prolonged analgesia, reduced opioid doses, and lower pain scores. Furthermore, peripheral dexamethasone in prolonging the analgesic duration and lowering opioid usage was better than the other adjuvants when used a single medication. All therapies significantly prolonged the analgesic duration and reduced the opioid dose of a single-shot ISB in shoulder arthroscopy compared with the placebo.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
X-MW and ZL provided substantial contributions to the conception or design of the work, acquisition, analysis, interpretation of the data, drafting of the manuscript, and critically revising the manuscript for important intellectual content. L-CL contributed substantially to the conception and design of the work, acquisition, analysis, interpretation of the data, drafting of the manuscript, and critically revising the manuscript for important intellectual content. G-HW agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. P-YS contributed substantially to the revision of the work and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. C-PG helped with the approval of the final version of the manuscript to be published. P-CS helped with the approval of the final version of the manuscript to be published. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by Bethune Charitable Foundation (ezmr2022-003) and Young Taishan Scholars (tsqn201812144).
Acknowledgments
We thank all the reviewers for their assistance and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1159216/full#supplementary-material
Abbreviations
CI, confidence interval; DEX, dexmedetomidine; DXM, dexamethasone; ISB, interscalene block; IV, intravenous; MD, mean difference; NMA, network meta-analysis; NRS, Numeric Rating Score; PN, perineural; RCT, randomized controlled trial; ROB, risk of bias; SD, standard deviation; SUCRA, surface under the cumulative ranking curve; VAS, Visual Analog Scale.
References
1. Kang RA, Jeong JS, Yoo JC, Lee JH, Gwak MS, Choi SJ, et al. Improvement in postoperative pain control by combined use of intravenous dexamethasone with intravenous dexmedetomidine after interscalene brachial plexus block for arthroscopic shoulder surgery: a randomised controlled trial. Eur J Anaesthesiol. (2019) 36:360–8. doi: 10.1097/EJA.0000000000000977
2. Morita S, Oizumi N, Suenaga N, Yoshioka C, Yamane S, Tanaka Y. Dexamethasone added to levobupivacaine prolongs the duration of interscalene brachial plexus block and decreases rebound pain after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. (2020) 29:1751–7. doi: 10.1016/j.jse.2020.04.019
3. Jung HS, Seo KH, Kang JH, Jeong J-Y, Kim Y-S, Han N-R. Optimal dose of perineural dexmedetomidine for interscalene brachial plexus block to control postoperative pain in patients undergoing arthroscopic shoulder surgery: A prospective, double-blind, randomized controlled study. Medicine (Baltimore). (2018) 97:e0440. doi: 10.1097/MD.0000000000010440
4. Kawanishi R, Yamamoto K, Tobetto Y, Nomura K, Kato M, Go R. Perineural but not systemic low-dose dexamethasone prolongs the duration of interscalene block with ropivacaine: a prospective randomized trial. Local Reg Anesth. (2014) 7:5–9. doi: 10.2147/LRA.S59158
5. Choi S, Rodseth R, Mccartney CJ. Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: a systematic review and meta-analysis of randomized trials. Br J Anaesth. (2014) 112:427–39. doi: 10.1093/bja/aet417
6. Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. (2010) 111:1548–51. doi: 10.1213/ANE.0b013e3181fa3095
7. Holland D, Amadeo RJJ, Wolfe S, Girling L, Funk F, Collister M, et al. Effect of dexamethasone dose and route on the duration of interscalene brachial plexus block for outpatient arthroscopic shoulder surgery: a randomized controlled trial. Can J Anaesth. (2018) 65:34–45. doi: 10.1007/s12630-017-0989-7
8. Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. (2012) 3:98–110. doi: 10.1002/jrsm.1044
9. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
10. Nielsen S, Degenhardt L, Hoban B. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. (2016) 25:733–7. doi: 10.1002/pds.3945
11. Li AM, Li X, Yang Z. Decompression and coflex interlaminar stabilisation compared with conventional surgical procedures for lumbar spinal stenosis: a systematic review and meta-analysis. Int J Surg. (2017) 40:60–7. doi: 10.1016/j.ijsu.2017.02.056
12. Brozek JL, Akl EA, Compalati E, Kreis J, Terracciano L, Fiocchi A, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3 An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. (2009) 64:669–77. doi: 10.1111/j.1398-9995.2009.02083.x
13. Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. (2005) 331:897–900. doi: 10.1136/bmj.331.7521.897
14. Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med. (2013) 11:159. doi: 10.1186/1741-7015-11-159
15. Salanti G, Marinho V, Higgins JP, A. case study of multiple-treatments meta-analysis demonstrates that covariates should be considered. J Clin Epidemiol. (2009) 62:857–64. doi: 10.1016/j.jclinepi.2008.10.001
16. Stojanovski KM. Bayesian methods in meta-analysis. EJC. (2006) 1:116–121. doi: 10.1201/b14674-20
17. Mavridis D, Salanti G, A. practical introduction to multivariate meta-analysis. Stat Methods Med Res. (2013) 22:133–58. doi: 10.1177/0962280211432219
18. van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. (2016) 7:80–93. doi: 10.1002/jrsm.1167
19. Nj W. Evidence Synthesis for Decision Making in Healthcare. New York, NY: John Wiley & Sons. (2012).
20. Mbuagbaw L, Rochwerg B, Jaeschke R, Heels-Andsell D, Alhazzani W, Thabane L, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. (2017) 6:79. doi: 10.1186/s13643-017-0473-z
21. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. (2011) 64:163–71. doi: 10.1016/j.jclinepi.2010.03.016
22. Page MJ, Shamseer L, Altman DG, Tetzlaff J, Sampson M, Tricco AC, et al. Epidemiology and Reporting Characteristics of Systematic Reviews of Biomedical Research: A Cross-Sectional Study. PLoS Med. (2016) 13:e1002028. doi: 10.1371/journal.pmed.1002028
23. Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS ONE. (2013) 8:e76654. doi: 10.1371/journal.pone.0076654
24. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. (2010) 29:932–44. doi: 10.1002/sim.3767
25. Rodrigues D, Amadeo RJJ, Wolfe S, Girling L, Funk F, Fidler K, et al. Analgesic duration of interscalene block after outpatient arthroscopic shoulder surgery with intravenous dexamethasone, intravenous dexmedetomidine, or their combination: a randomized-controlled trial. Can J Anaesth. (2021) 68:835–45. doi: 10.1007/s12630-021-01942-2
26. Woo JH, Lee HJ, Oh H-W, Lee JW, Baik HJ, Kim YJ. Perineural dexamethasone reduces rebound pain after ropivacaine single injection interscalene block for arthroscopic shoulder surgery: a randomized controlled trial. Reg Anesth Pain Med. (2021) 46:965–70. doi: 10.1136/rapm-2021-102795
27. Chalifoux F, Colin F, St-Pierre P, Godin N, Brulotte V. Low dose intravenous dexamethasone (4 mg and 10 mg) significantly prolongs the analgesic duration of single-shot interscalene block after arthroscopic shoulder surgery: a prospective randomized placebo-controlled study. Can J Anaesth. (2017) 64:280–9. doi: 10.1007/s12630-016-0796-6
28. Kataria S, Mitra S, Saroa R. A Randomized Double Blinded trial comparing dexmedetomidine with dexamethasone as an adjunct to ropivacaine in ultrasound guided interscalene block for arthroscopic shoulder surgery. Asian J Anesthesiol. (2019) 57:10–8. doi: 10.6859/aja.201903_57(1).0003
29. Chun EH, Kim YJ, Woo JH. Which is your choice for prolonging the analgesic duration of single-shot interscalene brachial blocks for arthroscopic shoulder surgery? intravenous dexamethasone 5 mg vs perineural dexamethasone 5 mg randomized, controlled, clinical trial. Medicine. (2016) 95:e3828. doi: 10.1097/MD.0000000000003828
30. Jadon A, Dixit S, Kedia SK, Chakraborty S, Agrawal A. Sinha N. Interscalene brachial plexus block for shoulder arthroscopic surgery: Prospective randomised controlled study of effects of 05% ropivacaine and 05% ropivacaine with dexamethasone. Indian J Anaesth. (2015) 59:171–6. doi: 10.4103/0019-5049.153039
31. Desmet M, Braems H, Reynvoet M. IV and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: a prospective, randomized, placebo-controlled study. Br J Anaesth. (2013) 111:445–52. doi: 10.1093/bja/aet109
32. Woo JH, Kim YJ, Kim DY. Dose-dependency of dexamethasone on the analgesic effect of interscalene block for arthroscopic shoulder surgery using ropivacaine 05%: A randomised controlled trial. Eur J Anaesthesiol. (2015) 32:650–5. doi: 10.1097/EJA.0000000000000213
33. McHardy PG, Singer O, Awad IT, Safa B, Henry PDG, Kiss A, et al. Comparison of the effects of perineural or intravenous dexamethasone on low volume interscalene brachial plexus block: a randomised equivalence trial. Br J Anaesth. (2020) 124:84–91. doi: 10.1016/j.bja.2019.08.025
34. Lin L, Chen C, Hou J, Yang P. A randomised controlled trial of intravenous Dexamethasone combined with interscalene brachial plexus blockade guided by ultrasound in shodder surgery. China Medical Herald. (2017) 14:67–70.
35. Margulis R, Francis J, Tischenkel B, Bromberg A, Pedulla D, Grtisenko K, et al. Comparison of dexmedetomidine and dexamethasone as adjuvants to ultra-sound guided interscalene block in arthroscopic shoulder surgery: a double-blinded randomized placebo-controlled study. Anesth Pain Med. (2021) 11:e117020. doi: 10.5812/aapm.117020
36. Vasconcelos MM, Pontes JPJ., Rodrigues Ad, Neto DRd, Alves RR, Silva FCdP, et al. Perineural dexamethasone in ultrasound-guided interscalene brachial plexus block with levobupivacaine for shoulder arthroscopic surgery in the outpatient setting: randomized controlled trial. Braz J Anesthesiol. (2020) 70:588–94. doi: 10.1016/j.bjane.2020.10.001
37. Sakae TM, Marchioro P, Schuelter-Trevisol F, Trevisol DJ. Dexamethasone as a ropivacaine adjuvant for ultrasound-guided interscalene brachial plexus block: a randomized, double-blinded clinical trial. J Clin Anesth. (2017) 38:133–6. doi: 10.1016/j.jclinane.2017.02.004
38. Yang X, Wang G, Xu C. Application of different doses of dexamethasone in brachial plexus block combined with general anesthesia in shoulder arthroscopy (2019).
39. Jin C, Wu J. Clinical application of dexamethasone combined with ropivacaine in ultrasound-guided intermuscular sulcus brachial plexus block (2019).
40. Lv J, Wu Q, Chen X, Yao S. Effects of intravenous dexamethasone combined with dexmedetomidine on ropivacaine brachial plexus block and plasma cortisol. Clin Anesthesiol. (2020) 36:842–6.
41. Shen Y, Chen H. Effect of ropivacaine combined with dexamethasone on postoperative pain after arthroscopic shoulder surgery (2021).
42. Yu D. Comparison of effects of different doses of Dexamethasone on brachial plexus block in patients undergoing arthroscopic shoulder surgery (2021).
43. Qian J, Wu S, Cheng H. Effect of dexmedetomidine combined with ropivacaine on brachial plexus block in patients undergoing rotator cuff repair (2018).
44. Feng Z, Fang Y, O D, Yang Y, Wang Z, Huang J. Clinical effect of dexmedetomidine and dexamethasone as adjuvants for brachial plexus block. J Kunming Med Univ. (2021) 42:68–75.
45. Aasboe V, Raeder JC, Groegaard B. Betamethasone reduces postoperative pain and nausea after ambulatory surgery. Anesth Analg. (1998) 87:319–23. doi: 10.1213/00000539-199808000-00015
46. Rasmussen SB, Saied NN, Bowens C. Duration of upper and lower extremity peripheral nerve blockade is prolonged with dexamethasone when added to ropivacaine: a retrospective database analysis. Pain Med. (2013) 14:1239–47. doi: 10.1111/pme.12150
47. Napierkowski DE, Parra-Sanchez I, Kurz A, Dalton JE, Brems JJ. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth. (2011) 107: 446–53. doi: 10.1093/bja/aer159
48. Albrecht E, Kern C, Kirkham KR, A. systematic review and meta-analysis of perineural dexamethasone for peripheral nerve blocks. Anaesthesia. (2015) 70:71–83. doi: 10.1111/anae.12823
49. Rahangdale R, Kendall MC, McCarthy RJ, Tureanu L, Jr RD, Weingart A, et al. The effects of perineural versus intravenous dexamethasone on sciatic nerve blockade outcomes: a randomized, double-blind, placebo-controlled study. Anesth Analg. (2014) 118:1113–9. doi: 10.1213/ANE.0000000000000137
50. Mattila K, Kontinen VK, Kalso E, Hynynen MJ. Dexamethasone decreases oxycodone consumption following osteotomy of the first metatarsal bone: a randomized controlled trial in day surgery. Acta Anaesthesiol Scand. (2010) 54:268–76. doi: 10.1111/j.1399-6576.2009.02126.x
51. Romundstad L, Breivik H, Niemi G, Helle A, Stubhaug A. Methylprednisolone intravenously 1 day after surgery has sustained analgesic and opioid-sparing effects. Acta Anaesthesiol Scand. (2004) 48:1223–31. doi: 10.1111/j.1399-6576.2004.00480.x
52. Kardash KJ, Sarrazin F, Tessler MJ, et al. Single-dose dexamethasone reduces dynamic pain after total hip arthroplasty. Anesth Analg. (2008) 106: 1253–7. doi: 10.1213/ANE.0b013e318164f319
53. Shrestha BR, Maharjan SK. Comparative study between bupivacaine heavy vs pethidine intrathecally to study early haemodynamic changes and postoperative analgesia in patients undergoing caesarean section. Kathmandu Univ Med J. (2007) 5:166–72. doi: 10.1016/j.ijoa.2004.04.006
54. Tandoc MN, Fan L, Kolesnikov S, Kruglov A, Nader ND. Adjuvant dexamethasone with bupivacaine prolongs the duration of interscalene block: a prospective randomized trial. J Anesth. (2011) 25:704–9. doi: 10.1007/s00540-011-1180-x
55. Weerink MAS, Struys MMRF, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. (2017) 56:893–913. doi: 10.1007/s40262-017-0507-7
56. Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. (2008) 109:502–11. doi: 10.1097/ALN.0b013e318182c26b
57. Fritsch G, Danninger T, Allerberger K, Tsodikov A, Felder TK, Kapeller M, et al. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med. (2014) 39:37–47. doi: 10.1097/AAP.0000000000000033
58. Obayah GM, Refaie A, Aboushanab O, Ibraheem N, Abdelazees M. Addition of dexmedetomidine to bupivacaine for greater palatine nerve block prolongs postoperative analgesia after cleft palate repair. Eur J Anaesthesiol. (2010) 27:280–4. doi: 10.1097/EJA.0b013e3283347c15
59. Abdallah FW, Dwyer T, Chan VWS, Niazi AU, Ogilvie-Harris DJ, Oldfield S, et al. IV and Perineural dexmedetomidine similarly prolong the duration of analgesia after interscalene brachial plexus block: a randomized, three-arm, triple-masked, placebo-controlled trial. Anesthesiology. (2016) 124:683–95. doi: 10.1097/ALN.0000000000000983
60. Holte K, Kehlet H. Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J Am Coll Surg. (2002) 195:694–712. doi: 10.1016/S1072-7515(02)01491-6
61. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. (2000) 21:55–89. doi: 10.1210/edrv.21.1.0389
62. Seidenari S, Di Nardo A, Mantovani L, Giannetti A. Parallel intraindividual evaluation of the vasoconstrictory action and the anti-allergic activity of topical corticosteroids. Exp Dermatol. (1997) 6:75–80. doi: 10.1111/j.1600-0625.1997.tb00150.x
63. Gaumann DM, Brunet PC, Jirounek P. Clonidine enhances the effects of lidocaine on C-fiber action potential. Anesth Analg. (1992) 74:719–25. doi: 10.1213/00000539-199205000-00017
64. Wessinger WD. Approaches to the study of drug interactions in behavioral pharmacology. Neurosci Biobehav Rev. (1986) 10:103–13. doi: 10.1016/0149-7634(86)90021-7
65. Tallarida RJ. Drug combinations: tests and analysis with isoboles. Curr Protoc Pharmacol. (2016) 72: 9–19. doi: 10.1002/0471141755.ph0919s72
66. Vorobeichik L, Brull R, Abdallah FW. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth. (2017) 118:167–81. doi: 10.1093/bja/aew411
67. Sharp DB, Wang X, Mendelowitz D. Dexmedetomidine decreases inhibitory but not excitatory neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Brain Res. (2014) 1574:1–5. doi: 10.1016/j.brainres.2014.06.010
68. Hussain N, Van den Langenbergh T, Sermer C, Fontes ML, Atrey A, Shaparin N, et al. Equivalent analgesic effectiveness between perineural and intravenous dexamethasone as adjuvants for peripheral nerve blockade: a systematic review and meta-analysis. Can J Anaesth. (2018) 65:194–206. doi: 10.1007/s12630-017-1008-8
69. Ma R, Wang X, Lu C, Li C, Cheng Y, Ding G, et al. Dexamethasone attenuated bupivacaine-induced neuron injury in vitro through a threonine-serine protein kinase B-dependent mechanism. Neuroscience. (2010) 167:329–42. doi: 10.1016/j.neuroscience.2009.12.049
70. Sugita K, Kobayashi S, Yokoo A, Inoue T. Intrathecal steroid therapy for post-traumatic visual disturbance. Neurochirurgia. (1983) 26:112–7. doi: 10.1055/s-2008-1053622
71. Benzon HT, Chew T-L, McCarthy RJ, Benzon HA, Walega DR. Comparison of the particle sizes of different steroids and the effect of dilution: a review of the relative neurotoxicities of the steroids. Anesthesiology. (2007) 106:331–8. doi: 10.1097/00000542-200702000-00022
Keywords: arthroscopic shoulder surgery, interscalene nerve block, adjuvants, dexamethasone, Bayesian network meta-analysis
Citation: Wei X-M, Liu Z, Lv L-C, Wu G-H, Sun P-Y, Gu C-P and Shi P-C (2023) Comparison of dexmedetomidine and dexamethasone as adjuvants to the ultrasound-guided interscalene nerve block in arthroscopic shoulder surgery: a systematic review and Bayesian network meta-analysis of randomized controlled trials. Front. Med. 10:1159216. doi: 10.3389/fmed.2023.1159216
Received: 05 February 2023; Accepted: 24 May 2023;
Published: 16 June 2023.
Edited by:
E. Wang, Xiangya Hospital, Central South University, ChinaReviewed by:
Guangyou Duan, Chongqing Medical University, ChinaAbhijit Nair, Ministry of Health, Oman
Copyright © 2023 Wei, Liu, Lv, Wu, Sun, Gu and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng-Cai Shi, c2hpcGVuZ2NhaTE5OTdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xiu-Min Wei
Xiu-Min Wei Zheng Liu1†
Zheng Liu1† Guang-Han Wu
Guang-Han Wu Chang-Ping Gu
Chang-Ping Gu Peng-Cai Shi
Peng-Cai Shi