
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med., 12 May 2023
Sec. Gastroenterology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1157389
This article is part of the Research TopicNew Technological Frontiers in the Diagnosis and Treatment of Gastrointestinal CancersView all 7 articles
Background: Anastomotic leakage is a serious complication after rectal cancer resection. Intraoperative use of indocyanine green fluorescence angiography (ICGFA) can help prevent anastomotic leakage, but its use is controversial. We conducted a systematic review and meta-analysis to determine the efficacy of ICGFA in reducing anastomotic leakage.
Methods: Relevant data and research published until September 30, 2022, was retrieved from the PubMed, Embase, and Cochrane Library databases, and the difference in the incidence of anastomotic leakage after rectal cancer resection between ICGFA and standard treatment was compared.
Results: This meta-analysis included 22 studies with a total of 4,738 patients. The results showed that ICGFA use during surgery decreased the incidence of anastomotic leakage after rectal cancer surgery [risk ratio (RR) = 0.46; 95% confidence interval (95% CI), 0.39–0.56; p < 0.001]. Simultaneously, in subgroup analyses for different regions, ICGFA was found to be used to reduce the incidence of anastomotic leakage after rectal cancer surgery in Asia (RR = 0.33; 95% CI, 0.23–0.48; p < 0.00001) and Europe (RR = 0.38; 95% CI, 0.27–0.53; p < 0.00001) but not in North America (RR = 0.72; 95% CI, 0.40–1.29; p = 0.27). Regarding different levels of anastomotic leakage, ICGFA reduced the incidence of postoperative type A anastomotic leakage (RR = 0.25; 95% CI, 0.14–0.44; p < 0.00001) but did not reduce the incidence of type B (RR = 0.70; 95% CI, 0.38–1.31; p = 0.27) and type C (RR = 0.97; 95% CI, 0.51–1.97; p = 0.93) anastomotic leakages.
Conclusion: ICGFA has been linked to a reduction in anastomotic leakage after rectal cancer resection. However, multicenter randomized controlled trials with larger sample sizes are required for further validation.
Rectal cancer morbidity and mortality are increasing (1). With the advancement in pathophysiology, current individualized treatments for rectal cancer include endoscopic, local surgical resection, systemic treatment, preoperative radiotherapy, local ablation treatment for metastatic tumors, extensive surgery for local and metastatic diseases, targeted therapy, palliative chemotherapy, and immunotherapy, whereas surgery is the cornerstone of curative intent treatment (1). However, rectal cancer patients may face many complications after resection, one of which is anastomotic leakage.
Despite significant advances in surgical techniques and perioperative management in recent years, the incidence of anastomotic leakage after rectal cancer surgery remains between 5 and 19% (2). The occurrence of anastomotic leakage will result in prolonged hospitalization, increased hospitalization costs, increased local recurrence rate, and shortened survival time (3–5). Studies have shown that the risk factors for anastomotic leakage have been determined by age, male gender, smoking, diabetes, previous radiotherapy and chemotherapy, intraoperative complications, anastomotic tension, and low perfusion (2, 6, 7). Among these, insufficient blood perfusion at the anastomosis plays an important role in the pathogenesis of anastomotic leakage (7).
Indocyanine green fluorescent angiography (ICGFA) is widely used in many surgical fields (8–10), including gastrointestinal surgery. The ICGFA allows the surgeon to visualize the blood supply of the intestinal canal and avoid inadequate perfusion of the anastomosis during colorectal surgery. The doctor injected diluted indocyanine green (ICG) into the venous system and monitored the development signal with a fluorescent laparoscope. When the ICG enters the anastomosis area to be observed, the doctor can dynamically observe the blood supply of the colorectal anastomosis with the fluorescent display. Therefore, intraoperative ICG use for intestinal anastomosis blood supply imaging could theoretically become a predictive test to evaluate anastomotic perfusion, reducing the risk of anastomotic leakage. Some meta-analyses have recently revealed that ICGFA can help reduce the incidence of anastomotic leakage after rectal cancer surgery (11–13). However, most of them are not convincing due to the limited number of good qualities RCT studies included and the small number of patients.
According to recently published research, we updated this meta-analysis to evaluate whether this technique can reduce the anastomotic leakage rate of rectal cancer patients after resection.
This systematic review and meta-analysis is based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statements (PRISMA) (14). Because this study is a meta-analysis, neither institutional review committee approval nor patient-informed consent was required.
We conducted a comprehensive and systematic search of electronic databases, including PubMed, Embase, and the Cochrane Library; retrieved relevant research published until September 30, 2022; and identified potential articles using the following keyword combinations: (“indocyanine green” OR “fluorescein angiography” OR “fluorescence imaging” OR “indocyanine green-sulfo-OSu” OR “ICG”) AND (“Rectal Neoplasm” OR “Rectal Tumor” OR “Rectal Cancer” OR “Rectum Cancer”). Simultaneously, we searched the reference list and previous comments in these studies for more comprehensive studies that could be included. Two authors (SJ-X and WJ-W) worked independently on this study.
Anastomotic leakage was defined as a defect in the anastomotic region where there is communication between the intraluminal and extraluminal chambers. Anastomotic leakage was graded by the International Research Group on Rectal Cancer based on its clinical symptoms. Type A had the mildest clinical symptoms, and type C had the most severe clinical symptoms (15). Type A anastomotic leakage is referred to as subclinical leakage or imaging leakage, whereas type B and C anastomotic leakages are referred to as clinical leakage or significant leakage.
The inclusion criteria of this study were as follows: (a) patients undergoing rectal cancer surgery; (b) the report result compares the incidence of anastomotic leakage between the ICGFA group and the control group; and (c) the study design was a randomized controlled trial (RCT), prospective trial, or retrospective trial.
The exclusion criteria were as follows: (a) the study was published in the form of comments, case reports, and letters; (b) the data were insufficient or could not be obtained from the author for meta-analysis; and (c) all inconsistent articles that were ruled by the third examiner (Y-L).
The two authors (SJ-X and LD-L) conducted independent research and were selected to extract data at the same time based on the inclusion and exclusion criteria. Each included article provided the following information: first author, publication year, research country, research design, and the number of anastomotic leakages.
After reading the full text of each included study, the two authors (LJ-M and LC-Y) independently evaluated the quality of the study using the Newcastle Ottawa Scale (NOS). The NOS includes the following four areas: patient selection quality, exposure determination, group comparability, and patient results. The total NOS score ranges from 0 to 9, with a score ≥ 6 indicating high quality.
The purpose of this study was to compare the incidence of anastomotic leakages in patients undergoing rectal cancer surgery with and without ICG. The secondary analysis was a subgroup analysis, which included the difference in the incidence of anastomotic leakages between regions and grades.
This study is primarily based on binary data. The risk ratio (RR) is used as the effect measure, with a 95% confidence interval. All statistical tests had a significance level of p < 0.05 (double-tailed). I2 statistics was used to determine heterogeneity, with results ranging from 0 to 100%. When I2 = 0%, heterogeneity was assumed as not observed; I2 = 25%, heterogeneity was low; I2 = 50%, heterogeneity was medium; and I2 = 75%, heterogeneity was high. When I2 < 50%, a fixed model effect was used. The random model effect was used for all other cases. This study was conducted using the Review Manager software version 5.3 (Nordic Cochrane Center, Cochrane Collaboration, London, UK).
The detailed process of literature retrieval and screening is shown in Figure 1. A total of 1,007 papers were retrieved. After reading the title and abstract, we excluded 531 studies because they did not meet our inclusion criteria. A total of 22 studies (16–37) met the inclusion criteria among the remaining 94 potential included studies. From 2013 to 2021, 22 studies involving 4,738 patients from 10 countries were published. The sample size of the study ranged from 38 to 657 people. Three RCTs, three prospective studies, and sixteen retrospective studies were conducted. We scored 22 studies from 5 to 9 on the NOS, with 19 (86.4%) rated as high quality. Table 1 shows the basic information included in the study.
The combined results of 22 studies revealed that the total anastomotic leakage rates of the ICG and non-ICG groups were 3.7 and 7.6%, respectively (RR = 0.46; 95% CI, 0.39–0.56; p < 0.001; Figure 2).
As in the summary analysis, a similar relationship was observed for grade A anastomotic leakages but not for grades B and C. Grade A anastomotic leakage rate was 2.4% in the ICG group and 7.9% in the non-ICG group (RR = 0.25; 95% CI, 0.14–0.44; p < 0.00001); grade B: 2.9 and 3.9%, respectively (RR = 0.70; 95% CI, 0.38–1.31; p = 0.27); and grade C: 2.5 and 2.8%, respectively (RR = 0.97; 95% CI, 0.51–1.97; p = 0.93; Figure 3).
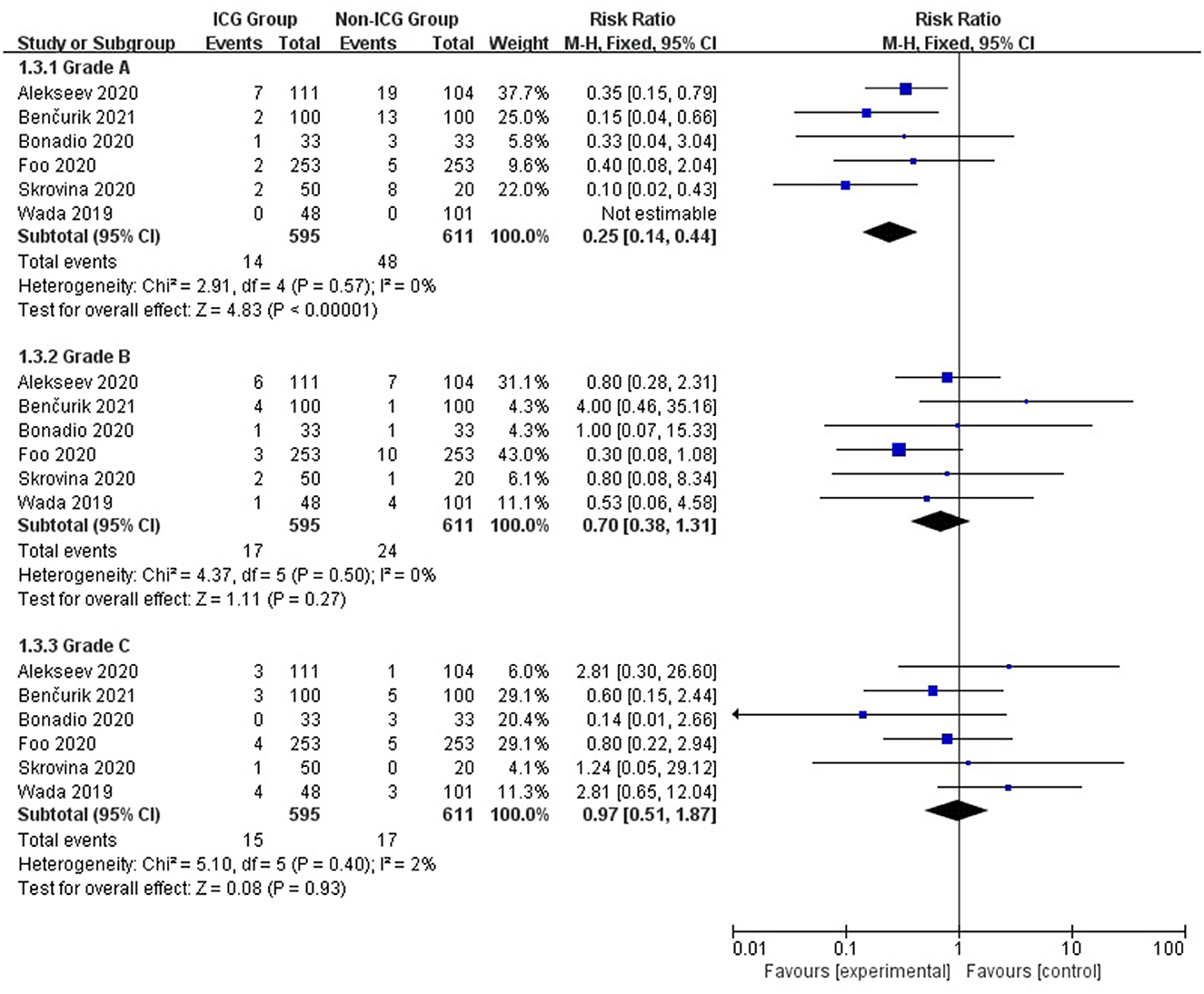
Figure 3. Forest plot of anastomotic leak grade occurrence in the ICG vs. non-ICG groups with sub-analysis.
The subgroup analysis of 22 studies from different regions revealed that the rate of anastomotic leakage in the ICG group was 3.2% in Asia compared with 11.4% in the non-ICG group (RR = 0.33; 95% CI, 0.23–0.48; p < 0.00001), 6.5 and 14.1% in Europe (RR = 0.38; 95% CI, 0.27–0.53; p < 0.00001), and 6.0 and 9.4% in North America (RR = 0.72; 95% CI, 0.40–1.29; p = 0.27; Figure 4).
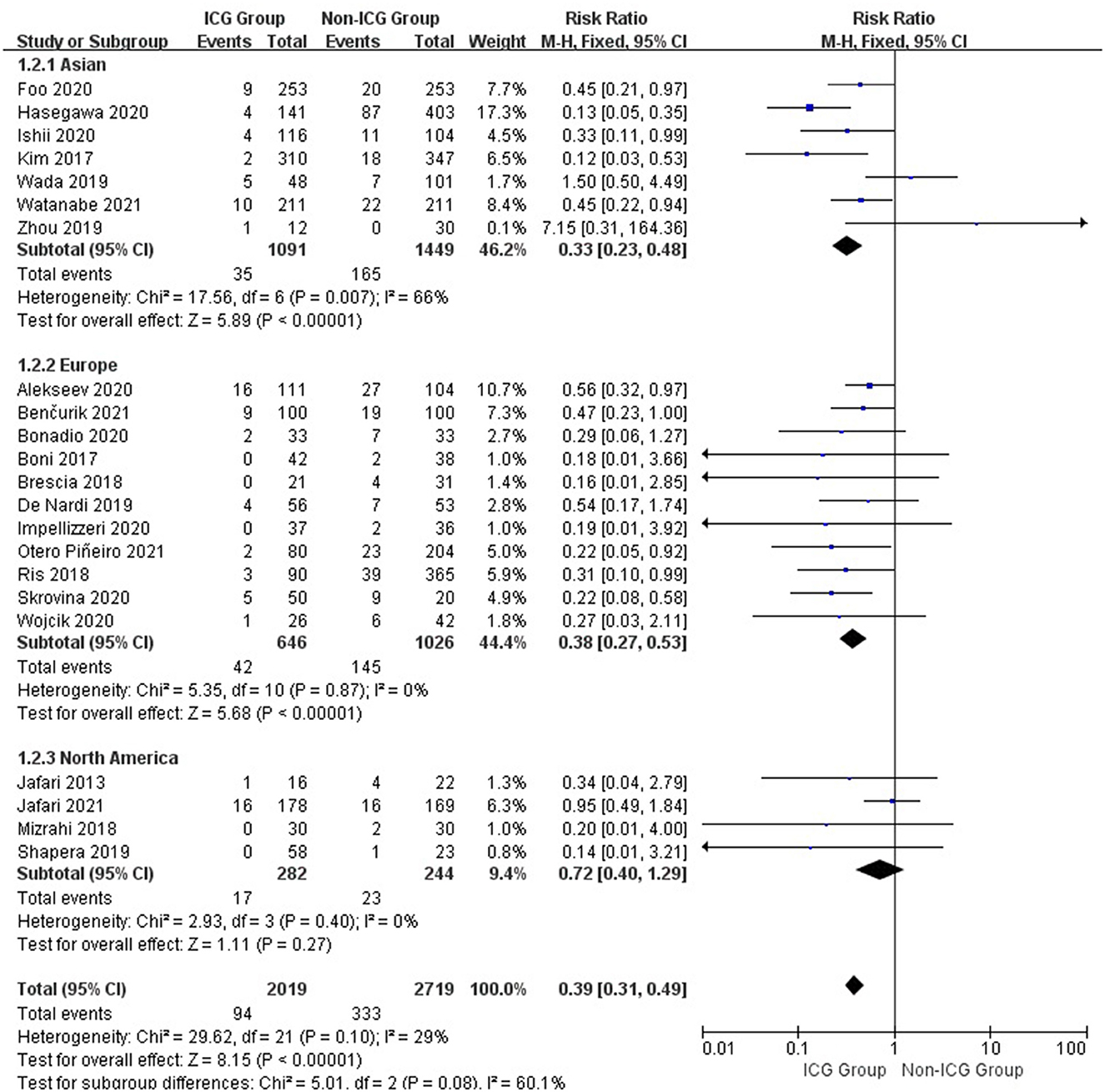
Figure 4. Forest plot of anastomotic leakage grade occurrence with different regions in the ICG vs. non-ICG groups with sub-analysis.
Funnel plots were used to assess potential publication bias in a meta-analysis of the relationship between ICG use and anastomotic leakages. The funnel plot is symmetrical, as shown in Figure 5, indicating that the risk of deviation published in this study is low.
The largest meta-analysis on the impact of ICGFA on anastomotic leakage in patients with rectal cancer surgery included 22 articles and 4,738 patients. The results of a pooled analysis of these patients revealed that the use of ICGFA during surgery was associated with a lower incidence of anastomotic leakage after rectal cancer resection. The results of a subgroup analysis of these patients revealed that the use of ICGFA during surgery in Asia and Europe was associated with a lower incidence of anastomotic leakage after rectal cancer resection and the use of ICGFA during surgery was associated with a lower incidence of grade A anastomotic leakage after rectal cancer resection.
The negative impact of anastomotic leakage after rectal cancer surgery cannot be ignored. Anastomotic leakage will result in prolonged hospitalization, increased hospitalization costs, increased local recurrence rate, and shortened survival time (38). Many studies have determined that anastomotic leakage is an independent risk factor for low long-term survival rate of patients with rectal cancer (39, 40). The study shows that anastomotic leakage risk factors have been determined in many situations, such as age, male gender, smoking, diabetes, previous radiotherapy and chemotherapy, intraoperative complications, anastomotic tension, and low perfusion. These risk factors are partly related to patients, tumors, and surgery. Some risk factors are objective and cannot be changed, whereas other risk factors can be changed. Anastomotic perfusion is one of the few variables that can be changed.
ICG is a biocompatible near-infrared contrast agent that can be excited by external light with a wavelength of 750–800 nm and emit near-infrared light with a longer wavelength, allowing tissues and organs to develop. Because Nagata first used ICG in colorectal surgery in 2006, this technology has demonstrated significant research value and promising application prospects in the auxiliary diagnosis and treatment of colorectal cancer (41, 42). It has been reported in the literature that ICG near-infrared imaging technology can improve the visualization of tumor lesions, improve the detection rate of lymph nodes, and reduce the incidence of anastomotic leakage in laparoscopic colorectal cancer surgery (42).
The ICG near-infrared imaging technique is used to evaluate the intestinal blood supply at the anastomotic site during colorectal cancer surgery. Before the proximal intestinal wall is disconnected, the operator can use clinical judgment to select the resection line under white light or visible light and mark the “pre-resection line” on the intestinal wall without damage. However, it should be noted that unipolar or bipolar electrical equipment should be used to burn the mark to avoid damaging the local blood supply of the intestinal wall and ultimately affecting the accuracy of the assessment. After the location of intestinal resection was determined, ICG was injected intravenously for the first time, and vascular perfusion was monitored using a near-infrared camera system. If the ICG vascular perfusion imaging is good within 60 s, the intestinal blood supply is likely to be adequate. ICG has a median development time of 35 (29–44) s and a duration of 3 min. Record the boundary between perfusion and non-perfusion tissues of the intestinal tube and compare it with the previously marked “pre-resection line” of the intestinal tube before cutting the intestinal wall along the ischemic line. If the blood perfusion at the “pre-resection line” of the intestinal tube is deemed insufficient, the “pre-resection line” at the proximal end of the intestinal tube should be moved to a position with adequate blood perfusion. ICG was injected intravenously again after intestinal anastomosis. The perfusion after anastomosis was evaluated using a fluorescence system, and the blood supply and appearance of the intestinal wall were observed to determine whether the surgical strategy should be changed and intestinal anastomosis performed again.
The use of ICGFA in assessing anastomotic perfusion has been concerned in recent years due to its relative ease of use, low cost, and satisfactory safety (17, 21). Recently published meta-analyses (11–13) showed that the risk of anastomotic leakage after rectal surgery in patients with ICGFA was significantly reduced, which was consistent with our results. However, due to the limited number of articles included, the majority of the studies are inconclusive. In comparison to these studies, the advantage of our meta-analysis lies in the inclusion of all relevant RCT, prospective, and retrospective studies, allowing us to analyze a larger sample size than previous meta-analyses and produce more convincing research results. Simultaneously, our research object is more specific and singular, which is patients with rectal cancer undergoing surgical treatment. In patients undergoing rectal cancer resection, the incidence of anastomotic leakage is higher than in patients undergoing colon cancer surgery. Therefore, this group is most likely to benefit from this intervention.
According to a summary analysis of all patients, the use of ICGFA during surgery is associated with a reduction of anastomotic leakage after rectal cancer resection (RR = 0.25; 95% CI, 0.14–0.44; p < 0.00001). We found that using ICGFA during surgery was associated with a reduction in the incidence of anastomotic leakage after rectal cancer resection in Asia and Europe (RR = 0.33; 95% CI, 0.23–0.48; p < 0.00001 and RR = 0.38; 95% CI, 0.27–0.53; p < 0.00001) but not in North America (RR = 0.72; 95% CI, 0.40–1.29; p = 0.27). We believe that this is due to fewer studies and a smaller sample size, which causes heterogeneity in the analysis of results. The use of ICGFA during surgery is associated with a reduction in the incidence of grade A anastomotic leakage after rectal cancer resection (RR = 0.25; 95% CI, 0.14–0.44; p < 0.00001), but there is no difference in the incidence of grades B and C anastomotic leakages (RR = 0.70; 95% CI, 0.38–1.31; p = 0.27 and RR = 0.97; 95% CI, 0.51–1.97; p = 0.93). Therefore, we can conclude that ICGFA cannot reduce the occurrence of type B and C anastomotic leakages after rectal cancer resection. Because both the ICGFA intervention and control groups had a low incidence of types B and C anastomotic leakage, ICGFA could not reduce the incidence of types B and C anastomotic leakage after rectal cancer resection.
At the same time, we recognize that this study has some limitations. First, the diagnosis of anastomotic leakage varied across the studies. Some studies were diagnosed solely through radiology, whereas others were diagnosed through physical examination and endoscopy as well. Second, the dose of ICG administered and timing of the included studies were inconsistent. Third, restricted by the articles included, this study cannot be analyzed according to the level of rectal cancer. Finally, the number of RCTs in the study is insufficient, which may increase the risk of deviation. These limitations may result in heterogeneity in the analysis of research findings.
In conclusion, the results of this systematic review and meta-analysis show that using ICGFA during rectal cancer surgery is associated with a significant reduction in AL. However, given the limitations mentioned above, more multicenter RCT studies with a large sample size are required in the future to demonstrate our research findings.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://pubmed.ncbi.nlm.nih.gov/.
SX: analysis, interpretation of data, and drafting the article or revising it critically for important intellectual content. WW: conception and design and final approval of the version to be published. LL, LM, LY, and YL: acquisition of data. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. (2019) 394:1467–80. doi: 10.1016/S0140-6736(19)32319-0
2. McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg. (2015) 102:462–79. doi: 10.1002/bjs.9697
3. Ptok H, Marusch F, Meyer F, Schubert D, Gastinger I, Lippert H, et al. Impact of anastomotic leakage on oncological outcome after rectal cancer resection. Br J Surg. (2007) 94:1548–54. doi: 10.1002/bjs.5707
4. Merkel S, Wang WY, Schmidt O, Dworak O, Wittekind C, Hohenberger W, et al. Locoregional recurrence in patients with anastomotic leakage after anterior resection for rectal carcinoma. Colorectal Dis. (2001) 3:154–60. doi: 10.1046/j.1463-1318.2001.00232.x
5. Ha GW, Kim JH, Lee MR. Oncologic impact of anastomotic leakage following colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg Oncol. (2017) 24:3289–99. doi: 10.1245/s10434-017-5881-8
6. Chadi SA, Fingerhut A, Berho M, DeMeester SR, Fleshman JW, Hyman NH, et al. Emerging trends in the etiology, prevention, and treatment of gastrointestinal anastomotic leakage. J Gastrointest Surg. (2016) 20:2035–51. doi: 10.1007/s11605-016-3255-3
7. Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V. Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum. (2000) 43:76–82. doi: 10.1007/BF02237248
8. Kusano M, Tajima Y, Yamazaki K, Kato M, Watanabe M, Miwa M. Sentinel node mapping guided by indocyanine green fluorescence imaging: a new method for sentinel node navigation surgery in gastrointestinal cancer. Dig Surg. (2008) 25:103–8. doi: 10.1159/000121905
9. Lin J, Lin LS, Chen DR, Lin KJ, Wang YF, Chang YJ. Indocyanine green fluorescence method for sentinel lymph node biopsy in breast cancer. Asian J Surg. (2020) 43:1149–53. doi: 10.1016/j.asjsur.2020.02.003
10. Lu J, Huang CM. Exploration and development of indocyanine green fluorescence applied in laparoscopic splenic hilum lymph node dissection for gastric cancer. Zhonghua Zhong Liu Za Zhi. (2019) 41:900–3. doi: 10.3760/cma.j.issn.0253-3766.2019.12.004
11. Pang HY, Chen XL, Song XH, Galiullin D, Zhao LY, Liu K, et al. Indocyanine green fluorescence angiography prevents anastomotic leakage in rectal cancer surgery: a systematic review and meta-analysis. Langenbecks Arch Surg. (2021) 406:261–71. doi: 10.1007/s00423-020-02077-6
12. Song M, Liu J, Xia D, Yao H, Tian G, Chen X, et al. Assessment of intraoperative use of indocyanine green fluorescence imaging on the incidence of anastomotic leakage after rectal cancer surgery: a PRISMA-compliant systematic review and meta-analysis. Tech Coloproctol. (2021) 25:49–58. doi: 10.1007/s10151-020-02335-1
13. Li Z, Zhou Y, Tian G, Liu Y, Jiang Y, Li X, et al. Meta-analysis on the efficacy of indocyanine green fluorescence angiography for reduction of anastomotic leakage after rectal cancer surgery. Am Surg. (2021) 87:1910–9. doi: 10.1177/0003134820982848
14. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
15. Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. (2010) 147:339–51 doi: 10.1016/j.surg.2009.10.012
16. Jafari MD, Lee KH, Halabi WJ, Mills SD, Carmichael JC, Stamos MJ, et al. The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc. (2013) 27:3003–8. doi: 10.1007/s00464-013-2832-8
17. Kim JC, Lee JL, Park SH. Interpretative guidelines and possible indications for indocyanine green fluorescence imaging in robot-assisted sphincter-saving operations. Dis Colon Rectum. (2017) 60:376–84. doi: 10.1097/DCR.0000000000000782
18. Boni L, Fingerhut A, Marzorati A, Rausei S, Dionigi G, Cassinotti E. Indocyanine green fluorescence angiography during laparoscopic low anterior resection: results of a case-matched study. Surg Endosc. (2017) 31:1836–40. doi: 10.1007/s00464-016-5181-6
19. Brescia A, Pezzatini M, Romeo G, Cinquepalmi M, Pindozzi F, Dall'Oglio A, et al. Indocyanine green fluorescence angiography: a new ERAS item. Updates Surg. (2018) 70:427–32. doi: 10.1007/s13304-018-0590-9
20. Mizrahi I, Abu-Gazala M, Rickles AS, Fernandez LM, Petrucci A, Wolf J, et al. Indocyanine green fluorescence angiography during low anterior resection for low rectal cancer: results of a comparative cohort study. Tech Coloproctol. (2018) 22:535–40. doi: 10.1007/s10151-018-1832-z
21. Ris F, Liot E, Buchs NC, Kraus R, Ismael G, Belfontali V, et al. Multicentre phase II trial of near-infrared imaging in elective colorectal surgery. Br J Surg. (2018) 105:1359–67. doi: 10.1002/bjs.10844
22. Shapera E, Hsiung RW. Assessment of anastomotic perfusion in left-sided robotic assisted colorectal resection by indocyanine green fluorescence angiography. Minim Invasive Surg. (2019) 2019:3267217. doi: 10.1155/2019/3267217
23. Watanabe J, Ishibe A, Suwa Y, Suwa H, Ota M, Kunisaki C, et al. Indocyanine green fluorescence imaging to reduce the risk of anastomotic leakage in laparoscopic low anterior resection for rectal cancer: a propensity score-matched cohort study. Surg Endosc. (2020) 34:202–8. doi: 10.1007/s00464-019-06751-9
24. De Nardi P, Elmore U, Maggi G, Maggiore R, Boni L, Cassinotti E, et al. Intraoperative angiography with indocyanine green to assess anastomosis perfusion in patients undergoing laparoscopic colorectal resection: results of a multicenter randomized controlled trial. Surg Endosc. (2020) 34:53–60. doi: 10.1007/s00464-019-06730-0
25. Wada T, Kawada K, Hoshino N, Inamoto S, Yoshitomi M, Hida K, et al. The effects of intraoperative ICG fluorescence angiography in laparoscopic low anterior resection: a propensity score-matched study. Int J Clin Oncol. (2019) 24:394–402. doi: 10.1007/s10147-018-1365-5
26. Zhou SC, Tian YT, Wang XW, Zhao CD, Ma S, Jiang J, et al. Application of indocyanine green-enhanced near-infrared fluorescence-guided imaging in laparoscopic lateral pelvic lymph node dissection for middle-low rectal cancer. World J Gastroenterol. (2019) 25:4502–11. doi: 10.3748/wjg.v25.i31.4502
27. Alekseev M, Rybakov E, Shelygin Y, Chernyshov S, Zarodnyuk I. A study investigating the perfusion of colorectal anastomoses using fluorescence angiography: results of the FLAG randomized trial. Colorectal Dis. (2020) 22:1147–53. doi: 10.1111/codi.15037
28. Bonadio L, Iacuzzo C, Cosola D, Cipolat Mis T, Giudici F, Casagranda B, et al. Indocyanine green-enhanced fluorangiography (ICGf) in laparoscopic extraperitoneal rectal cancer resection. Updates Surg. (2020) 72:477–82. doi: 10.1007/s13304-020-00725-6
29. Foo CC, Ng KK, Tsang J, Wei R, Chow F, Chan TY, et al. Colonic perfusion assessment with indocyanine-green fluorescence imaging in anterior resections: a propensity score-matched analysis. Tech Coloproctol. (2020) 24:935–42. doi: 10.1007/s10151-020-02232-7
30. Hasegawa H, Tsukada Y, Wakabayashi M, Nomura S, Sasaki T, Nishizawa Y, et al. Impact of intraoperative indocyanine green fluorescence angiography on anastomotic leakage after laparoscopic sphincter-sparing surgery for malignant rectal tumors. Int J Colorectal Dis. (2020) 35:471–80. doi: 10.1007/s00384-019-03490-0
31. Impellizzeri HG, Pulvirenti A, Inama M, Bacchion M, Marrano E, Creciun M, et al. Near-infrared fluorescence angiography for colorectal surgery is associated with a reduction of anastomotic leak rate. Updates Surg. (2020) 72:991–8. doi: 10.1007/s13304-020-00758-x
32. Skrovina M, Bencurik V, Martinek L, Machackova M, Bartos J, Andel P, et al. The significance of intraoperative fluorescence angiography in miniinvasive low rectal resections. Wideochir Inne Tech Maloinwazyjne. (2020) 15:43–8. doi: 10.5114/wiitm.2019.84851
33. Wojcik M, Doussot A, Manfredelli S, Duclos C, Paquette B, Turco C, et al. Intra-operative fluorescence angiography is reproducible and reduces the rate of anastomotic leak after colorectal resection for cancer: a prospective case-matched study. Colorectal Dis. (2020) 22:1263–70. doi: 10.1111/codi.15076
34. Ishii M, Hamabe A, Okita K, Nishidate T, Okuya K, Usui A, et al. Efficacy of indocyanine green fluorescence angiography in preventing anastomotic leakage after laparoscopic colorectal cancer surgery. Int J Colorectal Dis. (2020) 35:269–75. doi: 10.1007/s00384-019-03482-0
35. Benčurik V, Škrovina M, Martínek L, Bartoš J, Macháčková M, Dosoudil M, et al. Intraoperative fluorescence angiography and risk factors of anastomotic leakage in mini-invasive low rectal resections. Surg Endosc. (2021) 35:5015–23. doi: 10.1007/s00464-020-07982-x
36. Jafari MD, Pigazzi A, McLemore EC, Mutch MG, Haas E, Rasheid SH, et al. Perfusion assessment in left-sided/low anterior resection (PILLAR III): a randomized, controlled, parallel, multicenter study assessing perfusion outcomes with PINPOINT near-infrared fluorescence imaging in low anterior resection. Dis Colon Rectum. (2021) 64:995–1002. doi: 10.1097/DCR.0000000000002007
37. Otero-Piñeiro AM, de Lacy FB, Van Laarhoven JJ, Martín-Perez B, Valverde S, Bravo R, et al. The impact of fluorescence angiography on anastomotic leak rate following transanal total mesorectal excision for rectal cancer: a comparative study. Surg Endosc. (2021) 35:754–62. doi: 10.1007/s00464-020-07442-6
38. Kryzauskas M, Bausys A, Degutyte AE, Abeciunas V, Poskus E, Bausys R, et al. Risk factors for anastomotic leakage and its impact on long-term survival in left-sided colorectal cancer surgery. World J Surg Oncol. (2020) 18:205. doi: 10.1186/s12957-020-01968-8
39. Lu ZR, Rajendran N, Lynch AC, Heriot AG, Warrier SK. Anastomotic leaks after restorative resections for rectal cancer compromise cancer outcomes and survival. Dis Colon Rectum. (2016) 59:236–44. doi: 10.1097/DCR.0000000000000554
40. Wang S, Liu J, Wang S, Zhao H, Ge S, Wang W. Adverse effects of anastomotic leakage on local recurrence and survival after curative anterior resection for rectal cancer: a systematic review and meta-analysis. World J Surg. (2017) 41:277–84. doi: 10.1007/s00268-016-3761-1
41. Nagata K, Endo S, Hidaka E, Tanaka J, Kudo SE, Shiokawa A. Laparoscopic sentinel node mapping for colorectal cancer using infrared ray laparoscopy. Anticancer Res. (2006) 26:2307–11.
Keywords: anastomotic leakage, fluorescence angiography, indocyanine green, meta-analysis, rectal cancer
Citation: Xia S, Wu W, Luo L, Ma L, Yu L and Li Y (2023) Indocyanine green fluorescence angiography decreases the risk of anastomotic leakage after rectal cancer surgery: a systematic review and meta-analysis. Front. Med. 10:1157389. doi: 10.3389/fmed.2023.1157389
Received: 02 February 2023; Accepted: 21 April 2023;
Published: 12 May 2023.
Edited by:
Giuseppe Quero, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Akira Umemura, Iwate Medical University, JapanCopyright © 2023 Xia, Wu, Luo, Ma, Yu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenjiang Wu, MTA1MzY2MDY0NUBxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.