
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 17 April 2023
Sec. Family Medicine and Primary Care
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1153670
A correction has been applied to this article in:
Corrigendum: Risk factors and 180-day mortality of acute kidney disease in critically ill patients: a multi-institutional study
Background: Critically ill patients with acute kidney injury (AKI) have a poor prognosis. Recently, the Acute Disease Quality Initiative (ADQI) proposed to define acute kidney disease (AKD) as acute or subacute damage and/or loss of kidney function post AKI. We aimed to identify the risk factors for the occurrence of AKD and to determine the predictive value of AKD for 180-day mortality in critically ill patients.
Methods: We evaluated 11,045 AKI survivors and 5,178 AKD patients without AKI, who were admitted to the intensive care unit between 1 January 2001 and 31 May 2018, from the Chang Gung Research Database in Taiwan. The primary and secondary outcomes were the occurrence of AKD and 180-day mortality.
Results: The incidence rate of AKD among AKI patients who did not receive dialysis or died within 90 days was 34.4% (3,797 of 11,045 patients). Multivariable logistic regression analysis indicated that AKI severity, underlying early CKD, chronic liver disease, malignancy, and use of emergency hemodialysis were independent risk factors of AKD, while male gender, higher lactate levels, use of ECMO, and admission to surgical ICU were negatively correlated with AKD. 180-day mortality was highest among AKD patients without AKI during hospitalization (4.4%, 227 of 5,178 patients), followed by AKI with AKD (2.3%, 88 of 3,797 patients) and AKI without AKD (1.6%, 115 of 7,133 patients). AKI with AKD had a borderline significantly increased risk of 180-day mortality (aOR 1.34, 95% CI 1.00–1.78; p = 0.047), while patients with AKD but no preceding AKI episodes had the highest risk (aOR 2.25, 95% CI 1.71–2.97; p < 0.001).
Conclusion: The occurrence of AKD adds limited additional prognostic information for risk stratification of survivors among critically ill patients with AKI but could predict prognosis in survivors without prior AKI.
Acute kidney injury has been recognized as a major global health concern (1, 2). Due to the aging population and increase in associated comorbidities, the incidence of acute kidney injury (AKI) is growing among ICU patients (3). The pathophysiology of AKI is multifactorial and is also attributed to dysfunction in other organs, such that AKI is often involved in multiple organ failure syndrome (4). In the literature, the occurrence of AKI is associated with higher risk of multiple comorbidities, chronic kidney disease (CKD), and short-and long-term mortality (4–8). However, kidney damage may persist after an episode of AKI has ended. Recently, the Acute Disease Quality Initiative (ADQI) proposed to define acute kidney disease (AKD) as acute or subacute damage and/or loss of kidney function that lasts 7–90 days post AKI (9).
AKD can include patients with evolving kidney disease that might not fulfil the strict criteria for AKI, and patients with AKI who have prolonged kidney dysfunction but do not fulfil the criteria for CKD (10). AKD is a growing concern; several previous studies had shown that AKD without AKI, and AKD following AKI, are both associated with significantly worse outcomes compared to AKI patients with renal recovery (1, 2, 11–15). Nevertheless, the epidemiology of critically ill patients with AKD after AKI is still very limited. Furthermore, whether the occurrence of AKD adds additional prognostic information after AKI remains unclear.
To address the knowledge gaps in the current understanding of AKD, we conducted a multi-institutional study of critically ill patients in Taiwan. The purpose of this study was to identify critically ill patients who are likely to develop AKD, and to further examine whether the occurrence of AKD adds prognostic information in addition to AKI stage in predicting 180-day mortality.
We used the Chang Gung Research Database (CGRD) as the data source in this study. The CGRD is a collection of daily medical records that have been prospectively collected from seven branches of Chang Gung Memorial Hospital in Taiwan since January 2001 and covers an annual average of 500,000 emergency department visits, 8,500,000 outpatient visits, and over 280,000 admissions to 10,070 beds. In 2015, outpatient and inpatient records comprised 6.1 and 10.2% of the CGRD, respectively (16). The abundance of medical information available from the CGRD makes it a good source for the conduct of retrospective clinical studies (16–18). The CGRD collects the patient’s personal information, including gender, body weight, height, lifestyle, and birth date. In addition, laboratory findings (e.g., serum creatinine [sCr], blood nitrogen urea [BUN], etc.), results of imaging exams, and comprehensive information regarding every emergency visit, inpatient admission, and outpatient visit, are available in the CGRD. The International Classification of Diseases, 9th and 10th revision, Clinical Modification (ICD-9-CM and ICD-10-CM) codes were used to categorize patients’ underlying diseases and the reasons for admission and emergency and outpatient visits. This information was stored digitally. The chart number of each patient was securely encrypted to protect personal privacy and was only used to link data between different databases in the CGRD.
The study protocol is shown in Figure 1, and the data were retrospectively analyzed. All admissions to the intensive care unit (ICU) between January 2001 and May 2018 were obtained from the CGRD (n = 377,823). Subjects excluded from analysis were those with ICU admission for less than 2 days (n = 50,386), existing ESRD (n = 24,975), history of kidney transplant (n = 615), age outside the limits set in this study and/or missing age data (n = 2,050), and missing sCr data during the 180-day follow-up period (n = 269,145). Two cohorts were defined in this study. The first cohort consisted of ICU patients who had survived AKI (n = 11,045) and was used to explore the predictive covariates for the occurrence of AKD. The second cohort consisted of AKD patients without prior AKI during ICU admission. Patients from both cohorts were used to explore predictors of 180-day mortality in the following clinical scenarios: AKI without AKD, AKI with AKD stage 1–3, and AKD stage 1–3 with no AKI. AKI was diagnosed and classified according to the Kidney Disease: Improving Global Outcomes (KDIGO) definition, and stages were defined by the ratio of the lowest sCr value during the first 7 days of ICU admission to the highest sCr value within 3 months prior to ICU admission. Based on the results of our previous study, ratios of 1.5–2, 2–3, and >3 were used to classify AKI stage 1, 2, and 3, respectively (4). Patients who required urgent hemodialysis were also defined as having stage 3 AKI (19). The definition of AKD in this study was based on the consensus of the ADQI 16 workgroup in 2017. Fold increases of <1.5, 1.5–2, 2–3, and >3 in sCr level from baseline after AKI injury indicated AKD stage 0, 1, 2, and 3, respectively (10). Since sCr levels may fluctuate over a period of 7–90 days post AKI injury, the lowest sCr value during this period was used for the diagnosis of AKD. The 180-day mortality was defined as death of the patient, or discharge against medical advice due to being in a critically ill condition, at 90–180 days after diagnosis of AKI. To avoid potential confounding bias, patients undergoing dialysis and those who died within 90 days after AKI diagnosis were excluded. The entire study protocol was approved by the Institutional Review Board of the Chang Gung Medical Foundation (IRB No.: 201702274B0).
Diabetes mellitus (DM; 250, E10, E11), hypertension (401, 402, E10, E11), cardiovascular diseases (410, 427.31, 428, 430, 431, 433, 434, I21, I48.0, I48.2, I48.91, I50, I60, I61, I65 and I66), chronic liver diseases (571, K70), malignancies (140–175, 179–208, C00-C97 and D00-D48), and CKD (580–589, I12, I13, N00-N05, N07, N11, N14, N17,-N19 and Q61) were recognized as comorbidities if patients had at least two outpatient visits or one inpatient admission with these diagnosis codes within one year preceding the date of ICU admission (Supplementary Table S1). To assess the possible seasonal variations, the four seasons in Taiwan were divided into “spring to summer” (March to August) and “autumn to winter” (September to February). Laboratory data obtained within the first 7 days of ICU admission included sCr, serum sodium, serum albumin, fasting sugar, hemoglobin, platelet count, leukocytes, procalcitonin, c-reactive protein (CRP), lactate, B-type natriuretic peptide (BNP), total bilirubin, and prothrombin time. If multiple test results were available, the first result was used. The estimated glomerular filtration rate (eGFR) was calculated from the sCr value according to the Modification of Diet in Renal Disease equation, and the eGFR value was used to classify the CKD stages. Medication records were also collected, including angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), direct renin inhibitors (DRI), and calcium channel blockers (CCB). Patients who used these medications within one year preceding the ICU admission date were recognized as users. In addition, the qSOFA score was assessed to classify the initial disease severity at ICU admission (20). Shock and anemia were defined as mean arterial pressure ≤ 65 mmHg and hemoglobin ≤10 g/dl within 7 days before ICU admission, separately. The proportion of use of radiocontrast within 7 days before ICU admission was also obtained as the possible existence of radiocontrast-induced nephropathy. Additionally, ICU procedures that could influence renal function, including emergency hemodialysis, extracorporeal membrane oxygenation (ECMO), ventilator use, coronary artery bypass grafting (CABG), intra-aortic balloon pump (IABP), and other emergency surgeries, were recorded, as was the use of norepinephrine and dopamine during ICU admission.
Descriptive statistics were used to present the baseline differences between AKI patients with and without AKD. Categorical covariates were presented as numbers and proportions and were analyzed with χ2 tests. Continuous covariates were presented as medians with interquartile ranges (IQRs), and Mann–Whitney U tests were used to examine the differences between AKD and non-AKD patients. Univariable and multivariable logistic regression was used to evaluate the predictors for AKD occurrence among AKI patients (cohort 1), and to evaluate the odds ratios of 180-day mortality among the three different conditions in cohort 2, including AKD stage 0–3, AKI stage 0–3, and AKI without AKD, AKI with AKD, and AKD without AKI. The validity of the regression model predicting AKD, and 180-day mortality was examined by C-statistics. All statistical analyses were carried out using SAS v 9.1.3 (SAS Institute, Cary, NC) and results with value of p <0.05 were regarded as statistically significant.
Between 1 January 2001 and 31 May 2018, a total of 377,823 subjects were admitted to ICU, and 16,855 AKI events (55.1%) were identified among all eligible subjects. Within 90 days after ICU admission, 2,024 subjects received permanent dialysis and 3,786 subjects had died. A total of 11,045 AKI subjects entered the final analysis stage; stage 1 AKI was most prevalent (n = 7,284, 65.9%), followed by stage 3 (n = 2,152, 19.5%) and stage 2 AKI (n = 1,609, 14.6%). CKD was the most common comorbidity observed among all AKI patients (n = 6,081, 55.1%), and most had stage 3–5 CKD (n = 3,562, 32.2%). Most patients had hypertension (n = 5,617, 50.9%) and cardiovascular diseases (n = 4,400, 39.9%). During hospitalization, more than 90% of AKI patients were admitted to the non-surgical ICU (10,333, 93.6%), and more than 80% of patients were at low risk of multiple organ failure (qSOFA score 0–1 upon ICU admission, n = 8,993, 81.4%). However, nearly half of the patients needed ventilator support (n = 4,941, 44.7%). On the other hand, there were no differences in admission season, shock, anemia, or use of radiocontrast before admission.
After discharge, 7,248 AKI patients (65.6%) had completely recovered, whereas 3,797 patients were diagnosed with AKD stage 1–3 (34.4%, Table 1). AKD patients had a higher proportion of females than non-AKD patients (37.5% versus 31.9%, p < 0.001). AKD patients tended to be at higher AKI stages than non-AKD patients (55, 20, 25% versus 71.7, 11.7, and 16.6% for AKI stage 1, 2 and 3, respectively; p < 0.001). For comorbidities, a lower proportion of AKD patients had hypertension (46.6%) and cardiovascular diseases (37.2%) compared to non-AKD patients (53.1 and 41.2%, respectively, p < 0.001). However, compared to patients without AKD, there were more AKD patients with pre-existing stage 3–5 CKD (31.2% versus 34.2%, p < 0.001). AKD patients had marginally lower serum creatinine (1.47, IQR: 1.70 mg/dl) than non-AKD patients (1.52, IQR: 1.44 mg/dl, p < 0.001), and lower lactate levels (20, IQR: 25.9 mg/dl versus 23.7, IQR: 32.9 mg/dl; p < 0.001). No significant differences in medications were found between AKD and non-AKD patients. A greater proportion of AKD patients were admitted to the non-surgical ICU compared to non-AKD patients (95.3% versus 92.6%, p < 0.001), and more AKD patients were classified as low risk upon ICU admission (83.8% versus 80.2%, p < 0.001) (Table 2). Additionally, fewer AKD patients needed ventilator support (40.9% versus 46.7%, p < 0.001) or ECMO (4.0% versus 6.0%, p < 0.001). However, emergency hemodialysis was more common among AKD patients (4.8% versus 3.1%, p < 0.001), as was dopamine use (28.2% in AKD patients and 22.9% in non-AKD patients; p < 0.001).
Table 3 shows the unadjusted and adjusted odds ratio (OR, aOR) for AKD occurrence. When considering all covariates, a higher AKI stage was associated with the occurrence of AKD (aOR 2.15, 95% CI 1.92–2.41 for stage 2 AKI, p < 0.001; aOR 1.91, 95% CI 1.72–2.12 for stage 3 AKI, p < 0.001). Male gender was associated with a 22% lower risk of AKD occurrence (95% CI 0.72–0.85), and the older patients (aged ≥70 years) had a 1.16-fold higher risk (95% CI 1.06–1.27) than younger patients (aged <70 years). In addition, patients with early CKD had a 1.46-fold higher risk of AKD than patients with no pre-existing CKD (95% CI 1.23–1.73; p < 0.001). On the other hand, hypertension (aOR 0.83, 95% CI 0.76–0.91; p < 0.001) and cardiovascular diseases (aOR 0.91, 95% CI 0.83–0.99; p = 0.047) lowered the risk of AKD by 17 and 9%, respectively. Pre-existing chronic liver diseases (aOR 1.43, 95% CI 1.27–1.61; p < 0.001) and malignancies (aOR 1.30, 95% CI 1.14–1.47; p < 0.001) increased the risk of AKD occurrence by 43 and 30%, respectively. During ICU hospitalization, higher qSOFA scores (aOR 0.87, 95% CI 0.77–0.97; p = 0.017) and higher lactate levels (aOR 0.74, 95% CI 0.63–0.86; p < 0.001) were associated with lower risk of AKD. In addition, emergency hemodialysis (aOR 1.29, 95% CI 1.04–1.59; p = 0.019), and use of dopamine (aOR 1.14, 95% CI 1.03–1.25; p = 0.009) raised the risk of AKD by 29 and 14%, respectively, whereas ECMO (aOR 0.76, 95% CI 0.62–0.94; p = 0.012) and ventilator support (aOR 0.84, 95% CI 0.77–0.93; p < 0.001) decreased the risk of AKD.
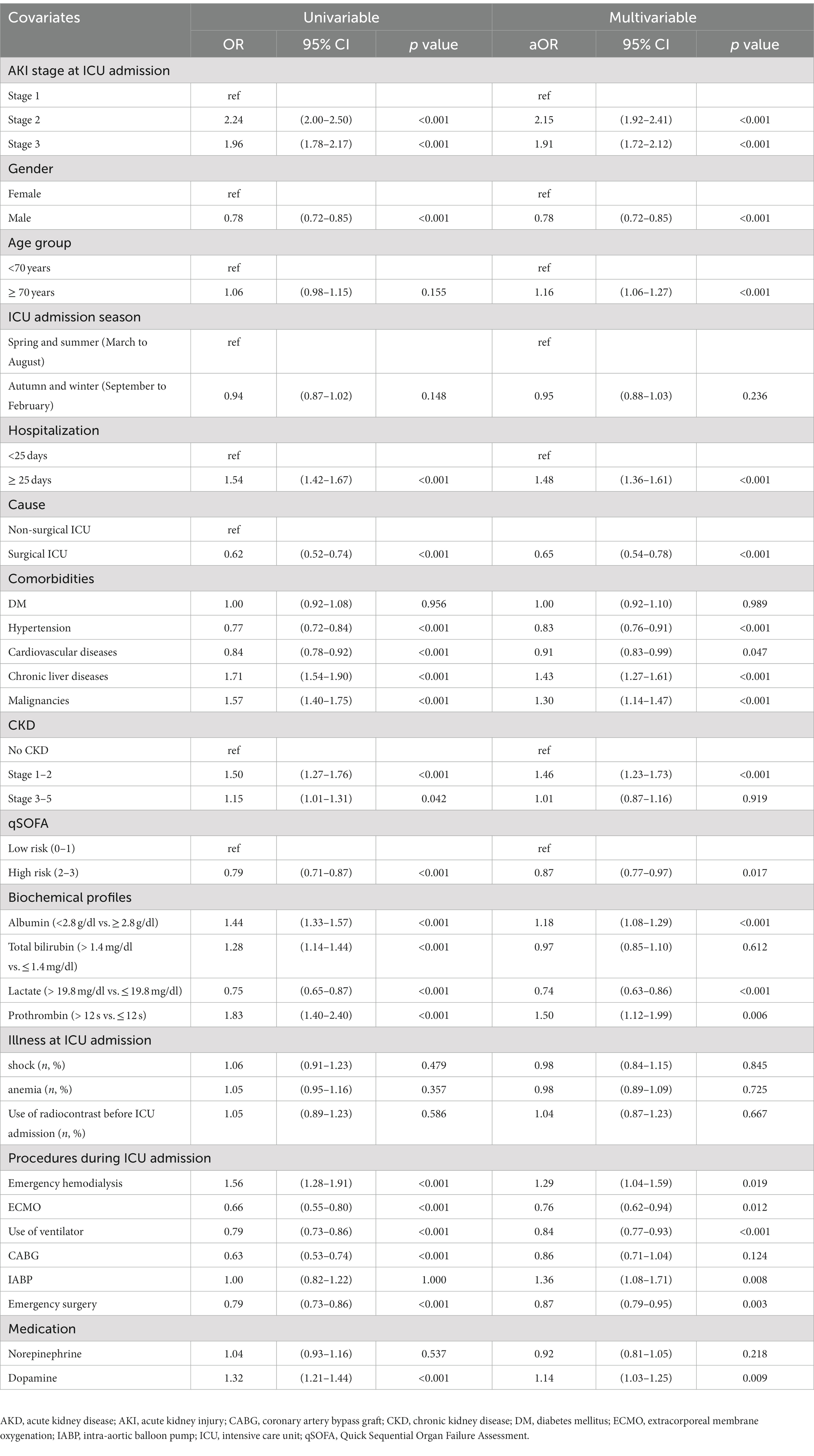
Table 3. Univariable and multivariate logistic regression of factors associated with the occurrence of AKD.
Figure 2 shows the 180-day outcomes associated with the various AKI/AKD conditions. In addition to 11,045 hospitalized AKI patients, we enrolled 5,178 patients without prior AKI events who experienced AKD within 90 days of ICU admission. The 180-day mortality rate appeared to be highest among patients with AKD without AKI events (n = 227, 4.4% of 5,178 patients), followed by AKD patients with AKI (n = 88, 2.3% of 3,797 patients) and AKI patients without AKD (n = 115, 1.6% of 7,248 patients, p < 0.001). A higher proportion of patients who died after 180 days consisted of those whose hospital stays exceeded 25 days and those with chronic liver diseases and malignancies, but fewer patients in this group had cardiovascular diseases (Supplementary Table S2). Also, a higher proportion of patients who survived at 180 days after ICU admission received emergency hemodialysis, ECMO, CABG, emergency surgery, and dopamine injection, whereas a lower proportion of surviving patients used ventilators (Supplementary Table S2). When considering the potential influence of covariates, the occurrence of AKI seemed to be negatively correlated with 180-day mortality (aOR 0.67, 95% CI 0.51–0.88, p = 0.003; aOR 0.60, 95% CI 0.39–0.92, p = 0.019; aOR 0.55, 95% CI 0.37–0.83, p = 0.004, for AKI stage 1–3, respectively) whereas AKD, especially stage 3, was a detrimental factor for 180-day mortality (aOR 1.96, 95% CI 1.25–3.06, p = 0.003) (Table 4). Compared to patients with AKI without AKD, patients who had no AKI but suffered from AKD after ICU admission had the highest risk of 180-day mortality (aOR 2.25, 95% CI 1.71–2.97; p < 0.001), even higher than that in patients with AKI and AKD (aOR 1.34, 95% CI 1.00–1.78; p = 0.047). The discrimination ability of the regression model for the prediction of 180-day mortality was within an acceptable range (area under curve = 0.702; 95% CI 0.667–0.737) (Figure 3).
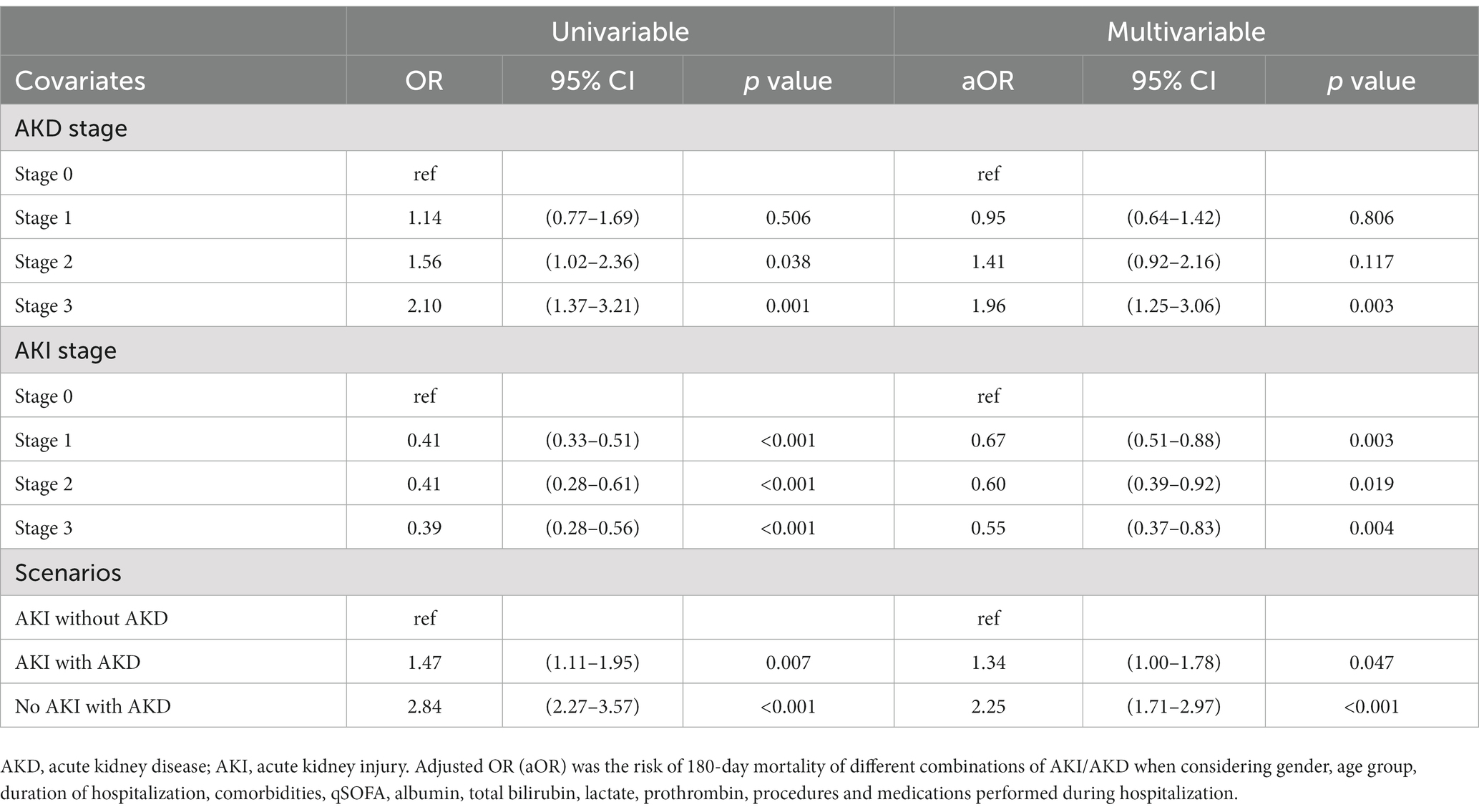
Table 4. Univariable and multivariate logistic regression of different combinations of AKD and AKI associated with 180-day mortality.
To study the clinical characteristics and outcomes of AKD, we analyzed 11,045 AKI survivors, and 5,178 AKD patients without prior AKI who were admitted to ICU. Several critical risk factors for the occurrence of AKD among AKI patients were identified. The main findings were as follows: first, the incidence of AKD after AKI was 22.5% (3,797 of 16,855). The overall 180-day mortality rate was 2.3% (88 of 3,797) in patients with AKD after AKI, and 4.4% in patients with AKD without AKI (227 of 5,178), which was consistent with previous studies (2, 12, 21–24). Second, several independent risk factors for the development of AKD were identified: severity of AKI, female gender, being older, preexisting early CKD, underlying chronic liver disease, malignancy, and the use of emergency hemodialysis, intra-aortic balloon pumps, dopamine, and longer hospitalization stays. In contrast, male gender, underlying hypertension, cardiovascular disease, higher qSOFA scores, higher lactate levels, the use of ECMO and ventilators, receiving emergency surgery, and admission to surgical ICU were negatively correlated with the development of AKD. Third, when compared to AKI without AKD, the occurrence of AKD after AKI, but not AKD without prior AKI, was associated with a significantly increased risk of 180-day mortality in critically ill patients.
Renal dysfunction impacts innate and adaptive immunity, autoregulation, and vasodilation response, and reduces tolerance to the side effects of drugs (4, 25, 26). Previous studies have proposed that pre-existing CKD can modify the risks of clinical outcomes subsequent to AKI (11, 27). Patients with preexisting CKD were more susceptible to various acute insults, possibly leading to oliguria, fluid overload, electrolyte, or acid–base disturbances, and uremia. Such patients might fulfill the criteria for dialysis or severe AKI when exposed to less severe damage (4). Our study demonstrated that preexisting CKD, the severity of AKI, and the use of emergency hemodialysis were independent risk factors for the development of AKD. On the other hand, the incidence of AKD in patients admitted to the surgical ICU (25.0%, 178 of 712) was much lower than in patients admitted to non-surgical ICU (35.0%, 3,619 of 10,333; p < 0.001). The patients who received emergency surgery (31.6%, 1,657 of 5,242) also had significantly lower incidence of AKD than the total patient cohort (34.4%, 3,797 of 11,045; p < 0.001). Our data were in line with evidence from previous studies showing that surgical etiology was an independent predictor of better outcomes, such as high survival and renal recovery (14, 28). Interestingly, patients with cardiovascular disease, high qSOFA scores, higher lactate levels, and who used ECMO and ventilators, also had significantly lower incidence of AKD in the current study. In the literature, the occurrence of AKD has been connected to the combined effects of structural kidney damage and less responsive renal repair (2). Considering that these are well-known risk factors for extremely grave outcomes, the paradoxically protective effect of these factors on the development of AKD might be attributed to the competing risk of death. This interpretation is consistent with previous reports (14, 22, 29–32).
AKD is considered an important transition period in the AKI-to-CKD continuum (33). The severity of AKD has been reported to be significantly correlated to increased risk of adverse outcomes (14, 15, 22, 34–36). However, several previous studies have reported conflicting results (22, 33, 37–39). Impressively, our study showed that most AKI survivors (55.5%, 7,248 of 13,069) experienced complete renal recovery and did not develop AKD within 7–90 days after the AKI episode. The occurrence of AKD added limited prognostic information for 180-day mortality after AKI (aOR 1.33, 95% CI 1.00–1.77, compared to AKI without AKD). Nevertheless, we found that critically ill patients who had no AKI but later developed AKD (aOR 2.25, 95% CI 1.71–2.97) had a greater risk than the patients who developed AKD after AKI (aOR 1.34, 95% CI 1.00–1.78; p = 0.047). These findings indicate that the presence of AKD may provide limited information for risk stratification of survivors among critically ill patients with AKI but could predict prognosis in survivors without AKI.
Based on these findings, we recommend continued monitoring of renal function even in critically ill patients who do not meet the diagnostic criteria for AKI after an acute renal insult. More attention should also be given to those who develop AKD without AKI (i.e., sCr levels <1.5 times baseline within 7 days of an episode that increased to >1.5 times baseline 7–90 days after the episode). The occurrence of AKD without AKI might signify disease progression of subclinical AKI, which is not detected by current sCr-based AKI criteria. Clinical physicians should incorporate the comprehensive kidney health assessments set out by the ADQI 22 working group into clinical practice for critically ill patients who do not meet criteria for AKI after an acute insult but are at risk of AKD (40). Targeted management using an intensive care program, including the avoidance of exposure to nephrotoxic agents and unnecessary drugs, and regular monitoring of renal function, might help reduce the incidence of AKD and the risk of subsequent adverse outcomes in critically ill patients surviving 7 days after an acute insult (41).
While our study yielded encouraging results, it is important to acknowledge its potential limitations. First, the patient population was heterogeneous, with varying causes of acute kidney injury (AKI), the severity of kidney damage, and accompanying chronic comorbidities. For this reason, we conducted a multivariable analysis to better understand these variables’ clinical impact. Second, due to the retrospective nature of our study, determining the exact onset time of each AKI episode was challenging. To address this, we defined the date of AKI onset as the day within the first seven days of ICU admission when the lowest serum creatinine (sCr) measurement was recorded. While this approach may not accurately capture the ongoing progression of AKI or incomplete renal recovery after AKI, recent research from the same database suggests that our approach likely had a low risk of bias (42). Nevertheless, subclinical AKI may be missed due to a lack of biomarkers other than sCr in the database (43–45). Third, community-acquired AKI may have been misdiagnosed as chronic kidney disease (CKD) due to our use of sCr data collected during admission, which could have led to an underestimation of the true incidence of AKI. Forth, information about blood transfusions was not available in our database. While the demand for blood transfusions may be low in this population, the lack of information on this variable may have influenced our outcomes. Finally, it is essential to note that logistic regression models have inherent limitations in their predictive accuracy, and the lack of external validation from an independent cohort is a significant concern. Also, the study only involved patients of a single ethnicity, which limits the generalizability of the findings to other hospitals with different patient populations. Future studies should aim to validate our findings in a separate cohort of patients to increase the reliability and generalizability of our results.
In the present study, approximately a quarter of critically ill AKI patients developed AKD, and another quarter died within 180 days. Our analyses showed that AKI stage 2–3, underlying early CKD, chronic liver disease, malignancy, and use of emergency hemodialysis were independently associated with increased risk of AKD development. Although the occurrence of AKD did not add prominent prognostic information for risk stratification of survivors among critically ill patients with AKI, it could predict prognosis in survivors without AKI.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Chang Gung Medical Foundation. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
H-CP and H-YC contributed to data conception, design, and interpretation. H-YC, H-MC, Y-TH, and H-CP contributed to collecting data and manuscript drafting. Y-CC, J-TF, and H-CP provided patient information, participated in the design and coordination, and helped draft the manuscript. H-YC, H-CP, and Y-CC provided intellectual content of the work and were involved in editing and revising the manuscript. All authors discussed, contributed to, and approved the final manuscript version.
We thank financial support from the Ministry of Science and Technology, Taiwan (MOST 107-2314-B-182A-019-MY3), and in part from the Chang Gung Medical Foundation (CMRPG1M0041, CMRPG1M0121, and CORPG1L0041).
The authors thank the statistical assistance and wish to acknowledge the support of the Maintenance Project of the Center for Big Data Analytics and Statistics (Grant No.: CLRPG3D0048) at Chang Gung Memorial Hospital for study design and monitor, data analysis and interpretation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1153670/full#supplementary-material
1. Lameire, NH, Levin, A, Kellum, JA, Cheung, M, Jadoul, M, Winkelmayer, WC, et al. Harmonizing acute and chronic kidney disease definition and classification: report of a kidney disease: improving global outcomes (KDIGO) consensus Conference. Kidney Int. (2021) 100:516–26. doi: 10.1016/j.kint.2021.06.028
2. Yan, P, Duan, XJ, Liu, Y, Wu, X, Zhang, NY, Yuan, F, et al. Acute kidney disease in hospitalized acute kidney injury patients. Peer J. (2021) 9:e11400. doi: 10.7717/peerj.11400
3. Hwang, S, Park, H, Kim, Y, Kang, D, Ku, HS, Cho, J, et al. Changes in acute kidney injury epidemiology in critically ill patients: a population-based cohort study in Korea. Ann Intensive Care. (2019) 9:65. doi: 10.1186/s13613-019-0534-7
4. Pan, HC, Wu, PC, Wu, VC, Yang, YF, Huang, TM, Shiao, CC, et al. A nationwide survey of clinical characteristics, management, and outcomes of acute kidney injury (AKI)–patients with and without preexisting chronic kidney disease have different prognoses. Medicine. (2016) 95:e4987. doi: 10.1097/MD.0000000000004987
5. Chawla, LS, Amdur, RL, Amodeo, S, Kimmel, PL, and Palant, CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. (2011) 79:1361–9. doi: 10.1038/ki.2011.42
6. Lai, TS, Wang, CY, Pan, SC, Huang, TM, Lin, MC, Lai, CF, et al. Risk of developing severe sepsis after acute kidney injury: a population-based cohort study. Crit Care. (2013) 17:R231. doi: 10.1186/cc13054
7. Wu, VC, Wu, CH, Huang, TM, Wang, CY, Lai, CF, Shiao, CC, et al. Long-term risk of coronary events after AKI. J Am Soc Nephrol. (2014) 25:595–605. doi: 10.1681/ASN.2013060610
8. Wang, WJ, Chao, CT, Huang, YC, Wang, CY, Chang, CH, Huang, TM, et al. The impact of acute kidney injury with temporary dialysis on the risk of fracture. J Bone Miner Res. (2014) 29:676–84. doi: 10.1002/jbmr.2061
9. Hoste, EAJ, Kellum, JA, Selby, NM, Zarbock, A, Palevsky, PM, Bagshaw, SM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. (2018) 14:607–25. doi: 10.1038/s41581-018-0052-0
10. Chawla, LS, Bellomo, R, Bihorac, A, Goldstein, SL, Siew, ED, Bagshaw, SM, et al. Acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat Rev Nephrol. (2017) 13:241–57. doi: 10.1038/nrneph.2017.2
11. Mizuguchi, KA, Huang, CC, Shempp, I, Wang, J, Shekar, P, and Frendl, G. Predicting kidney disease progression in patients with acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. (2018) 155:2455–2463.e5. doi: 10.1016/j.jtcvs.2018.01.093
12. Kofman, N, Margolis, G, Gal-Oz, A, Letourneau-Shesaf, S, Keren, G, Rozenbaum, Z, et al. Long-term renal outcomes and mortality following renal injury among myocardial infarction patients treated by primary percutaneous intervention. Coron Artery Dis. (2019) 30:87–92. doi: 10.1097/MCA.0000000000000678
13. James, MT, Levey, AS, Tonelli, M, Tan, Z, Barry, R, Pannu, N, et al. Incidence and prognosis of acute kidney diseases and disorders using an integrated approach to laboratory measurements in a universal health care system. JAMA Netw Open. (2019) 2:e191795. doi: 10.1001/jamanetworkopen.2019.1795
14. Matsuura, R, Iwagami, M, Moriya, H, Ohtake, T, Hamasaki, Y, Nangaku, M, et al. The clinical course of acute kidney disease after cardiac surgery: a retrospective observational study. Sci Rep. (2020) 10:6490. doi: 10.1038/s41598-020-62981-1
15. Hsu, CK, Wu, IW, Chen, YT, Tsai, TY, Tsai, FC, Fang, JT, et al. Acute kidney disease stage predicts outcome of patients on extracorporeal membrane oxygenation support. PLoS One. (2020) 15:e0231505. doi: 10.1371/journal.pone.0231505
16. Shao, SC, Chan, YY, Kao Yang, YH, Lin, SJ, Hung, MJ, Chien, RN, et al. The Chang gung research database-a multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol Drug Saf. (2019) 28:593–600. doi: 10.1002/pds.4713
17. Tsai, MS, Lin, MH, Lee, CP, Yang, YH, Chen, WC, Chang, GH, et al. Chang gung research database: a multi-institutional database consisting of original medical records. Biom J. (2017) 40:263–9. doi: 10.1016/j.bj.2017.08.002
18. Lee, CW, Yu, MC, Wang, CC, Lee, WC, Tsai, HI, Kuan, FC, et al. Liver resection for hepatocellular carcinoma larger than 10 cm: a multi-institution long-term observational study. World J Gastrointest Surg. (2021) 13:476–92. doi: 10.4240/wjgs.v13.i5.476
19. Kellum, JA, and Lameire, N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. (2013) 17:204. doi: 10.1186/cc11454
20. Wagner, T, Sinning, C, Haumann, J, Magnussen, C, Blankenberg, S, Reichenspurner, H, et al. qSOFA score is useful to assess disease severity in patients with heart failure in the setting of a heart failure unit (HFU). Front Cardiovasc Med. (2020) 7:574768. doi: 10.3389/fcvm.2020.574768
21. Federspiel, CK, Itenov, TS, Mehta, K, Hsu, RK, Bestle, MH, and Liu, KD. Duration of acute kidney injury in critically ill patients. Ann Intensive Care. (2018) 8:30. doi: 10.1186/s13613-018-0374-x
22. Chen, YT, Jenq, CC, Hsu, CK, Yu, YC, Chang, CH, Fan, PC, et al. Acute kidney disease and acute kidney injury biomarkers in coronary care unit patients. BMC Nephrol. (2020) 21:207. doi: 10.1186/s12882-020-01872-z
23. Xiao, YQ, Cheng, W, Wu, X, Yan, P, Feng, LX, Zhang, NY, et al. Novel risk models to predict acute kidney disease and its outcomes in a Chinese hospitalized population with acute kidney injury. Sci Rep. (2020) 10:15636. doi: 10.1038/s41598-020-72651-x
24. Tonon, M, Rosi, S, Gambino, CG, Piano, S, Calvino, V, Romano, A, et al. Natural history of acute kidney disease in patients with cirrhosis. J Hepatol. (2021) 74:578–83. doi: 10.1016/j.jhep.2020.08.037
25. Chawla, LS, and Kimmel, PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. (2012) 82:516–24. doi: 10.1038/ki.2012.208
26. Chawla, LS, Eggers, PW, Star, RA, and Kimmel, PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. (2014) 371:58–66. doi: 10.1056/NEJMra1214243
27. Sawhney, S, Mitchell, M, Marks, A, Fluck, N, and Black, C. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open. (2015) 5:e006497. doi: 10.1136/bmjopen-2014-006497
28. Pan, HC, Chen, YY, Tsai, IJ, Shiao, CC, Huang, TM, Chan, CK, et al. Accelerated versus standard initiation of renal replacement therapy for critically ill patients with acute kidney injury: a systematic review and meta-analysis of RCT studies. Crit Care. (2021) 25:5. doi: 10.1186/s13054-020-03434-z
29. Pan, HC, Jenq, CC, Tsai, MH, Fan, PC, Chang, CH, Chang, MY, et al. Risk models and scoring systems for predicting the prognosis in critically ill cirrhotic patients with acute kidney injury: a prospective validation study. PLoS One. (2012) 7:e51094. doi: 10.1371/journal.pone.0051094
30. Tsai, TY, Chien, H, Tsai, FC, Pan, HC, Yang, HY, Lee, SY, et al. Comparison of RIFLE, AKIN, and KDIGO classifications for assessing prognosis of patients on extracorporeal membrane oxygenation. J Formos Med Assoc. (2017) 116:844–51. doi: 10.1016/j.jfma.2017.08.004
31. Chang, CH, Chen, SW, Fan, PC, Lee, CC, Yang, HY, Chang, SW, et al. Sequential organ failure assessment score predicts mortality after coronary artery bypass grafting. BMC Surg. (2017) 17:22. doi: 10.1186/s12893-017-0219-9
32. Pan, HC, Huang, TM, Sun, CY, Chou, NK, Tsao, CH, Yeh, FY, et al. Predialysis serum lactate levels could predict dialysis withdrawal in type 1 cardiorenal syndrome patients. EClinicalMedicine. (2022) 44:101232. doi: 10.1016/j.eclinm.2021.101232
33. Peerapornratana, S, Priyanka, P, Wang, S, Smith, A, Singbartl, K, Palevsky, PM, et al. Sepsis-associated acute kidney disease. Kidney Int Rep. (2020) 5:839–50. doi: 10.1016/j.ekir.2020.03.005
34. Chen, YW, Wu, MY, Mao, CH, Yeh, YT, Chen, TT, Liao, CT, et al. Severe acute kidney disease is associated with worse kidney outcome among acute kidney injury patients. Sci Rep. (2022) 12:6492–9. doi: 10.1038/s41598-022-09599-7
35. Chen, JJ, Lee, TH, Kuo, G, Yen, CL, Chen, SW, Chu, PH, et al. Acute kidney disease after acute decompensated heart failure. Kidney Int Rep. (2022) 7:526–36. doi: 10.1016/j.ekir.2021.12.033
36. Wang, H, Lambourg, E, Guthrie, B, Morales, DR, Donnan, PT, and Bell, S. Patient outcomes following AKI and AKD: a population-based cohort study. BMC Med. (2022) 20:229. doi: 10.1186/s12916-022-02428-8
37. Mima, A, Tansho, K, Nagahara, D, and Tsubaki, K. Incidence of acute kidney disease after receiving hematopoietic stem cell transplantation: a single-center retrospective study. Peer J. (2019) 7:e6467. doi: 10.7717/peerj.6467
38. Flannery, AH, Li, X, Delozier, NL, Toto, RD, Moe, OW, Yee, J, et al. Sepsis-associated acute kidney disease and long-term kidney outcomes. Kidney Med. (2021) 3:507–514.e1. doi: 10.1016/j.xkme.2021.02.007
39. Chang, CH, Chen, SW, Chen, JJ, Chan, YH, Yen, CL, Lee, TH, et al. Incidence and transition of acute kidney injury, acute kidney disease to chronic kidney disease after acute type a aortic dissection surgery. J Clin Med. (2021) 10:4769. doi: 10.3390/jcm10204769
40. Ostermann, M, Zarbock, A, Goldstein, S, Kashani, K, Macedo, E, Murugan, R, et al. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus Conference: a consensus statement. JAMA Netw Open. (2020) 3:e2019209. doi: 10.1001/jamanetworkopen.2020.19209
41. Kashani, K, Rosner, MH, Haase, M, Lewington, AJP, O’Donoghue, DJ, Wilson, FP, et al. Quality improvement goals for acute kidney injury. Clin J Am Soc Nephrol. (2019) 14:941–53. doi: 10.2215/CJN.01250119
42. Lee, CC, Kuo, G, Chan, MJ, Fan, PC, Chen, JJ, Yen, CL, et al. Characteristics of and outcomes after dialysis-treated acute kidney injury, 2009-2018: a Taiwanese Multicenter study. Am J Kidney Dis. (in press). doi: 10.1053/j.ajkd.2022.08.022
43. Pan, HC, Huang, TM, Huang, CT, Sun, CY, Chen, YM, and Wu, VC. Urinary biomarkers can predict weaning from acute dialysis therapy in critically ill patients. Arch Pathol Lab Med. (2022) 146:1353–63. doi: 10.5858/arpa.2021-0411-OA
44. Yang, SY, Chiou, TT, Shiao, CC, Lin, HY, Chan, MJ, Wu, CH, et al. Nomenclature and diagnostic criteria for acute kidney injury–2020 consensus of the Taiwan AKI-task force. J Formos Med Assoc. (2021) 121:749–65. doi: 10.1016/j.jfma.2021.08.005
Keywords: acute kidney injury, acute kidney disease, chronic kidney disease, risk factor, survival
Citation: Pan H-C, Chen H-Y, Chen H-M, Huang Y-T, Fang J-T and Chen Y-C (2023) Risk factors and 180-day mortality of acute kidney disease in critically ill patients: A multi-institutional study. Front. Med. 10:1153670. doi: 10.3389/fmed.2023.1153670
Received: 29 January 2023; Accepted: 28 March 2023;
Published: 17 April 2023.
Edited by:
Redhwan Ahmed Al-Naggar, National University of Malaysia, MalaysiaReviewed by:
Nagarajan Muthialu, Great Ormond Street Hospital for Children NHS Foundation Trust, United KingdomCopyright © 2023 Pan, Chen, Chen, Huang, Fang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yung-Chang Chen, Y3ljMjM1NkBnbWFpbC5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.