- 1Department of Respiratory and Critical Care Medicine, Wuxi No.2 People’s Hospital (Jiangnan University Medical Center), Wuxi, China
- 2Department of Respiratory and Critical Care Medicine, The Wuxi People’s Hospital, Wuxi, China
- 3Department of Respiratory and Critical Care Medicine, The Xishan People’s Hospital of Wuxi City, Wuxi, China
- 4Department of Respiratory and Critical Care Medicine, The Wuxi Fifth People’s Hospital (Jiangnan University Medical Center), Wuxi, China
Purpose: More and more patients with community-acquired pneumonia have been detected with Chlamydia psittaci (C. psittaci) infected using metagenomic next-generation sequencing (mNGS). Previously, this was unheard of, and several patients presented with severe pneumonia and even required ECMO. We aimed to clarify the clinical characteristics of C. psittaci pneumonia and find out if there are any possible predictors of severe C. psittaci pneumonia.
Methods: In this retrospective study, we included all confirmed cases of C. psittaci pneumonia in Wuxi. Epidemiological, clinical, and radiological features, as well as laboratory data, were collected and analyzed.
Results: We enrolled 55 patients with C. psittaci pneumonia, with 30 (54.5%) having a history of exposure to birds or their internal organs. 50 (90.9%) patients were diagnosed by mNGS. Patients with C. psittaci pneumonia had many complications, among which, that deserve sufficient attention from clinicians were vascular embolic events (3, 5.5%). High fever was the most common clinical manifestation (41, 74.5%). The majority of patients had a significant increase in neutrophils ratio, neutrophils to lymphocytes ratio (NLR), rapid c-reactive protein, creatine kinase (CK), and lactate dehydrogenase (LDH), as well as a decrease in lymphocytes ratio, albumin, serum sodium, serum potassium, and serum phosphorus. Chest computed tomography scans revealed unilateral pneumonia (70.9%), consolidation (87.3%), air bronchogram (76.4%), and ground-glass opacity (69.1%). The neutrophil ratio, NLR, LDH, and CK were all factors that could identify severe pneumonia. Both AUCs exceeded 0.8; the respective 95% CIs were 0.715–0.944, 0.710–0.963, 0.677–0.937, and 0.718–0.950; all p < 0.05 (0.01, 0.001, 0.007, 0.007 respectively). The ORs were 10.057, 9.750, 10.057, and 9.667, respectively; the 95% CIs were 2.643–38.276, 2.339–40.649, and 2.643–38.276, respectively; all p-values were less than 0.05 (0.001, 0.002, 0.001, 0.001 respectively).
Conclusion: C. psittaci pneumonia is a very complex disease that changes all the time. Some patients showed severe pneumonia. Patients will have a poor prognosis if they are not treated promptly and effectively. We discovered that many clinical indicators were typical. Meanwhile, significant increases in neutrophil ratio, NLR, LDH, and CK predicted severe pneumonia. Timely detection of mNGS provided substantial help for clinical diagnosis and early treatment.
Background
Humans can be infected by Chlamydia psittaci (C. psittaci) via the respiratory tract through air or aerosols, resulting in atypical community-acquired pneumonia (CAP) (1). However, C. psittaci is challenging to be identified by traditional methods such as microbial culture or microscopic examination. C. psittaci pneumonia is often underdiagnosed or misdiagnosed in humans, with an estimated incidence rate of at least 1.03% (2).
Chlamydia psittaci infection causes severe pneumonia in some patients (3). The inflammatory reaction imbalance in their body would lead to cytokine storm (4), resulting in diffuse alveolar injury (DAD) and pulmonary capillary endothelial injury, causing acute respiratory distress syndrome (ARDS) (5). Early etiological diagnosis and timely and effective treatment can improve the prognosis of patients. In recent years, metagenomic next-generation sequencing (mNGS) has become increasingly essential for etiological diagnosis. The researchers analyze nucleic acids from bronchoalveolar lavage fluid (BALF), sputum, and other clinical samples, then create a biological reference database and rigorous sequence read mapping and finally identify pathogens like Mycobacterium tuberculosis (6–8). Currently, mNGS has gradually been used for the diagnosis of difficult-to-diagnose severe pneumonia (9). Additionally, mNGS can quickly and accurately identify most pathogens, especially atypical pathogens such as Legionella and C. psittaci.
With the increasing popularity of mNGS applications, more and more patients with CAP have been detected with C. psittaci infected. A multicenter prospective cohort study in China included 329 patients with severe community-acquired pneumonia (SCAP), 264 of whom were immunocompetent, and 8% of whom were infected with C. psittaci (10). Previously, this was unheard of. Nowadays, C. psittaci pneumonia has become a public health concern in China. According to a comprehensive review of recent papers, it has a variety of clinical features, and the severity of C. psittaci pneumonia ranges from mild flu-like symptoms to life-threatening diseases (1, 11). In this article, we attempt to provide an updated description of patients with C. psittaci pneumonia. It will deepen the clinicians’ understanding of the diagnosis and treatment of C. psittaci pneumonia, diagnose as soon as possible, treat in time, and improve the prognosis.
Materials and methods
Enrolled subjects
This retrospective study included 55 patients with C. psittaci pneumonia. The inclusion diagnostic criteria for C. psittaci pneumonia were as follows: (1) the diagnostic criteria of adult CAP (12); (2) identification of the C. psittaci gene fragments through mNGS analysis of the BALF, blood, or sputum, and met the criteria for a positive mNGS result (13); (3) positive results in C. psittaci—specific PCR (14, 15); (4) routine microbiological tests, including blood, sputum, and BALF culture were all negative (14, 15). (5) Respiratory failure, defined as a partial oxygen pressure is <60 mmHg or an oxygenation index <300 mm Hg (1 mm Hg = 0.133 kPa).
These patients were admitted from January 1, 2020, to December 31, 2022. These patients were diagnosed and treated at Wuxi Second People’s Hospital, Wuxi People’s Hospital, Xishan People’s Hospital of Wuxi City, and Wuxi Fifth People’s Hospital. Few patients with C. psittaci pneumonia in other hospitals in Wuxi; contacted their doctors to learn.
Procedures
We obtained epidemiological, demographic, clinical, laboratory, management, and outcome data from patients’ medical records. We acquired data from attending doctors, other healthcare providers, and patients, if data was missing from the records or clarification, was required. All data were checked by two physicians (YG and YW).
The C. psittaci infection was confirmed by mNGS for 50 BALF samples or positive PCR testing done by the Wuxi Centers for Disease Control and Prevention (CDC) for 5 BALF samples. One of the patients had BALF and blood mNGS testing. The NGS had mainly completed in the following institutions: the Shenzhen Huada Gene Technology Co. Ltd. (Shenzhen, China), Adicon (Hangzhou, China), Darui Diagnostics (Guangzhou, China), and Genskey (Shanghai, China), etc.
Blood, sputum, or endotracheal aspirates were obtained at admission to identify the possible causative bacteria or fungi. In addition, all patients underwent chest computed tomography (CT) and laboratory tests.
Outcomes
We describe the clinical data, demographics, epidemiological data (such as poultry contact history), primary medical conditions, signs, and symptoms on admission, chest CT findings, laboratory results, comorbidities, mNGS results, treatment received for C. psittaci pneumonia, etc. We conducted a statistical analysis of the severe and non-severe groups and discovered the predictive factors of the severe group.
Statistical analysis
We present continuous measurements as mean (SD) if they were normally distributed, median (IQR) if they were not, and categorical variables as count (%). The corresponding statistical analysis was used separately with a t-test, Kruskall–Wallis test, chi-square test, or Fisher’s exact test. In the case of laboratory results, we also assessed whether the measurements were outside the normal range. Risk factors were analyzed using binary logistic regression analysis. The reliability of the predicted value was analyzed using the receiver operating characteristic curve. We used SPSS (version 21.0), RStudio (version 1.4.1106.0), and MSTATA (V 0.5) for all analyses.
Results
Patient characteristics
Fifty-five patients with C. psittaci pneumonia were included in this study, three of whom were family members, and two others were mother and son.
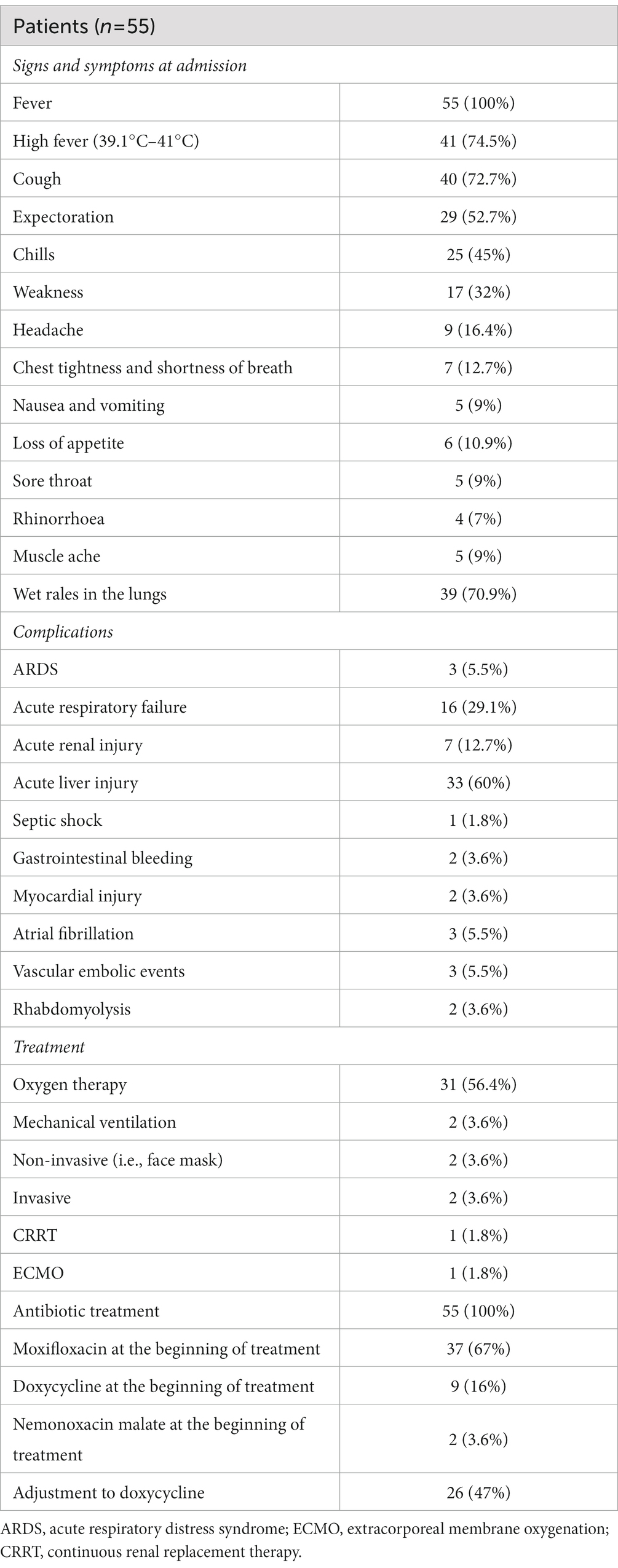
For the 55 patients, the mean age was 58 (SD13; range 31–87 years) years, and 56.4% of patients were male. More than half of the patients (54.5%) had a documented history of exposure to birds or their internal organs. The majority of patients had chronic diseases. The overall clinical features of the patients are summarized in Table 1.
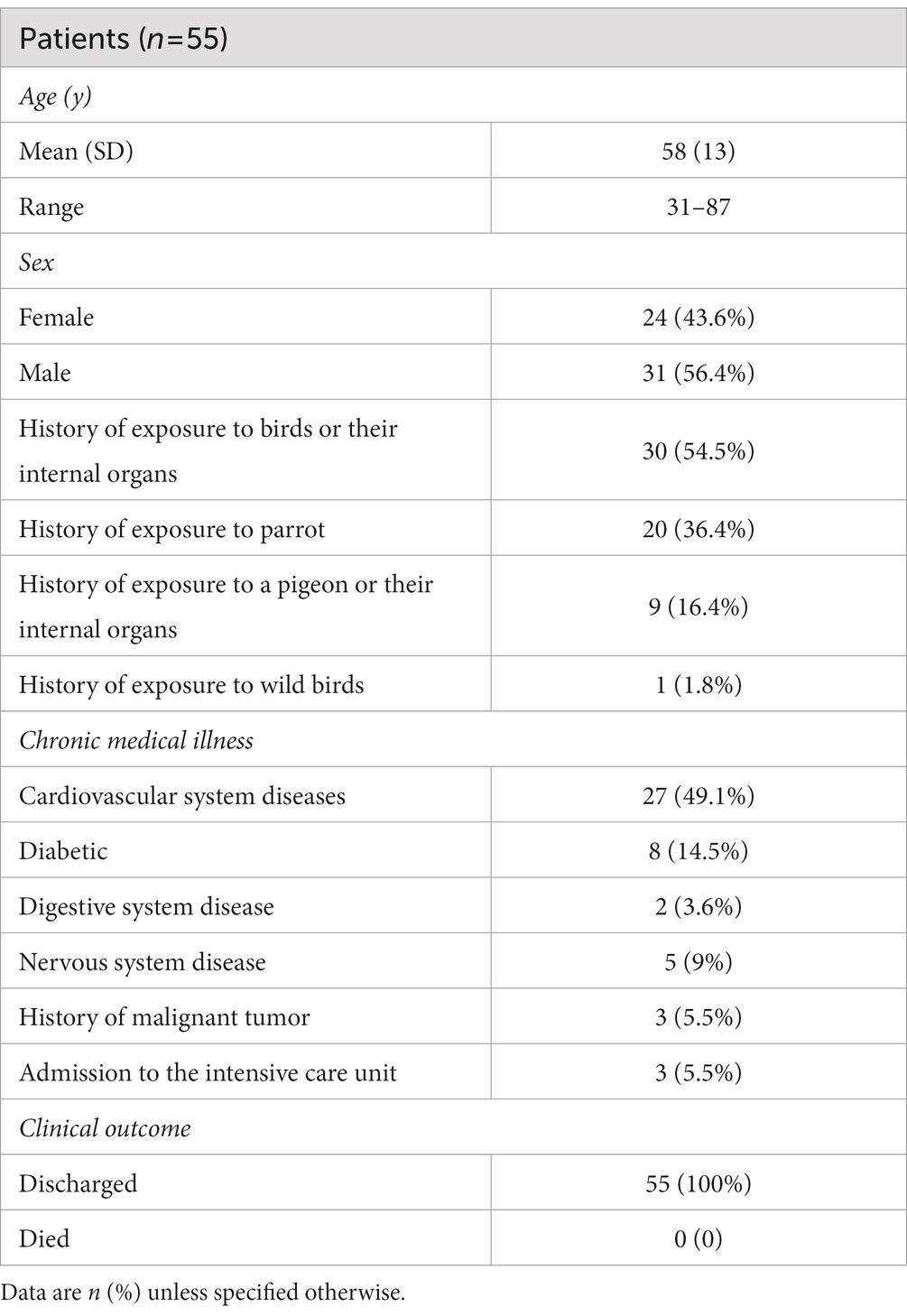
Table 1. Demographics, baseline characteristics, and clinical outcomes of 55 patients with Chlamydia psittaci pneumonia.
On admission, the most prominent symptom was fever, which occurred in almost all patients; most of them had a high fever (41, 74.5%). Other common symptoms were chills and weakness, cough, and expectoration, but the expectoration was only a little yellow-white sputum or white sputum. About 39 (70.9%) patients had wet rales in their lungs. All symptoms and signs are shown in Table 2.
The number of patients with complications was high. The most common was acute liver injury (33, 60%). That deserves sufficient attention from clinicians was vascular embolic events (3, 5.5%). Other complications included ARDS (3, 5.5%), severe lung injury (16, 29.1%), septic shock (1, 1.8%), and others, as shown in Table 2.
Laboratory findings
On admission, 37 (67.3%) patients had a neutrophil ratio above the normal range. Many patients’ lymphocyte ratios were lower than usual, and the overall neutrophil-to-lymphocyte ratio (NLR) was 8.25 ± 6.9. For the infection index, all patients had rapid c-reactive protein tests, and their median levels were 151.08 ± 70.35 mg/L. An erythrocyte sedimentation (ESR) test was conducted on 47 patients, 46 (97.9%) of whom had levels higher than usual, with a median of 64 ± 29 mm/h. Procalcitonin (PCT) levels were observed to be elevated in 17 (34%) of the 50 patients tested. The characteristics of all inflammatory indicators are listed in Table 3.
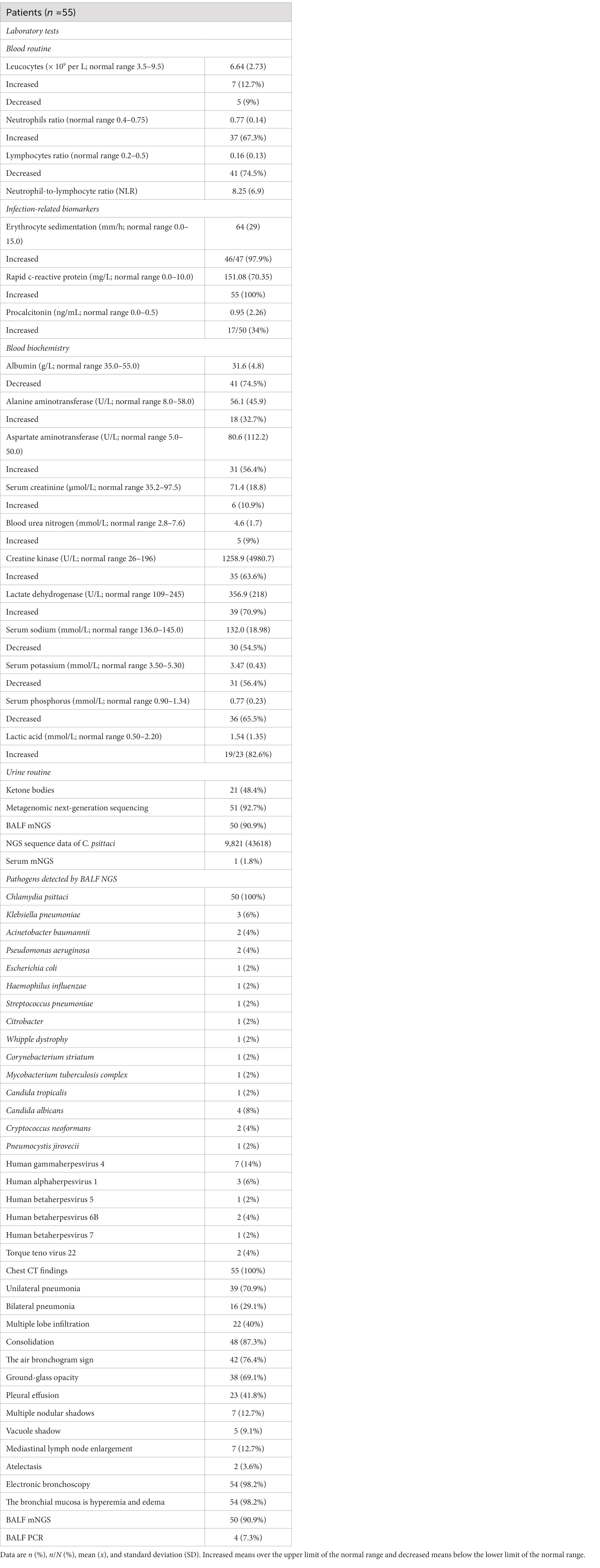
Table 3. Characteristics of laboratory tests, chest CT, and electronic bronchoscopies of 55 patients with C. psittaci pneumonia.
All patients had myocardial zymograms, and 35 (63.6%) had elevated creatine kinase (CK) levels, while 39 (70.9%) had increased lactate dehydrogenase (LDH), with one patient having abnormal CK levels (34,035 U/L) and LDH levels (1,273 U/L). Electrolyte abnormalities were prevalent in the majority of patients, with hyponatremia in 30 cases (54.5%), hypokalemia in 31 cases (56.4%), and hypophosphatemia in 36 cases (65.5%), one of whom also displayed abnormal hyponatremia (116.4 mmol/L) and hypokalemia (2.51 mmol/L). Forty-one (74.5%) patients had degrees of albumin reduction. Thirty-three patients had varying degrees of liver function abnormalities, with alanine aminotransferase (ALT) or aspartate aminotransferase (AST) above normal levels; no severe impairment occurred. Only 7 (12.7%) patients had elevated blood urea nitrogen (BUN) or serum creatinine, indicating renal function damage. In addition, lactic acid was increased in 19 patients (19/23, 82.6%), and ketone bodies were detected in 21 patients (48.4%). Which is shown in Table 3.
Chest CT findings
All patients underwent chest CT scans. According to chest CT, 39 (70.9%) patients showed unilateral pneumonia, with just 16 (29.1%) patients showing bilateral pneumonia and 22 (40%) patients showing multiple lobe infiltration. In 48 (87.3%) of the patients, consolidation was observed, and 42 of the patients were observed with air bronchograms as consolidation. The ground-glass opacity was observed in 38 patients (69.1%). Other signs included pleural effusion (23, 41.8%), multiple nodular shadows (7, 12.7%), vacuole shadow (5, 9.1%), mediastinal lymph nodes enlargement (7, 12.7%), and atelectasis (2, 3.6%). All imaging features were shown in Table 3 and Figure 1.
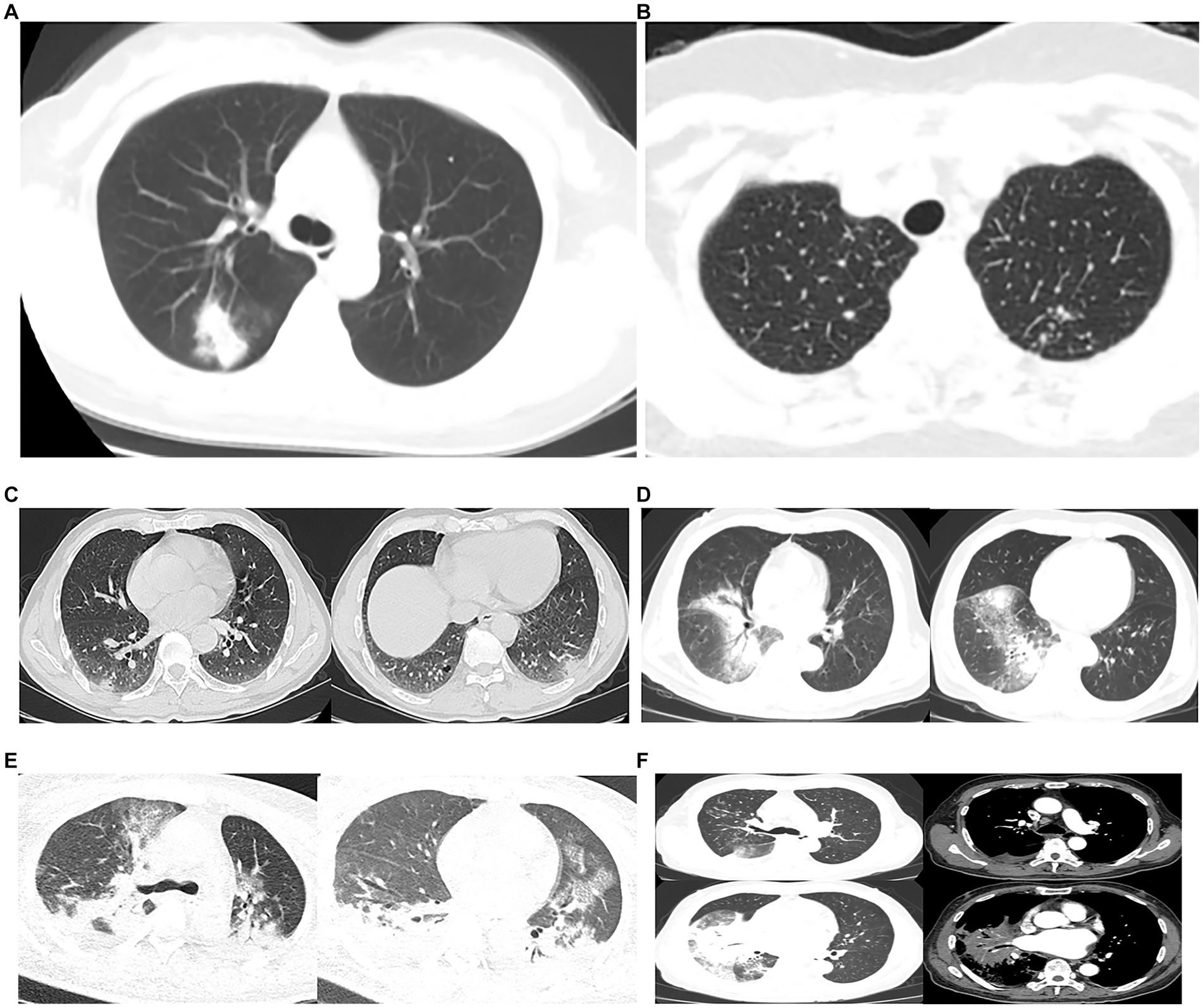
Figure 1. Chest CTs of 6 patients. (A), (B) and (C) were family members. (A) Consolidation and ground-glass opacity was seen in the right upper lung. (B) Multiple nodular shadows were seen in both lungs. (C) Patchy consolidation and vacuoles in the pleura of both lower lungs, and patchy indistinct shadows in the margin. (D) Large patchy consolidation and blurred shadows were seen in the right lung, and an air bronchogram sign was seen inside. (E) Severe pneumonia: extensive cloudy flocculent ground glass shadow in both lungs, lamellar consolidation shadow in each lobe of both lungs, air bronchogram sign in them, bilateral pleural effusion, bilateral lower lung atelectasis. (F) Severe pneumonia: patchy consolidation shadow could be seen in the right lung, and an air bronchogram sign could be seen inside, cloud flocculent ground glass shadow in the upper and lower lobe of the right lung, enlarged lymph nodes in the mediastinum, and a small amount of effusion in the right chest.
Etiological results
Among the 55 patients, two of whom were mother and son, and the disease occurred at the same time, the mother did not undergo bronchoscopy, while the other 54 patients underwent bronchoscopy (Table 3). Bronchoscopy revealed congestion and oedema of the bronchial mucosa. A total of 50 patients received mNGS testing of their BALF, and one patient received mNGS testing of both his BALF and blood. The median mNGS sequence number of C. psittaci for BALF samples was 9,821 (IQR 4-244,385). The mNGS sequence number in the blood sample was 4. All pathogenic bacteria detected by NGS are listed in Table 3. The BALF from four patients was sent to the Wuxi CDC for PCR testing for C. psittaci, and the results were positive. Three of them were family members, the household had two parrots, and the parrots were sick. The living environment was positive for C. psittaci based on PCR (Table 3).
Fifty four patients had a sputum culture. Only one patient in the ICU detected Klebsiella pneumonia, and the rest were normal flora or Candida albicans. A blood culture was performed on 35 patients, and the results were negative.
Treatment and outcome
All patients received antibiotic treatment. Before diagnosis, many patients received combination treatment, such as beta-lactam/beta-lactamase inhibitors or cephalosporins coupled with moxifloxacin or doxycycline. When C. psittaci was identified as the causative pathogen, doxycycline monotherapy was prescribed to most patients. An allergic response happened in two patients who began therapy with nemonoxacin malate after being adjusted to doxycycline, and the treatment was readjusted to nemonoxacin malate (Table 2).
Three of 55 patients were admitted to the intensive care unit, including two who used mechanical ventilation with an invasive ventilator and one who used a high-flow humidifier. Only one patient was treated with extracorporeal membrane oxygenation. Oxygen therapy was administered to 31 (56.4%) patients in general wards (Table 2).
There was no dead case in our research, which was a benefit. All patients were discharged with normal temperatures, no obvious respiratory symptoms, and normal laboratory results of the re-examination. Following follow-up, 50 patients’ chest CTs showed absorption of pulmonary inflammation, and five patients were informed of improvement via telephone.
Predictors for severe pneumonia
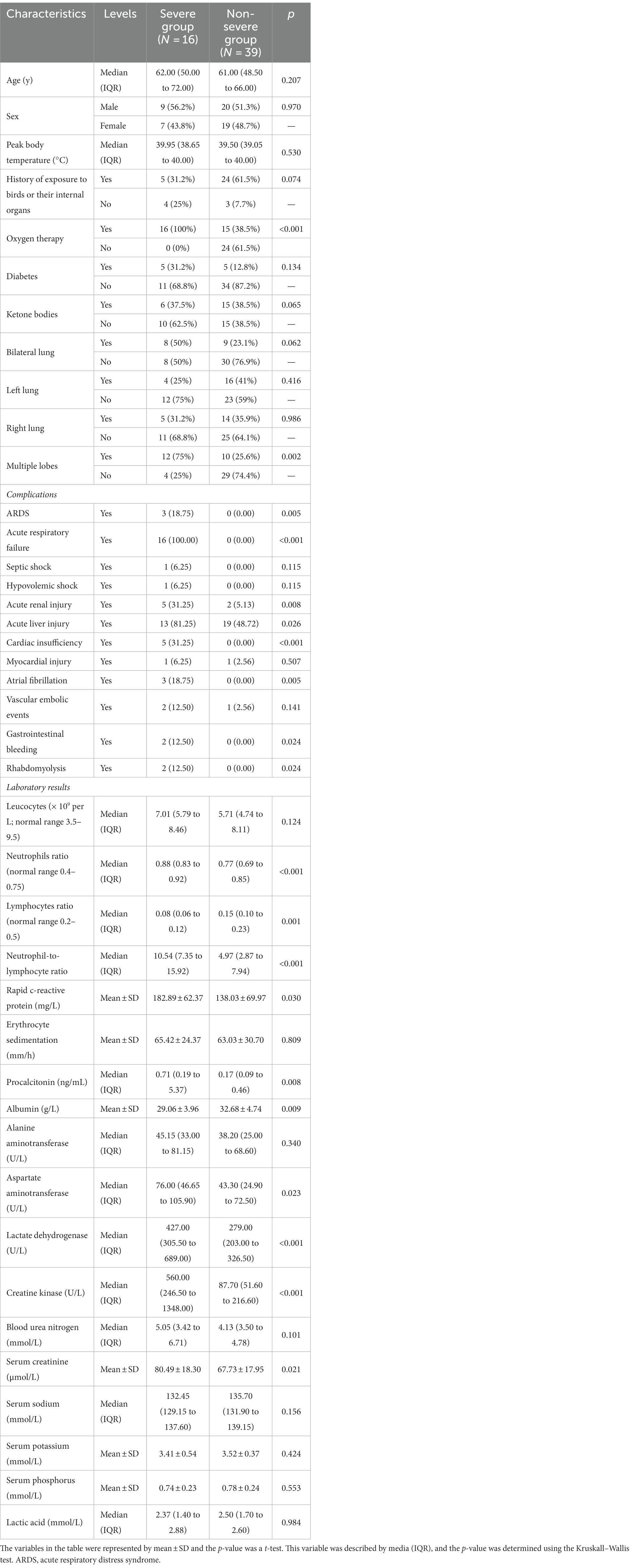
In our study, 16 of 55 cases of C. psittaci pneumonia met the criteria for severe pneumonia (12). Patients were divided into two categories, severe and non-severe, and the variables in both groups were statistically analyzed (Table 4). The severe group was found to have more complications than the non-severe group. And the treatment of ventilator or ECMO was only used in the severe group.
The majority of the severe group had bilateral lung inflammation (50% vs. 23.1%, p = 0.062) or multiple lobes infiltration (75% vs. 25.6%, p = 0.002), but the difference in bilateral lung inflammation was not statistically significant. The neutrophil ratio (0.88 vs. 0.77, p < 0.001), NLR (10.54 vs. 4.97, p < 0.001), rapid c-reactive protein (182.89 vs. 138.03, p = 0.030), PCT (0.71 vs. 0.17, p = 0.008), AST (76.00 vs. 43.30, p = 0.023), LDH (427.00 vs. 279.00, p < 0.001), CK (560.00 vs. 87.70, p < 0.001), and serum creatinine (80.49 vs. 67.73, p = 0.021) were significantly higher in the severe group than in the non-severe group, and the differences were statistically significant. The lymphocyte ratio and serum albumin level were lower in the severe group than in the non-severe group, and the differences were also statistically significant (0.08 vs. 0.15, p = 0.001; 29.06 vs. 32.68, p = 0.009). Which is shown in Table 4.
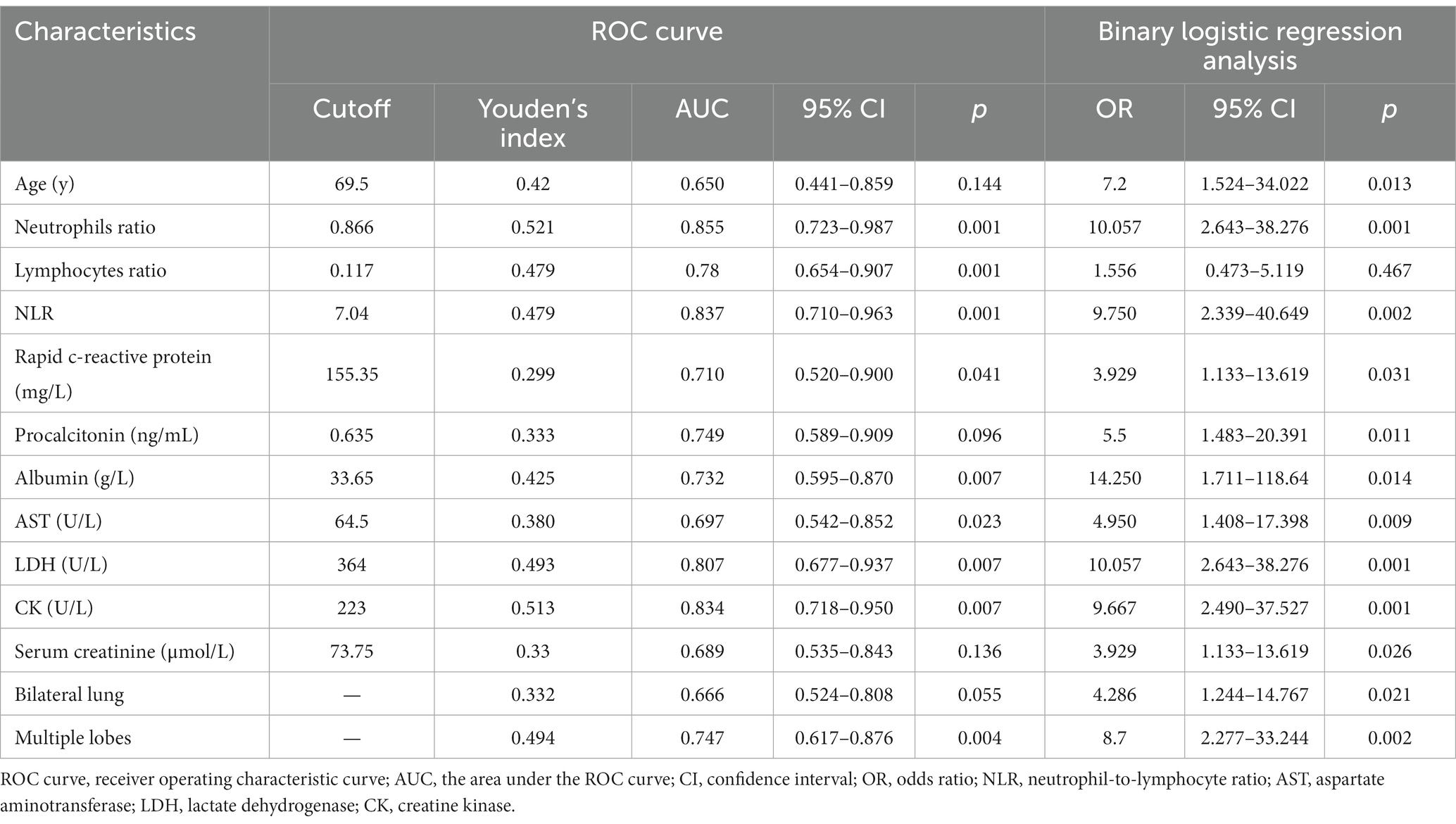
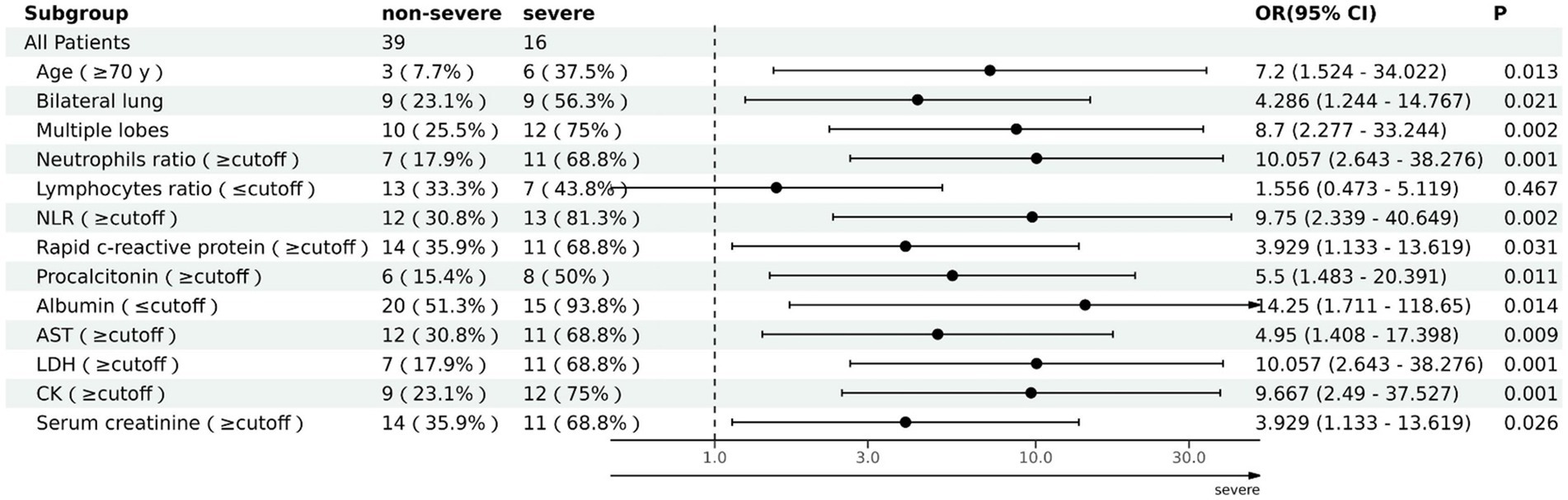
The ability of these 12 variables to predict the severe group was assessed using the receiver operating characteristic curve (ROC curve) (Table 5 and Figure 2). The ROC curve was employed to determine the cutoff values for each continuous variable, and the cutoff values were utilized to transform the variables into dichotomous variables. Besides, according to ROC curves, the age threshold for both groups was 70 years, which also transformed into a dichotomous variable, but its AUC was 0.650 and p > 0.05. The area under the ROC curve (AUC) of neutrophil ratio, NLR, LDH, and CK all surpassed 0.8 (0.855, 0.837, 0.807, and 0.834, respectively); 95% confidence intervals (CIs) were 0.715–0.944, 0.710–0.963, 0.677–0.937, and 0.718–0.950; all p-values were lower than 0.05 (0.01, 0.001, 0.007, 0.007 respectively). After that, binary logistic regression analysis showed that all 13 variables (including age) were risk factors for the severe group, but the lymphocyte ratio was not statistically significant (p = 0.467), while the others were statistically significant (p < 0.05) (Table 5). And the results were presented by the forest plot (Figure 3). Except for the lymphocyte ratio, the forest plot shows that the number of people in the severe group whose variables exceed the cutoff level is considerably higher than that in the non-severe group.
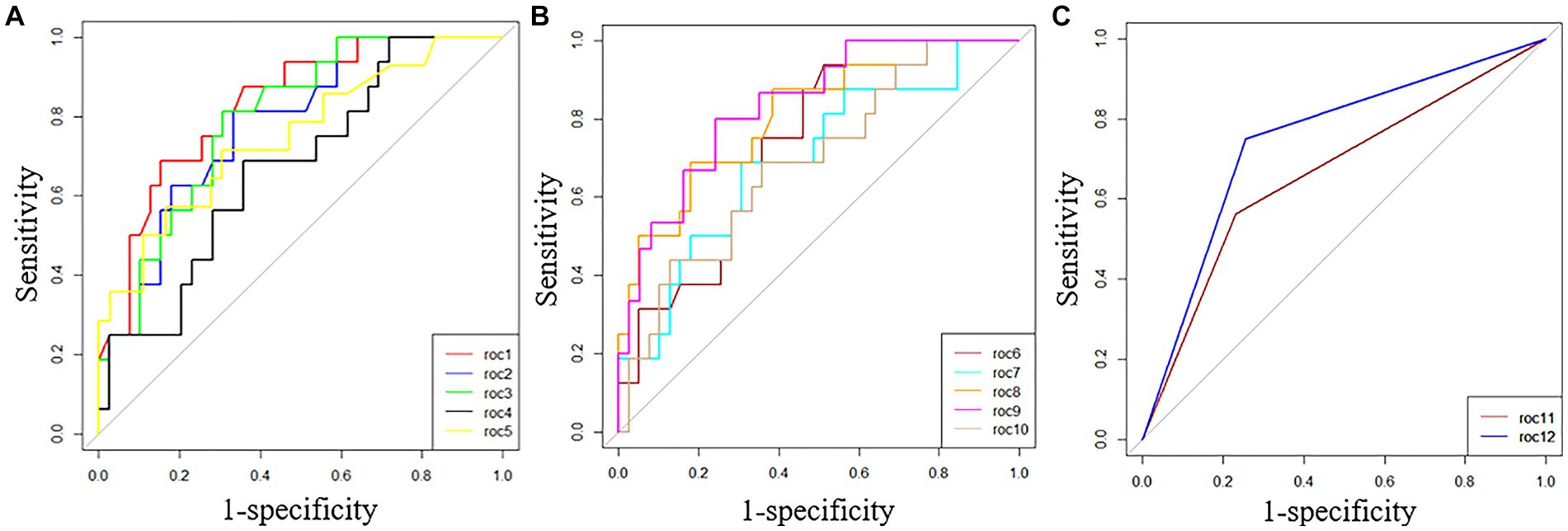
Figure 2. ROC curves of 12 variables predicted for the severe group. (A) roc1 = neutrophil ratio; roc2 = lymphocyte ratio; roc3 = NLR; roc4 = rapid c-reactive protein; roc5 = procalcitonin. (B) roc6 = serum albumin; roc7 = AST; roc8 = LDH; roc9 = CK; roc10 = serum creatinine. (C) roc11 = bilateral lung inflammation; roc12 = multiple lobes infiltration.
The factors that could identify severe pneumonia and with good identification ability were found by binary Logistic regression analysis and ROC curves. Those factors were neutrophil ratio, NLR, LDH, and CK, with respective ORs of 10.057, 9.750, 10.057, and 9.667; 95% CIs of 2.643–38.276, 2.339–40.649, 2.643–38.276, 2.490–37.527; and all were statistically significant (all p < 0.05; 0.001, 0.002, 0.001, 0.001 respectively).
Discussion
This is an extended retrospective study of the epidemiology and clinical characteristics of C. psittaci pneumonia. A total of 55 patients were examined at four major medical facilities in Wuxi. It presents the latest status of C. psittaci pneumonia infection in Wuxi, China. In this paper, we summarized the clinical characteristics of C. psittaci pneumonia, identified predictors of severe pneumonia, and pointed out that mNGS is a means to provide early diagnosis.
As everyone knows, C. psittaci pneumonia is rare, accounting for one to 8% of CAP cases (2, 16). However, with the increased use of mNGS in recent years, it has been discovered that the detection rate of C. psittaci in CAP patients is not low. We can find 14 cases with severe C. psittaci pneumonia diagnosed by mNGS (3) and 32 other patients diagnosed with C. psittaci pneumonia by mNGS during the COVID-19 pandemic (15). We have reason to suggest that physicians should be vigilant about atypical pneumonia caused by uncommon pathogens such as C. psittaci.
The clinical features of these patients in this retrospective study were not the same, but they had many symptoms in common. It was found that the most prominent symptom was fever, which occurred in all patients; most were high fever, their body temperature was over 39.1°C, and most with chills. Other typical symptoms of C. psittaci pneumonia include cough, expectoration, weakness, etc. This report is similar to previous ones (17). On the other hand, it differs from previous studies in that most patients had flu-like symptoms, such as fever, fatigue, muscle aches, headaches, and sore throats (7, 18). Aside from fever, other flu-like symptoms were uncommon in this study. The study found that only 12.7% of patients suffered from chest tightness and shortness of breath. The proportion was significantly lower than in other studies (1, 7). Different diagnostic classifications of dyspnea and treatment approaches may explain this.
When a patient with a high fever has a chest CT that shows patchy consolidation with an air bronchogram and ground-glass opacity, C. psittaci pneumonia is highly suspected. According to this study, 76.4% of patients had patchy consolidation with the air bronchogram sign and ground-glass opacity on chest CT, the consolidation performance reached 87.3%, and pleural effusion was not uncommon. Several imaging characteristics, such as consolidation of the chest with a bronchogram obtained via chest CT, have been reported (19). Most patients have bilateral, middle-lower lobe, or multilobar lesions, usually presenting as consolidation, and have pleural effusions (7). Moreover, bilateral lung consolidation and numerous lobe infiltration were observed primarily in severe cases. However, these signs differ from our understanding of atypical pathogen pneumonia, such as Chlamydia pneumoniae. In contrast to C. psittaci pneumonia, thickening of the bronchial walls is most common in M. pneumoniae pneumonia, followed by nodules (tree-in-bud and centrilobular) (81%) (20). Identifying these CT signs can help diagnose C. psittaci pneumonia.
The erythrocyte sedimentation rate, rapid C-reactive protein, CK, and LDH were significantly elevated in laboratory tests. It is known that CK is distributed in many tissues, mainly muscle tissue. High CK levels occur in C. psittaci pneumonia, but the mechanism is unclear, and high CK levels are an independent risk factor for severe C. psittaci pneumonia (11). Other studies have found that CK levels greater than 174 U/L are a risk factor for severe C. psittaci pneumonia (7). Our study found most patients had CK levels higher than 196 U/L, and one of the 55 patients had a significant increase, as high as 34,035 U/L, coupled with rhabdomyolysis in our study. We also found that LDH was significantly increased in most patients (70.9%). This discovery has also been proposed previously (7). The LDH level in the severe group was higher than in the non-severe group, and the difference was statistically significant. These are similar to previous studies (1). Additionally, we found from binomial logistic regression analysis and ROC curve analysis that significant increases in CK and LDH levels may indicate the severity of the disease and both may be predictors of severe pneumonia. This is a discovery that needs to be studied further in the future.
More than half of the patients in this study had electrolyte disorders, primarily manifesting as hypokalemia (54.5%), hyponatremia (56.4%), and hypophosphatemia (65.5%). Since there are few studies on electrolyte disorders, we are interested in learning whether more case reports can provide diagnostic information. Additionally, 21 patients (48.4%) were in ketosis, 5 of whom were diabetic.
It is consistent with the previous reports that the leukocyte increase is not noticeable (4), the neutrophil ratio and NLR increase, the lymphocyte ratio decreases, the PCT increases, the transaminase increases, the creatinine and urea nitrogen increases, and the hypoproteinemia increases, etc. (1, 7). However, in our study, further binomial logistic regression analysis and ROC curve analysis showed that both the increase of neutrophil ratio and NLR were risk factors of the severe group, with ORs of 10.057 and 9.750 respectively, both statistically significant, and AUCs of both were over 0.8, both also statistically significant. This result implies that neutrophil ratio and NLR elevation are indicators of severity and predictors of severe pneumonia.
When a CAP patient with a high fever has the above characteristics, such as a CT showing consolidation with an air bronchogram sign and ground-glass opacity, laboratory tests reveal a significant increase in rapid c-reactive protein, no significant increase in white blood cells, significant increases in CK and LDH, hypokalemia, hyponatremia, hypophosphatemia, and so on. In this patient, there is a high probability of C. psittaci pneumonia, and the history of bird exposure should be verified, although this history of bird exposure should be clarified at the initial visit. However, most patients dispute that we should follow up on their history during diagnosis and therapy and are encouraged to recall previous contact information. A study conducted in this study found that 30 patients had a history of exposure to pigeons or parrots or their internal organs. The research discovered that nearly all patients had had close contact with birds or poultry (16). Several reports showed that most patients had a history of poultry contact (7, 15). Among 116 patients studied, only 18 (15.5%) had a history of exposure (1). Sometimes it is difficult to determine the exposure history. As in our study, one patient denied any history of bird contact. However, after being diagnosed with C. psittaci Pneumonia, the patient replied that he walked in a park where pigeons were around. This may be an indirect exposure. It reminds us to ask about the patient’s hobbies and habits.
How is the diagnosis of C. psittaci pneumonia confirmed when clinical features and exposure history are highly suggestive? We know that conventional methods of diagnosing C. psittaci have poor sensitivity and specificity (21). To identify pathogenic bacteria, PCR or mNGS is needed. Nucleic acid PCR assays, which do not rely on culture techniques, are rapid and accurate, but these methods require a priori knowledge or assumptions about the pathogen type. mNGS has the main advantage of unbiased sampling, the ability to detect all potential pathogens in the same sample simultaneously and the avoidance of pre-defined detection ranges. Therefore, mNGS has a clear advantage in the diagnosis of unexplained and co-infected lower respiratory tract infections (22). For mNGS testing, BALF is usually used, but sputum or serum can also be tested if bronchoscopy is not an option. In this study, a patient admitted to the intensive care unit was too ill to undergo bronchoscopy in the early stages. Therefore, a serum mNGS test was performed, which revealed a C. psittaci infection. Following this test, the appropriate antibiotics were immediately adjusted, enabling the patient to survive and receive effective antimicrobial treatment. Additionally, the symptoms of C. psittaci pneumonia are not unique and can be seen in other diseases as well. Furthermore, not all patients can give a history of their exposure. As a result, the diagnosis is not straightforward. In this situation, mNGS is still required. Since mNGS is unbiased and hypothesis-free, it has been widely used in the diagnosis of respiratory tract infections (22). In the case of unexplained pneumonia, mNGS could help identify rare pathogens and shorten diagnosis time. mNGS assays in the lower respiratory tract or pleural effusion samples can provide a rapid and early diagnosis of severe M. pneumoniae pneumonia with ARDS (23). In contrast to traditional microbial culture methods, mNGS detects pathogens within 3 days (24). More evidence suggests that mNGS is an effective tool for diagnosing C. psittaci pneumonia (7, 25). But for some patients, mNGS testing is unaffordable due to its high cost.
Our study was fortunate to have no deaths among the patients. An intensive care unit admitted three patients, one of whom required tracheal intubation with mechanical ventilation and ECMO, and the outcome was positive. This could be due to the fast etiological diagnosis of these patients using mNGS, which allowed them to receive effective antibacterial therapy. The treatment of C. psittaci is straightforward. C. psittaci can be treated with tetracyclines, macrolides, and quinolones (26). In this study, each patient received either doxycycline, moxifloxacin, or nenofloxacin malate. A few patients with poor results on oral moxifloxacin were switched to oral doxycycline, and some patients with allergic reactions to oral doxycycline were switched to quinolones. Tetracycline-resistant chlamydia strains have been reported in recent years due to the widespread use of tetracycline in poultry (27, 28). A new generation of quinolones, moxifloxacin is highly effective against C. psittaci (1). However, the antibiotics must be personalized to the particular condition.
A total of three patients in our study had vascular embolism events, namely pulmonary embolism, cerebral artery thrombosis, and bilateral lower limb vein thrombosis. The literature search did not yield any relevant articles. It is unclear whether C. psittaci infections cause vascular emboli, and further observation and research are needed.
There are several limitations to this study. First, only 55 patients with confirmed C. psittaci pneumonia were included; those with strong clinical suspicion but no evidence of pathogens were excluded. As a consequence, some information may be missed. Second, most patients were diagnosed with BALF mNGS but did not receive standard laboratory tests. Not all patients can afford mNGS. Third, several factors regarding the use of medication, such as drug combinations, different types and manufacturers, and irregular application of antibiotics against C. psittaci, such as one patient being administered moxifloxacin and doxycycline consecutively, were included. These might lead to bias in the analysis of drug efficacy. Research on these drugs’ efficacy in C. psittaci pneumonia requires more in-depth prospective studies.
Conclusion
There were 55 hospitalized patients with confirmed C. psittaci pneumonia in this multi-centre case series in Wuxi, China. We found that many clinical indicators were typical. Meanwhile, significant increases in neutrophil ratio, NLR, LDH, and CK predicted severe pneumonia. Timely detection of mNGS provides substantial help for clinical diagnosis and early treatment. This provides clinicians with a better understanding of the disease and some useful information for the CDC to help them carry out effective epidemiological control measures.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
TB and SH: conception and design of the experiments. YG, DX, LB, XD, and CM: collection of clinical data. YG and DX: analysis of the data. YG and YW: writing the text of the main manuscript. LB and LL: preparation of tables and figures. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank all patients involved in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yang, M, Yang, DH, Yang, H, Ding, SZ, Liu, CH, Yin, HM, et al. Clinical characteristics of Chlamydia psittaci pneumonia infection in central south China. Infect Dis Ther. (2022) 11:1631–47. doi: 10.1007/s40121-022-00662-4
2. Hogerwerf, L, De Gier, B, Baan, B, and van der Hoek, W. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect. (2017) 145:3096–105. doi: 10.1017/S0950268817002060
3. Zhang, A, Xia, X, Yuan, X, Lv, Y, Liu, Y, Niu, H, et al. Clinical characteristics of 14 cases of severe Chlamydia psittaci pneumonia diagnosed by metagenomic next-generation sequencing: a case series. Medicine. (2022) 101:e29238. doi: 10.1097/MD.0000000000029238
4. Zhang, Z, Wang, P, Ma, C, Wang, J, Li, W, Quan, C, et al. Host inflammatory response is the major factor in the progression of Chlamydia psittaci pneumonia. Front Immunol. (2022) 13:929213. doi: 10.3389/fimmu.2022.929213
5. Ruaro, B, Salton, F, Braga, L, Wade, B, Confalonieri, P, Volpe, MC, et al. The history and mystery of alveolar epithelial type II cells: focus on their physiologic and pathologic role in lung. Int J Mol Sci. (2021) 22:2566. doi: 10.3390/ijms22052566
6. Chiu, CY, and Miller, SA. Clinical metagenomics. Nat Rev Genet. (2019) 20:341–55. doi: 10.1038/s41576-019-0113-7
7. Jiang, J, Yang, W, Wu, Y, Peng, W, Zhang, W, Pan, P, et al. Metagenomic next-generation sequencing for identifying pathogens in patients with rheumatic diseases and diffuse pulmonary lesions: a retrospective diagnostic study. Front Cell Infect Microbiol. (2022) 12:963611. doi: 10.3389/fcimb.2022.963611
8. Liu, X, Hou, XF, Gao, L, Deng, GF, Zhang, MX, Deng, QY, et al. Indicators for prediction of Mycobacterium tuberculosis positivity detected with bronchoalveolar lavage fluid. Infect Dis Poverty. (2018) 7:22. doi: 10.1186/s40249-018-0403-x
9. Zhou, JJ, Ding, WC, Liu, YC, Gao, YL, Xu, L, Geng, RL, et al. Diagnostic value of metagenomic next-generation sequencing for pulmonary infection in intensive care unit and non-intensive care unit patients. Front Cell Infect Microbiol. (2022) 12:929856. doi: 10.3389/fcimb.2022.929856
10. Wu, X, Li, Y, Zhang, M, Li, M, Zhang, R, Lu, X, et al. Etiology of severe community-acquired pneumonia in adults based on metagenomic next-generation sequencing: a prospective multicenter study. Infect Dis Ther. (2020) 9:1003–15. doi: 10.1007/s40121-020-00353-y
11. Su, S, Su, X, Zhou, L, Lin, P, Chen, J, Chen, C, et al. Severe Chlamydia psittaci pneumonia: clinical characteristics and risk factors. Ann Palliat Med. (2021) 10:8051–60. doi: 10.21037/apm-21-1502
12. Metlay, JP, Waterer, GW, Long, AC, Anzueto, A, Brozek, J, Crothers, K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. (2019) 200:e45–67. doi: 10.1164/rccm.201908-1581ST
13. Miao, Q, Ma, Y, Wang, Q, Pan, J, Zhang, Y, Jin, W, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. (2018) 67:S231–s40. doi: 10.1093/cid/ciy693
14. Chen, X, Cao, K, Wei, Y, Qian, Y, Liang, J, Dong, D, et al. Metagenomic next-generation sequencing in the diagnosis of severe pneumonias caused by Chlamydia psittaci. Infection. (2020) 48:535–42. doi: 10.1007/s15010-020-01429-0
15. Yin, Q, Li, Y, Pan, H, Hui, T, Yu, Z, Wu, H, et al. Atypical pneumonia caused by Chlamydia psittaci during the COVID-19 pandemic. Int J Infect Dis. (2022) 122:622–7. doi: 10.1016/j.ijid.2022.07.027
16. Wu, HH, Feng, LF, and Fang, SY. Application of metagenomic next-generation sequencing in the diagnosis of severe pneumonia caused by Chlamydia psittaci. BMC Pulm Med. (2021) 21:300. doi: 10.1186/s12890-021-01673-6
17. Knittler, MR, and Sachse, K. Chlamydia psittaci: update on an underestimated zoonotic agent. Pathog Dis. (2015) 73:1–15. doi: 10.1093/femspd/ftu007
18. Branley, JM, Weston, KM, England, J, Dwyer, DE, and Sorrell, TC. Clinical features of endemic community-acquired psittacosis. New Microbes New Infect. (2014) 2:7–12. doi: 10.1002/2052-2975.29
19. Tang, J, Tan, W, Luo, L, Xu, H, and Li, N. Application of metagenomic next-generation sequencing in the diagnosis of pneumonia caused by Chlamydia psittaci. Microbiol Spectr. (2022) 10:e0238421. doi: 10.1128/spectrum.02384-21
20. Miyashita, N, Nakamori, Y, Ogata, M, Fukuda, N, Yamura, A, Ishiura, Y, et al. Early identification of novel coronavirus (COVID-19) pneumonia using clinical and radiographic findings. J Infect Chemother. (2022) 28:718–21. doi: 10.1016/j.jiac.2022.02.005
21. Nieuwenhuizen, AA, Dijkstra, F, Notermans, DW, and van der Hoek, W. Laboratory methods for case finding in human psittacosis outbreaks: a systematic review. BMC Infect Dis. (2018) 18:442. doi: 10.1186/s12879-018-3317-0
22. Diao, Z, Han, D, Zhang, R, and Li, J. Metagenomics next-generation sequencing tests take the stage in the diagnosis of lower respiratory tract infections. J Adv Res. (2022) 38:201–12. doi: 10.1016/j.jare.2021.09.012
23. Ding, L, Zhao, Y, Li, X, Wang, R, Li, Y, Tang, X, et al. Early diagnosis and appropriate respiratory support for Mycoplasma pneumoniae pneumonia associated acute respiratory distress syndrome in young and adult patients: a case series from two centers. BMC Infect Dis. (2020) 20:367. doi: 10.1186/s12879-020-05085-5
24. Shi, CL, Han, P, Tang, PJ, Chen, MM, Ye, ZJ, Wu, MY, et al. Clinical metagenomic sequencing for diagnosis of pulmonary tuberculosis. J Infect. (2020) 81:567–74. doi: 10.1016/j.jinf.2020.08.004
25. Gu, L, Liu, W, Ru, M, Lin, J, Yu, G, Ye, J, et al. The application of metagenomic next-generation sequencing in diagnosing Chlamydia psittaci pneumonia: a report of five cases. BMC Pulm Med. (2020) 20:65. doi: 10.1186/s12890-020-1098-x
26. Kohlhoff, SA, and Hammerschlag, MR. Treatment of chlamydial infections: 2014 update. Expert Opin Pharmacother. (2015) 16:205–12. doi: 10.1517/14656566.2015.999041
27. Unterweger, C, Schwarz, L, Jelocnik, M, Borel, N, Brunthaler, R, Inic-Kanada, A, et al. Isolation of tetracycline-resistant Chlamydia suis from a pig herd affected by reproductive disorders and conjunctivitis. Antibiotics. (2020) 9:187. doi: 10.3390/antibiotics9040187
Keywords: Chlamydia psittaci pneumonia, severe pneumonia, metagenomic next-generation sequencing, retrospective analysis, clinical characteristics, predictors
Citation: Gao Y, Wu Y, Xu D, Bao L, Ding X, Lv L, Ma C, Bian T and Han S (2023) Chlamydia psittaci pneumonia in Wuxi, China: retrospective analysis of 55 cases and predictors of severe disease. Front. Med. 10:1150746. doi: 10.3389/fmed.2023.1150746
Edited by:
Patricia Khashayar, Ghent University, BelgiumReviewed by:
Valentina A. Feodorova, Federal Research Center for Virology and Microbiology, RussiaJie Chen, Shanghai Jiao Tong University, China
Copyright © 2023 Gao, Wu, Xu, Bao, Ding, Lv, Ma, Bian and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Bian, YmlhbnRhb3BoZEAxMjYuY29t; Shuguang Han, aHNnenhzQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Ying Gao
Ying Gao Yan Wu2†
Yan Wu2†