- 1Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States
- 2Department of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States
- 3Centre for Inflammation Research, University of Edinburgh, Edinburgh, United Kingdom
- 4Network for Improving Critical Care Systems and Training, Colombo, Sri Lanka
- 5Nat Intensive Care Surveillance-MORU, Colombo, Sri Lanka
- 6Kijabe Hospital, Kijabe, Kenya
- 7Clinical Research, Investigation, and Systems Modeling of Acute Illness Center, University of Pittsburgh, Pittsburgh, PA, United States
- 8Malawi-Liverpool-Wellcome Trust Clinical Research Programme, Blantyre, Malawi
- 9Division of Allergy, Pulmonary, and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, TN, United States
- 10Critical Illness, Brain Dysfunction, and Survivorship Center, Vanderbilt University Medical Center, Nashville, TN, United States
- 11Geriatric Research, Education, and Clinical Center, Tennessee Valley Healthcare System, Nashville, TN, United States
- 12Liverpool School of Tropical Medicine, Liverpool, United Kingdom
- 13University College London Hospitals, London, United Kingdom
- 14University Hospital-Kotelawala Defence University, Boralesgamuwa, Sri Lanka
- 15Department of Global Health and Social Medicine, Harvard Medical School, Boston, MA, United States
- 16Hypoxia Research Laboratory, University of California, San Francisco, San Francisco, CA, United States
- 17Center for Health Equity in Surgery and Anesthesia, University of California, San Francisco, San Francisco, CA, United States
- 18Department of Anesthesia and Perioperative Care, University of California, San Francisco, San Francisco, CA, United States
- 19Harvard Medical School, Boston, MA, United States
- 20Department of Surgery, Faculty of Health Sciences, Egerton University, Nakuru, Kenya
- 21Mahidol Oxford Tropical Medicine Research Unit, Bangkok, Thailand
- 22Mercy Ships, Lindale, TX, United States
- 23College of Medicine and Health Sciences, University of Rwanda, Kigali, Rwanda
- 24University Teaching Hospital of Kigali, Kigali, Rwanda
- 25Division of Pulmonary, Critical Care, Allergy, and Sleep Medicine, Department of Medicine, University of California, San Francisco, San Francisco, CA, United States
- 26Department of Anaesthesia, Aga Khan University, Nairobi, Kenya
- 27University Teaching Hospital of Butare, Butare, Rwanda
- 28Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States
Knowing the target oxygen saturation (SpO2) range that results in the best outcomes for acutely hypoxemic adults is important for clinical care, training, and research in low-income and lower-middle income countries (collectively LMICs). The evidence we have for SpO2 targets emanates from high-income countries (HICs), and therefore may miss important contextual factors for LMIC settings. Furthermore, the evidence from HICs is mixed, amplifying the importance of specific circumstances. For this literature review and analysis, we considered SpO2 targets used in previous trials, international and national society guidelines, and direct trial evidence comparing outcomes using different SpO2 ranges (all from HICs). We also considered contextual factors, including emerging data on pulse oximetry performance in different skin pigmentation ranges, the risk of depleting oxygen resources in LMIC settings, the lack of access to arterial blood gases that necessitates consideration of the subpopulation of hypoxemic patients who are also hypercapnic, and the impact of altitude on median SpO2 values. This process of integrating prior study protocols, society guidelines, available evidence, and contextual factors is potentially useful for the development of other clinical guidelines for LMIC settings. We suggest that a goal SpO2 range of 90-94% is reasonable, using high-performing pulse oximeters. Answering context-specific research questions, such as an optimal SpO2 target range in LMIC contexts, is critical for advancing equity in clinical outcomes globally.
Introduction
Guidelines for best-practice oxygen saturation (SpO2) targets in acutely hypoxemic adults are based entirely on evidence from high-income countries (HICs), which encompass only 16% of the world’s population (1–11). This can be problematic for clinical staff, clinical teachers, and researchers in low-income and lower-middle income countries (collectively LMICs) who are working to improve patient outcomes with oxygen therapy. As Chowdhury et al. note when examining a case of stroke care in an LMIC setting, “evidence-based standards of care cannot be separated from the contexts in which they are produced” (12). This is not simply a need to acknowledge that certain recommended interventions may not be available in all settings. Features of a given context may actually shift the risk-benefit balance of an intervention toward a different best-practice standard of care; differences in context could mean that a given intervention that leads to better outcomes in one setting could lead to neutral or worse outcomes in another. There is a specific example of this complexity in the sepsis literature, where fluid resuscitation volumes found to be life-saving or neutral in multiple HICs were found to be harmful in LMICs (13–18). The reasons for this may have to do with differences in the underlying etiologies of sepsis, timing of patient presentation, and lack of availability of ventilators to “rescue” patients from fluid overload (19).
Not only does all of the evidence on optimal SpO2 target ranges emanate from HICs, but also the evidence does not definitively point to an optimal SpO2 range (2–11, 20). Our research team is planning a trial of high flow versus standard flow oxygen delivery in five hospitals in three LMIC countries in sub-Saharan Africa (Kenya, Malawi, and Rwanda) (21). To ensure consistent practices in the two arms of the trial, we need to choose an SpO2 range to target for titrating oxygen therapy in both arms. The trial itself is based on the premise that context matters: the question of whether high flow oxygen is superior to standard flow has been explored in multiple HIC settings (22), but different epidemiology and resources may change the answer as to whether high flow oxygen should be used in LMIC settings for the best patient- and systems-level outcomes.
Methods
To identify the optimal SpO2 range for patients enrolled in the trial, we conducted a scoping literature review regarding SpO2 targets for patients with hypoxemia (23). We reviewed prior interventional trials in hypoxemic adults to determine the precedent of SpO2 target ranges set in research protocols. We examined SpO2 target guidelines from national and international respiratory and critical care organizations for patients with hypoxemia. We also looked at direct evidence from trials comparing patient outcomes using different SpO2 target ranges. We explored literature regarding context-specific considerations that could impact the choice of best-practice SpO2 range in LMICs, including the relative inaccuracy of pulse oximetry in patients with darker skin pigmentation and shock, the need to conserve oxygen resources for all patients (24, 25), the lack of consistent access to arterial blood gases, which necessitates consideration of hypercapnic patients, and the impact of altitude on SpO2 (26–28).
SpO2 ranges used in previous interventional trials
In previous ARDSNet trials evaluating mortality outcomes with interventions in patients with acute respiratory distress syndrome (ARDS), target oxygenation has been set at 88–95% (Table 1). The landmark trial of low tidal volume ventilation in ARDS chose this target range for study participants (29). The average level of arterial partial pressure of oxygen (PaO2; SpO2 not reported) reflects this and was similar between both groups when measured at days 1, 3, and 7 (range 73–77 mmHg). More recent studies assessing proning and paralysis in ARDS used this same 88–95% range without differences in measured PaO2 between groups (30, 31). These studies were all conducted in patients in HICs admitted to intensive care units with ARDS.
Other studies that assessed acute hypoxemic respiratory failure (AHRF) in non-intubated patients have used a higher SpO2 cutoff (Table 1). The FLORALI trial of high flow oxygen, non-invasive ventilation, and standard flow oxygen in acutely hypoxemic adults used a target SpO2 of ≥92%; the HIGH trial of high flow versus standard oxygen in immunocompromised adults with acute hypoxemia targeted ≥95% (32, 33). The Recovery-RS trial, which was conducted to assess optimal non-invasive respiratory support modalities in patients with COVID-19 and AHRF, did not set a specific oxygen saturation target and instead deferred to individual study site policies and clinical discretion (34).
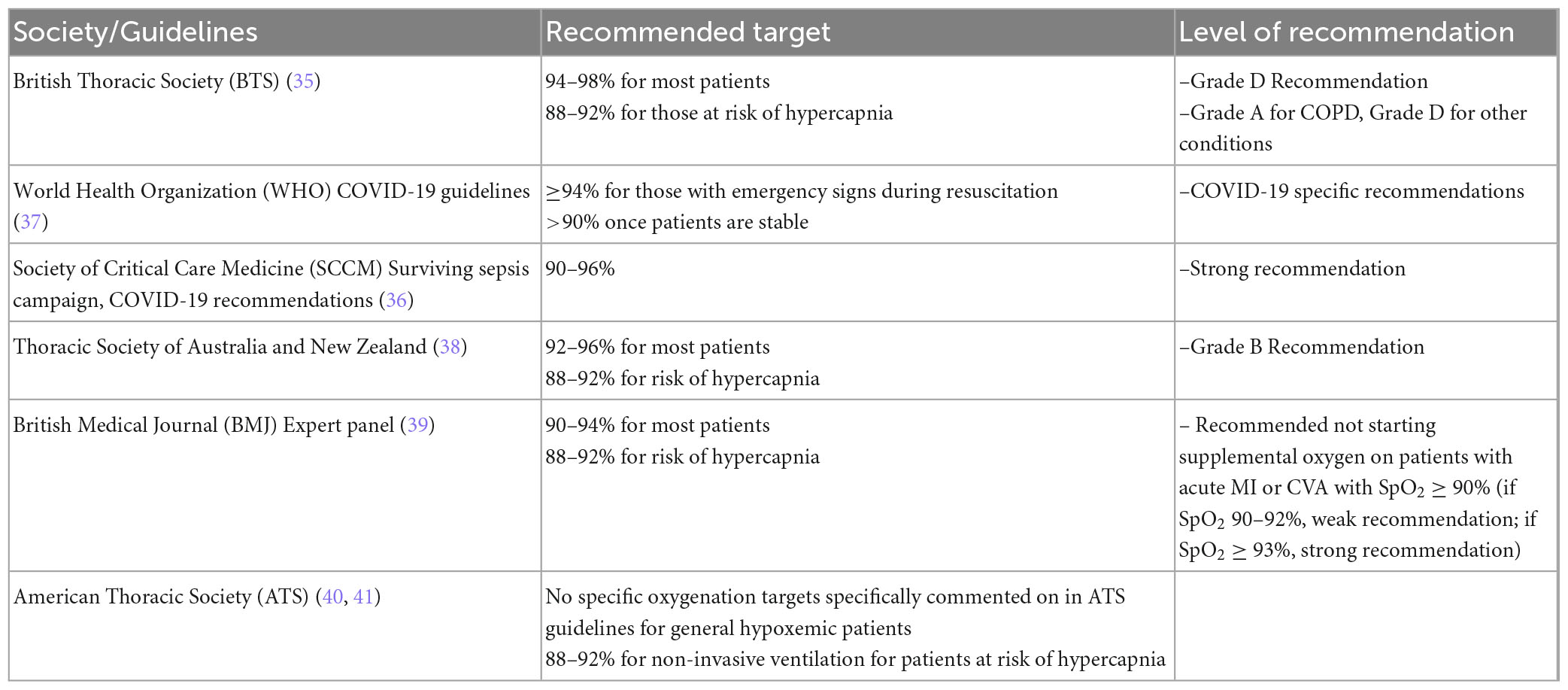
Society guidelines for SpO2 targets
Multiple professional societies provide recommendations and guidance for SpO2 targets for patients with AHRF (Table 2). The British Thoracic Society (BTS) guidelines recommend a higher oxygenation goal of 94–98%, except for patients at risk for hypercapnia, for which they recommend a goal of 88–92% (35). Other groups, including the World Health Organization (WHO), Society of Critical Care Medicine (SCCM) Surviving Sepsis campaign, and the Thoracic Society of Australia and New Zealand, advocate for a more conservative oxygenation goal typically of at least ≥90%, except again for patients at risk for hypercapnia, where they recommend 88–92% (36–38). In 2018, a British Medical Journal (BMJ) Expert panel evaluated data on oxygenation targets in multiple clinical scenarios, including respiratory failure, myocardial infarction, and acute stroke. The expert panel recommended a general oxygenation goal of 90–94% for most patients, with an adjusted goal of 88–92% for those at risk of hypercapnia (39). The American Thoracic Society (ATS) does not specifically cite an oxygenation target in their guidelines, with the exception of 88–92% for non-invasive ventilation for patients at risk of hypercapnia (40, 41).
Direct evidence for different SpO2 target ranges
An understanding of the potential risks of both hypoxemia and hyperoxia has driven investigations of the optimal SpO2 target range for different populations of patients. While we know that hypoxemia can result in tissue ischemia and death, we also know that hyperoxia can cause oxidative stress and inflammation, with the potential for negative clinical consequences (42–44). In critically ill (not necessarily hypoxemic) patients, a prior practice of allowing or even targeting hyperoxia to promote tissue oxygenation has been found to be harmful (42, 45). In acutely hypoxemic patients, the question is more complicated, as potentially harmful levels of FiO2 may be needed to reach “normoxia” in some patients; this raises the question of whether not only an avoidance of hyperoxia, but even pursuing some level of permissive hypoxemia, could be beneficial (43).
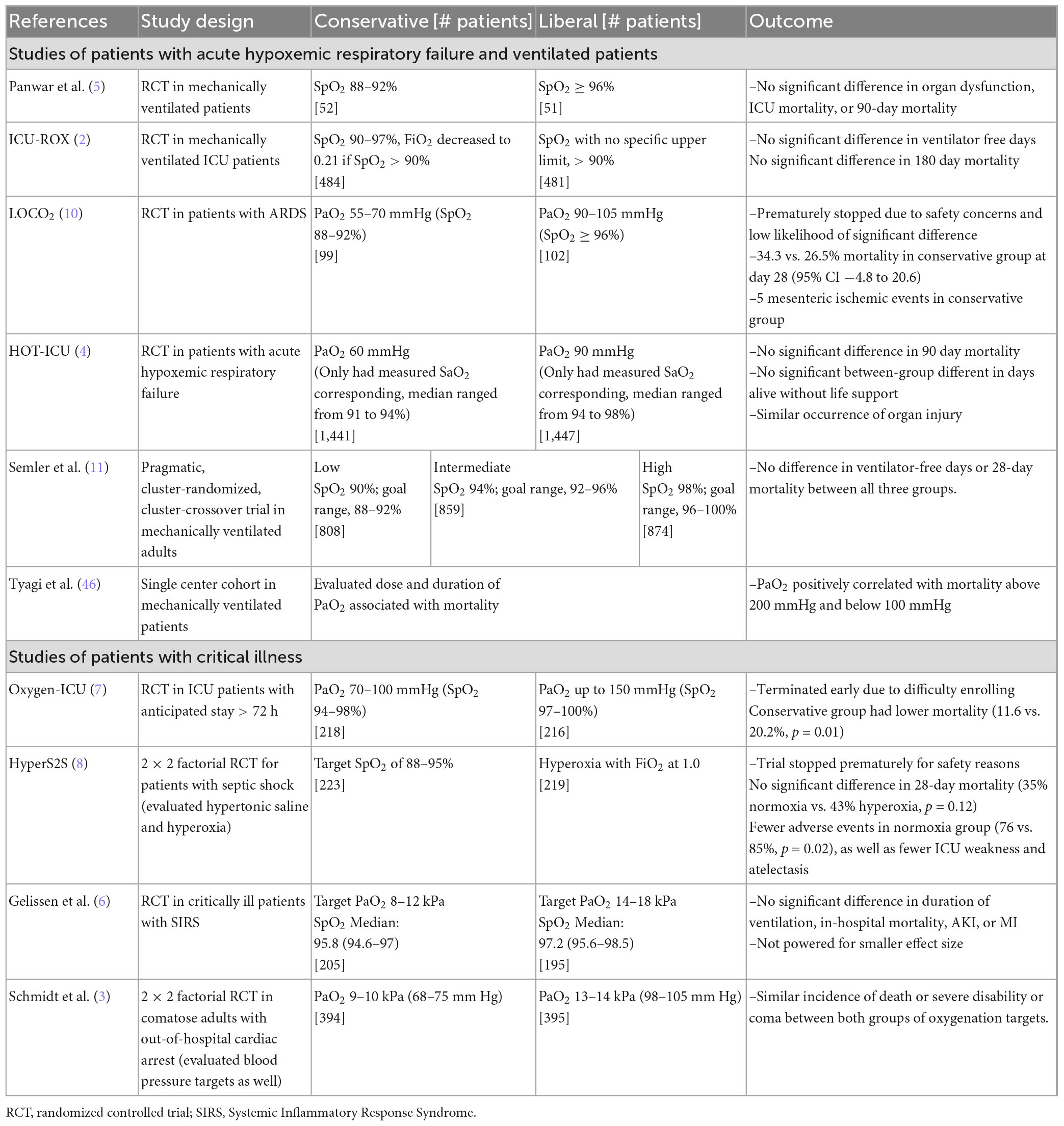
Many recent studies have assessed conservative versus liberal SpO2 goals in different patient populations, with the weight of evidence suggesting that lower (“conservative”) SpO2 targets are not harmful and may even produce superior outcomes as compared with higher (“liberal”) SpO2 targets in some cases. Table 3 outlines these studies, patient populations, and their specific comparisons, all of which occurred in HICs.
SpO2 goals in patients with acute hypoxemic respiratory failure or mechanically ventilated
In patients with acute hypoxemic respiratory failure, ARDS, and patients receiving mechanical ventilation, multiple recent studies have found no difference in outcomes between liberal and conservative oxygen targets (2, 4, 5). Semler et al. assessed outcomes in mechanically ventilated patients with three SpO2 target ranges [low (88–92%), intermediate (92–96%), or high (96–100%)], and found no significant difference in ventilator-free days or mortality between the three target groups (11).
Only one study of patients with respiratory failure, the LOCO2 study, suggested possible harm to lower oxygenation targets, in patients with ARDS. It compared conservative (PaO2 55–70 mmHg or SpO2 88–92%) and liberal (PaO2 90–105 mmHg or SpO2 ≥96%) oxygenation goals, and while the study was stopped early due to low likelihood of significant difference, there were 5 mesenteric ischemic events present in the conservative oxygenation group (10).
Tyagi et al. conducted a recent retrospective cohort study analyzing the relationship between the occurrence of hyperoxia with mortality in mechanically ventilated patients. They identified a U-shaped curve, where PaO2 was positively correlated with mortality below 100 mmHg and above 200 mmHg. This study found that exposure to severe hyperoxia, defined as PaO2 >200 mmHg, correlated with higher mortality (OR 1.29; 95% CI 1.04–1.59) (46).
A systematic review and meta-analysis by Cumpstey et al. included eight trials of mechanically ventilated patients (47). They found a possible increased long-term mortality with targeting hyperoxia versus normoxia, and no difference in outcomes with targeting relative hypoxemia versus normoxemia.
SpO2 goals in critically ill patients
As noted, studies have also been done in critically ill patients (not necessarily with acute hypoxemia or ventilated), largely focused on the question of benefits or harms with hyperoxia, and also comparing different target ranges for SpO2. These have generally confirmed either harm or neutrality with higher oxygen targets.
The HyperS2S study, which was a two-by-two factorial design study including an evaluation of normoxia versus hyperoxia in patients with septic shock, used the same oxygenation target of 88–95% in the normoxia group as the ARDS studies mentioned above. Compared to hyperoxia (using an FiO2 of 1.0 regardless of SpO2), the normoxia group had fewer adverse events and no significant difference in 28-day mortality (35% in normoxia vs. 43% in hyperoxia, p = 0.12) (8). The Oxygen-ICU trial in ICU patients with expected stay >72 h, was terminated early due to difficulty enrolling patients, but the study showed lower mortality in the conservative oxygenation group (SpO2 94–98%) compared to the “conventional” oxygenation group (SpO2 97–100%) (11.6% vs. 20.2%, p = 0.01) (7).
Gelissen et al. compared “low-normal” to “high-normal” oxygenation targets in ICU patients who met two or more criteria for the Systemic Inflammatory Response Syndrome (SIRS) (6). They found no significant different in organ dysfunction between the arms, though the study may have been underpowered to find a difference. Schmidt et al. examined restrictive and liberal oxygenation targets in comatose adults after out-of-hospital cardiac arrest, with a lower target in the liberal arm than Gelissen et al. (3). In this population of critically ill adults, they found no difference in death or severe disability or coma between the two arms.
The Improving Oxygen Therapy in Acute-Illness (IOTA) systematic review, a meta-analysis of 25 randomized controlled trials (RCTs) comparing liberal and conservative oxygen therapy in acutely ill adults, evaluated liberal and conservative oxygen strategies defined either by an FiO2 or target SpO2 value (9). This analysis found that a 1% increase in SpO2 was associated with a 25% increase in relative risk of in-hospital mortality. Overall, liberal oxygen strategies carried an increased relative risk for in-hospital mortality (1.21, 95% CI 1.03–1.43), 30-day mortality (1.14, 95% CI 1.01–1.29), and mortality at longest follow-up (1.10, 95% CI 1.00–1.20).
Across the several ranges of oxygenation targets and several populations examined, it appears that low-normal SpO2 targets and high-normal SpO2 targets result in similar outcomes. Supra-normal target ranges (hyperoxia) may be harmful.
Context-specific considerations in LMICs
Bias in pulse oximetry measurements
Pulse oximetry (SpO2) does not perfectly correlate with arterial oxygen saturation (SaO2) as measured by blood gas, and prior studies have found multiple potential sources of inaccuracy, particularly as patients become sicker, as denoted by rising lactate and hypoxia (48, 49). These include severe hypoxemia, low perfusion, patient movement, and severe anemia. The quality and appropriate use of the device also play a significant role (50).
The accuracy of pulse oximetry can also be influenced by skin pigmentation, leading to measurement bias. Studies have demonstrated discrepancies in oxygen saturation detected by pulse oximetry and true arterial oxygen saturation based on patients’ skin tone (51). Valbuena et al. evaluated discrepancies by race between pulse oximetry and arterial oxygen saturation among patients in medical and surgical wards. They found that, compared to white patients, Black patients had higher odds of having occult hypoxemia noted on arterial blood gas that was not detected by pulse oximetry, with occult hypoxemia defined as arterial blood oxygen saturation (SaO2) of <88% despite a pulse oximetry (SpO2) reading of ≥92% (52). Henry et al. found that, compared to white patients, Black, Asian, and American-Indian patients admitted to the ICU or undergoing surgery during hospitalization were more likely to experience occult hypoxemia; however, the differences were only significant for Black patients after adjustment (OR 1.65; 1.28–2.14; p < 0.001) (53). A systematic review and meta-analysis investigating this bias in pulse oximetry measurement was performed by Shi et al., and found that compared to standard SaO2 measurement, pulse oximetry overestimates oxygen saturation in people with higher levels of skin pigmentation (pooled mean bias 1.11%; 95% CI 0.29–1.93%) and people described as Black/African American (1.52%; 0.95–2.09%) (54).
Henry et al. also looked at clinical outcomes of these disparities, and found that occult hypoxemia was associated with increased odds of mortality in surgical (OR 2.96; 1.20–7.28; p = 0.019) and ICU patients (OR 1.36; 1.03–1.80; p = 0.033). Occult hypoxemia was associated with fewer hospital-free days in surgical patients (−2.5 days; −3.9 to −1.2 days; p < 0.001) but not ICU patients (0.4 days; −0.7 to 1.4 days; p = 0.500) (53). This is in agreement with other studies that have found that patients with occult hypoxemia defined in this case as SpO2 ≥88% despite SaO2 <88%, were at higher risk of organ dysfunction and mortality (55).
This bias in measurement can also influence delivery of medical interventions. Gottlieb et al. investigated how these discrepancies in pulse oximetry measurements translate into racial and ethnic disparities in supplemental oxygen administration. This study found that patients of Asian, Black, and Hispanic race and ethnicity were all associated with a higher SpO2 for a given hemoglobin oxygen saturation. Furthermore, Asian (coefficient, −0.291; 95% CI, −0.546 to −0.035; p = 0.03), Black (coefficient, −0.294; 95% CI, −0.460 to −0.128; p = 0.001) and Hispanic (coefficient, −0.242; 95% CI, −0.463 to −0.020; p = 0.03) race and ethnicity were associated with lower average oxygen delivery rates (56). When controlling for the discrepancy between average SpO2 and average hemoglobin oxygen saturation, race and ethnicity were not associated with oxygen delivery rate. In other words, Asian, Black, and Hispanic patients received less supplemental oxygen than white patients, and this bias was explained by differences in pulse oximeter performance. Fawzy et al. similarly found that inaccuracy of pulse oximetry by race and ethnicity was associated with significantly delayed or unrecognized eligibility for COVID-19 therapies among Black and Hispanic patients (57).
The studies above demonstrate that not only is there significant bias in measurement of oxygen saturation in individuals with darker skin tones, it also translates into poorer healthcare delivery and clinical outcomes for these patients, which further perpetuates health inequities. The literature also highlights that the magnitude of inaccuracy with different skin tones is variable and not well-quantified, differing by device and other patient factors, including low perfusion (49, 58). These studies were all performed in HICs, and there is a critical paucity of such data in LMICs. In our LMIC study settings of Kenya, Malawi, and Rwanda, most patients are Black. In addition, the inability to monitor oxygen saturation continuously or frequently in these settings also increases the risk of late identification of hypoxemia.
Oxygen conservation
While the optimal SpO2 target range is not known, we do know that high levels of SpO2 can be harmful for patients (46). In addition, the lack of adequate supplemental oxygen supply is still a significant barrier to the provision of adequate oxygen therapy in many LMICs (25). Geographical constraints to oxygen delivery and periods of high demand, among other logistical barriers, highlight the insufficient access to oxygen in many health systems across the world (59, 60). This is confirmed by World Health Organization (WHO) data that indicated that 35% of LMIC hospitals evaluated did not have any access to supplemental oxygen (61). While HIC settings generally do not need to consider the possibility that higher SpO2 target ranges could result in shortages of oxygen and therefore critical hypoxemia for a proportion of hypoxemic patients, this is a relevant consideration for LMIC settings. If unnecessarily high SpO2 targets result in an inability to provide oxygen to a proportion of hypoxemic patients, then this will result in worse outcomes overall for the population of hypoxemic patients. The risks of hyperoxia, coupled with the fact that supplemental oxygen is a scarce resource that can be depleted in LMIC settings, highlights the need to consider the full context and population consequences when deciding on an SpO2 target range. Prior studies have suggested that even small differences in SpO2 target ranges can have profound impacts on oxygen consumption (62). Semler et al. also demonstrated that lower SpO2 targets were associated with overall lower FiO2 despite no difference in hypoxemia (SpO2 <85%) (11). This demonstrates a lower SpO2 target could decrease overall oxygen resource utilization without carrying a risk for worse hypoxemia.
Lack of consistent access to arterial blood gases
Many LMIC sites do not have consistent access to arterial blood gases. This means that pulse oximetry is crucial for the ability to recognize and treat hypoxemia, even recognizing the limitations of pulse oximetry noted above (24, 63).
The lack of consistent access to arterial blood gases also means that LMIC sites will often not be able to identify the subpopulation of hypoxemic patients who also have hypercapnia. A chosen SpO2 target range must be safe for the subpopulation of hypoxemic patients who are also hypercapnic. Higher SpO2 targets in this population can dangerously worsen hypercapnia due to an increase in dead space, the Haldane effect, and a decrease in minute ventilation (64). For this population, as noted above, the accepted target range is 88–92% (35–41).
Altitude
The impact of altitude on median SpO2 in a healthy population is not specific to LMIC settings, but is another contextual factor that could impact the choice of SpO2 target range (26–28). Median SpO2 values decrease in healthy populations as altitude increases (27, 28). Four of our five study sites are located at an altitude above 1,500 m, and thus likely have a somewhat lower median SpO2 value for their healthy populations than sea-level locations. While it is not well-understood whether or how SpO2 targets should be adjusted based on the “normal” values for a population at a given altitude (26), sites at higher altitudes may want to choose lower SpO2 target ranges to account for the lower “normoxia” ranges of their own population.
SpO2 target range of 90–94%
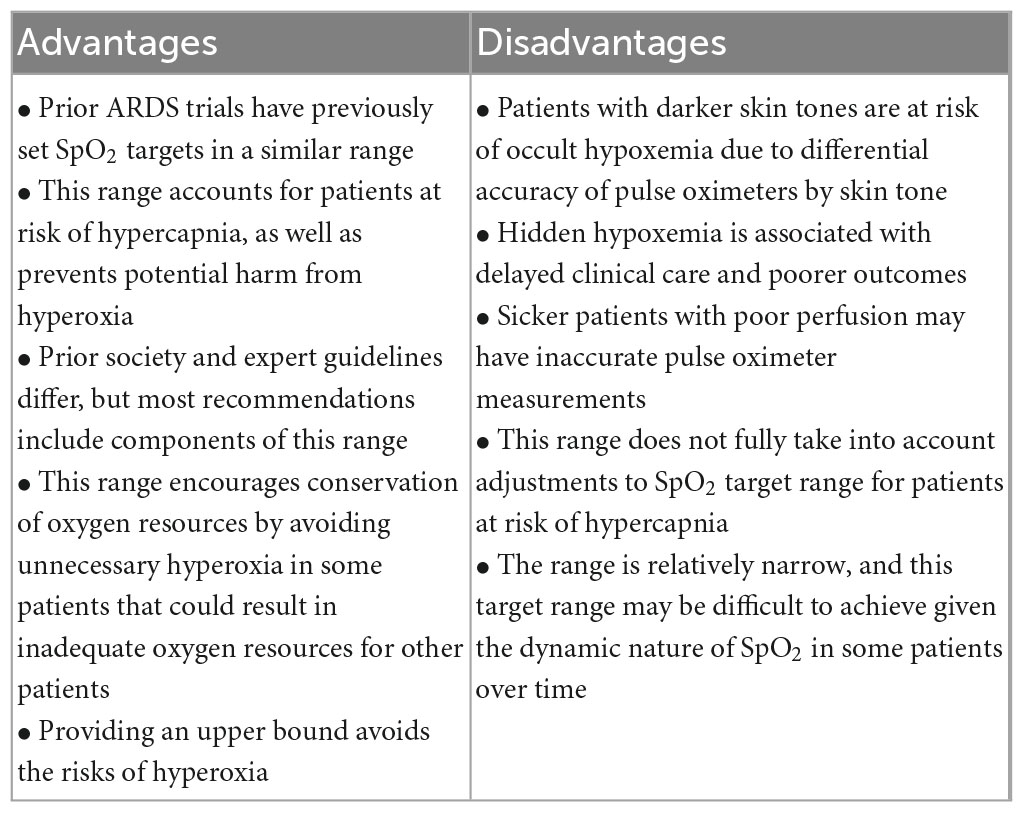
Based on the review of available evidence and discussion among investigators and clinicians across study sites, we preliminarily decided to use 90–94% as the target SpO2 range for our trial of high flow versus standard flow oxygen (Table 4). This was consistent with precedent from prior trials, multiple society guidelines, and the evidence we have for liberal versus conservative targets. Our choice of target SpO2 range of 90–94% also accounts for contextual factors, including pulse oximetry bias in patients with dark skin pigment, oxygen conservation goals, the need to consider hypercapnic patients, and the impact of altitude on the median SpO2 of a population. Recognizing that pulse oximeters have a range of performance characteristics, (65, 66) we are mitigating the risk of occult hypoxemia by using pulse oximeters that have expanded evidence for accurate performance with a range of skin pigmentation levels (67); locations with less-studied or poorly-performing pulse oximeters may want to choose a higher range.
Conclusion
The optimal SpO2 target range for acute hypoxemic adults has not been established by trial evidence. Landmark studies of hypoxemic respiratory failure have often used a range of 88–95%, and society guidelines recommend at least an SpO2 > 90% (88–92% for patients with hypercapnia). A lower SpO2 target might lead to occult hypoxemia and worse outcomes in patients with darker skin pigmentation or for patients in settings where monitoring may be limited. However, risks of hyperoxia and depletion of oxygen resources must be considered when recommending a target oxygenation goal. Inability to identify hypercapnic patients and the impact of altitude are also important considerations. Based on available evidence from HICs of outcomes between liberal and conservative oxygen targets, we preliminarily chose an SpO2 target of 90–94%, determined by an accurate pulse oximeter, as a reasonable SpO2 range that might mitigate risks from hyperoxia while avoiding hypoxemia and conserving oxygen in our study settings.
We used precedent from prior trials, society guideline recommendations, direct evidence from HIC trials, and the input of content and context experts in LMICs to determine a reasonable target SpO2 range for our LMIC contexts. Nonetheless, our work points to the need for ongoing critical care research in LMICs, to create robust evidence that arises from and is applicable to LMIC settings. Improving equity in evidence must include prioritization of research in diverse settings globally.
Author contributions
ER and TT conceived the review. AH and ER conducted the initial review and synthesis of data. AH and SG drafted the initial manuscript. All authors participated in discussions of the literature and contextual factors, reviewed and interpreted the studies included in the manuscript, and contributed toward the preparation of this manuscript.
Funding
This work was supported by Wellcome Trust, United Kingdom and Harvard Global Health Institute, United States.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. The World Bank. High income. (2023). Available online at: https://data.worldbank.org/country/XD (accessed January 3, 2023).
2. The ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group, Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, et al. Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med. (2020) 382:989–98. doi: 10.1056/nejmoa1903297
3. Schmidt H, Kjaergaard J, Hassager C, Mølstrøm S, Grand J, Borregaard B, et al. Oxygen targets in comatose survivors of cardiac arrest. N Engl J Med. (2022) 387:1467–76. doi: 10.1056/nejmoa2208686
4. Schjørring O, Klitgaard T, Perner A, Wetterslev J, Lange T, Siegemund M, et al. Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N Engl J Med. (2021) 384:1301–11. doi: 10.1056/NEJMoa2032510
5. Panwar R, Hardie M, Bellomo R, Barrot L, Eastwood G, Young P, et al. Conservative versus liberal oxygenation targets for mechanically ventilated patients. A pilot multicenter randomized controlled trial. Am J Respir Crit Care Med. (2016) 193:43–51. doi: 10.1164/rccm.201505-1019OC
6. Gelissen H, de Grooth H, Smulders Y, Wils E, de Ruijter W, Vink R, et al. Effect of low-normal vs high-normal oxygenation targets on organ dysfunction in critically ill patients: a randomized clinical trial. JAMA. (2021) 326:940–8. doi: 10.1001/jama.2021.13011
7. Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. (2016) 316:1583–9. doi: 10.1001/jama.2016.11993
8. Asfar P, Schortgen F, Boisramé-Helms J, Charpentier J, Guérot E, Megarbane B, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. (2017) 5:180–90. doi: 10.1016/S2213-2600(17)30046-2
9. Chu D, Kim L, Young P, Zamiri N, Almenawer S, Jaeschke R, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. (2018) 391:1693–705. doi: 10.1016/S0140-6736(18)30479-3
10. Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. (2020) 382:999–1008. doi: 10.1056/NEJMoa1916431
11. Semler M, Casey J, Lloyd B, Hastings P, Hays M, Stollings J, et al. Oxygen-saturation targets for critically ill adults receiving mechanical ventilation. N Engl J Med. (2022) 387:1759–69. doi: 10.1056/NEJMoa2208415
12. Chowdhury S, Laux T, Morse M, Jenks A, Stonington S, Jain Y. Democratizing evidence production - A 51-year-old man with sudden onset of dense hemiparesis. N Engl J Med. (2019) 381:1501–5. doi: 10.1056/NEJMp1907988
13. Pro C, Yealy D, Kellum J, Huang D, Barnato A, Weissfeld L, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. (2014) 370:1683–93. doi: 10.1056/nejmoa1401602
14. Mouncey P, Osborn T, Power G, Harrison D, Sadique M, Grieve R, et al. Protocolised Management In Sepsis (ProMISe): a multicentre randomised controlled trial of the clinical effectiveness and cost-effectiveness of early, goal-directed, protocolised resuscitation for emerging septic shock. Health Technol Assess. (2015) 19:1–150. doi: 10.3310/hta19970
15. ARISE Investigators, ANZICS Clinical Trials Group, Peake S, Delaney A, Bailey M, Bellomo R, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. (2014) 371:1496–506. doi: 10.1056/NEJMoa1404380
16. Andrews B, Semler M, Muchemwa L, Kelly P, Lakhi S, Heimburger D, et al. Effect of an early resuscitation protocol on in-hospital mortality among adults with sepsis and hypotension: a randomized clinical trial. JAMA. (2017) 318:1233–40. doi: 10.1001/jama.2017.10913
17. Maitland K, Babiker A, Kiguli S, Molyneux E. The FEAST trial of fluid bolus in African children with severe infection. Lancet. (2012) 379:613. doi: 10.1016/S0140-6736(12)60260-8
18. National Heart, Lung, and Blood Institute Prevention and Early Treatment of Acute Lung Injury Clinical Trials Network, Shapiro N, Douglas I, Brower R, Brown S, Exline M, et al. Early restrictive or liberal fluid management for sepsis-induced hypotension. N Engl J Med. (2023) 388:499–510. doi: 10.1056/NEJMoa2212663
19. Riviello E. Improving outcomes for severe sepsis in Africa. Crit Care Med. (2014) 42:2439–40. doi: 10.1097/CCM.0000000000000591
20. Urner M, Calfee C, Fan E. Titrating oxygen therapy in critically ill patients. JAMA. (2021) 326:911–3. doi: 10.1001/jama.2021.9843
21. Clinicaltrials.gov. Building respiratory support in east Africa through high flow versus standard flow oxygen evaluation (BREATHE). Bethesda, MD: National Library of Medicine (2023).
22. Rochwerg B, Granton D, Wang D, Helviz Y, Einav S, Frat J, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. (2019) 45:563–72. doi: 10.1007/s00134-019-05590-5
23. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 8:19–32. doi: 10.1080/1364557032000119616
24. Wick K, Matthay M, Ware L. Pulse oximetry for the diagnosis and management of acute respiratory distress syndrome. Lancet Respir Med. (2022) 10:1086–98. doi: 10.1016/S2213-2600(22)00058-3
25. Sutherland T, Musafiri S, Twagirumugabe T, Talmor D, Riviello ED. Oxygen as an essential medicine: under- and over-treatment of hypoxemia in low- and high-income nations. Crit Care Med. (2016) 44:e1015–6. doi: 10.1097/CCM.0000000000001912
26. Lam F, Subhi R, Houdek J, Schroder K, Battu A, Graham H. The prevalence of hypoxemia among pediatric and adult patients presenting to healthcare facilities in low- and middle-income countries: protocol for a systematic review and meta-analysis. Syst Rev. (2020) 9:67. doi: 10.1186/s13643-020-01326-5
27. Rojas-Camayo J, Mejia C, Callacondo D, Dawson J, Posso M, Galvan C, et al. Reference values for oxygen saturation from sea level to the highest human habitation in the Andes in acclimatised persons. Thorax. (2018) 73:776–8. doi: 10.1136/thoraxjnl-2017-210598
28. Subhi R, Smith K, Duke T. When should oxygen be given to children at high altitude? A systematic review to define altitude-specific hypoxaemia. Arch Dis Child. (2009) 94:6–10. doi: 10.1136/adc.2008.138362
29. Acute Respiratory Distress Syndrome Network, Brower R, Matthay M, Morris A, Schoenfeld D, Thompson BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. (2000) 342:1301–8. doi: 10.1056/NEJM200005043421801
30. Guérin C, Reignier J, Richard J, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. (2013) 368:2159–68. doi: 10.1056/NEJMoa1214103
31. National Heart, Lung, and Blood Institute PETAL Clinical Trials Network, Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. (2019) 380:1997–2008. doi: 10.1056/NEJMoa1901686
32. Frat J, Thille A, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. (2015) 372:2185–96. doi: 10.1056/nejmoa1503326
33. Azoulay E, Lemiale V, Mokart D, Nseir S, Argaud L, Pène F, et al. Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: the HIGH randomized clinical trial. JAMA. (2018) 320:2099–107. doi: 10.1001/jama.2018.14282
34. Perkins G, Ji C, Connolly B, Couper K, Lall R, Baillie J, et al. Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19: the RECOVERY-RS randomized clinical trial. JAMA. (2022) 327:546–58. doi: 10.1001/jama.2022.0028
35. O’Driscoll B, Howard L, Earis J, Mak V. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. (2017) 72:ii1–90. doi: 10.1136/thoraxjnl-2016-209729
36. Alhazzani W, Evans L, Alshamsi F, Møller M, Ostermann M, Prescott H, et al. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med. (2021) 49:e219–34. doi: 10.1097/CCM.0000000000004899
37. World Health Organization [WHO]. Clinical management of COVID-19: living guideline. (2022). Available online at: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2022-1 (accessed December 2022).
38. Barnett A, Beasley R, Buchan C, Chien J, Farah C, King G, et al. Thoracic society of Australia and New Zealand position statement on acute oxygen use in adults: ‘Swimming between the flags.’ Respirology. (2022) 27:262–76. doi: 10.1111/resp.14218
39. Siemieniuk R, Chu D, Kim L, Güell-Rous M, Alhazzani W, Soccal P, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. (2018) 363:k4169. doi: 10.1136/bmj.k4169
40. Rochwerg B, Brochard L, Elliott M, Hess D, Hill N, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. (2017) 50:1602426. doi: 10.1183/13993003.02426-2016
41. Fan E, Del Sorbo L, Goligher E, Hodgson C, Munshi L, Walkey A, et al. An official American thoracic society/European society of intensive care medicine/society of critical care medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. (2017) 195:1253–63. doi: 10.1164/rccm.201703-0548ST
42. Martin D, Grocott M. Oxygen therapy in critical illness: precise control of arterial oxygenation and permissive hypoxemia. Crit Care Med. (2013) 41:423–32. doi: 10.1097/CCM.0b013e31826a44f6
43. Damiani E, Donati A, Girardis M. Oxygen in the critically ill: friend or foe? Curr Opin Anaesthesiol. (2018) 31:129–35. doi: 10.1097/ACO.0000000000000559
44. Mart M, Sendagire C, Ely E, Riviello ED, Twagirumugabe T. Oxygen as an essential medicine. Crit Care Clin. (2022) 38:795–808. doi: 10.1016/j.ccc.2022.06.010
45. McIlroy D, Shotwell M, Lopez M, Vaughn M, Olsen J, Hennessy C, et al. Oxygen administration during surgery and postoperative organ injury: observational cohort study. BMJ. (2022) 379:e070941. doi: 10.1136/bmj-2022-070941
46. Tyagi S, Brown C, Dickson R, Sjoding M. Outcomes and predictors of severe hyperoxemia in patients receiving mechanical ventilation: a single-center cohort study. Ann Am Thorac Soc. (2022) 5:1338–45. doi: 10.1513/annalsats.202107-804oc
47. Cumpstey A, Oldman A, Martin D, Smith A, Grocott M. Oxygen targets during mechanical ventilation in the ICU: a systematic review and meta-analysis. Crit Care Explor. (2022) 4:e0652. doi: 10.1097/CCE.0000000000000652
48. Singh A. Comparative evaluation of accuracy of pulse oximeters and factors affecting their performance in a tertiary intensive care unit. J Clin Diagn Res. (2017) 11:OC05–8. doi: 10.7860/JCDR/2017/24640.9961
49. Gudelunas M, Lipnick M, Hendrickson C, Vanderburg S, Okunlola B, Auchus I, et al. Low perfusion and missed diagnosis of hypoxemia by pulse oximetry in darkly pigmented skin: a prospective study. medRxiv [Preprint]. (2022). doi: 10.1101/2022.10.19.22281282
50. Lipnick M, Feiner J, Au P, Bernstein M, Bickler P. The accuracy of 6 inexpensive pulse oximeters not cleared by the food and drug administration: the possible global public health implications. Anesth Analg. (2016) 123:338–45. doi: 10.1213/ANE.0000000000001300
51. Bickler P, Feiner J, Severinghaus J. Effects of skin pigmentation on pulse oximeter accuracy at low saturation. Anesthesiology. (2005) 102:715–9. doi: 10.1097/00000542-200504000-00004
52. Valbuena V, Seelye S, Sjoding M, Valley T, Dickson R, Gay S, et al. Racial bias and reproducibility in pulse oximetry among medical and surgical inpatients in general care in the Veterans health administration 2013-19: multicenter, retrospective cohort study. BMJ. (2022) 378:e069775. doi: 10.1136/bmj-2021-069775
53. Henry N, Hanson A, Schulte P, Warner N, Manento M, Weister T, et al. Disparities in hypoxemia detection by pulse oximetry across self-identified racial groups and associations with clinical outcomes. Crit Care Med. (2022) 50:204–11. doi: 10.1097/CCM.0000000000005394
54. Shi C, Goodall M, Dumville J, Hill J, Norman G, Hamer O, et al. The accuracy of pulse oximetry in measuring oxygen saturation by levels of skin pigmentation: a systematic review and meta-analysis. BMC Med. (2022) 20:267. doi: 10.1186/s12916-022-02452-8
55. Wong A, Charpignon M, Kim H, Josef C, de Hond A, Fojas J, et al. Analysis of discrepancies between pulse oximetry and arterial oxygen saturation measurements by race and ethnicity and association with organ dysfunction and mortality. JAMA Netw Open. (2021) 4:e2131674. doi: 10.1001/jamanetworkopen.2021.31674
56. Gottlieb E, Ziegler J, Morley K, Rush B, Celi L. Assessment of racial and ethnic differences in oxygen supplementation among patients in the intensive care unit. JAMA Intern Med. (2022) 182:849–58. doi: 10.1001/jamainternmed.2022.2587
57. Fawzy A, Wu T, Wang K, Robinson M, Farha J, Bradke A, et al. Racial and ethnic discrepancy in pulse oximetry and delayed identification of treatment eligibility among patients with COVID-19. JAMA Intern Med. (2022) 182:730–8. doi: 10.1001/jamainternmed.2022.1906
58. Moore K, Gudelunas K, Lipnick M, Bickler P, Hendrickson C. Pulse oximeter bias and inequities in retrospective studies–now what? Respir Care. (2022) 67:1633–6. doi: 10.4187/respcare.10654
59. Wandi F, Peel D, Duke T. Hypoxaemia among children in rural hospitals in Papua New Guinea: epidemiology and resource availability–a study to support a national oxygen programme. Ann Trop Paediatr. (2006) 26:277–84. doi: 10.1179/146532806X152791
60. Bradley B, Howie S, Chan T, Cheng Y. Estimating oxygen needs for childhood pneumonia in developing country health systems: a new model for expecting the unexpected. PLoS One. (2014) 9:e89872. doi: 10.1371/journal.pone.0089872
61. Vo D, Cherian M, Bianchi S, Noël L, Lundeg G, Taqdeer A, et al. Anesthesia capacity in 22 low and middle income countries. J Anesth Clin Res. (2012) 3:207–12. doi: 10.4172/2155-6148.1000207
62. Bourassa S, Bouchard P, Dauphin M, Lellouche F. Oxygen conservation methods with automated titration. Respir Care. (2020) 65:1433–42. doi: 10.4187/respcare.07240
63. Riviello ED, Kiviri W, Twagirumugabe T, Mueller A, Banner-Goodspeed V, Officer L, et al. Hospital incidence and outcomes of the acute respiratory distress syndrome using the kigali modification of the Berlin definition. Am J Respir Crit Care Med. (2016) 193:52–9. doi: 10.1164/rccm.201503-0584OC
64. Feller-Kopman D, Schwartzstein R. Mechanisms, causes, and effects of hypercapnia. UpToDate. (2022). Available online at: https://www.uptodate.com/contents/mechanisms-causes-and-effects-of-hypercapnia (accessed March 13, 2023).
65. Okunlola O, Lipnick M, Batchelder P, Bernstein M, Feiner J, Bickler P. Pulse oximeter performance, racial inequity, and the work ahead. Respir Care. (2022) 67:252–7. doi: 10.4187/respcare.09795
66. Openoximetry. Openoximetry. (2023). Available online at: https://openoximetry.org/oximeters/ (accessed January 3, 2023).
Keywords: LMICs, Africa, context, oxygen saturation targets, SpO2
Citation: Herbst A, Goel S, Beane A, Brotherton BJ, Dula D, Ely EW, Gordon SB, Haniffa R, Hedt-Gauthier B, Limbani F, Lipnick MS, Lyon S, Njoki C, Oduor P, Otieno G, Pisani L, Rylance J, Shrime MG, Uwamahoro DL, Vanderburg S, Waweru-Siika W, Twagirumugabe T and Riviello E (2023) Oxygen saturation targets for adults with acute hypoxemia in low and lower-middle income countries: a scoping review with analysis of contextual factors. Front. Med. 10:1148334. doi: 10.3389/fmed.2023.1148334
Received: 20 January 2023; Accepted: 27 March 2023;
Published: 17 April 2023.
Edited by:
Abele Donati, Marche Polytechnic University, ItalyReviewed by:
Elisa Damiani, Università Politecnica delle Marche, ItalyCopyright © 2023 Herbst, Goel, Beane, Brotherton, Dula, Ely, Gordon, Haniffa, Hedt-Gauthier, Limbani, Lipnick, Lyon, Njoki, Oduor, Otieno, Pisani, Rylance, Shrime, Uwamahoro, Vanderburg, Waweru-Siika, Twagirumugabe and Riviello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elisabeth Riviello, ZXJpdmllbGxAYmlkbWMuaGFydmFyZC5lZHU=
†These authors share last authorship
 Austin Herbst1
Austin Herbst1 Abi Beane
Abi Beane B. Jason Brotherton
B. Jason Brotherton Dingase Dula
Dingase Dula Rashan Haniffa
Rashan Haniffa Samuel Lyon
Samuel Lyon Luigi Pisani
Luigi Pisani Sky Vanderburg
Sky Vanderburg Wangari Waweru-Siika
Wangari Waweru-Siika Elisabeth Riviello
Elisabeth Riviello