
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 03 July 2023
Sec. Ophthalmology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1146529
This article is part of the Research Topic Progressive Keratoconus: Insights into Etiopathogenesis, Diagnosis, and Treatment View all 11 articles
Purpose: To explore and validate the utility of machine learning (ML) methods using a limited sample size to predict changes in visual acuity and keratometry 2 years following corneal crosslinking (CXL) for progressive keratoconus.
Methods: The study included all consecutive patients with progressive keratoconus who underwent CXL from July 2014 to December 2020, with a 2 year follow-up period before July 2022 to develop the model. Variables collected included patient demographics, visual acuity, spherical equivalence, and Pentacam parameters. Available case data were divided into training and testing data sets. Three ML models were evaluated based on their performance in predicting case corrected distance visual acuity (CDVA) and maximum keratometry (Kmax) changes compared to actual values, as indicated by average root mean squared error (RMSE) and R-squared (R2) values. Patients followed from July 2022 to December 2022 were included in the validation set.
Results: A total of 277 eyes from 195 patients were included in training and testing sets and 43 eyes from 35 patients were included in the validation set. The baseline CDVA (26.7%) and the ratio of steep keratometry to flat keratometry (K2/K1; 13.8%) were closely associated with case CDVA changes. The baseline ratio of Kmax to mean keratometry (Kmax/Kmean; 20.9%) was closely associated with case Kmax changes. Using these metrics, the best-performing ML model was XGBoost, which produced predicted values closest to the actual values for both CDVA and Kmax changes in testing set (R2 = 0.9993 and 0.9888) and validation set (R2 = 0.8956 and 0.8382).
Conclusion: Application of a ML approach using XGBoost, and incorporation of identifiable parameters, considerably improved variation prediction accuracy of both CDVA and Kmax 2 years after CXL for treatment of progressive keratoconus.
Corneal collagen crosslinking (CXL) has been extensively used in clinical management of keratoconus since Wollensak et al. (1) originally demonstrated in 2003 that CXL enhances corneal stiffness. Although the effect of CXL in halting the progression of keratoconus has been widely recognized, long-term (≥ 2 years) randomized controlled (RCT) studies still indicated that its failure rate was highly variable (0–28%) (2–5) depending on CXL procedure type, age, race, disease severity, and other factors.
If CXL surgery fails, the progression of keratoconus continues, and CXL retreatment and/or keratoplasty are necessary. In China, there is a shortage of corneal donor tissue (6) which might induce patients to delay treatment. Meanwhile, this increases the financial and psychological burden on the patient. Therefore, accurate prediction of the postoperative outcome prior to CXL could help patients choose a newly alternative treatment options, such as intraocular lens or intrastromal implantation (7).
The most commonly used definition of keratoconus progression is maximum keratometry (Kmax) increase ≥ 1.0 D or corrected distance visual acuity (CDVA) decrease >2 lines (8). High preoperative Kmax, thin corneas thickness, and atopic diseases were widely recognized as the risk factors for progression of keratoconus after CXL (9–12). Female gender, young age, pronounced optical aberrations, and the cone location were also found as the risk factors of the progression in 2 years (10–12).
Several studies have utilized liner regression approaches to predict treatment outcomes including CDVA and Kmax, but these approaches failed to produce sufficiently reliable predictive power (13–17). An early study used a multivariate regression statistical model to predict CDVA and Kmax for pediatric keratoconus patients (1 year follow-up) (13), and obtained a low predictive value model with CDVA (R2 = 0.45, p < 0.01) and Kmax (R2 = 0.15, p > 0.05). A prior study (14) demonstrated that CDVA could potentially be predicted for 1 year postoperative following CXL, but did not evaluate the model’s ability to predict Kmax. Similarly, other studies only provided the predictors such as baseline Kmax (15–17). However, the cornea biomechanics are not completely stable at 1 year following CXL.
Machine learning (ML) is a computer science discipline that utilizes algorithms and other approaches to automatically address complex problems that cannot easily be addressed by conventional data analysis means (18). Previous ML approaches have traditionally required very large datasets for training. With newly developed approaches, ML is now also suited for interrogation of small datasets with hundreds or dozens of variables using approaches such as few-shot learning (FSL) (19) and gradient boosting. The most widely used gradient boosting algorithms including categorical gradient boosting on decision trees (CatBoost), light gradient boosting machine (LightGBM), eXtreme gradient boosting (XGBoost), and Bayesian optimization (20).
Recently, ML has been used for keratoconus detection (21), classification (20), and candidacy for CXL treatment (23). However, no reports have used ML to predict the therapeutic outcome of CXL postoperatively. This study aimed to apply ML algorithms trained with a limited dataset to predict changes of visual acuity and keratometry 2 years following CXL for progressive keratoconus.
A retrospective medical chart review was conducted on all consecutive patients with progressive keratoconus who underwent CXL treatment between July 2014 and December 2020 at the Aier Eye Hospital of Wuhan University (Wuhan, Hubei province, China). Patients who returned for a follow-up visit at 2 years were included in the study. Data were collected using a convenient data management system supported by Empower Electronic Data Capture (EDC) system (https://empoweredc.com, Solution Inc., Shanghai, China).
An increase of at least 1 diopter (D) in maximum keratometry (Kmax) derived from computerized corneal topography during the preceding 12 months was required for inclusion. We enrolled keratoconus patients for all grades based on the Amsler-Krumeich keratoconus classification (24). Patients with previous refractive surgeries or corneal history of ocular surface or other eye disorders were excluded. In addition, patients whose data could not be reviewed for any reason were classified as being lost to follow-up and excluded from the study.
Patients were included regardless of their treatment protocols, which were not included in the prediction model. Two different treatment combinations were included in the study. When the thinnest corneal thickness of the eye was ≥ 450 μm, patients were undergo the high-fluence accelerated CXL (HF A-CXL). When the thinnest corneal thickness of the eye was < 450 μm, they were undergo the accelerated transepithelial CXL (A-TE CXL).
1. A-TE CXL: In the first step, 0.25% riboflavin (Paracel Part I, Avedro Inc., USA) containing 0.02% benzalkonium chloride (BAC) and 0.85% hydroxypropyl methyl cellulose (HPMC) was applied onto the cornea every 90 s for 4 min. Thereafter, part I solution was rinsed with 0.22% riboflavin (Paracel Part II, Avedro), and part II solution was instilled every 90 s over the next 6 min. UV-A was applied using the Avedro KXL System (Avedro Inc., Waltham, USA) with 30 mW/cm2 UV power for 8 min with a 1 s on/off cycle (7.2 J/cm2) (25).
2. HF A-CXL: The corneal epithelium was removed with a blunt knife in a 10 mm zone. CXL was then performed with 0.1% dextran-free riboflavin (VibeX Rapid, Avedro) instilled every 90 s for 10 min. Subsequently, it was placed under UA irradiation for 4 min at 30 mW/cm2 (7.2 J/cm2, Avedro) (26).
The operator verified irradiance prior to each treatment. The two CXL procedures are summarized in Table 1.
All patients received 0.5% levofloxacin drops four times daily for 3 days prior to surgery. Thirty minutes before surgery, patients received 2% pilocarpine (Sigma-Aldrich, St. Louis, MO, United States) and 0.4% oxybuprocaine hydrochloride (Bausch & Lomb Pty Ltd., NSW, Australia) drops three times, with 5 min between each administration.
At the end of the surgery, the corneal surface was dressed with a therapeutic soft contact lens (Bausch & Lomb Pty Ltd.) for at least 24 h until the epithelium had completely healed.
Twenty-six preoperative variables were recorded in all patients: sex, age, uncorrected visual acuity (UCVA, logarithm of the minimum angle of resolution [LogMAR] units), CDVA (LogMAR units), spherical equivalence (SE), flat keratometry (K1), steep keratometry (K2), mean keratometry (Kmean), astigmatism (Astig), eccentricity (ecc), maximum keratometry (Kmax), minimum corneal thickness (MCT), the most elevated points on the front corneal surfaces (F. Ele Th), the most elevated points on the back corneal surfaces (B. Ele Th), the index of surface variance (ISV), the index of vertical asymmetry (IVA), keratoconus index (KI), center keratoconus index (CKI), the index of height decentration (IHD), minimum radius of curvature (RMin), and Belin/Ambrósio final D value (BAD-D), which were measured by Pentacam (Oculus, Wetzlar, Germany). In addition, five incorporation parameters were also collected, including the ratio of K2 to K1 (K2/K1), the ratio of Kmax to Kmean (Kmax/Kmean), the ratio of Kmax to K1 (Kmax/K1), the ratio of Kmax to K2 (Kmax/K2), and the difference between Kmax and Kmean (Kmax-mean). All the exams were executed by the skilled examiners. The ‘quality specifications (QS)’ was used to evaluate the quality of Pentacam images. If the QS is not ‘OK’, the exams were executed more than two times. Finally, at least two experienced ophthalmologists validated the accuracy of the images and data.
Features that demonstrated the highest feature importance to the model on primary runs were included for further analysis. Histograms of each numerical attribute were generated to understand the distribution features across distinct values. Categorical features (sex) were encoded into a binary representation to enable machine learning (ML) readability of algorithms.
Patients who returned for a follow-up visit at 2 years before June 30, 2022 were included in the study to develop the model. Data processing and ML model development were performed in Python 3.9.7 using the pandas (version 1.3.4), numpy (version 1.20.3), and scikit-learn (version 1.0.2; [mode: sklearn.model_selection and sklearn.metrics]) packages. Three models were run by supervised ML methods, while examples of inputs (features chosen) and outputs (actual changes in CDVA and Kmax) were provided to models as training inputs to build an algorithm for future predictions including, CatBoost, LightGBM, and XGBoost.
The database was randomly split into two groups: 80% of data (n = 222) was used for model training, while the remaining 20% of the dataset (n = 55) was reserved to test the model’s predicted case value for CDVA and Kmax variation. The predicted values were compared to the actual case changes. Performance metrics included root mean squared error (RMSE) and R-squared (R2). Models were compared using a Nadeau and Bengio’s corrected resampled t-test (27, 28). Feature importance values were derived using prespecified methodology specific to the algorithms studied.
To validate the accuracy of the prediction model, we established a free website and used data from patients followed from July 2022 to December 2022. We use the same RMSE and R2 evaluations.
The workflow diagram detailing the data modeling process was showed in Figure 1. The website for validating the prediction model was showed in Figure 2.
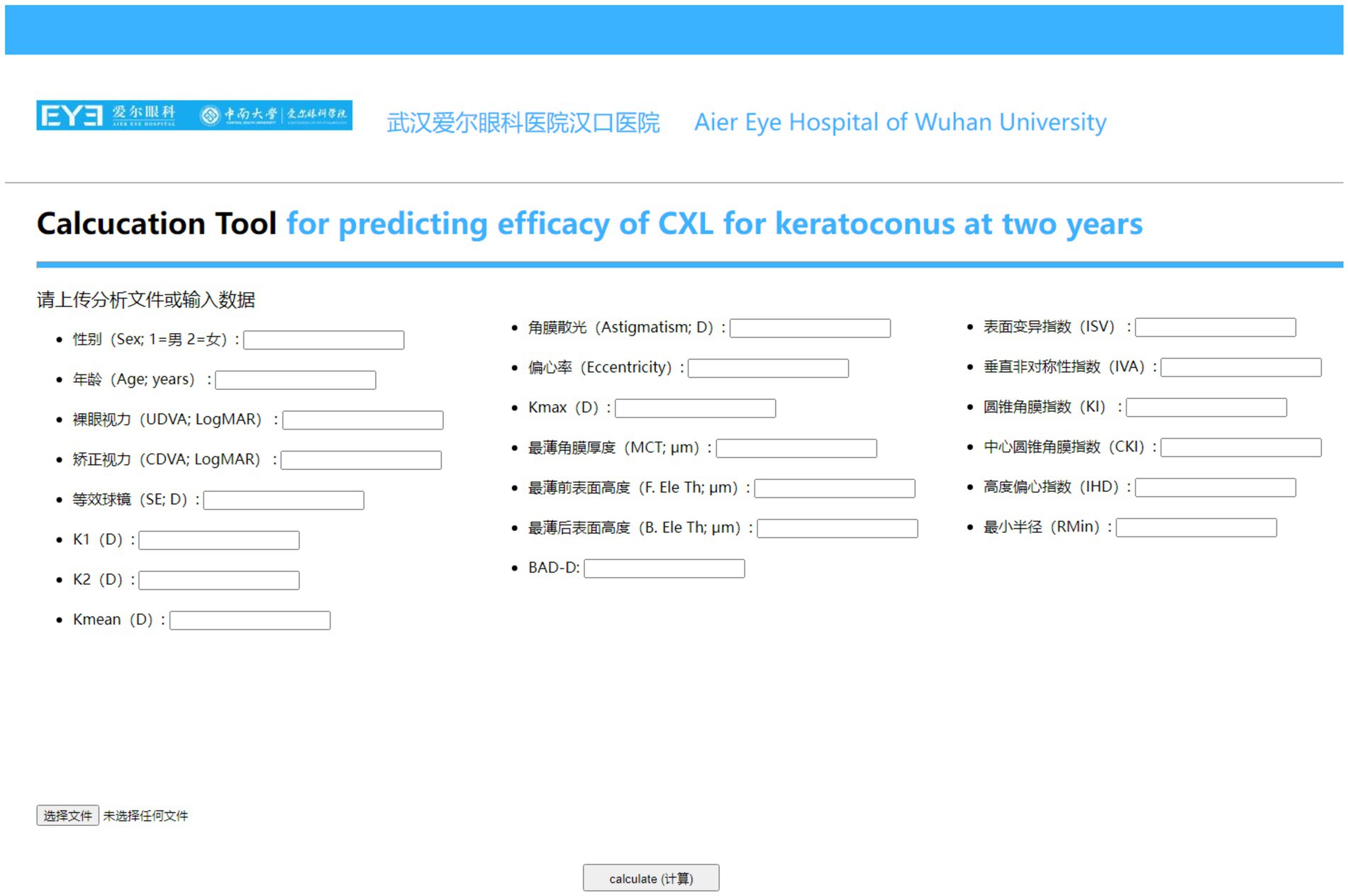
Figure 2. Online web-based calculator for predicting changes in CDVA and Kmax 2 years after CXL crosslinking for keratoconus.
Data were recorded for 405 eyes from 289 patients who were diagnosed with progressive keratoconus and underwent CXL treatment. Thirty-nine patients (49 eyes) who were followed up at different clinics, and 30 patients (36 eyes) did not complete the 2 year follow-up, were excluded. Finally, the study included 277 eyes from 195 patients in training and testing sets and 43 eyes from 35 patients in the validation set. The demographic and baseline data of all patients are summarized in Table 2.
Both CDVA and Kmax improved significantly over baseline at the 2 year follow-up. Average CDVA decreased by 0.08 from 0.27 to 0.19 LogMAR (range: − 0.8 to 0.7 LogMAR; p < 0.001), and average Kmax decreased by 1.06 D from 58.13 to 57.07 D (range: − 14.6 to 5.8 D; p < 0.001) in training and testing sets.
Feature importance was applied to select key features for improved performance. Baseline CDVA (26.7%), K2/K1 value (13.8%), and F. Ele Th (11.1%) were closely associated with case CDVA changes. The baseline Kmax/Kmean (20.9%), UDVA (14%), and ecc (10%) were closely associated with case changes of Kmax. The feature importance of each parameter is shown in Figure 3.
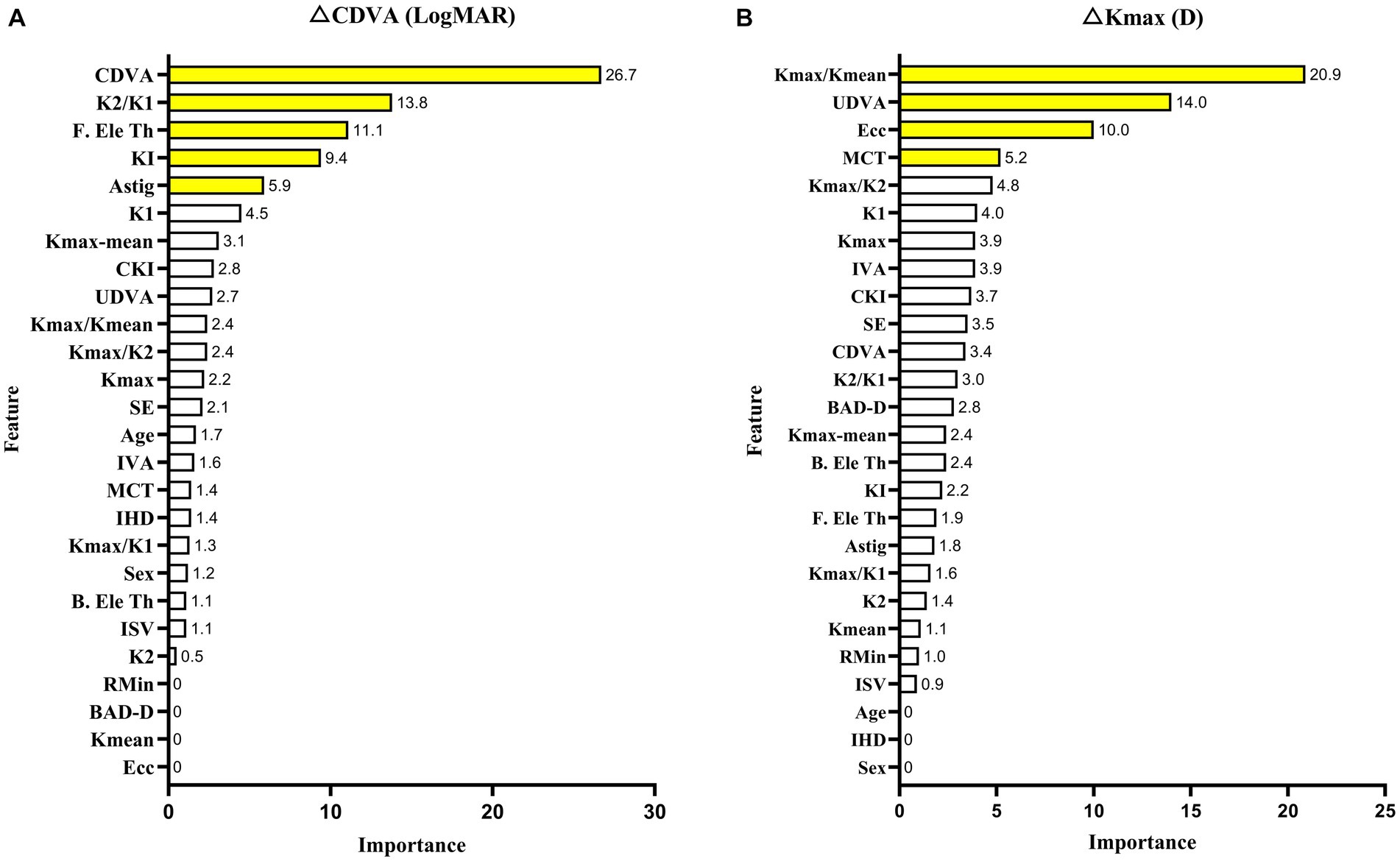
Figure 3. XGBoost model with the feature importance of each parameter in CDVA (A) and Kmax (B) changes with keratoconus 2 years after crosslinking.
All baseline features were used as training features to construct a baseline regression model, to predict changes in CDVA and Kmax 2 years after CXL while applying different algorithms. The XGBoost model demonstrated the best predictive ability in the training set (RMSE = 0.001 LogMAR and 0.013 D) compared to CatBoost and LightGBM. Therefore, XGBoost was selected for model and website building. Finally, our predictive model performed robustly in the testing set (R2 = 0.9991 and 0.9888). The performance of the three models in predicting changes of CDVA and Kmax with the training and testing dataset were showed in Table 3.
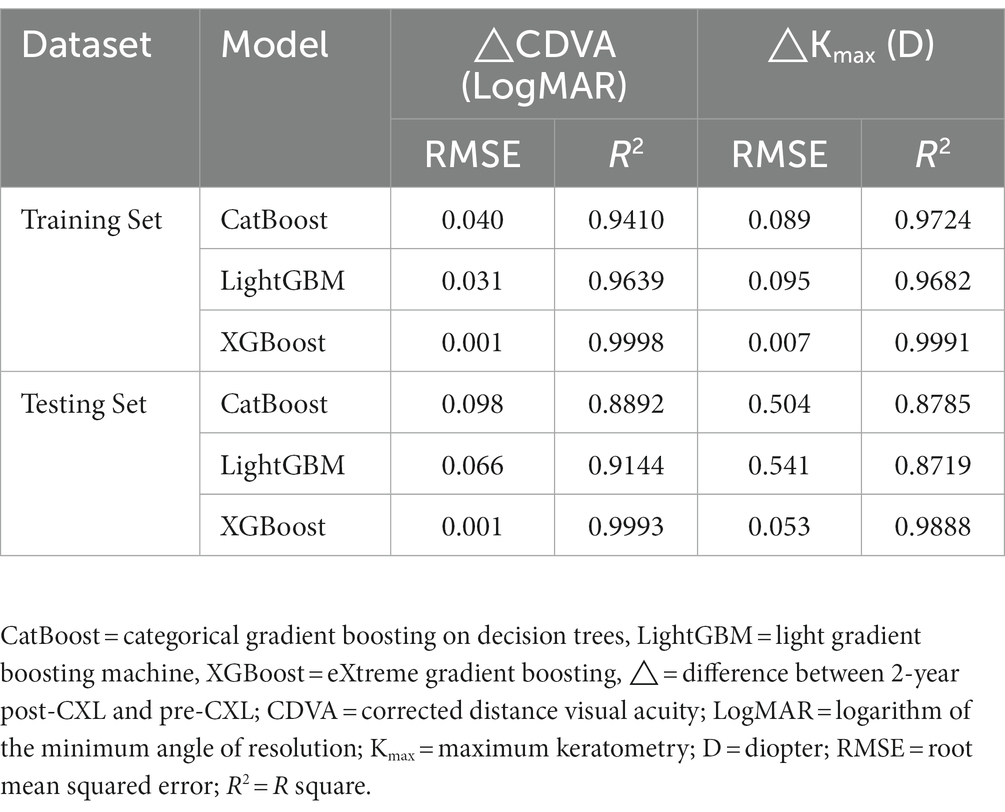
Table 3. Performance of three models with changes of CDVA and Kmax in training and testing data sets.
The CDVA also improved by 0.07 ± 0.21 LogMAR (p = 0.029) and Kmax decreased by 1.16 ± 2.22 D (p = 0.001) in the validation set. The validation for the model achieved RMSE of 0.066 LogMAR and 0.907 D, and R2 of 0.8956 and 0.8382, respectively. The scatterplot of the predicted values compared to actual value in validation set were showed in Figure 4 and the raw data was showed in Supplementary Table 1.
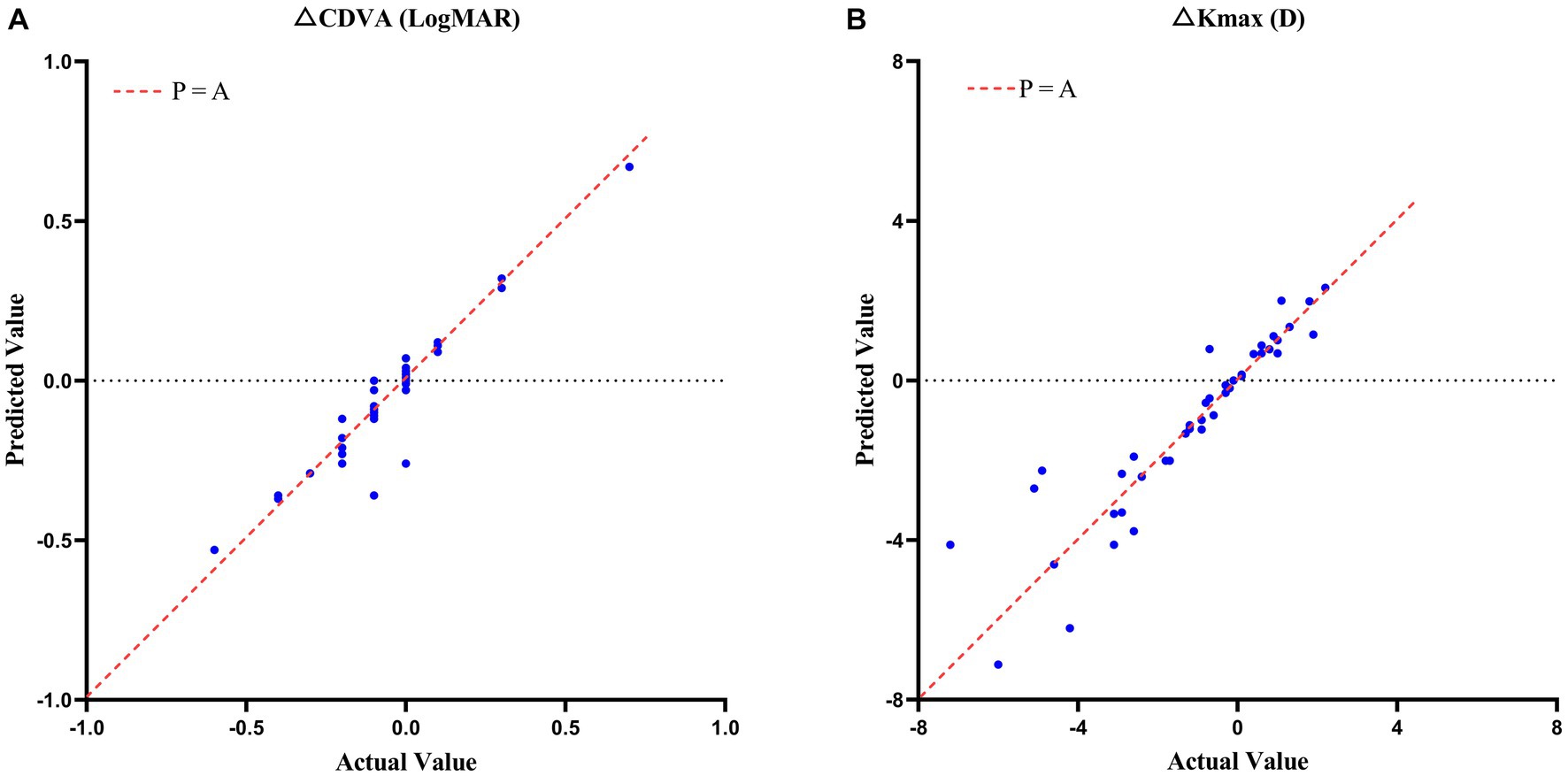
Figure 4. Scatterplots of the case predicted values compared to actual values in CDVA (A) and Kmax (B) changes with keratoconus 2 years after crosslinking in validation set.
Opacity of the corneal stroma at the central and paracentral areas occurred in two eyes of one patients (10 years) during the follow-up in HF A-CXL treatment group. The minimum stromal thickness after epithelial removal were 411 and 429 μm. The patient had a history of sunlight exposure early in the postoperative period. Thereafter, the corneal transparency was restored after treatment with 0.1% fluorometholone (Allergan, Irvine, CA) and corneal protection to avoid direct irritation from sunlight (Supplementary Figure 1). No infections or other adverse events were observed in slit-lamp examination.
To our knowledge, this is the first study to use ML to predict the 2 year efficacy of CXL for keratoconus. Classical regression analysis has used one regression equation (linear regression) or several equations (hierarchical regression) to explain outcomes. The algorithm we ultimately used was XGBoost, which is accomplished through a process known as boosting. Boosting is an iterative procedure that intelligently adds weak learners to the ensemble model. The new weak learners will focus on the unlearned and thus strengthen the ensemble (20). By increasing the iterative over time, XGBoost improves the accuracy of regression analysis. Further, the XGBoost model will overfit when the dataset is too large, even if lasso or ridge regression are used to filter variables. Therefore, XGBoost is commonly used for small datasets and is effective for this application (29–31). The present study tested a variety of machine learning algorithms to develop prediction models for changes in CDVA and Kmax 2 years following CXL for keratoconus, and XGBoost provided a superior models.
This research aimed to include five new incorporation variable indicators, including K2/K1, Kmax/Kmean, Kmax/K1, Kmax/K2, and Kmax-mean. Further, some combinations of factors were relevant for improving model accuracy. Baseline CDVA was the most significant contributing feature in the CDVA change model, which is consistent with previous studies (13, 14, 32). The resultant feature importance could be simply defined as the extent to which the feature is incorporated into the model. In addition, we discovered that K2/K1 is another key feature in predicting CDVA changes. We hypothesized that K2/K1 was more important to CDVA changes than other indicators because CDVA monitors the overall visual function of the eye, which is directly connected to the shape of the cornea. Accordingly, the K2/K1 ratio is indicative of the general regularity of the front surface of the cornea. Some other combined parameters have been applied to develop a prediction model for keratoconus. A prior study indicated that the ratio of anterior radius of curvature (ARC) to posterior radius of curvature (PRC) was linked to CDVA 1 year after CXL for keratoconus (11). The greatest obstacle to correcting ametropia for keratoconus is irregularity of the cornea. A new parameter developed by Pabolo et al., the K-factor (KF = K2 [K2–K1]) (33), was utilized to predict considerable improvement in CDVA after intracorneal ring segment implantation (ICRS). K2/K1 could determine the topographic form of the 3 mm of the central cornea, which contains the visual axis and is a crucial area for investigations focused on visual results. Similarity, KI and astigmatism (Astig) are two indicators of corneal regularity that can be used to evaluate corneal regularity, and account for 9.4 and 5.9%, respectively, of the feature importance outcomes in the CDVA change prediction model (Figure 3).
Concurrence of the highest elevated point on the front corneal surfaces (F. Ele Th) relative to the best fit sphere on the elevation maps was a novel important feature that could explain CDVA variations, consistent with a prior study (34). The study suggested that the difference in location between the most elevated areas on the corneal surfaces could be connected to biomechanical deterioration of the cornea. Corneal biomechanics could be connected to visual acuity, as the mechanical qualities of the cornea reflect its capacity to bear intraocular pressure. Furthermore, corneal curvature is intimately connected to visual acuity as described above.
According to prior studies, it is difficult to predict changes in Kmax after CXL for keratoconus (13). This is most likely because Kmax, as measured by Pentacam, simulates corneal morphology and calculates maximum curvature via three-dimensional reconstruction of the collected corneal Scheimpflug pictures, rather than using directly measured values (35). Hence, Kmax is influenced by an excessive number of variables. In the present study, we used a novel incorporation parameter, the baseline Kmax/Kmean ratio, to predict changes in Kmax. This parameter was much more predictive of final Kmax than was baseline Kmax. This could be because baseline Kmax is the maximum curvature of the anterior corneal surface, which indicates the absolute preoperative convexity of a point. However, after the crosslinking reaction, corneal rigidity increases, changing the biomechanical characteristics of the integrated cornea, thus there are additional factors impacting postoperative Kmax. Consistent with this notion, a recent study identified strong associations between corneal hysteresis (CH), corneal resistance factor (CRF), and Kmax in keratoconic eyes, but not in crosslinked eyes (36). Variations in the biomechanics of the whole cornea result in more complex changes in Kmax that are difficult to predict using preoperative data. Nevertheless, the Kmax/Kmean ratio maybe normalized for some unclear confounding factor, which reflects the convexity of the cornea, can be used to measure the overall qualities of the cornea.
It is presently unclear why baseline UDVA is a secondary-importance feature in the Kmax change prediction model. One possible explanation is that visual function and corneal structure are inextricably linked (37).
Eccentricity (ecc) was identified as another significant predictive factor for changes in Kmax, consistent with prior findings (13, 15, 38). Additionally, central cones have a larger degree of postoperative corneal flattening than do peripheral cones (38). This conclusion could be explained by several factors. The efficacy of CXL is decreased in eccentric cones because UV devices cannot be applied uniformly across the treatment zone. UV rays could scatter in the perimeter, with a weaker and inconsistent beam in peripherally located cones. The second potential contributor is that even with homogenous light energy, the treatment power was relatively low in the peripheral cornea (39). Accordingly, cones in the periphery could be exposed to less crosslinking power, making ecc a significant predictor of Kmax changes after CXL.
Minimum corneal thickness, the parameter of corneal thickness, was also of importance in predicting changes in Kmax after CXL, which was consistent with an earlier study demonstrating a link between MCT and Kmax variations (40). Corneal thinning in keratoconus results from defects in collagen lamellae caused by errors in the collagen lamellae manufacturing process (41). After stabilization with CXL, corneal collagen structural changes (42), primarily crimping (43), change corneal biomechanics. Therefore, using baseline data from MCT would enhance the prediction efficiency of Kmax.
Our pilot model still has several significant limitations that should be considered in its interpretation. First, even though XGBoost could effectively create a predictive model with a small dataset, our sample size was also limited, which could result in an unnecessarily complicated model. Second, parameters, such as atopic constitution, positive family history, and smoking, which did not have predictive potential in conventional linear regression analyses were excluded from the ML dataset (13, 16). However, XGBoost or other ML algorithms filter variables by lasso regression. The predictive potential of these parameters with ML approaches should be examined to determine their feature importance. Furthermore, a prior study found that keratoconus progression after CXL in one eye should be continuously monitored due to an increased chance of progression in the contralateral eye (44). This suggests that fellow eye data could also be incorporated into analysis.
Moreover, we found a pediatric patient who underwent HF A-CXL procedure has occurred the corneal opacity. Another study that used the same procedure to treat pediatric keratoconus patients did not report complications through 2 years postoperatively (45). Corneal haze following CXL has been reported in previous studies (46), but the reasons remain unclear at present. Potential reasons for this phenomenon are as follows: (1) more severe corneal ectasis caused by the fibroblast proliferation, which is more common in pediatric patients than in adults due to a more active proliferation response (47, 48); (2) the slow spontaneous crosslinking reactions triggered by residual riboflavin in the corneal stroma and UV-A rays in natural light (49); or (3) endothelial toxicity caused by reduced corneal thickness.
The study has several limitations that should be considered in its interpretation: the limited sample size, the multiplied CXL modalities, the various variable, the accuracy of pre-existing data, and the inherent biases introduced by retrospective analysis. Hence, these findings should be further confirmed by prospective trials with a longer follow-up period, larger sample size, and better variable selection.
Using an ML algorithm and incorporating identifiable parameters from historical case data improved prediction of case changes in CDVA and Kmax at 2 years after CXL for progressive keratoconus. These techniques could improve case accuracy and decrease patient treatment expenses. To improve the prediction model, more data sets and richer feature collections should be examined in further studies.
The data analyzed in this study is subject to the following licenses/restrictions: The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. Requests to access these datasets should be directed to Q-yZ, emVuZ3Fpbmd5YW4xOTcyQDE2My5jb20=.
The studies involving human participants were reviewed and approved by Ethics Committee of Aier Eye Hospital of Wuhan University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
YL conceived the study and was the major contributor in design and coordination, in collecting data, in analyzing data, in the web designing, and in writing the manuscript. DS and H-yW helped to collect the data. M-yQ made the web design. Q-yZ participated in surgeries and contributed in its design and coordination, and made the analysis and interpretation of data. All authors have read and approved the final manuscript.
This study received support from the scientific research project of the Health Commission of Wuhan (Grant No. WX20Q19), and the Research Fund of the Clinical Research Institute of Aier Eye Hospital Group (No. AR2110D22).
The authors acknowledge Guang Zhao from the Department of Statistics of Tongji Medical College, Huazhong University of Science and Technology for his help in statistical analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1146529/full#supplementary-material
1. Wollensak, G, Spoerl, E, and Seiler, T. Riboflavin/ultraviolet-A–induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. (2003) 135:620–7. doi: 10.1016/S0002-9394(02)02220-1
2. Wittig-Silva, C, Chan, E, Islam, FM, et al. A randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus: three-year results. Ophthalmology. (2014) 121:812–21. doi: 10.1016/j.ophtha.2013.10.028
3. Iqbal, M, Elmassry, A, Saad, H, Am Gad, A, Ibrahim, O, Hamed, N, et al. Standard cross-linking protocol versus accelerated and transepithelial cross-linking protocols for treatment of paediatric keratoconus: a 2-year comparative study. Acta Ophthalmol. (2020) 98:e352–62. doi: 10.1111/aos.14275
4. Lombardo, M, Serrao, S, Lombardo, G, and Schiano-Lomoriello, D. Two-year outcomes of a randomized controlled trial of transepithelial corneal crosslinking with iontophoresis for keratoconus. J Cataract Refract Surg. (2019) 45:992–1000. doi: 10.1016/j.jcrs.2019.01.026
5. Bikbova, G, and Bikbov, M. Standard corneal collagen crosslinking versus transepithelial iontophoresis-assisted corneal crosslinking, 24 months follow-up: randomized control trial. Acta Ophthalmol. (2016) 94:e600–6. doi: 10.1111/aos.13032
6. Zhong, W, Montana, M, Santosa, SM, Isjwara, ID, Huang, YH, Han, KY, et al. Angiogenesis and lymphangiogenesis in corneal transplantation—a review. Surv Ophthalmol. (2018) 63:453–79. doi: 10.1016/j.survophthal.2017.12.008
7. Santodomingo-Rubido, J, Carracedo, G, Suzaki, A, Villa-Collar, C, Vincent, SJ, and Wolffsohn, JS. Keratoconus: an updated review. Cont Lens Anterior Eye. (2022) 45:101559. doi: 10.1016/j.clae.2021.101559
8. Raiskup-Wolf, F, Hoyer, A, Spoerl, E, and Pillunat, LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. (2008) 34:796–801. doi: 10.1016/j.jcrs.2007.12.039
9. Lenk, J, Herber, R, Oswald, C, Spoerl, E, Pillunat, LE, and Raiskup, F. Risk factors for progression of keratoconus and failure rate after corneal cross-linking. J Refract Surg. (2021) 37:816–23. doi: 10.3928/1081597X-20210830-01
10. Sağlık, A, Özcan, G, and Uçakhan, Ö. Risk factors for progression following corneal collagen crosslinking in keratoconus. Int Ophthalmol. (2021) 41:3443–9. doi: 10.1007/s10792-021-01908-9
11. Sot, M, Gan, G, François, J, Chaussard, D, Da Costa, M, Luc, MS, et al. Risk factors for keratoconus progression after treatment by accelerated cross-linking (A-CXL): a prospective 24-month study. J Fr Ophtalmol. (2021) 44:863–72. doi: 10.1016/j.jfo.2020.08.040
12. Sarac, O, Caglayan, M, Cakmak, HB, and Cagil, N. Factors influencing progression of keratoconus 2 years after corneal collagen cross-linking in pediatric patients. Cornea. (2016) 35:1503–7. doi: 10.1097/ICO.0000000000001051
13. Wisse, RP, Godefrooij, DA, Soeters, N, Imhof, SM, and Van der Lelij, A. A multivariate analysis and statistical model for predicting visual acuity and keratometry one year after cross-linking for keratoconus. Am J Ophthalmol. (2014) 157:519–25.e1-2. doi: 10.1016/j.ajo.2013.11.001
14. Gilevska, F, Biscevic, A, Popovic Suic, S, Bohac, M, and Patel, S. Are changes in visual acuity and astigmatism after corneal cross-linking (CXL) in keratoconus predictable? Graefes Arch Clin Exp Ophthalmol. (2021) 259:2259–68. doi: 10.1007/s00417-021-05173-5
15. Tian, M, Jian, W, Zhang, X, Sun, L, Shen, Y, and Zhou, X. Predictive factors of the accelerated transepithelial corneal cross-linking outcomes in keratoconus. BMC Ophthalmol. (2022) 22:7. doi: 10.1186/s12886-021-02235-4
16. Godefrooij, DA, Boom, K, Soeters, N, Imhof, SM, and Wisse, RP. Predictors for treatment outcomes after corneal crosslinking for keratoconus: a validation study. Int Ophthalmol. (2017) 37:341–8. doi: 10.1007/s10792-016-0262-z
17. Badawi, AE, Abou Samra, WA, and El Ghafar, AA. Predictive factors of the standard cross-linking outcomes in adult keratoconus: one-year follow-up. J Ophthalmol. (2017) 2017:4109208. doi: 10.1155/2017/4109208
18. Rebala, G, Ravi, A, and Churiwala, S. Machine learning definition and basics In:. An Introduction to Machine Learning. Cham: Springer (2019) (pp. 1–17).
19. Wang, Y, Yao, Q, Kwok, JT, and Ni, LM. Generalizing from a few examples: A survey on few-shot learning[J]. ACM Comput Surv. (2020) 53:1–34. doi: 10.1145/3386252
20. Korstanje, J . Gradient boosting with XGBoost and LightGBM In:. Advanced Forecasting With Python. Berkeley, CA: Apress (2021)
21. Maile, H, Li, JO, Gore, D, Leucci, M, Mulholland, P, Hau, S, et al. Machine learning algorithms to detect subclinical keratoconus: systematic review. JMIR Med Inform. (2021) 9:e27363. doi: 10.2196/27363
22. Aatila, M, Lachgar, M, Hamid, H, and Kartit, A. Keratoconus severity classification using features selection and machine learning algorithms. Comput Math Methods Med. (2021) 2021:9979560–26. doi: 10.1155/2021/9979560
23. Kato, N, Masumoto, H, Tanabe, M, Sakai, C, Negishi, K, Torii, H, et al. Predicting keratoconus progression and need for corneal crosslinking using deep learning. J Clin Med. (2021) 10:844. doi: 10.3390/jcm10040844
24. McMahon, TT, Szczotka-Flynn, L, Barr, JT, Anderson, RJ, Slaughter, ME, Lass, JH, et al. A new method for grading the severity of keratoconus: the keratoconus severity score (KSS). Cornea. (2006) 25:794–800. doi: 10.1097/01.ico.0000226359.26678.d1
25. Cronin, B, Ghosh, A, and Chang, CY. Oxygen-supplemented transepithelial-accelerated corneal crosslinking with pulsed irradiation for progressive keratoconus: 1 year outcomes. J Cataract Refract Surg. (2022) 48:1175–82. doi: 10.1097/j.jcrs.0000000000000952
26. Mazzotta, C, Traversi, C, Caragiuli, S, and Rechichi, M. Pulsed vs continuous light accelerated corneal collagen crosslinking: in vivo qualitative investigation by confocal microscopy and corneal OCT. Eye (Lond). (2014) 28:1179–83. doi: 10.1038/eye.2014.163
27. Nadeau, C, and Bengio, Y. Inference for the generalization error. Mach Learn. (2003) 52:239–81. doi: 10.1023/A:1024068626366
28. Bouckaert, RR, and Frank, E. Evaluating the replicability of significance tests for comparing learning algorithms. Lecture Notes Comput Sci. (2004) 3056:3–12. doi: 10.1007/978-3-540-24775-3_3
29. Kan, J, Li, A, Zou, H, Chen, L, and Du, J. A machine learning based dose prediction of lutein supplements for individuals with eye fatigue. Front Nutr. (2020) 7:577923. doi: 10.3389/fnut.2020.577923
30. Zhao, X, Wu, S, Fang, N, Sun, X, and Fan, J. Evaluation of single-cell classifiers for single-cell RNA sequencing data sets. Brief Bioinform. (2020) 21:1581–95. doi: 10.1093/bib/bbz096
31. Xia, Y, Li, X, Chen, X, Lu, C, and Yu, X. Inferring retinal degeneration-related genes based on Xgboost. Front Mol Biosci. (2022) 9:843150. doi: 10.3389/fmolb.2022.843150
32. Tayfur, M, Ocak, SY, and Elcioglu, MN. Factors affecting visual gain after accelerated crosslinking in pediatric Keratoconic cases. Beyoglu Eye J. (2021) 6:267–71. doi: 10.14744/bej.2021.15046
33. Peña-García, P, Vega-Estrada, A, Barraquer, RI, Burguera-Giménez, N, and Alio, JL. Intracorneal ring segment in keratoconus: a model to predict visual changes induced by the surgery. Invest Ophthalmol Vis Sci. (2012) 53:8447–57. doi: 10.1167/iovs.12-10639
34. Sedaghat, MR, Momeni-Moghaddam, H, Piñero, DP, Akbarzadeh, R, Moshirfar, M, Bamdad, S, et al. Predictors of successful outcome following intrastromal corneal ring segments implantation. Curr Eye Res. (2019) 44:707–15. doi: 10.1080/02713683.2019.1594945
35. Motlagh, MN, Moshirfar, M, Murri, MS, Skanchy, DF, Momeni-Moghaddam, H, Ronquillo, YC, et al. Pentacam® corneal tomography for screening of refractive surgery candidates: A review of the literature, part I. Med Hypothesis Discov Innov Ophthalmol. (2019) 8:177–203.
36. Viswanathan, D, Kumar, NL, Males, JJ, and Graham, SL. Relationship of structural characteristics to biomechanical profile in Normal, Keratoconic, and crosslinked eyes. Cornea. (2015) 34:791–6. doi: 10.1097/ICO.0000000000000434
37. Amanzadeh, K, Elham, R, and Jafarzadepur, E. Effects of single-segment Intacs implantation on visual acuity and corneal topographic indices of keratoconus. J Curr Ophthalmol. (2017) 29:189–93. doi: 10.1016/j.joco.2016.10.004
38. De Angelis, F, Rateau, J, Destrieux, C, Patat, F, and Pisella, PJ. Facteurs prédictifs de bonne réponse au crosslinking d'un kératocône évolutif: résultats réfractifs et topographiques à un an postopératoire [Predictive factors for visual outcome after corneal collagen crosslinking treatment in progressive keratoconus: One-year refractive and topographic results]. J Fr Ophtalmol. (2015) 38:595–606. doi: 10.1016/j.jfo.2014.11.017
39. Greenstein, SA, Fry, KL, and Hersh, PS. Effect of topographic cone location on outcomes of corneal collagen cross-linking for keratoconus and corneal ectasia. J Refract Surg. (2012) 28:397–405. doi: 10.3928/1081597X-20120518-02
40. Greenstein, SA, Shah, VP, Fry, KL, and Hersh, PS. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. (2011) 37:691–700. doi: 10.1016/j.jcrs.2010.10.052
41. Takahashi, A, Nakayasu, K, Okisaka, S, and Kanai, A. Quantitative analysis of collagen fiber in keratoconus. Nippon Ganka Gakkai Zasshi. (1990) 94:1068–73.
42. Uçakhan, OO, Kanpolat, A, Ylmaz, N, and Ozkan, M. In vivo confocal microscopy findings in keratoconus. Eye Contact Lens. (2006) 32:183–91. doi: 10.1097/01.icl.0000189038.74139.4a
43. Bradford, SM, Mikula, ER, Juhasz, T, Brown, DJ, and Jester, JV. Collagen fiber crimping following in vivo UVA-induced corneal crosslinking. Exp Eye Res. (2018) 177:173–80. doi: 10.1016/j.exer.2018.08.009
44. Mimouni, M, Sorkin, N, Hatch, W, Slomovic, AR, KEI CXL Study Group, and Singal, N. Fellow eye as a predictor for keratoconus progression following accelerated corneal cross-linking. J Refract Surg. (2021) 37:186–91. doi: 10.3928/1081597X-20201229-02
45. Ozgurhan, EB, Kara, N, Cankaya, KI, Kurt, T, and Demirok, A. Accelerated corneal cross-linking in pediatric patients with keratoconus: 24-month outcomes. J Refract Surg. (2014) 30:843–9. doi: 10.3928/1081597X-20141120-01
46. Vinciguerra, P, Albé, E, Frueh, BE, Trazza, S, and Epstein, D. Two-year corneal cross-linking results in patients younger than 18 years with documented progressive keratoconus. Am J Ophthalmol. (2012) 154:520–6. doi: 10.1016/j.ajo.2012.03.020
47. Olivo-Payne, A, Abdala-Figuerola, A, Hernandez-Bogantes, E, Pedro-Aguilar, L, Chan, E, and Godefrooij, D. Optimal management of pediatric keratoconus: challenges and solutions. Clin Ophthalmol. (2019) 13:1183–91. doi: 10.2147/OPTH.S183347
48. Kotecha, A, Elsheikh, A, Roberts, CR, Zhu, H, and Garway-Heath, DF. Corneal thickness- and age-related biomechanical properties of the cornea measured with the ocular response analyzer. Invest Ophthalmol Vis Sci. (2006) 47:5337–47. doi: 10.1167/iovs.06-0557
Keywords: crosslinking (CXL) corneal collagen, machine learning, keratoconus, prediction model, XGBoost (extreme gradient boosting)
Citation: Liu Y, Shen D, Wang H-y, Qi M-y and Zeng Q-y (2023) Development and validation to predict visual acuity and keratometry two years after corneal crosslinking with progressive keratoconus by machine learning. Front. Med. 10:1146529. doi: 10.3389/fmed.2023.1146529
Received: 17 January 2023; Accepted: 16 June 2023;
Published: 03 July 2023.
Edited by:
Cristina Nicula, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaReviewed by:
Mehran Zarei-Ghanavati, Tehran University of Medical Sciences, IranCopyright © 2023 Liu, Shen, Wang, Qi and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing-yan Zeng, emVuZ3Fpbmd5YW4xOTcyQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.