
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Med., 18 May 2023
Sec. Geriatric Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1144156
This article is part of the Research TopicThe Role of Nutrition in Healthy AgingView all 10 articles
 Jeannette M. Beasley1,2*
Jeannette M. Beasley1,2* Emily A. Johnston2
Emily A. Johnston2 Mary Ann Sevick2,3
Mary Ann Sevick2,3 Melanie Jay2,3,4
Melanie Jay2,3,4 Erin S. Rogers3
Erin S. Rogers3 Hua Zhong3
Hua Zhong3 Sondra Zabar2
Sondra Zabar2 Eric Goldberg2
Eric Goldberg2 Joshua Chodosh2,3,4
Joshua Chodosh2,3,4In the Diabetes Prevention Program (DPP) randomized, controlled clinical trial, participants who were ≥ 60 years of age in the intensive lifestyle (diet and physical activity) intervention had a 71% reduction in incident diabetes over the 3-year trial. However, few of the 26.4 million American adults age ≥65 years with prediabetes are participating in the National DPP. The BRInging the Diabetes prevention program to GEriatric Populations (BRIDGE) randomized trial compares an in-person DPP program Tailored for Older AdulTs (DPP-TOAT) to a DPP-TOAT delivered via group virtual sessions (V-DPP-TOAT) in a randomized, controlled trial design (N = 230). Eligible patients are recruited through electronic health records (EHRs) and randomized to the DPP-TOAT or V-DPP-TOAT arm. The primary effectiveness outcome is 6-month weight loss and the primary implementation outcome is intervention session attendance with a non-inferiority design. Findings will inform best practices in the delivery of an evidence-based intervention.
Over one-quarter (29.2%) of US adults aged 65 and older have Type 2 diabetes (i.e., 15.9 million people), and the Centers for Disease Control and Prevention (CDC) estimated that an additional 26.4 million older adults had prediabetes, defined as fasting plasma glucose values of 100 to 125 mg/dl or hemoglobin A1C values of 5.7 to 6.4%, in 2019 (1). Evidence-based diabetes prevention strategies, such as the National Diabetes Prevention Program (DPP), reduce the risk of developing diabetes but remain underutilized. In the original Diabetes Prevention Program (DPP) study, the diet and physical activity intervention conferred a 71% reduction in risk of type 2 diabetes for the participants who were ≥60 years of age (n = 648) after 3 years of follow-up (2). Since the seminal DPP was established as efficacious (2), the intervention has been implemented in hospital, community, work, and other settings.
Despite this, just 14.6% of rural counties and 48.4% of urban counties in the nation have a DPP site. Barriers to DPP participation exist for both individuals and healthcare systems. Individuals face barriers to access, including travel to 22 in-person sessions, as well as cost if they elect to utilize a commercial online program. Older adults, in particular, face barriers to care, including transportation, costs, time burden, and limited physical function/reserve. Healthcare systems face significant cost burdens for training DPP facilitators and hosting the program (3). In the few studies that have reported on costs of administering the DPP, there was a 5-fold difference in cost per participant across studies, with a virtual program costing less than on-site delivery (4, 5). In the BRInging the Diabetes prevention program to GEriatric Populations (BRIDGE) trial, we will address these barriers by generating a DPP Tailored for Older Adults (DPP-TOAT) and delivered via Virtual sessions (V-DPP-TOAT).
Technology use among older adults is increasing. Almost two-thirds (63%) of adults aged 66–75 years use the Internet to access health information, including 49% of adults aged 75 and older (6). Furthermore, 69% of adults ≥65 have a mobile phone, including 56% of adults ≥75 years of age. Web-based interventions have effectively increased health knowledge (7) and physical activity among older adults (8–10). The proportion of older adults enrolling in internet-based programs will grow with the aging of younger cohorts who are more accustomed to depending on technology to meet their health care and other needs.
Delivering a DPP through a videoconferencing platform may extend the program’s reach into the older adult community that may otherwise lack access (11). The NYU Langone Health catchment area includes more than 7.2 million people, of whom nearly 1 million individuals are aged 65 and older. An intervention using technology to provide remote training and individualized feedback increases the likelihood of reproducibility outside of the NYU Langone Health network and sustainability over time (12). Virtual interventions do not require brick-and-mortar facilities or centralized staff; these highly scalable interventions can be delivered from any location. However, a recent behavioral weight loss trial reported that in-clinic group visits, but not telephone group visits, resulted in statistically significant greater weight loss at 24 months compared with traditional in-clinic individual visits (13). Lessons learned from the implementation evaluation of the BRIDGE DPP will provide the opportunity for other healthcare systems to offer the program to eligible patients. The study aims are to compare the effectiveness and implementation of V-DPP-TOAT versus DPP-TOAT within a large healthcare system.
We will conduct a type 1 hybrid (14), randomized, controlled trial of the BRInging the Diabetes prevention program to GEriatric Populations (BRIDGE) study, which will compare an in-person DPP Tailored for Older AdulTs (DPP-TOAT) to a DPP-TOAT delivered via group virtual sessions (V-DPP-TOAT; Figure 1). All randomized individuals (N = 230; 1:1 randomization) will receive 22 intervention sessions over the course of a year facilitated by a certified DPP lifestyle coach, but the delivery method will vary (virtual versus in-person).
Outcome assessments will be conducted at 0, 6, and 12 months at the clinic where we will assess weight, hemoglobin A1c, diet, and physical activity in both groups. At baseline, we will assess height, weight, waist circumference, and administer questionnaires to all participants. The trial design will adhere to the CONSORT checklist (see Appendix A), including an intention-to-treat analysis (15). Meticulous adherence to the study protocol and fidelity monitoring will ensure robust and unbiased results.
The NYU Langone Health patient population includes over 15,000 patients aged 65 or older with a diagnosis of prediabetes who meet the basic inclusion criteria. NYU Langone Health clinical providers utilize Epic, one of the country’s largest electronic health record platforms, and its patient portal (MyChart), which allows for direct outreach and bi-directional communication with patients. Using Epic, we will continuously identify eligible older adults with prediabetes. As in our prior work (16–19), patients will be recruited through both physician referral and proactive outreach to patients. For proactive outreach, we use Epic-generated lists of eligible patients based on our eligibility criteria. A research team member will send lists to patients’ primary care provider (PCP), who will subsequently identify any contraindications to participating. Potential participants will be sent a message through the patient portal (MyChart) and/or mailed a letter signed by the principal investigators (JB and JC) and medical director (EG) that describes the study and gives them the opportunity to opt out (i.e., request not to be contacted). We will then call potential participants to recruit, screen for eligibility, and schedule a baseline visit. Table 1 outlines eligibility criteria for study participation.
The study statistician (HZ), will generate randomized treatment allocation in a blinded environment using a Research Electronic Data Capture (REDCap, https://projectredcap.org/) randomization tool.
We will use the following strategies to encourage retention of participants in both arms of the study: (1) reminder of study visits and intervention sessions via email and phone call, (2) financial incentives following successful completion of study visits ($50 gift card for each baseline, 6, 12 month visits), (3) birthday cards, (4) certificates of completion and milestone emails.
As with most behavioral trials, it is difficult to mask participants to the intervention arm. To reduce bias, all outcome assessors will be masked to the intervention arm and participants will be asked not to disclose treatment allocation. To detect potential unmasking, we will ask outcome assessors about participant allocation after each post-randomization measurement visit.
Both programs will be composed of sixteen 60-min weekly/bi-weekly sessions followed by six 60-min monthly support sessions (see Appendix B) with a group size of 8–15 facilitated by a certified DPP lifestyle coach. The DPP intervention is based on Social Cognitive Theory (20), which focuses on the role played by self-referent thought in the maintenance of behavior change. The following describes the theoretical concepts threaded throughout the intervention sessions. Self-efficacy (e.g., the participant’s confidence in their ability to engage in healthier behavior) is derived from four major sources of information: (1) mastery experiences; (2) social modeling; (3) verbal persuasion; and (4) physiological states. Mastery experiences emphasize past successes; setting incremental, easily achievable goals; identifying modifiable barriers to healthy behavior; receiving positive feedback on goal achievement; and practicing problem solving skills around barriers to adherence. Social modeling enhances mastery when participants share their successes and help each other problem-solve around barriers they encounter. Verbal persuasion emphasizes the participant’s previous successes to demonstrate their capability (e.g., “As a result of your effort, you lost a pound last week. You can do it again.”) We will assist participants in recognizing physiologic benefits they experience from lifestyle change (e.g., more energy, better sleep, or BP control). Intervention materials, which are based on the 2021 PreventT2 lifestyle change program (21), will include:
• Participant Manual: An evidence-based manual describing detailed program content and personalized calorie and macronutrient recommendations to achieve the desired weight. Written at the sixth-grade reading level in a large (14 point) sans serif font, self-assessments and goal setting procedures will be included along with approaches to self-monitor food intake, physical activity, and cooking and meal pattern guides. To enhance self-identification with the program and for ongoing visual cues for program affiliation, we will provide tote bags inscribed with the institutional logo.
• Tailoring of content for aging: Based on our prior work (22), we have adapted group sessions to the unique needs of older adults. Resources include healthful eating strategies for dentition issues, changes in taste, and special attention to interactions between food and medications. Physical activity sessions provide adaptations for physical limitations, including chair exercises and strength training.
• Food Guide: Resources developed by the United States Department of Agriculture (23) providing tailored advice for older adults will be provided, along with instructions and tips for using programs such as Healthwatch 360 (24) to track calorie and nutrient intake compared to recommendations.
• Hearing Assistance: For participants in the in-person arm, we will offer a personal amplifier (PA; PockeTalker™) to all participants to address any self-reported or suspected hearing loss. A simple PA consists of an amplifier and microphone with the amplifier feeding a speaker’s voice directly into the wearer’s ears via headphones or ear buds. These devices reduce the difficulty with hearing in “difficult listening situations” by feeding the speaker’s amplified voice directly into the patient’s ear, while headphones or ear buds muffle external sounds (i.e., signal is louder than background noise level). These devices require no professional customization; simple volume and tuning controls allow for use directly out of the box. PAs enhance communication for those who have hearing loss in a variety of clinical settings (25–27). PAs were equivalent in performance to hearing aids in one randomized controlled trial (28), sound quality of PAs was preferred over hearing aids, improved physician-patient communication for elderly hospitalized patients (29) and understanding discharge information in an Emergency Department setting (30). Participants in the virtual arm will be offered ear buds to address any self-reported or suspected hearing loss.
• Videos: Each intervention session will also feature educational and behavioral coaching videos developed by our team or reputable organizations such as the National Institutes Health and VA MOVE (Weight Management Program, supported by VA’s National Center for Health Promotion and Disease Prevention) (31). The videos last 3–5 min, and will be used to anchor the discussion. Videos will also be available on the study website for participants to review as they desire.
• Smart Scales: We will provide participants with a Renpho® (Joicom Corporation, Eastvale, CA) Smart Scale for Body Weight, Digital Bathroom Scale for self-monitoring purposes and to monitor weight given the possibility of disruptions in in-person study visits due to COVID-19 pandemic restrictions. Renpho® Scales will transmit weights to researchers automatically using BlueTooth technology and have been used in other weight management studies. A research assistant will instruct each participant during the baseline visit on using the scale to report weekly weights, providing written instructions as well as a phone number and email address to obtain technical support as needed.
Intervention sessions will take place via videoconference rather than in-person, and we will encourage participants to use their preferred device to connect with the group (e.g., tablet, computer, and smartphone). Participants will be offered a tablet computer if they do not have an electronic device they’d like to use to connect. A research assistant will orient each participant to the tablet during the baseline visit, providing written instructions, a phone number and email address to obtain technical support as needed. Before or after the intervention session, participants will be assigned to breakout rooms where they will have a 1-on-1 visit with the lifestyle coach, report their weight, physical activity minutes and discuss action plan and goal achievement.
All interactions will be in-person and weights will be measured immediately before or after intervention sessions using a Renpho® scale.
In-person data collection will occur during study visits for both arms. Assessments will occur at: baseline (first in-person encounter), six, and 12 months (Tables 2, 3). Participants will meet with a research assistant masked to treatment assignment to complete surveys and to measure glycemia, weight, height, and waist circumference (baseline, six, and 12 months). Research staff will administer all surveys, entering data directly into a REDCap database. The DPP facilitator will record DPP attendance data.
Core measures for Aim 1 are displayed in Table 2.
We will compare the effect of the V-DPP-TOAT versus the DPP-TOAT interventions on weight and glycemia, which are both continuous outcomes. First, we will report means and standard deviations by randomization assignment and assess the assumption of normality in the outcomes. We will estimate the model using ordinary least squares regression
We will assess heterogeneity in outcomes among healthcare settings/clinics by estimating an intraclass correlation coefficient (ICC). If the heterogeneity cannot be ignored, we will use a multi-level model with nested random effects in the above equation to accommodate the correlations caused by healthcare settings/centers. Outcomes analysis will use an intention-to-treat principle, with a per-protocol analysis as a sensitivity analysis (41) employing R software (version 1.2.5019, R Foundation for Statistical Computing) for all analyses.
We will compare demographic and clinical covariates in participants with and without missing data to identify factors potentially contributing to missingness. We will use 10 multiple imputations from the original dataset using the predictive mean matching method substituting missing values within each impute.
To achieve 80% power to detect non-inferiority using a one-sided, two-sample t-test (lower bound), we will need 85 participants per group. The margin of non-inferiority is 5% of weight loss in the DPP-TOAT arm. The true efficacy difference between the 6-month weight loss is assumed to be 1.6 kg (weight loss of 5 kg in the V-DPP-TOAT arm and 6.6 kg in the in-person DPP-TOAT arm with standard deviation of 5 kg in each arm (42)). The significance level (alpha) of the test is 0.05. A total of 230 participants are needed, assuming 25% attrition at 6 months based on data from our work and others (22, 42, 43).
To determine the feasibility of generalizing the DPP-TOAT intervention to other clinics within the NYU Langone Health system in New York and, ultimately, to other states, we will evaluate the implementation of the program during the RCT. Based on previous work, we hypothesize that adherence to the V-DPP-TOAT will be greater than the DPP-TOAT, as measured by the number of group sessions completed by each participant (42). We will use the RE-AIM (reach, effectiveness, adoption, implementation, maintenance) framework (44) to assess quantitative implementation outcomes during the Aim 1 effectiveness trial (Table 3). We will use the Consolidated Framework for Implementation Research (CFIR) (45) to examine barriers/facilitators to implementation within five main domains (intervention characteristics, inner setting, outer setting, participant/stakeholder characteristics, and implementation process), ensuring that this intervention can be generalized and readily disseminated and implemented elsewhere.
• Reach: We define the reach of the intervention as the number (proportion) of individuals who are (a) eligible for the study based on electronic health record data, (b) exposed to recruitment, (c) who initially responded, and (d) who decided to participate. We will survey those who decline participation to evaluate reasons for not participating. We will evaluate factors associated with participation by comparing the demographic and clinical characteristics of patients reached by the intervention vs. those not reached.
• Effectiveness: We will explore potential outcome differences in subgroups (e.g., sex, BMI category). We will conduct descriptive exploratory data analyses to explore which components may contribute most to the improvement of the outcomes.
• Adoption: We will assess willingness to adopt the intervention if it is found to be effective among key settings serving older adults with prediabetes, including other healthcare systems (e.g., a public hospital setting, senior centers, and rural settings, identifying barriers/facilitators toward future adoption.
• Implementation: We will evaluate implementation fidelity and resource requirements. We will also evaluate the acceptability and feasibility of the intervention among participants and facilitators, as well as barriers to implementing the program during the trial and to inform future implementation efforts. We will collect data on time spent delivering the intervention by lifestyle coaches including number of sessions, cost of materials, and other program costs.
• Maintenance: We will assess long-term maintenance of primary clinical outcomes (weight maintenance and glycemia at 12 months post-baseline visit).
Research assistants keep detailed recruitment records using standardized forms to capture variables related to intervention reach described above (e.g., percent eligible, percent who enroll). Recordings of each session provide rich data related to implementation fidelity described above (e.g., number of group sessions completed, session length, topics covered during each session). Core measures for Aim 2 are listed in Table 3.
Aim 2 uses the RE-AIM framework to evaluate implementation of the V-DPP-TOAT and in-person DPP-TOAT interventions. We will compare the number of group sessions completed by each participant in the two arms using generalized linear models with count outcomes, because DPP session attendance is positively associated with weight loss (42, 46), and is a CDC recognition status benchmark (47). The proportion of participants who complete at least eight intervention sessions will be compared by logistic regression between the two arms to make our findings comparable to other DPP implementation studies (42, 46). However, our primary implementation outcome of attendance is independent of any threshold chosen in the number of sessions. We will test interaction terms between baseline covariates and treatment assignment to determine if there is effect modification by any of the baseline covariates.
If there are imbalances at baseline for any important participant health, demographic or other factors, we will adjust the models to take these imbalances into consideration. For the remaining analyses, we will conduct similar types of analyses, using logistic regression for categorical outcomes and linear regression models for continuous outcomes.
We will use mediation analysis to dissect the indirect effects of the treatment acting through the intermediate variables (social support, self-efficacy, and self-monitoring) on the primary outcome (attendance), and the direct effects of treatment on the outcome (attendance) not mediated through the mediators. For each of the three mediators, an overall score and subscale scores will be computed by summing relevant items. This analysis will help understand the underlying treatment mechanisms of behavior change. Specifically, we will use the principal stratification and structural mean models. We will assess the assumptions necessary for attaining identifiability of key parameters of the basic causal model and perform a series of sensitivity analyses. We will also investigate the interactions between baseline covariates and the mediation effects to determine whether any baseline factors modify the mediation effects (48, 49).
In order to inform cost for future implementations and policy makers (4), we will compare the sum of variable (e.g., operating costs, supply costs, percent time of intervention and staff) and fixed costs (e.g., space, equipment) between intervention arms (50).
Based on data from Lee 2018, we expect a higher proportion of V-DPP-TOAT participants will complete at least eight intervention sessions compared to DPP-TOAT participants (89 vs. 63%, respectively) (42). With 90 samples in each arm at 6 months, we achieve 98% power to detect the difference in session completion proportions of 26% at alpha = 0.05 (89 vs. 63% respectively) and 80% power to detect smaller difference of 19% (82 vs. 63% respectively).
All interviews will be recorded using Zoom (in-person sessions will use Zoom via conference room video and audio system) with transcripts saved. Data analysis from transcriptions will follow techniques of narrative analysis (51, 52) and a “constant comparison” analytic approach (53). To code transcripts, the research team will develop an initial set of codes, informed by the open-ended questions and the interviews’ guiding conceptual framework. For each core code, we will ultimately develop one or more “secondary codes” that represent either more specific or restricted aspects of the phenomenon for contextualization or to suggest underlying personal meanings. The secondary codes will vary in specificity or subtlety depending on the judged substantive value of additional refinements. The coding schema is a strategy for organizing and assimilating the large amount of data that the interviews will yield. To ensure that the coding is both valid (i.e., well grounded in the data) and reliable (consistent in meaning), the criteria for assigning a specific code to a block of text will be systematically developed and well documented. The resulting codebook will be refined and expanded upon to reflect and incorporate emerging insights throughout the coding process. All transcripts will be double-coded, and discrepancies will be resolved by consensus discussion.
The coded transcripts will be analyzed with Atlas.ti (Version 8, Berlin, Germany), a software package for qualitative data analysis. With Atlas.ti, concepts (constructs, themes), contextual factors, participant characteristics, behaviors and attitudes can be related to one another. This will be particularly important to examine, for example, how themes vary or are consistent across different participant subgroups. Frequency counts will be generated for core or secondary codes by identified subgroups, and the total data file.
We will use a fidelity checklist to monitor skill acquisition and quality of counseling. A study team member will monitor 10% of the recorded intervention sessions using a fidelity checklist. We will provide refresher training if fidelity checklist ratings average <80%. We will measure attendance, outcomes assessment completion, and diet and physical activity monitoring completion.
Dissemination. The RE-AIM and CFIR frameworks analyses will address generalizability and transportability to facilitate future dissemination.
Previous DPP studies have shown great benefit for older adult participants with reductions in incidence of diabetes of up to 71% (2). Barriers to attendance in older adults limit participation and barriers to engagement may include reduced hearing acuity in a group setting, reduced visual acuity and ability to read DPP materials, and reduced applicability of diet and physical activity recommendations. These barriers may be attenuated by adaptations made in the BRIDGE study.
This work will contribute to the growing body of implementation studies that at present, consist largely of commercial online DPP programs that engaged older adults (Table 4) (42, 46, 54, 55). Most recently, Omada’s randomized, controlled trial recruiting from primary care centers in Nebraska demonstrated meaningful reductions in weight and HbA1c (42, 46, 54, 56–58). However, none of the commercial programs provide facilitated theory-based group sessions. Data from our work and others suggests the social support provided by these sessions is critical for engagement and retention of older adults (22, 43, 59). Furthermore, existing programs do not offer adaptations for older adults for nutrition, physical activity, hearing impairment, and/or low vision. Due to the COVID-19 pandemic, Medicare allowed for virtual delivery of the DPP (60), and it is likely that demand for convenient, online programs will continue (61, 62). The goal of this project is to test the effectiveness of a virtual adaptation that will: (1) be accessible to those who cannot afford to pay for commercial programs; (2) provide social support by the facilitated group sessions; (3) reduce the barriers associated with in-person DPP programs; and (4) serve the unique needs of older adults.
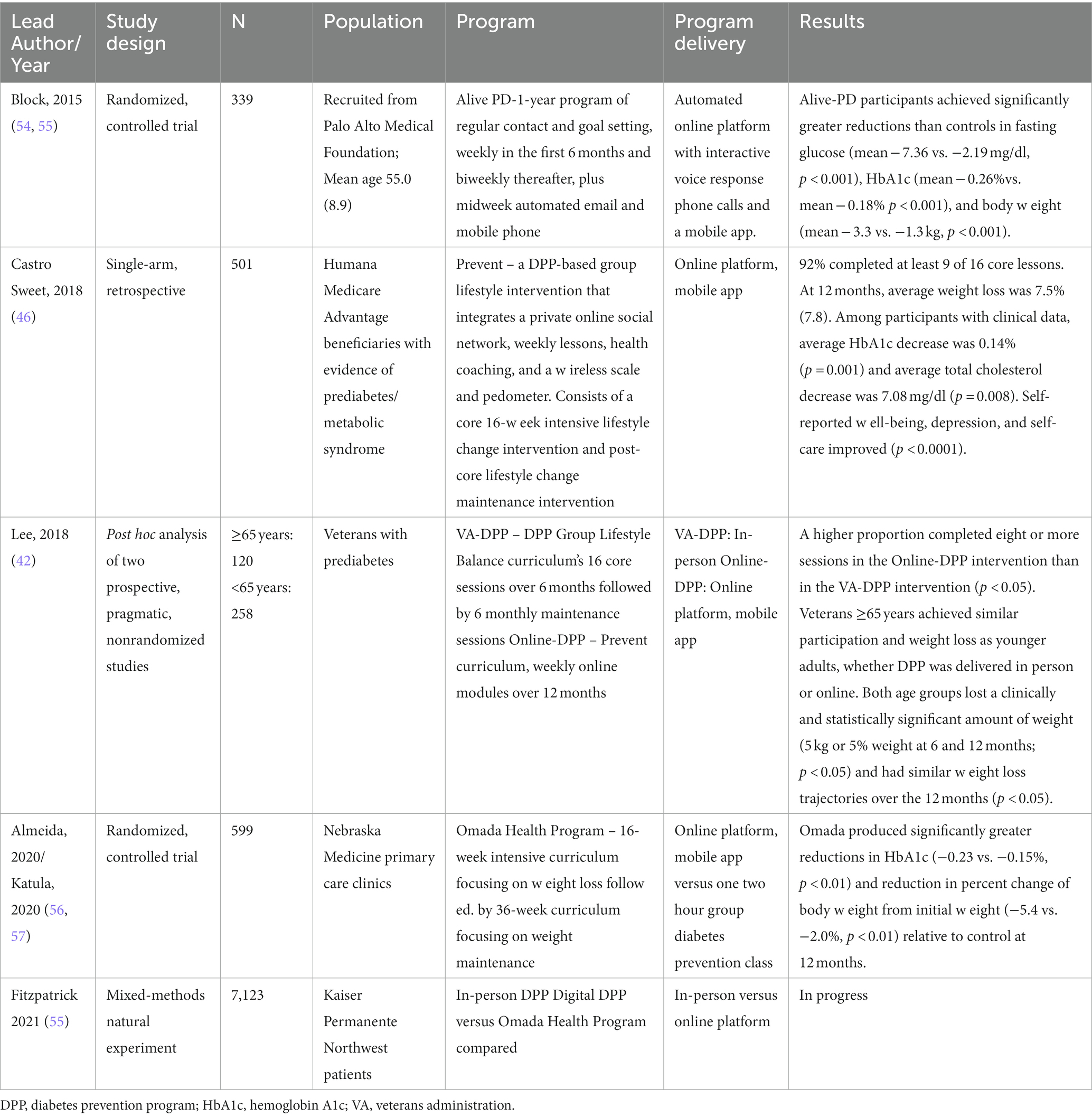
Table 4. Healthcare system implementations of online diabetes prevention programs (DPP) engaging older adults.
Our study has several strengths. The results of our trial can directly inform policy recommendations to Medicare that will have broad implications for older adults with prediabetes (63). Another major strength is our ability to recruit a diverse study population from broad socioeconomic backgrounds, which will help us to better understand how implementation may need to be tailored for different populations and settings. Our team has the breadth and depth of experience in conducting behavioral intervention trials and implementation to analyze results and interpret them for stakeholders to inform potential future adoption of the DPP by a large healthcare system. We have also identified several challenges and discussed ways to address these. First, trials often have challenges recruiting people from underrepresented groups. Our experience recruiting underserved populations from health care systems enhances our ability to meet recruitment targets. Second, there is inherent measurement error associated with self-reported dietary intake, but we do not expect the measurement error to differ between randomized groups, allowing for a valid, randomized comparison. Third, though using technology to engage participants can be challenging, technology use among older adults is increasing (64), and our prior and ongoing work suggests it is feasible and increases opportunities for broader dissemination of successful interventions.
This work will generate a high fidelity, easily implemented adaptation of a theory-based DPP for older adults in a virtual setting. This will increase access to an evidence-based prediabetes intervention tailored to older adults, providing opportunities to address multiple potential barriers for care (e.g., travel, need for care partners, social distancing due to COVID-19). We anticipate that including virtual social support will increase adherence and maintain effectiveness of the intervention. The information gained will inform best practices for other virtual health interventions both for the general population as well as for older adults.
Online/remote learning is rapidly becoming a critical piece of the new normal going forward for DPP programs. Findings will inform policy decisions regarding the use of virtual DPP programs to prevent diabetes among the Medicare population to improve best practices in the delivery of an evidence-based intervention, having the potential to help over 26.4 million people with prediabetes (1).
JB wrote the protocol, secured funding, and drafted the manuscript. EJ is a co-facilitator of the intervention sessions and contributed substantively to the development of the manuscript. MS provided expertise related to recruitment, retention, and group facilitation. MJ provided clinical insight to sample characteristics and interpretation of survey data. ER provided oversight of aim 2 measures and interpretation of pilot data to inform updating of implementation measures. HZ provided statistical guidance including power analyses and analytic plan. SZ and EG served as physician champions, recruited other physicians, and provided resources for intervention delivery. JC provided oversight of recruitment and retention and provided edits to the manuscript. All authors contributed to the article and approved the submitted version.
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R01 DK127916.
We thank BRIDGE study participants for their time and feedback.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1144156/full#supplementary-material
1. Centers for Disease Control and Prevention (2022). https://www.cdc.gov/diabetes/data/statistics-report/index.html. [Accessed May 8, 2023].
2. Knowler, WC, Barrett-Connor, E, Fowler, SE, Hamman, RF, Lachin, JM, Walker, EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. (2002) 346:393–403. doi: 10.1056/NEJMoa012512
3. Smith, KJ, Kuo, S, Zgibor, JC, McTigue, KM, Hess, R, Bhargava, T, et al. Cost effectiveness of an internet-delivered lifestyle intervention in primary care patients with high cardiovascular risk. Prev Med. (2016) 87:103–9. doi: 10.1016/j.ypmed.2016.02.036
4. Sun, Y, You, W, Almeida, F, Estabrooks, P, and Davy, B. The effectiveness and cost of lifestyle interventions including nutrition education for diabetes prevention: a systematic review and meta-analysi. J Acad Nutr Diet. (2017) 117:404–421.e36. doi: 10.1016/j.jand.2016.11.016
5. Vadheim, LM, Brewer, KA, Kassner, DR, Vanderwood, KK, Hall TO, Butcher, MK, et al. Effectiveness of a lifestyle intervention program among persons at high risk for cardiovascular disease and diabetes in a rural community. J Rural Health. (2010) 26:266–72. doi: 10.1111/j.1748-0361.2010.00288.x
6. Hall, AK, Bernhardt, JM, Dodd, V, and Vollrath, MW. The digital health divide: evaluating online health information access and use among older adults. Health Educ Behav. (2015) 42:202–9. doi: 10.1177/1090198114547815
7. Freund, O, Reychav, I, McHaney, R, Goland, E, and Azuri, J. The ability of older adults to use customized online medical databases to improve their health-related knowledge. Int J Med Inform. (2017) 102:1–11. doi: 10.1016/j.ijmedinf.2017.02.012
8. Peels, DA, Hoogenveen, RR, Feenstra, TL, Golsteijn, RH, Bolman, C, Mudde, AN, et al. Long-term health outcomes and cost-effectiveness of a computer-tailored physical activity intervention among people aged over fifty: modelling the results of a randomized controlled trial. BMC Public Health. (2014) 14:1099. doi: 10.1186/1471-2458-14-1099
9. Peels, DA, Bolman, C, Golsteijn, RH, de Vries, H, Mudde, AN, van Stralen, MM, et al. Long-term efficacy of a printed or a web-based tailored physical activity intervention among older adults. Int J Behav Nutr Phys Act. (2013) 10:104. doi: 10.1186/1479-5868-10-104
10. Peels, D, Mudde, A, Bolman, C, Golsteijn, R, de Vries, H, and Lechner, L. Correlates of the intention to implement a tailored physical activity intervention: perceptions of intermediaries. Int J Environ Res Public Health. (2014) 11:1885–903. doi: 10.3390/ijerph110201885
11. Madigan, E, Schmotzer, BJ, Struk, CJ, DiCarlo, CM, Kikano, G, Pina, IL, et al. Home health care with telemonitoring improves health status for older adults with heart failure. Home Health Care Serv Q. (2013) 32:57–74. doi: 10.1080/01621424.2012.755144
12. Singer, JP, Soong, A, Bruun, A, Bracha, A, Chin, G, Hays, SR, et al. A mobile health technology enabled home-based intervention to treat frailty in adult lung transplant candidates: a pilot study. Clin Transpl. (2018) 32:e13274. doi: 10.1111/ctr.13274
13. Befort, CA, VanWormer, JJ, Desouza, C, Ellerbeck, EF, Gajewski, B, Kimminau, KS, et al. Effect of behavioral therapy with in-clinic or telephone group visits vs in-clinic individual visits on weight loss among patients with obesity in rural clinical practice: a randomized clinical trial. JAMA. (2021) 325:363–72. doi: 10.1001/jama.2020.25855
14. Curran, GM, Bauer, M, Mittman, B, Pyne, JM, and Stetler, C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. (2012) 50:217–26. doi: 10.1097/MLR.0b013e3182408812
15. Schulz, KF, Altman, DG, and Moher, D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. (2010) 8:18. doi: 10.1186/1741-7015-8-18
16. Viglione, C, Bouwman, D, Rahman, N, Fang, Y, Beasley, JM, Sherman, S, et al. A technology-assisted health coaching intervention vs. enhanced usual care for primary care-based obesity treatment: a randomized controlled trial. BMC Obes. (2019) 6:4. doi: 10.1186/s40608-018-0226-0
17. Wittleder, S, Ajenikoko, A, Bouwman, D, Fang, Y, McKee, MD, Meissner, P, et al. Protocol for a cluster-randomized controlled trial of a technology-assisted health coaching intervention for weight management in primary care: the GEM (goals for eating and moving) study. Contemp Clin Trials. (2019) 83:37–45. doi: 10.1016/j.cct.2019.06.005
18. Sevick, MA, Woolf, K, Mattoo, A, Katz, SD, Li, H, St-Jules, DE, et al. The healthy hearts and kidneys (HHK) study: design of a 2x2 RCT of technology-supported self-monitoring and social cognitive theory-based counseling to engage overweight people with diabetes and chronic kidney disease in multiple lifestyle changes. Contemp Clin Trials. (2018) 64:265–73. doi: 10.1016/j.cct.2017.08.020
19. Popp, CJ, St-Jules, DE, Hu, L, Ganguzza, L, Illiano, P, Curran, M, et al. The rationale and design of the personal diet study, a randomized clinical trial evaluating a personalized approach to weight loss in individuals with pre-diabetes and early-stage type 2 diabetes. Contemp Clin Trials. (2019) 79:80–8. doi: 10.1016/j.cct.2019.03.001
20. Bandura, A. Human agency in social cognitive theory. Am Psychol. (1989) 44:1175–84. doi: 10.1037/0003-066X.44.9.1175
21. Centers for Disease Control and Prevention (2018). National DPP PreventT2 curricula and handouts. Available At: https://nationaldppcsc.cdc.gov/s/article/National-DPP-PreventT2-Curricula-and-Handouts [Accessed April 3, 2020].
22. Beasley, JM, Kirshner, L, Wylie-Rosett, J, Sevick, MA, DeLuca, L, and Chodosh, J. BRInging the diabetes prevention program to GEriatric populations (BRIDGE): a feasibility study. Pilot Feasibility Stud. (2019) 5:129. doi: 10.1186/s40814-019-0513-7
23. Build a Healthy Eating Routine as You Get Older (2021). Dietary Guidelines [Internet]. Available at: https://www.dietaryguidelines.gov/sites/default/files/2021-12/DGA_OlderAdults_FactSheet-508c.pdf [Accessed November 7, 2022].
24. HealthWatch360 (n.d.). Available at: https://healthwatch360.gbhealthwatch.com/ [Accessed November 7, 2022].
25. Wallhagen, MI, Pettengill, E, and Whiteside, M. Sensory impairment in older adults: part 1: hearing loss. Am J Nurs. (2006) 106:40–8. doi: 10.1097/00000446-200610000-00030
26. Palumbo, MV. Hearing access 2000. Increasing awareness of the hearing impaired. J Gerontol Nurs. (1990) 16:26–31. doi: 10.3928/0098-9134-19900901-09
27. Smith, AK, Jain, N, and Wallhagen, ML. Hearing loss in palliative care. J Palliat Med. (2015) 18:559–62. doi: 10.1089/jpm.2014.0367
28. Jerger, J, Chmiel, R, Florin, E, Pirozzolo, F, and Wilson, N. Comparison of conventional amplification and an assistive listening device in elderly persons. Ear Hear. (1996) 17:490–504. doi: 10.1097/00003446-199612000-00005
29. Fook, L, Morgan, R, Sharma, P, Adekoke, A, and Turnbull, CJ. The impact of hearing on communication. Postgrad Med J. (2000) 76:92–5. doi: 10.1136/pmj.76.892.92
30. Chodosh, J, Goldfeld, K, Weinstein, BE, Radcliffe, K, Burlingame, M, Dickson, V, et al. The HEAR-VA pilot study: hearing assistance provided to older adults in the emergency department. J Am Geriatr Soc. (2021) 69:1071–8. doi: 10.1111/jgs.17037
31. MOVE! (n.d.). Weight management program Available at: https://www.move.va.gov/ [Accessed November 7, 2022].
32. Bang, H, Edwards, AM, Bomback, AS, Ballantyne, CM, Brillon, D, Callahan, MA, et al. Development and validation of a patient self-assessment score for diabetes risk. Ann Intern Med. (2009) 151:775–83. doi: 10.7326/0003-4819-151-11-200912010-00005
33. Chew, LD, Bradley, KA, and Boyko, EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. (2004) 36:588–94.
34. Wallace, LS, Rogers, ES, Roskos, SE, Holiday, DB, and Weiss, BD. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. (2006) 21:874–7. doi: 10.1111/j.1525-1497.2006.00532.x
35. American Community Health Survey (2017-2021). United states census bureau. Available at: https://www.census.gov/acs/www/about/why-we-ask-each-question/computer/ [Accessed May 8, 2023].
36. Wittleder, S, Smith, S, Wang, B, Beasley, JM, Orstad, SL, Sweat, V, et al. Peer-assisted lifestyle (PAL) intervention: a protocol of a cluster-randomised controlled trial of a health-coaching intervention delivered by veteran peers to improve obesity treatment in primary care. BMJ Open. (2021) 11:e043013. doi: 10.1136/bmjopen-2020-043013
37. Cyranowski, JM, Zill, N, Bode, R, Butt, Z, Kelly, MA, Pilkonis, PA, et al. Assessing social support, companionship, and distress: National Institute of health (NIH) toolbox adult social relationship scales. Health Psychol. (2013) 32:293–301. doi: 10.1037/a0028586
38. Clark, MM, Abrams, DB, Niaura, RS, Eaton, CA, and Rossi, JS. Self-efficacy in weight management. J Consult Clin Psychol. (1991) 59:739–44. doi: 10.1037/0022-006X.59.5.739
39. Centers for Disease Control and Prevention (2021). Behavioral risk factor surveillance system. Available at: https://www.cdc.gov/brfss/index.html [Accessed May 8, 2023].
40. Hays, RD, Sherbourne, CD, and Mazel, RM. The RAND 36-item health survey 1.0. Health Econ. (1993) 2:217–27. doi: 10.1002/hec.4730020305
41. Schumi, J, and Wittes, JT. Through the looking glass: understanding non-inferiority. Trials. (2011) 12:106. doi: 10.1186/1745-6215-12-106
42. Lee, PG, Damschroder, LJ, Holleman, R, Moin, T, and Richardson, CR. Older adults and diabetes prevention programs in the veterans health administration. Diabetes Care. (2018) 41:2644–7. doi: 10.2337/dc18-1141
43. Kramer, MK, Vanderwood, KK, Arena, VC, Miller, RG, Meehan, R, Eaglehouse, YL, et al. Evaluation of a diabetes prevention program lifestyle intervention in older adults: a randomized controlled study in three senior/community centers of varying socioeconomic status. Diabetes Educ. (2018) 44:118–29. doi: 10.1177/0145721718759982
44. Glasgow, RE, Vogt, TM, and Boles, SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. (1999) 89:1322–7. doi: 10.2105/AJPH.89.9.1322
45. Damschroder, LJ, Reardon, CM, Widerquist, MAO, and Lowery, J. The updated consolidated framework for implementation research based on user feedback. Implement Sci. (2022) 17:75. doi: 10.1186/s13012-022-01245-0
46. Castro Sweet, CM, Chiguluri, V, Gumpina, R, Abbott, P, Madero, EN, Payne, M, et al. Outcomes of a digital health program with human coaching for diabetes risk reduction in a Medicare population. J Aging Health. (2017) 30:692–710. doi: 10.1177/0898264316688791
47. Centers for Disease Control and Prevention (2021). Diabetes prevention recognition: program standards and operating procedures. Available at: https://www.cdc.gov/diabetes/prevention/pdf/dprp-standards.pdf. [Accessed May 8, 2023].
48. Maracy, M, and Dunn, G. Estimating dose-response effects in psychological treatment trials: the role of instrumental variables. Stat Methods Med Res. (2011) 20:191–215. doi: 10.1177/0962280208097243
49. Landau, S, Emsley, R, and Dunn, G. Beyond total treatment effects in randomised controlled trials: baseline measurement of intermediate outcomes needed to reduce confounding in mediation investigations. Clin Trials. (2018) 15:247–56. doi: 10.1177/1740774518760300
50. Saldana, L, Chamberlain, P, Bradford, WD, Campbell, M, and Landsverk, J. The cost of implementing new strategies (COINS): a method for mapping implementation resources using the stages of implementation completion. Child Youth Serv Rev. (2014) 39:177–82. doi: 10.1016/j.childyouth.2013.10.006
51. Mishler, E. Research interviewing: Context and narrative. Cambridge, MA: Harvard University Press (1986).
53. Blumer, H. Symbolic interactions: Perspective and method. Englewood Cliffs, NJ: Prentice Hall (1969).
54. Block, G, Azar, KM, Romanelli, RJ, Block, TJ, Hopkins, D, Carpenter, HA, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and Mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. (2015) 17:e240. doi: 10.2196/jmir.4897
55. Fitzpatrick, SL, Mayhew, M, Catlin, CL, Firemark, A, Gruss, I, Nyongesa, DB, et al. Evaluating the implementation of digital and in-person diabetes prevention program in a large, integrated health system: natural experiment study design. Perm J. (2021) 26:21–31. doi: 10.7812/TPP/21.056
56. Block, G, Azar, KM, Block, TJ, Romanelli, RJ, Carpenter, H, Hopkins, D, et al. A fully automated diabetes prevention program, alive-PD: program design and randomized controlled trial protocol. JMIR Res Protoc. (2015) 4:e3. doi: 10.2196/resprot.4046
57. Almeida, FA, Michaud, TL, Wilson, KE, Schwab, RJ, Goessl, C, Porter, GC, et al. Preventing diabetes with digital health and coaching for translation and scalability (PREDICTS): a type 1 hybrid effectiveness-implementation trial protocol. Contemp Clin Trials. (2020) 88:105877. doi: 10.1016/j.cct.2019.105877
58. Katula, J, Dressler, E, Kittle, C, Almeida, F, Wilson, K, Michaud, T, et al., editors. (2020). Effects of a digital diabetes prevention program on HbA1c and body weight in prediabetes. Obesity week.
59. Venditti, EM, Wylie-Rosett, J, Delahanty, LM, Mele, L, Hoskin, MA, Edelstein, SL, et al. Short and long-term lifestyle coaching approaches used to address diverse participant barriers to weight loss and physical activity adherence. Int J Behav Nutr Phys Act. (2014) 11:16. doi: 10.1186/1479-5868-11-16
60. CMS (2020). Participants in the Medicare diabetes prevention program: CMS flexibilities to fight COVID-19. Available at: https://www.cms.gov/files/document/covid-medicare-diabetes-prevention-program.pdf [Accessed November 4, 2020].
61. Mann, DM, Chen, J, Chunara, R, Testa, PA, and Nov, O. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inform Assoc. (2020) 27:1132–5. doi: 10.1093/jamia/ocaa072
62. Chunara, R, Zhao, Y, Chen, J, Lawrence, K, Testa, PA, Nov, O, et al. Telemedicine and healthcare disparities: a cohort study in a large healthcare system in new York City during COVID-19. J Am Med Inform Assoc. (2021) 28:33–41. doi: 10.1093/jamia/ocaa217
63. Kalyani, RR, Golden, SH, and Cefalu, WT. Diabetes and aging: unique considerations and goals of care. Diabetes Care. (2017) 40:440–3. doi: 10.2337/dci17-0005
64. Anderson, M, and Perrin, A. (2017). Technology use among seniors: Pew Research Center Internet & Technology; Available at: http://www.pewinternet.org/2017/05/17/technology-use-among-seniors/. [Accessed May 8, 2023].
Keywords: diabetes prevention, older adults, nutrition, physical activity, lifestyle change, virtual, social support, implementation science
Citation: Beasley JM, Johnston EA, Sevick MA, Jay M, Rogers ES, Zhong H, Zabar S, Goldberg E and Chodosh J (2023) Study protocol: BRInging the Diabetes prevention program to GEriatric Populations. Front. Med. 10:1144156. doi: 10.3389/fmed.2023.1144156
Received: 13 January 2023; Accepted: 24 April 2023;
Published: 18 May 2023.
Edited by:
Roberta Zupo, University of Bari Aldo Moro, ItalyReviewed by:
Sathish Thirunavukkarasu, Emory University, United StatesCopyright © 2023 Beasley, Johnston, Sevick, Jay, Rogers, Zhong, Zabar, Goldberg and Chodosh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeannette M. Beasley, amJlYXNsZXlAbnl1LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.