- 1Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Saud bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia
- 2Biomedical Research Department, King Abdullah International Medical Research Center, Jeddah, Saudi Arabia
Introduction: Inflammation is known to contribute to the development of age-related macular degeneration (AMD). Several inflammatory indices derived from routine complete blood counts have been proposed as biomarkers in multiple disorders.
Methods: In this study, clinical and laboratory data were retrospectively collected from medical records to assess the aggregate index of systemic inflammation (AISI) and the systemic inflammatory response index (SIRI) as potential biomarkers of systemic inflammation in patients with early diagnosis of dry AMD.
Results: The study included 90 patients with dry AMD and 270 age/sex-matched patients with cataracts as a control group. There were no significant differences in the AISI and SIRI results between the cases and controls (p = 0.16 and 0.19, respectively).
Conclusion: This suggests that AISI and SIRI may be inadequate metrics for AMD or lack sensitivity in detecting inflammatory changes. Exploring other routine blood markers may help to identify and prevent the early stages of AMD.
Introduction
Age-related macular degeneration (AMD) is a chronic, progressive degenerative disease of the retina that is characterized by the loss of central vision. It is a leading cause of visual impairment and blindness in older adults (1). AMD is divided into early-stage, characterized by the presence of drusen and changes in the retinal pigment epithelium, and late-stage, which can be either neovascular (wet or exudative) or non-neovascular (atrophic, or non-exudative) (2).
Systemic inflammation may contribute to AMD development and progression, as some inflammatory diseases share risks and an inflammatory profile with AMD (3). The exact mechanisms by which inflammation contributes to the development of AMD are not fully understood, but it is thought to involve the production of reactive oxygen species and other oxidative stress-related factors that can damage the retina and contribute to the development of AMD. In addition, inflammation may stimulate the production of pro-angiogenic factors, which can promote the growth of new blood vessels in the retina and increase the risk of wet AMD (4, 5).
The examination of blood components, including inflammatory indices and genetic variations, has the potential to facilitate the early detection of AMD. For instance, elevated levels of inflammation-related biomarkers, such as C-reactive protein, lipids, and interleukin-6, have been found in patients with AMD compared to those without the disease (6–9). Certain genetic variations have also been linked to increasing the risk of developing AMD (10, 11). An elevated white blood cell count was linked to an increased risk of developing AMD (12).
Systemic inflammatory indices derived from complete blood count (CBC) tests, including the neutrophil/lymphocyte ratio (NLR), derived-NLR, platelet/lymphocyte ratio (PLR), monocyte/lymphocyte ratio (MLR), have received attention in recent years due to their low cost, accessibility, and predictive power for outcomes in several disorders including AMD (13–17).
The systemic inflammatory response index (SIRI) is calculated by multiplying the neutrophil and monocyte counts and then dividing the product by the lymphocyte count. Variations in SIRI values have been shown to be associated with clinical outcomes in a variety of cancers, including pancreatic (18), gallbladder (19), gastric (20), breast (21), and cervical cancers (22), as well as in COVID-19 patients (23). Similarly, the aggregate index of systemic inflammation (AISI) is calculated by multiplying the counts of neutrophils, monocytes, and platelets and then dividing the product by the lymphocyte count. While AISI has been studied relatively sparingly, recent research has investigated its relationship to neoplastic conditions such as non-small-cell lung cancer (24) and COVID-19 (23). The cells involved in both AISI and SIRI calculations are critical in maintaining a well-balanced immune system, which helps protect the body from harmful pathogens and diseases. However, it’s important to note that these cells can also produce pro-inflammatory substances that have been associated with various inflammatory diseases (25, 26).
This study aimed to assess the value of two CBC-derived indices, AISI and SIRI, in dry AMD since their diagnostic role remains to be elucidated. A literature search has revealed one study that assessed the levels of AISI and SIRI in individuals with AMD and was conducted on a cohort of men with Sardinian ancestry (27).
Methods
This study represents a secondary analysis of previously reported patients’ data (28). Briefly, Electronic health records at a tertiary care hospital (King Abdulaziz Medical City, Jeddah, Saudi Arabia) were enquired for new AMD patients (aged ≥50 years) with macular drusen in at least one eye, with or without signs of geographic atrophy, and other fundus characteristics. Exclusion criteria included wet AMD in the fellow eye, inflammatory ocular disorders, other ophthalmic conditions, malignancies, hematological and autoimmune disorders, chronic inflammatory disorders, leukocytosis (>11 × 103 cells/mm3), leukopenia (<4 × 103 cells/mm3), thrombocytosis (>450 × 103 cells/mm3), and thrombocytopenia (<150 × 103 cells/mm3). CBC results [Cell-Dyn Sapphire (Abbott Diagnostics Division, Santa Clara, CA)] were collected for cases and controls, and the AISI ((neutrophils × monocytes × platelets)/lymphocytes) and SIRI ((neutrophil × monocytes)/lymphocytes) ratios were calculated. Informed consent was obtained from all participants, and the study was conducted following the Declaration of Helsinki. Institutional review board approval was granted by the King Abdullah International Medical Research Center (#RJ20/106/J-SP21J/083/03). The control group for the study was composed of cataract individuals without a previous diagnosis of AMD. The sample size of the control group was determined to be three times the size of the AMD group, to increase the accuracy and statistical power of the study and reduce the potential for selection bias. Control subjects were randomly selected from the electronic health records, and their ages were closely matched to the mean age of the AMD group (within a range of 5 years). The chi-square test and t-test were used to analyze differences in demographics and blood result characteristics between the case and control groups. Multivariate analysis was conducted, taking into account age and sex, to further examine differences between the groups. The assumptions of the linear relationship and homogeneity of the regression slopes were checked and all models met these assumptions. All statistical analyses were performed using SAS software (version 9.4, SAS Institute Inc.), with p-values and confidence intervals at 95%.
Results
The study included 90 participants in the case group (22.55% of the total sample) and 270 participants in the control group (77.44%). The demographics and hematological parameters of the case and control groups are presented in Table 1. In the univariate analysis, the mean AISI for the case group was 471.18 ± 163.8 and 477.32 ± 239.5 for the control group. The mean SIRI for the case group was 1.99 ± 0.94 and 1.84 ± 0.93 for the control group. There were no statistically significant differences between the two groups (Table 1).

Table 1. Demographic and hematological parameters of dry age-related macular degeneration (AMD) cases and controls.
In the regression analysis (adjusted for age and sex, Table 2), the AISI was not significantly associated with the development of AMD compared to controls, with a beta coefficient of 0.16 (standard error 0.11, p = 0.16). Similarly, the SIRI was not significantly associated with the development of AMD compared to controls, with a beta coefficient of 0.14 (standard error 0.11, p = 0.19). These results suggest that AISI and SIRI are not significant predictors of AMD. In addition, these models showed that age and sex had no significant effects on the AISI and SIRI values in the case group compared to the control group.
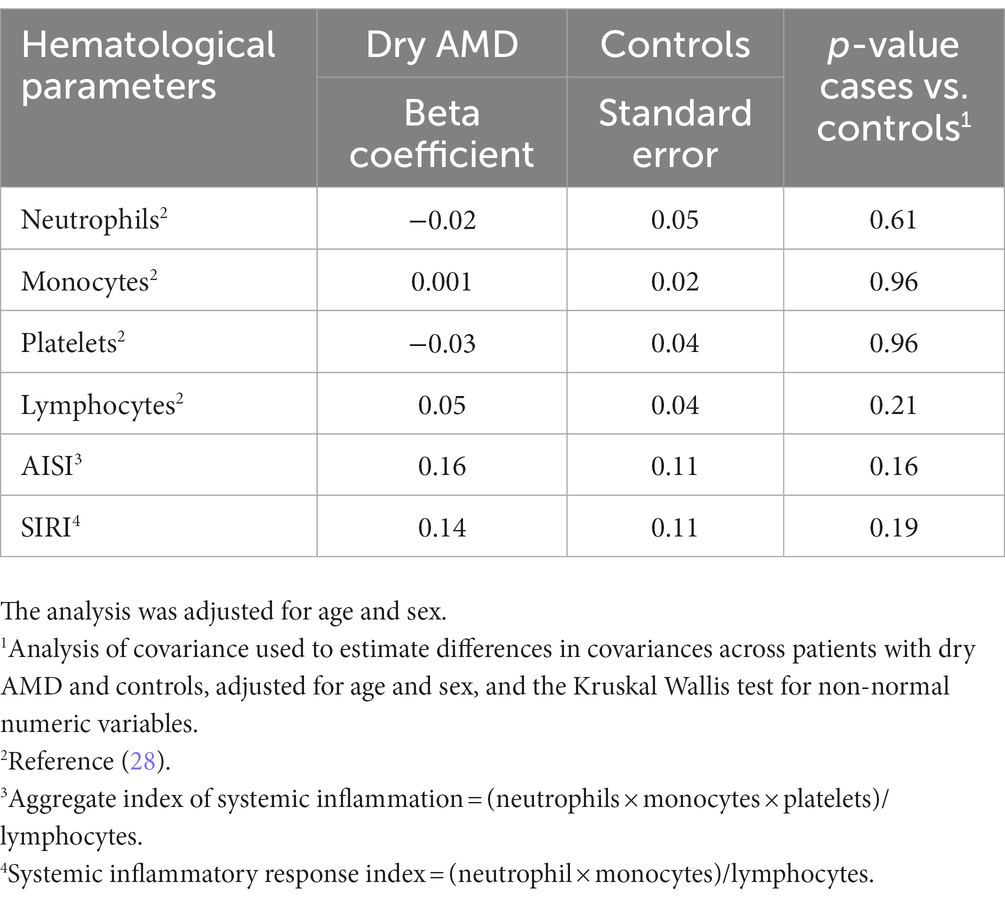
Table 2. Regression analysis to predict factors associated with age-related macular degeneration (AMD) compared to controls.
Discussion
Although AISI is thought to be a more precise indicator of inflammation than other indices that only consider fewer cell types, it has not been widely used or studied in the literature. The AISI index was evaluated in several conditions such as a prognostic marker for small cell lung carcinoma (29), identification of patients at risk of prolonged hospital stay in open elective thoracic surgery (30), and as a predictor for severity and intensive care unit admission in COVID-19 patients (31). Another study has highlighted the prognostic significance of AISI in predicting poor outcomes in patients with idiopathic pulmonary fibrosis (32). AISI is a measure of inflammation that takes into account several different types of cells involved in the immune response, including neutrophils, lymphocytes, and platelets, as well as the monocyte count. These cells play a role in producing proinflammatory substances such as cytokines, chemokines, enzymes, and reactive oxidative species, which can contribute to inflammation and the development of certain diseases (25). SIRI is another immune system biomarker that has been studied in various disorders. The SIRI index was first introduced by Qi et al. in a study of pancreatic cancer patients, where it was shown to be a useful predictor of prognosis (33). The SIRI is a measure that considers three types of white blood cells, neutrophils × monocytes/lymphocytes, to provide information about the overall balance of immune and inflammation activity in the body (33). Additional studies have shown that the SIRI may be able to predict survival in several types of cancer, including pancreatic cancer (18), gallbladder cancer (19), oral squamous cell carcinoma (34), and cervical cancer (22).
The purpose of this study was to assess the ability of the AISI and SIRI to detect AMD in the early stage of disease development. However, there were no significant differences in AISI and SIRI results between the case and control groups. This suggests that the metrics of AISI and SIRI may be inadequate in accurately assessing the inflammatory changes that are associated with AMD, or they may exhibit insufficient sensitivity in detecting such changes. The findings of this study are in line with those of a recent publication on AMD patients of Sardinian ancestry (27), which appears to be the only study on AISI and SIRI in AMD.
Previous studies have yielded conflicting results on the association between white blood cells and AMD, with some studies finding correlations between higher counts and increased risk of AMD (12, 35) while others did not (36, 37). More recent studies have looked at various blood components derived from routine CBC measurements to calculate inflammatory relevant indices in AMD patients. For instance, studies have reported higher levels of neutrophils and lower levels of lymphocytes in AMD patients compared to controls (27, 28, 38). Furthermore, it has been demonstrated that the NLR and MLR are significantly elevated in patients with wet AMD compared to individuals with dry AMD and healthy controls (39), with ratios indicating low-grade inflammation. These observations strongly suggest that alterations in CBC parameters could serve as potential markers of AMD-related inflammation. Neutrophils are the most abundant type of white blood cell in the body and play a role in acute and chronic inflammation, phagocytosis, and the release of anti-inflammatory mediators (40, 41). Lymphocytes are involved in both the initiation and resolution of inflammation and may be activated or suppressed in response to various signals. Lymphocyte infiltration plays a role in the initiation and progression of inflammatory responses, a key contributor to the tissue damage and functional impairment that occur in inflammatory disorders (41, 42). Monocytes are a type of white blood cell that is also involved in the immune response and inflammation (41, 43). A high PLR is considered a negative prognostic factor for inflammatory diseases, as an elevated platelet count can result in lymphopenia (44, 45). It is possible that neutrophils and lymphocytes play a more crucial role in the development of AMD than other cell types, and their influence could be “diluted” by taking into account multiple cell types in AISI and SIRI calculations. Additionally, it is also plausible that local inflammation in the retina may not be accurately reflected by systemic inflammatory indices, leading to an apparent disconnect between the presence of certain cell types and systemic inflammatory markers.
This study has some limitations that should be taken into account when interpreting the results. One of these limitations is the relatively small sample size, which may not be representative of the entire population and could lead to insufficient statistical power to detect certain trends or associations. Additionally, the study is retrospective in design, meaning that it looks back at past data and events, which may be subject to bias or error. Finally, the study was conducted at a single center, so the results may not be generalizable to other settings or populations. In addition, the control group in this study consists of cataract patients, which may have biased the results of the CBC analysis. A literature search revealed no prior studies regarding the association of cataractogenesis with AISI and SIRI. Also, it should be noted that inflammatory pathologies may play a role in the onset of cataracts (46, 47) and could also be associated with post-cataract surgery complications (48). This could have affected the accuracy of the AISI and the SIRI as potential biomarkers for the early detection of AMD. Therefore, it is important to consider these limitations when evaluating the findings of this study, and it would be beneficial for future research to confirm and build upon these results. Further research is needed to elucidate the underlying mechanisms that link immune-related cell types with AMD and to explore the potential limitations of using systemic inflammatory response indices to assess ocular inflammatory conditions.
Conclusion
This study found that the AISI and the SIRI are not effective biomarkers for the early detection of AMD. These results suggest that further research is needed to identify other potential biomarkers that can be used to identify and prevent the early stages of AMD.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of King Abdullah International Medical Research Center (protocol code# RJ20/106/J-SP21J/083/03).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by King Abdullah International Medical Research Center Ethics Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mitchell, P, Liew, G, Gopinath, B, and Wong, TY. Age-related macular degeneration. Lancet. (2018) 392:1147–59. doi: 10.1016/S0140-6736(18)31550-2
2. Wong, WL, Su, X, Li, X, Cheung, CMG, Klein, R, Cheng, CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. (2014) 2:e106–16. doi: 10.1016/S2214-109X(13)70145-1
3. Schnabolk, G. Systemic inflammatory disease and AMD comorbidity. Adv Exp Med Biol. (2019) 1185:27–31. doi: 10.1007/978-3-030-27378-1_5
4. Telander, DG. Inflammation and age-related macular degeneration (AMD). Semin Ophthalmol. (2011) 26:192–7. doi: 10.3109/08820538.2011.570849
5. Donoso, LA, Kim, D, Frost, A, Callahan, A, and Hageman, G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. (2006) 51:137–52. doi: 10.1016/j.survophthal.2005.12.001
6. Seddon, JM, Gensler, G, Milton, RC, Klein, ML, and Rifai, N. Association between C-reactive protein and age-related macular degeneration. J Am Med Assoc. (2004) 291:704. doi: 10.1001/jama.291.6.704
7. Nahavandipour, A, Krogh Nielsen, M, Sørensen, TL, and Subhi, Y. Systemic levels of interleukin-6 in patients with age-related macular degeneration: a systematic review and meta-analysis. Acta Ophthalmol. (2020) 98:434:444. doi: 10.1111/aos.14402
8. Semba, RD, Moaddel, R, Cotch, MF, Jonasson, F, Eiriksdottir, G, Harris, TB, et al. Serum lipids in adults with late age-related macular degeneration: a case-control study. Lipids Health Dis. (2019) 18:7. doi: 10.1186/s12944-018-0954-7
9. Wu, S, Hsu, LA, Teng, MS, Lin, JF, Chou, HH, Lee, MC, et al. Interactive effects of C-reactive protein levels on the association between APOE variants and triglyceride levels in a Taiwanese population. Lipids Health Dis. (2016) 15:94. doi: 10.1186/s12944-016-0262-z
10. Fritsche, LG, Igl, W, Bailey, JNC, Grassmann, F, Sengupta, S, Bragg-Gresham, JL, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. (2016) 48:134–43. doi: 10.1038/ng.3448
11. Warwick, A, and Lotery, A. Genetics and genetic testing for age-related macular degeneration review-article. Eye. (2018) 32:849–57. doi: 10.1038/eye.2017.245
12. Shankar, A, Mitchell, P, Rochtchina, E, Tan, J, and Wang, JJ. Association between circulating white blood cell count and long-term incidence of age-related macular degeneration: the Blue Mountains eye study. Am J Epidemiol. (2007) 166:393–402. doi: 10.1093/aje/kwm096
13. Sengul, EA, Artunay, O, Kockar, A, Afacan, C, Rasier, R, Gun, P, et al. Correlation of neutrophil/lymphocyte and platelet/lymphocyte ratio with visual acuity and macular thickness in age-related macular degeneration. Int J Ophthalmol. (2017) 10:754–9. doi: 10.18240/ijo.2017.05.16
14. Suppiah, A, Malde, D, Arab, T, Hamed, M, Allgar, V, Smith, AM, et al. The prognostic value of the neutrophil-lymphocyte ratio (NLR) in acute pancreatitis: identification of an optimal NLR. J Gastrointest Surg. (2013) 17:675–81. doi: 10.1007/s11605-012-2121-1
15. Torun, S, Tunc, BD, Suvak, B, Yildiz, H, Tas, A, Sayilir, A, et al. Assessment of neutrophil-lymphocyte ratio in ulcerative colitis: a promising marker in predicting disease severity. Clin Res Hepatol Gastroenterol. (2012) 36:491–7. doi: 10.1016/j.clinre.2012.06.004
16. Mertoglu, C, and Gunay, M. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as useful predictive markers of prediabetes and diabetes mellitus. Diabetes Metab Syndr. (2017) 11:S127–31. doi: 10.1016/j.dsx.2016.12.021
17. Ilhan, N, Daglioglu, MC, Ilhan, O, Coskun, M, Tuzcu, EA, Kahraman, H, et al. Assessment of neutrophil/lymphocyte ratio in patients with age-related macular degeneration. Ocul Immunol Inflamm. (2015) 23:287–90. doi: 10.3109/09273948.2014.921715
18. Topkan, E, Mertsoylu, H, Kucuk, A, Besen, AA, Sezer, A, Sezen, D, et al. Low systemic inflammation response index predicts good prognosis in locally advanced pancreatic carcinoma patients treated with concurrent chemoradiotherapy. Gastroenterol Res Pract. (2020) 2020:5701949. doi: 10.1155/2020/5701949
19. Sun, L, Hu, W, Liu, M, Chen, Y, Jin, B, Xu, H, et al. High systemic inflammation response index (SIRI) indicates poor outcome in gallbladder cancer patients with surgical resection: a single institution experience in China. Cancer Res Treat. (2020) 52:1199–210. doi: 10.4143/crt.2020.303
20. Li, S, Lan, X, Gao, H, Li, Z, Chen, L, Wang, W, et al. Systemic inflammation response index (SIRI), cancer stem cells and survival of localised gastric adenocarcinoma after curative resection. J Cancer Res Clin Oncol. (2017) 143:2455–68. doi: 10.1007/s00432-017-2506-3
21. Chen, L, Kong, X, Wang, Z, Wang, X, Fang, Y, and Wang, J. Pretreatment systemic inflammation response index in patients with breast cancer treated with neoadjuvant chemotherapy as a useful prognostic indicator. Cancer Manag Res. (2020) 12:1543–67. doi: 10.2147/CMAR.S235519
22. Chao, B, Ju, X, Zhang, L, Xu, X, and Zhao, Y. A novel prognostic marker systemic inflammation response index (SIRI) for operable cervical cancer patients. Front Oncol. (2020) 10:766. doi: 10.3389/fonc.2020.00766
23. Paliogiannis, P, Zinellu, A, Scano, V, Mulas, G, de Riu, G, Pascale, RM, et al. Laboratory test alterations in patients with COVID-19 and non COVID-19 interstitial pneumonia: a preliminary report. J Infect Dev Ctries. (2020) 14:685–90. doi: 10.3855/jidc.12879
24. Paliogiannis, P, Putzu, C, Cortinovis, D, Colonese, F, Canova, S, Fois, A, et al. Blood cell count indexes of systemic inflammation as predictive biomarkers of immunotherapy outcomes in advanced non-small-cell lung cancer. (2018).
25. Mittal, M, Siddiqui, MR, Tran, K, Reddy, SP, and Malik, AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. (2014) 20:1126–67. doi: 10.1089/ars.2012.5149
26. Nathan, C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. (2006) 6:173–82. doi: 10.1038/nri1785
27. Pinna, A, Porcu, T, D’Amico-Ricci, G, Dore, S, Boscia, F, Paliogiannis, P, et al. Complete blood cell count-derived inflammation biomarkers in men with age-related macular degeneration. Ocul Immunol Inflamm. (2019) 27:932–6. doi: 10.1080/09273948.2018.1485960
28. Naif, S, Majed, R, Mohieldin, E, Hanan, A, Lamis, A, and Maha, A. Neutrophil-lymphocyte ratios in dry age-related macular degeneration. Ocul Immunol Inflamm :1–6. doi: 10.1080/09273948.2022.2092752
29. Putzu, C, Cortinovis, DL, Colonese, F, Canova, S, Carru, C, Zinellu, A, et al. Blood cell count indexes as predictors of outcomes in advanced non-small-cell lung cancer patients treated with Nivolumab. Cancer Immunol Immunother. (2018) 67:1349–53. doi: 10.1007/s00262-018-2182-4
30. Paliogiannis, P, Ginesu, GC, Tanda, C, Feo, CF, Fancellu, A, Fois, AG, et al. Inflammatory cell indexes as preoperative predictors of hospital stay in open elective thoracic surgery. ANZ J Surg. (2018) 88:616–20. doi: 10.1111/ans.14557
31. Hamad, DA, Aly, MM, Abdelhameid, MA, Ahmed, SA, Shaltout, AS, Abdel-Moniem, AE, et al. Combined blood indexes of systemic inflammation as a Mirror to admission to intensive care unit in COVID-19 patients: a multicentric study. J Epidemiol Glob Health. (2022) 12:64–73. doi: 10.1007/s44197-021-00021-5
32. Zinellu, A, Paliogiannis, P, Sotgiu, E, Mellino, S, Mangoni, AA, Zinellu, E, et al. Blood cell count derived inflammation indexes in patients with idiopathic pulmonary fibrosis. Lung. (2020) 198:821–7. doi: 10.1007/s00408-020-00386-7
33. Qi, Q, Zhuang, L, Shen, Y, Geng, Y, Yu, S, Chen, H, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. (2016) 122:2158–67. doi: 10.1002/cncr.30057
34. Lin, J, Chen, L, Chen, Q, Zhuang, Z, Bao, X, Qian, J, et al. Prognostic value of preoperative systemic inflammation response index in patients with oral squamous cell carcinoma: propensity score-based analysis. Head Neck. (2020) 42:3263–74. doi: 10.1002/hed.26375
35. Yasuda, M, Kiyohara, Y, Hata, Y, Arakawa, S, Yonemoto, K, Doi, Y, et al. Nine-year incidence and risk factors for age-related macular degeneration in a defined Japanese population. The Hisayama study. Ophthalmology. (2009) 116:2135–40. doi: 10.1016/j.ophtha.2009.04.017
36. Wu, KHC, Tan, AG, Rochtchina, E, Favaloro, EJ, Williams, A, and Mitchell, P. Circulating inflammatory markers and hemostatic factors in age-related maculopathy: a population-based case-control study. Invest Ophthalmol Vis Sci. (2007) 48:1983–8. doi: 10.1167/iovs.06-0223
37. Gopinath, B, Flood, VM, Rochtchina, E, Wang, JJ, and Mitchell, P. Homocysteine, folate, vitamin B-12, and 10-y incidence of age-related macular degeneration. Am J Clin Nutr. (2013) 98:129–35. doi: 10.3945/ajcn.112.057091
38. Niazi, S, Krogh Nielsen, M, Sørensen, TL, and Subhi, Y. Neutrophil-to-lymphocyte ratio in age-related macular degeneration: a systematic review and meta-analysis. Acta Ophthalmol. (2019) 97:558–66. doi: 10.1111/aos.14072,566
39. Karahan, M, Hazar, L, Erdem, S, Ava, S, Dursun, ME, Demirtaş, AA, et al. Is there a relationship between hematological inflammatory parameters and age-related macular degeneration? Ther Adv Ophthalmol. (2021) 13:251584142110105. doi: 10.1177/25158414211010550
40. Herrero-Cervera, A, Soehnlein, O, and Kenne, E. Neutrophils in chronic inflammatory diseases. Cell Mol Immunol. (2022) 19:177–91. doi: 10.1038/s41423-021-00832-3
41. Kauppinen, A, Paterno, JJ, Blasiak, J, Salminen, A, and Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. (1765) 2016:1786. doi: 10.1007/s00018-016-2147-8
42. Sakai, Y, and Kobayashi, M. Lymphocyte “homing” and chronic inflammation. Pathol Int. (2015) 65:344–54. doi: 10.1111/pin.12294
43. Shi, C, and Pamer, EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. (2011) 11:762–74. doi: 10.1038/nri3070
44. Akboga, MK, Canpolat, U, Yayla, C, Ozcan, F, Ozeke, O, Topaloglu, S, et al. Association of Platelet to lymphocyte ratio with inflammation and severity of coronary atherosclerosis in patients with stable coronary artery disease. Angiology. (2016) 67:89–95. doi: 10.1177/0003319715583186
45. Lian, L, Xia, YY, Zhou, C, Shen, XM, Li, XL, Han, SG, et al. Application of platelet/lymphocyte and neutrophil/lymphocyte ratios in early diagnosis and prognostic prediction in patients with resectable gastric cancer. Cancer Biomark. (2015) 15:899–907. doi: 10.3233/CBM-150534
46. Worgul, BV, and Merriam, GR. The role of inflammation in radiation cataractogenesis. Exp Eye Res. (1981) 33:167–73. doi: 10.1016/S0014-4835(81)80065-6
47. Ainsbury, EA, Bouffler, SD, Dörr, W, Graw, J, Muirhead, CR, Edwards, AA, et al. Radiation cataractogenesis: a review of recent studies. Radiat Res. (2009) 172:1–9. doi: 10.1667/RR1688.1
Keywords: AMD, retina, macula, inflammation, biomarker, AISI, SIRI
Citation: Sannan NS (2023) Assessment of aggregate index of systemic inflammation and systemic inflammatory response index in dry age-related macular degeneration: a retrospective study. Front. Med. 10:1143045. doi: 10.3389/fmed.2023.1143045
Edited by:
Jiyang Cai, University of Oklahoma Health Sciences Center, United StatesReviewed by:
Yingbin Fu, Baylor College of Medicine, United StatesOyuna S. Kozhevnikova, Russian Academy of Sciences (RAS), Russia
Elizabeth Pearsall, University of Missouri Extension, United States
Copyright © 2023 Sannan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naif S. Sannan, c2FubmFubkBrc2F1LWhzLmVkdS5zYQ==
 Naif S. Sannan
Naif S. Sannan