
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med. , 07 March 2023
Sec. Nephrology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1143028
This article is part of the Research Topic Novel Insights and Prospects of Targeted Therapy in Kidney Diseases View all 8 articles
 Peter R. Corridon1,2,3*
Peter R. Corridon1,2,3*The rising global incidence of acute and chronic kidney diseases has increased the demand for renal replacement therapy. This issue, compounded with the limited availability of viable kidneys for transplantation, has propelled the search for alternative strategies to address the growing health and economic burdens associated with these conditions. In the search for such alternatives, significant efforts have been devised to augment the current and primarily supportive management of renal injury with novel regenerative strategies. For example, gene- and cell-based approaches that utilize recombinant peptides/proteins, gene, cell, organoid, and RNAi technologies have shown promising outcomes primarily in experimental models. Supporting research has also been conducted to improve our understanding of the critical aspects that facilitate the development of efficient gene- and cell-based techniques that the complex structure of the kidney has traditionally limited. This manuscript is intended to communicate efforts that have driven the development of such therapies by identifying the vectors and delivery routes needed to drive exogenous transgene incorporation that may support the treatment of acute and chronic kidney diseases.
Renal dysfunction can be acute, chronic, or end-stage, manifesting in several forms. The most prevalent cases arise from congenital disorders (1, 2); nephrotoxicity (3); ischemia–reperfusion injury (4, 5); systolic hypotension and hemorrhage (6); hypertension (7); trauma (8); essential mineral deficiencies (9); malignancies (10); diabetes (11, 12); and viral infections, as observed with the COVID-19 pandemic (13, 14). Paradoxically, hospitalization and the complex relationship between various forms of kidney injuries are additional factors that can contribute to renal dysfunction. For decades, clinicians have been aware of the risk of patients, with and without underlying kidney injury, developing hospital-acquired kidney malfunction (15). They have also been aware of the complex connection between acute kidney injury (AKI) and chronic kidney disease (CKD), whereby they are closely linked and likely promote one another. For instance, CKD is a reputed risk factor for developing AKI during hospitalization, while there is a growing body of evidence illustrating how AKI accelerates the progression of CKD in critically ill patients (16), particularly hospitalized COVID-19 patients (17).
From a global perspective, it is estimated that AKI affects approximately 13 million people annually, contributing to nearly 1.7 million annual deaths (18). Traditionally, AKI is a critical stage in injury progression because of its reversibility (19). In comparison, CKD affects over one-tenth of the general population worldwide (20), and eventually, these conditions contribute to 5–8 million patients with end-stage renal disease (ESRD) requiring renal replacement therapy (21). AKI is a critical stage in injury progression because of its reversibility (21). Beyond this stage, treatment options are limited to renal replacement therapy, as the dysfunction has progressed to either CKD or, unfortunately, ESRD. It was previously thought that AKI, a sudden reduction in renal function, was fully reversible in all patients (22). Nevertheless, recent research has gone against this notion based on studies conducted on individuals with reduced filtration capacities who are more prone to ESRD progression and mortality than a reversal of the condition (23, 24).
These facts highlight significant clinical problems that arise from acute and chronic disorders. Furthermore, from a financial perspective, these patients often require long-term hospitalization, which imposes substantial burdens on the healthcare systems related to the etiologies of these disorders and their complex and debilitating interconnected nature. Likewise, these conditions lead to enhanced levels of morbidity and reductions in quality of life. Overall, morbidity and mortality are expected to rise exponentially with the growing rates of diabetes and cardiovascular diseases. Given that current treatments are mainly preventive strategies and early detection and intervention can be difficult in asymptomatic patients with these conditions, there is a definite need for alternative strategies to address the growing prevalence and subtle progression of renal dysfunction and ultimately reduce the need for renal replacement therapy (5, 25–28).
In the search for such strategies, significant efforts are being devised to augment the present-day management of kidney disease using novel regenerative strategies. For example, gene- and cell-based approaches that utilize recombinant peptides/proteins, gene, cell, organoid, and RNAi technologies have shown promising outcomes primarily in experimental models (25). Accompanying efforts have also been devised to facilitate the development of efficient gene- and cell-based techniques. This article is intended to convey efforts that have advanced these alternative forms of therapy by highlighting vectorization and mechanisms that can elicit genetic modifications that may support the treatment of acute and chronic kidney diseases.
Various methods have been proposed to deliver exogenous genes to mammalian cells. For the kidney, attempts have been made to protect and repair renal function by targeting single genetic loci with purified protein products, plasmids, recombinant growth factors, and viruses encoding peptides and proteins. Intravenous doses of human growth factor (HGF), which has anti-fibrotic properties, have promoted kidney repair in rodents with CKD (29, 30). Injections of IL-18BP, a recombinant interleukin, improved renal function, restored tubular morphology, and decreased tubular necrosis and apoptosis in small animal models (31). Cell-based approaches conducted with intrarenal injections of human placenta-derived stem cells have also ameliorated damage in ischemia–reperfusion settings of AKI (32).
Single intravenous doses of plasmids encoding human growth factor (HGF) have also been shown to improve tissue regeneration and protect tubular epithelial cells from injury and apoptosis during acute renal failure (33). In such earlier studies, HGF also helped preserve renal structure in chronic injury models by activating matrix degradation and reducing fibrosis (34–36). Researchers have tested growth hormone-releasing hormone (GHRH) plasmid-based therapy in feline and canine chronic injury models. GHRH-treated animals displayed better levels of erythropoiesis, urea and creatinine clearances compared to controls (37), as well as more recent findings related to its therapeutic effect in CKD patients (38).
It has been well-established that adenovirus and adeno-associated virus vectors are two of the most efficient systems for transducing non-dividing cells (39) and have been used to target a variety of genetic loci. Other experimental studies have used adeno-based vectors for gene transfer. Lately, such vectors have displayed the long noncoding RNA-H19-derived attenuation of acute ischemic kidney injury (40) and the mediation of AKI to CKD progression (41). These vectors have also helped preserve renal microvascular morphology and suppress the progression of AKI via the upregulation of vascular endothelial growth factor (VEGF) and angiopoietin (42). Interestingly, the inhibition of VEGF also promoted structural and functional improvements in diabetes-induced chronic kidney disease (43, 44). These findings support the long-derived notion that repairing ischemic and toxic renal injuries depended critically on regulating a redundant, interactive network of cytokines and growth factors (45). Thus, it would be of value to devise a system that could reliably modulate gene expression levels to return kidney function to near-normal baseline levels without inducing viral-derived toxicity. However, despite its benefits regarding kidney function recovery, recombinant agents have short half-lives and require large doses (46). Further studies are needed to demonstrate consistent safety and effectiveness levels before these experimental techniques become clinical practice (47).
Cell therapy is another option to improve tissue/organ regeneration. Research efforts initially focused on cell transfer for bone marrow and organ transplantation, blood transfusion, and in vitro fertilization (48). Nowadays, this technique is being developed to facilitate the repair/replacement of damaged and lost compartments in solid organs. This regenerative strategy transplants cells, which deliver genes of interest, to targeted organs. To achieve this purpose, investigators use the following cells: stem or progenitor cells; mature, functional cells from humans or animals; and genetically modified and transdifferentiated cells (48–51). More recently, organoids, transdifferentiated three-dimensional cell clusters, arose as another promising option to enhance or restore kidney function (52–54).
Papazova et al. published a meta-analysis of CKD and cell therapies (55). This analysis demonstrated that more than half of all cell-based studies focused on the therapeutic effects of single intravenous doses of mesenchymal stem cells. About a third of the studies investigated the preventive benefits of such therapies, while half of the studies focused on their therapeutic benefits. For instance, in AKI animal models, mesenchymal stem cells improved renal function (56–58). Even though the specific mechanisms of action are still under investigation, these cells helped reduce renal fibrosis, improve remodeling, and promote neoangiogenesis (59). Kelly et al. also helped restore renal function using undifferentiated reprogrammed cells to generate sera amyloid A proteins in ischemia–reperfusion, plus gentamicin- and cisplatin-based nephrotoxicity acute injury rat models (60).
Additional efforts have also reported the successful differentiation of embryonic and induced pluripotent stem cells into tubular, glomerular, and whole nephron organoids (61–68). A greater understanding of the roles of key signaling pathways has also allowed investigators to differentiate stem cell niches into various lineages. We believe that shortly, organoids derived from patients’ cells will be able to repopulate decellularized renal scaffolds and printed tissues or even be injected back into the patients to restore their native dysfunction (69–71). Nevertheless, many technical (72–78) and ethical (79–87) issues still need to be solved in this field. It is well-established that embryonic stem cell technology offers hope for new therapies, yet societal and moral incongruences limit their use. Teratoma, a hallmark of pluripotency (89–91), is a significant concern after implantation. The ability to culture and manipulate human stem cells indefinitely while simultaneously governing their differentiation characteristics offers excellent possibilities for the future of medicine (92–94).
Another option within the growing arsenal of gene and cell therapy applications is RNA interference (RNAi). The discovery of mammalian RNAi is one of the most promising therapeutic strategies because it enables the silencing of any gene (95). RNAi is an advantageous technique, as it is easier to silence deficient and non-functional genes than replace them (96). Moreover, RNAi is the most practical approach thus far, as it is relatively low cost, highly specific, and can inhibit multiple genes of various pathways simultaneously (97). This technology can help identify complex genetic loci essential to human pathology.
RNAi is an endogenous process that allows cells to regulate their genetic activity. This process remains central to gene expression and the defense against mutagenesis generated from viral genes and transposons (98). The primary methods that induce exogenous RNAi-based gene silencing utilize micro-RNA (miRNA), small interfering RNA (siRNA), and small hairpin RNA (shRNA) systems. Since Napoli and Jorgensen first reported on this phenomenon in 1990 (99), there has been a growing interest in using RNAi technology to improve renal health (95). This interest has directed RNAi-based research focused on improving the study and management of kidney disease by identifying miRNA targets and AKI biomarkers. It has also prompted interest in improving the delivery of exogenous silencing mediators and siRNA and shRNA targets to either reduce or protect against renal injury. Currently, lipid nanoparticles are the most frequently used formulation to mediate silencing (100), and further work has been proposed to determine in vivo silencing efficiencies and investigate other small RNAs that can affect post-transcriptional gene silencing (101, 102).
From a diagnostic standpoint, several studies have provided fundamental insight into renal injury biomarkers. Valadi et al. showed that miRNAs recovered from urinary exosomes provide information about the kidney in standard and injury settings (103). Zhou et al. showed that miR-27b and miR-192 in these urinary vesicles could differentiate between glomerular and tubular damage (104). Also, from a therapeutic standpoint, exosomes containing miRNAs can enter recipient cells by membrane surface proteins. This phenomenon offers a new mechanism for cell–cell communication and gene delivery (105–111). In a study by Cantaluppi et al., microvesicles enriched with pro-angiogenic miR-126 and miR-296 were injected into the vein, enhanced tubular cell proliferation, and reduced apoptosis and leukocyte infiltration (112). In AKI settings, such silencing has demonstrated that the caspase-3 siRNA improved ischemic reperfusion (IR) injury with reduced caspase-3 expression and apoptosis, better renal oxygenation and acid–base homeostasis, and the silencing IKKβ using siRNA diminished inflammation and protected the kidneys against IR injury (113). Whereas, in a glomerulonephritic chronic injury model, MAPK1 suppression remarkably improved kidney function, reduced proteinuria, and ameliorated glomerular sclerosis (113).
RNAi therapy could be a valuable surrogate for treating patients with AKI by reducing the uptake of nephrotoxins, ameliorating immunologic response mechanisms, and downregulating harmful disease mediators (114–116). Such characteristics have prompted interest in the knockdown of dynamin-2 (Dyn2) and low-density lipoprotein-related protein 2 (LRP2). Dyn2 is a critical component of the endocytic pathway (117–119), and its knockdown blocks clathrin-coat-dependent endocytosis and coat-independent fluid phase probe uptake in several epithelial cell lines (120). In animal models, silencing LRP2 reduced gentamicin toxicity in proximal tubule epithelial cells (121–123). In a rat model of kidney transplantation, caudal vein administration of siRNAs, which targeted connective tissue growth factor (CTGF), decreased renal fibrosis (124). CTGF is an essential pro-fibrotic cofactor that is downstream from TGF-β. Electroporation also enhanced the delivery of siRNA targeted to TGF-β1, significantly reducing glomerular matrix deposition and proteinuria four and 6 weeks after anti-Thy-1 administration (124, 125).
In other studies, which have investigated the renotherapeutic potential of siRNA technology (126), siRNA sequences were systemically delivered to inhibit the expression of p53. This strategy significantly reduced ischemia-induced p53 upregulation and helped attenuate ischemic and cisplatin-induced AKI (127, 128). The oligonucleotides used to facilitate RNAi contained stabilizing modifications with a relatively low affinity for albumin and other plasma proteins. Such modifications diminished their hepatic distribution and degradation in serum and facilitated their renal clearance and endocytic tubular uptake (128). This fact limits the class of therapeutic siRNAs for such procedures because of the natural tendency of systemically delivered materials to accumulate within the liver.
In comparison, the expression of transgenic shRNA targeting the proapoptotic BIM gene prevented the development of polycystic kidney disease in BCl-2 deficient mice (129). However, the mortality rate in this study was high. Additional research is required to identify whether the high mortality rate was due to the sequence of the shRNA.
One major challenge to developing gene- and cell-based strategies is our need to understand their mechanisms of action. Regardless of the performance of recombinant peptides, DNA vectors, stem cells, and RNAi agents, mechanisms related to each approach still need to be uncovered (47, 69, 130–135). This gap in knowledge makes it difficult to optimize these techniques. Nevertheless, the basic principles for successful transgene expression have been documented (130–134, 136–142). All such therapies rely on efficiently delivering exogenous genes to widespread cellular targets. The techniques discussed earlier have achieved this by directly using DNA/RNA strands or inserting these molecules into gene transport vehicles. Once the genetic materials enter the nuclei, they either aid or inhibit the expression of the gene product(s) of interest in transformed cells and their progeny.
Likewise, the overall efficacy of RNAi in inducing gene silencing in any cell depends on the ability of the dsRNA reagent to access the subcellular compartment containing the RNA-induced silencing complex (RISC) and other components of the RNAi machinery (143, 144). This subcellular compartment is in the perinuclear region of the cytoplasm. However, if cell transplantation mediates transgene expression, the gene delivery process will rely on integrating the delivered cells, native cellular division, and intercellular communication. Furthermore, the goal is to facilitate gene expression/inhibition once exogenous cells are integrated into tissues and organs (145, 146).
For instance, previous work suggests that the effectiveness of gene therapies using adenoviral (147) and siRNA (148) vectors depends on the dose and timing of transgene administration. Such dependence drives variations in drug concentrations at the respective sites of the gene expression and silencing machinery.
It is, therefore, essential to understanding how effective concentrations within the cytoplasm affect therapeutic potency based on dosing and timing of transgene administrations. This factor is a topic of practical importance, as the mechanism(s) will determine the intracellular fate of exogenous transgenes from non-viral, viral, and cellular sources and aid the development of effectual medical strategies that can control the duration and extent of induced genetic traits. Alternatively, for approaches that focus on whole organ engineering and re-engineering, additional insights are needed into the mechanisms behind the successful repopulation of tissue and organ templates (65). Researchers must also determine the characteristics required to facilitate exogenous genetic and cellular harmony for viable transplantable kidneys before these findings can translate into clinical practice.
Over the past 30 years, many methods have been proposed to deliver exogenous genes and cells to target organs (32, 39, 46, 97, 100, 102, 130, 142, 149–157). From a fundamental viewpoint, these techniques seek to provide inexpensive and rapid alternatives to pronuclear microinjection-derived transgenic models and platforms for translational studies (121). However, a limiting step in this process is the need for more reliable delivery systems. Several reports have indicated inconsistent outcomes regarding the effectiveness of existing gene and cell transfer techniques. Studies in the kidney have illustrated this variability (155, 156, 158–164). In general, an in vivo gene and cell transfer system’s success relies on various factors. The factors include:
• the ability to deliver vectors to the target cells/organ;
• the time the target cell/organs take to express the exogenous materials; and
• the number of cells/organs that express the required phenotype.
Other essential factors are the resulting expression levels, cellular turnover rates, the reproducibility of the process, and the severity of the injury that may result from it (95, 130). Thus, most existing strategies remain experimental (165–168).
Researchers must consider organ morphology and function variations as crucial elements to improve the overall efficacy of delivery strategies (169, 170). Thus, efficient gene and cellular therapies for treating kidney diseases remain challenging (47, 171–175). The structure of the renal vasculature and its unique characteristics are prominent limiting factors. Systems focusing on proximal tubular epithelial cellular uptake could be helpful (175–177). However, a potential drawback to this technique is the variations in the glomerular permeability of different molecules (178–183). Likewise, the unknown degree to which these cells are accessible for gene delivery at the basolateral surface via the peritubular capillaries provides another level of complication. Studies using adenovirus vectors have demonstrated the need to improve our understanding of renal physiology and our ability to manipulate it.
Intra-arterial kidney injections, pre-chilled for extended periods, facilitated transgene expression within the cortical vasculature (184). Combining the pre-chilling treatment with vasodilators provided gene transfer in the outer medulla’s inner and outer strips (184). Other studies have successfully presented adenoviral vector delivery to rat glomerular and tubular compartments by infusions into the right renal artery (185, 186). This technique provided high levels of transgene expression for 2–4 weeks without causing significant damage (187, 188). Analogous concentrations of the same adenovirus vector were suspended in different volumes and delivered to the kidney via arterial injections and pelvic catheter infusions. This approach facilitated transgene expression in distinct kidney regions (188, 189). After injecting vectors into the aorta at a location proximal to the left renal artery, the investigators observed transgene expression only in proximal tubular cells.
Tail vein and retrograde ureteral adenovirus infusions that target aquaporin water channels also reported different expression levels, which depended on the transgene infusion site (130, 156). Aquaporin 1 transgenes were expressed in apical and basolateral membranes of proximal tubule epithelial cells in the renal cortex but not in the glomerulus, loop of Henle, or collecting duct. Conversely, ureteral and renal papilla transgene expression was reported through ureteral infusions. The researchers also reported less intense and patchy expression in cortical collecting ducts. Ashworth et al. (190) and Tanner et al. (161) explored the direct transfer of adenovirus vectors that carried transgenes into individual nephron segments using micropuncture techniques. They observed site-specific transgene expression within the injected tubules or vascular welling points. These results also demonstrated the utility of intravital fluorescent multiphoton microscopy to monitor protein expression in live animals directly. However, one limitation of the approach was that the injection sites were the only places where the investigators found transgene expression.
These studies further highlight the challenge of introducing genes into multiple renal cell types due to the intricate anatomy of the kidney, even when using the same type of vector. Results depend on the transgene infusion site, volume, and rate, as well as the organ temperature and the use of vasodilators. Hydroporation may address these challenges by increasing vascular permeability and thus efficiently delivering exogenous substances throughout the kidney. Hydrodynamic fluid delivery impacts fluid pressures within thin, stretchable capillaries (191, 192). The enhanced fluid flow generated from pressurized injections produces rapid and high fluctuations in blood circulation. Theoretically, it increases the permeability of the capillary endothelium and epithelial junctions by generating transient pores in plasma membranes that facilitate the cellular internalization of macromolecules of interest (47, 191, 193). The unique anatomy of the kidney provides various innate delivery pathways (artery, vein, and ureter) that may be ideal for hydrodynamic gene delivery. In our recent reports, this delivery method provided efficient and lengthy plasmid and viral expression in live rat kidneys (130, 142, 194) and facilitated protection against moderate forms of ischemia–reperfusion injury (154, 195–197). A summary of delivery methods and associated vectors is presented in Table 1.
The gene of interest is infused either systemically or directly into the kidney. Apart from the artery, vein, and ureter, direct infusions into the renal capsule and parenchyma using micro-needles (161, 190) or blunt-tip needles (157, 198) have also been proposed, along with indirect tail vein (191, 196, 199) and peritoneum (200, 201) gene delivery schemes. As indicated before, the success of these methods varies per the anatomical location of the targeted cells and the types of vectors used to support gene expression. These vectors include PRC-amplified DNA fragments; plasmid DNA; liposomes; polycations; viral vectors; and stem cells (130). If transformed cells act as gene vectors to promote transgene expression, they may be engineered with various anchoring or binding proteins/peptides to assist their integration into the tissue of interest (202). This process mimics endogenous viral capsid components, which mediate receptor binding and support entry into mammalian cells. As observed in some injured kidney animal models, local healing/regeneration factors facilitate the incorporation of exogenous renal cells delivered intravenously (55). An outline of transgene vector incorporation into the renal epithelium is presented in Figure 1.
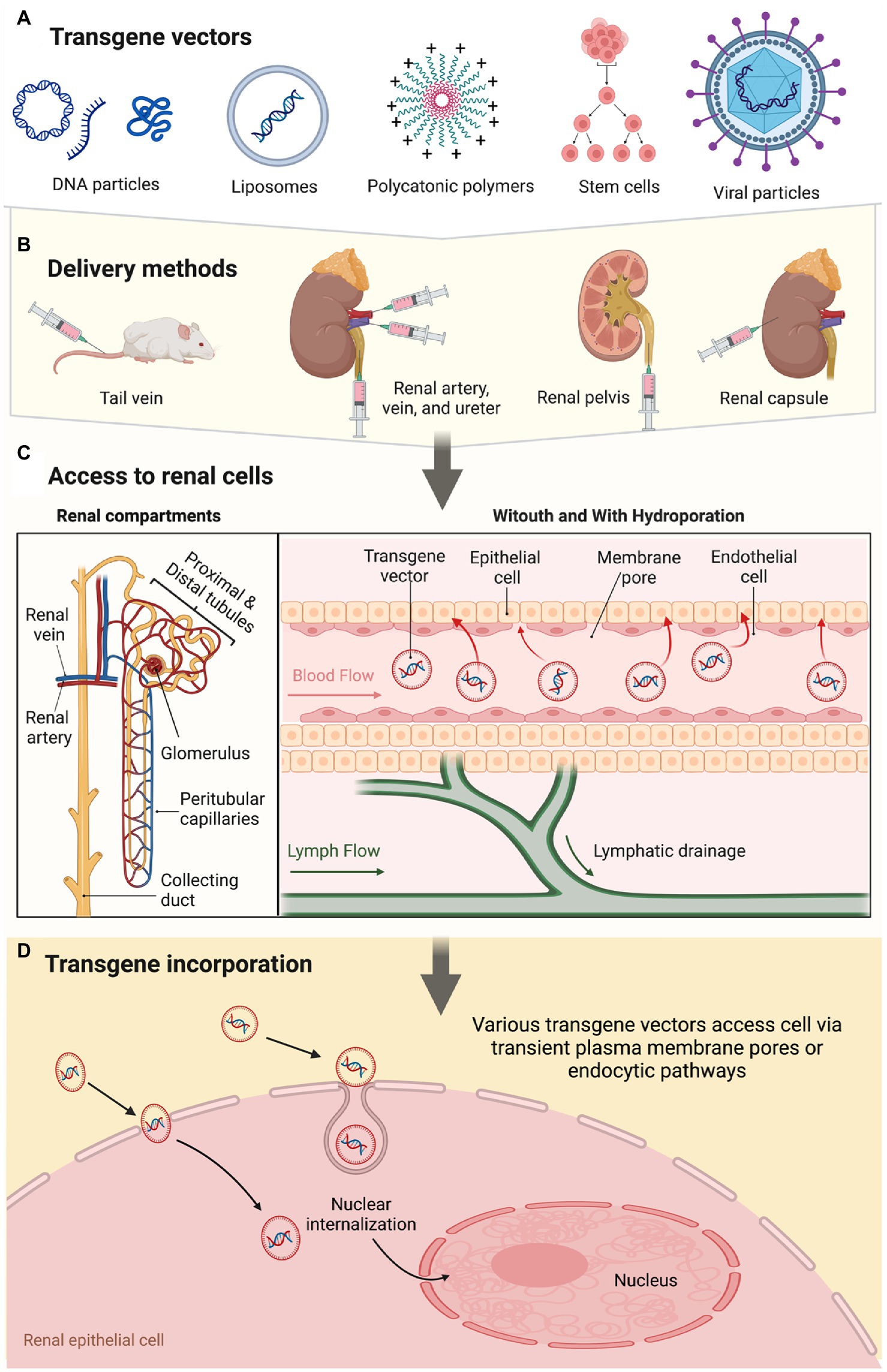
Figure 1. A schematic overview of the renal gene- and cell-based approaches highlights vectorization, delivery mode, and pathways supporting transgene incorporation and expression.
Apart from achieving successful genetic modifications, we must also focus on exogenous transgene delivery and expression effects. Such considerations relate to the levels of cellular toxicity and injury that may occur during and after the transfer process. Endo- and exonucleases efficiently degrade DNA fragments (203, 204). However, an overload of exogenous DNA fragmentation may stimulate Ca2+ endonuclease activity, degrade endogenous DNA, and mediate cell death (205). Similarly, plasmid DNA, prepared from bacteria, may induce unmethylated CpG motif toxicity that can trigger lower respiratory tract inflammatory responses (206). Oligonucleotide therapies have also been shown to stimulate immune system responses and induce hepatotoxicity and nephrotoxicity (207). Virus-induced toxic and immunogenic responses from high titers, protein overexpression, and capsid protein infections are also topics of significant concern (208). Long-term mutagenesis may also be an issue. Reports have shown such events using recombinant adenovirus systems (209, 210). Specifically, slow-transforming insertional mutagenesis may arise from retroviruses that incorporate into an organism’s genome (211), and in vivo stem cell quiescence can tamper with DNA repair mechanisms to further support mutagenesis (212).
There is a dire need to improve the clinical management of acute and chronic renal diseases. Preliminary outcomes in experimental models with kidney dysfunction managed by gene-based and cell-based approaches are promising. Recent findings echo the traditional need to address several challenges before these therapies become viable clinical options. Existing techniques provide a wide range of success rates and, in some instances, also induce harmful side effects. Thus, further research is needed to develop methods to induce transient or permanent modifications with minimal physiological interference or damage as we aim to improve the treatment of acute and chronic kidney diseases.
The author confirms being the sole contributor of this work and has approved it for publication.
This study was supported in part by the Khalifa University’s College of Medicine and Health Sciences and Grant Number: FSU-2020-25 and funding from RC2-2018-022 (HEIC) awarded to PC.
The author would like to thank Maja Corridon for reviewing the manuscript.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1.Isert, S, Müller, D, and Thumfart, J. Factors associated with the development of chronic kidney disease in children with congenital anomalies of the kidney and urinary tract. Front Pediatr. (2020) 8:298. doi: 10.3389/fped.2020.00298
2.Bergmann, C, Guay-Woodford, LM, Harris, PC, Horie, S, Peters, DJM, and Torres, VE. Polycystic kidney disease. Nat Rev Dis Primers. (2018) 4:50. doi: 10.1038/s41572-018-0047-y
3.al-Naimi, MS, Rasheed, HA, Hussien, NR, al-Kuraishy, HM, and al-Gareeb, AI. Nephrotoxicity: role and significance of renal biomarkers in the early detection of acute renal injury. J Adv Pharm Technol Res. (2019) 10:95–9. doi: 10.4103/japtr.JAPTR_336_18
4.Burek, M, Burmester, S, Salvador, E, Möller-Ehrlich, K, Schneider, R, Roewer, N, et al. Kidney ischemia/reperfusion injury induces changes in the drug transporter expression at the blood–brain barrier in vivo and in vitro. Front Physiol. (2020) 11:569881. doi: 10.3389/fphys.2020.569881
5.Pantic, I., Cumic, J, Dugalic, S, Petroianu, GA, and Corridon, PR, Gray level co-occurrence matrix and wavelet analyses reveal discrete changes in proximal tubule cell nuclei after mild acute kidney injury. (2022), Res Square [Preprint].
6.Qureshi, AI, Huang, W, Lobanova, I, Hanley, DF, Hsu, CY, Malhotra, K, et al. Systolic blood pressure reduction and acute kidney injury in Intracerebral hemorrhage. Stroke. (2020) 51:3030–8. doi: 10.1161/STROKEAHA.120.030272
7.Jankowski, J, Floege, J, Fliser, D, Böhm, M, and Marx, N. Cardiovascular disease in chronic kidney disease. Circulation. (2021) 143:1157–72. doi: 10.1161/CIRCULATIONAHA.120.050686
8.Jheong, J-H, Hong, S-K, and Kim, T-H. Acute kidney injury after trauma: risk factors and clinical outcomes. J Acute Care Surg. (2020) 10:90–5. doi: 10.17479/jacs.2020.10.3.90
9.Lombardi, Y, Ridel, C, and Touzot, M. Anaemia and acute kidney injury: the tip of the iceberg? Clin Kidney J. (2020) 14:471–3. doi: 10.1093/ckj/sfaa202
10.Malyszko, J, Tesarova, P, Capasso, G, and Capasso, A. The link between kidney disease and cancer: complications and treatment. Lancet. (2020) 396:277–87. doi: 10.1016/S0140-6736(20)30540-7
11.Forst, T, Mathieu, C, Giorgino, F, Wheeler, DC, Papanas, N, Schmieder, RE, et al. New strategies to improve clinical outcomes for diabetic kidney disease. BMC Med. (2022) 20:337. doi: 10.1186/s12916-022-02539-2
12.Alicic, R, and Nicholas, SB. Diabetic kidney disease Back in focus: management field guide for health care professionals in the 21st century. Mayo Clin Proc. (2022) 97:1904–19. doi: 10.1016/j.mayocp.2022.05.003
13.Adapa, S, Chenna, A, Balla, M, Merugu, GP, Koduri, NM, Daggubati, SR, et al. COVID-19 pandemic causing acute kidney injury and impact on patients with chronic kidney disease and renal transplantation. J Clin Med Res. (2020) 12:352–61. doi: 10.14740/jocmr4200
14.Geetha, D, Kronbichler, A, Rutter, M, Bajpai, D, Menez, S, Weissenbacher, A, et al. Impact of the COVID-19 pandemic on the kidney community: lessons learned and future directions. Nat Rev Nephrol. (2022) 18:724–37. doi: 10.1038/s41581-022-00618-4
15.Hsu, RK, and Hsu, C-y. CKD increases risk of acute kidney injury during hospitalization. Nat Clin Pract Nephrol. (2008) 4:408–8. doi: 10.1038/ncpneph0850
16.Hsu, RK, and Hsu, CY. The role of acute kidney injury in chronic kidney disease. Semin Nephrol. (2016) 36:283–92. doi: 10.1016/j.semnephrol.2016.05.005
17.Bell, JS, James, BD, al-Chalabi, S, Sykes, L, Kalra, PA, and Green, D. Community- versus hospital-acquired acute kidney injury in hospitalised COVID-19 patients. BMC Nephrol. (2021) 22:269. doi: 10.1186/s12882-021-02471-2
18.Minja, NW, Akrabi, H, Yeates, K, and Kilonzo, KG. Acute kidney injury and associated factors in intensive care units at a tertiary Hospital in Northern Tanzania. Can J Kidney Health Dis. (2021) 8:20543581211027971. doi: 10.1177/20543581211027971
19.Tanemoto, F, and Mimura, I. Therapies targeting epigenetic alterations in acute kidney injury-to-chronic kidney disease transition. Pharmaceuticals. (2022) 15:123. doi: 10.3390/ph15020123
20.Kovesdy, CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. (2022) 12:7–11. doi: 10.1016/j.kisu.2021.11.003
21.Lv, JC, and Zhang, LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. (2019) 1165:3–15. doi: 10.1007/978-981-13-8871-2_1
22.Goyal, A., Daneshpajouhnejad, Parnaz, Hashmi, Muhammad F., Bashir, Khalid, and John, Bini K. (2022). Acute kidney injury (nursing) in StatPearls: Treasure Island (FL), 68, 159.
23.Palevsky, PM. Endpoints for clinical trials of acute kidney injury. Nephron. (2018) 140:111–5. doi: 10.1159/000493203
24.Muroya, Y, He, X, Fan, L, Wang, S, Xu, R, Fan, F, et al. Enhanced renal ischemia-reperfusion injury in aging and diabetes. Am J Physiol Ren Physiol. (2018) 315:F1843–f1854. doi: 10.1152/ajprenal.00184.2018
25.Corridon, PR, Wang, X, Shakeel, A, and Chan, V. Digital technologies: advancing individualized treatments through gene and Cell therapies, Pharmacogenetics, and disease detection and diagnostics. Biomedicine. (2022) 10:2445. doi: 10.3390/biomedicines10102445
26.Pantic, IV, Shakeel, A, Petroianu, GA, and Corridon, PR. Analysis of vascular architecture and parenchymal damage generated by reduced blood perfusion in Decellularized porcine kidneys using a gray level co-occurrence matrix. Front Cardio Med. (2022) 9:797283. doi: 10.3389/fcvm.2022.797283
27.Pantic, I, Paunovic, J, Cumic, J, Valjarevic, S, Petroianu, GA, and Corridon, PR. Artificial neural networks in contemporary toxicology research. Chem Biol Interact. (2023) 369:110269. doi: 10.1016/j.cbi.2022.110269
28.Davidovic, LM, Cumic, J, Dugalic, S, Vicentic, S, Sevarac, Z, and Petroianu, G. Gray-level Co-occurrence matrix analysis for the detection of discrete, ethanol-induced, structural changes in cell nuclei: an artificial intelligence approach. Microsc. Microanal. (2022) 28:265–271. doi: 10.1017/S1431927621013878
29.Oka, M, Sekiya, S, Sakiyama, R, Shimizu, T, and Nitta, K. Hepatocyte growth factor–secreting Mesothelial cell sheets suppress progressive fibrosis in a rat model of CKD. J Am Soc Nephrol. (2019) 30:261–76. doi: 10.1681/ASN.2018050556
30.Flaquer, M, Franquesa, M, Vidal, A, Bolaños, N, Torras, J, Lloberas, N, et al. Hepatocyte growth factor gene therapy enhances infiltration of macrophages and may induce kidney repair in db/db mice as a model of diabetes. Diabetologia. (2012) 55:2059–68. doi: 10.1007/s00125-012-2535-z
31.Hirooka, Y, and Nozaki, Y. Interleukin-18 in inflammatory kidney disease. Front Med. (2021) 8:8. doi: 10.3389/fmed.2021.639103
32.Kim, JH, Yang, H, Kim, MW, Cho, KS, Kim, DS, Yim, HE, et al. The delivery of the recombinant protein cocktail identified by stem cell-derived Secretome analysis accelerates kidney repair after renal ischemia-reperfusion injury. Front Bioeng Biotechnol. (2022) 10:10. doi: 10.3389/fbioe.2022.848679
33.Dai, C, Yang, J, and Liu, Y. Single injection of naked plasmid encoding hepatocyte growth factor prevents cell death and ameliorates acute renal failure in mice. J Am Soc Nephrol. (2002) 13:411–22. doi: 10.1681/ASN.V132411
34.Mihajlovic, M, Wever, KE, van der Made, TK, de Vries, RBM, Hilbrands, LB, and Masereeuw, R. Are cell-based therapies for kidney disease safe? A systematic review of preclinical evidence. Pharmacol Ther. (2019) 197:191–211. doi: 10.1016/j.pharmthera.2019.01.004
35.Liu, Y, Rajur, K, Tolbert, E, and Dworkin, LD. Endogenous hepatocyte growth factor ameliorates chronic renal injury by activating matrix degradation pathways. Kidney Int. (2000) 58:2028–43. doi: 10.1111/j.1523-1755.2000.00375.x
36.Zhou, D, Tan, RJ, Fu, H, and Liu, Y. Wnt/β-catenin signaling in kidney injury and repair: a double-edged sword. Lab Investig. (2016) 96:156–67. doi: 10.1038/labinvest.2015.153
37.Brown, PA, Bodles-Brakhop, AM, Pope, MA, and Draghia-Akli, R. Gene therapy by electroporation for the treatment of chronic renal failure in companion animals. BMC Biotechnol. (2009) 9:4. doi: 10.1186/1472-6750-9-4
38.Rieger, AC, Bagno, LL, Salerno, A, Florea, V, Rodriguez, J, Rosado, M, et al. Growth hormone-releasing hormone agonists ameliorate chronic kidney disease-induced heart failure with preserved ejection fraction. Proc Natl Acad Sci U S A. (2021) 118:e2019835118. doi: 10.1073/pnas.2019835118
39.Wang, AY, Peng, PD, Ehrhardt, A, Storm, TA, and Kay, MA. Comparison of adenoviral and adeno-associated viral vectors for pancreatic gene delivery in vivo. Hum Gene Ther. (2004) 15:405–13. doi: 10.1089/104303404322959551
40.Haddad, G, Kölling, M, Wegmann, UA, Dettling, A, Seeger, H, Schmitt, R, et al. Renal AAV2-mediated overexpression of long non-coding RNA H19 attenuates ischemic acute kidney injury through sponging of microRNA-30a-5p. J Am Soc Nephrol. (2021) 32:323–41. doi: 10.1681/ASN.2020060775
41.Dong, X, Cao, R, Li, Q, and Yin, L. The long noncoding RNA-H19 mediates the progression of fibrosis from acute kidney injury to chronic kidney disease by regulating the miR-196a/Wnt/β-catenin signaling. Nephron. (2022) 146:209–19. doi: 10.1159/000518756
42.Qin, Z, Li, X, Yang, J, Cao, P, Qin, C, Xue, J, et al. VEGF and Ang-1 promotes endothelial progenitor cells homing in the rat model of renal ischemia and reperfusion injury. Int J Clin Exp Pathol. (2017) 10:11896–908.
43.Tanabe, K, Maeshima, Y, Sato, Y, and Wada, J. Antiangiogenic therapy for diabetic nephropathy. Biomed Res Int. (2017) 2017:5724069–12. doi: 10.1155/2017/5724069
44.Tao, Q-R, Chu, YM, Wei, L, Tu, C, and Han, YY. Antiangiogenic therapy in diabetic nephropathy: a double-edged sword. Mol Med Rep. (2021) 23:260. doi: 10.3892/mmr.2021.11899
45.Torras, J, Cruzado, JM, Herrero-Fresneda, I, and Grinyo, JM. Gene therapy for acute renal failure. Contrib Nephrol. (2008) 159:96–108. doi: 10.1159/000125614
46.AlQahtani, AD, O’Connor, D, Domling, A, and Goda, SK. Strategies for the production of long-acting therapeutics and efficient drug delivery for cancer treatment. Biomed Pharmacother. (2019) 113:108750. doi: 10.1016/j.biopha.2019.108750
47.Rubin, JD, and Barry, MA. Improving molecular therapy in the kidney. Mol Diagn Ther. (2020) 24:375–96. doi: 10.1007/s40291-020-00467-6
48.Hunt, CJ. Cryopreservation of human stem cells for clinical application: a review. Transfus Med Hemother. (2011) 38:107–23. doi: 10.1159/000326623
49.Shakeel, A, and Corridon, PR. Mitigating challenges and expanding the future of vascular tissue engineering—are we there yet? Front Physiol. (2023) 13:1079421. doi: 10.3389/fphys.2022.1079421
50.Wang, X, Chan, V, and Corridon, PR. Decellularized blood vessel development: current state-of-the-art and future directions. Front Bioeng Biotechnol. (2022) 10:951644. doi: 10.3389/fbioe.2022.951644
51.Wang, X, Chan, V, and Corridon, PR. Acellular tissue-engineered vascular grafts from polymers: methods, achievements, characterization, and challenges. Polymers. (2022) 14:4825. doi: 10.3390/polym14224825
52.Geuens, T, van Blitterswijk, CA, and LaPointe, VLS. Overcoming kidney organoid challenges for regenerative medicine. NPJ Regen Med. (2020) 5:8. doi: 10.1038/s41536-020-0093-4
53.Khoshdel-Rad, N, Ahmadi, A, and Moghadasali, R. Kidney organoids: current knowledge and future directions. Cell Tissue Res. (2022) 387:207–24. doi: 10.1007/s00441-021-03565-x
54.Liu, M, Cardilla, A, Ngeow, J, Gong, X, and Xia, Y. Studying kidney diseases using Organoid models. Front Cell Develop Biol. (2022) 10:845401. doi: 10.3389/fcell.2022.845401
55.Papazova, DA, Oosterhuis, NR, Gremmels, H, van Koppen, A, Joles, JA, and Verhaar, MC. Cell-based therapies for experimental chronic kidney disease: a systematic review and meta-analysis. Dis Model Mech. (2015) 8:281–93. doi: 10.1242/dmm.017699
56.Wang, J, Lin, Y, Chen, X, Liu, Y, and Zhou, T. Mesenchymal stem cells: a new therapeutic tool for chronic kidney disease. Front Cell Develop Biol. (2022) 10:910592. doi: 10.3389/fcell.2022.910592
57.Eirin, A, and Lerman, LO. Mesenchymal stem/stromal cell–derived extracellular vesicles for chronic kidney disease: are we there yet? Hypertension. (2021) 78:261–9. doi: 10.1161/HYPERTENSIONAHA.121.14596
58.Wong, CY. Current advances of stem cell-based therapy for kidney diseases. World J Stem Cells. (2021) 13:914–33. doi: 10.4252/wjsc.v13.i7.914
59.Missoum, A. Recent updates on Mesenchymal stem cell based therapy for acute renal failure. Curr Urol. (2019) 13:189–99. doi: 10.1159/000499272
60.Kelly, KJ, Kluve-Beckerman, B, Zhang, J, and Dominguez, JH. Intravenous cell therapy for acute renal failure with serum amyloid a protein-reprogrammed cells. Am J Physiol Ren Physiol. (2010) 299:F453–64. doi: 10.1152/ajprenal.00050.2010
61.Fatehullah, A, Tan, SH, and Barker, N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. (2016) 18:246–54. doi: 10.1038/ncb3312
62.Little, MH. Generating kidney tissue from pluripotent stem cells. Cell Death Dis. (2016) 2:16053. doi: 10.1038/cddiscovery.2016.53
63.Morizane, R, Lam, AQ, Freedman, BS, Kishi, S, Valerius, MT, and Bonventre, JV. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol. (2015) 33:1193–200. doi: 10.1038/nbt.3392
64.Garreta, E, Nauryzgaliyeva, Z, and Montserrat, N. Human induced pluripotent stem cell-derived kidney organoids toward clinical implementations. Curr Opinion Biomed Eng. (2021) 20:100346. doi: 10.1016/j.cobme.2021.100346
65.Corridon, PR. Intravital microscopy datasets examining key nephron segments of transplanted decellularized kidneys. Sci Data. (2022) 9:561. doi: 10.1038/s41597-022-01685-9
66.Pantic, IV, Shakeel, A, Petroianu, GA, and Corridon, PR. Analysis of vascular architecture and parenchymal damage generated by reduced blood perfusion in Decellularized porcine kidneys using a gray level co-occurrence matrix. Front Cardiovasc Med. (2022) 9:797283. doi: 10.3389/fcvm.2022.797283
67.Ciampi, O, Bonandrini, B, Derosas, M, Conti, S, Rizzo, P, Benedetti, V, et al. Engineering the vasculature of decellularized rat kidney scaffolds using human induced pluripotent stem cell-derived endothelial cells. Sci Rep. (2019) 9:8001. doi: 10.1038/s41598-019-44393-y
68.de Haan, MJA, Witjas, FMR, Engelse, MA, and Rabelink, TJ. Have we hit a wall with whole kidney decellularization and recellularization: a review. Curr Opinion Biomed Eng. (2021) 20:100335. doi: 10.1016/j.cobme.2021.100335
69.Corridon, PR, Ko, IK, Yoo, JJ, and Atala, A. Bioartificial kidneys. Curr Stem Cell Rep. (2017) 3:68–76. doi: 10.1007/s40778-017-0079-3
70.Corridon, PR. In vitro investigation of the impact of pulsatile blood flow on the vascular architecture of decellularized porcine kidneys. Sci Rep. (2021) 11:16965. doi: 10.1038/s41598-021-95924-5
71.Khan, R, Khraibi, A, Dumée, LF, and Corridon, PR. From waste to wealth: Repurposing slaughterhouse waste for xenotransplantation. Front. Bioeng. Biotechnol. (2023). doi: 10.3389/fbioe.2023.1091554
72.Becerra, J, Santos-Ruiz, L, Andrades, JA, and Marí-Beffa, M. The stem cell niche should be a key issue for cell therapy in regenerative medicine. Stem Cell Rev Rep. (2011) 7:248–55. doi: 10.1007/s12015-010-9195-5
73.Haworth, R, and Sharpe, M. The issue of immunology in stem cell therapies: a pharmaceutical perspective. Regen Med. (2015) 10:231–4. doi: 10.2217/rme.14.50
74.Jalil, RA, Neng, LC, and Kofidis, T. Challenges in deriving and utilizing stem cell-derived endothelial cells for regenerative medicine: a key issue in clinical therapeutic applications. J Stem Cells. (2011) 6:93–9.
75.Lepperdinger, G, Brunauer, R, Jamnig, A, Laschober, G, and Kassem, M. Controversial issue: is it safe to employ mesenchymal stem cells in cell-based therapies? Exp Gerontol. (2008) 43:1018–23. doi: 10.1016/j.exger.2008.07.004
76.Lin, SZ. Era of stem cell therapy for regenerative medicine and cancers: an introduction for the special issue of pan Pacific symposium on stem cells and cancer research. Cell Transplant. (2015) 24:311–2. doi: 10.3727/096368915X686814
77.Sell, S. Adult stem cell plasticity: introduction to the first issue of stem cell reviews. Stem Cell Rev. (2005) 1:001–8. doi: 10.1385/SCR:1:1:001
78.Wade, N. An old question becomes new again: stem cell issue causes debate over the exact moment life begins. N Y Times Web. (2001):A20.
79.Brown, M. No ethical bypass of moral status in stem cell research. Bioethics. (2013) 27:12–9. doi: 10.1111/j.1467-8519.2011.01891.x
80.Cohen, CB, Brandhorst, B, Nagy, A, Leader, A, Dickens, B, Isasi, RM, et al. The use of fresh embryos in stem cell research: ethical and policy issues. Cell Stem Cell. (2008) 2:416–21. doi: 10.1016/j.stem.2008.04.002
81.Cote, DJ, Bredenoord, AL, Smith, TR, Ammirati, M, Brennum, J, Mendez, I, et al. Ethical clinical translation of stem cell interventions for neurologic disease. Neurology. (2017) 88:322–8. doi: 10.1212/WNL.0000000000003506
82.Habets, MG, van Delden, JJ, and Bredenoord, AL. The inherent ethical challenge of first-in-human pluripotent stem cell trials. Regen Med. (2014) 9:1–3. doi: 10.2217/rme.13.83
83.Lo, B, and Parham, L. Ethical issues in stem cell research. Endocr Rev. (2009) 30:204–13. doi: 10.1210/er.2008-0031
84.Manzar, N, Manzar, B, Hussain, N, Hussain, MFA, and Raza, S. The ethical dilemma of embryonic stem cell research. Sci Eng Ethics. (2013) 19:97–106. doi: 10.1007/s11948-011-9326-7
85.Mauron, A, and Jaconi, ME. Stem cell science: current ethical and policy issues. Clin Pharmacol Ther. (2007) 82:330–3. doi: 10.1038/sj.clpt.6100295
86.Schuklenk, U. How not to win an ethical argument: embryo stem cell research revisited. Bioethics. (2008) 22:ii–iii. doi: 10.1111/j.1467-8519.2008.00640.x
87.Suckiel, E. Human embryonic stem cell research: a critical survey of the ethical issues. Adv Pediatr Infect Dis. (2008) 55:79–96. doi: 10.1016/j.yapd.2008.07.017
88.Cho, SJ, Kim, SY, Jeong, HC, Cheong, H, Kim, D, Park, SJ, et al. Repair of ischemic injury by pluripotent stem cell based cell therapy without teratoma through selective photosensitivity. Stem Cell Reports. (2015) 5:1067–80. doi: 10.1016/j.stemcr.2015.10.004
89.Tang, C, Weissman, IL, and Drukker, M. The safety of embryonic stem cell therapy relies on teratoma removal. Oncotarget. (2012) 3:7–8. doi: 10.18632/oncotarget.434
90.Zhang, W. Teratoma formation: a tool for monitoring pluripotency in stem cell research. StemBook. (2014). doi: 10.3824/stembook.1.53.1
91.de Los Angeles, A, Ferrari, F, Xi, R, Fujiwara, Y, Benvenisty, N, Deng, H, et al. Hallmarks of pluripotency. Nature. (2015) 525:469–78. doi: 10.1038/nature15515
92.Dhawan, AP, D'Alessandro, B, and Fu, X. Optical imaging modalities for biomedical applications. IEEE Rev Biomed Eng. (2010) 3:69–92. doi: 10.1109/RBME.2010.2081975
93.Zakrzewski, W, Dobrzyński, M, Szymonowicz, M, and Rybak, Z. Stem cells: past, present, and future. Stem Cell Res Ther. (2019) 10:68. doi: 10.1186/s13287-019-1165-5
94.Mousaei Ghasroldasht, M, Seok, J, Park, HS, Liakath Ali, FB, and al-Hendy, A. Stem cell therapy: from idea to clinical practice. Int J Mol Sci. (2022) 23:2850. doi: 10.3390/ijms23052850
95.Lien, YH, and Lai, LW. Renal gene transfer: nonviral approaches. Mol Biotechnol. (2003) 24:283–94. doi: 10.1385/MB:24:3:283
96.Agrawal, N, Dasaradhi, PVN, Mohmmed, A, Malhotra, P, Bhatnagar, RK, and Mukherjee, SK. RNA interference: biology, mechanism, and applications. Microbiol Mol Biol Rev. (2003) 67:657–85. doi: 10.1128/MMBR.67.4.657-685.2003
97.Chen, X, Mangala, LS, Rodriguez-Aguayo, C, Kong, X, Lopez-Berestein, G, and Sood, AK. RNA interference-based therapy and its delivery systems. Cancer Metastasis Rev. (2018) 37:107–24. doi: 10.1007/s10555-017-9717-6
98.Gupta, S, Verfaillie, C, Chmielewski, D, Kren, S, Eidman, K, Connaire, J, et al. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol. (2006) 17:3028–40. doi: 10.1681/ASN.2006030275
99.Sen, GL, and Blau, HM. A brief history of RNAi: the silence of the genes. FASEB J. (2006) 20:1293–9. doi: 10.1096/fj.06-6014rev
100.Bondue, T, van den Heuvel, L, Levtchenko, E, and Brock, R. The potential of RNA-based therapy for kidney diseases. Pediatr Nephrol. (2023) 38:327–44. doi: 10.1007/s00467-021-05352-w
101.Kameda, S, Maruyama, H, Higuchi, N, Iino, N, Nakamura, G, Miyazaki, J, et al. Kidney-targeted naked DNA transfer by retrograde injection into the renal vein in mice. Biochem Biophys Res Commun. (2004) 314:390–5. doi: 10.1016/j.bbrc.2003.12.107
102.Lam, JK, Chow, MYT, Zhang, Y, and Leung, SWS. siRNA versus miRNA as therapeutics for gene silencing. Mol Ther Nucleic Acids. (2015) 4:e252. doi: 10.1038/mtna.2015.23
103.Thorling, CA, Dancik, Y, Hupple, CW, Medley, G, Liu, X, Zvyagin, AV, et al. Multiphoton microscopy and fluorescence lifetime imaging provide a novel method in studying drug distribution and metabolism in the rat liver in vivo. J Biomed Opt. (2011) 16:086013. doi: 10.1117/1.3614473
104.Schena, FP, Serino, G, and Sallustio, F. MicroRNAs in kidney diseases: new promising biomarkers for diagnosis and monitoring. Nephrol Dial Transplant. (2014) 29:755–63. doi: 10.1093/ndt/gft223
105.Saal, S, and Harvey, SJ. MicroRNAs and the kidney: coming of age. Curr Opin Nephrol Hypertens. (2009) 18:317–23. doi: 10.1097/MNH.0b013e32832c9da2
106.Aslan, C, Kiaie, SH, Zolbanin, NM, Lotfinejad, P, Ramezani, R, Kashanchi, F, et al. Exosomes for mRNA delivery: a novel biotherapeutic strategy with hurdles and hope. BMC Biotechnol. (2021) 21:20. doi: 10.1186/s12896-021-00683-w
107.Zhang, Y, Liu, Q, Zhang, X, Huang, H, Tang, S, Chai, Y, et al. Recent advances in exosome-mediated nucleic acid delivery for cancer therapy. J Nanobiotechnol. (2022) 20:279. doi: 10.1186/s12951-022-01472-z
108.Herrmann, IK, Wood, MJA, and Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. (2021) 16:748–59. doi: 10.1038/s41565-021-00931-2
109.Lv, LL, Wu, WJ, Feng, Y, Li, ZL, Tang, TT, and Liu, BC. Therapeutic application of extracellular vesicles in kidney disease: promises and challenges. J Cell Mol Med. (2018) 22:728–37. doi: 10.1111/jcmm.13407
110.Xiang, H, Zhang, C, and Xiong, J. Emerging role of extracellular vesicles in kidney diseases. Front Pharmacol. (2022) 13:985030. doi: 10.3389/fphar.2022.985030
111.Corrêa, RR, Juncosa, EM, Masereeuw, R, and Lindoso, RS. Extracellular vesicles as a therapeutic tool for kidney disease: current advances and perspectives. Int J Mol Sci. (2021) 22:5787. doi: 10.3390/ijms22115787
112.Cantaluppi, V, Gatti, S, Medica, D, Figliolini, F, Bruno, S, Deregibus, MC, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. (2012) 82:412–27. doi: 10.1038/ki.2012.105
113.Yang, C, Zhang, C, Zhao, Z, Zhu, T, and Yang, B. Fighting against kidney diseases with small interfering RNA: opportunities and challenges. J Transl Med. (2015) 13:39. doi: 10.1186/s12967-015-0387-2
114.Miller, RP, Tadagavadi, RK, Ramesh, G, and Reeves, WB. Mechanisms of Cisplatin nephrotoxicity. Toxins. (2010) 2:2490–518. doi: 10.3390/toxins2112490
115.Pabla, N, and Dong, Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. (2008) 73:994–1007. doi: 10.1038/sj.ki.5002786
116.Peres, LA, and da Cunha, AD Jr. Acute nephrotoxicity of cisplatin: molecular mechanisms. J Bras Nefrol. (2013) 35:332–40. doi: 10.5935/0101-2800.20130052
117.BOSCH, B, GRIGOROV, B, SENSERRICH, J, CLOTET, B, DARLIX, J, MURIAUX, D, et al. A clathrin-dynamin-dependent endocytic pathway for the uptake of HIV-1 by direct T cell-T cell transmission. Antivir Res. (2008) 80:185–93. doi: 10.1016/j.antiviral.2008.06.004
118.Marina-García, Ń, Franchi, L, Kim, YG, Hu, Y, Smith, DE, Boons, GJ, et al. Clathrin- and dynamin-dependent endocytic pathway regulates muramyl dipeptide internalization and NOD2 activation. J Immunol. (2009) 182:4321–7. doi: 10.4049/jimmunol.0802197
119.Wiejak, J, Surmacz, L, and Wyroba, E. Dynamin- and clathrin-dependent endocytic pathway in unicellular eukaryote paramecium. Biochem Cell Biol. (2004) 82:547–58. doi: 10.1139/o04-098
120.McFarland, MJ, Bardell, TK, Yates, ML, Placzek, EA, and Barker, EL. RNA interference-mediated knockdown of dynamin 2 reduces endocannabinoid uptake into neuronal dCAD cells. Mol Pharmacol. (2008) 74:101–8. doi: 10.1124/mol.108.044834
121.Hall, AM, Rhodes, GJ, Sandoval, RM, Corridon, PR, and Molitoris, BA. In vivo multiphoton imaging of mitochondrial structure and function during acute kidney injury. Kidney Int. (2013) 83:72–83. doi: 10.1038/ki.2012.328
122.Mahadevappa, R, Nielsen, R, Christensen, EI, and Birn, H. Megalin in acute kidney injury: foe and friend. Am J Physiol Ren Physiol. (2014) 306:F147–54. doi: 10.1152/ajprenal.00378.2013
123.Nagai, J, Saito, M, Adachi, Y, Yumoto, R, and Takano, M. Inhibition of gentamicin binding to rat renal brush-border membrane by megalin ligands and basic peptides. J Control Release. (2006) 112:43–50. doi: 10.1016/j.jconrel.2006.01.003
124.Stokman, G, Qin, Y, Rácz, Z, Hamar, P, and Price, LS. Application of siRNA in targeting protein expression in kidney disease. Adv Drug Deliv Rev. (2010) 62:1378–89. doi: 10.1016/j.addr.2010.07.005
125.Ren, Y, du, C, Yan, L, Wei, J, Wu, H, Shi, Y, et al. CTGF siRNA ameliorates tubular cell apoptosis and tubulointerstitial fibrosis in obstructed mouse kidneys in a Sirt1-independent manner. Drug Des Devel Ther. (2015) 9:4155–71. doi: 10.2147/DDDT.S86748
126.Thompson, JD, Kornbrust, DJ, Foy, JWD, Solano, ECR, Schneider, DJ, Feinstein, E, et al. Toxicological and pharmacokinetic properties of chemically modified siRNAs targeting p53 RNA following intravenous administration. Nucleic Acid Ther. (2012) 22:255–64. doi: 10.1089/nat.2012.0371
127.Imamura, R, Isaka, Y, Sandoval, RM, Ori, A, Adamsky, S, Feinstein, E, et al. Intravital two-photon microscopy assessment of renal protection efficacy of siRNA for p53 in experimental rat kidney transplantation models. Cell Transplant. (2010) 19:1659–70. doi: 10.3727/096368910X516619
128.Molitoris, BA, Dagher, PC, Sandoval, RM, Campos, SB, Ashush, H, Fridman, E, et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol. (2009) 20:1754–64. doi: 10.1681/ASN.2008111204
129.Bouillet, P, Robati, M, Bath, M, and Strasser, A. Polycystic kidney disease prevented by transgenic RNA interference. Cell Death Differ. (2005) 12:831–3. doi: 10.1038/sj.cdd.4401603
130.Corridon, PR, Rhodes, GJ, Leonard, EC, Basile, DP, Gattone, VH II, Bacallao, RL, et al. A method to facilitate and monitor expression of exogenous genes in the rat kidney using plasmid and viral vectors. Am J Physiol Ren Physiol. (2013) 304:F1217–29. doi: 10.1152/ajprenal.00070.2013
131.Duvshani-Eshet, M, Haber, T, and Machluf, M. Insight concerning the mechanism of therapeutic ultrasound facilitating gene delivery: increasing cell membrane permeability or interfering with intracellular pathways? Hum Gene Ther. (2014) 25:156–64. doi: 10.1089/hum.2013.140
132.Felgner, JH, Kumar, R, Sridhar, CN, Wheeler, CJ, Tsai, YJ, Border, R, et al. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem. (1994) 269:2550–61. doi: 10.1016/S0021-9258(17)41980-6
133.Grandinetti, G, Smith, AE, and Reineke, TM. Membrane and nuclear permeabilization by polymeric pDNA vehicles: efficient method for gene delivery or mechanism of cytotoxicity? Mol Pharm. (2012) 9:523–38. doi: 10.1021/mp200368p
134.Wickham, T. A novel approach and a novel mechanism for stealthing gene delivery vehicles. Mol Ther. (2000) 2:103–4. doi: 10.1006/mthe.2000.0112
135.Davis, L, and Park, F. Gene therapy research for kidney diseases. Physiol Genomics. (2019) 51:449–61. doi: 10.1152/physiolgenomics.00052.2019
136.Ang, D, Nguyen, QV, Kayal, S, Preiser, PR, Rawat, RS, and Ramanujan, RV. Insights into the mechanism of magnetic particle assisted gene delivery. Acta Biomater. (2011) 7:1319–26. doi: 10.1016/j.actbio.2010.09.037
137.Choi, HS, Kim, HH, Yang, JM, and Shin, S. An insight into the gene delivery mechanism of the arginine peptide system: role of the peptide/DNA complex size. Biochim Biophys Acta. (2006) 1760:1604–12. doi: 10.1016/j.bbagen.2006.09.011
138.Elnaggar, R, Hanawa, H, Liu, H, Yoshida, T, Hayashi, M, Watanabe, R, et al. The effect of hydrodynamics-based delivery of an IL-13-Ig fusion gene for experimental autoimmune myocarditis in rats and its possible mechanism. Eur J Immunol. (2005) 35:1995–2005. doi: 10.1002/eji.200425776
139.Liu, H, Hanawa, H, Yoshida, T, Elnaggar, R, Hayashi, M, Watanabe, R, et al. Effect of hydrodynamics-based gene delivery of plasmid DNA encoding interleukin-1 receptor antagonist-Ig for treatment of rat autoimmune myocarditis: possible mechanism for lymphocytes and noncardiac cells. Circulation. (2005) 111:1593–600. doi: 10.1161/01.CIR.0000160348.75918.CA
140.McKay, T, Reynolds, P, Jezzard, S, Curiel, D, and Coutelle, C. Secretin-mediated gene delivery, a specific targeting mechanism with potential for treatment of biliary and pancreatic disease in cystic fibrosis. Mol Ther. (2002) 5:447–54. doi: 10.1006/mthe.2002.0560
141.Simeoni, F, Morris, MC, Heitz, F, and Divita, G. Insight into the mechanism of the peptide-based gene delivery system MPG: implications for delivery of siRNA into mammalian cells. Nucleic Acids Res. (2003) 31:2717–24. doi: 10.1093/nar/gkg385
142.Corridon, PR, Karam, SH, Khraibi, AA, Khan, AA, and Alhashmi, MA. Intravital imaging of real-time endogenous actin dysregulation in proximal and distal tubules at the onset of severe ischemia-reperfusion injury. Sci Rep. (2021) 11:8280. doi: 10.1038/s41598-021-87807-6
143.Filipowicz, W. RNAi: the nuts and bolts of the RISC machine. Cells. (2005) 122:17–20. doi: 10.1016/j.cell.2005.06.023
144.Pratt, AJ, and MacRae, IJ. The RNA-induced silencing complex: a versatile gene-silencing machine. J Biol Chem. (2009) 284:17897–901. doi: 10.1074/jbc.R900012200
145.Zhang, X, Edwards, JP, and Mosser, DM. The expression of exogenous genes in macrophages: obstacles and opportunities. Methods Mol Biol. (2009) 531:123–43. doi: 10.1007/978-1-59745-396-7_9
146.Arrighi, N. (ed.). “3 - Stem Cells at the Core of Cell Therapy,” in Stem Cells. Amsterdam: Elsevier. (2018). 73–100.
147.Crystal, RG. Adenovirus: the first effective in vivo gene delivery vector. Hum Gene Ther. (2014) 25:3–11. doi: 10.1089/hum.2013.2527
148.Manfredsson, FP, Lewin, AS, and Mandel, RJ. RNA knockdown as a potential therapeutic strategy in Parkinson's disease. Gene Ther. (2006) 13:517–24. doi: 10.1038/sj.gt.3302669
149.Katz, MG, Fargnoli, AS, Williams, RD, and Bridges, CR. Gene therapy delivery systems for enhancing viral and nonviral vectors for cardiac diseases: current concepts and future applications. Hum Gene Ther. (2013) 24:914–27. doi: 10.1089/hum.2013.2517
150.Nayerossadat, N, Maedeh, T, and Ali, PA. Viral and nonviral delivery systems for gene delivery. Adv Biomed Res. (2012) 1:27. doi: 10.4103/2277-9175.98152
151.Kamimura, K, Suda, T, Zhang, G, and Liu, D. Advances in gene delivery systems. Pharmaceut Med. (2011) 25:293–306. doi: 10.2165/11594020-000000000-00000
152.Bhattacharya, S, Saini, M, Bisht, D, Rana, M, Bachan, R, Gogoi, SM, et al. Lentiviral-mediated delivery of classical swine fever virus Erns gene into porcine kidney-15 cells for production of recombinant ELISA diagnostic antigen. Mol Biol Rep. (2019) 46:3865–76. doi: 10.1007/s11033-019-04829-0
153.Huang, S, Ren, Y, Wang, X, Lazar, L, Ma, S, Weng, G, et al. Application of ultrasound-targeted microbubble destruction–mediated exogenous gene transfer in treating various renal diseases. Hum Gene Ther. (2018) 30:127–38. doi: 10.1089/hum.2018.070
154.Kolb, AL, Corridon, PR, Zhang, S, Xu, W, Witzmann, FA, Collett, JA, et al. Exogenous gene transmission of Isocitrate dehydrogenase 2 mimics ischemic preconditioning protection. J Am Soc Nephrol. (2018) 29:1154–64. doi: 10.1681/ASN.2017060675
155.Nakamura, A, Imaizumi, A, Niimi, R, Yanagawa, Y, Kohsaka, T, and Johns, EJ. Adenoviral delivery of the beta2-adrenoceptor gene in sepsis: a subcutaneous approach in rat for kidney protection. Clin Sci (Lond). (2005) 109:503–11. doi: 10.1042/CS20050088
156.Verkman, AS, and Yang, B. Aquaporin gene delivery to kidney. Kidney Int. (2002) 61:S120–4. doi: 10.1046/j.1523-1755.2002.0610s1120.x
157.Imai, E, and Isaka, Y. Strategies of gene transfer to the kidney. Kidney Int. (1998) 53:264–72. doi: 10.1046/j.1523-1755.1998.00768.x
158.Boletta, A, Benigni, A, Lutz, J, Remuzzi, G, Soria, MR, and Monaco, L. Nonviral gene delivery to the rat kidney with polyethylenimine. Hum Gene Ther. (1997) 8:1243–51. doi: 10.1089/hum.1997.8.10-1243
159.Kapturczak, MH, Chen, S, and Agarwal, A. Adeno-associated virus vector-mediated gene delivery to the vasculature and kidney. Acta Biochim Pol. (2005) 52:293–9. doi: 10.18388/abp.2005_3442
160.Ramirez-Gordillo, D, Trujillo-Provencio, C, Knight, VB, and Serrano, EE. Optimization of gene delivery methods in Xenopus laevis kidney (A6) and Chinese hamster ovary (CHO) cell lines for heterologous expression of Xenopus inner ear genes. In Vitro Cell Dev Biol Anim. (2011) 47:640–52. doi: 10.1007/s11626-011-9451-2
161.Tanner, GA, Sandoval, RM, Molitoris, BA, Bamburg, JR, and Ashworth, SL. Micropuncture gene delivery and intravital two-photon visualization of protein expression in rat kidney. Am J Physiol Ren Physiol. (2005) 289:F638–43. doi: 10.1152/ajprenal.00059.2005
162.Wang, Y, Cui, H, Li, K, Sun, C, Du, W, Cui, J, et al. A magnetic nanoparticle-based multiple-gene delivery system for transfection of porcine kidney cells. PLoS One. (2014) 9:e102886. doi: 10.1371/journal.pone.0102886
163.Xing, Y, Pua, EC, Lu, X, and Zhong, P. Low-amplitude ultrasound enhances hydrodynamic-based gene delivery to rat kidney. Biochem Biophys Res Commun. (2009) 386:217–22. doi: 10.1016/j.bbrc.2009.06.020
164.Zhu, G, Nicolson, AG, Zheng, XX, Strom, TB, and Sukhatme, VP. Adenovirus-mediated beta-galactosidase gene delivery to the liver leads to protein deposition in kidney glomeruli. Kidney Int. (1997) 52:992–9. doi: 10.1038/ki.1997.421
165.Friedmann, T. A new serious adverse event in a gene therapy study. Mol Ther. (2007) 15:1899–900. doi: 10.1038/sj.mt.6300328
166.Gonçalves, GAR, and Paiva, RMA. Gene therapy: advances, challenges and perspectives. Einstein. (2017) 15:369–75. doi: 10.1590/s1679-45082017rb4024
167.Tremblay, JP, Annoni, A, and Suzuki, M. Three decades of clinical gene therapy: from experimental technologies to viable treatments. Mol Ther. (2021) 29:411–2. doi: 10.1016/j.ymthe.2021.01.013
168.Goswami, R, Subramanian, G, Silayeva, L, Newkirk, I, Doctor, D, and Chawla, K. Gene therapy leaves a vicious cycle. Front Oncol. (2019) 9:297. doi: 10.3389/fonc.2019.00297
169.Cai, N, Lai, AC-K, Liao, K, Corridon, PR, Graves, DJ, and Chan, V. Recent advances in fluorescence recovery after photobleaching for decoupling transport and kinetics of biomacromolecules in cellular physiology. Polymers. (2022) 14:1913. doi: 10.3390/polym14091913
170.Shaya, J, Corridon, PR, al-Omari, B, Aoudi, A, Shunnar, A, Mohideen, MIH, et al. Design, photophysical properties, and applications of fluorene-based fluorophores in two-photon fluorescence bioimaging: a review. J Photochem Photobiol C: Photochem Rev. (2022) 52:100529. doi: 10.1016/j.jphotochemrev.2022.100529
171.Hickson, LJ, Eirin, A, and Lerman, LO. Challenges and opportunities for stem cell therapy in patients with chronic kidney disease. Kidney Int. (2016) 89:767–78. doi: 10.1016/j.kint.2015.11.023
172.Moore, MAS, Leonard, JP, Flasshove, M, Bertino, J, Gallardo, H, and Sadelain, M. Gene therapy - the challenge for the future. Ann Oncol. (1996) 7:53–8. doi: 10.1093/annonc/7.suppl_2.53
173.Oshimura, M, Kazuki, Y, and Uno, N. Challenge toward gene-therapy using iPS cells for Duchenne muscular dystrophy. Rinsho Shinkeigaku. (2012) 52:1139–42. doi: 10.5692/clinicalneurol.52.1139
174.Sousa, F, Passarinha, L, and Queiroz, JA. Biomedical application of plasmid DNA in gene therapy: a new challenge for chromatography. Biotechnol Genet Eng Rev. (2010) 26:83–116.
175.Stokman, MF, Renkema, KY, Giles, RH, Schaefer, F, Knoers, NVAM, and van Eerde, AM. The expanding phenotypic spectra of kidney diseases: insights from genetic studies. Nat Rev Nephrol. (2016) 12:472–83. doi: 10.1038/nrneph.2016.87
176.Chen, P, Chen, BK, Mosoian, A, Hays, T, Ross, MJ, Klotman, PE, et al. Virological synapses allow HIV-1 uptake and gene expression in renal tubular epithelial cells. J Am Soc Nephrol. (2011) 22:496–507. doi: 10.1681/ASN.2010040379
177.Gusella, GL, Fedorova, E, Marras, D, Klotman, PE, and Klotman, ME. In vivo gene transfer to kidney by lentiviral vector. Kidney Int. (2002) 61:S32–6. doi: 10.1046/j.1523-1755.2002.0610s1032.x
178.Deen, WM. What determines glomerular capillary permeability? J Clin Invest. (2004) 114:1412–4. doi: 10.1172/JCI23577
179.Deen, WM, Lazzara, MJ, and Myers, BD. Structural determinants of glomerular permeability. Am J Physiol Ren Physiol. (2001) 281:F579–96. doi: 10.1152/ajprenal.2001.281.4.F579
180.Drumond, MC, and Deen, WM. Structural determinants of glomerular hydraulic permeability. Am J Phys. (1994) 266:F1–F12. doi: 10.1152/ajprenal.1994.266.1.F1
181.Ishida, R, Kami, D, Kusaba, T, Kirita, Y, Kishida, T, Mazda, O, et al. Kidney-specific Sonoporation-mediated gene transfer. Mol Ther. (2016) 24:125–34. doi: 10.1038/mt.2015.171
182.Li, P, Gao, Y, Zhang, J, Liu, Z, Tan, K, Hua, X, et al. Renal interstitial permeability changes induced by microbubble-enhanced diagnostic ultrasound. J Drug Target. (2013) 21:507–14. doi: 10.3109/1061186X.2013.776053
183.Reiser, J, Gupta, V, and Kistler, AD. Toward the development of podocyte-specific drugs. Kidney Int. (2010) 77:662–8. doi: 10.1038/ki.2009.559
184.Appledorn, DM, Seregin, S, and Amalfitano, A. Adenovirus vectors for renal-targeted gene delivery. Contrib Nephrol. (2008) 159:47–62. doi: 10.1159/000125581
185.Ye, X, Jerebtsova, M, Liu, XH, Li, Z, and Ray, PE. Adenovirus-mediated gene transfer to renal glomeruli in rodents. Kidney Int. (2002) 61:S16–23. doi: 10.1046/j.1523-1755.2002.0610s1016.x
186.Ye, X, Liu, XH, Li, Z, and Ray, PE. Efficient gene transfer to rat renal glomeruli with recombinant adenoviral vectors. Hum Gene Ther. (2001) 12:141–8. doi: 10.1089/104303401750061203
187.Imai, E. Gene therapy approach in renal disease in the 21st century. Nephrol Dial Transplant. (2001) 16:26–34. doi: 10.1093/ndt/16.suppl_5.26
188.Moullier, P, Friedlander, G, Calise, D, Ronco, P, Perricaudet, M, and Ferry, N. Adenoviral-mediated gene transfer to renal tubular cells in vivo. Kidney Int. (1994) 45:1220–5. doi: 10.1038/ki.1994.162
189.Brunetti-Pierri, N, Stapleton, GE, Palmer, DJ, Zuo, Y, Mane, VP, Finegold, MJ, et al. Pseudo-hydrodynamic delivery of helper-dependent adenoviral vectors into non-human primates for liver-directed gene therapy. Mol Ther. (2007) 15:732–40. doi: 10.1038/sj.mt.6300102
190.Ashworth, SL, Sandoval, RM, Tanner, GA, and Molitoris, BA. Two-photon microscopy: visualization of kidney dynamics. Kidney Int. (2007) 72:416–21. doi: 10.1038/sj.ki.5002315
191.Suda, T, and Liu, D. Hydrodynamic gene delivery: its principles and applications. Mol Ther. (2007) 15:2063–9. doi: 10.1038/sj.mt.6300314
192.Collett, JA, Corridon, PR, Mehrotra, P, Kolb, AL, Rhodes, GJ, Miller, CA, et al. Hydrodynamic isotonic fluid delivery ameliorates moderate-to-severe ischemia-reperfusion injury in rat kidneys. J Am Soc Nephrol. (2017) 28:2081–92. doi: 10.1681/ASN.2016040404
193.Zhang, G, Gao, X, Song, YK, Vollmer, R, Stolz, DB, Gasiorowski, JZ, et al. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. (2004) 11:675–82. doi: 10.1038/sj.gt.3302210
194.Oyama, N, Kawaguchi, M, Itaka, K, and Kawakami, S. Efficient messenger RNA delivery to the kidney using renal pelvis injection in mice. Pharmaceutics. (2021) 13:1810. doi: 10.3390/pharmaceutics13111810
195.Corridon, P. Hydrodynamic delivery of mitochondrial genes in vivo protects against moderate ischemia-reperfusion injury in the rat kidney. FASEB J. (2014) 28. doi: 10.1096/fasebj.28.1_supplement.690.17
196.Woodard, LE, Cheng, J, Welch, RC, Williams, FM, Luo, W, Gewin, LS, et al. Kidney-specific transposon-mediated gene transfer in vivo. Sci Rep. (2017) 7:44904. doi: 10.1038/srep44904
197.Corridon, PR. Enhancing the expression of a key mitochondrial enzyme at the inception of ischemia-reperfusion injury can boost recovery and halt the progression of acute kidney injury. Front Physiol. (2023). doi: 10.3389/fphys.2023.1024238
198.Imai, E. Gene therapy for the treatment of renal disease: prospects for the future. Curr Opin Nephrol Hypertens. (1997) 6:496–503. doi: 10.1097/00041552-199709000-00015
199.Bonamassa, B, Hai, L, and Liu, D. Hydrodynamic gene delivery and its applications in pharmaceutical research. Pharm Res. (2011) 28:694–701. doi: 10.1007/s11095-010-0338-9
200.Rocca, CJ, Ur, SN, Harrison, F, and Cherqui, S. rAAV9 combined with renal vein injection is optimal for kidney-targeted gene delivery: conclusion of a comparative study. Gene Ther. (2014) 21:618–28. doi: 10.1038/gt.2014.35
201.Zincarelli, C, Soltys, S, Rengo, G, and Rabinowitz, JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. (2008) 16:1073–80. doi: 10.1038/mt.2008.76
202.Yao, J, Fan, Y, Li, Y, and Huang, L. Strategies on the nuclear-targeted delivery of genes. J Drug Target. (2013) 21:926–39. doi: 10.3109/1061186X.2013.830310
203.Lovett, ST. The DNA exonucleases of Escherichia coli. EcoSal Plus. (2011) 4. doi: 10.1128/ecosalplus.4.4.7
204.Dermić, D. Functions of multiple exonucleases are essential for cell viability, DNA repair and homologous recombination in recD mutants of Escherichia coli. Genetics. (2006) 172:2057–69. doi: 10.1534/genetics.105.052076
205.Villalba, M, Ferrari, D, Bozza, A, del Senno, L, and di Virgilio, F. Ionic regulation of endonuclease activity in PC12 cells. Biochem J. (1995) 311:1033–8. doi: 10.1042/bj3111033
206.Schwartz, DA, Quinn, TJ, Thorne, PS, Sayeed, S, Yi, AK, and Krieg, AM. CpG motifs in bacterial DNA cause inflammation in the lower respiratory tract. J Clin Invest. (1997) 100:68–73. doi: 10.1172/JCI119523
207.Frazier, KS. Antisense oligonucleotide therapies: the promise and the challenges from a toxicologic pathologist's perspective. Toxicol Pathol. (2015) 43:78–89. doi: 10.1177/0192623314551840
208.Tenenbaum, L, Lehtonen, E, and Monahan, PE. Evaluation of risks related to the use of adeno-associated virus-based vectors. Curr Gene Ther. (2003) 3:545–65. doi: 10.2174/1566523034578131
209.Thomas, CE, Ehrhardt, A, and Kay, MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. (2003) 4:346–58. doi: 10.1038/nrg1066
210.Maggio, I, Holkers, M, Liu, J, Janssen, JM, Chen, X, and Gonçalves, MAFV. Adenoviral vector delivery of RNA-guided CRISPR/Cas9 nuclease complexes induces targeted mutagenesis in a diverse array of human cells. Sci Rep. (2014) 4:5105. doi: 10.1038/srep05105
211.Uren, AG, Kool, J, Berns, A, and van Lohuizen, M. Retroviral insertional mutagenesis: past, present and future. Oncogene. (2005) 24:7656–72. doi: 10.1038/sj.onc.1209043
Keywords: acute kidney disease, chronic kidney disease, gene therapy, cell therapy, renal
Citation: Corridon PR (2023) Still finding ways to augment the existing management of acute and chronic kidney diseases with targeted gene and cell therapies: Opportunities and hurdles. Front. Med. 10:1143028. doi: 10.3389/fmed.2023.1143028
Received: 12 January 2023; Accepted: 17 February 2023;
Published: 07 March 2023.
Edited by:
Shan Mou, Shanghai Jiao Tong University, ChinaReviewed by:
Darukeshwara Joladarashi, Temple University, United StatesCopyright © 2023 Corridon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter R. Corridon, cGV0ZXIuY29ycmlkb25Aa3UuYWMuYWU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.