- Department of Anesthesiology, Hubei Key Laboratory of Geriatric Anesthesia and Perioperative Brain Health, Wuhan Clinical Research Center for Geriatric Anesthesia, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: Diabetes mellitus is an independent risk factor for postoperative complications. It has been reported that insulin-treated diabetes is associated with increased postoperative mortality compared to non-insulin-treated diabetes after cardiac surgery; however, it is unclear whether this finding is applicable to non-cardiac surgery.
Objective: We aimed to assess the effects of insulin-treated and non-insulin-treated diabetes on short-term mortality after non-cardiac surgery.
Methods: Our study was a systematic review and meta-analysis of observational studies. PubMed, CENTRAL, EMBASE, and ISI Web of Science databases were searched from inception to February 22, 2021. Cohort or case-control studies that provided information on postoperative short-term mortality in insulin-treated diabetic and non-insulin-treated diabetic patients were included. We pooled the data with a random-effects model. The Grading of Recommendations, Assessment, Development, and Evaluation system was used to rate the quality of evidence.
Results: Twenty-two cohort studies involving 208,214 participants were included. Our study suggested that insulin-treated diabetic patients was associated with a higher risk of 30-day mortality than non-insulin-treated diabetic patients [19 studies with 197,704 patients, risk ratio (RR) 1.305; 95% confidence interval (CI), 1.127 to 1.511; p < 0.001]. The studies were rated as very low quality. The new pooled result only slightly changed after seven simulated missing studies were added using the trim-and-fill method (RR, 1.260; 95% CI, 1.076–1.476; p = 0.004). Our results also showed no significant difference between insulin-treated diabetes and non-insulin-treated diabetes regarding in-hospital mortality (two studies with 9,032 patients, RR, 0.970; 95% CI, 0.584–1.611; p = 0.905).
Conclusion: Very-low-quality evidence suggests that insulin-treated diabetes was associated with increased 30-day mortality after non-cardiac surgery. However, this finding is non-definitive because of the influence of confounding factors.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021246752, identifier: CRD42021246752.
1. Introduction
Diabetes comprises a group of metabolic disorders that is characterized by hyperglycemia, and it is a rapidly growing health problem worldwide, affecting the quality of life; diabetes had the ninth highest global mortality rate in 2010 (1–3). The International Diabetes Federation estimated that the prevalence of diabetes among adult women and men was 8.4 and 8.9%, respectively, in 2017. It is estimated the prevalence of diabetes in men and women would have increased to 9.9% by 2045 (4). The proportion of patients with diabetes who were undergoing cardiac surgery and non-cardiac surgery increased from 12.3% in 1995 to 21.2% in 2009 (5), and from 20.3% in 2004 to 25.4% in 2013 (6), respectively.
Compared to patients without diabetes, those with diabetes have a higher risk of postoperative complications, including postoperative pneumonia, wound complications, delayed wound healing, unplanned readmission, unplanned reoperation, and extended length of hospital stay (7–14). Diabetes can be divided into insulin-treated diabetes and non-insulin-treated diabetes according to the treatment regimen (15). Insulin-treated diabetes is not necessarily type 1 diabetes; it may also be type 2 diabetes that cannot be controlled with oral hypoglycemic drugs (16). Therefore, insulin-treated diabetes may be an indicator of the degree of progression of type 2 diabetes.
Meta-studies have shown that patients with insulin-treated diabetes have a higher short-term postoperative mortality risk compared to those with non-insulin-treated diabetes during cardiac surgery (17, 18). However, no such meta-analysis has been performed for non-cardiac surgery. According to a report, diabetes treated with insulin alone was an independent risk factor for death 30 days after surgery compared with diabetes treated with oral hypoglycemic drugs alone (19). The Revised Cardiac Risk Index included insulin-treated diabetes as a predictor of cardiac risk after non-cardiac surgery (20). Notably, although studies have reported higher short-term postoperative mortality in insulin-treated diabetes than in non-insulin-treated diabetes, there was no correlation between insulin-treated diabetes and postoperative mortality in multivariate logistic regression analysis (21–23). Another study also showed that in elderly patients with coronary artery disease or heart failure, insulin exposure 3 months before surgery was not associated with 30-day mortality after non-cardiac surgery (24). One study held the opposite view (25); among patients with coronary artery disease, diabetes treated with oral hypoglycemic agents was associated with a higher 2-year all-cause mortality than diabetes treated with insulin after non-cardiac surgery (25). To date, there is disagreement regarding postoperative mortality between insulin-treated diabetes and non-insulin-treated diabetes in non-cardiac surgery. Insulin-treated diabetes has been associated with more diabetes-related comorbidities and coexisting medical conditions than non-insulin-treated diabetes, and diabetes-related comorbidities and coexisting medical conditions were independent risk factors for death 30 days after non-cardiac surgery (19, 22, 26).
Therefore, we hypothesized that in non-cardiac surgery, insulin-treated diabetes would not be associated with postoperative mortality compared with non-insulin-treated diabetes after controlling for confounding factors. Patients with insulin-treated diabetes and those with non-insulin-treated diabetes were grouped according to long-term hypoglycemic regimens before admission. Considering that the hypoglycemic regimens of patients with diabetes may change after surgery, we determined the outcome as short-term postoperative mortality, including 30-day mortality and in-hospital mortality. In summary, we performed a meta-analysis of observational studies that assessed the effect of insulin-treated diabetes and non-insulin-treated diabetes on short-term mortality after non-cardiac surgery.
2. Methods
This study was conducted according to the Meta-analyses of Observational Studies in Epidemiology (MOOSE) (27). This study was registered with PROSPERO (CRD42021246752).
2.1. Data sources and search strategy
We comprehensively searched PubMed, EMBASE, CENTRAL (Cochrane Library), and Web of Science databases from inception to February 22, 2021. The retrieval strategies for each database are described in Supplementary Digital Content 1 (see Text document, Supplementary Digital Content 1, which demonstrates search strategies). The reference lists of relevant reviews were also identified.
2.2. Inclusion and exclusion criteria
We included studies that met each of the following criteria: (1) cohort or case-control studies; (2) inclusion of patients with diabetes who were undergoing non-cardiac surgery; (3) availability of information on 30-day mortality or in-hospital mortality regarding insulin-treated diabetes and non-insulin-treated diabetes; (4) insulin-treated diabetes and non-insulin-treated diabetes grouped according to long-term hypoglycemic regimens before admission; (5) insulin-treated diabetes referred to patients with diabetes who were receiving long-term insulin treatment before admission, including a combination of insulin and non-insulin therapy; and (6) non-insulin-treated diabetes referred to patients with diabetes who were receiving long-term non-insulin treatment before admission, excluding those who received only diet or lifestyle modifications.
We excluded studies that met any of the following criteria: (1) patients were not grouped according to pre-admission diabetes treatment regimens; (2) after contacting the authors three times, the relative risk (RR) of death and its 95% confidence interval (CI) or the odds ratio (OR) and its 95% CI or the number of deaths were not available; and (3) duplicate publications, comprising duplicate publications in different languages, duplicate publications of the same data in the same database, and articles published using the same data in the same research. Duplicate publications in different languages were translated into English using online translation software and were included as one study. For articles that collected the same data from the same database and led to repeated publications, priority was given to studies that had controlled for confounding factors. If the effect size adjusted for confounding factors was unavailable, then the latest published study was selected. For articles published using the same data in the same study, all articles were comprehensively analyzed and included as one study.
There were no language restrictions on search strategies. Non-English articles were translated into English using an online translation software.
2.3. Study selection
Two reviewers (RS and JJ) independently screened the titles and abstracts after using document management software to remove duplicate articles. Full-text analyses of studies that met the inclusion criteria were conducted. Finally, the included studies were determined based on the inclusion and exclusion criteria. Any differences were resolved through discussion.
2.4. Data extraction
Regarding the studies to be included, RS and JJ extracted the following data from the studies: the first author, publication year, study type, surgery type, demographic information of patients with insulin-treated diabetes and non-insulin-treated diabetes, RR or OR of postoperative mortality, and adjusted factors. Any differences were resolved through discussion. We extracted the death outcomes of different surgical types reported in a study.
2.5. Quality assessment
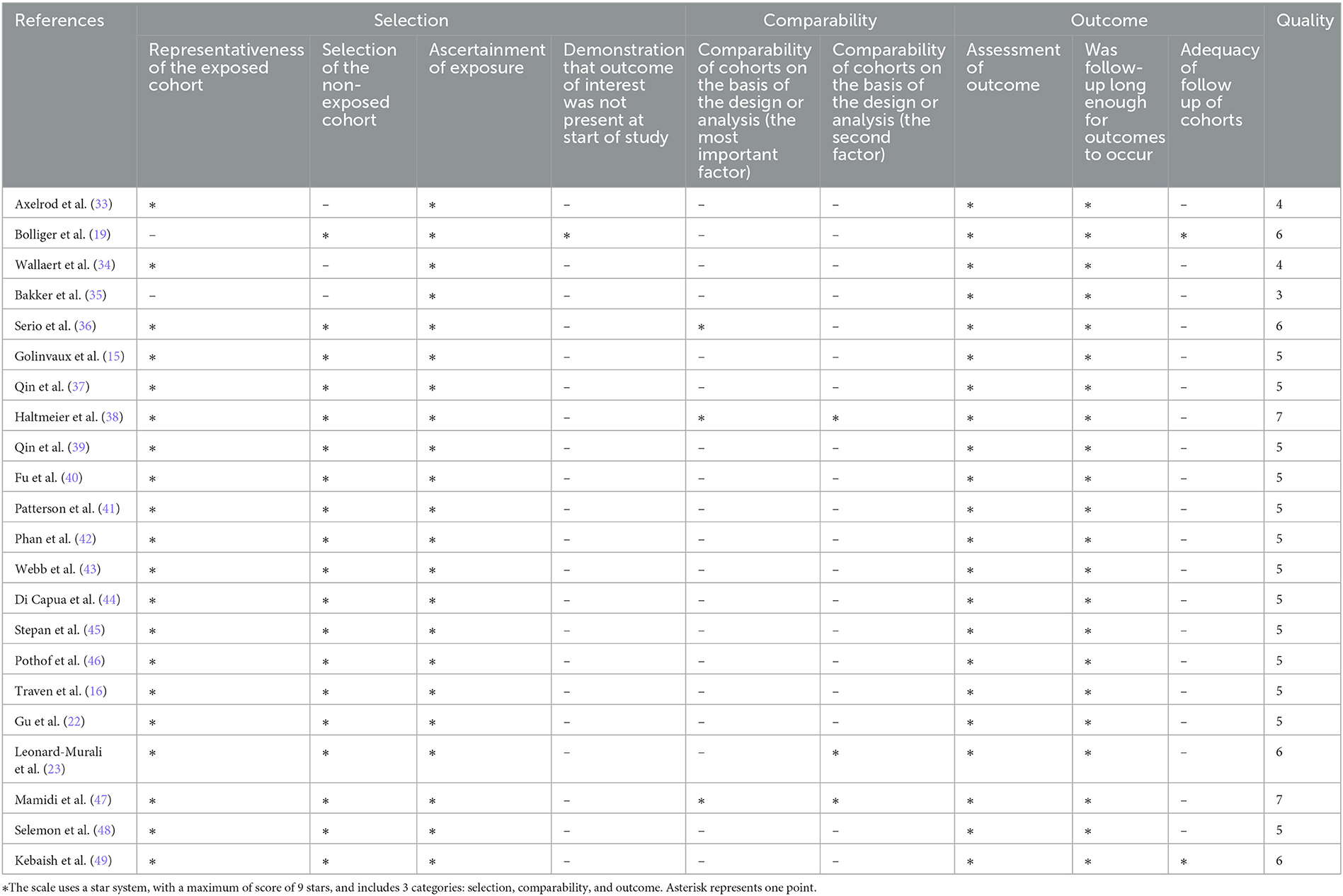
The Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of the studies (28). A study with a score of ≥7 points was considered high quality (29, 30). In the comparability score criteria, complications and comorbidities of patients with diabetes at admission were considered the most important confounding factors. Age and American Society of Anesthesiologists (ASA) scores were considered the second most important confounding factors.
2.6. Data analyses
In the present study, 30-day mortality was the primary outcome, and hospital mortality was the secondary outcome. We chose the adjusted RR or OR of death to pool the data. Since the 30-day and hospital mortality after non-cardiac surgery are very low, the OR can be considered an approximate estimate of RR. Therefore, for studies in which the adjusted RR was unavailable, the adjusted OR was extracted. For studies in which neither the adjusted RR nor OR was available, we directly extracted or calculated the crude RR or OR to pool data. Although one study reported the outcome of interest, the number of deaths in both cohorts was zero. Since the RR or OR of this study could not be calculated, the data of this study was not pooled with the data of other studies. However, clinical heterogeneity was unavoidable. Therefore, a random-effects model using the DerSimonian-Laird method was used to pool the data.
A standard chi-squared test with a significance level of α = 0.1 and the I2 statistic were used to assess the magnitude of heterogeneity. P ≤ 0.1 or I2 ≥ 50% was considered to indicate significant heterogeneity. The source of heterogeneity was explored using subgroup and sensitivity analyses. We conducted subgroup analysis by age, surgery type, RR/OR type, NOS comparability score, and mortality type. Sensitivity analysis was performed by restricting the meta-analysis to studies with a NOS score of ≥7 points and studies that controlled for complications and comorbidities of patients with diabetes based on the design or analysis. We excluded studies one by one to assess the impact of a single study. We used the funnel plot, Begg's test, and Egger's test to evaluate publication bias. If publication bias seemed present, we used the trim-and-fill method to describe its potential impact (31). All statistical analyses were performed with STATA version 15.0. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to rate the level of evidence (32).
3. Results
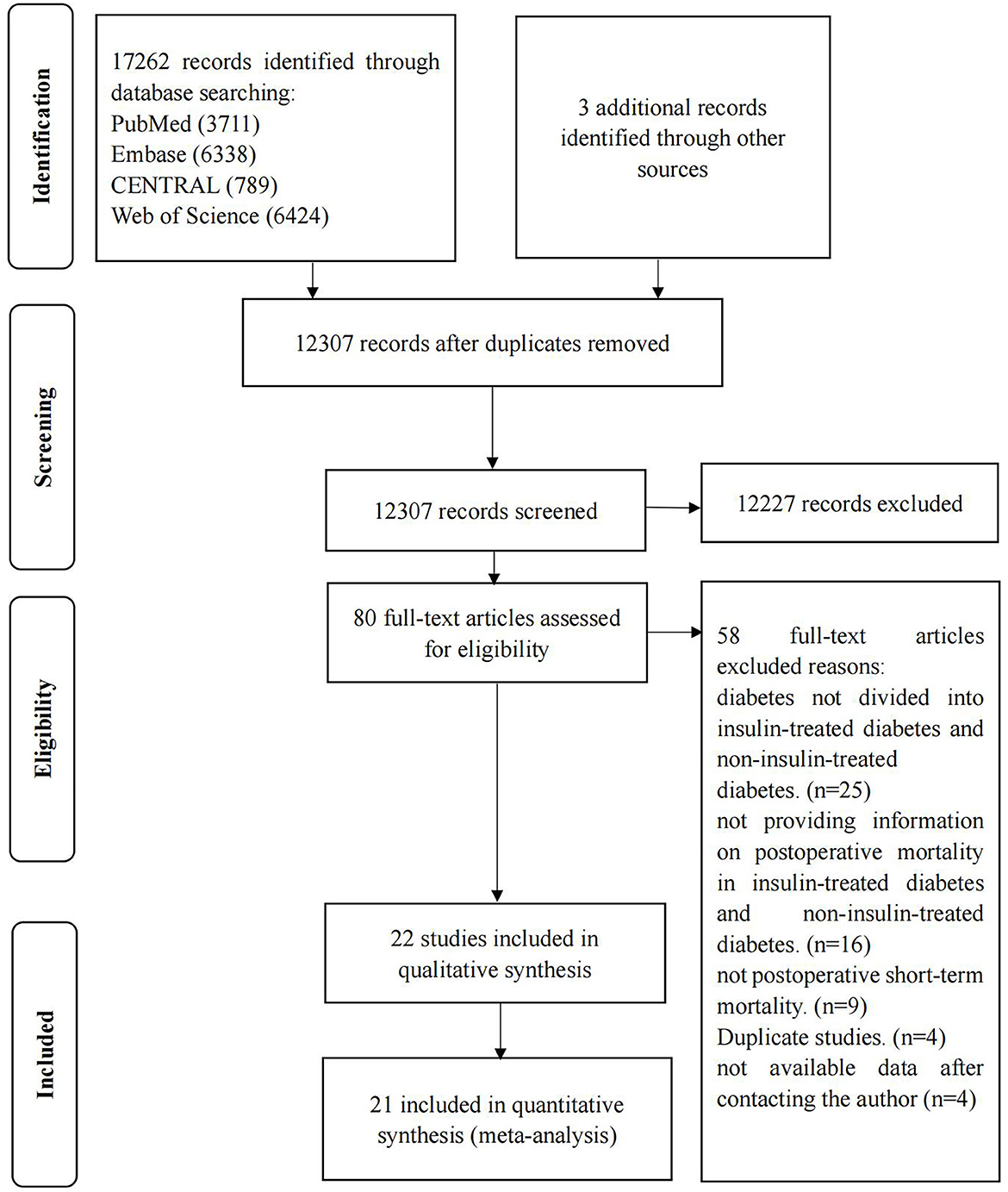
A total of 17,265 studies were retrieved, and 22 studies were eventually included (15, 16, 19, 22, 23, 33–49). Details of the screening process are shown in Figure 1. The excluded articles through full-text evaluation and the reasons for exclusion can be found in Supplemental Digitary Content 2 (see Text document, Supplementary Digital Content 2, which demonstrates excluded studies).
3.1. Characteristics of the included studies
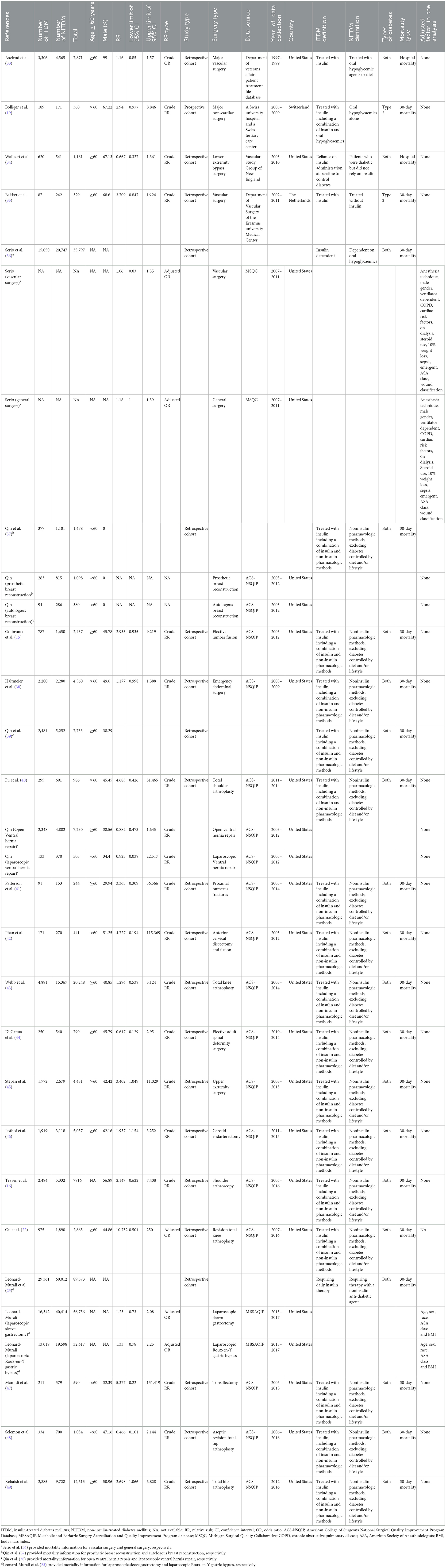
Twenty-two studies were included, involving 208,214 patients with diabetes, of which 70,806 were insulin-treated and 137,408 were non-insulin-treated. The average age of participants in 15 studies was over 60 years (15, 19, 22, 33–35, 38–41, 43–46, 49), while the average age of participants in four studies was <60 years (37, 42, 47, 48). We were unable to determine the average age in three studies (16, 23, 36). The type of surgery in one study was breast reconstruction surgery; therefore, only female patients were included (37). Another study was based on the Department of Veterans Affairs Patient Treatment File database; therefore, 99% of the study population was male (33). In the remaining studies, the proportion of men ranged from 29.94 to 68.6%. Two studies in the study population only included patients with type 2 diabetes (19, 35). Detailed characteristics of the study are presented in Table 1.
The NOS scores of the studies ranged from 4 to 7 points. Most of them scored five points. Only two studies were rated as high quality (38, 47). The details of the NOS scores are shown in Table 2. Regarding the comparability score, only three studies controlled for the most important confounding factor (36, 38, 47), and only three studies controlled for the second most important confounding factor (23, 38, 47).
3.2. Thirty-day mortality
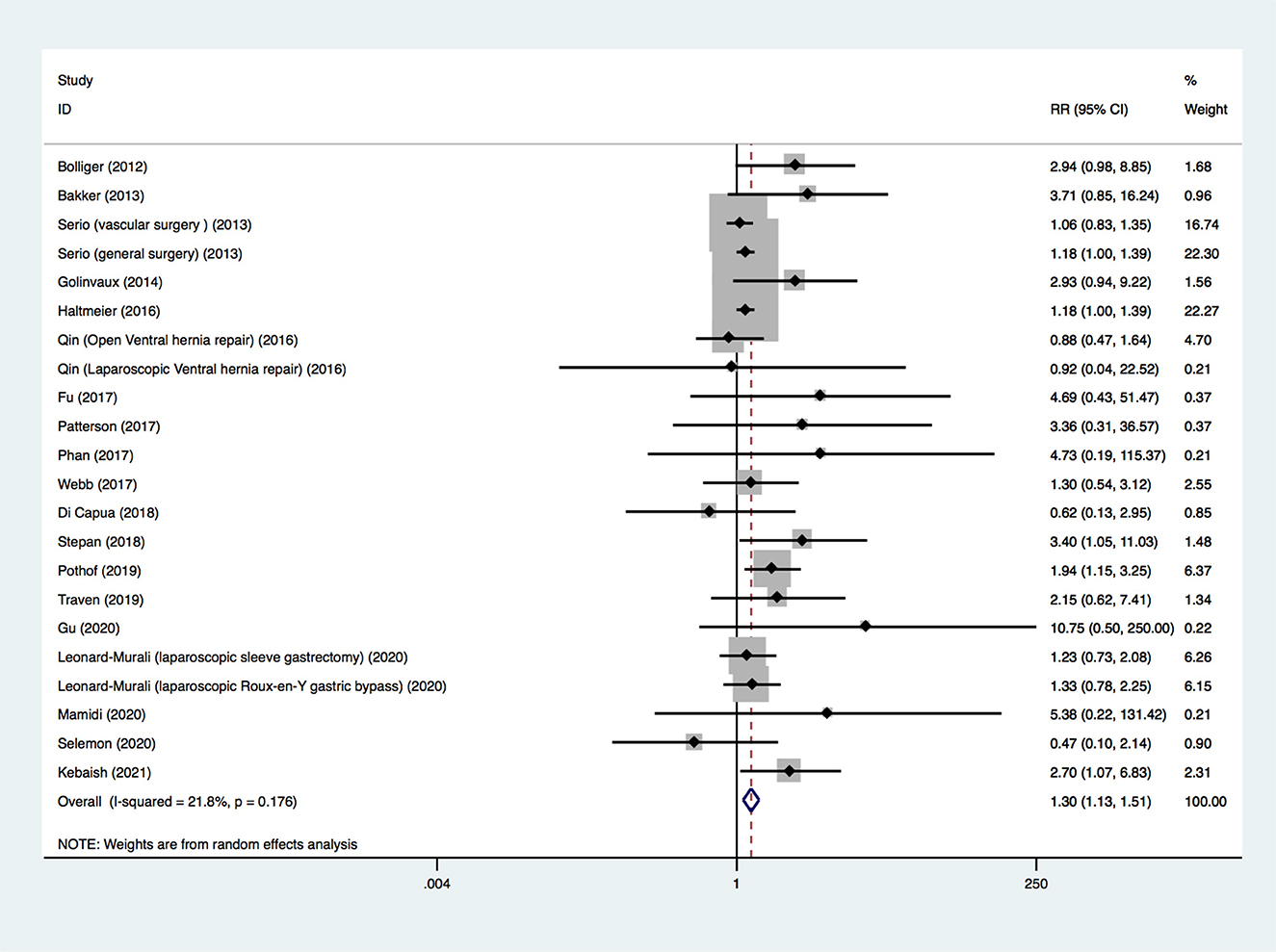
The 20 included studies provided information on the outcome of 30-day mortality. Because four studies provided death outcomes for different types of surgery (23, 36, 37, 39), there were 24 groups of postoperative death data. Because the death outcomes of insulin-treated diabetes and non-insulin-treated diabetes in one study were both zero events (37), the data from this study could not be combined with data from other studies. Finally, 22 sets of data (19 studies, 206,736 participants) were pooled. Among these 22 sets, five were the adjusted OR, and the remaining 17 were the crude RR. A random-effects model was used to pool all the data, and a significant difference was found (RR, 1.305; 95% CI, 1.127–1.511; p < 0.001; Figure 2), suggesting that insulin-treated diabetes had a higher risk of 30-day mortality compared to non-insulin-treated diabetes. The quality of evidence was rated as very low. The details of the GRADE summary of the findings are shown in Supplementary Digital Content 3 (see Table, Supplementary Digital Content 3, which demonstrates GRADE).
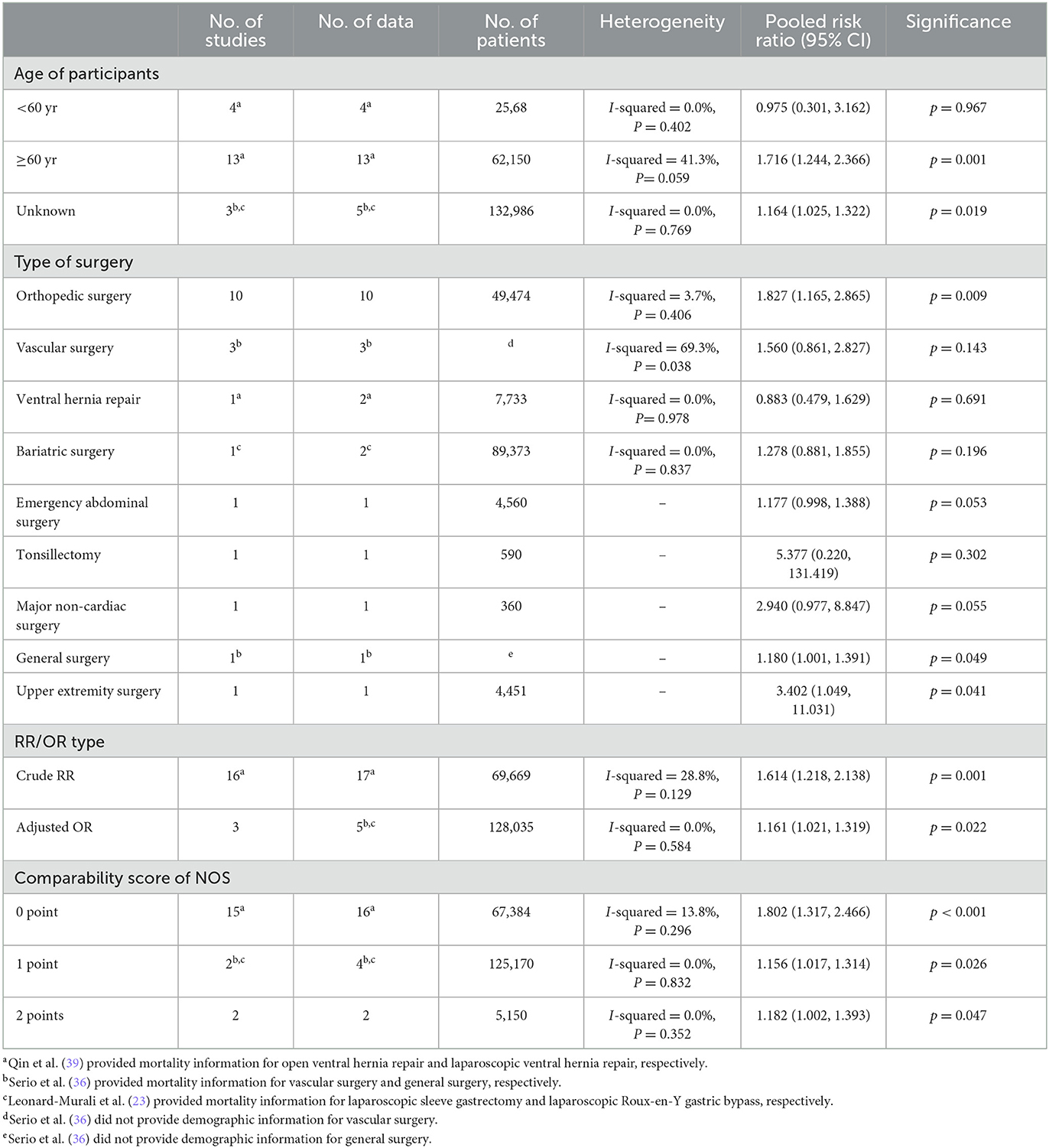
We conducted subgroup analyses by the age of participants, type of surgery, RR/OR type, and comparability score of NOS (Supplemental Digital Content 4). In the subgroups of aged ≥ 60 years, orthopedic surgery, general surgery, upper extremity surgery, crude RR, adjusted OR, comparability score of 0-point, 1-point and 2-point, the results showed a higher risk of 30-day mortality of insulin-treated diabetes compared to non-insulin-treated diabetes. In the subgroups of aged <60 years, vascular surgery, ventral hernia repair, bariatric surgery, emergency abdominal surgery, tonsillectomy, major non-cardiac surgery, the results showed a similar 30-day mortality between insulin-treated diabetes and non-insulin-treated diabetes.
The details of the subgroup analysis are presented in Table 3.
We also conducted sensitivity analysis. Limiting the analysis to studies with a NOS score ≥ 7, the results were consistent with the original analysis (RR, 1.182; 95% CI, 1.002–1.393; p = 0.047). Limiting the analysis to the studies that controlled for complications and comorbidities of patients with diabetes based on the design or analysis, the results were consistent with the original analysis (RR, 1.157; 95% CI, 1.042–1.286; p = 0.006). The included studies were excluded one by one, and no single study had a noticeable influence on the total combined effect size (see Text document, Supplementary Digital Content 4, which demonstrates sensitivity analysis).
The publication bias was evaluated. The funnel plot appeared asymmetrical (see Text document, Supplementary Digital Content 5, which demonstrates funnel plot). Significant publication bias was detected using Egger's test (bias = 0.88; 95% CI, 0.31–1.44; p = 0.004). After using the trim-and-fill method, the new pooled effect size from the random-effects model was similar to the primary result (RR, 1.260; 95% CI, 1.076–1.476; p = 0.004). A new funnel plot after adding seven simulated missing studies is presented in Supplementary Digital Content 6 (see Text document, Supplementary Digital Content 6, which demonstrates new funnel plot).
3.3. Hospital mortality
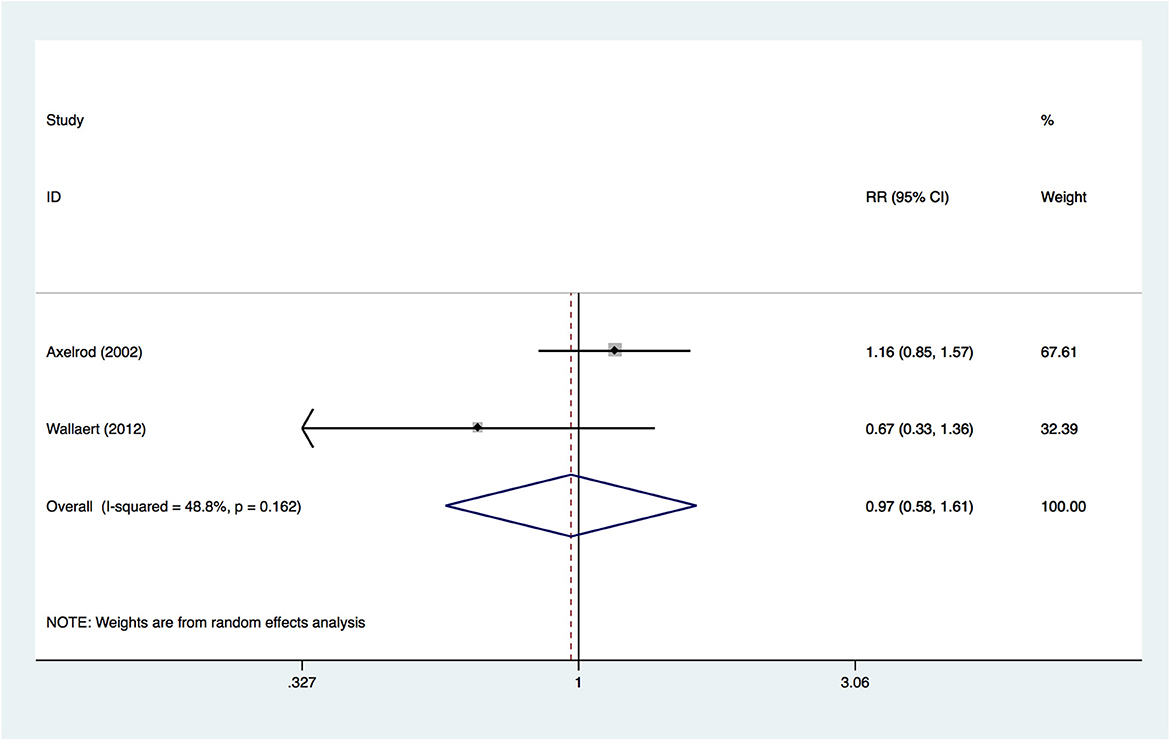
Two included studies provided information on the outcome of hospital mortality. A random-effects model was used to pool the data, and no significant difference was found (RR, 0.970; 95% CI, 0.584–1.611; p = 0.905; Figure 3) between insulin-treated diabetes and non-insulin-treated diabetes, suggesting that the two groups had a similar hospital mortality. The quality of evidence was rated as very low. The details of the GRADE summary of the findings are shown in Supplementary Digital Content 3 (see Table, Supplementary Digital Content 3, which demonstrates GRADE).
As only two studies were included in the outcome, we did not conduct subgroup analysis, sensitivity analysis or evaluate publication bias.
4. Discussion
Our study demonstrated that compared with non-insulin-treated diabetes, insulin-treated diabetes was associated with a higher risk of 30-day mortality, but a similar risk of in-hospital mortality after surgery. The quality of the evidence was rated as very low.
4.1. Comparison with the published literature
Our findings are consistent with two previously published systematic reviews of cardiac surgery that found that insulin-treated diabetes had a significantly higher risk of short-term mortality (<1 year) after percutaneous coronary intervention (18) and a significantly higher risk of short-term mortality (≤ 30 days) after coronary artery bypass surgery compared with non-insulin-treated diabetes (17).
Although all the included studies provided postoperative death outcomes for insulin-treated diabetes and non-insulin-treated diabetes, only seven studies specifically compared postoperative mortality outcomes between insulin-treated and non-insulin-treated diabetes (19, 22, 23, 33, 36, 38, 47). Only one study found that insulin-treated diabetes was associated with increased postoperative mortality compared to non-insulin-treated diabetes (19). However, in this study, the relationship only held for insulin-only diabetes, and did not include diabetes treated both with and without insulin.
It has also been reported that neither diabetes nor insulin exposure is an independent risk factor for death at 30 days after non-cardiac surgery in 65-year-old patients with coronary artery disease or heart failure (24). Among studies with longer follow-up times after non-cardiac surgery, some studies found insulin-treated diabetes to be an independent risk factor for postoperative death compared with non-insulin-treated diabetes (50, 51), while one study found no association (52). One study found that oral hypoglycemic therapy, but not insulin therapy, was associated with non-cardiac mortality at 2 years in patients at coronary risk (25).
4.2. Strengths
Our study had several strengths. First, to our knowledge, it is the first systematic review comparing short-term postoperative mortality between insulin-treated diabetes and non-insulin-treated diabetes in non-cardiac surgery. Second, we screened 17,265 articles and finally included 22 studies that comprised 208,214 patients with diabetes; thus, our study comprised a notably large number of patients. Third, no significant heterogeneity was found in our study (I2= 22.5%, p = 0.159). Although there was significant publication bias, the new pooled result was only slightly changed after adding the simulated missing study using the trim-and-fill method, suggesting that the effect of publication bias on the result might be small. Fourth, of the studies we included, only two included type 2 diabetes (19, 35). The remaining 21 studies did not clarify the type of diabetes. The patients were grouped based on prehospital hypoglycemic regimens. This shows that our conclusions are practical.
4.3. Implications for clinicians, policy, and research
Our study showed that insulin-treated diabetes and non-insulin-treated diabetes had a similar risk of in-hospital mortality after surgery. However, only two studies were included in the outcome, thus, the findings may be not reliable. Our study also showed that in non-cardiac surgery, the 30-day mortality of insulin-treated patients with diabetes was higher than that of non-insulin-treated diabetes patients. This suggests that prehospital hypoglycemic regimens might be one of the bases for the preoperative risk stratification of patients with diabetes. However, it must be noted that because of the generally low quality of the originally included studies, this conclusion may be unreliable. In particular, only three studies controlled the bias of comorbidities and complications of patients with diabetes in the study design or data analysis. Only one of the originally included studies found that type 2 diabetes treated with insulin alone was an independent risk factor for short-term postoperative death compared with diabetes treated with oral hypoglycemic agents alone. However, the results of the study might be explained by its inclusion of more patients with a history of myocardial infarction, more patients with a history of coronary artery bypass graft, more patients with a history of heart failure, and a higher ASA grade in the insulin-only group. Surprisingly, insulin-treated diabetes was still associated with increased postoperative mortality but with a reduced effect size in our sensitivity analyses that included only studies that controlled for comorbidities and complications and only high-quality studies. This suggests on the one hand that confounders caused an overestimation of the effect size, and on the other hand, long-term prehospital hypoglycemic regimens might indeed influence short-term postoperative mortality. This might be related to the long-term cardiovascular protective effects of oral hypoglycemic agents (53–55).
In published models predicting postoperative cardiac complications for non-cardiac surgery, the Revised Cardiac Risk Index included preoperative treatment with insulin as a risk factor (20), while the Geriatric-Sensitive Perioperative Cardiac Risk Index included diabetes as a risk factor (56). Although it is still not clear whether the hypoglycemic regimen or the severity of the patient's condition leads to increased short-term postoperative mortality, our current results still tended to stratify diabetes patients according to prehospital hypoglycemic regimens and assign insulin-treated diabetes with a higher risk level.
Sensitivity and subgroup analyses with adjusted effect sizes showed that insulin-treated diabetes was an independent risk factor. However, in the subgroup analysis of surgical types, only the subgroup of orthopedic surgery and vascular surgery could be further analyzed, while the number of studies on other surgical types was too small to be further analyzed. In the vascular surgery subgroup, insulin-treated diabetes was not associated with increased 30-day mortality. This suggests that there is a need to further distinguish the risks of long-term hypoglycemic regimens between different surgical types. There was no association between insulin-treated diabetes and 30-day mortality in the subgroup of age <60 years. This indicates that our results may not be applicable to all populations. It is also possible that the severity of diabetes might be similar in patients undergoing vascular surgery or in patients aged <60 years. However, our current research was unable to answer this question; thus, well-designed and high-quality research are required to answer these questions in the future.
5. Limitation
The most notable limitation of our study is that the quality of most of the originally included studies was not high. Of the 22 studies, only two were rated as high-quality studies. Diabetes-related complications and comorbidities were independent risk factors for non-cardiac postoperative 30-day mortality (26). Age and ASA grade were also listed as risk factors for major adverse cardiovascular events and mortality within 30 days after surgery (56–58). Therefore, we regarded diabetes-related complications and comorbidities as the most important confounding factors, while age and ASA grade were the second most important confounding factors. Of the 22 studies included, only three controlled for diabetes-related complications and comorbidities, and only three controlled for age and ASA classification. The evidence quality rating was very low. This showed that the results obtained are uncertain.
In the subgroup analysis, the results of the vascular surgery and age <60 subgroups were not consistent with the original analysis. Thus, our study conclusions may not be applicable to all surgery types and populations.
6. Conclusion
Our study showed that insulin-treated diabetes was associated with an increased risk of 30-day mortality after non-cardiac surgery compared with non-insulin-treated diabetes. The quality of the evidence was rated as very low. As it is difficult to rule out the influence of confounding factors, we believe that the results obtained are not reliable. Well-designed, high-quality studies that controls for important confounding factors is needed to investigate the impact of prehospital hypoglycemic regimens on short-term mortality after non-cardiac surgery.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JJ and SW: methodology and writing—original draft preparation. RS and SL: data curation and writing—review and editing. YZ and ZZ: methodology and writing—editing. JB: data curation and funding acquisition. AL: funding acquisition and supervision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by China National Key R&D (Program No. 2020YFC2009002) and grants from the National Natural Science Foundation of China (Grant Nos. 81771159 and 81974160 to AL, 82001161 to JB).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1142490/full#supplementary-material
References
1. Nyberg ST, Fransson EI, Heikkila K, Ahola K, Alfredsson L, Bjorner JB, et al. Job strain as a risk factor for type 2 diabetes: a pooled analysis of 124,808 men and women. Diabetes Care. (2014) 37:2268–75. doi: 10.2337/dc13-2936
2. Preiser JC, Provenzano B, Mongkolpun W, Halenarova K, Cnop M. Perioperative management of oral glucose-lowering drugs in the patient with type 2 diabetes. Anesthesiology. (2020) 133:430–8. doi: 10.1097/ALN.0000000000003237
3. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. (2012) 380:2095–128. doi: 10.1016/S0140-6736(12)61728-0
4. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. (2018) 138:271–81. doi: 10.1016/j.diabres.2018.02.023
5. Taylor AH, Mitchell AE, Mitchell IM, A. 15-year study of the changing demographics and infection risk in a new UK cardiac surgery unit. Interact Cardiovasc Thorac Surg. (2012) 15:390–4. doi: 10.1093/icvts/ivs278
6. Newman JD, Wilcox T, Smilowitz NR, Berger JS. Influence of diabetes on trends in perioperative cardiovascular events. Diabetes Care. (2018) 41:1268–74. doi: 10.2337/dc17-2046
7. Vannini P, Ciavarella A, Olmi R, Flammini M, Moroni A, Galuppi V, et al. Diabetes as pro-infective risk factor in total hip replacement. Acta Diabetol Lat. (1984) 21:275–80. doi: 10.1007/BF02642901
8. Sharma A, Muir R, Johnston R, Carter E, Bowden G, Wilson-MacDonald J. Diabetes is predictive of longer hospital stay and increased rate of complications in spinal surgery in the UK. Ann R Coll Surg Engl. (2013) 95:275–9. doi: 10.1308/003588413X13511609958299
9. Lopez-de-Andres A, Hernandez-Barrera V, Martinez-Huedo MA, Villanueva-Martinez M, Jimenez-Trujillo I, Jimenez-Garcia R. Type 2 diabetes and in-hospital complications after revision of total hip and knee arthroplasty. PLoS ONE. (2017) 12:e0183796. doi: 10.1371/journal.pone.0183796
10. Worley N, Buza J, Jalai CM, Poorman GW, Day LM, Vira S, et al. Diabetes as an independent predictor for extended length of hospital stay and increased adverse post-operative events in patients treated surgically for cervical spondylotic myelopathy. Int J Spine Surg. (2017) 11:10. doi: 10.14444/4010
11. Lanzetti RM, Lupariello D, Venditto T, Guzzini M, Ponzo A, De Carli A, et al. The role of diabetes mellitus and BMI in the surgical treatment of ankle fractures. Diabetes Metab Res Rev. (2018) 34. doi: 10.1002/dmrr.2954
12. Liu JW, Ahn J, Raspovic KM, Liu GT, Nakonezny PA, Lavery LA, et al. Increased rates of readmission, reoperation, and mortality following open reduction and internal fixation of ankle fractures are associated with diabetes mellitus. J Foot Ankle Surg. (2019) 58:470–4. doi: 10.1053/j.jfas.2018.09.023
13. Lopez-de-Andres A, Perez-Farinos N, de Miguel-Diez J, Hernandez-Barrera V, Jimenez-Trujillo I, Mendez-Bailon M, et al. Type 2 diabetes and postoperative pneumonia: An observational, population-based study using the Spanish Hospital Discharge Database, 2001-2015. PLoS ONE. (2019) 14:e0211230. doi: 10.1371/journal.pone.0211230
14. Ma CM, Liu Q, Li ML Ji MJ, Zhang JD, Zhang BH, et al. The effects of type 2 diabetes and postoperative pneumonia on the mortality in inpatients with surgery. Diabetes Metab Syndr Obes. (2019) 12:2507–13. doi: 10.2147/DMSO.S232039
15. Golinvaux NS, Varthi AG, Bohl DD, Basques BA, Grauer JN. Complication rates following elective lumbar fusion in patients with diabetes: insulin dependence makes the difference. Spine. (2014) 39:1809–16. doi: 10.1097/BRS.0000000000000506
16. Traven SA, Reeves RA, Walton ZJ, Woolf SK, Slone HS. Insulin dependence is associated with increased medical complications and mortality after shoulder arthroscopy. Arthroscopy. (2019) 35:1316–21. doi: 10.1016/j.arthro.2018.11.059
17. Munnee K, Bundhun PK, Quan H, Tang Z. Comparing the clinical outcomes between insulin-treated and non-insulin-treated patients with type 2 diabetes mellitus after coronary artery bypass surgery: a systematic review and meta-analysis. Medicine. (2016) 95:e3006. doi: 10.1097/MD.0000000000003006
18. Bundhun PK Li N, Chen MH. Adverse cardiovascular outcomes between insulin-treated and non-insulin treated diabetic patients after percutaneous coronary intervention: a systematic review and meta-analysis. Cardiovasc Diabetol. (2015) 14:135. doi: 10.1186/s12933-015-0300-6
19. Bolliger D, Seeberger MD, Lurati Buse G, Christen P, Seeberger E, Ruppen W, et al. The influence of pre-admission hypoglycaemic therapy on cardiac morbidity and mortality in type 2 diabetic patients undergoing major non-cardiac surgery: a prospective observational study. Anaesthesia. (2012) 67:149–57. doi: 10.1111/j.1365-2044.2011.06963.x
20. Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. (1999) 100:1043–9. doi: 10.1161/01.CIR.100.10.1043
21. Karamanos E, Sivrikoz E, Beale E, Chan L, Inaba K, Demetriades D. Effect of diabetes on outcomes in patients undergoing emergent cholecystectomy for acute cholecystitis. World J Surg. (2013) 37:2257–64. doi: 10.1007/s00268-013-2086-6
22. Gu A, Wei C, Robinson HN, Sobrio SA, Liu J, Sculco TP, et al. Postoperative complications and impact of diabetes mellitus severity on revision total knee arthroplasty. J Knee Surg. (2020) 33:228–34. doi: 10.1055/s-0038-1677542
23. Leonard-Murali S, Nasser H, Ivanics T, Shakaroun D, Genaw J. Perioperative outcomes of Roux-en-Y gastric bypass and sleeve gastrectomy in patients with diabetes mellitus: an analysis of the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) Database. Obes Surg. (2020) 30:111–8. doi: 10.1007/s11695-019-04175-x
24. Hanninen M, McAlister FA, Bakal JA, van Diepen S, Ezekowitz JA. Neither diabetes nor glucose-lowering drugs are associated with mortality after noncardiac surgery in patients with coronary artery disease or heart failure. Can J Cardiol. (2013) 29:423–8. doi: 10.1016/j.cjca.2012.07.004
25. Jeger RV, Seeberger MD, Keller U, Pfisterer ME, Filipovic M. Oral hypoglycemics: increased postoperative mortality in coronary risk patients. Cardiology. (2007) 107:296–301. doi: 10.1159/000099065
26. Yeh C-C, Liao C-C, Chang Y-C, Jeng L-B, Yang H-R, Shih C-C, et al. Adverse outcomes after noncardiac surgery in patients with diabetes: a nationwide population-based retrospective cohort study. Diabetes Care. (2013) 36:3216–21. doi: 10.2337/dc13-0770
27. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
28. Wells BS, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed February 1, 2021).
29. Yuhara H, Steinmaus C, Cohen SE, Corley DA, Tei Y, Buffler PA. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am J Gastroenterol. (2011) 106:1911–21; quiz 22. doi: 10.1038/ajg.2011.301
30. Yang Y, Zhang D, Feng N, Chen G, Liu J, Chen G, et al. Increased intake of vegetables, but not fruit, reduces risk for hepatocellular carcinoma: a meta-analysis. Gastroenterology. (2014) 147:1031–42. doi: 10.1053/j.gastro.2014.08.005
31. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
32. Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res. (2004) 4:38. doi: 10.1186/1472-6963-4-38
33. Axelrod DA, Upchurch GR Jr, DeMonner S, Stanley JC, Khuri S, Daley J, et al. Perioperative cardiovascular risk stratification of patients with diabetes who undergo elective major vascular surgery. J Vasc Surg. (2002) 35:894–901. doi: 10.1067/mva.2002.123681
34. Wallaert JB, Nolan BW, Adams J, Stanley AC, Eldrup-Jorgensen J, Cronenwett JL, et al. The impact of diabetes on postoperative outcomes following lower-extremity bypass surgery. J Vasc Surg. (2012) 56:1317–23. doi: 10.1016/j.jvs.2012.04.011
35. Bakker EJ, Valentijn TM, van de Luijtgaarden KM, Hoeks SE, Voute MT, Goncalves FB, et al. Type 2 diabetes mellitus, independent of insulin use, is associated with an increased risk of cardiac complications after vascular surgery. Anaesth Intens Care. (2013) 41:584–90. doi: 10.1177/0310057X1304100515
36. Serio S, Clements JM, Grauf D, Merchant AM. Outcomes of diabetic and nondiabetic patients undergoing general and vascular surgery. ISRN Surg. (2013) 2013:963930. doi: 10.1155/2013/963930
37. Qin C, Vaca E, Lovecchio F, Ver Halen JP, Hansen NM, Kim JY. Differential impact of non-insulin-dependent diabetes mellitus and insulin-dependent diabetes mellitus on breast reconstruction outcomes. Breast Cancer Res Treat. (2014) 146:429–38. doi: 10.1007/s10549-014-3024-5
38. Haltmeier T, Benjamin E, Beale E, Inaba K, Demetriades D. Insulin-treated patients with diabetes mellitus undergoing emergency abdominal surgery have worse outcomes than patients treated with oral agents. World J Surg. (2016) 40:1575–82. doi: 10.1007/s00268-016-3469-2
39. Qin C, Souza J, Aggarwal A, Kim JY. Insulin dependence as an independent predictor of perioperative morbidity after ventral hernia repair: a National Surgical Quality Improvement Program analysis of 45,759 patients. Am J Surg. (2016) 211:11–7. doi: 10.1016/j.amjsurg.2014.08.046
40. Fu MC, Boddapati V, Dines DM, Warren RF, Dines JS, Gulotta LV. The impact of insulin dependence on short-term postoperative complications in diabetic patients undergoing total shoulder arthroplasty. J Shoulder Elbow Surg. (2017) 26:2091–6. doi: 10.1016/j.jse.2017.05.027
41. Patterson DC, Shin JI, Andelman SM, Olujimi V, Parsons BO. Increased risk of 30-day postoperative complications for diabetic patients following open reduction-internal fixation of proximal humerus fractures: an analysis of 1391 patients from the American College of Surgeons National Surgical Quality Improvement Program database. JSES Open Access. (2017) 1:19–24. doi: 10.1016/j.jses.2017.03.006
42. Phan K, Kim JS, Lee N, Kothari P, Cho SK. Impact of insulin dependence on perioperative outcomes following anterior cervical discectomy and fusion. Spine. (2017) 42:456–64. doi: 10.1097/BRS.0000000000001829
43. Webb ML, Golinvaux NS, Ibe IK, Bovonratwet P, Ellman MS, Grauer JN. Comparison of perioperative adverse event rates after total knee arthroplasty in patients with diabetes: insulin dependence makes a difference. J Arthroplasty. (2017) 32:2947–51. doi: 10.1016/j.arth.2017.04.032
44. Di Capua J, Lugo-Fagundo N, Somani S, Kim JS, Phan K, Lee NJ, et al. Diabetes mellitus as a risk factor for acute postoperative complications following elective adult spinal deformity surgery. Global Spine J. (2018) 8:615–21. doi: 10.1177/2192568218761361
45. Stepan JG, Boddapati V, Sacks HA, Fu MC, Osei DA, Fufa DT. Insulin dependence is associated with increased risk of complications after upper extremity surgery in diabetic patients. J Hand Surg Am. (2018) 43:745–54 e4. doi: 10.1016/j.jhsa.2018.06.006
46. Pothof AB, O'Donnell TFX, Swerdlow NJ, Liang P, Li C, Varkevisser RRB, et al. Risk of insulin-dependent diabetes mellitus in patients undergoing carotid endarterectomy. J Vasc Surg. (2019) 69:814–23. doi: 10.1016/j.jvs.2018.05.250
47. Mamidi IS Li L, Jones JW, Lee R, Rana MS, Reilly BK. Impact of diabetes mellitus following tonsillectomy in adults: A National Surgical Quality Improvement Program Analysis. Ann Otol Rhinol Laryngol. (2021) 130:682–8. doi: 10.1177/0003489420967041
48. Selemon NA, Gu A, Malahias MA, Fassihi SC, Chen AZ, Adriani M, et al. Insulin-dependent diabetes mellitus is an independent risk factor for postoperative complications in aseptic revision total hip arthroplasty. Hip Int. (2022) 32:213–20. doi: 10.1177/1120700020945221
49. Kebaish KJ, Puvanesarajah V, Rao S, Zhang B, Ottesen TD, Grauer JN, et al. Diabetes status affects odds of body mass index-dependent adverse outcomes after total hip arthroplasty. J Am Acad Orthop Surg. (2021) 29:71–7. doi: 10.5435/JAAOS-D-20-00028
50. Lee TC, Lee YL, Chen JC, Chen CH, Ho PS. Impact of type 2 diabetes on postoperative outcome after hip fracture: nationwide population-based study in Taiwan. BMJ Open Diabetes Res Care. (2020) 8:e000843. doi: 10.1136/bmjdrc-2019-000843
51. Ting CT, Chen RC, Chen CC, Liu MH, Chu D, Kuo NW. Diabetes worsens the surgical outcomes in cirrhotic patients with hepatocellular carcinoma. Tohoku J Exp Med. (2012) 227:73–81. doi: 10.1620/tjem.227.73
52. Ortved M, Petersen PB, Jorgensen CC, Kehlet H, Lundbeck Foundation Centre for Fast-track H, Knee Replacement Collaborative Group. Postoperative morbidity and mortality in diabetic patients after fast-track hip and knee arthroplasty: a prospective follow-up cohort of 36,762 procedures. Anesth Analg. (2021) 133:115–22. doi: 10.1213/ANE.0000000000005248
53. Bianchi C, Miccoli R, Daniele G, Penno G, Del Prato S. Is there evidence that oral hypoglycemic agents reduce cardiovascular morbidity/mortality? Yes Diabetes Care. (2009) 32(Suppl. 2):S342–8. doi: 10.2337/dc09-S336
54. Kassem SA, Raz I. Is there evidence that oral hypoglycemic agents reduce cardiovascular morbidity or mortality? No Diabetes Care. (2009) 32(Suppl. 2):S337–41. doi: 10.2337/dc09-S335
55. Liang H, Ding X, Li L, Wang T, Kan Q, Wang L, et al. Association of preadmission metformin use and mortality in patients with sepsis and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Crit Care. (2019) 23:50. doi: 10.1186/s13054-019-2346-4
56. Alrezk R, Jackson N, Al Rezk M, Elashoff R, Weintraub N, Elashoff D, et al. Derivation and validation of a geriatric-sensitive perioperative cardiac risk index. J Am Heart Assoc. (2017) 6:e006648. doi: 10.1161/JAHA.117.006648
57. Gupta PK, Gupta H, Sundaram A, Kaushik M, Fang X, Miller WJ, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. (2011) 124:381–7. doi: 10.1161/CIRCULATIONAHA.110.015701
Keywords: postoperative mortality, diabetes mellitus, hypoglycemic regimens, non-cardiac surgery, insulin
Citation: Jiang J, Wang S, Sun R, Zhao Y, Zhou Z, Bi J, Luo A and Li S (2023) Postoperative short-term mortality between insulin-treated and non-insulin-treated patients with diabetes after non-cardiac surgery: a systematic review and meta-analysis. Front. Med. 10:1142490. doi: 10.3389/fmed.2023.1142490
Received: 11 January 2023; Accepted: 13 April 2023;
Published: 02 May 2023.
Edited by:
Pranav Kumar Prabhakar, Lovely Professional University, IndiaCopyright © 2023 Jiang, Wang, Sun, Zhao, Zhou, Bi, Luo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiyong Li, c2hpeW9uZ2xpQGh1c3QuZWR1LmNu
†These authors have contributed equally to this work
 Jie Jiang†
Jie Jiang† Rao Sun
Rao Sun Yilin Zhao
Yilin Zhao Jiangjiang Bi
Jiangjiang Bi Ailin Luo
Ailin Luo Shiyong Li
Shiyong Li