
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 31 March 2023
Sec. Hematology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1140217
This article is part of the Research Topic Hematopoietic Stem Cell Transplantation: Back to the Future View all 7 articles
 Wen-hui Gao1†
Wen-hui Gao1† Jia-yan Zhu1†
Jia-yan Zhu1† Li-ning Wang1†
Li-ning Wang1† Ming Wan2
Ming Wan2 Ling Wang1
Ling Wang1 Raynier Devillier3
Raynier Devillier3 Jie-ling Jiang1*
Jie-ling Jiang1* Didier Blaise3*
Didier Blaise3* Jiong Hu1*
Jiong Hu1*Introduction: Post-transplantation cyclophosphamide (PT-Cy) use is a recent graft-versus-host disease (GVHD) prophylaxis strategy for patients undergoing allogeneic stem cell transplantation (allo-HSCT). PT-Cy combined with two immunosuppressants is now widely used after haplo-identical (haplo) and HLA-matched peripheral blood stem cell (PBSC) transplantations with promising GVHD and relapsefree survival (GRFS) probabilities. Although appealing, these results may benefit from improvement notably outside matched sibling donor transplantation, and should be investigated in various ethnic populations.
Methods: Therefore, we report our experience of GVHD prophylaxis regimen combining PT-Cy and tacrolimus with addition of post-engraftment low-dose anti-thymocyte globulin (ATG) in allogeneic stem cell transplantation from haplo-identical donors (Haplo). Sixtyseven patients were included in the analysis. All patients received myeloablative or intensified sequential conditioning regimen.
Results: The median follow-up was 521 (range, 10~991) days. The cumulative incidences of 100-day grade II-IV acute GVHD was 14.9±4.4%, and no case of grade III-IV acute GVHD was documented. The cumulative incidences of 2-yearchronic GVHD and moderate-to-severe chronic GVHD were 25.4±5.4% and 11.9±4%, respectively. The non-relapse mortality at day+100 and 2year were 7.5±3.2% and 9.0±3.5%, respectively. The cumulative incidence of relapse at 2year was 16±6.4%. The 2-year probability of DFS and OS were 73.8% (95%CI, 61.5~88.4%) and 72.5% (95% CI, 57.1~92.1%), respectively. The 2-year GRFS was estimated as 63.6% (95%CI, 50.6~80%).
Discussion: Our results suggested that a combination of PT-Cy, tacrolimus, and low-dose post-engraftment ATG was a promising GVHD prophylaxis with low incidence of acute GVHD in the haplo-transplantation setting.
Graft-versus-host disease (GVHD) remains as a major complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT) (1). The standard GVHD prophylaxis strategy is mostly based on the use of calcineurin inhibitors (CNI) or combined with other immunosuppressants (IS) (2, 3). Recently, the post-transplantation cyclophosphamide (PT-Cy) regimen has become an important approach for GVHD prophylaxis, not only in the context of allo-HSCT from HLA-matched related donors (MSD) or HLA-matched unrelated donors (MUD) but also from haplo-identical donors (Haplo) (3–6).
Although PT-Cy can be used as a single agent for GVHD prophylaxis in allogeneic bone marrow transplantation (BMT) from MSD donor (7–9), the addition of other IS seems essential in other situations, especially when using peripheral blood stem cells (PBSCs) (10). Ruggeri et al. (10) documented that there was no difference in the risk of grade II-IV acute GVHD (aGVHD) between PT-Cy only and PT-Cy combined with other IS. However, the incidence of extensive chronic GVHD (cGVHD) was significantly higher among patients receiving PT-Cy only (18%) or PT-Cy with one other IS (20%) than those receiving PT-Cy with two other IS (8.5%). Furthermore, PT-Cy with two additional IS did not affect the incidence of relapse or NRM but improved both OS and GRFS. Besides, addition of anti-thymoglobulin (ATG) prior to transplantation did not affect the relapse rate in the multivariate analysis (10).
In a previous phase II clinical trial, we reported that the incidences of grade II-IV aGVHD and moderate-to-severe cGVHD (mod/sev cGVHD) were 39 and 24%, respectively, in adult patients with lymphoid malignancies receiving PT-Cy and CNI as GVHD prophylaxis, using mobilized PBSCs as grafts (11). To further reduce the incidence of aGVHD, we added a single dose of 2.5 mg/kg ATG after documentation of neutrophil engraftment in case of MUD or Haplo donors. Initial analysis showed a low incidence (9.1%) of grade II-IV aGVHD as previously reported (12).
In this study, we analyzed the outcomes of patients included in three prospective clinical trials (NCT 04118075, NCT 04269811, and NCT 04897139) using the same GVHD prophylaxis regimen focusing on the aGVHD in patients with haplo donor transplantation.
This study was approved by the Human Ethics Committee of Rui Jin Hospital. It was conducted in accordance with the Declaration of Helsinki. The patients were treated at the Blood and Marrow Transplantation Center, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine. The first 67 adult patients consecutively enrolled in these clinical trials were included in the analysis. The eligibility criteria for allo-HSCT were as follows: (1) adult patients (18–65 years) with hematological malignancies, including acute leukemia, chronic myelomonocytic leukemia(CMML), Myeloid sarcoma, lymphoma, and high-risk MDS; (2) patients receiving their first allo-HSCT from Haplo donors; (3) patients with ECOG ≤3, normal renal and hepatic function (serum creatinine ≤133 μmol/L, serum bilirubin ≤34 μmol/L, serum alanine aminotransferase or aspartate aminotransferase <3 times the upper normal limit), cardiac left ventricular ejection fraction ≥50%, and normal pulmonary function tests (including forced expiratory volume in 1 min) at transplantation; (4) negative serology for hepatitis B, C, and human immunodeficiency virus; and (5) patients providing written informed consent prior to allo-HSCT.
For lymphoid malignancies, patients received etoposide 400 mg/m2/d from day −8 to day −7, fludarabine (FLU) 30 mg/m2/d from day −6 to day −2, intravenous busulfan (BU) at 1.6 mg/kg every 12 h in six doses over 3 days (from day −6 to day −5) and melphalan (Mel) 50 mg/m2/d from day −3 to day −2. For AML and high-risk MDS, patients received FLU 30 mg/m2/d from day −6 to day −2, melphalan (Mel) 50 ~ 70 mg/m2/d from day −3 to day −2, and intravenous BU at 1.6 mg/kg every 12 h in four doses over 2 days (from day −6 to day −5). For relapse or refractory AML, patients initially received CLAE chemotherapy as debulking treatment, which consisted of 5 mg/m2/d cladribine and 1.5 g/m2/day cytarabine for 5 days (day −20 to day −16) and etoposide 200 mg/m2/d for 3 days (from day −16 to day −14). With an interval of 7 days, patients received a reduced conditioning regimen of FLU 30 mg/m2/day from day −6 to day −2 and intravenous BU at 1.6 mg/kg every 12 h in six doses over 3 days (from day −6 to day −4). In patients with ECOG ≥2, the doses of BU and Mel were reduced accordingly, as shown in Figure 1. BU was administered by infusion over 3 h, and the dose of BU was based on actual body weight (ABW) or adjusted ideal body weight (AIBW, AIBW = ideal body weight (IBW) + 25% (ABW-IBW)) for overweight patients. Granulocyte colony-stimulating factor (G-CSF)-mobilized PBSCs were infused on day 0. GVHD prophylaxis consisted of cyclophosphamide 50 mg/kg on day +3 and day +4, followed by tacrolimus 1.5 mg twice daily, starting on day 5 adjusted with target therapeutic trough level of 5 ~ 15 ng/ml. A single dose of 2.5 mg/kg ATG was administered in patients on day +15 after documentation of neutrophil engraftment. If neutrophil engraft was delayed, the dose of ATG was postponed to day +22 or afterwards likewise, as shown in Figure 1.
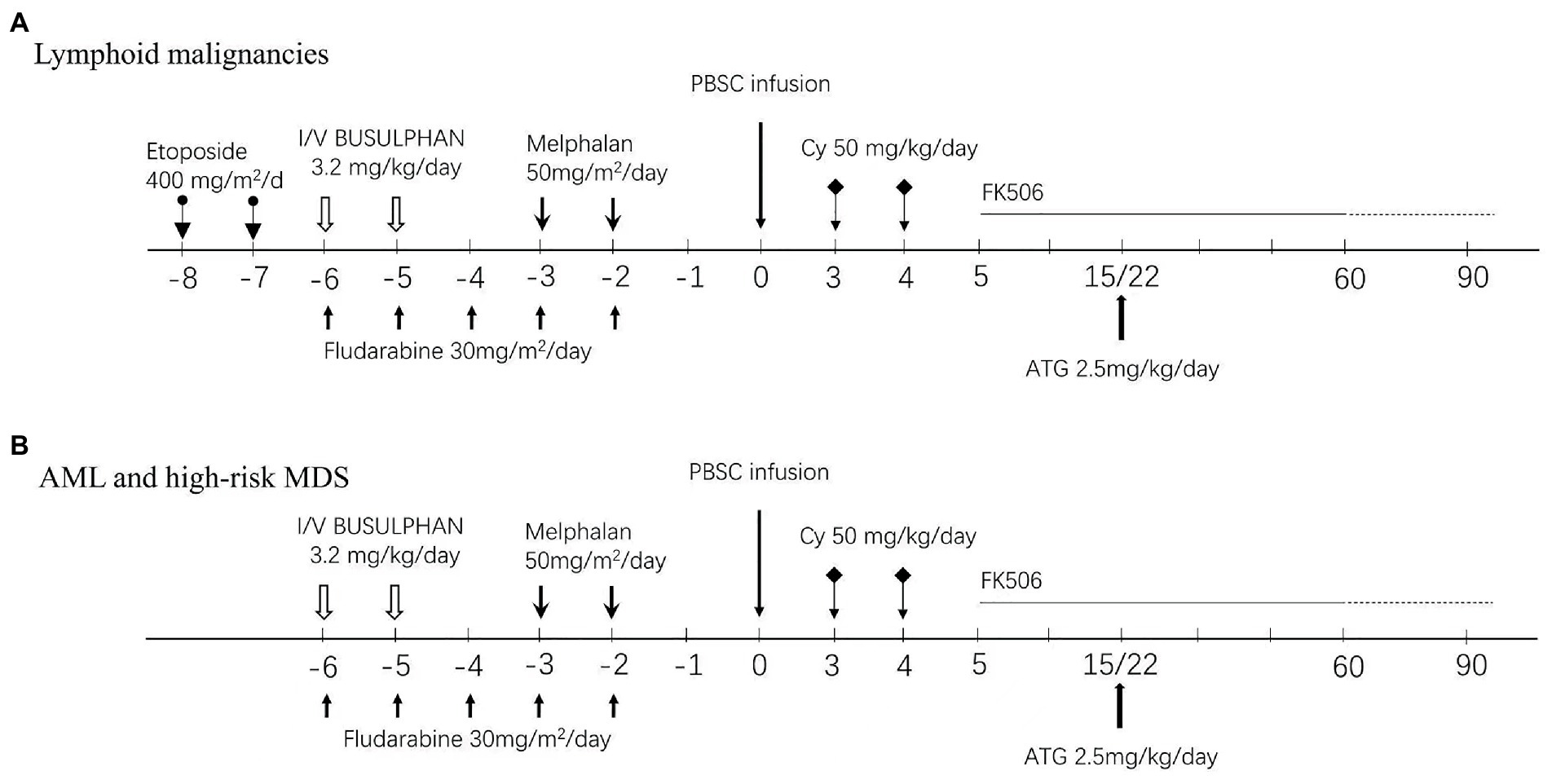
Figure 1. Conditioning regimens and GVHD prophylaxis protocol. (A) Lymphoid malignancies. (B) AML/high-risk MDS and CMML/Myeloid sacroma.
In the case of grade II-IV aGVHD, first line treatment was methylprednisolone 1 ~ 2 mg/kg/day (i.v.) only. If no response was observed, ruxolitinib or basiliximab was added as second line treatment. For cytomegalovirus (CMV) prophylaxis, ganciclovir was administered on day −9 to day −2. After transplantation, pre-emptive treatment of ganciclovir was based on weekly monitoring and administered if CMV DNAemia level > 1 × 104 IU/mL. Additionally, Epstein–Barr virus (EBV) DNA was monitored weekly, and preemptive rituximab was used if EBV-DNA level was >1 × 105 IU/ml to prevent EBV-associated post-transplantation lymphoproliferative disease (PTLD). Regarding the prophylaxis of invasive fungal disease (IFD), risk adapted escalation strategy was used as described in our previous study (12). All patients without previous probable or proven IFD received fluconazole (i.v.) as IFD prophylaxis in the laminar airflow unit. After patients were discharged from the BMT units, fluconazole (p.o) was administered on day 100 in patients undergoing transplantation from MSD without aGVHD. For all patients with MUD or haplo donors and all patients with aGVHD requiring steroid treatment, the prophylaxis was escalated to voriconazole or posaconazole until day 100. For patients with previous documented probable or proven IFD, caspofungin or voriconazole was administered as secondary prevention at the BMT unit, and voriconazole or posaconazole was used sequentially until day 100. Moreover, sulfamethoxazole was administered every other day, from day of engraftment to 100 days after transplantation to prevent pneumocystis. All other supportive care were according to the standard protocol of Rui Jin Hospital.
The primary endpoint of the study was the incidence of grade II-IV aGVHD at day +100. Secondary endpoints were engraftment rate, cumulative incidence of grade III-IV aGVHD and NRM at day +100, cumulative incidence of cGVHD, moderate-to-severe cGVHD, relapse, disease-free survival (DFS), overall survival (OS), and GVHD and relapse-free survival (GRFS) at 2 year. Definitions of neutrophil and platelet engraftment, NRM, relapse, DFS, and OS were previously reported (12). Full donor chimerism was defined as ≥95% leukocytes of donor origin in peripheral blood or marrow samples. Mixed chimerism was defined as >5% but <95% leukocytes of donor origin (13). GRFS events were defined as primary engraftment failure, grade III-IV aGVHD, moderate-to-severe cGVHD, and mild cGVHD requiring systemic immunosuppressive treatment, disease relapse, or death from any cause during follow-up after allo-HSCT.
The immune reconstitution in terms of peripheral lymphocytes population test was performed regularly after transplantation. The distributions of peripheral CD3+, CD4+, CD8+, NK and CD19+ cells were analyzed by FACSCanto II flow cytometer (Becton Dickinson, United States) labeled with monoclonal antibodies (FITC/PE/Percp/APC/PE-Cy7/APC-Cy7) and the absolute number of cells were calculated based on peripheral blood count and the percentage of cell populations.
The sample size was calculated using the one arm binomial. The null hypothesis was set as the incidence of grade II-IV GVHD ≥30%, whereas the alternate hypothesis was incidence of grade II-IV GVHD ≤15% with one-sided significance of 0.05 and power of 0.8. The incidences of aGVHD, cGVHD, relapse, and NRM were estimated with cumulative incidence functions. The OS, DFS, and GRFS were calculated using the Kaplan–Meier method. The cumulative incidences of relapse and death of any causes were calculated as competing risks (14). Data on patients who were alive in CR were censored at last follow-up on December 31, 2021. The statistical analyses were performed using SPSS 26.0 and R statistical software.
The demographic data of patients included in the analysis are summarized in Table 1. The median age was 45 years (range, 18 ~ 63 years). Fifty-nine patients had myeloid malignancies, including AML (n = 39), high-risk MDS (n = 18), chronic myelomonocytic leukemia (CMML, n = 1), and myeloid sarcoma (MS, n = 1). Eight patients were diagnosed with Lymphoid malignancies acute lymphoblastic leukemia, Hodgkin’s lymphoma at the time of allo-HSCT, 49 patients were in complete remission (n = 48) or partial remission (n = 1), and 18 patients had active disease. All patients have been followed up for a median of 521 days (range, 10 ~ 991 days); whereas for alive patients, the median follow-up was 527 days (range 319 ~ 991 days). The treatment flow is illustrated in Figure 2.
The median number of mononuclear cells (MNC) and CD34+ cells infused were 7.29 × 108/kg (range, 4.06 ~ 13.3 × 108/kg) and 7.87 × 106/kg (range, 3.87 ~ 15.9 × 106/kg), respectively. Two patients died before engraftment (as shown in Figure 2). The engraftment was documented in 63 patients with a median of 15 (range, 11–22) days for neutrophil engraftment and 16 (range, 9–38) days for platelet engraftment, respectively. Among these patients, the chimerism analysis of MNC and CD3+ T cells in the bone marrow was performed on day 28 based on the PCR of short tandem repeat (STR) unique for donor or recipient. All patients achieved full donor chimerism (≥99%) both in MNC and CD3+ T cells. Two other patients experienced primary graft failure. One patient successfully underwent salvage second allo-HSCT from another haplo-donor, whereas the other patient died of an infection during the work-up for the second allo-HSCT from an alternative donor (Table 2).
Peripheral blood lymphocytes were detected at fixed time-points. On day +180 and + 270, median CD3+, CD4+, CD8+, CD56/CD16+ and CD19+ cell counts were 918 (352–3,856) and 1,308(201–3,996), 156 (79–549) and 265 (48–628), 703 (221–3,525) and 976 (150–3,495), 277 (71–860) and 193 (22–1,196), 186 (12–843) and 235 (2–715)/μl, respectively. Since +270 days after transplantation, the median of CD4 + cell counts were above 200/μL and reached nearly 400/μL on +365 days (as shown in Figure 3).
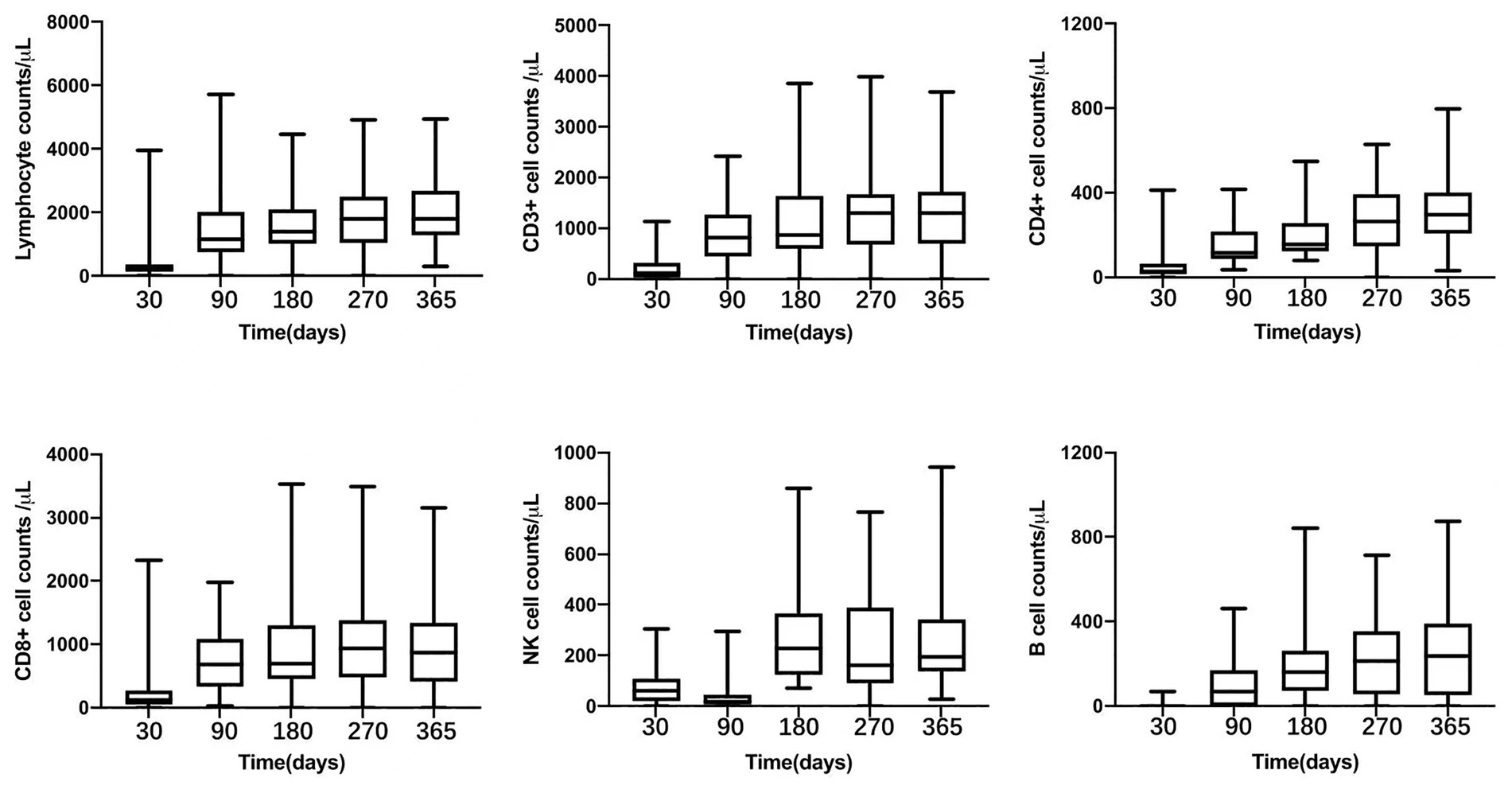
Figure 3. Peripheral lymphocyte counts after transplantation: total lymphocyte oucnt, CD3+, CD4+, CD8+, NK cell, and B lymphocyte.
For GVHD prophylaxis, five patients did not receive ATG due to early death before engraftment (n = 2), primary engraftment failure (n = 2), and multiple complications at engraftment, including persistent fever with unknown origin and progressive hemorrhagic cystitis (n = 1). Based on the time of neutrophil engraftment, 62 patients received ATG, as planned, on day+15 (n = 38) or day+22 (n = 24).
Overall, four and four patients developed grade I and II aGVHD, respectively. Therefore, the cumulative incidences of all grade and II-IV aGVHD at day+100 were 14.9 ± 4.4% and 7.5 ± 3.2%, respectively (Figure 4A). No cases of grade III-IV aGVHD were documented. Among four patients with grade II aGVHD, one patient had isolated skin aGVHD, two patients had isolated gut involvement, and another one patient had both skin and gut involved. Notably, all these patients achieved complete response after standard dose corticosteroid treatment (methylprednisolone with 1 ~ 2 mg/kg/d), and no steroid-resistant GVHD was documented.
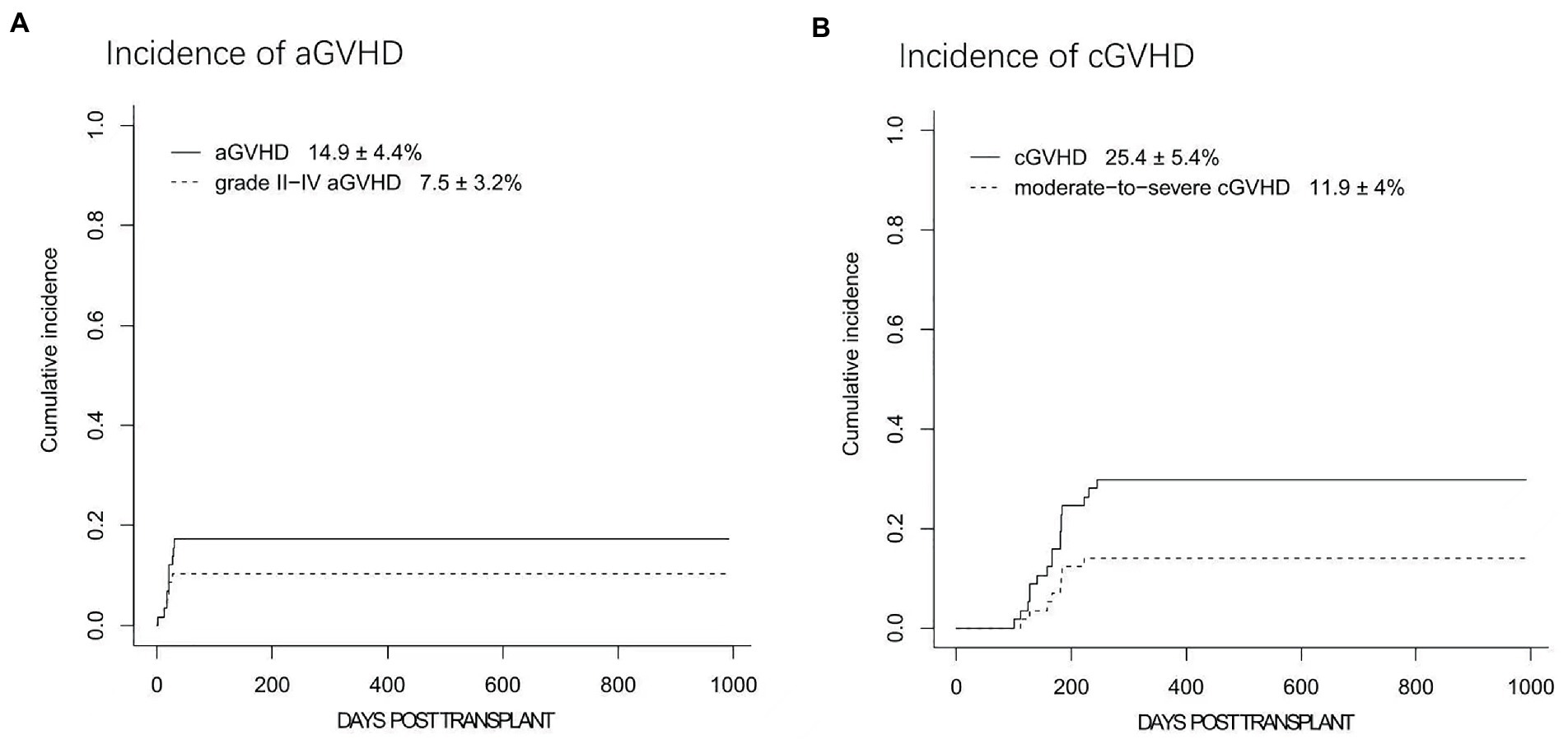
Figure 4. The cumulative incidences (CIs) of acute graft-versus-host disease (aGVHD) and chronic graft-versus-host disease (cGVHD). (A) The CIs of grades I-IV aGVHD and grades II-IV aGVHD. (B) The 2-year CIs of cGVHD and moderate to severe cGVHD.
Nine patients developed mild cGVHD with skin or mucosa involvement. Mod/sev cGVHD was documented in 8 patients. Among them, 7 patients had skin, liver and mucosa involved, whereas the other patient was diagnosed with bronchiolitis obliterans (BO) together with skin and liver cGVHD. Seven patients with moderate cGVHD responded to systemic corticosteroids combined with tacrolimus or sirolimus therapy. The patient with BO remained well with steroid and tacrolimus therapy after pulmonary transplantation. Overall, the 1-year incidences of cGVHD and mod/sev cGVHD were 25.4 ± 5.4% and 11.9 ± 4.0%, respectively (Figure 4B) while the 2-year cGVHD remained as 25.4 ± 5.4%.
Most patients experienced febrile neutropenia and were well controlled after hematological reconstitution. Eleven patients had documented pneumonia: five patients with possible aspergillus pneumonia, three patients with bacterial pulmonary infection, and one patient with pneumocystis carinii pneumonia. Within 100 days, CMV reactivation was observed in 34 (50.7%) patients, and five cases of CMV disease were documented, which included two cases of pneumonia and three cases of hemorrhagic cystitis. The median time to CMV reactivation was 40 (range, 12 ∼ 90) days post-transplantation. EBV reactivation was documented in 4 (6.0%) patients on days+40, +41, +102 and + 287, respectively, after transplantation. All four patients responded to dose reduction of tacrolimus (n = 2) or recovered spontaneously without any specific intervention (n = 2). No EBV-associated PTLD was documented. Besides, four (6.0%) patients developed hemorrhagic cystitis; three of these cases were due to CMV and the other was due to BK virus (Table 3).
At the last follow-up, seven patients relapsed. The cases included refractory T lymphoblastic leukemia/lymphoma transplanted in remission after salvage chemotherapy (n = 1, day+190), CR1 FLT3-ITD + AML (n = 1, day+249), refractory/relapse AML (n = 2, day+463 and + 657), and MDS-EB1 with TP53 mutation (n = 1, day+516), high risk AML (n = 1,day+318), high risk ALL (n = 1, day+437). Therefore, the 1-year cumulative incidence of relapse (CIR) was 4.5 ± 2.5% while the overall 2-year CIR was 16% ± 6.4% (Figure 5A and Supplementary Table S1). Eventually, four patients died of disease, and the other three patients were undergoing treatment with Inotuzumab-ozogamicin and second allo-HSCT as salvage therapy.
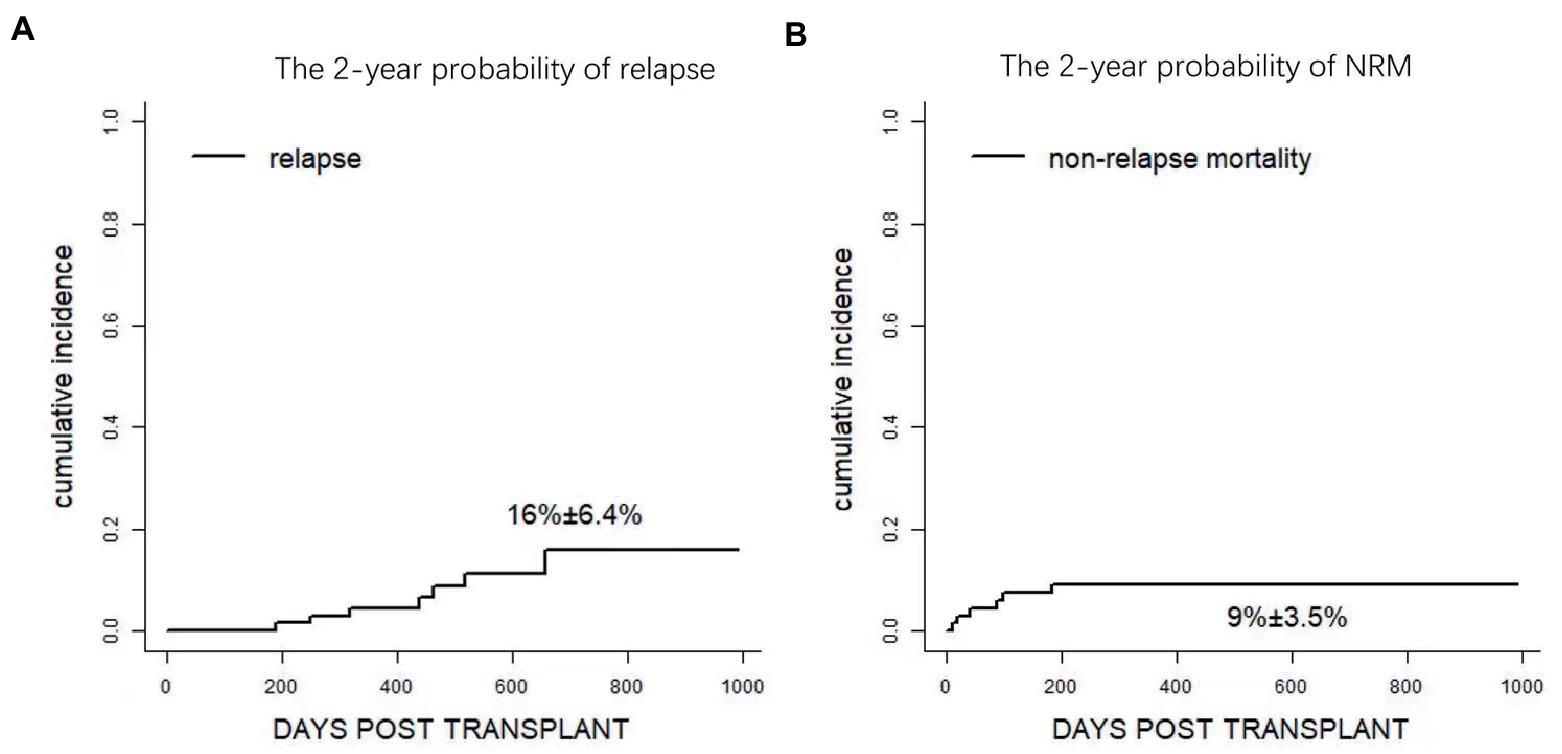
Figure 5. Clinical outcomes after Haplo-PBSCT. (A) The 2-year probability of relapse. (B) The 1 year probability of non-relapse mortality (NRM).
Regarding NRM, a total of 7 patients died of transplantation-related complications, including bacterial infection in neutropenia (n = 3), viral encephalitis (n = 1), heart failure (n = 1), and pulmonary infection (n = 2, as shown in Table 2). NRM at day+100 and 2 year were 7.5 ± 3.2% and 9.0 ± 3.5%, respectively (Figure 5B and Supplementary Table S1). Notably, no patient died of aGVHD or related complications at the last follow-up.
The 2-year probabilities of DFS and OS were 73.8% (95%CI, 61.5 ~ 88.4%) and 72.5% (95% CI, 57.1 ~ 92.1%), respectively (Figures 6A,B). Overall, 47 patients remained alive and free of relapse, graft failure, III-IV aGVHD, and mod/sev cGVHD with 2-year GRFS of 63.6% (95%CI, 50.6 ~ 80%, Figure 6C and Supplementary Table S1).
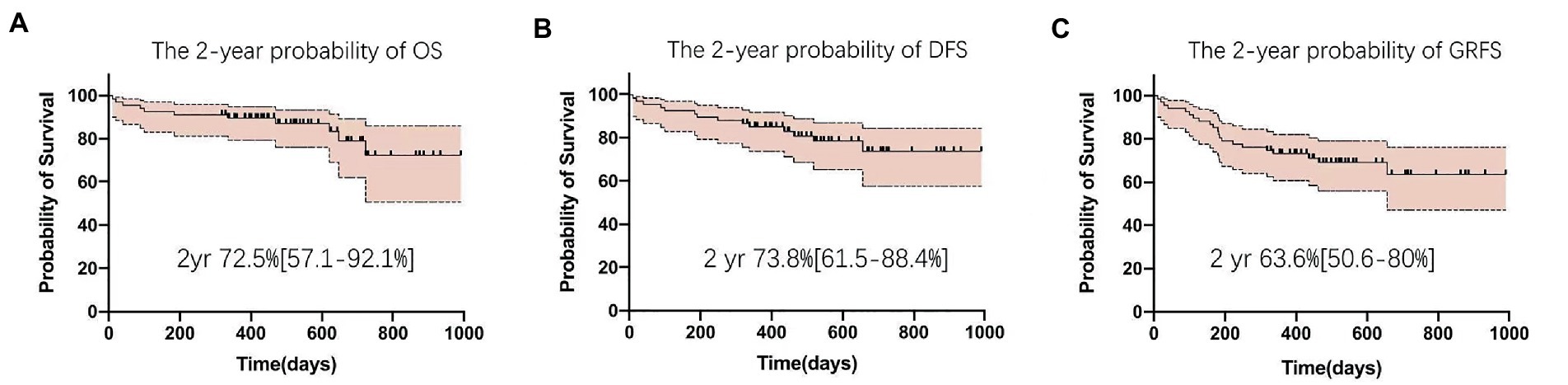
Figure 6. Clinical outcomes after Haplo-PBSCT. (A) The 2-year probability of disease-free survival (DFS). (B) The 2-year probability of overall survival (OS). (C) The 2-year probability of GVHD and relapse-free survival (GRFS).
GVHD is a major complication of allo-HSCT, particularly when MUD and Haplo donors are involved. CNIs remain the mainstay of GVHD prophylaxis. Previous studies demonstrated the superiority of combining CNI with ATG for prophylaxis of aGVHD and cGVHD in MUD, Haplo, and even MSD settings (15–17).
PT-Cy was also established as an important GVHD prophylaxis strategy. Previous clinical studies demonstrated that PT-Cy combined with other IS, such as CNI, further decreased the incidence of GVHD (10, 18–21). PT-Cy combined with two IS significantly reduced extensive cGVHD and improved the OS and GRFS, on the condition that patients underwent allo-HSCT from MSD or MUD donors (10). Moreover, addition of in vivo T cell depletion agent, ATG, to PT-Cy was independently associated with reduced risk of cGVHD and extensive cGVHD. Both strategies did not increase the relapse rate (22, 23). In the retrospective analysis, ATG (2.5 mg/kg on day −1 or day −2 to day −1) combined with PT-Cy decreased incidence of aGVHD to 12% (p = 0.029) and incidence of NRM to 8% (p = 0.005) with an improved OS (p = 0.029) (23). Various strategies were devised to combine ATG and PT-Cy in GVHD prophylaxis as summarized in Table 4 (24–26). Law et al. (24) reported a protocol of ATG 4.5 mg/kg from day −3 to day −1, followed by PT-Cy 50 mg/kg from day +3 to day +4 and cyclosporine for the prevention of graft rejection and GVHD with PBSCs as grafts. At day 100, the cumulative incidences of aGVHD of any grade and grade III-IV were 38.3 and 5.2%, respectively. No case of mod/sev cGVHD was recorded, and the incidence of mild cGVHD was 15.5%. Wang et al. (25) reported a protocol of high dose ATG (10 mg/kg from day −5 to day −2) and cyclosporine, followed by low-dose PT-Cy (14.5 mg/kg from day +3 to day +4) for the prevention GVHD in patients with haplo donors. Contrarily, other studies reported the outcome of low dose ATG (4.5 ~ 5 mg/kg) combined with single dose of PT-Cy (50 mg/kg on day +3) or standard PT-Cy combined with MMF/CsA in haplo-setting (Table 4) (26, 27). Both studies reported that the incidences of grade III-IV aGVHD (6.9 ~ 16) and mod/sev cGVHD (16%) were low.
In our center, we used a different approach to combine PT-Cy with post-engraft low-dose ATG. The rationale was based on our previous phase II study using PTCy-CsA as GVHD prophylaxis in patients undergoing allo-HSCT with PBSC and myeloablative conditioning. The incidence of aGVHD remained high in MUD or Haplo donor. To tackle the issue, we replaced the CsA with tacrolimus and add a single low-dose of ATG (2.5 mg/kg) after neutrophil engraftment in haplo settings. The rationale for post-engraftment ATG was to avoid the potential impact of immunosuppression drugs used before PTCy. In our previous report, the overall incidence of grade II-IV aGVHD was 9.1 ± 6.1% with 1-year mod/sev cGVHD at 4.6 ± 4.4% in patients undergoing PBSC transplantation in lymphoid malignancies (12). In this study, we extended the analysis to three prospective clinical trials using the same GVHD prophylaxis protocol in patients with myeloid and lymphoid malignancies receiving haplo-donor transplantation only. We observed a low incidence of grade II-IV aGVHD (7.5 ± 3.2%) and no case of III-IV aGVHD at day 100. Besides, all patients with grade II aGVHD responded well to first-line treatment, and no case of steroid-resistant aGVHD was documented. Additionally, the 1-year incidences of cGVHD and mod/sev cGVHD were acceptable. This was the first attempt to add low-dose ATG after engraftment to the GVHD prophylaxis and the short-term outcome was satisfactory with low incidence of aGVHD and particularly no patient died of aGVHD.
More recently, very low incidence of aGVHD was reported using new strategy such as T cell co-stimulation blockade by CTLA4Ig in both MUD or haplo donor transplantation (28, 29). In haplo setting, for patients receiving prophylactic donor lymphocyte (pDLI) infusion with CTLA4Ig based GVHD prophylaxis (n = 75), the incidences of aGVHD was low at 9.6% while the NRM and disease progression were low as 4 and 15.7%, respectively. Overall, the 2-year GRFS was significantly improved to 76.6%. Based on these promising data, randomize studies are warranted to confirm the superiority of T cell co-stimulation blockade or PTCy combined with post-engraftment low-dose ATG to conventional PTCy strategy.
One major concern of this enhanced GVHD prophylaxis was the potential infection associated with immunosuppression. We observed a relative high incidence of CMV reactivation (~50%), which was slightly lower than the GVHD prophylaxis with high-dose ATG (10 mg/kg) or at least comparable to that of protocols with moderate dose of ATG (4.5 ~ 5.0 mg/kg) combined with PT-Cy (Table 4). Regarding EBV reactivation and associated PTLD, we observed a low incidence of EBV reactivation, and all these cases were clinically non-significant. The overall incidence of other events of infection was not significantly increased, compared to that of our historical control.
This study had few limitations, which included the small sample and relatively short follow-up period. cGVHD and long-term outcomes, particularly risk of late relapse should be further evaluated. Besides, several other questions, such as the optimal dose and timing of ATG, which were beyond the reach of this study, warrant future clinical trials.
In conclusion, this study demonstrated that standard PT-Cy combined with tacrolimus and additional low-dose post-engraftment ATG in myeloablative Haplo allo-HSCT with PBSCs as grafts was feasible with satisfactory short-term outcomes, in terms of low incidence of aGVHD and NRM.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Human Ethics Committee of Rui Jin Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JH, DB, and J-lJ designed the clinical trial and revised the manuscript. W-hG, J-yZ, and L-nW collected the clinical data and wrote the manuscript. LW also helped to collect the clinical data. MW and RD did the statistical analysis. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1140217/full#supplementary-material
1. Hill, L, Alousi, A, Kebriaei, P, Mehta, R, Rezvani, K, and Shpall, E. New and emerging therapies for acute and chronic graft versus host disease. Ther Adv Hematol. (2018) 9:21–46. doi: 10.1177/2040620717741860
2. Luznik, L, and Fuchs, EJ. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res. (2010) 47:65–77. doi: 10.1007/s12026-009-8139-0
3. Robinson, TM, O’Donnell, PV, Fuchs, EJ, and Luznik, L. Haploidentical bone marrow and stem cell transplantation: experience with posttransplantation cyclophosphamide. Semin Hematol. (2016) 53:90–7. doi: 10.1053/j.seminhematol.2016.01.005
4. Luznik, L, Bolanos-Meade, J, Zahurak, M, Chen, AR, Smith, BD, Brodsky, R, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. (2010) 115:3224–30. doi: 10.1182/blood-2009-11-251595
5. McCurdy, SR, Kasamon, YL, Kanakry, CG, Bolaños-Meade, J, Tsai, HL, Showel, MM, et al. Comparable composite endpoints after HLA-matched and HLA-haploidentical transplantation with posttransplantation cyclophosphamide. Haematologica. (2017) 102:391–400. doi: 10.3324/haematol.2016.144139
6. Mielcarek, M, Furlong, T, O’Donnell, PV, Storer, BE, McCune, JS, Storb, R, et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA- matched mobilized blood cell transplantation. Blood. (2016) 127:1502–8. doi: 10.1182/blood-2015-10-672071
7. Kanakry, CG, Tsai, H-L, Bolanos-Meade, J, Smith, BD, Gojo, I, Kanakry, JA, et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood. (2014) 124:3817–27. doi: 10.1182/blood-2014-07-587477
8. Kanakry, CG, O’Donnell, PV, Furlong, T, de Lima, MJ, Wei, W, Medeot, M, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. (2014) 32:3497–505. doi: 10.1200/JCO.2013.54.0625
9. Bradstock, KF, Bilmon, I, Kwan, J, Micklethwaite, K, Blyth, E, Deren, S, et al. Single-agent high-dose cyclophosphamide for graft-versus-host disease prophylaxis in human leukocyte antigen-matched reduced-intensity peripheral blood stem cell transplantation results in an unacceptably high rate of severe acute graft-versus-host disease. Biol Blood Marrow Transplant. (2015) 21:941–4. doi: 10.1016/j.bbmt.2015.01.020
10. Ruggeri, A, Labopin, M, Bacigalupo, A, Afanasyev, B, Cornelissen, JJ, Elmaagacli, A, et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Oncol. (2018) 11:40–9. doi: 10.1186/s13045-018-0586-4
11. Wang, L, Wang, L, Fan, X, Tang, W, and Hu, J. Fludarabine and intravenous busulfan conditioning with post-transplantation cyclophosphamide for allogeneic peripheral stem cell transplantation for adult patients with lymphoid malignancies: a prospective single-arm phase II study. Front Med. (2020) 15:108–15. doi: 10.1007/s11684-019-0730-8
12. Jiang, JL, Gao, WH, Wang, LN, Wan, M, Wang, L, and Hu, J. Post-transplantation cyclophosphamide, tacrolimus and low-dose ATG as GVHD prophylaxis for allogeneic peripheral stem cell transplantation for adult patients with lymphoid malignancies: a single arm phase II study. Front Med. (2021) 8:630160. doi: 10.3389/fmed.2021.630160
13. Chang, YJ, Zhao, XY, Xu, LP, Zhang, XH, Wang, Y, Han, W, et al. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol. (2015) 8:84. doi: 10.1186/s13045-015-0182-9
14. Gooley, TA, Leisenring, W, Crowley, J, and Storer, BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. (1999) 18:695–706. doi: 10.1002/(SICI)1097-0258(19990330)18:6<695::AID-SIM60>3.0.CO;2-O
15. Soiffer, RJ, Kim, HT, McGuirk, J, Horwitz, ME, Johnston, L, Patnaik, MM, et al. Prospective, randomized, double-blind, phase III clinical trial of anti–T-lymphocyte globulin to assess impact on chronic graft-versus-host disease–free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. J Clin Oncol. (2017) 35:4003–11. doi: 10.1200/JCO.2017.75.8177
16. Walker, I, Panzarella, T, Couban, S, Couture, F, Devins, G, Elemary, M, et al. Pretreatment with anti-thymocyte globulin versus no antithymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. (2016) 17:164–73. doi: 10.1016/S1470-2045(15)00462-3
17. Walker, I, Panzarella, T, Couban, S, Couture, F, Devins, G, Elemary, M, et al. Addition of anti-thymocyte globulin to standard graft-versus-host disease prophylaxis versus standard treatment alone in patients with haematological malignancies undergoing transplantation from unrelated donors: final analysis of a randomised, openlabel, multicentre, phase 3 trial. Lancet Haematol. (2020) 7:e100–11. doi: 10.1016/S2352-3026(19)30220-0
18. Hess, BT, Gao, F, DiPersio, JF, Westervelt, P, Vij, R, Uy, GL, et al. Use of post-transplant cyclophosphamide (PTCy) with mycophenolate mofetil and tacrolimus in HLA matched allogeneic hematopoietic cell transplant is safe and associated with acceptable transplant outcomes. Am Soc Hematol. (2015) 126:1950. doi: 10.1182/blood.V126.23.1950.1950
19. Shah, MV, Saliba, RM, Rondon, G, Chen, J, Soebbing, D, Rus, I, et al. Pilot study using post-transplant cyclophosphamide (PTCy), tacrolimus and mycophenolate GVHD prophylaxis for older patients receiving 10/10 HLA-matched unrelated donor hematopoietic stem cell transplantation. Bone Marrow Transplant. (2019) 54:601–6. doi: 10.1038/s41409-018-0367-2
20. Gaballa, S, Ge, I, El Fakih, R, Brammer, JE, Kongtim, P, Tomuleasa, C, et al. Results of a 2-arm, phase 2 clinical trial using posttransplantation cyclophosphamide for the prevention of graft-versus-host disease in haploidentical donor and mismatched unrelated donor hematopoietic stem cell transplantation. Cancer. (2016) 122:3316–26. doi: 10.1002/cncr.30180
21. Moiseev, IS, Pirogova, OV, Alyanski, AL, Babenko, EV, Gindina, TL, Darskaya, EI, et al. Graft-versus-host disease prophylaxis in unrelated peripheral blood stem cell transplantation with posttransplantation cyclophosphamide, tacrolimus, and mycophenolate mofetil. Biol Blood Marrow Transplant. (2016) 22:1037–42. doi: 10.1016/j.bbmt.2016.03.004
22. Sun, X, Yang, J, Cai, Y, Wan, LP, Huang, CM, Qiu, HY, et al. Low-dose antithymocyte globulin plus low-dose posttransplant cyclophosphamide combined with cyclosporine and mycophenolate mofetil for prevention of graft-versus-host disease after HLA-matched unrelated donor peripheral blood stem cell transplantation. Bone Marrow Transplant. (2021) 56:2423–31. doi: 10.1038/s41409-021-01358-y
23. Xu, X, Yang, J, Cai, Y, Li, S, Niu, J, Zhou, K, et al. Low dose antithymocyte globulin with low dose posttransplant cyclophosphamide (low dose ATG/PTCy) can reduce the risk of graft versus-host disease as compared with standard-dose anti-thymocyte globulin in haploidentical peripheral hematopoietic stem cell transplantation combined with unrelated cord blood. Bone Marrow Transplant. (2021) 56:705–8. doi: 10.1038/s41409-020-01047-2
24. Law, AD, Salas, MQ, Lam, W, Michelis, FV, Thyagu, S, Kim, DDH, et al. Reduced-intensity conditioning and dual T lymphocyte suppression with antithymocyte globulin and post-transplant cyclophosphamide as graft-versus-host disease prophylaxis in Haploidentical hematopoietic stem cell transplants for hematological malignancies. Biol Blood Marrow Transplant. (2018) 24:2259–64. doi: 10.1016/j.bbmt.2018.07.008
25. Wang, Y, Wu, DP, Liu, QF, Xu, LP, Liu, KY, Zhang, XH, et al. Low-dose post- transplant cyclophosphamide and anti-thymocyte globulin as an effective strategy for GVHD prevention in haploidentical patients. J Hematol Oncol. (2019) 12:88. doi: 10.1186/s13045-019-0781-y
26. Yang, J, Jiang, J, Cai, Y, Li, S, Wan, L, Zhu, J, et al. Low-dose antithymocyte globulin plus low-dose posttransplant cyclophosphamide as graft-versus-host disease prophylaxis in haploidentical peripheral blood stem cell transplantation combined with unrelated cord blood for patients with hematologic malignancies: a prospective, phase II study. Bone Marrow Transplant. (2019) 54:1049–57. doi: 10.1038/s41409-018-0382-3
27. Peric, Z, Mohty, R, Bastos, J, Brissot, E, Battipaglia, G, Belhocine, R, et al. Thiotepa and antithymocyte globulin-based conditioning prior to haploidentical transplantation with posttransplant cyclophosphamide in high-risk hematological malignancies. Bone Marrow Transplant. (2020) 55:763–72. doi: 10.1038/s41409-019-0726-7
28. Jaiswal, SR, Bhakuni, P, Bhagawati, G, Aiyer, HM, Soni, M, Sharma, N, et al. CTLA4Ig-primed donor lymphocyte infusions following haploidentical transplantation improve outcome with a distinct pattern of early immune reconstitution as compared to conventional donor lymphocyte infusions in advanced hematological malignancies. Bone Marrow Transplant. (2021) 56:185–94. doi: 10.1038/s41409-020-01002-1
Keywords: PTCy, GVHD, haplo donors, peripheral blood stem cell transplantation, anti-thymocyte globulin
Citation: Gao W-h, Zhu J-y, Wang L-n, Wan M, Wang L, Devillier R, Jiang J-l, Blaise D and Hu J (2023) Post-transplantation cyclophosphamide combined with tacrolimus and low-dose post-engraftment anti-thymoglobulin as GVHD prophylaxis for patients undergoing peripheral blood stem cell transplantation from haploidentical family donor: A single center analysis. Front. Med. 10:1140217. doi: 10.3389/fmed.2023.1140217
Received: 08 January 2023; Accepted: 13 March 2023;
Published: 31 March 2023.
Edited by:
Erden Atilla, Fred Hutchinson Cancer Research Center, United StatesReviewed by:
Saurabh Chhabra, Mayo Clinic Arizona, United StatesCopyright © 2023 Gao, Zhu, Wang, Wan, Wang, Devillier, Jiang, Blaise and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie-ling Jiang, ampsMTIyMjNAcmpoLmNvbS5jbg==; Didier Blaise, QkxBSVNFREBpcGMudW5pY2FuY2VyLmZy; Jiong Hu, aGoxMDcwOUByamguY29tLmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.