- 1Department of Health Emergency, Changzhou Center for Disease Control and Prevention, Changzhou, China
- 2Department of Obstetrics and Gynecology, Changzhou Maternity and Child Health Care Hospital, Changzhou Medical Center, Nanjing Medical University, Nanjing, China
Objective: As the only hospital-based national surveillance spot of birth defects (BDs) in Changzhou city located in the economically developed eastern part of China, Changzhou Maternal and Child Health Care Hospital has encountered serious challenges in BD prevention. This study aimed to describe the epidemiology of total BDs born in the hospital from 2014 to 2018.
Methods: The data were collected from the national hospital-based birth defect surveillance system. BD prevalence was calculated by Poisson distribution. Trends of prevalence and the associations regarding information with BDs were analyzed by Poisson regression.
Results: The reported prevalence of total BDs was 313.92 (95% confidence interval [CI]: 299.59–328.76) per 10,000 perinatal infants (PIs), while the perinatal prevalence of BD was 160.19 (95% CI: 150.00–170.89) per 10,000 PIs. A remarkable uptrend in the prevalence of BDs was noticed with a prevalence rate ratio (PRR) of 1.09 (95% CI: 1.04–1.14) and 1.13 (95% CI: 1.09–1.16), respectively. Congenital heart disease (CHD), cleft lip with or without cleft palate (CL/P), congenital malformation of the kidney (CMK), polydactyly, Down syndrome (DS), cystic hygroma, neural tube defect (NTD), and congenital talipes equinovarus (CTE) were common types of total BDs. Mothers living in the urban area (PRR = 1.67, 95% CI:1.50–1.87), male fetuses (PRR = 1.16, 95% CI: 1.05–1.28), and maternal age younger than 20 (PRR = 2.28, 95% CI: 1.60–3.25) and 25 years (PRR = 1.41, 95% CI: 1.22–1.63) or older than 35 years (PRR = 1.18, 95% CI: 1.00–1.40) were risk factors for BD occurrence.
Conclusion: The reported prevalence of total BDs was nearly two times higher than the perinatal prevalence of BDs in PIs, and the ranks of total BDs and BDs in PIs were different. Mothers living in the urban area, male fetuses, and maternal ages younger than 25 or older than 35 years were risk factors for BD incidence. Thus, improving prenatal examination technology, expanding the surveillance time quantum of BDs, and keeping maternal health may be warranted.
1. Introduction
Birth defects (BDs), also known as congenital anomalies, are structural, functional, or metabolic anomalies that occur during intrauterine life and can be identified prenatally, at birth, or sometimes later in infancy (1). BDs have caused high morbidity and mortality of fetuses and infants and significant economic burden to both families and society and have been a global public health issue (2). The World Health Organization (WHO) has reported the prevalence of BDs at 47.2, 55.7, and 64.2 per 1,000 live births in developed, middle-income, and low-income countries, respectively (3). The estimated prevalence of BDs in China has been ~40–60 per 1000 live births, which is close to the level of middle-income countries (4). Meanwhile, the prevalence and rank of BDs have undergone tremendous changes. In the recent century, a significant increase in BD prevalence has been noticed in China, Korea, and Uganda (5–7). The prevalence of several BD subgroups, such as congenital heart disease (CHD), increased significantly, while the prevalence of neural tube defect (NTD) substantially decreased. Changes in prevalence caused rank alteration of BDs (6). Effective detection and full understanding of BDs are useful ways to prevent them, and describing the epidemiology of BDs can aid in implementing and evaluating preventive interventions (8).
There are two types of national surveillance systems in China (9). One is the hospital-based surveillance system, which is used to track the total number of BDs, including those in born children or terminated pregnancies. However, for higher requirements of medical level and longer monitoring time quantum of surveillance hospitals, most hospitals in China are still monitoring BDs in perinatal infants (PIs are infants aged between 28 weeks of gestation and 7 days after birth). Thus far, many studies have described the epidemiology of BDs in PIs of China (5, 10), but few had information regarding total BDs, which could underestimate the prevalence of BDs, especially regarding certain major prenatally diagnosed malformations (10). Therefore, it is urgent to evaluate total BDs.
Changzhou Maternal and Child Health Care Hospital is the only specialized hospital of obstetrics in Changzhou city, where >10,000 neonates are delivered every year. Meanwhile, as the only national hospital-based birth defect surveillance spot in Changzhou, the hospital reports total BDs of the whole gestational period in the national surveillance system. This study aimed at investigating and comparing the epidemiology of BDs in PIs and total BDs by using data from the national hospital-based birth defect surveillance system from 2014 to 2018.
2. Materials and methods
2.1. Study population
All pregnant women who delivered in Changzhou Maternal and Child Health Care Hospital between 2014 and 2018 were monitored. Live births within 7 days, stillbirth, and termination of pregnancy (ToP) at any gestational age following the perinatal diagnosis of BDs were recorded. Pregnancy records were anonymized. Participants' consent forms were achieved when doctors filled in the “Birth Defects Registration Form” after their delivery. The study complied with the World Medical Association Declaration of Helsinki and was approved by the Ethics Committee of Changzhou Maternity and Child Health Care Hospital.
2.2. Criteria of BD diagnosis
The criteria for BD diagnosis were based on the “Maternal and Child Health Monitoring Manual in China”. Anomalies were diagnosed by physical and auxiliary examinations, such as prenatal ultrasonography, and by professional obstetric or neonatal doctors of Changzhou Maternal and Child Health Care Hospital. Complex anomalies were diagnosed through expert consultation. In the surveillance system, 24 types of BDs are registered in detail, and other types of BDs are classified as “others” (10). Only types of BDs that ranked the first 16th were shown in this study, while the other types of BDs were rare. In addition, cleft lip with cleft palate and cleft lip without cleft palate were merged as cleft lip with or without cleft palate (CL/P). Congenital malformation of the kidney (CMK), cystic hygroma, single umbilical artery, congenital atresia of the intestine, subcutaneous edema, visceral inversion, holoprosencephaly, spine arrangement disorder, congenital club hands, Klinefelter's syndrome, and gastrointestinal obstruction were separated from others. BDs were coded according to the International Classification of Disease and Related Health Problems, 10th Revision (ICD-10) (9).
2.3. Data collection and quality control
The National Health and Family Planning Commission has formulated the surveillance data, including the “Birth Defects Registration Form” of livebirth, stillbirth, and ToP of the whole gestational period with BDs and a quarterly table with information on PIs. These data were collected and completed by experienced obstetricians and pediatricians of the hospital. Cases diagnosed by auxiliary examination needed to be confirmed by clinicians after delivery or elective termination. Every case form noted the maternal and neonatal information and BD diagnosis, among other data. Each quarterly table included the number of PIs in each maternal age group, the number of stillbirths, neonatal deaths, and BDs in PIs in each quarter. The case form and the quarterly table were reported both on paper and online. The staff of the Tianning Maternal and Child Health and Family Planning Service Center received and input all the information to the surveillance system monthly and audited them quarterly. The staff of the Changzhou Maternal and Child Health Care Hospital received and audited the information quarterly. Quality controls of the data were examined once every quarter at the district level, half-yearly at the city level, and yearly at the province level to ensure completeness and accuracy of the data and reduction of underreported errors.
2.4. Statistical analysis
The prevalence and 95% confidence interval (CI) of BDs were calculated by Poisson distribution. In prevalence calculation, the denominator remained the same: the number of PIs. For perinatal prevalence, the numerator was the number of BDs in PIs, and for reported prevalence, the numerator was the number of total BDs regardless of gestational age. The prevalence of 16 leading BDs was also calculated and ranked in descending order. Univariate Poisson regression was performed to identify the changing trends of BD prevalence by year, and multivariable Poisson regression was used to detect associations between regarding characteristics and BDs. R version 4.1.3 (the Comprehensive R Archive Network: http://cran.r-project.org) was used for the data analysis. A P-value of <0.05 was considered statistically significant.
3. Results
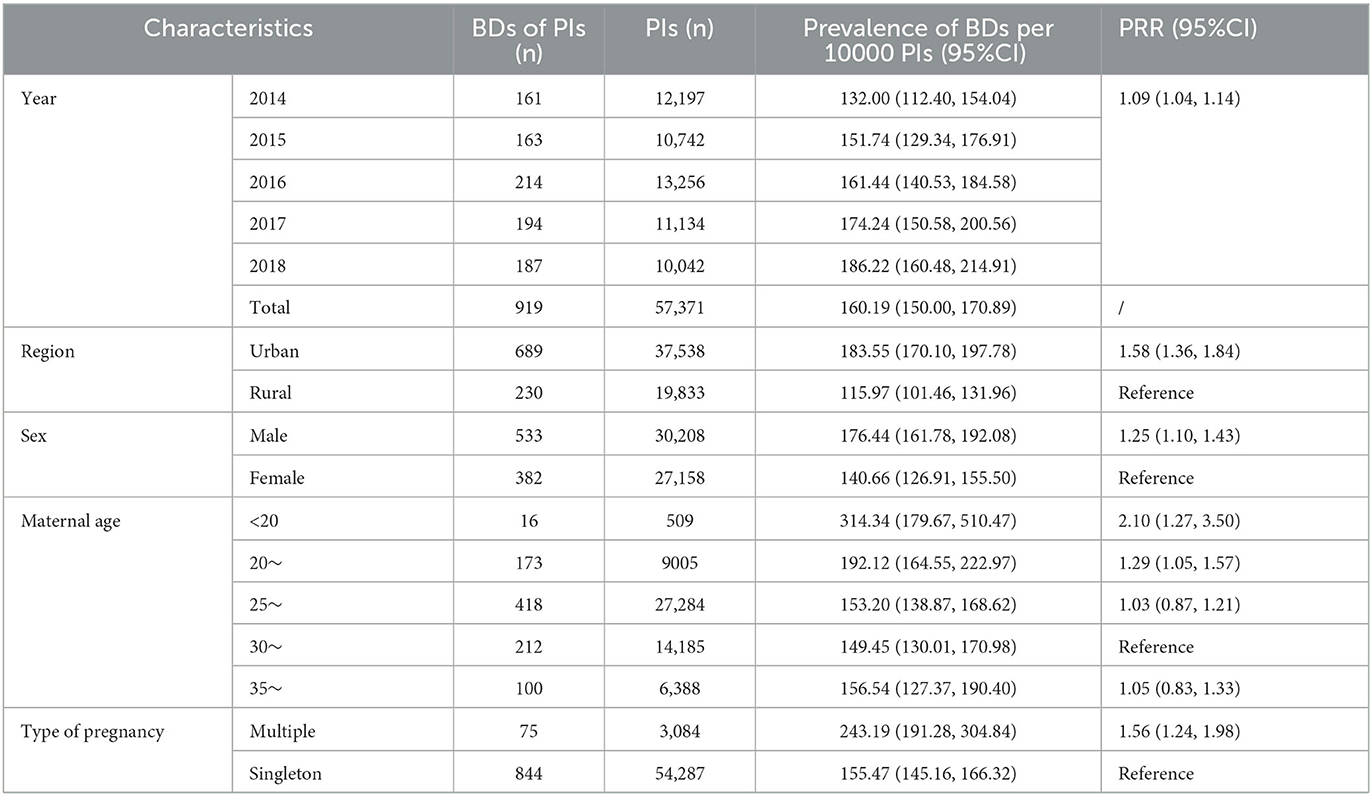
As shown in Table 1, during the study period, 57,371 PIs were registered, and 919 BDs in PIs were diagnosed, resulting in a perinatal prevalence of 160.19 (95% CI: 150.00–170.89) per 10,000 PIs. Univariate Poisson regression showed that the perinatal prevalence of BDs increased significantly during the 5-year study period (prevalence rate ratio [PRR] = 1.09, 95% CI: 1.04–1.14). The perinatal prevalence of BDs in the urban area was significantly higher than that in the rural area (183.55 vs. 115.97 per 10,000 PIs, PRR = 1.58, 95% CI: 1.36–1.84). The perinatal prevalence of BDs in male fetuses was significantly higher than that in female fetuses (176.44 vs. 140.66 per 10000 PIs, PRR = 1.25, 95% CI: 1.10–1.43). The perinatal prevalence of BDs in mothers aged 30–34 years was the lowest in this study; thus, it was set as a reference. The perinatal prevalence of BDs in mothers aged <20 years 20–24 years was significantly higher than that in the reference group (314.34 vs. 149.45 per 10,000 PIs, PRR = 2.10, 95% CI: 1.27–3.50 and 192.12 vs. 149.45 per 10000 PIs, PRR = 1.29, 95% CI: 1.05–1.57, respectively). However, a significant difference between the perinatal prevalence of BDs in mothers aged >35 years and that in the reference group was not identified. In addition, the perinatal prevalence of BDs in multiple births was significantly higher than that in singletons (243.19 vs. 155.47 per 10000 PIs, PRR = 1.56, 95% CI: 1.24–1.98).
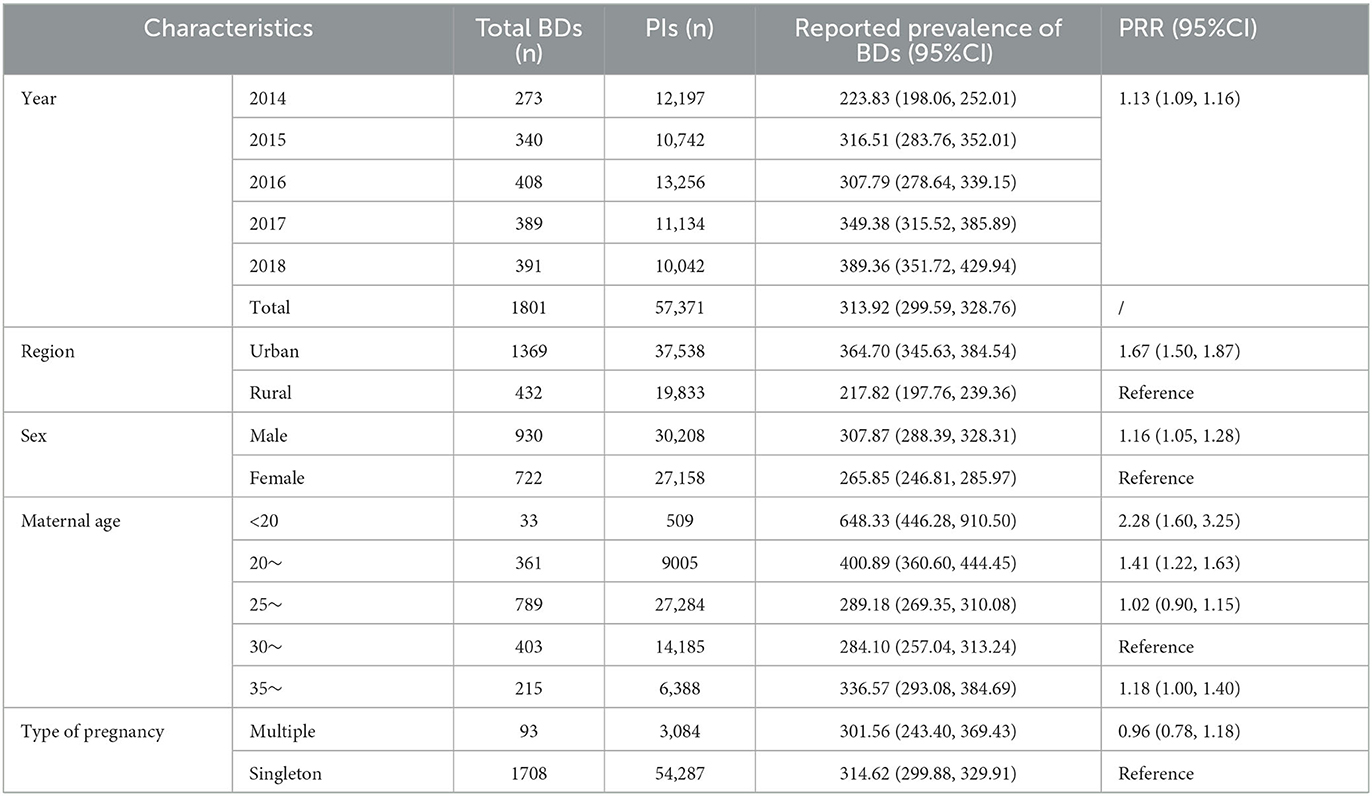
After combining the data on BDs at <28 weeks of gestation, the reported prevalence of total BDs analyzed from years and potential risk factors is summarized in Table 2. The reported prevalence of total BDs was 313.92 (95% CI: 299.59–328.76) per 10,000 PIs, nearly two times higher than perinatal prevalence. The reported prevalence of total BDs increased remarkably during the study period (PRR = 1.13, 95% CI: 1.09–1.16). In addition, the reported prevalence of total BDs was significantly higher in the urban versus rural areas (364.70 vs. 217.82 per 10000 PIs, PRR = 1.67, 95% CI: 1.50–1.87) and in male fetuses versus female fetuses (307.87 vs. 265.85 per 10000 PIs, PRR = 1.16, 95% CI: 1.05–1.28). Furthermore, with reported prevalence in mothers aged 30–34 years set as the reference group, PRRs were 2.28 (95% CI: 1.60–3.25) in mothers aged <20 years and 1.41 (95% CI: 1.22–1.63) in mothers aged 20–24 years. Moreover, the difference between the reported prevalence of BDs in mothers aged > 35 years and the reference group was moderate (PRR = 1.18, 95% CI: 1.00–1.40). However, a significant difference between multiple births and singletons was not identified.
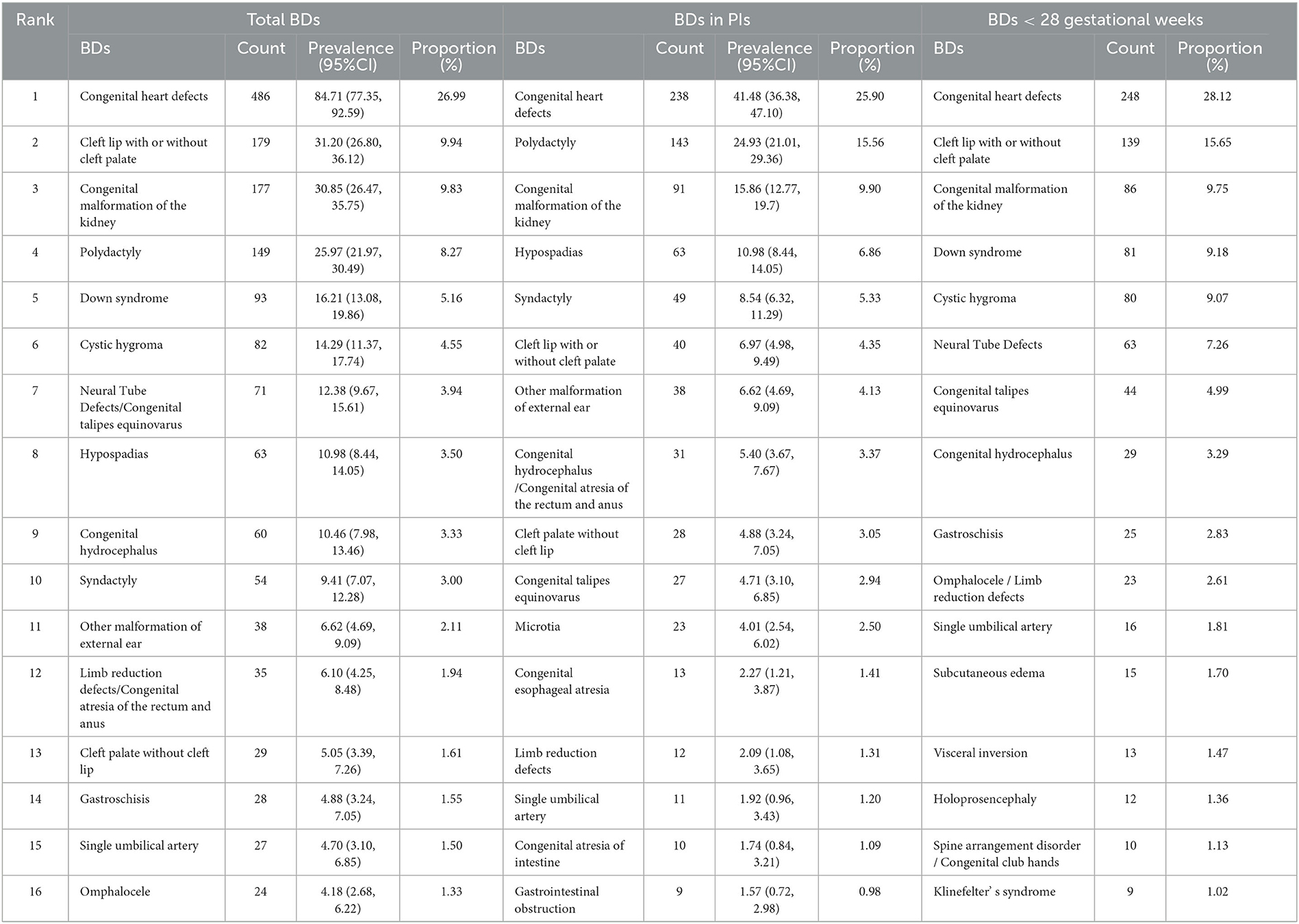
Table 3 shows the rank, prevalence, and proportion of different types of BDs. During the study period, 10 major subtypes of total BDs were CHD, CL/P, CMK, polydactyly, Down syndrome (DS), cystic hygroma, NTD/congenital talipes equinovarus (CTE), hypospadias, congenital hydrocephalus, and syndactyly. CHD, CL/P, CMK, DS, cystic hygroma, NTD, CTE, congenital hydrocephalus, gastroschisis, and omphalocele/limb reduction defects (LRDs) were the 10 most common subtypes of BDs among fetuses aged <28 weeks of gestation. However, among the PIs, the 10 most common subtypes of BDs were CHD, polydactyly, CMK, hypospadias, syndactyly, CL/P, other malformation of external ear (OMEE), congenital hydrocephalus/congenital atresia of the rectum and anus, cleft palate without a cleft lip, and CTE, which were different from those in total BDs.
4. Discussion
In the current study, the perinatal prevalence of BDs was 160.19 per 10,000 PIs, which is higher than the average level in Changzhou city (71.509, 2014–2018) (5) and Longgang district of Shenzhen (134.3, 2003–2009) (11) but lower than that in Hunan province (191.84, 2005–2014) (10). The reported prevalence of total BDs was 313.92 per 10,000 PIs, which is higher than that in Norway (290, 1980–2012) (12), and the average level of Jiangsu province (135.53, 2010–2014) (13) but lower than that in Korea (446.3, 2008–2014) (6). The discrepancy mentioned above might be caused by differences in regions, monitoring time quantum, and types of BDs included in the studies (6). Furthermore, treating more complicated pregnant women and having fewer underreported errors might be the reason for this higher prevalence than that of the average level of Changzhou city (5) and Jiangsu province (13). In addition, the apparent upward trend in the prevalence of BDs was detected during the study period, which is the same as that in Korea (6) and Uganda (7), which might be caused by environmental pollution, diagnostic technique improvement, and fewer underreported errors (5, 7). Therefore, avoiding exposure to environmental pollutants and improving diagnosis skills are critical to prevent, detect, and treat BDs on time (14).
As shown in this study, the reported prevalence of total BDs (313.92) was nearly two times higher than the perinatal prevalence of BDs in PIs (160.19), meaning it underestimates the total BDs by 50% if only BDs in PIs were monitored. This limitation has been reported by a Japanese study (15). Meanwhile, sustained surveillance after birth also detected some neonatal diseases, such as congenital hypothyroidism, which probably could not be found among PIs (9). Meanwhile, as shown in Table 3, except for polydactyly, the first seven types of BDs were the same between BDs at <28 weeks of gestation and total BDs but different from those in PIs, which proved that the reported disease spectrum of BDs was erroneous if only BDs in PIs were monitored. In addition, most BDs in PIs were less harmful or treatable. As researchers from the United States have noted, advancing diagnosis skills allow for BD detection before 28 weeks of gestation, and most pregnant women with severe BDs might choose the ToP (16). This attitude to BDs has also been found among women in Hong Kong, China (17). Additionally, in this study, it was estimated that nearly half of CHD, more than half of CL/P and CTE, and almost all lethal and residual BDs, such as DS and cystic hygroma, resulted in ToP before 28 weeks of gestation, according to the number of total BDs and BDs in PIs. Thus, expanding the time quantum of BD surveillance from conception is essential to estimate the exact epidemiology of BDs to develop better prevention measures. Moreover, it is necessary to enhance the technology of prenatal diagnosis to reduce the number of infants born with severe BDs and carefully plan medical care before and after birth, to improve survival rates and quality of life of treatable cases (18).
Similar to the findings of several studies all over the world, CHD ranked first among total BDs (6, 10, 19). A systematic review including 260 studies has revealed that the reported prevalence of CHD globally continues to increase, and the prevalence of CHD in Asia is higher than that in Europe and America (20). This increase is attributed to the advance and accessibility of detection methods such as perinatal B-mode ultrasonography (21). Potential risk factors also include air pollution and toxic chemicals (22). CL/P ranked second among total BDs in this study. The global average prevalence of CL/P has been approximately 0.794‰, with geographical and ethnic variation (23). CL/P imposes serious physical and mental problems on pediatric patients and a huge financial burden on their families and society (24). The etiology of CL/P may involve genetic factors, environmental factors, such as maternal smoking, alcohol consumption, or exposure to pesticides during the first trimester, and gene–environment interactions (25). The third highest type of BDs in this study was CMK. Some studies have suggested that the high incidence of CMK might be attributed to complex interactions between genetic and environmental factors, such as maternal obesity or diabetes, maternal drug intake, and chlorination disinfection byproduct in drinking water (6, 26). Polydactyly was also a common type of BD in this study, and it was the most common BD in PIs of Changzhou city (5). Gestational hypertension, maternal infectious disease, paternal smoking, and genetic factors have been associated with the incidence of polydactyly (27). The fifth highest type of BDs was DS in this study. DS is a genetic disorder of the chromosomes, with an estimated birth prevalence of 14 per 10,000 live births (28). It leads to early miscarriage, fetal death, learning disabilities, and other health concerns (29). Some studies have shown an uptrend in the incidence and termination among fetuses with DS. Meanwhile, researchers have indicated that advanced maternal age, male fetuses, and developed area were associated with the incidence of DS (30). Cystic hygroma ranked sixth among all types of BDs in this study. Cystic hygroma is a malformation of the lymphatic system with an incidence of 12.5 per 10,000 pregnancies. In total, 50% of cystic hygroma is associated with chromosomal abnormalities, mainly Turner syndrome, and 40% of cystic hygroma occurs in genetic syndromes, meaning that 90% of cases face fetal death, while the remaining 10% of cases probably have a good prognosis (31). NTD and CTE ranked seventh in this study. NTD is a structural disorder of the central nervous system and is a major cause of perinatal mortality, child morbidity, and disabilities. NTD is primarily a folate deficiency disease (32). Its incidence in China is 10 times higher than that in the United States and Europe (33). CTE affects 1–3 in 1,000 live births and occurs twice as often in male fetuses (34). Family history, twin births, and maternal alcohol consumption have been reported as risk factors for CTE (35). In addition, similar to other research in China, the reported prevalence of LRD was also high in this research (36). Thalidomide and pre-gestational diabetes are risk factors for LRD while taking folate before and/or during pregnancy is associated with a lower risk of offspring LRD (37). Thus, improvement of prenatal examination, avoidance of exposure to environmental pollutants and pathogenic microorganisms, normal maternal blood pressure and blood glucose maintenance, fertility at an appropriate age, maternal drug application under the guidance of doctors, folate/multivitamin supplements before and during pregnancy, and balanced medical resources are effective measures to prevent and control BDs on time.
It was found that urban area and male fetuses were risk factors for BDs, which is consistent with a previous study (10). The prevalence of higher BDs among male fetuses might be explained by the higher susceptibility of the Y chromosome than the X chromosome and more detectable external genital deformities in male fetuses (38, 39). Stronger overall health awareness, increased accessibility to prenatal examination, and more serious environmental pollution in urban areas might cause higher BD prevalence (6, 10). In addition, compared with the reported prevalence of BDs in mothers aged 30–34 years, the reported prevalence of BDs in mothers aged <20 or 20–24 years was higher in this study. As reported, young maternal age (<20 years) means an increase in accidental pregnancy and ToP, which is a risk factor for BD (40). Meanwhile, maternal age of <25 years is associated with an elevated risk of polydactyly (41). Different from the result in PIs, a moderate association between advanced maternal age (> 35 years) and the reported prevalence of total BDs was detected, which is similar to another study in China (10). On the one hand, advanced maternal age (35–40 years) has been associated with BDs such as CHD and CL/P (42). On the other hand, advanced maternal age indicated a higher possibility of serious BDs, such as chromosome aberration (43). Severe BDs in pregnant women aged > 35 years are generally ToP, and this might illustrate why the association between advanced maternal age and BD incidence in PIs could not be detected to some extent. Although multiple births were demonstrated as a risk factor for BDs in PIs, a significant association between multiple births and total BDs was not detected. A retrospective cohort study reported that there was no difference in the risk of BDs between multiple births and singleton (44). Therefore, the association between multiple births and the risk of BDs warrants further study.
There were several limitations in this study. First, this was a single-hospital study. However, more than one-third of pregnant women in Changzhou city gave birth at Changzhou Maternal and Child Health Care Hospital every year; hence, the data were representative. Second, different types of BDs have different etiology and pathogenesis; therefore, it is better to collect background information on demographic and social economic status and explore risk factors for certain types of BDs. Third, the denominator of the reported prevalence of BDs only included PIs. As the numbers of births before 28 weeks of gestation are smaller compared with those of births after 28 weeks of gestation, the impact on the reported prevalence is expected to be minimal (32). Fourth, early BDs could not document extremely for miscarriage, which has been extremely rare for these studies.
5. Conclusion
In summary, a remarkable uptrend in the prevalence of BDs was noticed from 2014 to 2018. The reported prevalence of total BDs was nearly two times higher than the perinatal prevalence of BDs in PIs. CHD, CL/P, CMK, polydactyly, DS, cystic hygroma, NTD, and CTE were normal types of BDs of the whole gestational period. Urban area, male fetuses, and maternal age <25 or >35 years were risk factors for BD occurrence. Improving prenatal examination technology, expanding the surveillance time quantum of BDs in the surveillance system, avoiding exposure to environmental pollutants and pathogenic microorganisms, maintaining maternal health, fertility at an appropriate age, nutrition supplements, and balanced medical resources are effective measures to timely prevent and control BDs. Meanwhile, multi-center research including background information is also needed in future studies.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Requests to access these datasets should be directed to YZ, MTM0MTYyODA3NEBxcS5jb20=.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Changzhou Maternal and Child Health Care Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
YZ designed the study, conducted statistical analysis, conceptualized, and wrote the initial draft of the manuscript. DY revised the manuscript and investigation. XM and HZ inputted and audited the data. LW summarized the data and wrote the initial draft of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Young Talent Science and Technology Project of the Changzhou Municipal Health Commission (QN201943) and the Young Talents Project of the Changzhou Municipal Health Commission (CZQM2020104). The funder was not involved in any part of the study process.
Acknowledgments
The authors thank obstetricians and pediatricians of Changzhou Maternal and Child Health Care Hospital and Tianning Maternal and Child Health and Family Planning Service Center.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rida SM, El-Hawash SAM, Fahmy HTY, Hazzaa AA, El-Meligy MMM. Primary health care approaches for prevention and control of congenital and genetic disorders: report of a WHO meeting, Cairo, Egypt, 6-8 December 1999. Cheminform. (2014) 45:5920–2. doi: 10.1002/chin.201442029
2. Kancherla V, Oakley GP, Brent RL. Urgent global opportunities to prevent birth defects. Semin Fetal Neonatal Med. (2014) 19:153–60. doi: 10.1016/j.siny.2013.11.008
3. Christianson A HC, Modell B. The Foundation of March of Dimes in American: Global Report on birth defects. New York: March of Dimes Birth Defects Foundation. (2006).
4. Ministry of Health of the People's Repub. China action to improve constitution of birth population quality and reduce birth defects and disabilities (2002–2010). Chin J Reprod Health. (2011) 13:98–101. doi: 10.3969/j.issn.1671-878X.2002.03.002
5. Zhou Y, Mao X, Zhou H, Qin Z, Wang L, Cai Z, et al. Epidemiology of birth defects based on a birth defect surveillance system in Southern Jiangsu, China, 2014-2018. J Matern Fetal Neonatal Med. (2022) 35:745–51. doi: 10.1080/14767058.2020.1731459
6. Ko JK, Lamichhane DK, Kim HC, Leem JH. Trends in the prevalences of selected birth defects in Korea (2008–2014). Int J Environ Res Public Health. (2018) 15:923. doi: 10.3390/ijerph15050923
7. Mumpe-Mwanja D, Barlow-Mosha L, Williamson D, Valencia D, Serunjogi R, Kakande A, et al. A hospital-based birth defects surveillance system in Kampala, Uganda. BMC Pregnancy Childbirth. (2019) 19:372. doi: 10.1186/s12884-019-2542-x
8. Zaganjor I, Sekkarie A, Tsang BL, Williams J, Razzaghi H, Mulinare J, et al. Describing the prevalence of neural tube defects worldwide: a systematic literature review. PLoS ONE. (2016) 11:e0151586. doi: 10.1371/journal.pone.0151586
9. Zhou Y, Mao X, Zhou H, Wang L, Qin Z, Cai Z, et al. Birth defects data from population-based birth defects surveillance system in a district of Southern Jiangsu, China, 2014–2018. Front Public Health. (2020) 8:378. doi: 10.3389/fpubh.2020.00378
10. Xie D, Yang T, Liu Z, Wang H. Epidemiology of birth defects based on a birth defect surveillance system from 2005 to 2014 in Hunan Province, China. PLoS ONE. (2016) 11:e0147280. doi: 10.1371/journal.pone.0147280
11. Yang M, Zhang S, Du Y. Epidemiology characteristics of birth defects in Shenzhen city during 2003 to 2009, China. J Matern Fetal Neonatal Med. (2015) 28:799–803. doi: 10.3109/14767058.2014.932767
12. Morris JK, Springett AL, Greenlees R, Loane M, Addor MC, Arriola L, et al. Trends in congenital anomalies in Europe from 1980 to 2012. PLoS One. (2018) 13:e0194986. doi: 10.1371/journal.pone.0194986
13. Lu Y, Ning W. Analysis of Birth Defect Surveillance in Jiangsu Province, 2010–2014. Matern Child Health J. (2016) 31:1579–82. doi: 10.7620/zgfybj.j.issn.1001
14. Bravo-Valenzuela NJ, Peixoto AB, Araujo Júnior E. Prenatal diagnosis of congenital heart disease: a review of current knowledge. Indian Heart J. (2018) 70:150–64. doi: 10.1016/j.ihj.2017.12.005
15. Kondo A, Akada S, Akiyama K, Arakawa M, Ichi S, Inamoto Y, et al. Real prevalence of neural tube defects in Japan: how many of such pregnancies have been terminated? Congenit Anom (Kyoto). (2019) 59:118–24. doi: 10.1111/cga.12333
16. Tsai GJ, Cameron CA, Czerwinski JL, Mendez-Figueroa H, Peterson SK, Noblin SJ, et al. Attitudes towards prenatal genetic counseling, prenatal genetic testing, and termination of pregnancy among Southeast and East Asian women in the United States. J Genet Couns. (2017) 26:1041–58. doi: 10.1007/s10897-017-0084-9
17. Leung TN, Ching Chau MM, Chang JJ, Leung TY, Fung TY, Lau TK, et al. Attitudes towards termination of pregnancy among Hong Kong Chinese women attending prenatal diagnosis counselling clinic. Prenat Diagn. (2004) 24:546–51. doi: 10.1002/pd.950
18. Castellanos DA, Lopez KN, Salemi JL, Shamshirsaz AA, Wang Y, Morris SA. Trends in preterm delivery among singleton gestations with critical congenital heart disease. J Pediatr. (2020) 222:28–34.e4. doi: 10.1016/j.jpeds.2020.03.003
19. Ekure EN, Kalu N, Sokunbi OJ, Kruszka P, Olusegun-Joseph AD, Ikebudu D, et al. Clinical epidemiology of congenital heart disease in Nigerian children, 2012–2017. Birth Defects Res. (2018) 110:1233–40. doi: 10.1002/bdr2.1361
20. Liu Y, Chen S, Zühlke L, Black GC, Choy MK Li N, et al. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. (2019) 48:455–63. doi: 10.1093/ije/dyz009
21. Jullien S. Newborn pulse oximetry screening for critical congenital heart defects. BMC Pediatr. (2021) 21:305. doi: 10.1186/s12887-021-02520-7
22. Wang Y, Wang L, Sun Y, Wu M, Ma Y, Yang L, et al. Prediction model for the risk of osteoporosis incorporating factors of disease history and living habits in physical examination of population in Chongqing, Southwest China: based on artificial neural network. BMC Public Health. (2021) 21:991. doi: 10.1186/s12889-021-11002-5
23. Tanaka SA, Mahabir RC, Jupiter DC, Menezes JM. Updating the epidemiology of cleft lip with or without cleft palate. Plast Reconstr Surg. (2012) 129:511e−8e. doi: 10.1097/PRS.0b013e3182402dd1
24. Tao HX, Shi JY, Lin YS, Yin B, Shi B, Jia ZL, et al. Rs9891446 in NTN1 is associated with right-side cleft lip in Han Chinese population. Arch Oral Biol. (2022) 141:105485. doi: 10.1016/j.archoralbio.2022.105485
25. Zhou Y, Zhu W, Shi B, Jia Z. Association between platelet-derived growth factor-C single nucleotide polymorphisms and nonsyndromic cleft lip with or without cleft palate in Western Chinese population. West China J Stomatol. (2020) 38:364–70. doi: 10.7518/hxkq.2020.04.002
26. Lee KS, Choi YJ, Cho J, Lee H, Lee H, Park SJ, et al. Environmental and genetic risk factors of congenital anomalies: an umbrella review of systematic reviews and meta-analyses. J Korean Med Sci. (2021) 36:e183. doi: 10.3346/jkms.2021.36.e183
27. Zou Q. Risk Factors and Genetic Study of Polydactyly and Syndactyly. Tianjin: Tianjin Medical University. (2020).
28. Aivazidis S, Coughlan CM, Rauniyar AK, Jiang H, Liggett LA, Maclean KN, et al. The burden of trisomy 21 disrupts the proteostasis network in Down syndrome. PLoS ONE. (2017) 12:e0176307. doi: 10.1371/journal.pone.0176307
29. Laignier MR, Lopes-Júnior LC, Santana RE, Leite FMC, Brancato CL. Down Syndrome in Brazil: occurrence and associated factors. Int J Environ Res Public Health. (2021) 18:11954. doi: 10.3390/ijerph182211954
30. Jaruratanasirikul S, Kor-Anantakul O, Chowvichian M, Limpitikul W, Dissaneevate P, Intharasangkanawin N, et al. A population-based study of prevalence of Down syndrome in Southern Thailand. World J Pediatr. (2017) 13:63–9. doi: 10.1007/s12519-016-0071-5
31. Sharma C, Jhirwal M, Shekhar S, Singh P. Prenatal evaluation of cystic hygroma- is there a need to revise the protocol? Taiwan J Obstet Gynecol. (2021) 60:581. doi: 10.1016/j.tjog.2021.03.039
32. Liu J, Zhang L, Li Z, Jin L, Zhang Y, Ye R, et al. Prevalence and trend of neural tube defects in five counties in Shanxi province of Northern China, 2000 to 2014. Birth Defects Res A Clin Mol Teratol. (2016) 106:267–74. doi: 10.1002/bdra.23486
33. Williams J, Mai CT, Mulinare J, Isenburg J, Flood TJ, Ethen M, et al. Updated estimates of neural tube defects prevented by mandatory folic Acid fortification—United States, 1995–2011. MMWR Morb Mortal Wkly Rep. (2015) 64:1–5. doi: 10.2307/24856282
34. Sharon-Weiner M, Sukenik-Halevy R, Tepper R, Fishman A, Biron-Shental T, Markovitch O. Diagnostic accuracy, work-up, and outcomes of pregnancies with clubfoot detected by prenatal sonography. Prenat Diagn. (2017) 37:754–63. doi: 10.1002/pd.5077
35. Cardy AH, Sharp L, Torrance N, Hennekam RC, Miedzybrodzka Z. Is there evidence for aetiologically distinct subgroups of idiopathic congenital talipes equinovarus? A case-only study and pedigree analysis. PLoS ONE. (2011) 6:e17895. doi: 10.1371/journal.pone.0017895
36. Liu J, Li Z, Ye R, Ren A, Liu J. Folic acid supplementation and risk for congenital limb reduction defects in China. Int J Epidemiol. (2019) 48:2010–7. doi: 10.1093/ije/dyz130
37. Klungsøyr K, Nordtveit TI, Kaastad TS, Solberg S, Sletten IN, Vik AK. Epidemiology of limb reduction defects as registered in the Medical Birth Registry of Norway, 1970–2016: Population based study. PLoS ONE. (2019) 14:e0219930. doi: 10.1371/journal.pone.0219930
38. Deng X, Berletch JB, Nguyen DK, Disteche CM, X. chromosome regulation: diverse patterns in development, tissues and disease. Nat Rev Genet. (2014) 15:367–78. doi: 10.1038/nrg3687
39. Sokal R, Tata LJ, Fleming KM. Sex prevalence of major congenital anomalies in the United Kingdom: a national population-based study and international comparison meta-analysis. Birth Defects Res A Clin Mol Teratol. (2014) 100:79–91. doi: 10.1002/bdra.23218
40. Alimohamadi Y, Taghdir M, Sepandi M. Statistical data analysis of the risk factors of Neonatal Congenital Hypothyroidism in Khuzestan Province, Iran. Data Brief. (2018) 21:2510–4. doi: 10.1016/j.dib.2018.11.113
41. Yu M, Ping Z, Zhang S, He Y, Dong R, Guo X. The survey of birth defects rate based on birth registration system. Chin Med J (Engl). (2015) 128:7–14. doi: 10.4103/0366-6999.147785
42. Luo YL, Cheng YL, Gao XH, Tan SQ Li JM, Wang W, et al. Maternal age, parity and isolated birth defects: a population-based case-control study in Shenzhen, China. PLoS ONE. (2013) 8:e81369. doi: 10.1371/journal.pone.0081369
43. Heinke D, Isenburg JL, Stallings EB, Short TD, Le M, Fisher S, et al. Prevalence of structural birth defects among infants with Down syndrome, 2013-2017: a US population-based study. Birth Defects Res. (2021) 113:189–202. doi: 10.1002/bdr2.1854
Keywords: birth defects, epidemiology, hospital-based, surveillance, risk factors
Citation: Zhou Y, Yang D, Mao X, Zhou H and Wang L (2023) Epidemiology of birth defects in a national hospital-based birth defect surveillance spot in Southern Jiangsu, China, 2014–2018. Front. Med. 10:1138946. doi: 10.3389/fmed.2023.1138946
Received: 06 January 2023; Accepted: 09 August 2023;
Published: 12 September 2023.
Edited by:
Tim S. Nawrot, University of Hasselt, BelgiumReviewed by:
Tadesse Gure Eticha, Haramaya University, EthiopiaMahmudur Rahman, Southern Cross University, Australia
Copyright © 2023 Zhou, Yang, Mao, Zhou and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Wang, bGl3YW5nODBAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Ying Zhou
Ying Zhou Di Yang2†
Di Yang2† Hua Zhou
Hua Zhou