- 1Department of Gynecology and Obstetrics, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, Shaanxi, China
- 2Department of Ophthalmology, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, Shaanxi, China
Background: Vaginal microecology has a definite influence on human papillomavirus (HPV) infection and clearance, but the specific correlation is still controversial. This research aimed to investigate the differences in the vaginal microenvironment of different types of HPV infection and also provide data supporting clinical diagnosis and treatment.
Methods: According to strict inclusion and exclusion criteria, the case data of 2,358 female patients who underwent vaginal microecology and HPV-DNA tests at the same time in the Department of Obstetrics and Gynecology of the First Affiliated Hospital of Xi'an Jiaotong University from May 2021 to March 2022 were retrospectively analyzed. The population was divided into two groups: an HPV-positive group and an HPV-negative group. HPV-positive patients were further classified into HPV16/18-positive group and HPV other subtypes positive group. The vaginal microecology of HPV-infected patients was analyzed using the chi-square test, Fisher's exact test, and logistic regression.
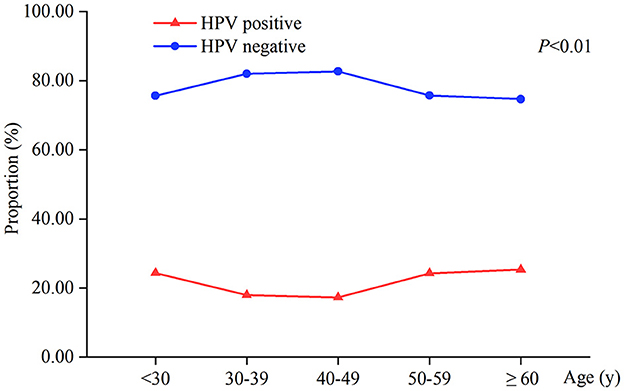
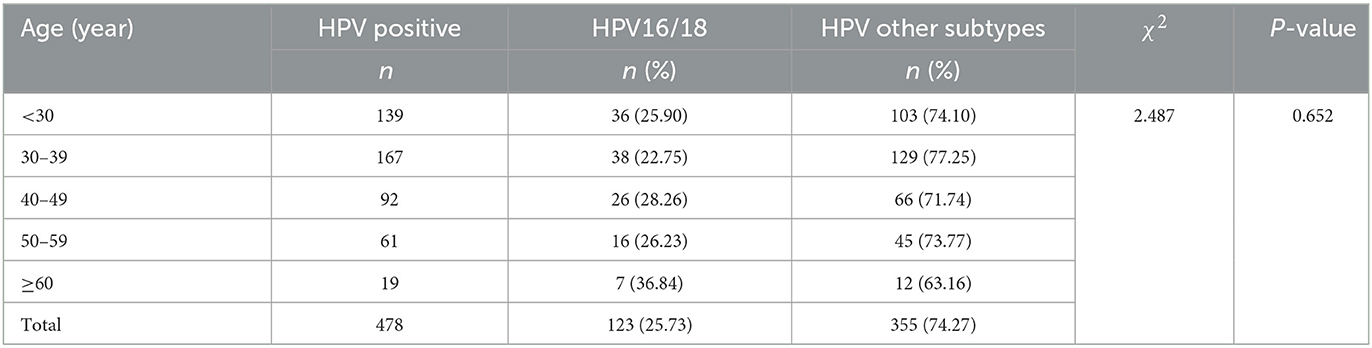
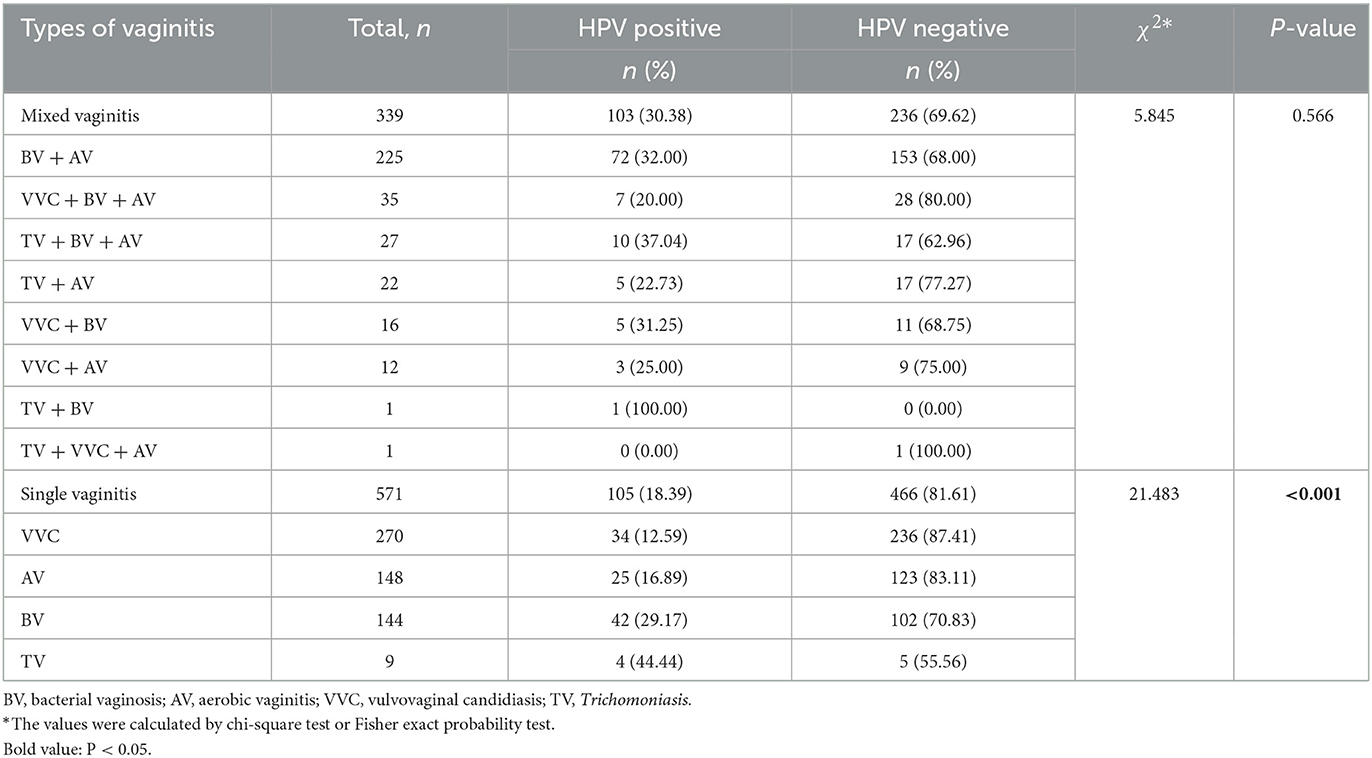
Results: Among the 2,358 female patients, the HPV infection rate was 20.27% (478/2,358), of which the HPV16/18 infection rate was 25.73% (123/478), and the HPV other subtypes infection rate was 74.27% (355/478). The difference in HPV infection rates between the age groups was statistically significant (P < 0.01). The prevalence of mixed vaginitis was 14.37% (339/2,358), with bacterial vaginosis (BV) paired with aerobic vaginitis (AV) accounting for the majority (66.37%). The difference in HPV infection rates among mixed vaginitis was not statistically significant (P > 0.05). The prevalence of single vaginitis was 24.22% (571/2,358), with the most frequent being vulvovaginal Candidiasis (VVC; 47.29%, 270/571), and there was a significant difference in HPV infection rates among single vaginitis (P < 0.001). Patients with BV had a higher risk of being positive for HPV16/18 (OR: 1.815, 95% CI: 1.050–3.139) and other subtypes (OR: 1.830, 95% CI: 1.254–2.669). Patients with Trichomoniasis were at higher odds of other HPV subtype infections (OR: 1.857, 95% CI: 1.004–3.437). On the contrary, patients with VVC had lower odds of becoming infected with other HPV subtypes (OR: 0.562, 95% CI: 0.380–0.831).
Conclusion: There were disparities in HPV infection among different age groups; therefore, we should pay attention to the prevention and treatment of susceptible individuals. BV and Trichomoniasis are linked to HPV infection; hence, restoring the balance of vaginal microecology could assist in the prevention of HPV infection. As a protective factor for other HPV subtype infections, VVC may provide new insights into the development of immunotherapeutic therapies.
Introduction
In 2020, cervical cancer had the fourth highest incidence and mortality rate among female patients, with an estimated 604,000 new cases and 342,000 deaths worldwide (1). High-risk human papillomavirus (HR-HPV) persistent infection, especially HPV16/18 infection, is considered to be a key factor in the occurrence of cervical cancer. Although most HPV infectious cases can be cleared in the first 2 years (2), the factors that promote HPV persistence and trigger carcinogenic pathways are not fully understood. In recent years, vaginal microecology has become a research hotspot for various gynecological diseases, and a variety of vaginal microbiota and related inflammation has been found to be potential drivers of HPV infection and disease severity (3). Previous studies have revealed that bacterial vaginosis (BV) and decreased Lactobacilli are associated with an increased risk of HPV infection (4, 5), while the relationships between Trichomoniasis, vulvovaginal Candidiasis (VVC), and other vaginal microdysbiosis and HPV infection are controversial (5–7). After collecting the results of vaginal microecology examinations, HPV-DNA tests, and related clinical data, this study aimed to clarify the correlation between vaginal microecology and HPV infection, so as to provide solid guidance for reducing the odds of HPV infection and preventing cervical cancer.
Materials and methods
Population screening
From May 2021 to March 2022, a total of 2,358 patients from the physical examination center, assisted reproductive technology center, and gynecology clinics were enrolled in this study. They underwent vaginal microecology and HPV-DNA tests during the same period at the First Affiliated Hospital of Xi'an Jiaotong University, Shaanxi Province, China. The median [interquartile range (IQR)] age of participants was 35 (28–42) years. The inclusion criteria are as follows: participants who (1) had a sexual history; (2) had no gynecological examination or vaginal ultrasound within 24 h; (3) are at a non-menstrual stage; and (4) had accepted HPV-DNA tests in our hospital. The exclusion criteria are as follows: participants who (1) are pregnant or lactating; (2) had sexual intercourse or used a drug in the vagina within the last 3 days; (3) used antibiotics within 1 month; (4) had accompanying autoimmune diseases or being under immunosuppressive treatment; (5) are complicated with serious medical or surgical diseases; (6) had the history of cervical lesion or HPV infection treatment, including medical and surgical treatments; and (7) had the history of HPV vaccination. The flow chart of the screening process is shown in the Supplementary Figure 1. This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China.
Detection methods
Two sterile cotton swabs were used to collect secretions from the upper 1/3 of the vagina; the swabs were placed in test tubes for immediate testing. A special brush for HPV-DNA testing was placed into the cervical canal and rotated 3–5 times; the brush was removed and placed into a specimen tube containing cell preservation solution, then the tube cap was closed and the samples were sent for testing.
Testing and diagnosis
One of the sample cotton swabs was used to make two slide preparations, one to microscopically observe Trichomoniasis and perform a Donders score using the physiological saline wet sheet method; and the other was used to observe the density and diversity of flora, pseudohyphae, spores, Nugent score, and Lactobacilli grading, as viewed microscopically under oil after drying, fixing, and gram staining. We used the Vaginitis Combined Test Kit provided by Jiangsu Shuoshi Biotechnology Co., Ltd., to detect vaginal microbial function and inflammatory response indicators using the specimens on the second set of cotton swabs, including pH value, leukocyte esterase (LE), neuraminidase (SNA), β-glucuronidase (GUS), and β-N-acetylglucosaminidase (NAG). Evaluation and diagnosis were based on the Vaginal Microecology Evaluation System v.2016 (8) as follows: (1) Normal vaginal microecology: density and diversity grades were II–III, the dominant bacteria were Lactobacilli, no pathogenic microorganisms were detected, vaginal pH value was 3.8–4.5, Lactobacilli grade was I–IIa, and inflammatory response indicators were negative; (2) BV: Nugent score ≥7 points; (3) VVC: fungal spores or pseudohyphae could be found microscopically under oil; (4) aerobic vaginitis (AV): according to the clinical manifestations and microscopic Donders score ≥3 points; (5) Trichomoniasis: a large number of white blood cells and active Trichomoniasis under the microscope; (6) Mixed vaginitis: two or more types of vaginitis existing at the same time; and (7) Microbial function and inflammatory response indicators could be determined according to the test kit.
DNA from cervical cells was extracted for hybridization and color development. A total of 15 high-risk HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, and 68) and 6 low-risk HPV (6, 11, 26, 73, 81, and 82) types can be detected by HPV-DNA detection kit provided by Jiangsu Shuoshi Biotechnology Co., Ltd. The evaluation and diagnosis results were classified into (1) “HPV-positive”: one or more of the above-mentioned HPV subtypes were detected and divided into “HPV16/18-positive group” and “HPV other subtype positive group”; and (2) “HPV-negative”: none of the above HPV subtypes were detected.
Statistical methods
SPSS 25.0 and Origin 2019 software were used for statistical analysis and graphing, respectively. Quantitative data were expressed as median (IQR) and qualitative data were described by absolute and relative indices. The chi-square test, Fisher's exact probability test, or univariate logistic regression analysis were used for the comparison of factors between the groups, the multivariate logistic regression analysis was carried out with significant results, and the OR value and 95% CI were calculated. Statistical tests were two-sided; P < 0.05 was considered statistically significant.
Results
HPV infection at different age ranges
The total HPV infection rate of 2,358 patients was 20.27% (478/2,358). There was a statistically significant difference in HPV infection between the different age groups (P < 0.01). The HPV-positive rates of women aged <30, 50–59, and ≥60 years were 24.34%, 24.30%, and 25.33%, respectively, higher than 17.29%−17.98% of women aged 30–49 years (Table 1, Figure 1).
HPV-positive types at different age distributions
The incidence of HPV16/18-positive was 25.73% (123/478), while the rate of HPV other subtypes was 74.27% (355/478). The difference in HPV16/18-positive rate between different age groups was not statistically significant (P > 0.05). HPV16/18 infection was most common in women aged ≥60 years, with a positive rate of 36.84%; the lowest positive rate was in the 30–39 years group (22.75%). HPV other subtypes infection was more common in women aged 30–39 years, and the positive rate was 77.25%. On average, the infection rates of HPV other subtypes in different age groups were three times higher than that of HPV16/18 (Table 2, Figure 2).
HPV infection in patients with different types of vaginitis
Among the 2,358 patients, 471 cases had normal vaginal microecology; there were 1,887 cases of abnormalities, including 339 cases of mixed vaginitis, 571 cases of single vaginitis, and 977 cases of vaginal microdysbiosis of an unknown pathogen. BV + AV accounted for the largest number (66.37%, 225/339) in patients with mixed vaginitis. In mixed vaginitis, the difference in HPV infection rates was not statistically significant (P > 0.05). VVC was the most common single vaginitis (47.29%, 270/571), and there was a significant difference in HPV infection rates among single vaginitis (P < 0.001; Table 3).
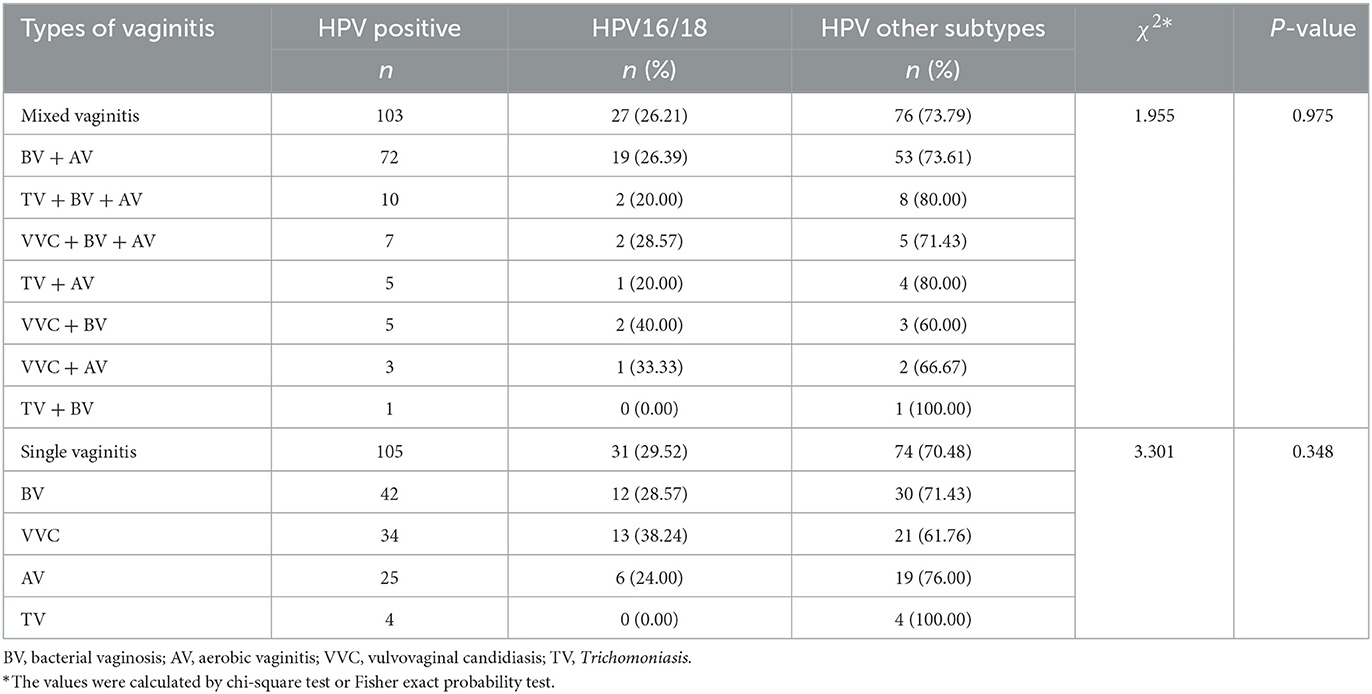
Different types of vaginitis in HPV-positive patients
HPV-positive patients with mixed vaginitis and single vaginitis accounted for 21.55% (103/478) and 21.97% (105/478), respectively. BV+AV was the most common mixed vaginitis in HPV-positive patients (69.90%, 72/103), and BV was the most common single vaginitis (40.00%, 42/105). There was no significant difference in the HPV16/18 infection rate among different types of vaginitis either for mixed vaginitis or single vaginitis (P > 0.05; Table 4).
Determinants of HPV16/18 positive
Table 5 shows distributions of patient characteristics according to the status HPV16/18 positive, using univariable and multivariable logistic regression. The differences in the incidence of BV and abnormal Lactobacilli grade were statistically significant between HPV16/18-positive and HPV-negative groups (P < 0.05). On the multivariable logistic regression, women with BV (OR: 1.815, 95% CI: 1.050–3.139) had higher odds of being HPV16/18 positive.
Determinants of HPV other subtypes positive
Table 6 shows distributions of patient characteristics according to HPV other subtypes positive by univariable and multivariable logistic regression. There were significant differences in Trichomoniasis, VVC, BV, AV, abnormal SNA, and Lactobacilli grade between HPV other subtypes positive and HPV-negative groups (P < 0.05). Multivariate logistic regression showed that patients with Trichomoniasis (OR: 1.857, 95% CI: 1.004–3.437) or BV (OR: 1.830, 95% CI: 1.254–2.669) had higher odds of being positive for HPV other subtypes; on the contrary, patients with VVC (OR: 0.562, 95% CI: 0.380–0.831) had lower odds of becoming infected with HPV other subtypes.
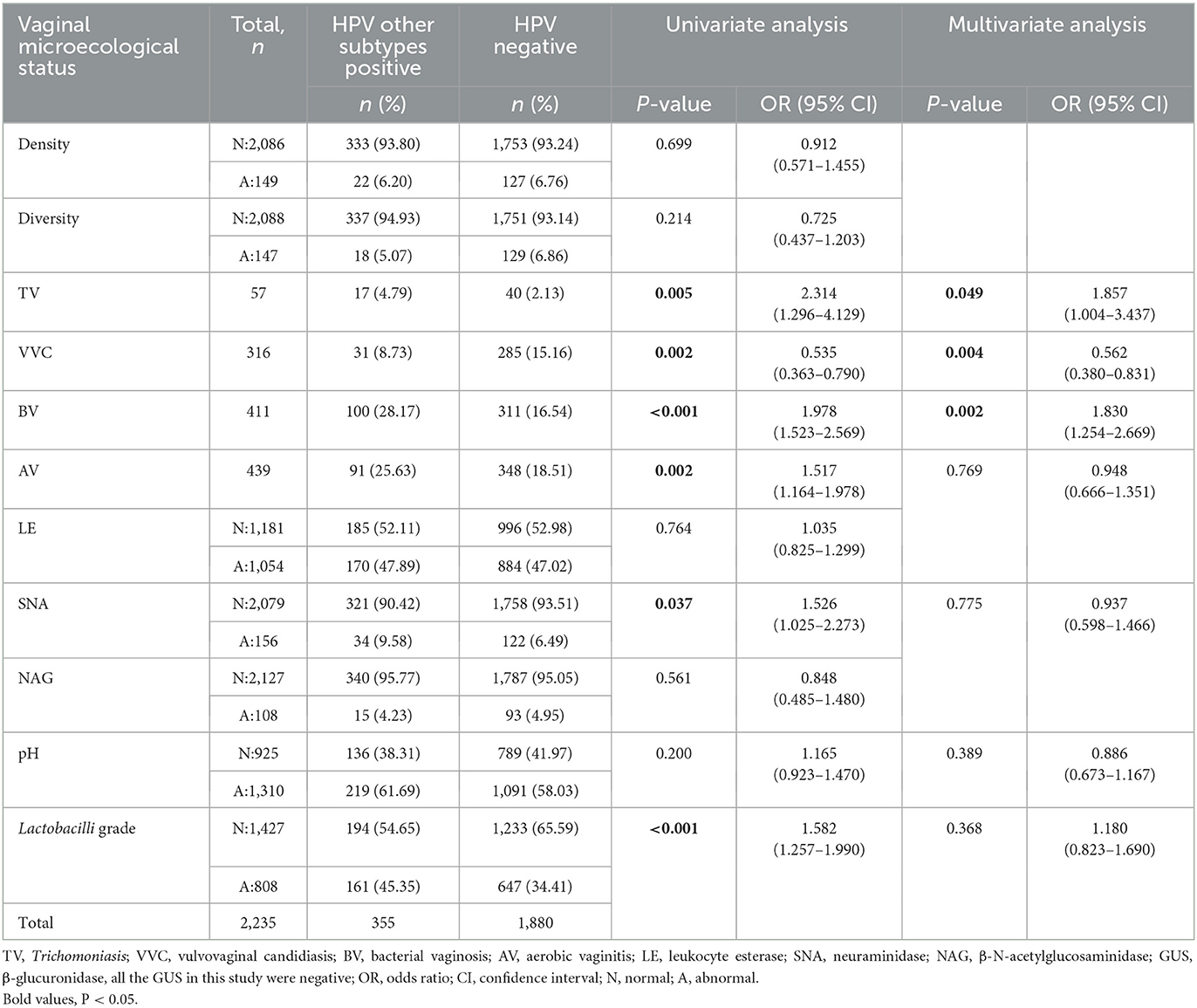
Table 6. The vaginal microecological status between HPV other subtypes positive and HPV-negative groups.
Discussion
In a 2021 study of 36 types of cancer in 186 countries, researchers found that cervical cancer was the most common cancer in 23 countries and was the leading cause of cancer death in 36 countries (1); it has remained a globally important public health issue. At present, the significance of HR-HPV infection, especially HPV16/18 persistent infection, for cervical cancer has been recognized by most clinicians. Our study showed that the HPV infection rate was 20.27%, a decrease from 22.97% in the same region previously (9), which may be related to higher education, widespread HPV vaccination, or an increase in average socioeconomic level (10, 11). HPV infection rate was higher in women aged <30 and ≥50 years; this was consistent with the previously reported double-peak distribution of HPV infection rates, which was considered to be related to the frequent sexual life, smoking, and oral hormonal contraceptive use in young women (11, 12), as well as the changes in sexual behavior, low autoimmunity, and the reactivation of menopausal latent HPV in older women (13, 14).
In this study, HPV16/18 infection rate was 25.73%, and it was more common in women ≥60 years, which was similar to findings in a previous study (9). Zhang et al. (15) found that the incidence of cervical cancer for women aged >60 years was 18.52%, while it was 0.99% for those aged ≤30 years, suggesting the existence of substantial cervical cancer risk for older women. We compared the vaginal microecology of patients with HPV16 single-type infection and those with HPV16 combined multiple-type infection in our research. However, we found no significant difference between them. Similarly, there was no statistically significant difference in the vaginal microecological status between the HPV18 single-type infection group and the HPV18 combined multiple-type infection group. We also found an interesting feature that the infection rates of HPV other subtypes in different age groups were, on average, three times higher than that of HPV16/18. This may be caused by differing invasiveness in various HPV types (16) and some cross-protection between HPV 16, 18, 6, 11, 31, and 45 (17). The reasons for this unique characteristic are not clear and should be investigated further. Although the HPV infection rate in young women is high, most infections are transient (2). From the specific age curve of cervical cancer, the incidence increased from the age of 25 years (18). Through a balanced decision analysis of the pros and cons, cervical cancer screening is strongly recommended by the American Cancer Society (ACS) from age 25 years using the primary HPV test (19).
Mixed vaginitis is the simultaneous presence of at least two types of vaginitis, contributing to an abnormal vaginal milieu. Nevertheless, the signs and symptoms of mixed vaginitis are often atypical, and treatment is complicated in contrast to single-type vaginitis (20). Owing to differences in race, region, detection technology, and methods, the incidence of mixed vaginitis is reported to vary greatly, from 4.44 to 35.06% (20, 21); meanwhile, the result in our study was 14.37%, of which BV combined with AV was the most common type (66.37%). Currently, little is known about the vaginal pathophysiology of mixed vaginitis. Studies have found that there were synergistic effects between different pathogens, allowing them to form multi-strain biofilms and to further lead to refractory infections and recurrences (21, 22). Bacteria and/or fungi can coexist within a host and influence each other via synergistic or antagonistic interactions (23). Furthermore, HPV infection can change host immunity or mucosal metabolism so that the genital tract microenvironment changes, making it easier for other infectious diseases to take hold (16). The latest consensus on mixed vaginitis also mentions that the issue of simultaneous infection of the cervix and vagina needs to be addressed while paying attention to the mixed infection of various vaginal inflammations, including Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma, and HPV (24).
The novelty of this study is that it investigates the correlation between mixed vaginitis and HPV infection, an area that has limited research. Research considered that mixed vaginitis was related to HPV infection by having higher species diversity and significantly fewer Lactobacilli (25). However, our study did not find any differences in mixed vaginitis among the different groups of HPV. Further basic research is needed to verify and clarify this correlation in the future.
Since AV was first proposed in 2002 (26), the research has gradually increased. Some researchers believe that AV is not related to HPV infection (27), while others consider that the infection status of AV patients is related to HR-HPV susceptibility of cervical epithelial cells (28). Meanwhile, studies also found no statistical relationship between moderate-to-severe AV and HR-HPV infection, but it may be involved in the progression of HR-HPV-infected women to cervical intraepithelial neoplasia and cancer (29); in turn, this was related to the inhibition of innate immune response due to their chronic inflammatory features. The AV-associated interleukin (IL) profile was consistent with that found in patients with HR-HPV infection progressing to cancer. Our study found that, compared with the HPV negative group, the infection rate of AV in the HPV other subtypes positive group was higher, but it was not an independent risk factor for the other subtypes positive group. Because of the high proportion (68.51%) of AV patients combined with the other vaginitis in this study, and mainly combined with BV, we suggest that different pathogens influenced each other, which may have affected the interpretation of their correlation. Increasingly, studies have shown a higher incidence of AV-related mixed vaginitis (30, 31), and the relationship between AV itself and HPV infection requires further investigation.
Previous studies have shown that the prevalence of HR-HPV is higher in patients with BV (32). Furthermore, a prospective study has revealed that BV is associated with persistent HPV infection, and the HPV clearance rate is reduced in women with BV (33), which is consistent with our results. It may be that an endogenous mixed infection caused by decreased Lactobacilli in the vagina, increased Gardnerella, and other anaerobic bacteria in BV increases the content of mucinolytic enzymes in the vagina and disrupts the local mucosal barrier, promoting viral adhesion, invasion, and even integration into the host genome, thereby increasing susceptibility to HPV infection. At the same time, anaerobic bacteria can produce ammonia and carcinogenic nitrites, resulting in abnormal lesions of the cervical epithelium (34).
The main type of single vaginitis varied widely owing to region, ethnicity, and population origin (5, 29). VVC was the most common vaginitis in our study. There is still controversy about the relationship between VVC and HPV infection; while VVC has been reported to not be related to HPV infection (5), another study found that VVC had a high incidence in the HPV infection group (34), which may be related to the inflammation and the increased mucosal permeability of the invasive enzymes caused by infection. However, a meta-analysis also found that VVC may be a protective factor against HPV infection (35); similarly, our study showed that VVC could reduce the risk of infection in HPV other subtypes. Candida albicans exhibits colonization; it is usually a harmless member of the native microbiota (23), and can promote T-cell proliferation and enhance immune response (35), which gives protection against HPV other subtypes. Moreover, researchers found that a skin test agent extracted from C. albicans and used as an adjuvant for a new HPV therapeutic vaccine can induce the immune response of HPV-specific CD8+ T cells and Th1 CD4+ T cells and can also induce humoral immunity (36). This indicates that C. albicans is a potential immunotherapeutic agent and could be employed in the development of new vaccines or the treatment of HPV infection and even other diseases.
Trichomoniasis is also a common sexually transmitted disease, associated with multiple health consequences in men and women. Our study found that Trichomoniasis can increase the risk of infection of HPV other subtypes, consistent with previous studies (37). This may be owing to the release of lytic enzymes by Trichomoniasis, which reduces the protective mucus layer of the vagina, leading to the development of epithelial cell microlesions, increasing the erosiveness of other subtypes of HPV, and allowing their DNA to integrate into host cells. This inflammatory process can also destroy the basal layer of the cervical epithelium, thus promoting the continued presence of HPV in cervical–vaginal epithelial tissue (38).
It has been shown that HPV infection is associated with an increase in floral diversity and a decrease in the number of Lactobacilli in the vaginal microecological environment (25). Our study found that the diversity of microbiota was not related to HPV infection; however, Lactobacilli grade and SNA were associated with infection of HPV other subtypes. We believe that the presence of normal Lactobacilli plays an important role in maintaining the acidic environment of the vagina and preventing the invasion of pathogenic microorganisms. Nevertheless, Lactobacilli still cannot produce sufficient protective effect against HPV16/18. As the main pathogen of BV, the detection rate of Gardnerella was significantly increased in HR-HPV-positive women (32); meanwhile, Gardnerella secretes SNA while elevated SNA concentration was associated with increased risk for cervical lesion and HR-HPV persistent infection (39, 40). A case–control study found that there was an interaction effect with abnormities of GUS and SNA on CIN2/3 in the HPV16-negative group, while there was an interaction effect with abnormities of GUS and SNA on CIN in the HPV16-positive group (41). Studies have also shown that the difference in positive rates of GUS between the HPV-positive and HPV-negative groups was not statistically significant (42). Furthermore, few studies have addressed the correlation between NAG and HPV infections. Therefore, further investigations are needed to determine the correlation among NAG, GUS, and HPV infections.
This is a cross-sectional study. To clarify the correlation between vaginal microbiota and different types of HPV infection, it is necessary to expand the sample size in multiple centers and design prospective studies, which is also a common limitation of this study and most other related studies. In addition, we all know that the vaginal microbiome is an intricate and dynamic microecosystem that constantly undergoes fluctuations during the female menstrual cycle and the woman's entire life (43, 44), which may influence the conclusion of the study. It is another limitation of this study.
In conclusion, our study from a hospital in northwest China found differences in HPV infection rates within different age ranges, which offers insights into the prevention of infection at different ages. Vaginal infectious diseases play an important role in HPV infection, and patients with BV or Trichomoniasis should be alerted to this interaction. Meanwhile, exploring the mechanism of action of VVC may assist in the development of new vaccines or immunotherapeutic agents.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
R-fA: study concept and design and critical revision. FF and Y-mH: data acquisition. FF, Y-mH, and L-yW: data analysis and interpretation. YZ, P-pL, and YG: manuscript preparation. All authors contributed to the article and approved the submitted version.
Funding
This retrospective study was funded by the National Natural Science Foundation of China (No. 81972428).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1138507/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. (2007) 195:1582–9. doi: 10.1086/516784
3. Ntuli L, Mtshali A, Mzobe G, Liebenberg LJ, Ngcapu S. Role of immunity and vaginal microbiome in clearance and persistence of human papillomavirus infection. Front Cell Infect Microbiol. (2022) 12:927131. doi: 10.3389/fcimb.2022.927131
4. Łaniewski P, Cui H, Roe DJ, Barnes D, Goulder A, Monk BJ, et al. Features of the cervicovaginal microenvironment drive cancer biomarker signatures in patients across cervical carcinogenesis. Sci Rep. (2019) 9:7333. doi: 10.1038/s41598-019-43849-5
5. Zhang D, Li T, Chen L, Zhang X, Zhao G, Liu Z. Epidemiological investigation of the relationship between common lower genital tract infections and high-risk human papillomavirus infections among women in Beijing, China. PLoS ONE. (2017) 12:e0178033. doi: 10.1371/journal.pone.0178033
6. Hu SY, Tsang SH, Chen F, Pan QJ, Zhang WH, Hong Y, et al. Association between common vaginal infections and cervical non-human papillomavirus (HPV) 16/18 infection in HPV-vaccinated women. J Infect Dis. (2021) 223:445–51. doi: 10.1093/infdis/jiaa384
7. Zheng JJ, Miao JR, Wu Q, Yu CX, Mu L, Song JH. Correlation between HPV-negative cervical lesions and cervical microenvironment. Taiwan J Obstet Gynecol. (2020) 59:855–61. doi: 10.1016/j.tjog.2020.08.002
8. Cooperative Cooperative Group of Infectious Disease Chinese Chinese Society of Obstetrics and Gynecology Chinese Medical Association. Expert consensus on the clinical application of vaginal microecology test. Zhonghua Fu Chan Ke Za Zhi. (2016) 51:721–3. doi: 10.3760/cma.j.issn.0529-567X.2016.10.001
9. Lin X, Chen L, Zheng Y, Yan F, Li J, Zhang J, et al. Age-specific prevalence and genotype distribution of human papillomavirus in women from Northwest China. Cancer Med. (2022) 11:4366–73. doi: 10.1002/cam4.4732
10. Bouvard V, Wentzensen N, Mackie A, Berkhof J, Brotherton J, Giorgi-Rossi P, et al. The IARC perspective on cervical cancer screening. N Engl J Med. (2021) 385:1908–18. doi: 10.1056/NEJMsr2030640
11. Chen Z, Lin H, Zheng J, Cai L, Chen Z, Li J, et al. Epidemiological study of HPV infection in 40,693 women in Putian: a population study based on screening for high-risk HPV infection. BMC Infect Dis. (2022) 22:893. doi: 10.1186/s12879-022-07893-3
12. Torres-Poveda K, Ruiz-Fraga I, Madrid-Marina V, Chavez M, Richardson V. High risk HPV infection prevalence and associated cofactors: a population-based study in female ISSSTE beneficiaries attending the HPV screening and early detection of cervical cancer program. BMC Cancer. (2019) 19:1205. doi: 10.1186/s12885-019-6388-4
13. Brotherton JM, Hawkes D, Sultana F, Malloy MJ, Machalek DA, Smith MA, et al. Age-specific HPV prevalence among 116,052 women in Australia's renewed cervical screening program: a new tool for monitoring vaccine impact. Vaccine. (2019) 37:412–6. doi: 10.1016/j.vaccine.2018.11.075
14. Althoff KN, Paul P, Burke AE, Viscidi R, Sangaramoorthy M, Gravitt PE. Correlates of cervicovaginal human papillomavirus detection in perimenopausal women. J Womens Health. (2009) 18:1341–6. doi: 10.1089/jwh.2008.1223
15. Zhang Q, Zhao M, Cao D, Wei X, Wang L, Li Y, et al. Assessment of the effectiveness of HPV16/18 infection referred for colposcopy in cervical cancer screening in Northwest of China. J Med Virol. (2018) 90:165–71. doi: 10.1002/jmv.24902
16. McBride AA. Human papillomaviruses: diversity, infection and host interactions. Nat Rev Microbiol. (2022) 20:95–108. doi: 10.1038/s41579-021-00617-5
17. Thomas KK, Hughes JP, Kuypers JM, Kiviat NB, Lee SK, Adam DE, et al. Concurrent and sequential acquisition of different genital human papillomavirus types. J Infect Dis. (2000) 182:1097–102. doi: 10.1086/315805
18. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. (2020) 8:e191–203. doi: 10.1016/S2214-109X(19)30482-6
19. Fontham ETH, Wolf AMD, Church TR, Etzioni R, Flowers CR, Herzig A, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. (2020) 70:321–46. doi: 10.3322/caac.21628
20. Qi W, Li H, Wang C, Li H, Zhang B, Dong M, et al. Recent advances in presentation, diagnosis and treatment for mixed vaginitis. Front Cell Infect Microbiol. (2021) 11:759795. doi: 10.3389/fcimb.2021.759795
21. Xiao B, Disi A, Qin H, Mi L, Zhang D. Correlation analysis of vaginal microbiome changes and bacterial vaginosis plus vulvovaginal candidiasis mixed vaginitis prognosis. Front Cell Infect Microbiol. (2022) 12:860589. doi: 10.3389/fcimb.2022.860589
22. Xie Y, Feng Y, Li W, Zhan F, Huang G, Hu H, et al. Revealing the disturbed vaginal micobiota caused by cervical cancer using high-throughput sequencing technology. Front Cell Infect Microbiol. (2020) 10:538336. doi: 10.3389/fcimb.2020.538336
23. Lohse MB, Gulati M, Johnson AD, Nobile CJ. Development and regulation of single- and multi-species Candida albicans biofilms. Nat Rev Microbiol. (2018) 16:19–31. doi: 10.1038/nrmicro.2017.107
24. Cooperative Cooperative Group of Infectious Disease Chinese Chinese Society of Obstetrics and Gynecology Chinese Medical Association. Consensus on the diagnosis and treatment of mixed vaginitis (2021 edition). (2021) 56:15–18. doi: 10.3760/cma.j.cn112141-20200603-00472
25. Lee JE, Lee S, Lee H, Song YM, Lee K, Han MJ, et al. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS ONE. (2013) 8:e63514. doi: 10.1371/journal.pone.0063514
26. Donders GG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G, Spitz B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. BJOG. (2002) 109:34–43. doi: 10.1111/j.1471-0528.2002.00432.x
27. Jahic M, Mulavdic M, Hadzimehmedovic A, Jahic E. Association between aerobic vaginitis, bacterial vaginosis and squamous intraepithelial lesion of low grade. Med Arch. (2013) 67:94–6. doi: 10.5455/medarh.2013.67.94-96
28. Salambanga C, Zohoncon TM, Traoré IMA, Ouedraogo RA, Djigma WF, Ouédraog C, et al. High prevalence of high-risk human papillomavirus (HPV) infection among sexually active women in Ouagadougou. Med Sante Trop. (2019) 29:302–5. doi: 10.1684/mst.2019.0920
29. Vieira-Baptista P, Lima-Silva J, Pinto C, Saldanha C, Beires J, Martinez-de-Oliveira J, et al. Bacterial vaginosis, aerobic vaginitis, vaginal inflammation and major Pap smear abnormalities. Eur J Clin Microbiol Infect Dis. (2016) 35:657–64. doi: 10.1007/s10096-016-2584-1
30. Zhang T, Xue Y, Yue T, Xiong L, Wang X, Wang W, et al. Characteristics of aerobic vaginitis among women in Xi'an district: a hospital-based study. BMC Womens Health. (2020) 20:138. doi: 10.1186/s12905-020-00997-5
31. Li M, Zeng Z, Feng H, Cao Y, Zhang Q, Lv T, et al. Accurate 16S absolute quantification sequencing revealed vaginal microecological composition and dynamics during mixed vaginitis treatment with Fufang FuRong effervescent suppository. Front Cell Infect Microbiol. (2022) 12:883798. doi: 10.3389/fcimb.2022.883798
32. Lin W, Zhang Q, Chen Y, Dong B, Xue H, Lei H, et al. Changes of the vaginal microbiota in HPV infection and cervical intraepithelial neoplasia: a cross-sectional analysis. Sci Rep. (2022) 12:2812. doi: 10.1038/s41598-022-06731-5
33. Guo YL, You K, Qiao J, Zhao YM, Geng L. Bacterial vaginosis is conducive to the persistence of HPV infection. Int J STD AIDS. (2012) 23:581–4. doi: 10.1258/ijsa.2012.011342
34. Kero K, Rautava J, Syrjänen K, Grenman S, Syrjänen S. Association of asymptomatic bacterial vaginosis with persistence of female genital human papillomavirus infection. Eur J Clin Microbiol Infect Dis. (2017) 36:2215–9. doi: 10.1007/s10096-017-3048-y
35. Liang Y, Chen M, Qin L, Wan B, Wang H. A meta-analysis of the relationship between vaginal microecology, human papillomavirus infection and cervical intraepithelial neoplasia. Infect Agent Cancer. (2019) 14:29. doi: 10.1186/s13027-019-0243-8
36. Wang X, Che Y, Chen B, Zhang Y, Nakagawa M, Wang X. Evaluation of immune responses induced by a novel human papillomavirus type 16 E7 peptide-based vaccine with Candida skin test reagent as an adjuvant in C57BL/6 mice. Int Immunopharmacol. (2018) 56:249–60. doi: 10.1016/j.intimp.2018.01.037
37. Menon S, Broeck DV, Rossi R, Ogbe E, Harmon S, Mabeya H. Associations between vaginal infections and potential high-risk and high-risk human papillomavirus genotypes in female sex workers in Western Kenya. Clin Ther. (2016) 38:2567–77. doi: 10.1016/j.clinthera.2016.10.005
38. Belfort IKP, Cunha APA, Mendes FPB, Galvão-Moreira LV, Lemos RG, de Lima Costa LH, et al. Trichomonas vaginalis as a risk factor for human papillomavirus: a study with women undergoing cervical cancer screening in a northeast region of Brazil. BMC Womens Health. (2021) 21:174. doi: 10.1186/s12905-021-01320-6
39. Meng D, Song L, Qi Z, Wang J, Liu H, Lyu YJ, et al. [Prognosis of high-risk HPV infection and its influences by vaginal micro-environmental factors]. Zhonghua Liu Xing Bing Xue Za Zhi. (2021) 42:1103–7. doi: 10.3760/cma.j.cn112338-20200829-01107
40. Govinden G, Parker JL, Naylor KL, Frey AM, Anumba DOC, Stafford GP. Inhibition of sialidase activity and cellular invasion by the bacterial vaginosis pathogen Gardnerella vaginalis. Arch Microbiol. (2018) 200:1129–33. doi: 10.1007/s00203-018-1520-4
41. Li L, Ding L, Gao T, Lyu Y, Wang M, Song L, et al. Association between vaginal micro-environment disorder and cervical intraepithelial neoplasia in a community based population in China. J Cancer. (2020) 11:284–91. doi: 10.7150/jca.35022
42. Zheng JJ, Song JH, Yu CX, Wang F, Wang PC, Meng JW. Difference in vaginal microecology, local immunity and HPV infection among childbearing-age women with different degrees of cervical lesions in Inner Mongolia. BMC Womens Health. (2019) 19:109. doi: 10.1186/s12905-019-0806-2
43. Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UM, Zhong X, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. (2012) 4:132ra152. doi: 10.1126/scitranslmed.3003605
Keywords: vaginal microecology, human papillomavirus (HPV), mixed vaginitis, bacterial vaginosis, vulvovaginal candidiasis
Citation: Feng F, Hou Y-m, Zhang Y, Wang L-y, Li P-p, Guo Y and An R-f (2023) Correlation analysis of vaginal microecology and different types of human papillomavirus infection: a study conducted at a hospital in northwest China. Front. Med. 10:1138507. doi: 10.3389/fmed.2023.1138507
Received: 30 January 2023; Accepted: 08 May 2023;
Published: 01 June 2023.
Edited by:
Jose Eleuterio Junior, Federal University of Ceara, BrazilReviewed by:
Bingbing Xiao, Peking University, ChinaPeipei Liu, Chinese Academy of Sciences (CAS), China
Copyright © 2023 Feng, Hou, Zhang, Wang, Li, Guo and An. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui-fang An, YW5ydWlmYW5nOTM2QDE2My5jb20=
†ORCID: Rui-fang An orcid.org/0000-0001-6501-1016
 Fang Feng
Fang Feng Yue-min Hou1
Yue-min Hou1 Rui-fang An
Rui-fang An