
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 24 July 2023
Sec. Pulmonary Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1135570
This article is part of the Research Topic Respiratory Support: Clinical Applications and the Novel Future View all 16 articles
A commentary has been posted on this article:
Commentary: Comparative efficacies of various corticosteroids for preventing postextubation stridor and reintubation: a systematic review and network meta-analysis
 I-Jung Feng1†
I-Jung Feng1† Jia-Wei Lin2†
Jia-Wei Lin2† Chih-Cheng Lai3
Chih-Cheng Lai3 Kuo-Chen Cheng4,5
Kuo-Chen Cheng4,5 Chin-Ming Chen6,7
Chin-Ming Chen6,7 Chien-Ming Chao8
Chien-Ming Chao8 Ying-Ting Wang9
Ying-Ting Wang9 Shyh-Ren Chiang4,10*
Shyh-Ren Chiang4,10* Kuang-Ming Liao11*
Kuang-Ming Liao11*Objectives: We assessed the efficacies of various corticosteroid treatments for preventing postexubation stridor and reintubation in mechanically ventilated adults with planned extubation.
Methods: We searched the Pubmed, Embase, the Cochrane databases and ClinicalTrial.gov registration for articles published through September 29, 2022. Only randomized controlled trials (RCTs) that compared the clinical efficacies of systemic corticosteroids and other therapeutics for preventing postextubation stridor and reintubation were included. The primary outcome was postextubation stridor and the secondary outcome was reintubation.
Results: The 11 assessed RCTs reported 4 nodes: methylprednisolone, dexamethasone, hydrocortisone, and placebo, which yielded 3 possible pairs for comparing the risks of post extubation stridor and 3 possible pairs for comparing the risks of reintubation. The risk of postextubation stridor was significantly lower in dexamethasone- and methylprednisolone-treated patients than in placebo-treated patients (dexamethasone: OR = 0.39; 95% CI = 0.22–0.70; methylprednisolone: OR = 0.22; 95% CI = 0.11–0.41). The risk of postextubation stridor was significantly lower in methylprednisolone-treated patients than in hydrocortisone-treated: OR = 0.24; 95% CI = 0.08–0.67) and dexamethasone-treated patients: OR = 0.55; 95% CI = 0.24–1.26). The risk of reintubation was significantly lower in dexamethasone- and methylprednisolone-treated patients than in placebo-treated patients: (dexamethasone: OR = 0.34; 95% CI = 0.13–0.85; methylprednisolone: OR = 0.42; 95% CI = 0.25–0.70). Cluster analysis showed that dexamethasone- and methylprednisolone-treated patients had the lowest risks of stridor and reintubation. Subgroup analyses of patients with positive cuff-leak tests showed similar results.
Conclusions: Methylprednisolone and dexamethasone were the most effective agents against postextubation stridor and reintubation.
Endotracheal intubation is a common procedure for critically ill patients with acute respiratory failure that requires mechanical ventilation (MV) in an intensive care unit (ICU) or for patients under general anesthesia undergoing surgery in the operating room. Although endotracheal intubation is usually uneventful, it can lead to tracheal rupture, pneumothorax or pneumomediastinum, tongue necrosis, or soft palate injury (1–5). Laryngeal edema is another complication of endotracheal intubation, and it might be a cause of postextubation stridor and subsequent reintubation (6). Most important, laryngeal edema-associated extubation failure can prolong an ICU or hospital stay, increase mortality and morbidity, and add to medical costs. Thus, it is important to avoid laryngeal edema-associated postextubation stridor and reintubation failure.
Several interventions—parenterally administering corticosteroids, nebulizing epinephrine, and administering an inhaled helium/oxygen mixture—have been developed to prevent postextubation stridor and reintubation (6). Corticosteroids are supposed to reduce both inflammation and edema, and to exert their effect on preventing postextubation stridor and reintubation. Moreover, several meta-analyses (7–9) have shown that administering prophylactic corticosteroids before planned extubation significantly reduced the incidence rates of postextubation stridor and reintubation in adults. In studies that used methylprednisolone, dexamethasone, hydrocortisone, and placebo (10–19), however, corticosteroid anti-inflammation potencies differed. No prior study has specifically compared these four interventions. We hypothesized that different corticosteroids would have different effects against postexubation stridor and reintubation. Therefore, we did this network meta-analysis to assess the efficacies of these interventions.
This network meta-analysis (NMA) was based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) extension statement (20). The protocol of this study was prospectively registered at PROSPERO (registration number: CRD42022362888). All clinical studies were identified in a systematic review of the literature through September 29, 2022 in the Pubmed, Embase, the Cochrane databases and ClinicalTrial.gov registration. We used the following search terms: “intubation,” “intratracheal,” “laryngeal edema,” “airway obstruction,” “stridor,” “post extubation larynx* edema*,” steroid*,” “corticosteroid*,” ‘glucocorticoid*,” “prednisone,” “prednisolone,” “methylprednisolone,” “dexamethasone,” “cortisone,” “hydrocortisone,” and “adult” (Appendix 1). Only randomized clinical trials (RCTs) that compared the efficacy of systemic corticosteroid treatments and other interventions against postextubation stridor and reintubation in MV adults scheduled for extubation were included. We also searched for additional eligible studies in the reference lists of retrieved articles and relevant meta-analyses. To avoid bias, two reviewers (CCL and JWL) independently searched and examined publications. Any discrepancies were judged and determined by third author (IJF).
The following data were extracted from every included study: year of publication, study design, sample size, gender ratio, treatment regimens of corticosteroids and other interventions, cumulative doses (mg), frequency of administration, and incidence rates of postextubation stridor and reintubation. For RCTs that used medians and interquartile ranges as measures of central tendency and dispersion, we estimated means and standard deviations (SDs) (21).
The primary outcome was overall postextubation stridor and reintubation. Subgroup analyses of patients with positive cuff-leak tests were also performed.
The quality of the analyzed RCTs and the risk of bias were assessed using the Cochrane Risk of Bias Assessment tool (22). We assessed seven quality domains: sequence generation, allocation concealment, participant masking, personnel masking, outcome assessor masking, incomplete outcome data, and reporting bias. Other biases were also assessed. Finally, we judged the quality of each domain as (a) low risk of bias, (b) unclear risk of bias, or (c) high risk of bias.
We used STATA 15.0 (StataCorp, College Station, TX, USA), R (version 4.2.1) and R package “netmeta” to do a frequentist network meta-analysis (23). Because of the diversity of the task-related characteristics of the included RCTs, we used a random-effects model. The heterogeneity variable of all comparisons was assumed to be a single τ2 value. The required property of transitivity was also assumed.
We used a design-by-treatment interaction model for global tests and side-splitting model for local tests to evaluate inconsistencies between direct and indirect evidence. Network plots show direct comparisons of investigated outcomes, and odds ratios (ORs) and 95% confidence intervals (95% CIs) show network effect-size estimates of pairwise comparisons.
A surface under the cumulative ranking curve (SUCRA) presents the likely rank order of each investigated treatment. To evaluate the joint ranking of postextubation stridor and reintubation both clinical outcomes, cluster analysis was performed. The optimal number of resulting cluster was defined by having maximum intra-cluster similarity and minimize inter-cluster similarity (24). Finally, we assessed publication bias using Egger's test.
We initially identified 308 studies. After excluding 44 duplicate articles, 264 articles were screened, 135 of which were excluded on the basis of the title and abstract. After excluding study on non-adult patients (n = 135), non-RCT (n = 30), the 99 remaining articles underwent a full-text review to assess their eligibility. Finally, a total of eleven RCTs (10–19, 25) fulfilled the inclusion criteria were selected for our network meta-analysis (Figure 1; Table 1). Six RCTs (11, 12, 16–19) were done in Taiwan, three (13–15) in France, one (25) in India, and one (10) in Pakistan. Six RCTs (10–12, 17–19) used the cuff leak test (CLT) to select high-risk patients. Dexamethasone was the most commonly used corticosteroid (n = 5), (10, 13, 17, 18, 25) then methylprednisolone (n = 4), (11, 12, 14, 15) and, finally, hydrocortisone (n = 2) (16, 19). Follow-up observation durations were 24 h (13, 14, 16, 25) and 48 h (10–12, 17, 18). Routes of administration, doses, and frequencies of administration differed (Appendix 2). Four studies (11, 13, 15, 16) used one injection before extubation, but five studies (10, 12, 17–19) used corticosteroid for 24 h before extubation. One RTC (25) intravenously injected one group of patients with dexamethasone and treated another group with nebulized budesonide 1 h before the scheduled extubation, and then every 12 h for 48 h after the extubation.
Among enrolled studies, four reported nodes were methylprednisolone, dexamethasone, hydrocortisone and placebo, resulting in 3 possible pairs for comparing the risks of post extubation stridor and 3 possible pairs for comparing the risks of reintubation. Most comparisons with direct evidence are from dexamethasone or methylprednisolone and placebo. Regarding the risk of postextubation stridor, five studies compared the effects between dexamethasone and placebo. Four and one studies used methylprednisolone and hydrocortisone as study agents, respectively (Figure 2A). Regarding the risk of reintubation, each four studies compared the effect between dexamethasone or methylprednisolone and placebo. Two studies used compared hydrocortisone with placebo (Figure 2B).
The risk of bias in each domain of each study was low (Figure 3). Control group in Gaussorhues et al.'s study (15) received nothing. This was hard to prevent participants and experimenters to keep unaware the group assignment during study period. High risks were marked in the bias evaluation report. Because no closed-loop was formed for the network of collected postextubation stridor-related literature, inconsistency was not examined here; however, the literature showed a significant publication bias (Egger's test: p = 0.013). In contrast, there was no publication bias (Egger's test: p = 0.623) in the collected reintubation-related literature.
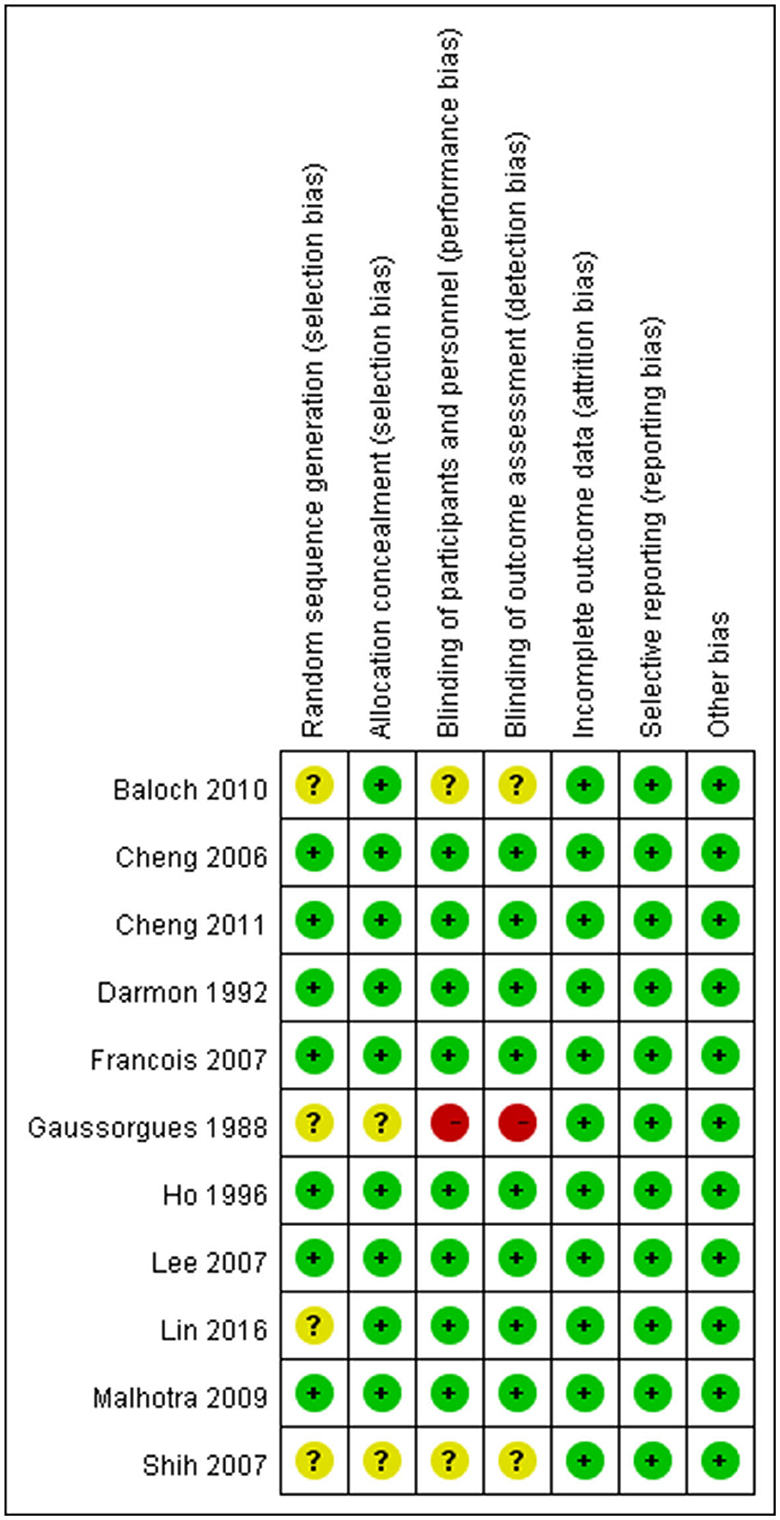
Figure 3. Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
NMAs showed that the risk of postextubation stridor was significantly lower in dexamethasone- and methylprednisolone-treated patients than in placebo-treated patients (dexamethasone: OR: 0.39; 95% CI: 0.22-0.70; methylprednisolone: OR: 0.22; 95% CI: 0.11-0.41) (Table 2A; Figure 4).
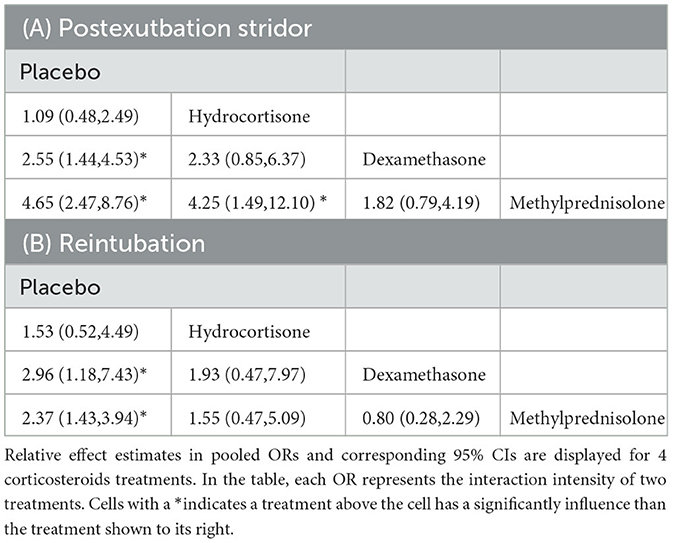
Table 2. Preventive efficiencies for postextubation stridor (A) and reintubation (B) calculated for each pair of corticosteroids as per the results of the network meta-analyses.
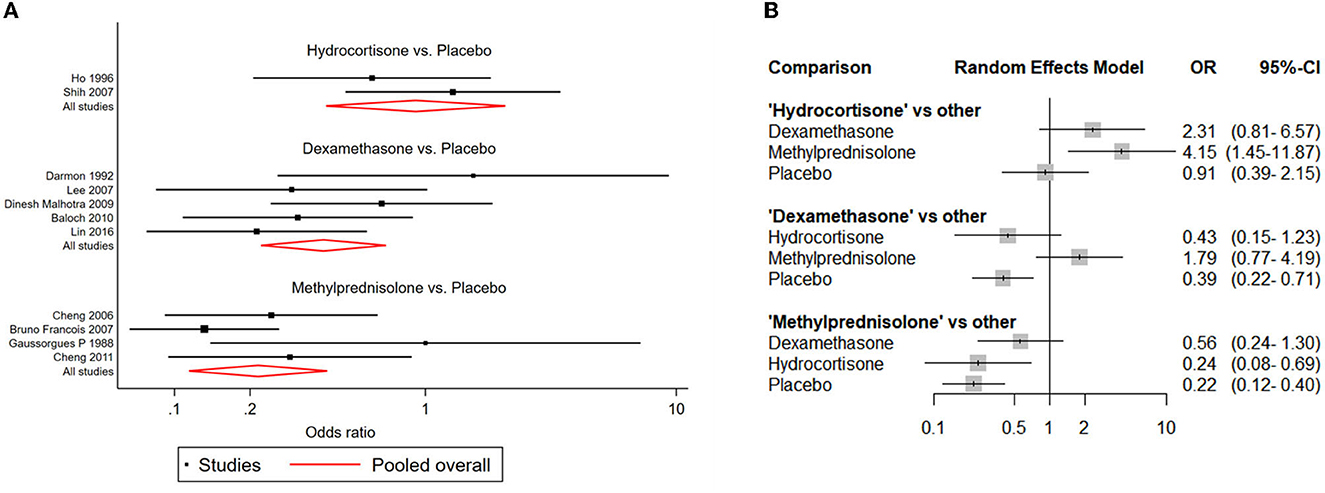
Figure 4. Network forest plots of postextubation stridor. (A) Forest plot of individual studies grouped by comparison with direct evidence. ORs and corresponding 95% CIs were displayed. (B) Forest plot of summarized association between different corticosteroids and risk of postextubation stridor.
Compared with placebo, both dexamethasone and methylprednisolone were found significantly associated with lower risk of reintubation (dexamethasone: OR: 0.34; 95% CI: 0.13-0.85; methylprednisolone: OR: 0.42; 95% CI: 0.25-0.70) (Table 2B; Figure 5).
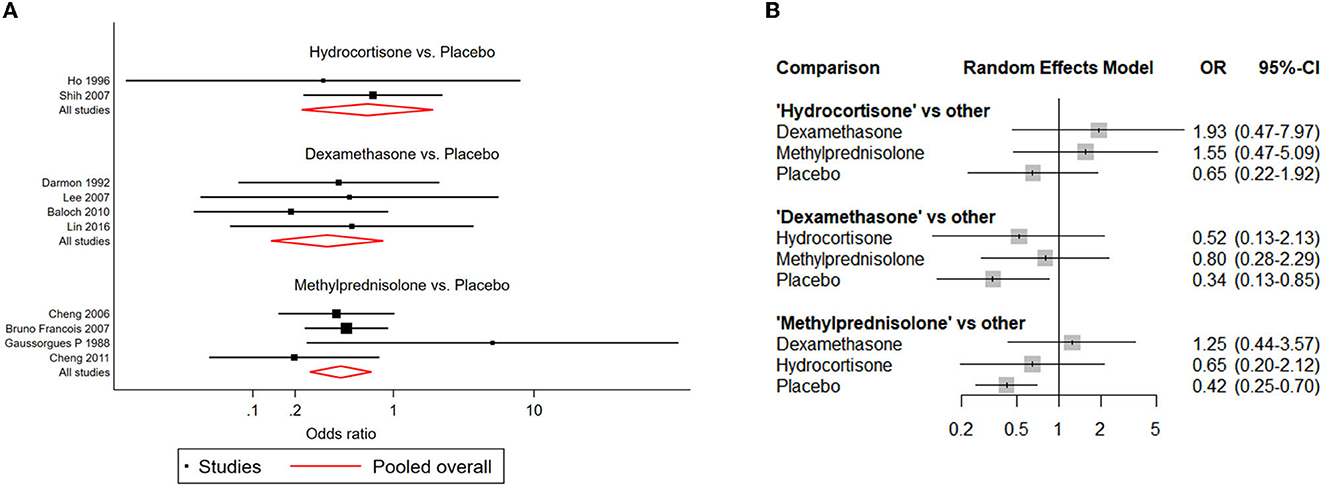
Figure 5. Network forest plots of reintubation. (A) Forest plot of individual studies grouped by comparison with direct evidence. ORs and corresponding 95% CIs were displayed. (B) Forest plot of summarized association between different corticosteroids and risk of reintubation.
For preventing postextubation stridor, methylprednisolone had the largest surface under the cumulative ranking curve (SUCRA) (97.6), followed by dexamethasone (66.9), hydrocortisone (21.6), and placebo (13.9). For reducing the risk of reintubation, dexamethasone had the largest SUCRA (80.7), followed by methylprednisolone (70.7), hydrocortisone (41.0), and placebo (7.6) (Figure 6). According to the SUCRA ranking outcomes, methylprednisolone and dexamethasone were considered in one with the most potent intervention cluster (Figure 6).
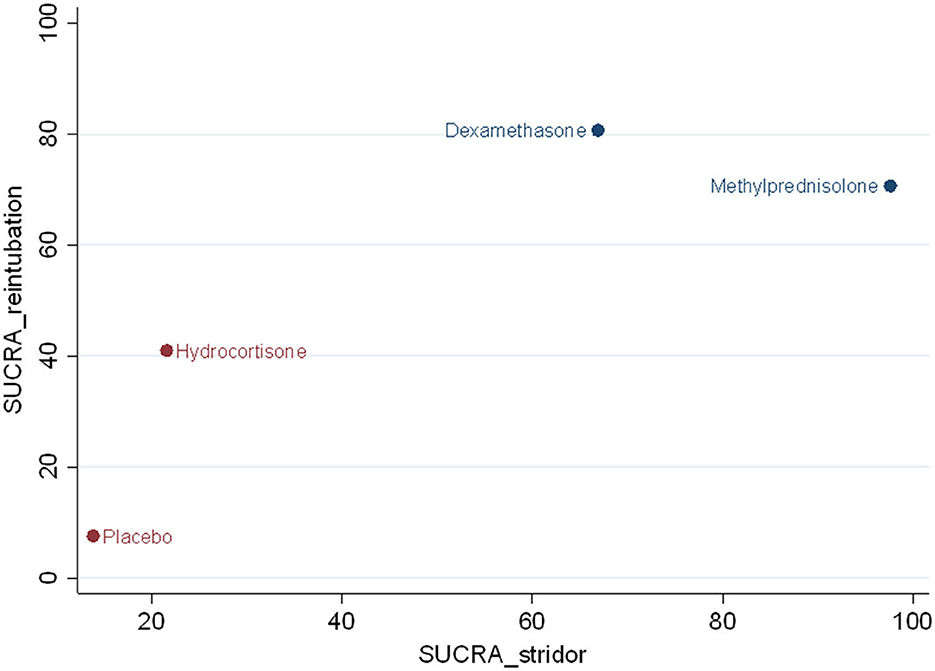
Figure 6. Clustered ranking plot. The SUCRA value for each agent for preventing effect of postextubation stridor and reintubation.
For patients with positive CLTs, both dexamethasone and methylprednisolone were associated with a significantly lower risk of postextubation stridor than was placebo (dexamethasone: OR:0.26; 95% CI:0.14–0.50; SUCRA: 75.1; methylprednisolone: OR:0.26; 95% CI:0.13–0.55; SUCRA: 74.8). Moreover, both corticosteroids were associated with a significantly lower risk of reintubation than was placebo (dexamethasone: OR:0.31; 95% CI:0.10–0.94; SUCRA: 74.0; methylprednisolone: OR:0.32; 95% CI:0.14–0.69; SUCRA: 74.8) methylprednisolone, respectively.
This is the first network meta-analysis of 11 RCTs with a total of 2,371 patients that compare the efficacies of three commonly used corticosteroids (dexamethasone, methylprednisolone, and hydrocortisone) against postextubation stridor and reintubation in patients with a scheduled extubation. This analysis provided useful information on the choice of appropriate corticosteroid formula for preventing postextubation stridor and reintubation in this population. Of three kinds of corticosteroid, dexamethasone and methylprednisolone were the most efficacious corticosteroids in this network meta-analysis. Methylprednisolone was ranked highest in the preventing postextubation stridor, and in contrast, hydrocortisone was ranked lowest. Regarding reintubation, dexamethasone and methylprednisolone was ranked much higher than hydrocortisone. A similar trend was observed in the subgroup analysis of high risk patients selected using cuff-leak test. Overall, this network meta-analysis also found that methylprednisolone and dexamethasone were associated with significantly lower risk of postextubation stridor and reintubation than placebo among overall population and the subgroups selected with cuff-leak test. In summary, we found that methylprednisolone and dexamethasone are the most potent kind of corticosteroids in the preventing postextubation stridor and reintubation and their prophylactic use can effectively reduce the risk of postextubation stridor and reintubation before elective extubation among patients with mechanical ventilation. In contrast, the usefulness of hydrocortisone was limited in this clinical condition. All these findings should suggest the better role of methylprednisolone and dexamethasone than hydrocortisone in this clinical entity.
Our findings in this network meta-analysis can be explained by the different anti-inflammatory activities of three corticosteroids. Corticosteroids differ in their relative amount of anti-inflammatory potency. Dexamethasone and methylprednisolone have much higher anti-inflammatory potency than hydrocortisone. In general, 25 mg of hydrocortisone is equivalent to 5 mg of methylprednisolone, and 1 mg of dexamethasone according to their relative anti-inflammatory potency (28). Therefore, methylprednisolone and dexamethasone are supposed to more effectively reduce inflammatory laryngeal edema and further significantly lower the risk of postextubation stridor and reintubation than hydrocorticosone. We suggest using methylprednisolone (20 mg ever 6 h) and dexamethasone (5 mg ever 6 h) before extubation.
Our study has some limitations. First, the regimens of the corticosteroid treatments are different in the selected RCTs, which might lead to different preventive effects. Second, the number of enrolled studies is small; thus, we cannot further assess the effects of the different regimens of corticosteroids. Additional studies are needed to investigate their confounding effects. Third, some mechanisms such as publication bias and other forms of reporting bias might have impact on our interpretation of the results. Several possible confounding factors, such as sex, duration of intubation, and the size of the endotracheal tube were not assessed in this meta-analysis. There are some potential biases, heterogeneity or generalizability in meta-analyses. The meta-analysis combines the results of various types of study design to generate an overall effect size. If there are significant heterogeneities in these studies, the focus should shift from the summary effect to the dispersion itself. In addition, meta-analysis focused on the main outcomes and its results can be generalizable to particular group of patients but not another.
However, we could not do a subgroup analysis because so few trials reported separate results based on sex, duration of intubation, and the size of the endotracheal tube. We did not evaluate the risk of adverse effects caused by corticosteroids.
Although the risk of corticosteroid-related complications, e.g., fluid retention, infection, gastrointestinal bleeding, and hyperglycemia are low in additional investigations are needed to assess the possibility of negative side effects. Finally, because studies were not administering equipotent doses of steroids, methylprednisolone and dexamethasone are more effective than hydrocortisone and this may be a significant confounder.
The comparison between different corticosteroids are statistical rather than direct clinical comparisons. However, we clarify the selection of studies, pre-specification of the pooled analysis, the risk of bias in each domain of each study was low and the outcome of individual studies in relation to the pooled result. We believe the validity and interpretation of pooled analyses.
Our findings indicate that methylprednisolone and dexamethasone are the most effective corticosteroids for preventing postextubation stridor and reintubation. Nevertheless, future large-scale clinical studies are needed to confirm our findings.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
C-CL, C-MChe, and I-JF: concepts and design. J-WL, K-CC, S-RC, and I-JF: analysis and interpretation of data. C-MCha and C-CL: drafting of the manuscript. C-MChe, K-ML, and Y-TW: critical revision of the manuscript. I-JF: statistical analysis. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1135570/full#supplementary-material
1. Khan RM, Sharma PK, Kaul N. Barotrauma: a life-threatening complication of fiberoptic endotracheal intubation in a neonate. Paediatr Anaesth. (2010) 20:782–4. doi: 10.1111/j.1460-9592.2010.03360.x
2. Kuhn MA, Zeitler DM, Myssiorek DJ. Tongue necrosis: a rare complication of oral intubation. Laryngoscope. (2010) 120 Suppl 4:S159. doi: 10.1002/lary.21623
3. Pham Q, Lentner M, Hu A. Soft palate injuries during orotracheal intubation with the videolaryngoscope. Ann Otol Rhinol Laryngol. (2017) 126:132–7. doi: 10.1177/0003489416678008
4. Sucato DJ, Girgis M. Bilateral pneumothoraces, pneumomediastinum, pneumoperitoneum, pneumoretroperitoneum, and subcutaneous emphysema following intubation with a double-lumen endotracheal tube for thoracoscopic anterior spinal release and fusion in a patient with idiopathic scoliosis. J Spinal Disord Tech. (2002) 15:133–8. doi: 10.1097/00024720-200204000-00007
5. Xu X, Xing N, Chang Y, Du Y, Li Z, Wang Z, et al. Tracheal rupture related to endotracheal intubation after thyroid surgery: a case report and systematic review. Int Wound J. (2016) 13:268–71. doi: 10.1111/iwj.12291
6. Wittekamp BHJ, van Mook WNKA, Tjan DHT, Zwaveling JH, Bergmans DCJJ. Clinical review: post-extubation laryngeal edema and extubation failure in critically ill adult patients. Crit Care. (2009) 13:233. doi: 10.1186/cc8142
7. Fan T, Wang G, Mao B, Xiong Z, Zhang Y, Liu X, et al. Prophylactic administration of parenteral steroids for preventing airway complications after extubation in adults: meta-analysis of randomised placebo controlled trials. BMJ. (2008) 337:a1841. doi: 10.1136/bmj.a1841
8. McCaffrey J, Farrell C, Whiting P, Dan A, Bagshaw SM, Delaney AP. Corticosteroids to prevent extubation failure: a systematic review and meta-analysis. Intensive Care Med. (2009) 35:977–86. doi: 10.1007/s00134-009-1473-9
9. Kuriyama A, Umakoshi N, Sun R. Prophylactic corticosteroids for prevention of postextubation stridor and reintubation in adults: a systematic review and meta-analysis. Chest. (2017) 151:1002–10. doi: 10.1016/j.chest.2017.02.017
10. Baloch R, Jakhrani N, Lal AS, Mehmood NA. Role of dexamethasone for prevention of post-extubation airway obstruction in critically ill adult patients. J Surg Pak [International]. (2010) 15:3–8. Available online at: http://old.jsp.org.pk/Issues/JSP%2015(1)%20Jan%20March%202010/Rohina%20N%20Baloch%20OA.pdf
11. Cheng KC, Chen CM, Tan CK, Chen HM, Lu CL, Zhang H. Methylprednisolone reduces the rates of postextubation stridor and reintubation associated with attenuated cytokine responses in critically ill patients. Minerva Anestesiol. (2011) 77:503–9.
12. Cheng KC, Hou CC, Huang HC, Lin SC, Zhang H. Intravenous injection of methylprednisolone reduces the incidence of postextubation stridor in intensive care unit patients. Crit Care Med. (2006) 34:1345–50. doi: 10.1097/01.CCM.0000214678.92134.BD
13. Darmon JY, Rauss A, Dreyfuss D, Bleichner G, Elkharrat D, Schlemmer B, et al. Evaluation of risk factors for laryngeal edema after tracheal extubation in adults and its prevention by dexamethasone: a placebo-controlled, double-blind, multicenter study. Anesthesiology. (1992) 77:245–51. doi: 10.1097/00000542-199208000-00004
14. François B, Bellissant E, Gissot V, Desachy A, Normand S, Boulain T, et al. 12-h pretreatment with methylprednisolone versus placebo for prevention of postextubation laryngeal oedema: a randomised double-blind trial. Lancet. (2007) 369:1083–9. doi: 10.1016/S0140-6736(07)60526-1
15. Gaussorgues P, Boyer F, Piperno D, Gerard M, Leger P, Robert D. Do corticosteroids prevent postextubation laryngeal edema? prospective study of 276 adults. Crit Care Med. (1988) 16:649. doi: 10.1097/00003246-198806000-00021
16. Ho LI, Harn HJ, Lien TC, Hu PY, Wang JH. Postextubation laryngeal edema in adults. risk factor evaluation and prevention by hydrocortisone. Intensive Care Med. (1996) 22:933–6. doi: 10.1007/BF02044118
17. Lee CH, Peng MJ, Wu CL. Dexamethasone to prevent postextubation airway obstruction in adults: a prospective, randomized, double-blind, placebo-controlled study. Crit Care. (2007) 11:R72. doi: 10.1186/cc5957
18. Lee C-H, Peng M-J, Wu C-L. Comparison of high-and low-dose dexamethasone for preventing postextubation airway obstruction in adults: a prospective, randomized, double blind, placebo-controlled study. Int J Gerontol. (2016) 10:11–6. doi: 10.1016/j.ijge.2015.10.002
19. Cheng KC, Chen CM, Tan CK, Lin SC, Chen HM, Zhang H. Methylprednisolone reduces the incidence of postextubation stridor associated with downregulation of IL-6 in critical ill patients. Am J Respir Crit Care Med. (2007) 7:A593. doi: 10.1097/01.CCM.0000262548.97035.1C
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
21. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
22. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
23. Balduzzi S, Rücker G, Nikolakopoulou A, Papakonstantinou T, Salanti G, Efthimiou O, et al. netmeta: an R package for network meta-analysis using frequentist methods. J Stat Softw. (2023) 106:1–40. doi: 10.18637/jss.v106.i02
24. Jung Y, Park H, Du DZ, Drake BL. A decision criterion for the optimal number of clusters in hierarchical clustering. J Global Optimization. (2003) 25:91–111. doi: 10.1023/A:1021394316112
25. Malhotra D, Gurcoo S, Qazi S, Gupta S. Randomized comparative efficacy of dexamethasone to prevent postextubation upper airway complications in children and adults in ICU. Indian J Anaesth. (2009) 53:442–9.
26. Shih CM, Chen W, Tu CY, Chen HJ, Lee JC, Tsai WK, et al. Multiple injections of hydrocortisone for the prevention of post-extubation stridor in acute respiratory failure. Am J Respir Crit Care Med. (2007) A593.
27. Lin CY, Cheng KH, Kou LK, Lee CH. Comparison of high-and low-dose dexamethasone for preventing postextubation airway obstruction in adults: a prospective, randomized, double blind, placebo-controlled study. Int J Gerontol. (2016) 10:11–6.
Keywords: dexamethasone, hydrocortisone, methylprednisolone, network meta-analysis, postextubation stridor, reintubation
Citation: Feng I-J, Lin J-W, Lai C-C, Cheng K-C, Chen C-M, Chao C-M, Wang Y-T, Chiang S-R and Liao K-M (2023) Comparative efficacies of various corticosteroids for preventing postextubation stridor and reintubation: a systematic review and network meta-analysis. Front. Med. 10:1135570. doi: 10.3389/fmed.2023.1135570
Received: 01 January 2023; Accepted: 23 June 2023;
Published: 24 July 2023.
Edited by:
Jun Duan, First Affiliated Hospital of Chongqing Medical University, ChinaReviewed by:
Hong Weng, Wuhan University, ChinaCopyright © 2023 Feng, Lin, Lai, Cheng, Chen, Chao, Wang, Chiang and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kuang-Ming Liao, YWJjODg3MEB5YWhvby5jb20udHc=; Shyh-Ren Chiang, Q2hpYW5nc3JAZ21haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.