- 1Department of Internal Medicine, Texas Tech University Health Sciences Center El Paso, El Paso, TX, United States
- 2Paul L. Foster School of Medicine, El Paso, TX, United States
Portal Vein Thrombosis (PVT), a common complication of advanced liver disease, is defined as an obstruction of the portal vein due to thrombus formation that can extend to the superior mesenteric and splenic veins. It was believed that PVT occurred predominantly due to prothrombotic potential. However, recent studies have shown that decreased blood flow related to portal hypertension appears to increase PVT risk as per Virchow’s triad. It is well known that there is a higher incidence of PVTs in cirrhosis with a higher MELD and Child Pugh score. The controversy for management of PVTs in cirrhotics lies in the individualized assessment of risks versus benefits of anticoagulation, since these patients have a complex hemostatic profile with both bleeding and procoagulant propensities. In this review, we will systematically compile the etiology, pathophysiology, clinical features, and management of portal vein thrombosis in cirrhosis.
Introduction
In a study conducted between 1970 and 1982 on 23,796 autopsies revealed a portal vein thrombosis (PVT) prevalence of 1% wherein 28% of the subjects had cirrhosis, 23% of the subjects had primary hepatobiliary malignancy and 44% of the subjects had secondary hepatobiliary malignancy (1). The incidence of PVT in cirrhosis is unclear but, ranges from 4.4% to 15.8% (2). In a prospective study of 369 cirrhotic patients, the incidence of PVT was 1.6% at 1 year, 6% at 3 years and 8.3% at 5 years (3).
Portal Vein Thrombosis (PVT) is defined as a partial or complete obstruction of the portal vein due to thrombus formation (4). Although, there are several causes for PVT development whether it may local or systemic, they usually occur in concurrence to one another. A meta-analysis which included 2,436 cirrhotic patients suggests that PVT may increase mortality and ascites (5). In this review, we will discuss the etiopathogenesis, clinical features, diagnosis, management, and prevention of portal vein thrombosis in cirrhosis.
Etiopathogenesis
The etiopathogenesis of portal vein thrombosis (PVT) in cirrhosis is dynamic. Increased PVT risk is associated with decreased blood flow in relation to portal hypertension as per Virchow’s triad. The liver has a major role in the hemostatic system as it synthesizes most of the coagulation factors and proteins involved in fibrinolysis. It also synthesizes thrombopoietin which is responsible for platelet production. Consequently, acute and chronic liver disease have a profound impact on the hemostatic system. All three major components of the hemostatic process are disturbed in patients with cirrhosis. The derangements occur both on the procoagulant and the anticoagulation processes, leaving cirrhotic patients in a state of rebalanced hemostasis that can be altered in either direction in response to insults.
The extent of certain parameters correlating with PVT in cirrhosis has been shown in literature. Previously, PVT formation has been postulated as a consequence to a hypercoagulable and hyperinflammatory state due to the decrease of anticoagulants such as protein C and protein S with higher concentrations of certain factors such as Von Willebrand Factor (vWF) and factor VIII. Indeed, these risk factors are significant for systemic DVT formation. However, these same factors do not contribute to the formation of PVT. It was believed previously that the portal vein propagated PVT formation through the composition of an excessively inflammatory/hypercoagulable vascular bed. This belief was challenged in a prospective cohort study that showed that hypercoagulability and increased levels of inflammatory markers in the systemic circulation were not predictive of PVT development (6).
One risk factor for systemic DVT formation in the general public is non-O blood types. This is secondary to a hypercoagulable state due to increased von Willebrand Factor (VWF)/factor VIII levels in these individuals. This association is not present in non-O blood type patients with cirrhosis who develop PVT. A retrospective analysis of two large cohorts of cirrhosis patients with PVT found that non-O blood types was not a significant risk factor for PVT formation (7). In the first large cohort prospective study to address the risk factors for non-tumoral PVT development in cirrhotic patients, it was found that the severity of portal hypertension, history of variceal bleeding, low platelet count, and low portal blood flow velocity were the only significant factors contributing to PVT formation (8). It found that acquired or genetic hypercoagulable states were not significant risk factors. The dissociation of genetic hypercoagulability predisposing as a risk factor for PVT formation was further confirmed in a longitudinal prospective study (9).
The hemodynamic changes that occur in portal vein blood flow contribute in the formation of PVT in cirrhotic patients. As the severity of cirrhosis progresses, the intrahepatic vascular resistance increases proportionally and results in the clinical appearance of portal hypertension Consequentially, the increase in portal pressures leads to a compensatory splanchnic arteriolar vasodilation and the formation of porto-collateral vessels. The dilatory effect of the portal vein with shunting of blood away via the newly formed porto-collateral vessels leads to a substantial reduction of portal blood flow and portal flow velocity. The role of decreased portal flow velocity in the formation of PVT was demonstrated in a published prospective study of 100 cirrhotic patients in 2009 (10). This study found a flow velocity below 15 cm/s as a significant risk factor for the formation of PVT in cirrhotic patients. This parameter has been confirmed as a risk factor in other retrospective and prospective studies, therefore, establishing decreased portal flow velocity as a significant risk factor in the development of PVT in cirrhosis (3, 11, 12).
The role of endothelial intravascular vessel wall damage in hemostasis is well understood outside of the splanchnic territory. Although the territory is venous, the pathogenesis of thrombosis differs from that of systemic venous thrombosis. Most of information concerning the pathogenesis of venous thrombosis is systemic. This is largely because the splanchnic venous territory is vastly inaccessible (13, 14). During periods of stress, the endothelial cells can become damaged and express a pro-coagulant phenotype which propagates the initial formation of thrombus (15). The endothelial pro-coagulant phenotype has shown an upregulation of certain markers as the advancement of cirrhosis leads to a significant risk factor for PVT formation. Many studies have shown the upregulation of P-selectin (16, 17) and vWF (18, 19) in dysfunctional endothelium of the portal vein signifying a contributing factor for PVT. A study with 20 cirrhotic patients found elevated vWF in the portal venous circulation in comparison to the peripheral venous circulation, as well as increased levels of endothelial secreted Factor VIII (20). The significance of portal vein endothelial damage and upregulation of procoagulant factors is further supported by circulating factors such as sulphated glycosaminoglycans (GAGs), MP, annexin V+, CD62+, thrombomodulin and t-PAIC (21, 22). Although, the endothelial vessel wall damage plays a role in the formation of PVT, the exact mechanism is not completely elucidated and requires further research (10). The damage of the endothelial vessel wall from increased severity of portal hypertension also increases intimal hyperplasia. The association between formation of PVT secondary to upregulation of procoagulant factors, intimal hyperplasia, and increased severity of portal hypertension is not well understood. In sight of recent studies showing hypercoagulable states not significantly correlating with development of PVT in cirrhosis confirms that PVT and DVT/PE are two distinct disease processes with a distinct pathogenesis (3, 6, 7, 9). Thus, further elucidation of the pathophysiology of PVT formation is needed in literature.
The composition of a thrombosis in the portal vein is quite enigmatic in the sense that it can recanalize in the absence of treatment unlike systemic venous thrombi (10). A recent study analyzed 16 prospective and 64 retrospective portal vein segments from cirrhotic patients using histology and electron microscopy demonstrated that not only was the PVT contributing to the occlusion of the portal vein lumen, but rather the thickening of the tunica intima was also present (23). This appearance resembled intimal fibrosis and demonstrated that the thrombus formation found in the portal vein is more complex when compared to systemic venous thrombi. In addition, in 1/3rd of the cases they found fibrinogen-rich blood clots which was distinct to those described in deep vein thrombi or arterial clots (23, 24). A study showed reduced incidence of PVT in patients given prophylactic ATIII and danaparoid sodium with liver cirrhosis and portal hypertension after splenectomy demonstrates that the etiopathogenesis of a thrombosis differs substantially in comparison to a systemic thrombosis and thus must be treated differently (25).
The formation of PVT in liver cirrhosis is associated with certain risk factors. A retrospective study with 98 cirrhotic patients with PVT and 101 cirrhosis without PVT demonstrated risk factors that contribute to the formation of PVT in cirrhotic patients. This study demonstrated an increase occurrence of PVT in advanced cirrhotic patients which confirmed previous studies. In this study it was demonstrated that patients with hyperglycaemia, hypoalbuminemia, anemia, hyperbilirubinemia, and elevated INR (international normalised ratio) levels, were statistically more likely to have thromboses compared with those of the controls (26, 27). Another significant risk factor leading to PVT that was seen in patient within this study was having HBV which was also seen in a similar study (28). These risk factors have been associated with PVT formation in patients with liver cirrhosis and portal hypertension which have been confirmed in prior studies (29–31). In sight of the many studies demonstrating risk factors for the etiopathogenesis of PVT development in cirrhosis, a meta-analysis of these studies would provide further insight on those that are truly significant.
Clinical features
The prevalence of PVT formation is generally low in the general population, but increased in individuals who have advanced liver cirrhosis with portal HTN. Studies have shown that the prevalence of PVT in cirrhosis with portal HTN increases proportionally with the severity of the disease (2, 32, 33). One study showed that 219 cirrhotic patients awaiting liver transplantation had an overall prevalence PVT that was 15.9% (2), supporting the reported prevalence range of 8%–25% of PVT in advanced liver cirrhosis found in similar studies (34, 35).
The clinical presentation of PVT is classified based on certain criteria. This includes whether the PVT is acute or subacute or chronic; occlusive vs. nonocclusive; benign vs. malignant, and intrahepatic vs. extrahepatic (2, 36–41). Depending on the criteria that is fulfilled on initial presentation determines the severity of symptoms. For example, a partially occluded acute portal vein thrombosis may be asymptomatic and present with nonspecific symptoms. In comparison, a completely occluded thrombosis in an acute phase can present with acute or progressive abdominal pain with signs of decompensation of chronic liver disease in the form of variceal bleeding, worsening ascites, bloody diarrhea, peritonitis, intestinal ischemia, or portal cholangiopathy. In a cirrhotic patient, sudden clinical deterioration such as the development of bacterial peritonitis can indicate the formation of an acute PVT through the unknown pathogenetic interaction of bacterial translocation, decreased portal blood flow velocity, and intimal hyperplasia of the portal vein wall. Intestinal infarction is a significant risk factor when the propagation of the thrombus extends to the superior mesenteric vein, mesenteric arches, and/or splenic vein (42).
The severity of portal hypertension in association with the extension of a portal vein thrombosis in cirrhotic patients correlates to an increased risk of complications. A cirrhotic patient with a PVT has greater than a threefold bleeding risk in comparison to a similar patient without a PVT irrespective of the use of endoscopic hemostasis or surgical shunting (43). In an acute complete occlusion of the portal vein, hepatic arterial vasodilatation typically preserves liver function (38, 42). Following a period of 3–5 weeks, the obstructed PVT is bypassed through the formation of venous collateral which is known as a portal cavernoma (39, 42).
Two separate studies showed that PVT in cirrhosis delayed the time to endoscopically fix esophageal varices, while also serving as an indicating factor of decompensation with poor diagnosis (44, 45). Contrary to these findings, two studies demonstrated no association between PVT development and prognosis (46, 47). A partial PVT that spontaneously resolved demonstrated a potential indication for improvement in liver function, but did not contribute to clinical outcome of a cirrhotic patient (48, 49). In comparison to benign, chronic PVT formation with a mortality rate of less than 10%, malignant PVT formation in the presence of liver cirrhosis secondary to HCC increases the mortality rate by 26% (50, 51). This demonstrates that PVT in liver cirrhosis increases the mortality risk of a patient irrespective of inciting factors.
Diagnosis
There are many classifications of portal vein thrombosis. The Yerdel Classification is the most widely used because it can predict outcomes, correlates with surgical technique and with complication rates (33).
(i) Grade I: Partial PVT wherein the thrombus occupies less than 50% of the diameter.
(ii) Grade II: The obstruction occupies greater than 50% of the vessel lumen with or without minimal extension into the superior mesenteric veins.
(iii) Grade III: Complete obstruction of the portal vein with extension into the proximal part of the superior mesenteric vein.
(iv) Grade IV: Complete thrombosis of the portal vein along with proximal and distal parts of the superior mesenteric veins.
There are several different diagnostic modalities to evaluate for portal vein thrombosis as listed below:
Ultrasonography
Ultrasonography and doppler ultrasonography are typically first-line imaging modalities to evaluate for PVT in cirrhosis with doppler ultrasonography having greater than 75% sensitivity (44). The identification of a thrombus, absent visualization of the hepatic veins, collateral veins along with caudate lobe hypertrophy, and a caudate vein with greater than 3 mm diameter (44). An acute thrombus typically shows heterogenous material in the vessel lumen although it can also be hypoechoic or isoechoic (45). In contrast, a chronic thrombus will show increased hyper-echogenicity due to fibrinous composition (46). A thrombus can also show an absence of blood flow partially or completely in a vessel lumen on a color doppler or have an increase in vessel diameter of greater than 13 mm (45). In a study conducted in 1991 on the efficacy of color doppler imaging in PVT revealed that color doppler ultrasonography had a sensitivity of 89%, specificity of 92%, and an accuracy of 92% (47).
Contrast enhanced ultrasonography (CEUS) is another useful tool to evaluate for portal vein thrombosis wherein microbubble contrast is injected intravenously for easier detection of the blood flow. There is a higher sensitivity (90.9%) and specificity (100%) of detection when compared to color doppler ultrasonography (48, 49). CEUS also has the highest rates of detection and characterization when compared to CT imaging, ultrasonography, and color doppler ultrasonography (52).
Computed tomography scan (CT scan)
On an unenhanced CT, fresh thrombi are typically hyperdense whereas the chronic thrombi can go undetected unless calcifications are present (53). In contrast-enhanced CT imaging, the lumen of the thrombosed vein does not enhance when compared to other vascular structures with periportal enhancement in some cases likely secondary to proliferation of the vasa vasorum of the portal vein (54). Dual-energy multidetector CT scan with iodine quantification has an even better performance for differentiating benign from malignant PVT based on the iodine-uptake assessment (53).
In a study conducted on 174 cirrhotic patients, it was noted that patients with a larger portal vein diameter especially greater than 12.5 mm on CT angiography had a higher risk of PVT development (55).
Magnetic resonance imaging (MRI)
In MRIs, rapid vascular flow creates a lack of signal on T1 or T2 enhanced images whereas slow or stagnant flow secondary to thrombi will create a bright intraluminal signal (54). Post contrast MRIs, Gadoxetic Acid-enhanced MR Imaging, and subtraction imaging are helpful in differentiating benign versus malignant portal vein thrombi in cirrhotics based on enhancement in arterial, portal venous, and delayed phases whereas diffusion weighted MRI has a low sensitivity to differentiate the two (56–58).
Gadoxetic acid-enhanced MRI is superior to contrast enhanced CT in terms of sensitivity and specificity in the detection of PVTs in patients with HCC meeting the Milan criteria. Hence, it is recommended that all patients who were not considered to be ideal liver transplant candidates due to the presence of PVT on contrast enhanced CT should undergo gadoxetic acid-enhanced MRI to confirm the presence of PVT (59).
MR angiography has multiple advantages when compared to ultrasonography with respect to having an unrestricted field of view, insensitivity to bowel gas or body habitus, and accommodate multiple views not limited by acoustic windows (60). MRIs which include Magnetic resonance angiography (MRA) have a very high sensitivity and specificity in detecting PVTs at 100% and 98–100%, respectively, (60, 61).
Angiography
Angiography is a less commonly used method due to its invasive nature where in contrast is administered with concurrent use of vasodilators to optimize for venous enhancement and opacification. This is not a commonly used method but, still used to evaluate the status of the portal venous system, patency of vessels, vascular pressures, collateral flow pathways, and thrombi (54).
Management
When considering treatment, several factors need to be taken into consideration. Some studies suggest that PVT is associated with increased decompensation and mortality risk, while others indicate that it merely indicates cirrhosis progression. Bleeding remains a feared complication. In transplant candidates, the presence of PVT affects surgical technique and may affect survival.
Anticoagulation
Anticoagulation is typically administered in a non-cirrhotic portal vein thrombosis (62), whereas in cirrhotic patients, there is controversy surrounding the need for anticoagulation as many patients have varices which render them prone to gastrointestinal bleeds. Major indications for anticoagulation in cirrhotic PVT include acute symptomatic PVT, liver transplant candidates and in individuals with thrombus extension to the mesenteric veins. Two meta-analyses have stated that in cirrhotic PVT, the rate of portal vein recanalization, complete portal vein recanalization, and thrombus progression after anticoagulation therapy is much higher when compared to untreated cirrhotic PVT (63, 64). Approximately, two thirds of cirrhotic patients with PVT achieved portal vein recanalization after anticoagulation and approximately half of that subset of patients achieved complete portal vein recanalization (63). Although bleeding complications are feared, when examining bleeding events in treatment versus control groups, those who were anticoagulated had less incidence of bleeding events. This may correlate with the reduction in portal pressure in those treated with anticoagulation therapy.
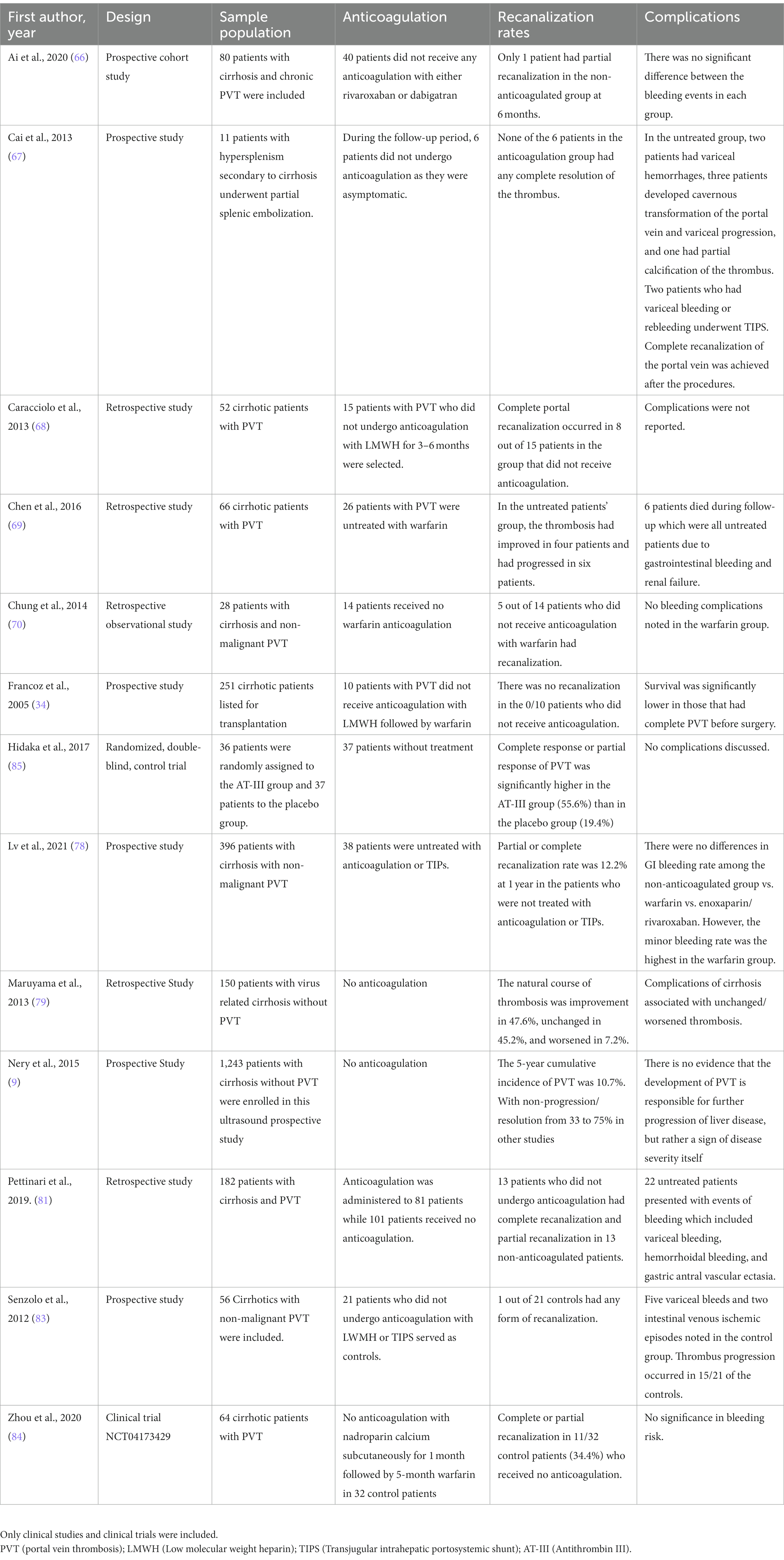
In a meta-analysis conducted on the effect of anticoagulation in portal vein thrombosis, it was noted that 71% of the patients underwent portal vein recanalization in the anticoagulation-treated group and 42% underwent portal vein recanalization without anticoagulation. The rates included both partial and complete portal vein recanalization. Despite this, only 53% of the anticoagulant treated groups underwent complete portal vein recanalization when compared to the 33% in the non-anticoagulation group in six of the studies. This shows that anticoagulation does not guarantee but, may only increase chances of complete portal vein recanalization. The meta-analysis also reported no significant differences in major or minor bleeding events between anticoagulation vs. non-anticoagulation treated groups in six of the studies (65). Several studies have described the effects of anticoagulation and its complications which are listed in Table 1. Case reports, case series, and foreign language manuscripts were excluded in Table 1.
Choice of anticoagulation agent
Low molecular weight heparin (LMWH) and direct oral anticoagulants (DOACS) are considered safe in patients with cirrhosis and PVT. The drug selection needs to be individualized and adverse effects need to be discussed with the patient. Warfarin therapy has a narrow therapeutic window and the baseline elevation of the PT (prothrombin time) and INR in these patients creates difficulty for monitoring and dosing. Although, LMWH does not require monitoring, it does however include only injections which may be uncomfortable for the patient but, has potentially lesser side effects.
A meta-analysis on DOACs reported that the pooled rate of PVT recanalization among cirrhotic patients was 87.3% versus 44.1% in DOACs versus vitamin K antagonists (VKA) respectively. DOACs were associated with an overall lower pooled risk of major bleeding events when compared to VKAs but, have similar pooled risks of variceal bleeding and death (86). Rivaroxaban is contraindicated in patients with Child Pugh Group B and C cirrhosis with potential to be hepatotoxic (87–89). Apixaban has no warnings against its use in patients with Child Pugh Group A and B cirrhosis (87, 88). Edoxaban has both hepatic and renal clearance whereas dabigatran has predominantly renal clearance (90, 91). As per a review published in 2019, DOACs and LMWH may be safer and more efficacious than warfarin. To a large extent, it appears that DOACs may be safer and a more convenient option in PVT in cirrhotics all for the exception of rivaroxaban due to its hepatotoxic potential (89). Historically, warfarin and LMWH has been preferred due to familiarity and reversal agents. However, it is important to keep in mind that DOACs also have reversal agents in the event of major bleeding events. Despite this, further randomized clinical trials need to be conducted to assess safety and efficacy of DOACs in PVT in cirrhosis and to establish a new standard of care.
The AGA (American Gastroenterological Association Institute) guidelines recommend anticoagulation over no anticoagulation for the treatment of acute or subacute PVT in patients with cirrhosis (92). The EASL (European Association for the Study of the Liver) guidelines also have mentioned that anticoagulation have led to partial or complete recanalization of PVT in cirrhotic patients but, have not formally recommended this as larger randomized clinical trials would be needed to assess for morbidity and mortality (93). Although, EASL guidelines have not formally recommended DOACs for the treatment of PVT, they do recommend using DOACs in the treatment of deep vein thrombosis/pulmonary embolism (DVT/PE) in patients with Child-Pugh class A cirrhosis. They recommend using DOACs with caution in patients with Child-Pugh class B cirrhosis and in those with a creatinine clearance of less than 30 ml/min and advise against DOACs in Child-Pugh class C cirrhosis (93). This could be applied to the treatment for PVT as well although, data is limited. The AASLD (American Association for the Study of the Liver) guidelines have stated treatment for PVT is weak due to lack of clinical trials and the indication for anticoagulation should be dependent on the patient while considering the expected benefits for the patient and decreasing risk for clot extension (44). The AASLD guidelines also state that the non-portal hypertensive bleeding rates among cirrhotics compared to the general population on therapeutic anticoagulation appear to be similar with portal hypertensive bleeding rates among cirrhotics appear to be unchanged by anticoagulation (44).
Based on the guidelines mentioned above and the studies summarized in Table 1, it can be stated that anticoagulation for PVT in cirrhotics should not be feared and may even be recommended especially in the cases of acute and subacute PVT. Individualized bleeding risks should be analyzed prior to initiation of anticoagulation such as evaluating for portal hypertensive gastropathy, unbanded varices, etc. The type of anticoagulation used must be individualized based on their class of cirrhosis, renal function, and compliance with injectables and INR monitoring. Patients can be followed up closely to evaluate for medication related adverse effects, thrombus progression or bleeding complications.
Table 2 summarizes studies regarding the natural history of portal vein thrombosis in patients who did not undergo any form of intervention.
Transjugular intrahepatic portosystemic shunt (TIPS)
Transjugular intrahepatic portosystemic shunt (TIPS) is a procedure that involves inserting a stent into the portal veins to recanalize the portal vein and reduce portal hypertension especially in patients with severe portal hypertensive symptoms such as recurrent gastrointestinal bleeding and refractory ascites (94). With the development of real-time visualization of the portal vein during TIPS, PVT is no longer considered as an absolute contraindication to TIPS placement (95). A retrospective study comparing the Yerdel grade of PVT showed that anticoagulation had an association with worsening of portomesenteric thrombosis when compared to TIPS (96). This study revealed that 72%–78% of TIPS patients, 27%–29% of anticoagulated patients, and 10%–17% of untreated patients at early and late follow-up showed an improvement in thrombus burden (96).
There was another study that evaluated the efficacy of TIPS in combination with anticoagulation or antiplatelet therapy which revealed that warfarin was superior to aspirin or clopidogrel in achieving partial or complete recanalization of PVT (97). A retrospective study analyzed data from 189 patients who underwent TIPS on chronic PVT depicted that there was a significant reduction in portal vein pressure, decreased rebleeding rates, and no significant different in hepatic encephalopathy (98). Angiojet thrombus aspiration technology has also been used simultaneously during TIPS which was studied on 63 patients with acute PVT and resulted in a 100% success rate. There were only 2 cases of biliary tract injuries and two cases of intrahepatic arteriovenous fistula as postprocedural complications. During the follow-up period, 74.61% had complete portal vein recanalization and 20.63% had partial recanalization (99).
A recently published randomized, controlled trial (2018) compared TIPS with covered stents versus endoscopic band ligation (EBL) plus propranolol for the prevention of variceal rebleeding among patients with cirrhosis and PVT (100). A total of 29 cirrhotic patients (94% Child-Pugh class A or B) with PVT and a recent variceal bleed (6 weeks) were randomly allotted to TIPS intervention (n = 24) versus the EBL and propranolol group (n = 25), respectively (100). The primary endpoint was variceal rebleeding (100). The study found that variceal rebleeding was significantly less frequent in the TIPS group (15% vs. 45% at 1 year and 25% versus 50% at 2 years, respectively; HR = 0.28, 95% CI 0.10 to 0.76, p = 0.008) (100). Hence, TIPS placement in patients with decompensated cirrhosis and PVT was more effective than EBL and propranolol combined for the prevention of rebleeding. Although, this did not improve translate into improved overall survival.
Another randomized, controlled trial (2015) randomly assigned 73 patients to either receive TIPS (n = 37) or EBL plus propranolol (n = 36) (101). This shows that TIPS may be more effective than EBL plus propranolol in preventing recurrent esophageal variceal bleeding (101). The 2-year probability of remaining free of variceal bleed in advanced cirrhosis with PVT was higher in the TIPS group (77.8%) than in the EBL group (42.9%) (value of p = 0.002) (101).
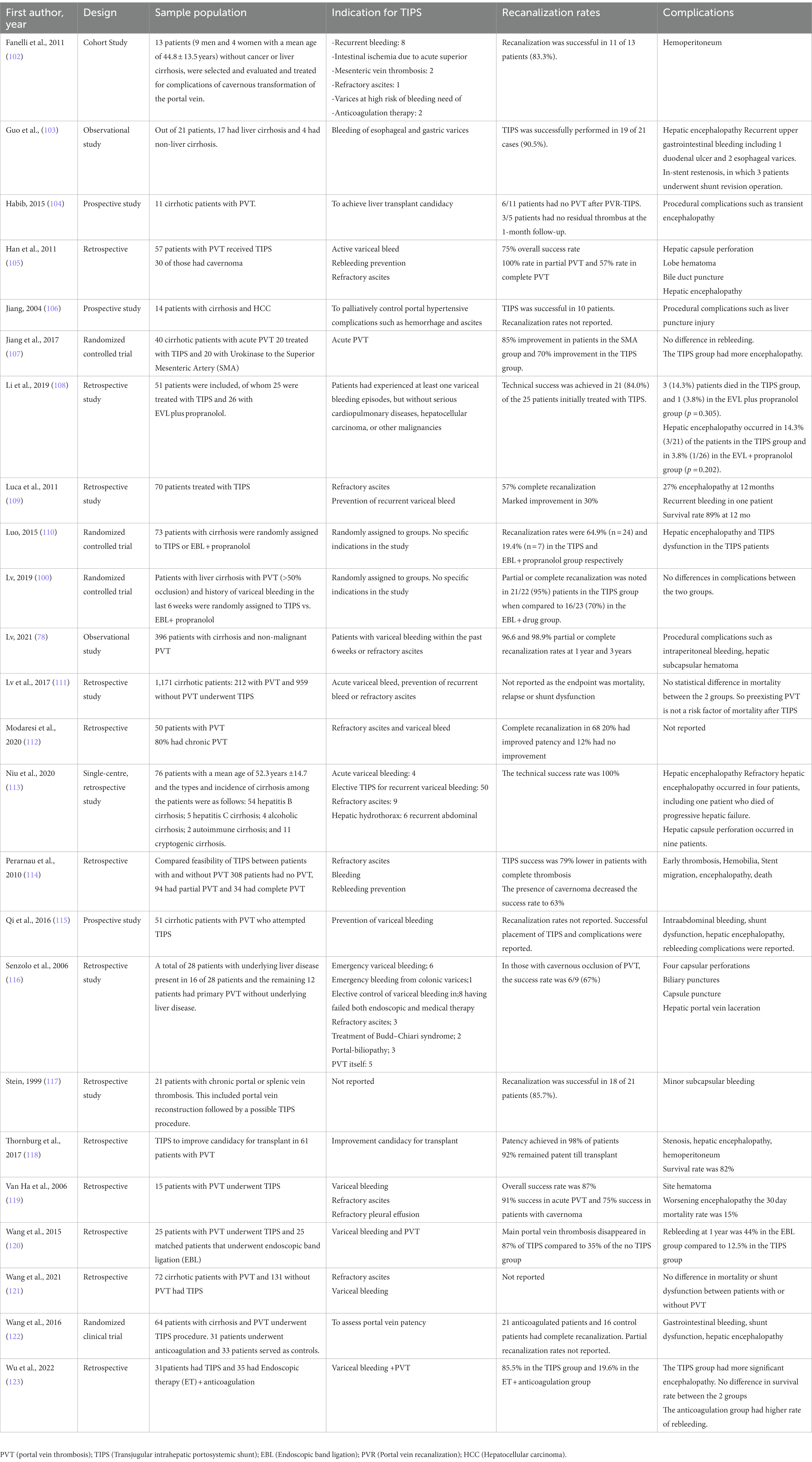
The AASLD guidance recommends portal vein recanalization (PVR) followed by TIPS in liver transplant candidates with chronic PVT which impedes the physiological anastomosis between graft and host portal vein (44). The AASLD guidance also recommends PVR followed by TIPS in patients with chronic PVT and recurrent bleeding and/or recurrent ascites which is not medically or endoscopically manageable (44). The various studies and clinical trial describing TIPS in cirrhotic patients with PVT are described in Table 3.
Thrombolysis
Endovascular thrombolysis is performed using multihole infusion catheters when anticoagulation alone is insufficient. They are commonly performed in conjunction to TIPS placement or mechanical thrombectomy. There are two major methods of thrombolysis which include the transvenous method wherein fibrinolytic agents such as tissue plasminogen activator or heparin are injected into the PVT directly. The other method is by using an ultrasound-accelerated infusion catheter wherein a fibrinolytic agent is injected into the clot and the ultrasound waves would additionally disrupt the clot integrity and aid in better resolution of the thrombus (124, 125). Thrombolysis typically achieves partial recanalization only and is likely more beneficial if combined with another technique such as thrombectomy (126). Several contraindications exist for thrombolysis which include a recent stroke, gastrointestinal bleeding, recent orthopedic, cranial, or spinal trauma, and the presence of an intracranial tumor (124, 125).
Mechanical thrombectomy
Mechanical thrombectomy is the restoration of flow within the main portal vein using balloon thrombectomy, rheolytic thrombectomy, or suction thrombectomy (127). Balloon thrombectomy is a procedure wherein a balloon is inflated past the clot which is subsequently retracted over the guidewire to pull the clot into a patent vein and then washed away. Rheolytic therapy uses high-velocity saline jets for the destruction of the thrombus. Suction thrombectomy involves using vacuum-based tools to suction the clot (127). Other methods such as an aspiration mechanical thrombectomy used simultaneously during TIPS is also another effective and safe treatment of PVT (128). A recently published study describes the successful resolution of PVT using large bore thrombectomy in conjunction with the Inari FlowTriever device during or after TIPS placement (129).
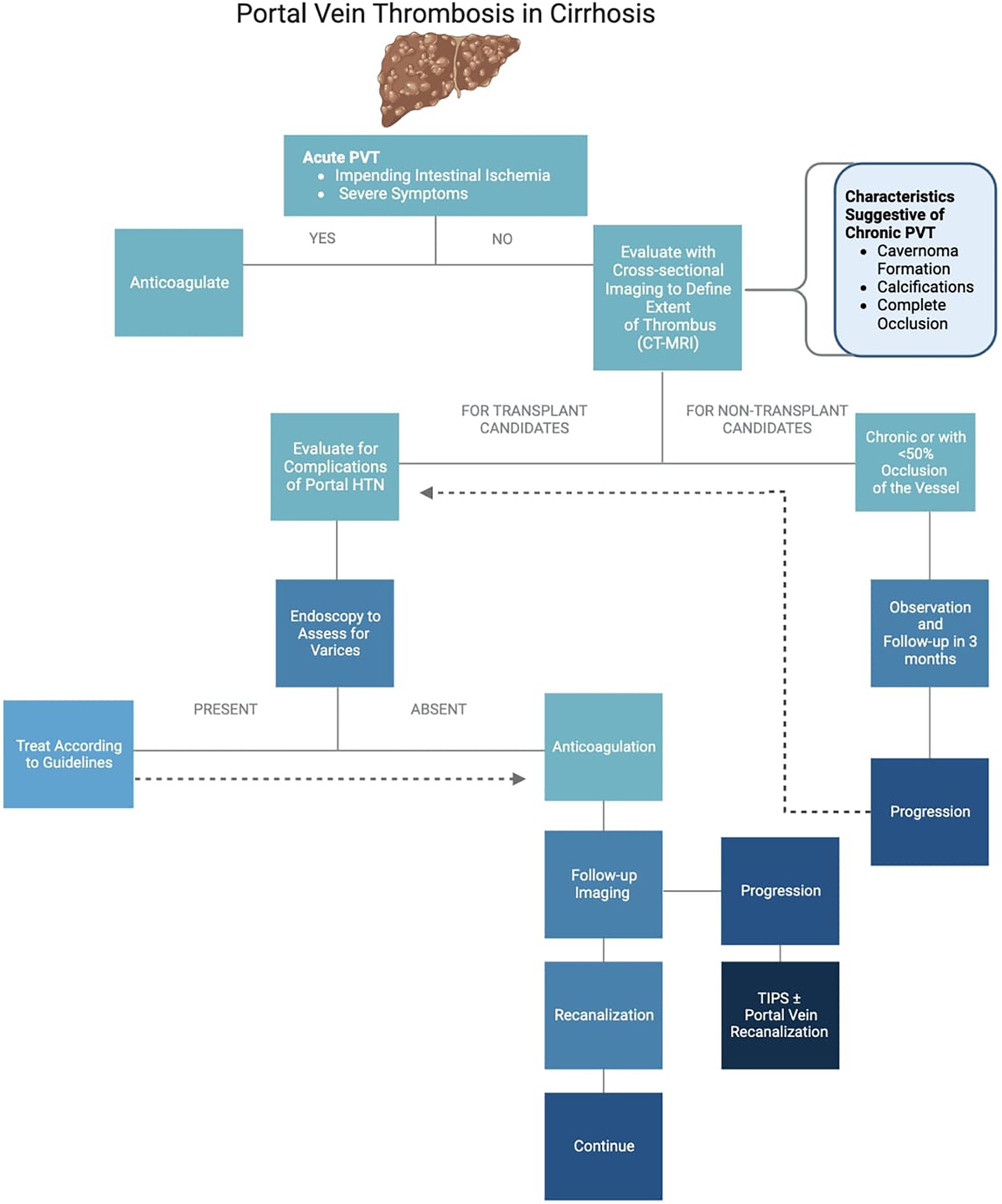
Treatment algorithm of portal vein thrombosis
The suggested treatment algorithm of PVT is as described in Figure 1.
Prevention
The prevention of PVT in cirrhosis is still an area that is currently undergoing research. A randomized controlled trial studying 70 cirrhotic patients showed that PVT was prevented at 96 weeks in the group that received 4,000 IU of enoxaparin versus 10 out of 36 controls developed PVT (130). Another study stratified risk of PVT based on antithrombin levels where high and highest risk cirrhotic patients received antithrombin III concentrates and danaparoid sodium which resulted in a low incidence of PVT (25). Another randomized controlled trial showed that warfarin is more effective than aspirin in preventing PVT after a laparoscopic splenectomy in cirrhotics while also providing hepato- and nephroprotective effects (131).
Conclusion
This review intends to evade the fear of anticoagulation and interventional strategies in patients with cirrhosis. It was written to enhance the knowledge of managing portal vein thrombosis in cirrhosis as it may help in reducing portal hypertensive complications. Despite the guidance on the management of PVT, an individualized assessment of risks vs. benefits is necessary when deciding between different treatment strategies.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ogren, M, Bergqvist, D, Björck, M, Acosta, S, Eriksson, H, and Sternby, NH. Portal vein thrombosis: prevalence, patient characteristics and lifetime risk: a population study based on 23,796 consecutive autopsies. World J Gastroenterol. (2006) 12:2115–9. doi: 10.3748/wjg.v12.i13.2115
2. Amitrano, L, Anna Guardascione, M, Brancaccio, V, Margaglione, M, Manguso, F, Iannaccone, L, et al. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol. (2004) 40:736–41. doi: 10.1016/j.jhep.2004.01.001
3. Turon, F, Driever, EG, Baiges, A, Cerda, E, García-Criado, Á, Gilabert, R, et al. Predicting portal thrombosis in cirrhosis: a prospective study of clinical, ultrasonographic and hemostatic factors. J Hepatol. (2021) 75:1367–76. doi: 10.1016/j.jhep.2021.07.020
4. Ponziani, FR, Zocco, MA, Campanale, C, Rinninella, E, Tortora, A, di Maurizio, L, et al. Portal vein thrombosis: insight into physiopathology, diagnosis, and treatment. World J Gastroenterol. (2010) 16:143–55. doi: 10.3748/wjg.v16.i2.143
5. Stine, JG, Shah, PM, Cornella, SL, Rudnick, SR, Ghabril, MS, Stukenborg, GJ, et al. Portal vein thrombosis, mortality and hepatic decompensation in patients with cirrhosis: a meta-analysis. World J Hepatol. (2015) 7:2774–80. doi: 10.4254/wjh.v7.i27.2774
6. Driever, EG, Magaz, M, Adelmeijer, J, Turon, F, Baiges, A, Olivas, P, et al. The portal vein in patients with cirrhosis is not an excessively inflammatory or hypercoagulable vascular bed, a prospective cohort study. J Thromb Haemost. (2022) 20:2075–82. doi: 10.1111/jth.15797
7. Scheiner, B, Northup, PG, Gruber, AB, Semmler, G, Leitner, G, Quehenberger, P, et al. The impact of ABO blood type on the prevalence of portal vein thrombosis in patients with advanced chronic liver disease. Liver Int. (2020) 40:1415–26. doi: 10.1111/liv.14404
8. Turon, F, Driever, EG, Baiges, A, Cerda, E, García-Criado, Á, Gilabert, R, et al. Predicting portal thrombosis in cirrhosis: A prospective study of clinical, ultrasonographic and hemostatic factors. J Hepatol. (2021) 75:1367–76. doi: 10.1016/j.jhep.2021.07.020
9. Nery, F, Chevret, S, Condat, B, de Raucourt, E, Boudaoud, L, Rautou, PE, et al. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study. Hepatology. (2015) 61:660–7. doi: 10.1002/hep.27546
10. Anton, A, Campreciós, G, Pérez-Campuzano, V, Orts, L, García-Pagán, JC, and Hernández-Gea, V. The pathophysiology of portal vein thrombosis in cirrhosis: getting deeper into Virchow’s triad. J Clin Med. (2022) 11:800. doi: 10.3390/jcm11030800
11. Abdel-Razik, A, Mousa, N, Elhelaly, R, and Tawfik, A. De-novo portal vein thrombosis in liver cirrhosis: risk factors and correlation with the model for end-stage liver disease scoring system. Eur J Gastroenterol Hepatol. (2015) 27:585–92. doi: 10.1097/MEG.0000000000000325
12. Stine, JG, Wang, J, Shah, PM, Argo, CK, Intagliata, N, Uflacker, A, et al. Decreased portal vein velocity is predictive of the development of portal vein thrombosis: A matched case-control study. Liver Int. (2018) 38:94–101. doi: 10.1111/liv.13500
13. Wang, M, Hao, H, Leeper, NJ, and Zhu, L. Thrombotic regulation from the endothelial cell perspectives. Arterioscler Thromb Vasc Biol. (2018) 38:e90–5. doi: 10.1161/ATVBAHA.118.310367
14. Yau, JW, Teoh, H, and Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. (2015) 15:130. doi: 10.1186/s12872-015-0124-z
15. Poredos, P, and Jezovnik, MK. Endothelial dysfunction and venous thrombosis. Angiology. (2018) 69:564–7. doi: 10.1177/0003319717732238
16. Ferroni, P, Mammarella, A, Martini, F, Paoletti, V, Cardarello, CM, Labbadia, G, et al. Increased soluble P-Selectin levels in hepatitis C virus-related chronic hepatitis. Correlation with viral load. J Investig Med. (2001) 49:407–12. doi: 10.2310/6650.2001.33785
17. Raparelli, V, Basili, S, Carnevale, R, Napoleone, L, del Ben, M, Nocella, C, et al. Low-grade endotoxemia and platelet activation in cirrhosis. Hepatology. (2017) 65:571–81. doi: 10.1002/hep.28853
18. Wannhoff, A, Müller, OJ, Friedrich, K, Rupp, C, Klöters-Plachky, P, Leopold, Y, et al. Effects of increased von Willebrand factor levels on primary hemostasis in thrombocytopenic patients with liver cirrhosis. PLoS One. (2014) 9:e112583. doi: 10.1371/journal.pone.0112583
19. Lisman, T, Bongers, TN, Adelmeijer, J, Janssen, HL, de Maat, MP, de Groot, PG, et al. Elevated levels of von Willebrand factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology. (2006) 44:53–61. doi: 10.1002/hep.21231
20. Praktiknjo, M, Trebicka, J, Carnevale, R, Pastori, D, Queck, A, Ettorre, E, et al. Von Willebrand and factor VIII Portosystemic circulation gradient in cirrhosis: implications for portal vein thrombosis. Clin Transl Gastroenterol. (2020) 11:e00123. doi: 10.14309/ctg.0000000000000123
21. Mooberry, MJ, and Key, NS. Microparticle analysis in disorders of hemostasis and thrombosis. Cytometry A. (2016) 89:111–22. doi: 10.1002/cyto.a.22647
22. Mei, H, Jiang, Y, Luo, L, Huang, R, Su, L, Hou, M, et al. Evaluation the combined diagnostic value of TAT, PIC, tPAIC, and sTM in disseminated intravascular coagulation: A multi-center prospective observational study. Thromb Res. (2019) 173:20–6. doi: 10.1016/j.thromres.2018.11.010
23. Driever, EG, von Meijenfeldt, FA, Adelmeijer, J, de Haas, RJ, van den Heuvel, MC, Nagasami, C, et al. Nonmalignant portal vein thrombi in patients with cirrhosis consist of intimal fibrosis with or without a fibrin-rich thrombus. Hepatology. (2022) 75:898–911. doi: 10.1002/hep.32169
24. Lippi, G, and Favaloro, EJ. Venous and arterial Thromboses: two sides of the same coin? Semin Thromb Hemost. (2018) 44:239–48. doi: 10.1055/s-0037-1607202
25. Kawanaka, H, Akahoshi, T, Itoh, S, Iguchi, T, Harimoto, N, Uchiyama, H, et al. Optimizing risk stratification in portal vein thrombosis after splenectomy and its primary prophylaxis with antithrombin III concentrates and danaparoid sodium in liver cirrhosis with portal hypertension. J Am Coll Surg. (2014) 219:865–74. doi: 10.1016/j.jamcollsurg.2014.07.939
26. Cagin, YF, Bilgic, Y, Berber, İ, Yildirim, O, Erdogan, MA, Firat, F, et al. The risk factors of portal vein thrombosis in patients with liver cirrhosis. Exp Ther Med. (2019) 17:3189–94. doi: 10.3892/etm.2019.7300
27. Ponziani, FR, Zocco, MA, Garcovich, M, D’Aversa, F, Roccarina, D, and Gasbarrini, A. What we should know about portal vein thrombosis in cirrhotic patients: a changing perspective. World J Gastroenterol. (2012) 18:5014–20. doi: 10.3748/wjg.v18.i36.5014
28. Lertpipopmetha, K, and Auewarakul, CU. High incidence of hepatitis B infection-associated cirrhosis and hepatocellular carcinoma in the southeast Asian patients with portal vein thrombosis. BMC Gastroenterol. (2011) 11:66. doi: 10.1186/1471-230X-11-66
29. Weber, A, Krebs, S, Lenhardt, C, Wagenpfeil, S, Schmid, RM, and Schulte-Frohlinde, E. Correlation of routinely used coagulation parameters and presence of portal vein thrombosis in patients with liver cirrhosis. Hepatol Res. (2009) 39:882–7. doi: 10.1111/j.1872-034X.2009.00531.x
30. Chen, H, Qi, X, He, C, Yin, Z, Fan, D, and Han, G. Coagulation imbalance may not contribute to the development of portal vein thrombosis in patients with cirrhosis. Thromb Res. (2013) 131:173–7. doi: 10.1016/j.thromres.2012.11.003
31. Stotts, MJ, Wentworth, BJ, and Northup, PG. Management of Portal Vein Thrombosis in cirrhosis. Semin Liver Dis. (2021) 41:79–86. doi: 10.1055/s-0040-1722260
32. Okuda, K, Ohnishi, K, Kimura, K, Matsutani, S, Sumida, M, Goto, N, et al. Incidence of portal vein thrombosis in liver cirrhosis. An angiographic study in 708 patients. Gastroenterology. (1985) 89:279–86. doi: 10.1016/0016-5085(85)90327-0
33. Yerdel, MA, Gunson, B, Mirza, D, Karayal??in, K, Olliff, S, Buckels, J, et al. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation. (2000) 69:1873–81. doi: 10.1097/00007890-200005150-00023
34. Francoz, C, Belghiti, J, Vilgrain, V, Sommacale, D, Paradis, V, Condat, B, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut. (2005) 54:691–7. doi: 10.1136/gut.2004.042796
35. Rodríguez-Castro, KI, Porte, RJ, Nadal, E, Germani, G, Burra, P, and Senzolo, M. Management of nonneoplastic portal vein thrombosis in the setting of liver transplantation: a systematic review. Transplantation. (2012) 94:1145–53. doi: 10.1097/TP.0b013e31826e8e53
36. Flores, B, Trivedi, HD, Robson, SC, and Bonder, A. Hemostasis, bleeding and thrombosis in liver disease. J Transl Sci. (2017) 3. doi: 10.15761/JTS.1000182
37. Intagliata, NM, Caldwell, SH, and Tripodi, A. Diagnosis, development, and treatment of portal vein thrombosis in patients with and without cirrhosis. Gastroenterology. (2019) 156:1582–99.e1. doi: 10.1053/j.gastro.2019.01.265
38. Primignani, M. Portal vein thrombosis, revisited. Dig Liver Dis. (2010) 42:163–70. doi: 10.1016/j.dld.2009.08.003
39. Hoekstra, J, and Janssen, HL. Vascular liver disorders (II): portal vein thrombosis. Neth J Med. (2009) 67:46–53.
40. Sogaard, KK, Astrup, LB, Vilstrup, H, and Gronbaek, H. Portal vein thrombosis; risk factors, clinical presentation and treatment. BMC Gastroenterol. (2007) 7:34. doi: 10.1186/1471-230X-7-34
41. Condat, B, Vilgrain, V, Asselah, T, O’Toole, D, Rufat, P, Zappa, M, et al. Portal cavernoma-associated cholangiopathy: a clinical and MR cholangiography coupled with MR portography imaging study. Hepatology. (2003) 37:1302–8. doi: 10.1053/jhep.2003.50232
42. Mantaka, A, Augoustaki, A, Kouroumalis, EA, and Samonakis, DN. Portal vein thrombosis in cirrhosis: diagnosis, natural history, and therapeutic challenges. Ann Gastroenterol. (2018) 31:315–29. doi: 10.20524/aog.2018.0245
43. D’Amico, G, and de Franchis, R, Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. (2003) 38:599–612. doi: 10.1053/jhep.2003.50385
44. Northup, PG, Garcia-Pagan, JC, Garcia-Tsao, G, Intagliata, NM, Superina, RA, Roberts, LN, et al. Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. (2021) 73:366–413. doi: 10.1002/hep.31646
45. Minoda, AM, Cadete, RBF, Teixeira, SR, Muglia, VF, Elias Junior, J, and de Melo-Leite, AF. The ABCD of portal vein thrombosis: a systematic approach. Radiol Bras. (2020) 53:424–9. doi: 10.1590/0100-3984.2019.0109
46. Jha, RC, Khera, SS, and Kalaria, AD. Portal vein thrombosis: imaging the Spectrum of disease with an emphasis on MRI features. AJR Am J Roentgenol. (2018) 211:14–24. doi: 10.2214/AJR.18.19548
47. Tessler, FN, Gehring, BJ, Gomes, AS, Perrella, RR, Ragavendra, N, Busuttil, RW, et al. Diagnosis of portal vein thrombosis: value of color Doppler imaging. AJR Am J Roentgenol. (1991) 157:293–6. doi: 10.2214/ajr.157.2.1853809
48. Ricci, P, Cantisani, V, Biancari, F, Drudi, FM, Coniglio, M, di Filippo, A, et al. Contrast-enhanced color Doppler US in malignant portal vein thrombosis. Acta Radiol. (2000) 41:470–3. doi: 10.1080/028418500127345703
49. Chammas, MC, Oliveira, AC, D’Ávilla, MJ, Moraes, PH, and Takahashi, MS. Characterization of malignant portal vein thrombosis with contrast-enhanced ultrasonography. Ultrasound Med Biol. (2019) 45:50–5. doi: 10.1016/j.ultrasmedbio.2018.09.009
50. Pirisi, M, Avellini, C, Fabris, C, Scott, C, Bardus, P, Soardo, G, et al. Portal vein thrombosis in hepatocellular carcinoma: age and sex distribution in an autopsy study. J Cancer Res Clin Oncol. (1998) 124:397–400. doi: 10.1007/s004320050189
51. Takizawa, D, Kakizaki, S, Sohara, N, Sato, K, Takagi, H, Arai, H, et al. Hepatocellular carcinoma with portal vein tumor thrombosis: clinical characteristics, prognosis, and patient survival analysis. Dig Dis Sci. (2007) 52:3290–5. doi: 10.1007/s10620-007-9808-2
52. Rossi, S, Ghittoni, G, Ravetta, V, Torello Viera, F, Rosa, L, Serassi, M, et al. Contrast-enhanced ultrasonography and spiral computed tomography in the detection and characterization of portal vein thrombosis complicating hepatocellular carcinoma. Eur Radiol. (2008) 18:1749–56. doi: 10.1007/s00330-008-0931-z
53. Ascenti, G, Sofia, C, Mazziotti, S, Silipigni, S, D’Angelo, T, Pergolizzi, S, et al. Dual-energy CT with iodine quantification in distinguishing between bland and neoplastic portal vein thrombosis in patients with hepatocellular carcinoma. Clin Radiol. (2016) 71:938.e1–9. doi: 10.1016/j.crad.2016.05.002
54. Abbitt, PL. Portal vein thrombosis: imaging features and associated etiologies. Curr Probl Diagn Radiol. (1992) 21:115–47. doi: 10.1016/0363-0188(92)90036-F
55. Dong, G, Huang, XQ, Zhu, YL, Ding, H, Li, F, and Chen, SY. Increased portal vein diameter is predictive of portal vein thrombosis development in patients with liver cirrhosis. Ann Transl Med. (2021) 9:289. doi: 10.21037/atm-20-4912
56. Gawande, R, Jalaeian, H, Niendorf, E, Olgun, D, Krystosek, L, Rubin, N, et al. MRI in differentiating malignant versus benign portal vein thrombosis in patients with hepatocellular carcinoma: value of post contrast imaging with subtraction. Eur J Radiol. (2019) 118:88–95. doi: 10.1016/j.ejrad.2019.07.008
57. Kim, JH, Lee, JM, Yoon, JH, Lee, DH, Lee, KB, Han, JK, et al. Portal vein thrombosis in patients with hepatocellular carcinoma: diagnostic accuracy of Gadoxetic acid-enhanced MR imaging. Radiology. (2016) 279:773–83. doi: 10.1148/radiol.2015150124
58. Ahn, JH, Yu, JS, Cho, ES, Chung, JJ, Kim, JH, and Kim, KW. Diffusion-weighted MRI of malignant versus benign portal vein thrombosis. Korean J Radiol. (2016) 17:533–40. doi: 10.3348/kjr.2016.17.4.533
59. Bae, JS, Lee, JM, Yoon, JH, Jang, S, Chung, JW, Lee, KB, et al. How to best detect portal vein tumor thrombosis in patients with hepatocellular carcinoma meeting the Milan criteria: Gadoxetic acid-enhanced MRI versus contrast-enhanced CT. Liver Cancer. (2020) 9:293–307. doi: 10.1159/000505191
60. Finn, JP, Kane, RA, Edelman, RR, Jenkins, RL, Lewis, WD, Muller, M, et al. Imaging of the portal venous system in patients with cirrhosis: MR angiography vs duplex Doppler sonography. AJR Am J Roentgenol. (1993) 161:989–94. doi: 10.2214/ajr.161.5.8273643
61. Shah, TU, Semelka, RC, Voultsinos, V, Elias Jr, J, Altun, E, Pamuklar, E, et al. Accuracy of magnetic resonance imaging for preoperative detection of portal vein thrombosis in liver transplant candidates. Liver Transpl. (2006) 12:1682–8. doi: 10.1002/lt.20873
62. Plessier, A, Darwish-Murad, S, Hernandez-Guerra, M, Consigny, Y, Fabris, F, Trebicka, J, et al. Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology. (2010) 51:210–8. doi: 10.1002/hep.23259
63. Qi, X, De Stefano, V, Li, H, Dai, J, Guo, X, and Fan, D. Anticoagulation for the treatment of portal vein thrombosis in liver cirrhosis: a systematic review and meta-analysis of observational studies. Eur J Intern Med. (2015) 26:23–9. doi: 10.1016/j.ejim.2014.12.002
64. Hepatobiliary Disease Study Group, Chinese Society of Gastroenterology, Chinese Medical Association. Consensus for management of portal vein thrombosis in liver cirrhosis (2020, Shanghai). J Dig Dis. (2021) 22:176–86. doi: 10.1111/1751-2980.12970
65. Loffredo, L, Pastori, D, Farcomeni, A, and Violi, F. Effects of anticoagulants in patients with cirrhosis and portal vein thrombosis: A systematic review and meta-analysis. Gastroenterology. (2017) 153:480–7.e1. doi: 10.1053/j.gastro.2017.04.042
66. Ai, MH, Dong, WG, Tan, XP, Xu, L, Xu, C, Zhang, Q, et al. Efficacy and safety study of direct-acting oral anticoagulants for the treatment of chronic portal vein thrombosis in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. (2020) 32:1395–400. doi: 10.1097/MEG.0000000000001846
67. Cai, M, Zhu, K, Huang, W, Meng, X, He, K, Zhou, B, et al. Portal vein thrombosis after partial splenic embolization in liver cirrhosis: efficacy of anticoagulation and long-term follow-up. J Vasc Interv Radiol. (2013) 24:1808–16. doi: 10.1016/j.jvir.2013.08.018
68. Caracciolo, G, Garcovich, M, Zocco, M, Ainora, M, Roccarina, D, Annicchiarico, BE, et al. Clinical outcome of partial portal vein thrombosis in cirrhotic patients: to observe or to treat? Dig Liver Dis. (2013) 45:S171. doi: 10.1016/S1590-8658(13)60485-5
69. Chen, H, Liu, L, Qi, X, He, C, Wu, F, Fan, D, et al. Efficacy and safety of anticoagulation in more advanced portal vein thrombosis in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. (2016) 28:82–9. doi: 10.1097/MEG.0000000000000482
70. Chung, JW, Kim, GH, Lee, JH, Ok, KS, Jang, ES, Jeong, SH, et al. Safety, efficacy, and response predictors of anticoagulation for the treatment of nonmalignant portal-vein thrombosis in patients with cirrhosis: a propensity score matching analysis. Clin Mol Hepatol. (2014) 20:384–91. doi: 10.3350/cmh.2014.20.4.384
71. Cui, SB, Shu, RH, Yan, SP, Wu, H, Chen, Y, Wang, L, et al. Efficacy and safety of anticoagulation therapy with different doses of enoxaparin for portal vein thrombosis in cirrhotic patients with hepatitis B. Eur J Gastroenterol Hepatol. (2015) 27:914–9. doi: 10.1097/MEG.0000000000000351
72. de Gottardi, A, Trebicka, J, Klinger, C, Plessier, A, Seijo, S, Terziroli, B, et al. Antithrombotic treatment with direct-acting oral anticoagulants in patients with splanchnic vein thrombosis and cirrhosis. Liver Int. (2017) 37:694–9. doi: 10.1111/liv.13285
73. Hanafy, AS, Bassiony, MA, and Basha, MAA. Management of HCV-related decompensated cirrhosis with direct-acting antiviral agents: who should be treated? Hepatol Int. (2019) 13:165–72. doi: 10.1007/s12072-019-09933-8
74. Hum, J, Shatzel, JJ, Jou, JH, and Deloughery, TG. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis. Eur J Haematol. (2017) 98:393–7. doi: 10.1111/ejh.12844
75. Intagliata, NM, Henry, ZH, Maitland, H, Shah, NL, Argo, CK, Northup, PG, et al. Direct Oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. (2016) 61:1721–7. doi: 10.1007/s10620-015-4012-2
76. Khan, FY, Habas, E, Sulaiman TO, Hamid, OA, Abdalhadi, A, Khalaf, A, et al. Risk factors, clinical presentation, diagnosis, and treatment outcomes of portal vein thrombosis: A five-year hospital-based study from Qatar. J Clin Med Res. (2022) 14:209–17. doi: 10.14740/jocmr4718
77. la Mura, V, Braham, S, Tosetti, G, Branchi, F, Bitto, N, Moia, M, et al. Harmful and beneficial effects of anticoagulants in patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol. (2018) 16:1146–52.e4. doi: 10.1016/j.cgh.2017.10.016
78. Lv, Y, Bai, W, Li, K, Wang, Z, Guo, W, Luo, B, et al. Anticoagulation and transjugular intrahepatic portosystemic shunt for the management of portal vein thrombosis in cirrhosis: a prospective observational study. Am J Gastroenterol. (2021) 116:1447–64. doi: 10.14309/ajg.0000000000001194
79. Maruyama, H, Takahashi, M, Shimada, T, and Yokosuka, O. Emergency anticoagulation treatment for cirrhosis patients with portal vein thrombosis and acute variceal bleeding. Scand J Gastroenterol. (2012) 47:686–91. doi: 10.3109/00365521.2012.674972
80. Nagaoki, Y, Aikata, H, Daijyo, K, Teraoka, Y, Shinohara, F, Nakamura, Y, et al. Efficacy and safety of edoxaban for treatment of portal vein thrombosis following danaparoid sodium in patients with liver cirrhosis. Hepatol Res. (2018) 48:51–8. doi: 10.1111/hepr.12895
81. Pettinari, I, Vukotic, R, Stefanescu, H, Pecorelli, A, Morelli, M, Grigoras, C, et al. Clinical impact and safety of anticoagulants for portal vein thrombosis in cirrhosis. Official journal of the American College of Gastroenterology. ACG. (2019) 114:258–66. doi: 10.1038/s41395-018-0421-0
82. Risso, A, Stradella, D, Martini, S, Rizzetto, M, and Salizzoni, M. Liver transplantation in cirrhotic patients with portal vein thrombosis: A single Centre experience. Dig Liver Dis. (2014) 46:e40. doi: 10.1016/j.dld.2014.01.093
83. Senzolo, M, Sartori, TM, Rossetto, V, Burra, P, Cillo, U, Boccagni, P, et al. Prospective evaluation of anticoagulation and transjugular intrahepatic portosistemic shunt for the management of portal vein thrombosis in cirrhosis. Liver Int. (2012) 32:919–27. doi: 10.1111/j.1478-3231.2012.02785.x
84. Zhou, T, Sun, X, Zhou, T, Li, Y, Chen, X, Cheng, B, et al. Efficacy and safety of Nadroparin calcium-warfarin sequential anticoagulation in portal vein thrombosis in cirrhotic patients: A randomized controlled trial. Clin Transl Gastroenterol. (2020) 11:e00228. doi: 10.14309/ctg.0000000000000228
85. Hidaka, H, Kokubu, S, Sato, T, Katsushima, S, Izumi, N, Igura, T, et al. Antithrombin III for portal vein thrombosis in patients with liver disease: A randomized, double-blind, controlled trial. Hepatol Res. (2018) 48:E107–16. doi: 10.1111/hepr.12934
86. Koh, JH, Liew, ZH, Ng, GK, Liu, HT, Tam, YC, de Gottardi, A, et al. Efficacy and safety of direct oral anticoagulants versus vitamin K antagonist for portal vein thrombosis in cirrhosis: A systematic review and meta-analysis. Dig Liver Dis. (2022) 54:56–62. doi: 10.1016/j.dld.2021.07.039
87. Turco, L, de Raucourt, E, Valla, D-C, and Villa, E. Anticoagulation in the cirrhotic patient. JHEP Rep. (2019) 1:227–39. doi: 10.1016/j.jhepr.2019.02.006
88. Intagliata, NM, Davitkov, P, Allen, AM, Falck-Ytter, YT, and Stine, JG. AGA technical review on coagulation in cirrhosis. Gastroenterology. (2021) 161:1630–56. doi: 10.1053/j.gastro.2021.09.004
89. Weinberg, EM, Palecki, J, and Reddy, KR. Direct-acting Oral anticoagulants (DOACs) in cirrhosis and cirrhosis-associated portal vein thrombosis. Semin Liver Dis. (2019) 39:195–208. doi: 10.1055/s-0039-1679934
90. Camm, AJ, and Bounameaux, H. Edoxaban: a new oral direct factor xa inhibitor. Drugs. (2011) 71:1503–26. doi: 10.2165/11595540-000000000-00000
91. Graff, J, and Harder, S. Anticoagulant therapy with the oral direct factor Xa inhibitors rivaroxaban, apixaban and edoxaban and the thrombin inhibitor dabigatran etexilate in patients with hepatic impairment. Clin Pharmacokinet. (2013) 52:243–54. doi: 10.1007/s40262-013-0034-0
92. O’Shea, RS, Davitkov, P, Ko, CW, Rajasekhar, A, Su, GL, Sultan, S, et al. AGA clinical practice guideline on the Management of Coagulation Disorders in patients with cirrhosis. Gastroenterology. (2021) 161:1615–27.e1. doi: 10.1053/j.gastro.2021.08.015
93. EASL clinical practice guidelines on prevention and management of bleeding and thrombosis in patients with cirrhosis. J Hepatol. (2022) 76:1151–84. doi: 10.1016/j.jhep.2021.09.003
94. Zhang, JB, Chen, J, Zhou, J, Wang, XM, Chen, S, Chu, JG, et al. Systematic review and meta-analysis of trans-jugular intrahepatic portosystemic shunt for cirrhotic patients with portal vein thrombosis. World J Clin Cases. (2021) 9:5179–90. doi: 10.12998/wjcc.v9.i19.5179
95. Rajesh, S, George, T, Philips, CA, Ahamed, R, Kumbar, S, Mohan, N, et al. Transjugular intrahepatic portosystemic shunt in cirrhosis: an exhaustive critical update. World J Gastroenterol. (2020) 26:5561–96. doi: 10.3748/wjg.v26.i37.5561
96. Zhan, C, Prabhu, V, Kang, SK, Li, C, Zhu, Y, Kim, S, et al. Comparison of non-Tumoral portal vein thrombosis Management in Cirrhotic Patients: TIPS versus anticoagulation versus no treatment. J Clin Med. (2021) 10:2316. doi: 10.3390/jcm10112316
97. Wan, YM, Li, YH, Wu, HM, Xu, ZY, Xu, Y, Yang, LH, et al. Portal vein thrombosis before and after transjugular intrahepatic portosystemic shunt placement: an observational study (STROBE compliant). Medicine (Baltimore). (2017) 96:e8498. doi: 10.1097/MD.0000000000008498
98. Sun, XY, Wang, GC, Wang, J, Huang, GJ, and Zhang, CQ. Transjugular intrahepatic portosystemic shunt is effective in patients with chronic portal vein thrombosis and variceal bleeding. Hepatobiliary Pancreat Dis Int. (2021) 20:128–36. doi: 10.1016/j.hbpd.2020.12.016
99. Zhang, DB, Zhang, KW, Lu, DH, Li, WX, Xu, RT, Li, K, et al. Analysis of the short-and medium-term curative effect of TIPS approach combined with AngioJet thrombus aspiration technology treatment in acute portal vein thrombosis. Zhonghua Gan Zang Bing Za Zhi. (2021) 29:754–8. doi: 10.3760/cma.j.cn501113-20200804-00435
100. Lv, Y, Qi, X, He, C, Wang, Z, Yin, Z, Niu, J, et al. Covered TIPS versus endoscopic band ligation plus propranolol for the prevention of variceal rebleeding in cirrhotic patients with portal vein thrombosis: a randomised controlled trial. Gut. (2018) 67:2156–68. doi: 10.1136/gutjnl-2017-314634
101. Luo, X, Wang, Z, Tsauo, J, Zhou, B, Zhang, H, and Li, X. Advanced cirrhosis combined with portal vein thrombosis: a randomized trial of TIPS versus endoscopic band ligation plus propranolol for the prevention of recurrent esophageal Variceal bleeding. Radiology. (2015) 276:286–93. doi: 10.1148/radiol.15141252
102. Fanelli, F, Angeloni, S, Salvatori, FM, Marzano, C, Boatta, E, Merli, M, et al. Transjugular intrahepatic portosystemic shunt with expanded-polytetrafuoroethylene-covered stents in non-cirrhotic patients with portal cavernoma. Dig Liver Dis. (2011) 43:78–84. doi: 10.1016/j.dld.2010.06.001
103. Guo, FF, Wu, ZY, Zhou, PL, and Han, XW. Transjugular intrahepatic portosystemic shunt for the treatment cavernous transformation of the portal vein with vareceal bleeding. Zhonghua Yi Xue Za Zhi. (2020) 100:387–90. doi: 10.3760/cma.j.issn.0376-2491.2020.05.014
104. Habib, A, Desai, K, Hickey, R, Thornburg, B, Vouche, M, Vogelzang, RL, et al. Portal vein recanalization-transjugularintrahepatic portosystemic shunt using the transsplenic approach to achieve transplant candidacy in patients with chronic portal vein thrombosis. J Vasc Interv Radiol. (2015) 26:499–506. doi: 10.1016/j.jvir.2014.12.012
105. Han, G, Qi, X, He, C, Yin, Z, Wang, J, Xia, J, et al. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with symptomatic portal hypertension in liver cirrhosis. J Hepatol. (2011) 54:78–88. doi: 10.1016/j.jhep.2010.06.029
106. Jiang, ZB, Shan, H, Shen, XY, Huang, MS, Li, ZR, Zhu, KS, et al. Transjugular intrahepatic portosystemic shunt for palliative treatment of portal hypertension secondary to portal vein tumor thrombosis. World J Gastroenterol. (2004) 10:1881–4. doi: 10.3748/wjg.v10.i13.1881
107. Jiang, TT, Luo, XP, Sun, JM, and Gao, J. Clinical outcomes of transcatheter selective superior mesenteric artery urokinase infusion therapy vs transjugular intrahepatic portosystemic shunt in patients with cirrhosis and acute portal vein thrombosis. World J Gastroenterol. (2017) 23:7470–7. doi: 10.3748/wjg.v23.i41.7470
108. Li, LN, Sun, XY, Wang, GC, Tian, XG, Zhang, MY, Jiang, KT, et al. Transjugular intrahepatic portosystemic shunt prevents rebleeding in cirrhotic patients having cavernous transformation of the portal vein without improving their survival. J Dig Dis. (2019) 20:89–96. doi: 10.1111/1751-2980.12702
109. Luca, A, Miraglia, R, Caruso, S, Milazzo, M, Sapere, C, Maruzzelli, L, et al. Short- and long-term effects of the transjugular intrahepatic portosystemic shunt on portal vein thrombosis in patients with cirrhosis. Gut. (2011) 60:846–52. doi: 10.1136/gut.2010.228023
110. Luo, X, Wang, Z, Tsauo, J, Zhou, B, Zhang, H, and Li, X. Advanced cirrhosis combined with portal vein thrombosis: A randomized trial of TIPS versus endoscopic band ligation plus propranolol for the prevention of recurrent esophageal Variceal bleeding. Radiology. (2015) 276:286–93. doi: 10.1148/radiol.15141252
111. Lv, Y, He, C, Wang, Z, Guo, W, Wang, J, Bai, W, et al. Association of Nonmalignant Portal Vein Thrombosis and Outcomes after Transjugular intrahepatic Portosystemic shunt in patients with cirrhosis. Radiology. (2017) 285:999–1010. doi: 10.1148/radiol.2017162266
112. Modaresi Esfeh, J, and Ansari-Gilani, K. Transjugular intrahepatic portosystemic shunt in cirrhotic patients with portal vein thrombosis. Eur J Gastroenterol Hepatol. (2020) 32:762–3. doi: 10.1097/MEG.0000000000001680
113. Niu, XK, Das, SK, Wu, HL, and Chen, Y. Computed tomography-based score model/nomogram for predicting technical and midterm outcomes in transjugular intrahepatic portosystemic shunt treatment for symptomatic portal cavernoma. World J Clin Cases. (2020) 8:887–99. doi: 10.12998/wjcc.v8.i5.887
114. Perarnau, JM, Baju, A, DʼAlteroche, L, Viguier, J, and Ayoub, J. Feasibility and long-term evolution of TIPS in cirrhotic patients with portal thrombosis. Eur J Gastroenterol Hepatol. (2010) 22:1093–8. doi: 10.1097/MEG.0b013e328338d995
115. Qi, X, He, C, Guo, W, Yin, Z, Wang, J, Wang, Z, et al. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with variceal bleeding in liver cirrhosis: outcomes and predictors in a prospective cohort study. Liver Int. (2016) 36:667–76. doi: 10.1111/liv.12929
116. Senzolo, M, Tibbals, J, Cholongitas, E, Triantos, CK, Burroughs, AK, and Patch, D. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with and without cavernous transformation. Aliment Pharmacol Ther. (2006) 23:767–75. doi: 10.1111/j.1365-2036.2006.02820.x
117. Stein, M, and Link, DP. Symptomatic spleno-mesenteric-portal venous thrombosis: recanalization and reconstruction with endovascular stents. J Vasc Interv Radiol. (1999) 10:363–71. doi: 10.1016/S1051-0443(99)70044-8
118. Thornburg, B, Desai, K, Hickey, R, Hohlastos, E, Kulik, L, Ganger, D, et al. Pretransplantation portal vein recanalization and Transjugular intrahepatic Portosystemic shunt creation for chronic portal vein thrombosis: final analysis of a 61-patient cohort. J Vasc Interv Radiol. (2017) 28:1714–21.e2. doi: 10.1016/j.jvir.2017.08.005
119. Van Ha, TG, Hodge, J, Funaki, B, Lorenz, J, Rosenblum, J, Straus, C, et al. Transjugular intrahepatic portosystemic shunt placement in patients with cirrhosis and concomitant portal vein thrombosis. Cardiovasc Intervent Radiol. (2006) 29:785–90. doi: 10.1007/s00270-005-0090-4
120. Wang, Z, Zhao, H, Wang, X, Zhang, H, Jiang, M, Tsauo, J, et al. Clinical outcome comparison between TIPS and EBL in patients with cirrhosis and portal vein thrombosis. Abdom Imaging. (2015) 40:1813–20. doi: 10.1007/s00261-014-0320-9
121. Wang, HL, Lu, WJ, Zhang, YL, Nie, CH, Zhou, TY, Zhou, GH, et al. Comparison of Transjugular intrahepatic Portosystemic shunt in the treatment of cirrhosis with or without portal vein thrombosis: A retrospective study. Front Med (Lausanne). (2021) 8:737984. doi: 10.3389/fmed.2021.737984
122. Wang, Z, Jiang, MS, Zhang, HL, Weng, NN, Luo, XF, Li, X, et al. Is post-TIPS anticoagulation therapy necessary in patients with cirrhosis and portal vein thrombosis? A randomized controlled trial. Radiology. (2016) 279:943–51. doi: 10.1148/radiol.2015150369
123. Wu, W, Zhang, H, Zeng, Z, Wang, X, and Kong, D. Comparison of transjugular intrahepatic portosystemic with endoscopic treatment plus anticoagulation for esophageal variceal bleeding and portal vein thrombosis in liver cirrhosis. Scand J Gastroenterol. (2022) 57:1494–502. doi: 10.1080/00365521.2022.2094724
124. Ju, C, Li, X, Gadani, S, Kapoor, B, and Partovi, S. Portal vein thrombosis: diagnosis and endovascular management. Rofo. (2022) 194:169–80. doi: 10.1055/a-1642-0990
125. Chamarthy, MR, Anderson, ME, Pillai, AK, and Kalva, SP. Thrombolysis and Transjugular intrahepatic Portosystemic shunt creation for acute and subacute portal vein thrombosis. Tech Vasc Interv Radiol. (2016) 19:42–51. doi: 10.1053/j.tvir.2016.01.005
126. Hollingshead, M, Burke, CT, Mauro, MA, Weeks, SM, Dixon, RG, and Jaques, PF. Transcatheter thrombolytic therapy for acute mesenteric and portal vein thrombosis. J Vasc Interv Radiol. (2005) 16:651–61. doi: 10.1097/01.RVI.0000156265.79960.86
127. Seedial, SM, Mouli, SK, and Desai, KR. Acute portal vein thrombosis: current trends in medical and endovascular management. Semin Intervent Radiol. (2018) 35:198–202. doi: 10.1055/s-0038-1660798
128. Zhang, WW, Ren, JZ, Han, XW, Chen, PF, Li, FZ, Kuang, DL, et al. Clinical application and efficacy of TIPS combined with AngioJet mechanical thrombectomy for liver cirrhosis with extensive portal vein thrombosis. Zhonghua Yi Xue Za Zhi. (2020) 100:533–7. doi: 10.3760/cma.j.issn.0376-2491.2020.07.011
129. Sullivan, IW, Fonseca, A, Brown, M, Ness, J, Borge, M, Amin, P, et al. Large bore portal vein thrombectomy: an Inari FlowTriever case series. Cardiovasc Intervent Radiol. (2022) 46:136–141. doi: 10.1007/s00270-022-03286-w
130. Villa, E, Cammà, C, Marietta, M, Luongo, M, Critelli, R, Colopi, S, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. (2012) 143:1253–60.e4. doi: 10.1053/j.gastro.2012.07.018
Keywords: portal vein, cirrhosis, anticoagulation, thrombosis, portal hypertension, bleeding, rebalanced hemostasis
Citation: Prakash S, Bies J, Hassan M, Mares A and Didia SC (2023) Portal vein thrombosis in cirrhosis: A literature review. Front. Med. 10:1134801. doi: 10.3389/fmed.2023.1134801
Edited by:
Carlos Jerjes-Sanchez, Tecnológico de Monterrey, MexicoReviewed by:
Ton Lisman, University Medical Center Groningen, NetherlandsYong Lv, Air Force Medical University, China
Copyright © 2023 Prakash, Bies, Hassan, Mares and Didia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. Claudia Didia, U2NsYXVkaWEuRGlkaWFAdHR1aHNjLmVkdQ==
 Swathi Prakash
Swathi Prakash Jared Bies
Jared Bies Mariam Hassan1
Mariam Hassan1 S. Claudia Didia
S. Claudia Didia