- 1Department of Ophthalmology, Pallas Klinik, Olten, Switzerland
- 2Department of Ophthalmology, University Hospital Essen, University Duisburg-Essen, Essen, Germany
- 3Department of Ophthalmology, San Raffaele Scientific Institute, University Vita-Salute, Milan, Italy
- 4Faculty of Medicine, University of Bern, Bern, Switzerland
- 5Department of Ophthalmology at St. Franziskus Hospital, Muenster, Germany
- 6Department of Hematology, University Hospital Basel, University of Basel, Basel, Switzerland
- 7Faculty of Medicine, University of Basel, Basel, Switzerland
Graft-versus-host disease (GVHD) is characterized by tissue inflammation in the host following an allogeneic hematopoietic cell transplantation (HCT). The pathophysiology is complex and only incompletely understood yet. Donor lymphocyte interaction with the histocompatibility antigens of the host plays a crucial role in the pathogenesis of the disease. Inflammation may affect multiple organs and tissues, e.g., the gastrointestinal tract, liver, lung, fasciae, vaginal mucosa, and the eye. Subsequently, alloreactive donor-derived T and B lymphocytes may lead to severe inflammation of the ocular surface (i.e., cornea and conjunctiva) and the eyelids. Furthermore, fibrosis of the lacrimal gland may lead to severe dry eye. This review focuses on ocular GVHD (oGVHD) and provides an overview of current challenges and concepts in the diagnosis and management of oGVHD. Ophthalmic manifestations, diagnostic procedures, grading of severity and recommendations for ophthalmic examination intervals are provided. Management of ocular surface disease with lubricants, autologous serum eye drops, topical anti-inflammatory agents and systemic treatment options are described based on the current evidence. Ocular surface scarring and corneal perforation are severe complications of oGVHD. Therefore, ophthalmic screening and interdisciplinary treatment approaches are highly relevant to improve the quality of life of patients and to prevent potentially irreversible visual loss.
1. Introduction
Graft-versus-host disease (GVHD) is a severe complication after allogeneic hematopoietic cell transplantation (HCT). Tissue inflammation in the host due to donor lymphocyte interaction with the histocompatibility antigens of the host may lead to a high morbidity and even mortality in these patients. This review focuses on ocular GVHD (oGVHD) and provides an overview of current challenges and concepts in the diagnosis and management of oGVHD.
1.1. Definition of GVHD
Allogeneic HCT offers the best chance of cure for several malignant hematological as well as non-malignant disorders like bone marrow failure, hemoglobinopathies or immunodeficiencies. Currently. over 30,000 allogeneic HCT are performed annually worldwide with over 18,000 in Europe in 2020 (1).
GVHD is one of the most important causes for non-relapse mortality post-transplantation. The current understanding of the pathophysiologic concepts and therapeutic targets has tremendously expanded during the last 20 years and recently been summarized in three excellent reviews (2–4). Chronic GVHD is the most common long-term complication after allogeneic HCT with an important impact on survival, morbidity, and quality of life. Traditionally, acute and chronic GVHD was differentiated depending on the time of the initial manifestation before or after 100 days post-transplant. These criteria were revised in the 2005 and 2014 National Institute of Health (NIH) Consensus Conference, introducing new criteria/definition for acute and chronic GVHD (5–7). Acute GVHD is defined as an immediate multi-organ inflammatory syndrome following HCT primarily affecting the skin, liver, and digestive tract, whereas chronic GVHD is a pleiotropic, multi-organ syndrome characterized by tissue inflammation and fibrosis that involves multiple sites including the skin, lungs, liver, gastrointestinal tract, mouth, genitalia, and eyes (5–8). Accordingly, the diagnosis of chronic GVHD requires at least one diagnostic sign of chronic GVHD or a distinctive manifestation plus a pertinent biopsy or another test (e.g., Schirmer test, evaluation by an ophthalmologist) showing or confirming chronic GVHD (Table 1).
1.2. Epidemiology of GVHD
After the first HCT in 1968 survival rates have increased in the last decades, due to human leukocyte antigen (HLA) matching, continuously improved preconditioning protocols and immunosuppressive regimen (7, 9, 10). Both, acute and chronic GVHD occur in about 30%–70% of patients after HCT depending on transplant regimens and GVHD prophylaxis strategies (7, 11). A variety of risk factors for GVHD related to donor as well as to recipients’ characteristics have been identified. The most important are the degree of histocompatibility, the source of hematopoietic progenitor cells, sex mismatch (transplantation from female donor to male recipient), the intensity of conditioning and immunosuppression, the age of donor and recipient and for chronic GVHD prior acute GVHD (2, 3, 8, 12–14).
1.3. Definition of ocular GVHD
Different criteria for the diagnosis of oGVHD have been proposed in the last decades (8). The original NIH criteria defined new onset of dry eye after HCT documented by low Schirmer test values with a mean value of both eyes <5 mm at 5 min or a new onset of keratoconjunctivitis sicca by slit-lamp examination with mean values of 6 to 10 mm at 5 min on the Schirmer test as sufficient for the diagnosis of chronic oGVHD if accompanied by distinctive manifestations in at least one other organ (6). An international consensus group proposed criteria based on Ocular Surface Disease Index (OSDI), Schirmer test score without anesthesia, corneal fluorescein staining and conjunctival injection (15). A score of 4–5 and ≥ 6 indicates probable or definite oGVHD, accordingly (15).
1.4. Epidemiology of ocular GVHD
Acute GVHD has been reported in 40%–50% of HCT patients (16). Ocular affection in acute GVHD is quite rare and has been reported in about 7.2% after HCT (17, 18). On the other hand, occurrence of chronic oGVHD was observed in 30%–60% in the further course after HCT (19, 20), and in 60%–90% of patients with systemic GVHD (7, 10, 19, 21, 22). Lower incidences have been found in Asian studies (23–25). The mean latency of oGVHD after HCT is about 1.5 years (26). Cumulative increase of incidences over time after HCT has been reported, with a prevalence of 16% by 100 days and 35% after 2 years (21). In children, symptoms consistent with chronic oGVHD have been found at highly variating rates from 4% up to 62% (27–33). In a large prospective study, a total of 29.4% of patients with chronic oGVHD were identified using the NIH consensus criteria (34).
2. Pathophysiology of GVHD and ocular GVHD
Pre-clinical animal models have been critical not only in understanding the immune mechanisms of systemic but also oGHVD (35–37). Acute and chronic GVHD are immune-mediated diseases involving a variety of immune cells such as macrophages, T cells and B cells (11, 19, 38, 39). Figure 1 depicts the immunological activation leading to ocular surface inflammation and lacrimal gland fibrosis. Self-reactive T cells (CD4+ and CD8+), deriving from the donor, are insufficiently deleted in the thymus (defective central tolerance) and in the lymph nodes (defective peripheral tolerance). These T cell mediated immune response is directed against host antigens as major (MHC) and minor (miHAG) histocompatibility antigens (40). The response is driven mainly by differences in host and donor antigen expression, e.g., by HLA mismatch (41, 42). But even in HLA-matched HCT, differences in polymorphic minor histocompatibility antigens (miHAs) and specific miHAs may trigger GVHD (43, 44). Imbalance between effector and regulatory T cells functions triggers the inflammatory cascades (11, 45–47). Although also B cells and antigen-presenting cells (APC) are involved, donor T cells are probably the predominant factor in the orchestration of systemic and ocular disease (48). In oGVHD, activation of APC, differentiation, proliferation and activation of donor T cells, and activation of B cells with release of pro-inflammatory cytokines currently are supposed to induce and maintain inflammation in the ocular surface, to activate fibroblasts and dendritic cells in the lacrimal gland finally leading to lacrimal tissue fibrosis (49, 50). However, tissue damage in oGHVD is not limited to the ocular surface and the lacrimal gland. Recent pre-clinical and clinical studies have shown that ocular adnexa are involved and Meibomian gland and ocular surface damage correlate with each other (51).
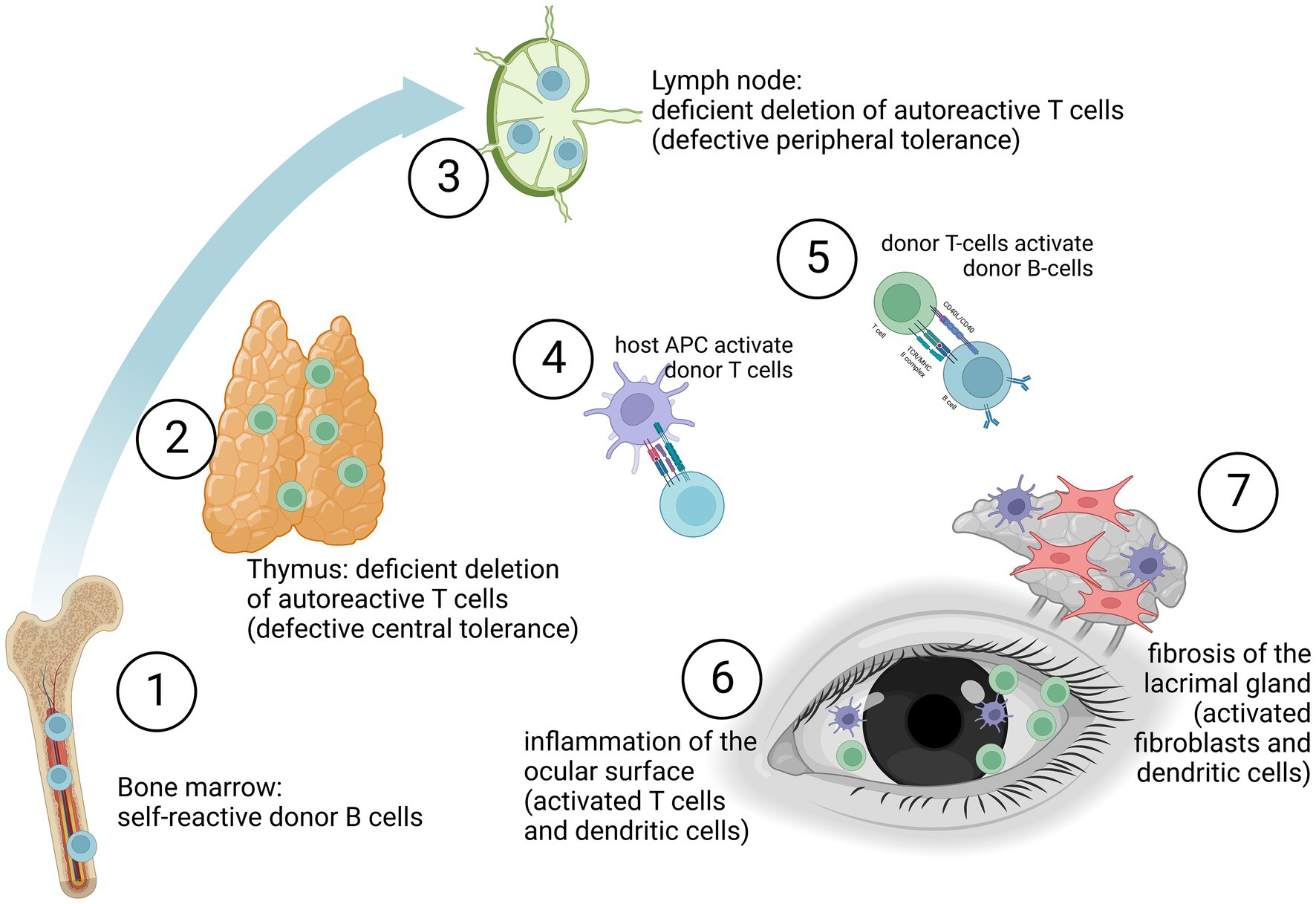
Figure 1. Graft-versus-host disease may be due to self-reactive donor B cells (1), deficient deletion of autoreactive donor T cells in the thymus (2) or deficient deletion of autoreactive donor T cells in the lymph nodes (3). Especially antigen-presenting cell (APC) driven activation of donor T cells (4) but also B cells (5) lead to an inflammation of the ocular surface (6). Furthermore, activation of fibroblasts by APCs (e.g., dendritic cells) induces fibrosis of the lacrimal gland (7). (The figure was created with biorender.com.)
3. Risk factors for the occurrence of ocular GVHD
A variety of risk factors associated with the onset of oGVHD have been reported (52), e.g., previous acute GVHD (21, 25), use of peripheral blood stem cells (25, 53), transplantation from a female donor to a male recipient (21, 54), absence of anti-thymocyte globulin prophylaxis (25), larger number of organs and tissues involved with GVHD (25, 55), and non-Caucasian and EBV-seropositive donors (56). Other risk factors are mismatch of HLA antigens, higher donor or recipient ages, and diabetes mellitus (25, 57). Increased occurrence of oGVHD has been found in patients with involvement of the skin (20, 21, 58), oral mucosa (20, 58), liver (56), or gastrointestinal tract and pulmonal involvement in chronic GVHD (25). Furthermore, ethnicity may have an impact, with Caucasians being at lower risk than Asians (56). Cord blood cell transplants (53), in vitro or in vivo T cell depletion or posttransplant cyclophosphamide lower the risk for GVHD. Dry eye and Meibomian gland disease before HCT may also be a risk factor for oGVHD, or worsen after GVHD (59–62).
4. Grading of ocular GVHD
Several grading systems have been proposed for oGVHD, which are based to varying degrees on findings by ophthalmologists or patient-reported symptoms. The international chronic oGVHD Consensus group (ICCGVHD) introduced criteria for the diagnosis of chronic oGVHD, based on scores calculated by ocular surface disease index (OSDI), Schirmer test without anesthesia, corneal fluorescein staining, conjunctival injection and the presence or absence of systemic GVHD (15, 63). On the other hand, the NIH chronic GVHD consensus group eye score system classifies oGVHD according to the degree of symptoms of dry eye (grade 1: mild dry eye symptoms not affecting activities of daily living (ADL) OR asymptomatic signs of keratoconjunctivitis sicca; grade 2: moderate dry eye symptoms partially affecting ADL (requiring drops >3x per day or punctal plugs), without vision impairment; grade 3: severe dry eye symptoms significantly affecting ADL (special eyewear to relieve pain) or unable to work because of ocular symptoms or loss of vision caused by keratoconjunctivitis sicca) (6, 7). A subsequent study aimed for validation of the suggested measurement scales. Herein, clinician or patient-reported changes in eye symptoms with calculated changes in 5 candidate scales (NIH eye score, patients-reported global rating of eye symptoms, Lee eye subscale, Ocular Surface Disease Index (OSDI), and Schirmer test) were compared. The results supported the use of the NIH eye score as a sensitive measures of eye symptom changes in clinical trials assessing treatment of chronic GVHD (64). Subsequently, the NIH chronic GVHD diagnosis and staging system criteria were refined with emphasis placed on usage of lubricant eye drops for dryness symptoms (65). Further scoring systems have been proposed by Robinson et al. based on exemplary photographs for everted upper and lower eyelids showing the different grades of conjunctival inflammation associated with chronic oGVHD (66). Furthermore, the ICCGVHD has proposed a grading system for conjunctival involvement (15, 67).
5. Recommendations for screening
Importantly, risk factors for ocular involvement have been investigated. In children, multiorgan GVHD involvement including skin and lung disease, and patients with ocular discomfort are at increased risk for eye involvement (27). However, as a significant number of GVHD patients do not exploit overt symptoms of eye involvement, regular ophthalmic screenings are recommended.
For early diagnosis of oGVHD, comprehensive ophthalmic evaluations by ophthalmologists are generally recommended before and after allogeneic HCT (68). In the acute phase, intervals corresponding to disease severity are recommended.
Chronic oGVHD may significantly influence quality of life (22). However, symptoms of chronic oGVHD may be subtle. Onset of any eye symptoms should prompt ophthalmic evaluation. More severe ocular surface damage at baseline indicates an increased risk to subsequent worsening and impaired vision (69). Therefore, prevalent ocular surface alterations and dry eye states should be evaluated in advance. Taken previous considerations, screening should be instituted at 3 (at the latest 6) months following transplantation (70, 71), and annually afterwards. Importantly, the screening intervals should be adapted to disease severity. There are no specific symptoms of oGHVD that allow a reliable differentiation from “simple” dry eye disease or lacrimal gland damage by total body irradiation. Therefore, any worsening or new manifestation of dry eye symptoms and/or worsening or new onset of ocular surface disease in patients after HCT should be evaluated and monitored closely.
Simple self-testing may further be critical for screening. For ocular discomfort testing, the ocular surface disease index (OSDI) questionnaire – considering vision-related function, ocular symptoms, and environmental triggers—may be used (72), and daily lubricant use reported. According to a recent study, the OSDI questionnaire is a valid screening test for oGVHD in transplant clinics and for patients’ self-monitoring (73). Thus, screening intervals may be adjusted based on the results from the OSDI questionnaire. The OSDI and other questionnaires are described in more detail in section 6.3.
6. Diagnosis of ocular GVHD
6.1. Ocular symptoms and findings
In the absence of overt ocular symptoms and signs during the acute disease stage, diagnosis may be delayed. Disease may partially mimic other immune-mediated inflammatory processes of the ocular surface. While no pathognomonic symptoms or clinical signs of oGVHD have been defined, certain combinations of findings are frequently present, and are provided within several recent publications (15, 67; Table 2). Key features of disease are new onset of refractory dry eye, being the most frequent manifestation (40%–70%), and secondary ocular surface damage (52). Patients suffer from diverse symptoms of the autoinflammatory reaction (particularly dry eye), including irritation, pain, burning, dryness, itchiness, blurred vision, foreign body sensation, photophobia, and redness (70, 74, 75). Visual disturbance may be the consequence from corneal higher order aberrations resulting from corneal pathology (76).
Severe ocular discomfort from dry eye, corneal epitheliopathy by means of fluorescein staining and vision loss are resulting in impaired quality of life (22). Patients with oGVHD had worse quality of life than patients without ocular involvement (77). In clinical studies, symptoms are quantified using validated QOL instruments such as Ocular Surface Disease Index (OSDI), National Eye Institute Visual Function Questionnaire (NEI-VFQ-25), and Symptom Assessment in Dry Eye (SANDE). Respective studies show that disease impact on QOL was comparable to herpetic uveitis or retinal vein occlusion (22).
By en-face evaluation, photophobia, pseudoptosis, frequent blinking or periorbital hyperpigmentation may be seen. Findings at the lid margin are common in oGVHD. Blepharitis and Meibomian gland dysfunction (50%) are probably the first signs of disease. Subsequently, atrophy, irregularity and keratinization of the eyelid margin may occur.
Conjunctival involvement mostly manifests as hyperemia (Figures 2A,B) and chemosis. Qualitative and quantitative alterations of the tear film are common, probably with serosanguineous exudation (78). In severe course, pseudo-membrane formation may be observed. Conjunctival fibrosis and subsequent scarring (Figures 2B,C) may not only result in loss of goblet cells, but also to entropion, distichiasis and trichiasis. Therefore, thorough subtarsal inspection is mandatory to determine the pathology also under the upper lid. Indeed, subtarsal fibrosis may correlate with worsening of corneal epitheliopathy. Inflammation and staining of the superior tarsal and bulbar conjunctiva with alteration of the superior limbal epithelium may be present (superior limbal keratoconjunctivitis; SLK-like appearance). The ICCGVHD grading system for conjunctival involvement in oGVHD is shown in Table 3 (15, 67).
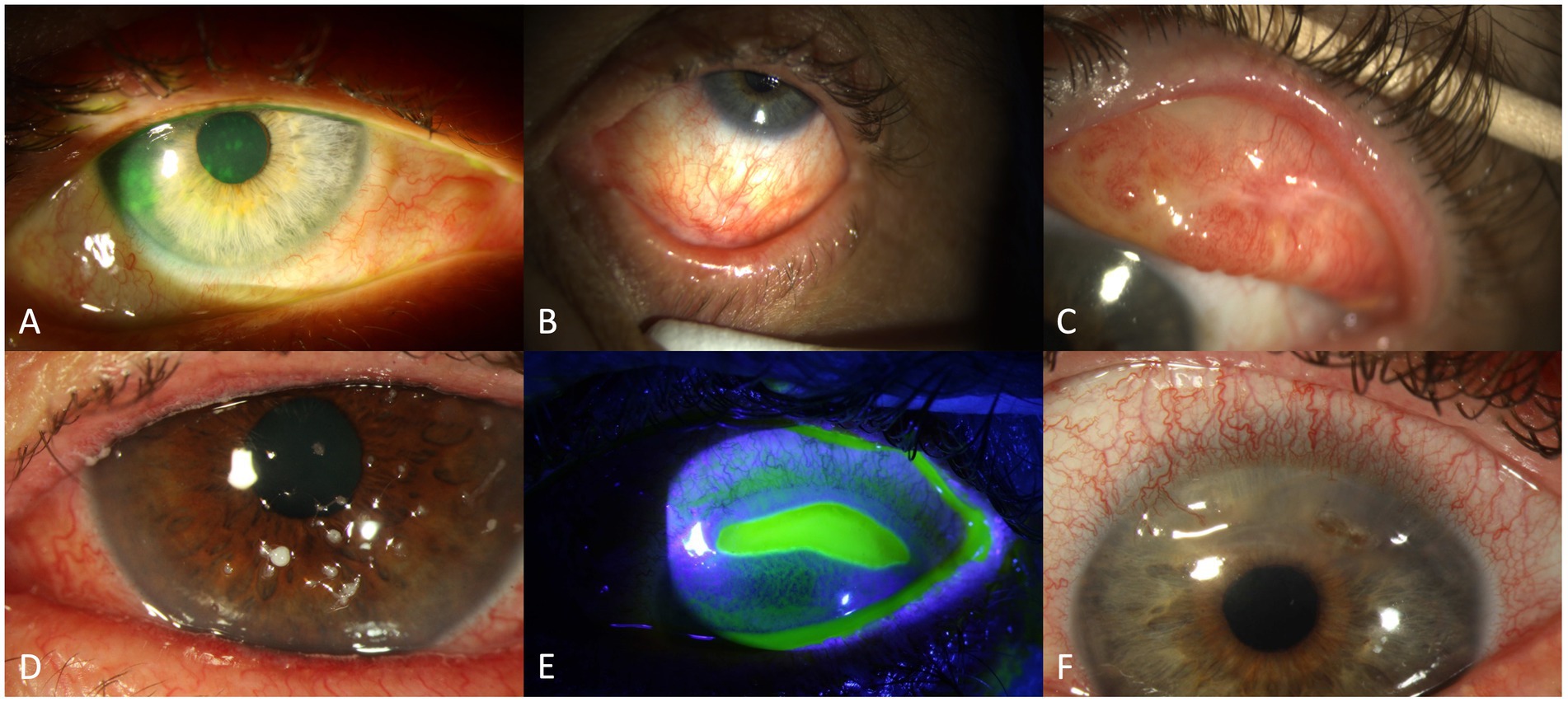
Figure 2. Findings in oGVHD: conjunctival hyperaemia and corneal staining (A), conjunctival scarring/fibrosis (B), conjunctival hyperaemia and symblepharon (C), filamentary keratitis (D), sterile corneal ulceration (E) and corneal melting with perforation (F).
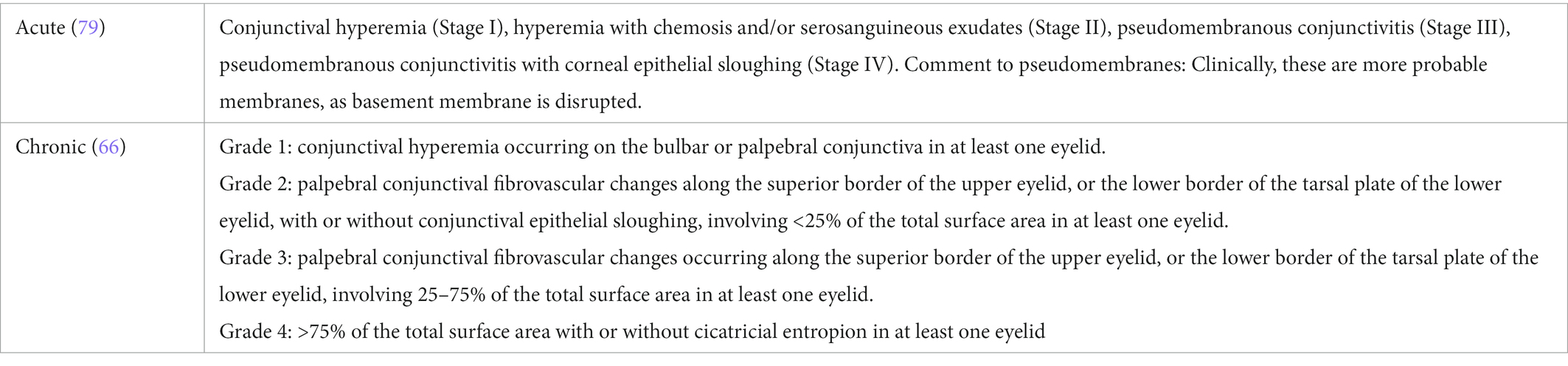
Table 3. Grading of conjunctival disease in ocular graft versus host disease according to the international chronic oGVHD consensus group (15, 67).
Morphological abnormalities of the cornea involve punctate keratopathy (Figure 2A), erosions, or filamentary keratitis (Figure 2D) in the more severe cases. Further, limbal stem cell deficiency, Bowman abnormalities, stromal thinning, ulceration (Figure 2E), scarring, calcification and neovascularization may appear. Corneal perforation (Figure 2F) may be secondary to epithelial barrier dysfunction and microorganisms (herpes simplex virus or bacteria), or as sterile “melt” probably in the setting of immunosuppression (80). According to previous reports, corneal ulceration or perforation is found in about 5% of cases (69).
Further, signs of episcleritis or scleritis, secondary cataract (10%, mostly from steroids), or glaucoma (also including steroid-induced ocular hypertension) may appear (81). Within a cohort of 635 patients undergoing HCT, 7.6% had secondary posterior eye segment complications, e.g., retinal hemorrhage, cytomegalovirus retinitis, or uveitis (40, 82).
6.2. Diagnostic techniques
A thorough ophthalmological examination is essential in patients with (suspected) oGVHD (83). For assessing the course of disease and response to treatment, a standardized documentation of ocular findings should be performed (Table 4). Assessing ocular findings at baseline before HCT and during follow-up visits allow to early detect worsening of the ocular surface (52, 68, 71, 84). A minimal set of data as visual acuity, slit lamp findings and intraocular pressure should be collected at each visit. Further investigations should be performed as appropriate.
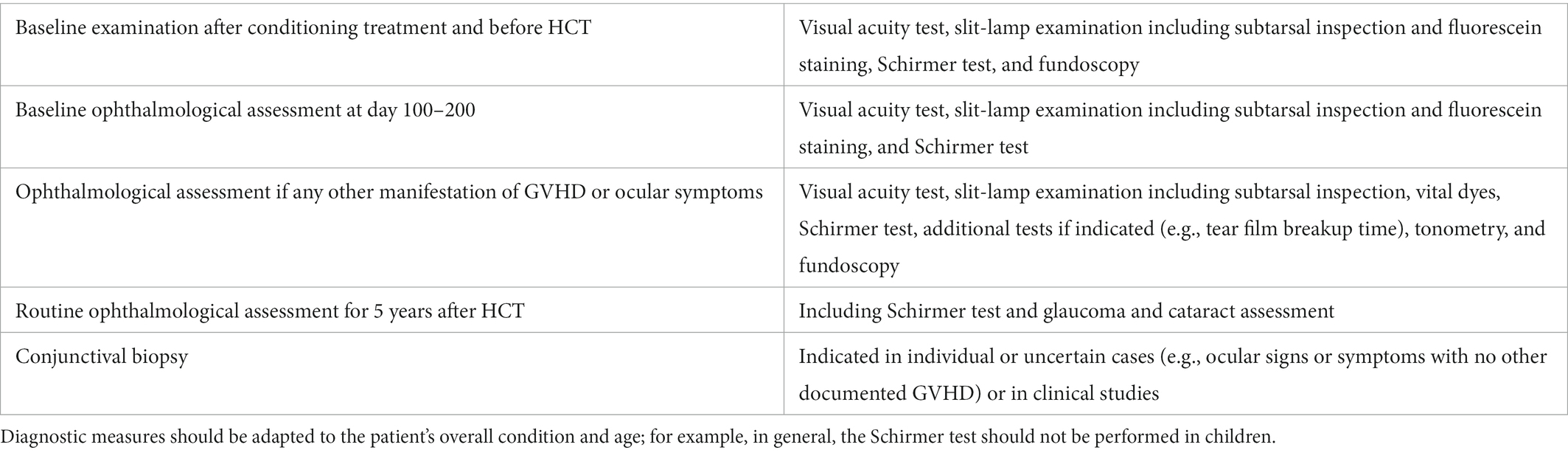
Table 4. Consensus Conference Proposal for diagnostic measures for assessment of ocular GVHD (75).
The Schirmer test I (without topical anesthesia) and II (with prior topical anesthesia) allows to assess the tear production during a defined time of 5 min. A folded filter paper strip is placed in the temporal third of the lower lid margin and the length of the wetting is measured (52). The Schirmer test without anesthesia is also included in the oGVHD (ICCGVHD) consensus group diagnostic criteria (67). While the Schirmer test is useful for diagnosing disease, it was removed from scoring recommendations, as values were not useful for follow-up due to poor correlation with symptom change (64). Due to its low reproducibility it has been removed in the revision of the 2005 NIH criteria and not been included in the 2014 NIH severity scoring, nor in the 2016 Japanese and Asian diagnostic criteria for dry eye disease (23, 63, 85).
Esthesiometry allows to assess the corneal sensitivity, which may be decreased due to pre-conditioning irradiation and neurotrophic keratopathy in patients with oGVHD (86–89).
Impairment of conjunctival and/or corneal epithelial integrity can be depicted with vital dye staining. Fluorescein is commonly used to evaluate the corneal staining according to the Oxford grading scheme (Figure 3) and/or the NEI grading for corneal and conjunctival staining (Figure 4) (52, 74, 92). Fluorescein dye is disclosing any disruption in superficial cell tight junctions, or defective glycocalyx of damaged epithelial cells (52). Additional dyes as Bengal rosa or lyssamine green can additionally be used in selected patients (90).
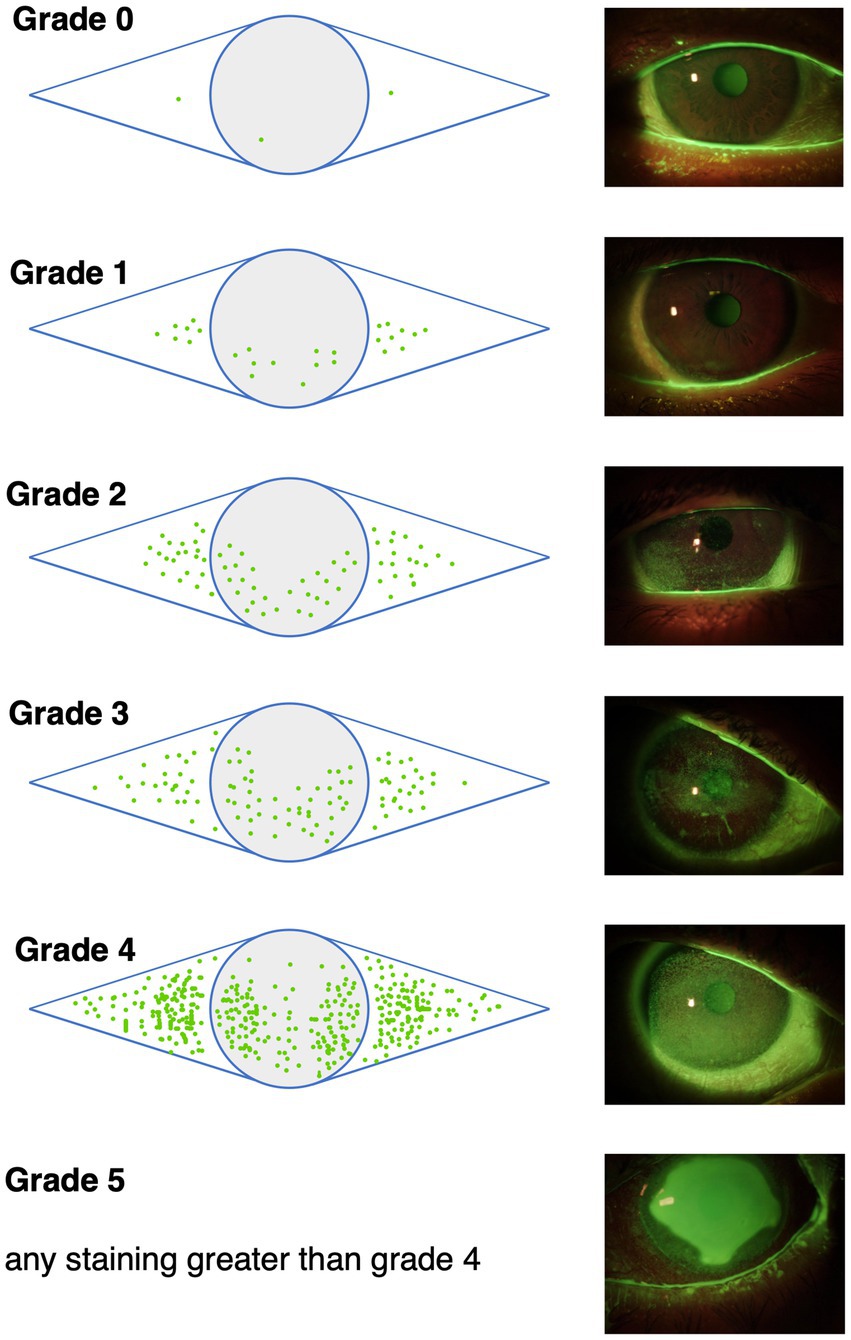
Figure 3. The Oxford grading scheme differentiates 5 grades of corneal and conjunctival fluoresceine staining. Image adapted from Bron et al. (90).
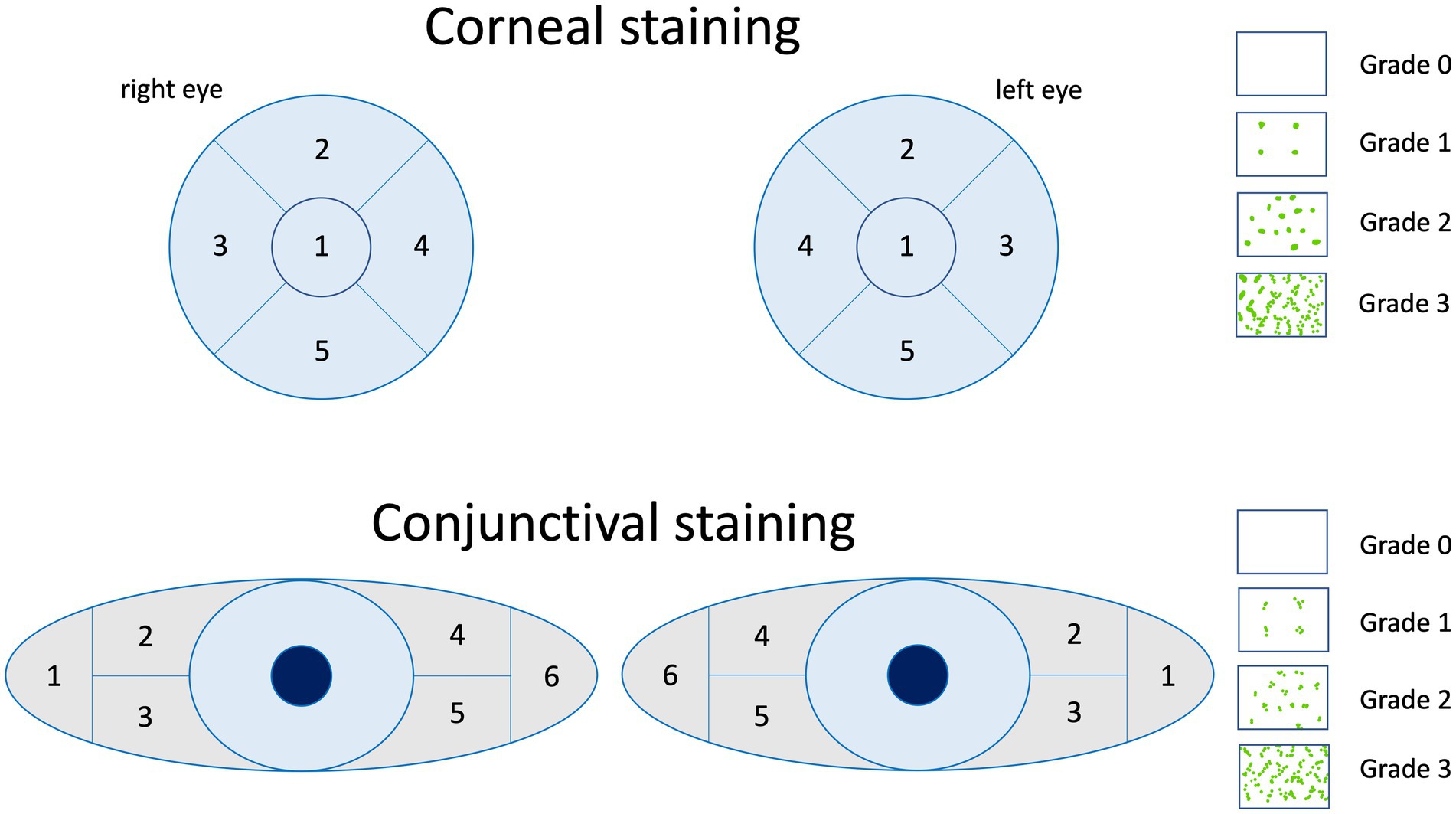
Figure 4. The NEI grading for corneal and conjunctival staining of the ocular surface is a standardized grading system that is summed up by the grading of 0 to 3 of each sector. Image adapted from Lemp et al. (91).
After fluorescein installation, the tear film break-up-time (TBUT) can be evaluated at the slit lamp (52, 70). A decreased TBUT indicates qualitative tear film impairment primarily due to Meibomian gland dysfunction (93).
Tear film osmolarity measurements reveal increased values in oGVHD (74, 94–96) and may be used as an additional factor in therapeutic decisions (19). The tear film osmolarity is also used in the ICCGVHD criteria (23, 95).
Meibomian gland imaging enables the assessment of Meibomian glands, which are often impaired in patients with oGVHD (52, 97–99).
In patients with keratitis, viral and/or microbial tests from corneal smears should be considered to identify viral (mainly by herpes simplex or varicella zoster virus), bacterial or fungal keratitis. The risk for infectious keratitis may be increased in patients under corticosteroid treatment.
In vivo confocal microscopy can be used as a diagnostic tool in patients with oGVHD to image epithelial cell density, epithelial dendritic cells and other inflammatory cells, subtarsal fibrosis and conjunctival changes (23, 52, 59, 99–103).
The use of anterior segment photography may be considered to document ocular findings (e.g., staining of ocular surface, conjunctival scarring/fibrosis, blepharitis). It may especially be useful for follow-up comparison of clinical course (75, 104).
Conjunctival impression cytology enables identification of epithelial cell necrosis, keratinization, goblet cells loss and also HLA-DR expression (83, 105, 106). As an alternative, Brush cytology is also a minimally invasive procedure to harvest ocular surface epithelium and inflammatory cells and to monitor pathological progress (88, 107), but interpretation might be difficult due to mechanical alteration of the harvested cells.
Tear film biomarkers (cytokines) can either directly be measured with specific antigen tests (e.g., MMP-9) (108) or (currently mainly for research purpose and not in clinical routine) by performing proteomics from tear fluid or tear-film soaked Schirmer stripes (52, 109). In eyes with oGVHD a variety of cytokines are differently expressed. Especially nucleic acid binding and cytoskeletal proteins are upregulated, while the most extensively downregulated proteins belong to an array of classes including transfer and receptor proteins, enzyme modulators, and hydrolases (109).
Tear flow cytometry is a novel approach, currently used mainly for research purpose, that allows differentiation of cells non-invasively from tear samples (51).
Histopathology may confirm the diagnosis of oGVHD. However lacrimal gland biopsies should not be performed routinely due to the increased risk of further impairment of its function. Previous investigations found mononuclear infiltration, loss of acinar lobules and fibrosis of the lacrimal gland in oGVHD (11, 110, 111). Also, conjunctival biopsies are not performed routinely but may be considered in selected patients, e.g., to rule out malignancy. In conjunctival specimen of oGVHD, lymphocyte exocytosis, vacuolization of the basal epithelium, and epithelial cell necrosis, similar to changes that are observed in other organs, have been found (11, 110, 111). Furthermore, T cells—probably driving alloreactivity in GVHD—have been found in conjunctival biopsies (112).
6.3. Questionnaires
Ocular surface inflammation and dryness may have a relevant impact on the quality of life and activities of daily living in patients with oGVHD (22). Different validated questionnaires are used to quantify symptoms, to assess the burden of disease and to track response to treatment (11, 22). The Ocular Surface Disease Index (OSDI), consisting of 12 patient-related questions of dry eye, and the Symptom Assessment in Dry Eye (SANDE) are commonly used questionnaires to assess symptoms in these patients (22, 72, 113–115). Alternatively, or additionally, the glaucoma symptom scale (GSS) may be used (116). On the other hand, the National Eye Institute Visual Function Questionnaire (NEI-VFQ-25) allows to assess vision related quality of life (22). Saboo et al. evaluated patients with oGVHD using the NEI-VFQ-25, OSDI and SANDE questionnaires and found a relevant impact of this disease on quality of life, that is comparable to other eye diseases as for example herpetic uveitis (22).
7. Treatment of ocular GVHD/management of complications
The primary aim of treating oGVHD is to maintain vision and quality of life by improving lubrification of the ocular surface (tear film quantity and quality), reducing ocular surface inflammation and preserving corneal epithelium integrity (5). The evidence for different treatments has recently been reviewed by Inamoto et al. (52).
7.1. Lubrication
An intensive lubrication for dry and inflamed ocular surface is essential in oGVHD (5, 83, 117). A variety of artificial tears, viscous eye drops, and viscous ointments are available and only limited data on specific preferences for oGVHD is available. In any case, preservative-free formulations should be preferred to avoid the negative impact of preservatives on the epithelium, especially if applied at high frequencies (118). Hyaluronic acid eye drops allow stabilization of the tear film and improvement of epithelial wound healing, ocular symptoms, and visual acuity (53, 117). Increasing the lubrification may also reduce the concentrations of proinflammatory cytokines on the ocular surface (5, 119). Mucolytic eye drops, i.e., topical N-acetylcysteine 5%–10%, should be considered in filamentary keratitis, which is often observed in eyes with a very dry ocular surface (5, 120).
7.2. Topical anti-inflammatory treatment
Reducing ocular surface inflammation is a key concept in the management of oGVHD. Topical corticosteroids are effective in treating dry eye in these patients (52, 66, 75). However, due to their probable adverse effects and risks, their application over a longer time periods, or at high dosages and/or with highly potent formulations should be avoided, or regular ophthalmological checks (intervals depending on corticosteroid dosage and duration, eye pressure and lens status) be instituted. Potential risks include cataract formation, infections, ocular hypertension/glaucoma, impaired epithelialization and impaired corneal wound healing (5, 11, 66). Nevertheless, they are used commonly in oGVHD patients (5, 52, 121, 122). However, topical corticosteroids are not able to sufficiently control oGVHD in about half of the patients (7). Low-dose/−less potent topical corticosteroids or their analogs seem to be less effective in patients with oGVHD compared to dry eye patients without oGVHD (11, 123). As an anti-inflammatory treatment option, cyclosporine (CsA) eye drops are used in patients with treatment refractory dry eye disease. CsA acts as a calcineurin inhibitor and suppresses T-cell activation (11, 124), and its efficacy has also been proven in patients with oGVHD (11, 125). Hereby, it reduces ocular surface inflammation, increases conjunctival goblet cell density and tear production and improves symptoms of dry eye (5, 75, 125–129). If treatment is initiated before HCT, it probably reduces the risk for oGVHD manifestation (130). However, a reduced tolerance (burning sensation) of topical CsA may limit its use in some patients. Furthermore, tacrolimus eye drops or ointment have been studied in patients with oGVHD, probably allowing corticosteroid sparing (11, 131–133). Tacrolimus ointment may also be applied to the eyelids as an off-label treatment. Although topical non-steroidal anti-inflammatory drugs (NSAIDs) are also used in oGVHD, there is no evidence for their efficacy.
7.3. Autologous serum eye drops
Based on several uncontrolled trials in oGVHD and in analogy to other forms of dry eye disease, autologous serum eye drops are also used in patients with oGVHD, especially in severe cases (86, 117, 134). Although the exact mechanism of action is not known, the high concentration of several growth factors combined with anti-inflammatory effects are suggested to improve healing of epithelial defects (129, 135, 136). Systemically applied cyclosporin A or mycophenolic acid might also be detectable in serum eye drops (137) and could contribute to the observed beneficial effect. Patients impaired condition to donate blood (poor venous access, severe anemia, active infection, low body weight, cardiovascular comorbidities) as well as regulatory restrictions are potential obstacles that prevent access to this therapy. Other options that have been reported are allogeneic serum eye drops (136), cord blood sera (117, 138, 139) and platelet lysate (116, 140). None of these options have become more widely available yet due to a couple of logistics and regulatory reasons.
7.4. Control of evaporation
Improving the lipid layer of the tear film with viscous eye drops and ointments, improving the Meibomian gland outflow with eyelid massage and eventually lipid sprays reduce evaporation of the tear film. The evidence for eyelid massage in oGVHD is low and the mechanical friction might even be counterproductive in oGVHD with affection of the corneal epithelium. Occlusive eye wear (52, 141) and an improvement of environmental factors as air humidity may also be helpful (117, 142).
7.5. Increase of tear and mucin production
Systemic treatment with oral muscarinic agonists as pilocarpine or cevimeline may increase tear production (117, 143, 144). As adjuvant treatment approaches, secretagogue eye drops as diquafosol and rebamipide may be used in patients with oGVHD (52, 101, 145). They stimulate secretion of aqueous and mucin and improve wound healing of the corneal surface (5, 101, 146).
7.6. Reduction of tear drainage
Reduction of the lacrimal drainage is a further approach to improve the tear film (11). Here, collagen or silicone punctal plugs (Figure 5A) may be inserted into the lacrimal ducts, or permanent punctal occlusion by thermal cauterization may be considered (147, 148). It has been speculated that reducing the tear drainage might result in a pooling of pro-inflammatory cytokines and increase damage of the ocular surface and patient discomfort (149). Positive effects of punctal occlusion predominate in the clinical situation (86, 147).
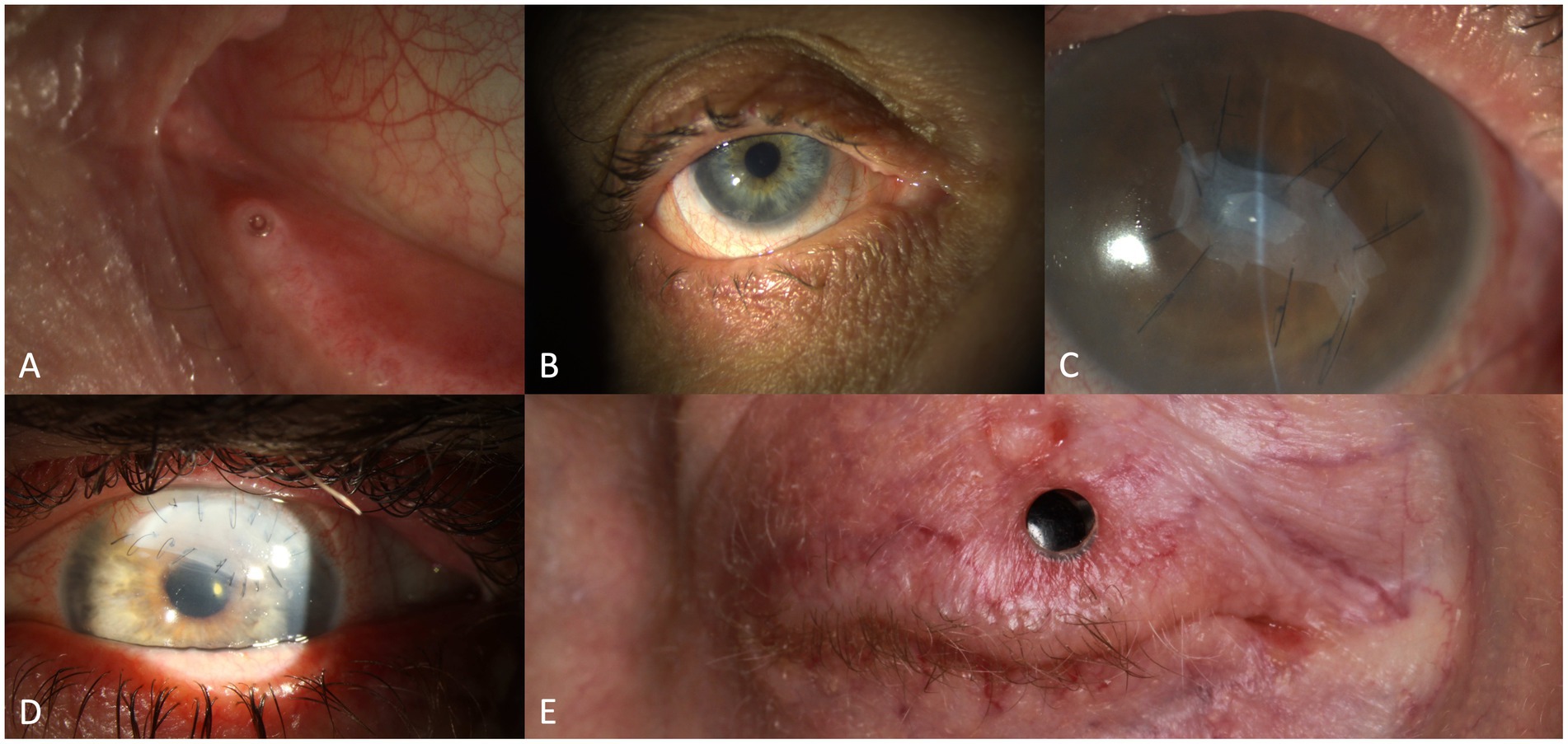
Figure 5. Therapeutic interventions in eyes with oGVHD: silicone punctal plug (A), scleral lens (B), amniotic membrane transplantation (C), lamellar keratoplasty with loosening of the sutures (D), transpalpebral osteo-odonto-keratoprosthesis (E).
7.7. Scleral lenses
The use of scleral lenses (Figure 5B) in patients with severe oGVHD has been shown to reduce ocular symptoms and especially ocular pain (83, 150) and improve visual acuity due to their uniform surface (11, 83, 107, 119, 150–155). These gas-permeable lenses cover most of the ocular surface, vault the cornea and limbus providing a fluid reservoir between the cornea and the lens (83). Furthermore, they protect the ocular surface from mechanical “scratching” from blinking (83). In a study by Schornack et al., most patients were still on scleral lenses after a 32-month observation period, indicating a relevant patient satisfaction (152). High costs, inadequate fitting, discomfort with blinking may be potential drawbacks (119). As an alternative to scleral lenses also soft contact lenses have been investigated in oGVHD (156), but may potentially bear a higher risk of infection (83).
7.8. Prevention of infectious disease
Especially in eyes with severe oGVHD, epithelial defects or even corneal melting may occur, due to the very dry and inflamed ocular surface. In this situation, infectious prophylaxis with topical antibiotics should be taken into consideration (19). In patients with extended wear of contact lenses (especially soft contact lenses and topical corticosteroid treatment) topical antibiotic prophylaxis should be considered (157). Furthermore, topical antibiotic ointments or eye drops but also systemic tetracyclines (e.g., doxycycline or minocycline) may be considered in patients with blepharitis as a sign of bacterial superinfection of the eyelids (11, 52, 75, 158).
7.9. Systemic treatment
Systemic treatment of oGVHD is absolutely indicated if severe oGVHD cannot be controlled with topical treatment alone.
High dose corticosteroids (methylprednisolone 1 mg/kg) remain the mainstay of initial systemic treatment of chronic GVHD, either given alone or in combination with calcineurin inhibitors, especially in high-risk disease (159). Second line treatment is indicated in case of steroid-refractory chronic GHVD with an increasing number of treatment options (160). Up to now, there is no standard yet (161). Levels of evidence for efficacy and treatment costs vary considerably and numbers of patients reported for eye response are usually low (162). Extracorporeal photopheresis (ECP) has been reported to resolve or improve eye manifestation in 30% compared to 7% with standard therapy alone by Flowers et al. (163). Other studies could confirm these results in similar or higher magnitude. Recently, ruxolitinib (Janus kinase 1/2 inhibitor; FDA and EMA) and belumosudil (inhibitor of Rho-associated coiled-coil-containing protein kinase 2; FDA) have been approved for treatment of steroid-refractory chronic GVHD. Both have been shown to be effective in a proportion of patients with oGVHD. In the randomized open-label REACH3 trial overall response was 26% with ruxolitinib versus 10.8% with best available treatment (164). Belumosudil was studied in the phase 2 ROCKstar trial mainly in patients with advanced, steroid-refractory chronic GVHD with a remarkable overall response rate of 42% (14% complete responses, 28% partial remissions) (165). In contrast, there are no conclusive data with the third FDA-approved agent ibrutinib (inhibitor of Bruton’s tyrosine kinase) in oGVHD (166). Other agents that are frequently used are sirolimus (mTOR inhibitor), bortezomib (proteosome inhibitor), imatinib (tyrosine kinase inhibitor) and low-dose methotrexate (162). However, there are no randomized controlled trials that evaluated the effect of systemic treatment specifically on oGHVD, or that investigated superiority of one agent to another.
Several new systemic therapeutic principles are tested in preclinical studies including bromodomain inhibitors (167) and SYK inhibition by entospletinib (168).
7.10. Antifibrotic treatment
Currently, no specific treatment strategy is available for fibrosis. Given the pathophysiology of chronic oGVHD, anti-inflammatory and anti-fibrotic treatment regiments might be beneficial. Topically, corticosteroids may have some local antifibrotic effect, but clinical relevance is unknown, and risks do not justify prolonged application. TGF-b signaling inhibition (tranilast) may be useful (169, 170). In contrast to topically applied agents, systemic DMARDs therapy is commonly recommended for severe oGVHD not properly responding to topical agents, as untoward side effects may occur. Agents such as corticosteroids and steroid sparing agents may be applied, including ciclosporin, tacrolimus, sirolimus, mycophenolate mofetil, and particularly B cell blockade with rituximab. Case reports document the value of amniotic membrane transplantation (AMT) for preventing excessive fibrosis (171).
8. Surgical management of complications
No data exist on how often surgical treatment for complications of chronic oGVHD is necessary. This section gives an overview of different surgical interventions for the most common complications of oGVHD.
8.1. Cauterization of lacrimal punctum
Punctal occlusion with punctal plugs has been shown to be safe to treat severe dry eye in oGVHD (147) and is often used. In rare cases plugs are not supported or extruded repeatedly. In such situations permanent surgical occlusion is possible. Yaguchi et al. described their method of punctal cauterization with a high-temperature sterile disposable cautery device in 23 puncta from 10 oGVHD patients (148). They achieved a 100% anatomical success without recanalization after 1 year and reported no surgical complications. Several other methods for surgical punctal occlusion in other etiologies of dry eye disease have been described, including thermal cautery, diathermy, laser coagulation and punctal suturing (172–177).
8.2. Tarsorrhaphy and botulinum toxin
Inflammation and tear deficiency in oGVHD can lead to severe corneal ulcerations (87, 178). In such situations, temporal or complete temporary tarsorrhaphies or botulinum toxin A induced protective ptosis (179) are good options to protect the cornea and gain time when systemic immunomodulatory treatment is initiated or escalated and not fully effective yet. Yeh et al. described a patient with oGVHD in whom even tarsorrhaphy and amniotic membrane transplantation (AMT) were not enough, and eventually the eye had to be eviscerated (180).
8.3. Amniotic membrane transplantation, cyanoacrylate glue or conjunctival (Gundersen) flap
Amniotic membrane transplantation (Figure 5C) is a surgical procedure that may help to prevent or stop corneal melting by reconstructing the ocular surface and supporting the epithelialization of the cornea (181–184). Epithelial recovery and suppression of inflammation may be achieved due to the contained cytokines and growth factors, additionally the amnion membrane acts as a mechanical barrier for frictional forces (184–187). Indeed, AMT has also successfully been used in progressive corneal ulcers in oGVHD patients (171, 188–190). However, only limited data are available about its success rate up to now. In deep corneal ulcers or descemetocele with pending perforation, cyanoacrylate glue may be an option to avoid or delay more invasive corneal surgery (80, 189). Conjunctival (Gundersen) flap may be another option to cover a corneal ulcer or a fresh corneal transplant. Xu et al. described four oGVHD patients in whom they combined tectonic penetrating keratoplasty with conjunctival flaps (191). Furthermore, Pellegrini et al. reported on one patient receiving a Gundersen flap for impending perforation in their case series of 283 patients with HCT (192).
8.4. Keratoplasty and keratoprosthesis
Despite intensive topical and systemic treatment and tarsorrhaphy and/or AMT, corneal perforations might still occur in severe oGVHD. In such situations, keratoplasties might be required. One option is to perform an urgent tectonic keratoplasty (Figure 5D) with the primary aim of saving the eye and gaining time to escalate the anti-inflammatory treatment. Another possibility is to perform a penetrating keratoplasty with the aim of restoring vision and globe integrity at the same time. Corneal transplant diameters from only few millimeters to large may be used for such keratoplasties depending on the individual need. Sinha et al. determined that the prevalence of corneal perforation in patients with oGVHD was 3.7% (193). Zhang et al. reported 14 corneal perforations in patients with oGVHD during an observation period of 59 years at 4 large centers (80). They all were initially glued and 8 needed penetrating keratoplasty, which had diameters of 2 to 9.5 mm. The best corrected visual acuity outcomes at last visit were 20/100 or better in 5 patients (36%), and hand motion or worse in 7 patients (50%) (80). Xu et al. reviewed 198 oGVHD patients within an observation period of 9 years and identified 9 eyes of 7 patients with corneal perforation necessitating penetrating keratoplasty (trepanation diameters of 2 to 8 mm were used). Only two eyes of two patients achieved a final best corrected visual acuity of 20/100 or better (191). Sometimes even repeat keratoplasty cannot prevent perforations and re-establish functional visual acuity, reason why we had to perform a through-lid Osteo-Odonto-Keratoprosthesis (OOKP; Figure 5E) in one patient (194) and Osteo-Keratoprosthesis (OKP) in another. The outcome was successful in both patients with a best corrected visual acuity of 20/32 or better. Liu et al. mentioned one oGVHD patient in their 10-years review on 36 patients with OOKP (195). Furthermore, Orive Bañuelos et al. also described an oGVHD patient who received a Boston keratoprosthesis Type II after several corneal perforations with repeated keratoplasties. As a further complication, probably related to the keratoprosthesis surgery, two cyclophotocoagulations had to performed. The final visual acuity was 20/20 but the visual field revealed glaucoma related damage (196). OOKP and OKP are high risk procedures that are not commonly performed but might sometimes be the last resort to restore vision in selected patients.
8.5. Cicatricial entropion repair and fornix reconstruction
Chronic conjunctival inflammation and subepithelial fibrosis, are often found in oGVHD and can eventually lead to progressive conjunctival scarring with entropion and trichiasis. In combination with keratoconjunctivitis sicca these complications can be devastating for the ocular surface, reason why cicatricial entropion and trichiasis have to be treated without delay (197). Komai et al. described the cultivated oral mucosal epithelial transplantation (COMET) as a method to treat fornix shortening/symblepharon in different chronic cicatrizing conjunctival diseases (198). One of their patients suffered from oGVHD and was successfully treated with this surgical method (198). Dulz et al. described a 7-year-old boy who developed a massive bilateral cicatricial entropion with trichiasis 5 years after HCT. They performed bilateral lamellar splitting via an eyelid crease and gray line incision. Cryocoagulation of persistent trichiatic lashes was additionally performed (199). Kheirkhah et al. utilized a combined approach with mucous membrane transplantation from the lower lip covering it with AMT for their series of symblepharon, among which was also a successfully treated oGVHD patient (200).
8.6. Cataract surgery
Cataracts frequently develop in patients after HCT. This is probably a side effect of the treatments with corticosteroids or total body irradiation, and not due to GVHD directly. The long-term use of topical corticosteroids, particularly when given at higher dosages increases the risk for cataract formation. In patients with oGVHD inactivity of the ocular surface inflammation and optimal stabilization of the dry eye disease is required before surgery (201), and a good peri-operative management is critical. Bae et al. described 77 cataract surgeries in 42 patients suffering from oGVHD. Out of these patients, 19 postoperatively developed punctate keratopathy, that was being treated with artificial tears or autologous serum drops; another 7 eyes developed corneal epithelial defects, requiring non-steroidal anti-inflammatory eye drops, and another 3 eyes had cystoid macular edema (202). These findings are supported by others, additionally reporting on corneal melts and perforation after surgery (203–207). Taken together, oGVHD patients require close post-operative monitoring and prolonged anti-inflammatory treatment.
9. Novel approaches and outlook
As described previously, the clinical manifestations of oGVHD are the result of various structural and functional changes in lacrimal and Meibomian glands, eye lids, quantitative and qualitative alterations of the tear film and damage of the ocular surface. It is likely that the contribution of each of this component to active oGVHD differs between individuals. Symptoms might manifest after the damage has already been set. Hence, a standardized ocular assessment and documentation as part of the posttransplant follow up as well as the identification of specific biomarkers might allow a better understanding of the pathophysiology of oGVHD, an earlier diagnosis in the future (47) and potentially also to identify eyes at risk for severe complications. Ophthalmologists should be constant members of multidisciplinary teams providing posttransplant care. More efficient treatments that prevent or treat inflammation and enable regeneration of the dysfunctional ocular surface, lacrimal glands and Meibomian glands are needed. Pre-clinical animal models of GVHD enable developing and investigating new treatments (208). During the last decade, the number of interventional studies in oGVHD has slowly increased. Most of them are single center trials of topical treatments involving limited patient numbers. Randomized controlled trials of topical and systemic treatment options in patients with oGVHD are urgently needed and could expand our current knowledge considerably.
Anti-inflammatory drugs as tocilizumab and sarilumab, that impact the IL-6 pathway, are promising as they have been shown to be beneficial in animal models of oGVHD (117, 209, 210). Furthermore, Janus kinase (JAK) inhibitors either alone or in combination with tyrosine kinase (SYK) inhibition are a further interesting option as an early intervention that had a favorable effect in a pilot study (211). Belumosudil is another promising new approach even in heavily pretreated chronic GVHD (165). It will be important to study the therapeutic potential of this drug on oGVHD in earlier lines of treatment because of its anti-inflammatory and antifibrotic action.
Innovative options coming from basic research and/or animal studies, like ATR type I antagonist, VAP-1 inhibitor, phenyl butyric acid, tranilast, heavy chain-hyaluronan/pentraxin 3 (HC-HA/PTX3), ABT-263 and vitamin A-coupled liposomes containing HSP4 siRNA reversed the changes seen in oGVHD (117). In a pilot trial pooled human immunoglobulin eye drops were promising for treating oGVHD (212). A variety of further ongoing trials in oGVHD investigate the potential of other therapeutic approaches, e.g., topical fibrinogen-depleted human platelet lysate, brimonidine nanoemulsion, rhDNase eye drops as well as different types of contact lenses (23).
10. Conclusion
A better understanding of the pathophysiology of oGVHD, definition of standardized diagnostic criteria, introduction of grading systems, increasing experience with different topical and systemic treatments, but also with tools as, e.g., punctal plugs or scleral lenses, has improved the management of this disease. Nevertheless, oGVHD still has a relevant impact on the quality of life of HCT survivors. Severe and potentially blinding complications as corneal perforations cannot always be prevented. There is a high need for randomized controlled trials comparing the efficacy of different treatment regimens and supporting measures.
Author contributions
CT, AH, and DG contributed to conceptualization. CT, AH, and JH performed the literature research. CT, AH, JH, and DG wrote individual chapters of the manuscript. DG, CT, and EM provided slit lamp photographs. CT produced the figures. All authors contributed to the article and approved the submitted version.
Funding
Open access funding was provided by University of Bern.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Passweg, JR, Baldomero, H, Chabannon, C, Corbacioglu, S, de la Cámara, R, Dolstra, H, et al. Impact of the SARS-CoV-2 pandemic on hematopoietic cell transplantation and cellular therapies in Europe 2020: a report from the EBMT activity survey. Bone Marrow Transplant. (2022) 57:742–52. doi: 10.1038/s41409-022-01604-x
2. Zeiser, R, and Blazar, BR. Acute graft-versus-host disease—biologic process, prevention, and therapy. N Engl J Med. (2017a) 377:2167–79. doi: 10.1056/NEJMra1609337
3. Zeiser, R, and Blazar, BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med. (2017b) 377:2565–79. doi: 10.1056/NEJMra1703472
4. Hill, GR, Betts, BC, Tkachev, V, Kean, LS, and Blazar, BR. Current concepts and advances in graft-versus-host disease immunology. Annu Rev Immunol. (2021) 39:19–49. doi: 10.1146/annurev-immunol-102119-073227
5. Bruscolini, A, Gharbiya, M, Sacchetti, M, Plateroti, R, Plateroti, R, Moramarco, A, et al. Involvement of ocular surface in graft-versus-host disease: An update from immunopathogenesis to treatment. J Cell Physiol. (2021) 236:6190–9. doi: 10.1002/jcp.30304
6. Filipovich, AH, Weisdorf, D, Pavletic, S, Socie, G, Wingard, JR, Lee, SJ, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. diagnosis and staging working group report. Biol Blood Marrow Transplant. (2005) 11:945–56. doi: 10.1016/j.bbmt.2005.09.004
7. Jagasia, MH, Greinix, HT, Arora, M, Williams, KM, Wolff, D, Cowen, EW, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. the 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. (2015) 21:389–401.e1. doi: 10.1016/j.bbmt.2014.12.001
8. Giannaccare, G, Pellegrini, M, Bernabei, F, Scorcia, V, and Campos, E. Ocular surface system alterations in ocular graft-versus-host disease: all the pieces of the complex puzzle. Graefes Arch Clin Exp Ophthalmol. (2019) 257:1341–51. doi: 10.1007/s00417-019-04301-6
9. Hahn, T, McCarthy, PL, Hassebroek, A, Bredeson, C, Gajewski, JL, Hale, GA, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. (2013) 31:2437–49. doi: 10.1200/JCO.2012.46.6193
10. Aldebasi, T, Bashir, R, Gangadharan, S, Shaheen, NA, Alhussain, B, Almudhaiyan, T, et al. Incidence of ocular manifestations in patients with graft versus host disease after allogeneic stem cell transplant in Riyadh, Saudi Arabia. Int J Ophthalmol. (2022) 15:1149–56. doi: 10.18240/ijo.2022.07.16
11. Carreno-Galeano, JT, Dohlman, TH, Kim, S, Yin, J, and Dana, R. A review of ocular graft-versus-host disease: pathophysiology, clinical presentation and management. Ocul Immunol Inflamm. (2021) 29:1190–9. doi: 10.1080/09273948.2021.1939390
12. Serapicos, P, Kim, C, Barros, SL, IMB, MSJ, Hiyane, MI, Barbosa de Sousa, L, et al. Tear film immunological profile in patients with ocular graft versus host disease. Ocul Immunol Inflamm. (2022) 1-9:1–9. doi: 10.1080/09273948.2022.2046794
13. Arora, M, Klein, JP, Weisdorf, DJ, Hassebroek, A, Flowers, ME, Cutler, CS, et al. Chronic GVHD risk score: a Center for International Blood and Marrow Transplant Research analysis. Blood. (2011) 117:6714–20. doi: 10.1182/blood-2010-12-323824
14. Heldal, D, Tjønnfjord, G, Brinch, L, Albrechtsen, D, Egeland, T, Steen, R, et al. A randomised study of allogeneic transplantation with stem cells from blood or bone marrow. Bone Marrow Transplant. (2000) 25:1129–36. doi: 10.1038/sj.bmt.1702422
15. Ogawa, Y, Kim, SK, Dana, R, Clayton, J, Jain, S, Rosenblatt, MI, et al. International chronic ocular graft-vs-host-disease (GVHD) consensus group: proposed diagnostic criteria for chronic GVHD (part I). Sci Rep. (2013) 3:3419. doi: 10.1038/srep03419
16. Choi, SW, Levine, JE, and Ferrara, JL. Pathogenesis and management of graft-versus-host disease. Immunol Allergy Clin N Am. (2010) 30:75–101. doi: 10.1016/j.iac.2009.10.001
17. Claes, K, and Kestelyn, P. Ocular manifestations of graft versus host disease following bone marrow transplantation. Bull Soc Belge Ophtalmol. (2000) 277:21–6.
18. Hirst, LW, Jabs, DA, Tutschka, PJ, Green, WR, and Santos, GW. The eye in bone marrow transplantation. I Clinical study. Arch Ophthalmol. (1983) 101:580–4. doi: 10.1001/archopht.1983.01040010580010
19. Shikari, H, Antin, JH, and Dana, R. Ocular graft-versus-host disease: a review. Surv Ophthalmol. (2013) 58:233–51. doi: 10.1016/j.survophthal.2012.08.004
20. Westeneng, AC, Hettinga, Y, Lokhorst, H, Verdonck, L, van Dorp, S, and Rothova, A. Ocular graft-versus-host disease after allogeneic stem cell transplantation. Cornea. (2010) 29:758–63. doi: 10.1097/ICO.0b013e3181ca321c
21. Jacobs, R, Tran, U, Chen, H, Kassim, A, Engelhardt, BG, Greer, JP, et al. Prevalence and risk factors associated with development of ocular GVHD defined by NIH consensus criteria. Bone Marrow Transplant. (2012) 47:1470–3. doi: 10.1038/bmt.2012.56
22. Saboo, US, Amparo, F, Abud, TB, Schaumberg, DA, and Dana, R. Vision-related quality of life in patients with ocular graft-versus-host disease. Ophthalmology. (2015) 122:1669–74. doi: 10.1016/j.ophtha.2015.04.011
23. Nair, S, Vanathi, M, Mukhija, R, Tandon, R, Jain, S, and Ogawa, Y. Update on ocular graft-versus-host disease. Indian J Ophthalmol. (2021) 69:1038–50. doi: 10.4103/ijo.IJO_2016_20
24. Nair, S, Vanathi, M, Mahapatra, M, Seth, T, Kaur, J, Velpandian, T, et al. Tear inflammatory mediators and protein in eyes of post allogenic hematopoeitic stem cell transplant patients. Ocul Surf. (2018) 16:352–67. doi: 10.1016/j.jtos.2018.04.007
25. Na, KS, Yoo, YS, Mok, JW, Lee, JW, and Joo, CK. Incidence and risk factors for ocular GVHD after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. (2015) 50:1459–64. doi: 10.1038/bmt.2015.187
26. Lin, X, and Cavanagh, HD. Ocular manifestations of graft-versus-host disease: 10 years’ experience. Clin Ophthalmol. (2015) 9:1209–13. doi: 10.2147/OPTH.S84704
27. Hébert, M, Archambault, C, Doyon, C, Ospina, LH, and Robert, MC. Risk factors for ocular involvement in pediatric graft-versus-host disease. Cornea. (2021) 40:1158–64. doi: 10.1097/ICO.0000000000002659
28. Fahnehjelm, KT, Törnquist, AL, and Winiarski, J. Dry-eye syndrome after allogeneic stem-cell transplantation in children. Acta Ophthalmol. (2008) 86:253–8. doi: 10.1111/j.1600-0420.2007.01120.x
29. Suh, DW, Ruttum, MS, Stuckenschneider, BJ, Mieler, WF, and Kivlin, JD. Ocular findings after bone marrow transplantation in a pediatric population. Ophthalmology. (1999) 106:1564–70. doi: 10.1016/S0161-6420(99)90454-2
30. Bradfield, YS, Kushner, BJ, and Gangnon, RE. Ocular complications after organ and bone marrow transplantation in children. J AAPOS. (2005) 9:426–32. doi: 10.1016/j.jaapos.2005.06.002
31. Kinori, M, Bielorai, B, Souroujon, D, Hutt, D, Ben-Bassat Mizrachi, I, and Huna-Baron, R. Ocular complications in children after hematopoietic stem cell transplantation without total body irradiation. Graefes Arch Clin Exp Ophthalmol. (2015) 253:1397–402. doi: 10.1007/s00417-015-2964-8
32. Kalinina Ayuso, V, Hettinga, Y, van der Does, P, Boelens, JJ, Rothova, A, and de Boer, J. Ocular complications in children within 1 year after hematopoietic stem cell transplantation. JAMA Ophthalmol. (2013) 131:470–5. doi: 10.1001/jamaophthalmol.2013.2500
33. Ng, JS, Lam, DS, Li, CK, Chik, KW, Cheng, GPM, Yuen, PMP, et al. Ocular complications of pediatric bone marrow transplantation. Ophthalmology. (1999) 106:160–4. doi: 10.1016/S0161-6420(99)90023-4
34. Cuvelier, GDE, Nemecek, ER, Wahlstrom, JT, Kitko, CL, Lewis, VA, Schechter, T, et al. Benefits and challenges with diagnosing chronic and late acute GVHD in children using the NIH consensus criteria. Blood. (2019) 134:304–16. doi: 10.1182/blood.2019000216
35. Herretes, S, Ross, DB, Duffort, S, Barreras, H, Yaohong, T, Saeed, AM, et al. Recruitment of donor T cells to the eyes during ocular GVHD in recipients of MHC-matched allogeneic hematopoietic stem cell transplants. Invest Ophthalmol Vis Sci. (2015) 56:2348–57. doi: 10.1167/iovs.14-15630
36. Perez, VL, Barsam, A, Duffort, S, Urbieta, M, Barreras, H, Lightbourn, C, et al. Novel scoring criteria for the evaluation of ocular graft-versus-host disease in a preclinical allogeneic hematopoietic stem cell transplantation animal model. Biol Blood Marrow Transplant. (2016) 22:1765–72. doi: 10.1016/j.bbmt.2016.07.012
37. Levy, RB, Mousa, HM, Lightbourn, CO, Shiuey, EJ, Latoni, D, Duffort, S, et al. Analyses and correlation of pathologic and ocular cutaneous changes in murine graft versus host disease. Int J Mol Sci. (2021) 23:184. doi: 10.3390/ijms23010184
38. Hong, YQ, Wan, B, and Li, XF. Macrophage regulation of graft-vs-host disease. World J Clin Cases. (2020) 8:1793–805. doi: 10.12998/wjcc.v8.i10.1793
39. Welniak, LA, Blazar, BR, and Murphy, WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. (2007) 25:139–70. doi: 10.1146/annurev.immunol.25.022106.141606
40. Mirza, N, Zierhut, M, Korn, A, Bornemann, A, Vogel, W, Schmid-Horch, B, et al. Graft versus self (GvS) against T-cell autoantigens is a mechanism of graft-host interaction. Proc Natl Acad Sci U S A. (2016) 113:13827–32. doi: 10.1073/pnas.1609118113
41. Milosevic, S, Bachnick, B, Karim, K, Bornkamm, GW, Witter, K, Gerbitz, A, et al. Identification of MHC II-restricted minor histocompatibility antigens after HLA-identical stem-cell transplantation. Transplantation. (2010) 90:1030–5. doi: 10.1097/TP.0b013e3181f5470c
42. Kawase, T, Morishima, Y, Matsuo, K, Kashiwase, K, Inoko, H, Saji, H, et al. High-risk HLA allele mismatch combinations responsible for severe acute graft-versus-host disease and implication for its molecular mechanism. Blood. (2007) 110:2235–41. doi: 10.1182/blood-2007-02-072405
43. Goulmy, E, Schipper, R, Pool, J, Blokland, E, Falkenburg, F, Vossen, J, et al. Mismatches of minor histocompatibility antigens between HLA-identical donors and recipients and the development of graft-versus-host disease after bone marrow transplantation. N Engl J Med. (1996) 334:281–5. doi: 10.1056/NEJM199602013340501
44. de Bueger, M, Bakker, A, Van Rood, JJ, Van der Woude, F, and Goulmy, E. Tissue distribution of human minor histocompatibility antigens. Ubiquitous versus restricted tissue distribution indicates heterogeneity among human cytotoxic T lymphocyte-defined non-MHC antigens. J Immunol. (1992) 149:1788–94. doi: 10.4049/jimmunol.149.5.1788
45. Cohen, JL, and Boyer, O. The role of CD4+CD25hi regulatory T cells in the physiopathogeny of graft-versus-host disease. Curr Opin Immunol. (2006) 18:580–5. doi: 10.1016/j.coi.2006.07.007
46. Bastian, D, Wu, Y, Betts, BC, and Yu, XZ. The IL-12 cytokine and receptor family in graft-vs.-host disease. Front Immunol. (2019) 10:988. doi: 10.3389/fimmu.2019.00988
47. Wolff, D, Radojcic, V, Lafyatis, R, Cinar, R, Rosenstein, RK, Cowen, EW, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The (2020) highly morbid forms report. Transplant Cell Ther. (2021) 27:817–35. doi: 10.1016/j.jtct.2021.06.001
48. Newman, RG, Ross, DB, Barreras, H, Herretes, S, Podack, ER, Komanduri, KV, et al. The allure and peril of hematopoietic stem cell transplantation: overcoming immune challenges to improve success. Immunol Res. (2013) 57:125–39. doi: 10.1007/s12026-013-8450-7
49. Antin, JH, and Ferrara, JL. Cytokine dysregulation and acute graft-versus-host disease. Blood. (1992) 80:2964–8. doi: 10.1182/blood.V80.12.2964.2964
50. Ferrara, JL, Cooke, KR, and Teshima, T. The pathophysiology of acute graft-versus-host disease. Int J Hematol. (2003) 78:181–7. doi: 10.1007/BF02983793
51. Perez, VL, Mousa, HM, Soifer, M, Beatty, C, Sarantopoulos, S, Saban, DR, et al. Meibomian gland dysfunction: a route of ocular graft-versus-host disease progression that drives a vicious cycle of ocular surface inflammatory damage. Am J Ophthalmol. (2022) 247:42–60. doi: 10.1016/j.ajo.2022.09.009
52. Inamoto, Y, Valdés-Sanz, N, Ogawa, Y, Alves, M, Berchicci, L, Galvin, J, et al. Ocular graft-versus-host disease after hematopoietic cell transplantation: expert review from the late effects and quality of life working committee of the CIBMTR and transplant complications working party of the EBMT. Bone Marrow Transplant. (2019) 54:662–73. doi: 10.1038/s41409-018-0340-0
53. Uchino, M, Ogawa, Y, Uchino, Y, Mori, T, Okamoto, S, and Tsubota, K. Comparison of stem cell sources in the severity of dry eye after allogeneic haematopoietic stem cell transplantation. Br J Ophthalmol. (2012) 96:34–7. doi: 10.1136/bjophthalmol-2011-300514
54. Kamoi, M, Ogawa, Y, Uchino, M, Tatematsu, Y, Mori, T, Okamoto, S, et al. Donor-recipient gender difference affects severity of dry eye after hematopoietic stem cell transplantation. Eye. (2011) 25:860–5. doi: 10.1038/eye.2011.73
55. Jeppesen, H, Sengeløv, H, Eriksson, F, Kiilgaard, JF, Andersen, ST, Lindegaard, J, et al. Chronic ocular graft-versus-host disease after allogeneic haematopoietic stem cell transplantation in Denmark—factors associated with risks and rates in adults according to conditioning regimen. Bone Marrow Transplant. (2021) 56:144–54. doi: 10.1038/s41409-020-0993-3
56. Wang, JC, Teichman, JC, Mustafa, M, O’Donnell, H, Broady, R, and Yeung, SN. Risk factors for the development of ocular graft-versus-host disease (GVHD) dry eye syndrome in patients with chronic GVHD. Br J Ophthalmol. (2015) 99:1514–8. doi: 10.1136/bjophthalmol-2014-306438
57. Inamoto, Y, Petriček, I, Burns, L, Chhabra, S, DeFilipp, Z, Hematti, P, et al. Non-GVHD ocular complications after hematopoietic cell transplantation: expert review from the late effects and quality of life working committee of the CIBMTR and transplant complications working party of the EBMT. Bone Marrow Transplant. (2019) 54:648–61. doi: 10.1038/s41409-018-0339-6
58. Khan, R, Nair, S, Seth, T, Mishra, P, Mahapatra, M, Agarwal, T, et al. Ocular graft versus host disease in allogenic haematopoetic stem cell transplantation in a tertiary care Centre in India. Indian J Med Res. (2015) 142:543–8. doi: 10.4103/0971-5916.171280
59. Ban, Y, Ogawa, Y, Ibrahim, OM, Tatematsu, Y, Kamoi, M, Uchino, M, et al. Morphologic evaluation of meibomian glands in chronic graft-versus-host disease using in vivo laser confocal microscopy. Mol Vis. (2011) 17:2533–43.
60. Engel, LA, Wittig, S, Bock, F, Sauerbier, L, Scheid, C, Holtick, U, et al. Meibography and meibomian gland measurements in ocular graft-versus-host disease. Bone Marrow Transplant. (2015) 50:961–7. doi: 10.1038/bmt.2015.72
61. Appenteng Osae, E, and Steven, P. Meibomian gland dysfunction in ocular graft vs. host disease: a need for pre-clinical models and deeper insights. Int J Mol Sci. (2021) 22:3516. doi: 10.3390/ijms22073516
62. Gehlsen, U, Stern, ME, Franklin, J, Tahmaz, V, Hallek, M, Holtick, U, et al. Desiccating stress significantly increases the risk for chronic ocular graft-versus-host-disease. Transplant Cell Ther. (2022) 28:782.e1–7. doi: 10.1016/j.jtct.2022.07.027
63. Ogawa, Y, Dana, R, Kim, S, Jain, S, Rosenblatt, MI, Perez, VL, et al. Multicenter prospective validation study for international chronic ocular graft-versus-host disease consensus diagnostic criteria. Ocul Surf. (2022) 277:S1542. doi: 10.1016/j.jtos.2022.09.002
64. Inamoto, Y, Chai, X, Kurland, BF, Cutler, C, Flowers, ME, Palmer, JM, et al. Validation of measurement scales in ocular graft-versus-host disease. Ophthalmology. (2012) 119:487–93. doi: 10.1016/j.ophtha.2011.08.040
65. Kerep, AZ, Broome, J, Pirsl, F, Curtis, LM, Steinberg, SM, Mitchell, SA, et al. Impact of the 2014 NIH chronic graft-versus-host disease scoring criteria modifications assessed in a large cohort of severely affected patients. Bone Marrow Transplant. (2019) 54:76–84. doi: 10.1038/s41409-018-0224-3
66. Robinson, MR, Lee, SS, Rubin, BI, Wayne, AS, Pavletic, SZ, Bishop, MR, et al. Topical corticosteroid therapy for cicatricial conjunctivitis associated with chronic graft-versus-host disease. Bone Marrow Transplant. (2004) 33:1031–5. doi: 10.1038/sj.bmt.1704453
67. Rapoport, Y, Freeman, T, Koyama, T, Engelhardt, BG, Jagasia, M, Savani, BN, et al. Validation of international chronic ocular graft-versus-host disease (GVHD) group diagnostic criteria as a chronic ocular GVHD-specific metric. Cornea. (2017) 36:258–63. doi: 10.1097/ICO.0000000000001109
68. Lee, SJ, Wolff, D, Kitko, C, Koreth, J, Inamoto, Y, Jagasia, M, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 response criteria working group report. Biol Blood Marrow Transplant. (2015) 21:984–99. doi: 10.1016/j.bbmt.2015.02.025
69. Sinha, S, Singh, RB, Dohlman, TH, Wang, M, Taketani, Y, Yin, J, et al. Prevalence of persistent corneal epithelial defects in chronic ocular graft-versus-host disease. Am J Ophthalmol. (2020) 218:296–303. doi: 10.1016/j.ajo.2020.05.035
70. Ogawa, Y, Okamoto, S, Wakui, M, Watanabe, R, Yamada, M, Yoshino, M, et al. Dry eye after haematopoietic stem cell transplantation. Br J Ophthalmol. (1999) 83:1125–30. doi: 10.1136/bjo.83.10.1125
71. Majhail, NS, Rizzo, JD, Lee, SJ, Aljurf, M, Atsuta, Y, Bonfim, C, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Rev Bras Hematol Hemoter. (2012) 34:109–33. doi: 10.5581/1516-8484.20120032
72. Schiffman, RM, Christianson, MD, Jacobsen, G, Hirsch, JD, and Reis, BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. (2000) 118:615–21. doi: 10.1001/archopht.118.5.615
73. Yang, J, Zhao, W, Liao, Y, Wu, S, Li, J, Jin, L, et al. Ocular surface disease index questionnaire as a sensitive test for primary screening of chronic ocular graft-versus-host disease. Ann Transl Med. (2022) 10:855. doi: 10.21037/atm-21-6946
74. Pathak, M, Diep, PP, Lai, X, Brinch, L, Ruud, E, and Drolsum, L. Ocular findings and ocular graft-versus-host disease after allogeneic stem cell transplantation without total body irradiation. Bone Marrow Transplant. (2018) 53:863–72. doi: 10.1038/s41409-018-0090-z
75. Dietrich-Ntoukas, T, Cursiefen, C, Westekemper, H, Eberwein, P, Reinhard, T, Bertz, H, et al. Diagnosis and treatment of ocular chronic graft-versus-host disease: report from the German-Austrian-Swiss consensus conference on clinical practice in chronic GVHD. Cornea. (2012) 31:299–310. doi: 10.1097/ICO.0b013e318226bf97
76. Shimizu, E, Aketa, N, Yazu, H, Uchino, M, Kamoi, M, Sato, Y, et al. Corneal higher-order aberrations in eyes with chronic ocular graft-versus-host disease. Ocul Surf. (2020) 18:98–107. doi: 10.1016/j.jtos.2019.10.005
77. Sun, YC, Chai, X, Inamoto, Y, Pidala, J, Martin, PJ, Flowers, MED, et al. Impact of ocular chronic graft-versus-host disease on quality of life. Biol Blood Marrow Transplant. (2015) 21:1687–91. doi: 10.1016/j.bbmt.2015.05.020
78. Kerty, E, Vigander, K, Flage, T, and Brinch, L. Ocular findings in allogeneic stem cell transplantation without total body irradiation. Ophthalmology. (1999) 106:1334–8. doi: 10.1016/S0161-6420(99)00720-4
79. Jabs, DA, Wingard, J, Green, WR, Farmer, ER, Vogelsang, G, and Saral, R. The eye in bone marrow transplantation. III Conjunctival graft-vs-host disease. Arch Ophthalmol. (1989) 107:1343–8. doi: 10.1001/archopht.1989.01070020413046
80. Zhang, CY, Farooq, AV, Harocopos, GJ, Sollenberger, EL, Hou, JH, Bouchard, CS, et al. Corneal perforation in ocular graft-versus-host disease. Am J Ophthalmol Case Rep. (2021) 24:101224. doi: 10.1016/j.ajoc.2021.101224
81. Kaya, AH, Namdaroğlu, S, Kayıkcı, Ö, Merdin, A, Batgi, H, İskender, D, et al. Impact of guideline-driven approach in follow-up of long-term complications after allogeneic hematopoietic cell transplant: single center experience. Exp Clin Transplant. (2020) 18:359–67. doi: 10.6002/ect.2018.0007
82. Yoo, YS, Na, KS, Shin, JA, Park, YH, and Lee, JW. Posterior eye segment complications related to allogeneic hematopoietic stem cell transplantation. Retina. (2017) 37:135–43. doi: 10.1097/IAE.0000000000001122
83. Balasubramaniam, SC, Raja, H, Nau, CB, Shen, JF, and Schornack, MM. Ocular graft-versus-host disease: a review. Eye Contact Lens. (2015) 41:256–61. doi: 10.1097/ICL.0000000000000150
84. Flowers, ME, and Martin, PJ. How we treat chronic graft-versus-host disease. Blood. (2015) 125:606–15. doi: 10.1182/blood-2014-08-551994
85. Tsubota, K, Yokoi, N, Shimazaki, J, Watanabe, H, Dogru, M, Yamada, M, et al. New perspectives on dry eye definition and diagnosis: a consensus report by the Asia dry eye society. Ocul Surf. (2017) 15:65–76. doi: 10.1016/j.jtos.2016.09.003
86. Ogawa, Y, Okamoto, S, Mori, T, Yamada, M, Mashima, Y, Watanabe, R, et al. Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Bone Marrow Transplant. (2003) 31:579–83. doi: 10.1038/sj.bmt.1703862
87. Singh, RB, Yuksel, E, Sinha, S, Wang, S, Taketani, Y, Luznik, Z, et al. Prevalence of neurotrophic keratopathy in patients with chronic ocular graft-versus-host disease. Ocul Surf. (2022) 26:13–8. doi: 10.1016/j.jtos.2022.07.001
88. Wang, Y, Ogawa, Y, Dogru, M, Tatematsu, Y, Uchino, M, Kamoi, M, et al. Baseline profiles of ocular surface and tear dynamics after allogeneic hematopoietic stem cell transplantation in patients with or without chronic GVHD-related dry eye. Bone Marrow Transplant. (2010) 45:1077–83. doi: 10.1038/bmt.2009.312
89. Versura, P, Profazio, V, Buzzi, M, Stancari, A, Arpinati, M, Malavolta, N, et al. Efficacy of standardized and quality-controlled cord blood serum eye drop therapy in the healing of severe corneal epithelial damage in dry eye. Cornea. (2013) 32:412–8. doi: 10.1097/ICO.0b013e3182580762
90. Bron, AJ, Evans, VE, and Smith, JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. (2003) 22:640–50. doi: 10.1097/00003226-200310000-00008
91. Lemp, MA. Report of the National eye Institute/industry workshop on clinical trials in dry eyes. CLAO J. (1995) 21:221–32.
92. Wolffsohn, JS, Arita, R, Chalmers, R, Djalilian, A, Dogru, M, Dumbleton, K, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. (2017) 15:539–74. doi: 10.1016/j.jtos.2017.05.001
93. Giannaccare, G, Bonifazi, F, Sessa, M, Dan, E, Arpinati, M, Fresina, M, et al. Ocular surface analysis in hematological patients before and after allogeneic hematopoietic stem cell transplantation: implication for daily clinical practice. Eye. (2017) 31:1417–26. doi: 10.1038/eye.2017.78
94. Na, KS, Yoo, YS, Hwang, KY, Mok, JW, and Joo, CK. Tear Osmolarity and ocular surface parameters as diagnostic markers of ocular graft-versus-host disease. Am J Ophthalmol. (2015) 160:143–9.e1. doi: 10.1016/j.ajo.2015.04.002
95. Schargus, M, Meyer-ter-Vehn, T, Menrath, J, Grigoleit, GU, and Geerling, G. Correlation between tear film Osmolarity and the disease score of the international chronic ocular graft-versus-host-disease consensus Group in Hematopoietic Stem Cell Transplantation Patients. Cornea. (2015) 34:911–6. doi: 10.1097/ICO.0000000000000494
96. Berchicci, L, Iuliano, L, Miserocchi, E, Bandello, F, and Modorati, G. Tear osmolarity in ocular graft-versus-host disease. Cornea. (2014) 33:1252–6. doi: 10.1097/ICO.0000000000000283
97. Arita, R, Minoura, I, Morishige, N, Shirakawa, R, Fukuoka, S, Asai, K, et al. Development of definitive and reliable grading scales for Meibomian gland dysfunction. Am J Ophthalmol. (2016) 169:125–37. doi: 10.1016/j.ajo.2016.06.025
98. Robin, M, Liang, H, Baudouin, C, and Labbé, A. In vivo Meibomian gland imaging techniques: a review of the literature. J Fr Ophtalmol. (2020) 43:e123–31. doi: 10.1016/j.jfo.2019.11.003
99. Kheirkhah, A, Coco, G, Satitpitakul, V, and Dana, R. Subtarsal fibrosis is associated with ocular surface Epitheliopathy in graft-versus-host disease. Am J Ophthalmol. (2018) 189:102–10. doi: 10.1016/j.ajo.2018.02.020
100. Shimizu, S, Sato, S, Taniguchi, H, Shimizu, E, He, J, Hayashi, S, et al. Observation of chronic graft-versus-host disease mouse model cornea with in vivo confocal microscopy. Diagnostics. (2021) 11:1515. doi: 10.3390/diagnostics11081515
101. Shamloo, K, Barbarino, A, Alfuraih, S, and Sharma, A. Graft versus host disease-associated dry eye: role of ocular surface mucins and the effect of Rebamipide, a mucin Secretagogue. Invest Ophthalmol Vis Sci. (2019) 60:4511–9. doi: 10.1167/iovs.19-27843
102. He, J, Ogawa, Y, Mukai, S, Saijo-Ban, Y, Kamoi, M, Uchino, M, et al. In vivo confocal microscopy evaluation of ocular surface with graft-versus-host disease-related dry eye disease. Sci Rep. (2017) 7:10720. doi: 10.1038/s41598-017-10237-w
103. Tepelus, TC, Chiu, GB, Maram, J, Huang, J, Chopra, V, Sadda, SVR, et al. Corneal features in ocular graft-versus-host disease by in vivo confocal microscopy. Graefes Arch Clin Exp Ophthalmol. (2017) 255:2389–97. doi: 10.1007/s00417-017-3759-x
104. Dietrich-Ntoukas, T, and Steven, P. Ocular graft-versus-host disease. Ophthalmologe. (2015) 112:1027–38. doi: 10.1007/s00347-015-0149-9
105. Hosseini, H, Kumar, PV, Geramizadeh, B, Nowroozizadeh, B, and Ramzi, M. Conjunctival scrape cytology findings in patients with chronic graft-versus-host disease following allogeneic bone marrow transplantation. Acta Cytol. (2010) 54:272–6. doi: 10.1159/000325034
106. Vanathi, M, Kashyap, S, Khan, R, Seth, T, Mishra, P, Mahapatra, M, et al. Ocular surface evaluation in allogenic hematopoietic stem cell transplantation patients. Eur J Ophthalmol. (2014) 24:655–66. doi: 10.5301/ejo.5000451
107. Johnson, NL. Ocular graft-versus-host disease after allogeneic transplantation. Clin J Oncol Nurs. (2013) 17:621–6. doi: 10.1188/13.CJON.621-626
108. Berchicci, L, Aragona, E, Arrigo, A, Marchese, A, Miserocchi, E, Bandello, F, et al. Conjunctival matrix Metalloproteinase-9 clinical assessment in early ocular graft versus host disease. J Ophthalmol. (2021) 2021:9958713:1–7. doi: 10.1155/2021/9958713
109. Gerber-Hollbach, N, Plattner, K, O'Leary, OE, Jenoe, P, Moes, S, Drexler, B, et al. Tear film proteomics reveal important differences between patients with and without ocular GvHD after allogeneic hematopoietic cell transplantation. Invest Ophthalmol Vis Sci. (2018) 59:3521–30. doi: 10.1167/iovs.18-24433
110. Shulman, HM, Kleiner, D, Lee, SJ, Morton, T, Pavletic, SZ, Farmer, E, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: II. Pathology working group report. Biol Blood Marrow Transplant. (2006) 12:31–47. doi: 10.1016/j.bbmt.2005.10.023
111. Nassar, A, Tabbara, KF, and Aljurf, M. Ocular manifestations of graft-versus-host disease. Saudi J Ophthalmol. (2013) 27:215–22. doi: 10.1016/j.sjopt.2013.06.007
112. Shen, Z, Ma, J, Peng, R, et al. Biomarkers in ocular graft-versus-host disease: implications for the involvement of B cells. Transplant Cell Ther. (2022):S2666.
113. Riemens, A, te Boome, LCJ, Kalinina Ayuso, V, Kuiper, JJW, Imhof, SM, Lokhorst, HM, et al. Impact of ocular graft-versus-host disease on visual quality of life in patients after allogeneic stem cell transplantation: questionnaire study. Acta Ophthalmol. (2014) 92:82–7. doi: 10.1111/aos.12047
114. Westekemper, H, Scholz, SL, Thomasen, H, Halfwassen, C, and Steuhl, KP. Ocular graft versus host disease: corneal complications. Ophthalmologe. (2017) 114:697–702. doi: 10.1007/s00347-017-0488-9
115. Okumura, Y, Inomata, T, Iwata, N, Sung, J, Fujimoto, K, Fujio, K, et al. A review of dry eye questionnaires: measuring patient-reported outcomes and health-related quality of life. Diagnostics. (2020) 10:E559. doi: 10.3390/diagnostics10080559
116. Pezzotta, S, del Fante, C, Scudeller, L, Rossi, GC, Perotti, C, Bianchi, PE, et al. Long-term safety and efficacy of autologous platelet lysate drops for treatment of ocular GvHD. Bone Marrow Transplant. (2017) 52:101–6. doi: 10.1038/bmt.2016.221
117. Ogawa, Y, Kawakami, Y, and Tsubota, K. Cascade of inflammatory, fibrotic processes, and stress-induced senescence in chronic GVHD-related dry eye disease. Int J Mol Sci. (2021) 22:6114. doi: 10.3390/ijms22116114
118. Figus, M, Agnifili, L, Lanzini, M, Brescia, L, Sartini, F, Mastropasqua, L, et al. Topical preservative-free ophthalmic treatments: an unmet clinical need. Expert Opin Drug Deliv. (2021) 18:655–72. doi: 10.1080/17425247.2021.1860014
119. Espana, EM, Shah, S, Santhiago, MR, and Singh, AD. Graft versus host disease: clinical evaluation, diagnosis and management. Graefes Arch Clin Exp Ophthalmol. (2013) 251:1257–66. doi: 10.1007/s00417-013-2301-z
120. Trindade, M, Rodrigues, M, Pozzebon, ME, Aranha, FJP, Colella, MP, Fernandes, A, et al. A plethora of ocular surface manifestations in a multidisciplinary ocular graft-versus-host disease unit. Sci Rep. (2022) 12:15926. doi: 10.1038/s41598-022-19990-z
121. Ruutu, T, Gratwohl, A, de Witte, T, Afanasyev, B, Apperley, J, Bacigalupo, A, et al. Prophylaxis and treatment of GVHD: EBMT–ELN working group recommendations for a standardized practice. Bone Marrow Transplant. (2014) 49:168–73. doi: 10.1038/bmt.2013.107
122. Munir, SZ, and Aylward, J. A review of ocular graft-versus-host disease. Optom Vis Sci. (2017) 94:545–55. doi: 10.1097/OPX.0000000000001071
123. Yin, J, Kheirkhah, A, Dohlman, T, Saboo, U, and Dana, R. Reduced efficacy of low-dose topical steroids in dry eye disease associated with graft-versus-host disease. Am J Ophthalmol. (2018) 190:17–23. doi: 10.1016/j.ajo.2018.03.024
124. Dastjerdi, MH, Hamrah, P, and Dana, R. High-frequency topical cyclosporine 0.05% in the treatment of severe dry eye refractory to twice-daily regimen. Cornea. (2009) 28:1091–6. doi: 10.1097/ICO.0b013e3181a16472
125. Kiang, E, Tesavibul, N, Yee, R, Kellaway, J, and Przepiorka, D. The use of topical cyclosporin a in ocular graft-versus-host-disease. Bone Marrow Transplant. (1998) 22:147–51. doi: 10.1038/sj.bmt.1701304
126. Lelli, GJ, Musch, DC, Gupta, A, Farjo, QA, Nairus, TM, and Mian, SI. Ophthalmic cyclosporine use in ocular GVHD. Cornea. (2006) 25:635–8. doi: 10.1097/01.ico.0000208818.47861.1d
127. Sall, K, Stevenson, OD, Mundorf, TK, and Reis, BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA phase 3 study group. Ophthalmology. (2000) 107:631–9. doi: 10.1016/S0161-6420(99)00176-1
128. Wang, Y, Ogawa, Y, Dogru, M, Kawai, M, Tatematsu, Y, Uchino, M, et al. Ocular surface and tear functions after topical cyclosporine treatment in dry eye patients with chronic graft-versus-host disease. Bone Marrow Transplant. (2008) 41:293–302. doi: 10.1038/sj.bmt.1705900
129. Sun, YC, Inamoto, Y, Wang, RK, Lee, SJ, Hung, KF, and Shen, TT. The disposable bandage soft contact lenses therapy and anterior segment optical coherence tomography for management of ocular graft-versus-host disease. BMC Ophthalmol. (2021) 21:271.
130. Cantú-Rodríguez, OG, Vázquez-Mellado, A, González-Treviño, JL, et al. Cyclosporine a for the prevention of ocular graft versus host disease in allogeneic hematopoietic stem cell transplant recipients is safe and feasible. Acta Haematol. (2020) 143:425–31. doi: 10.1159/000502405
131. Abud, TB, Amparo, F, Saboo, US, di Zazzo, A, Dohlman, TH, Ciolino, JB, et al. A clinical trial comparing the safety and efficacy of topical tacrolimus versus methylprednisolone in ocular graft-versus-host disease. Ophthalmology. (2016) 123:1449–57. doi: 10.1016/j.ophtha.2016.02.044
132. Jung, JW, Lee, YJ, Yoon, SC, Kim, TI, Kim, EK, and Seo, KY. Long-term result of maintenance treatment with tacrolimus ointment in chronic ocular graft-versus-host disease. Am J Ophthalmol. (2015) 159:519–27.e1. doi: 10.1016/j.ajo.2014.11.035
133. Ryu, EH, Kim, JM, Laddha, PM, Chung, ES, and Chung, TY. Therapeutic effect of 0.03% tacrolimus ointment for ocular graft versus host disease and vernal keratoconjunctivitis. Korean J Ophthalmol. (2012) 26:241–7. doi: 10.3341/kjo.2012.26.4.241
134. Tahmaz, V, Gehlsen, U, Sauerbier, L, Holtick, U, Engel, L, Radojska, S, et al. Treatment of severe chronic ocular graft-versus-host disease using 100% autologous serum eye drops from a sealed manufacturing system: a retrospective cohort study. Br J Ophthalmol. (2017) 101:bjophthalmol-2015-307666–326. doi: 10.1136/bjophthalmol-2015-307666
135. Pezzotta, S, Del Fante, C, Scudeller, L, Cervio, M, Antoniazzi, ER, and Perotti, C. Autologous platelet lysate for treatment of refractory ocular GVHD. Bone Marrow Transplant. (2012) 47:1558–63. doi: 10.1038/bmt.2012.64
136. Na, KS, and Kim, MS. Allogeneic serum eye drops for the treatment of dry eye patients with chronic graft-versus-host disease. J Ocul Pharmacol Ther. (2012) 28:479–83. doi: 10.1089/jop.2012.0002
137. Tahmaz, V, Wiesen, MHJ, Gehlsen, U, Sauerbier, L, Stern, ME, Holtick, U, et al. Detection of systemic immunosuppressants in autologous serum eye drops (ASED) in patients with severe chronic ocular graft versus host disease. Graefes Arch Clin Exp Ophthalmol. (2021) 259:121–8. doi: 10.1007/s00417-020-04865-8
138. Yoon, KC, Jeong, IY, Im, SK, Park, YG, Kim, HJ, and Choi, J. Therapeutic effect of umbilical cord serum eyedrops for the treatment of dry eye associated with graft-versus-host disease. Bone Marrow Transplant. (2007) 39:231–5. doi: 10.1038/sj.bmt.1705566
139. Jones, L, Downie, LE, Korb, D, Benitez-del-Castillo, JM, Dana, R, Deng, SX, et al. TFOS DEWS II management and therapy report. Ocul Surf. (2017) 15:575–628. doi: 10.1016/j.jtos.2017.05.006
140. Valentini, CG, Nuzzolo, ER, Orlando, N, Metafuni, E, Bianchi, M, Chiusolo, P, et al. Cytokine profile of autologous platelet-derived eye drops in patients with ocular chronic graft-versus-host disease. Vox Sang. (2016) 110:189–92. doi: 10.1111/vox.12325
141. Korb, DR, and Blackie, CA. Using goggles to increase periocular humidity and reduce dry eye symptoms. Eye Contact Lens. (2013) 39:273–6. doi: 10.1097/ICL.0b013e3182960ff9
142. Steven, P, Faust, C, Holtick, U, Scheid, C, Tahmaz, V, Stern, ME, et al. Adverse environmental conditions are a risk factor for ocular GvHD after allogeneic hematopoietic stem cell transplantation.[letter]. Bone Marrow Transplant. (2020) 55:1851–3. doi: 10.1038/s41409-020-0824-6
143. Agha-Hosseini, F, Mirzaii-Dizgah, I, Ghavamzadeh, L, Ghavamzadeh, A, and Tohidast-Acrad, Z. Effect of pilocarpine hydrochloride on unstimulated whole saliva flow rate and composition in patients with chronic graft-versus-host disease (cGVHD). Bone Marrow Transplant. (2007) 39:431–4. doi: 10.1038/sj.bmt.1705621
144. Barnett, BP, and Afshari, NA. Dupilumab-associated mucin deficiency (DAMD). Transl Vis Sci Technol. (2020) 9:29. doi: 10.1167/tvst.9.3.29
145. Yamane, M, Ogawa, Y, Fukui, M, Kamoi, M, Uchino, M, Saijo-Ban, Y, et al. Long-term topical Diquafosol Tetrasodium treatment of dry eye disease caused by chronic graft-versus-host disease: a retrospective study. Eye Contact Lens. (2018) 44:S215–20. doi: 10.1097/ICL.0000000000000455
146. Kashima, T, Itakura, H, Akiyama, H, and Kishi, S. Rebamipide ophthalmic suspension for the treatment of dry eye syndrome: a critical appraisal. Clin Ophthalmol. (2014) 8:1003–10. doi: 10.2147/OPTH.S40798
147. Sabti, S, Halter, JP, Braun Fränkl, BC, and Goldblum, D. Punctal occlusion is safe and efficient for the treatment of keratoconjunctivitis sicca in patients with ocular GvHD. Bone Marrow Transplant. (2012) 47:981–4. doi: 10.1038/bmt.2011.205
148. Yaguchi, S, Ogawa, Y, Kamoi, M, Uchino, M, Tatematsu, Y, Ban, Y, et al. Surgical management of lacrimal punctal cauterization in chronic GVHD-related dry eye with recurrent punctal plug extrusion. Bone Marrow Transplant. (2012) 47:1465–9. doi: 10.1038/bmt.2012.50
149. Behrens, A, Doyle, JJ, Stern, L, Chuck, RS, McDonnell, PJ, Azar, DT, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. (2006) 25:900–7. doi: 10.1097/01.ico.0000214802.40313.fa
150. Jacobs, DS, and Rosenthal, P. Boston scleral lens prosthetic device for treatment of severe dry eye in chronic graft-versus-host disease. Cornea. (2007) 26:1195–9. doi: 10.1097/ICO.0b013e318155743d
151. Magro, L, Gauthier, J, Richet, M, Robin, M, Nguyen, S, Suarez, F, et al. Scleral lenses for severe chronic GvHD-related keratoconjunctivitis sicca: a retrospective study by the SFGM-TC. Bone Marrow Transplant. (2017) 52:878–82. doi: 10.1038/bmt.2017.9
152. Schornack, MM, Baratz, KH, Patel, SV, and Maguire, LJ. Jupiter scleral lenses in the management of chronic graft versus host disease. Eye Contact Lens. (2008) 34:302–5. doi: 10.1097/ICL.0b013e318188e205
153. Takahide, K, Parker, PM, Wu, M, Hwang, WYK, Carpenter, PA, Moravec, C, et al. Use of fluid-ventilated, gas-permeable scleral lens for management of severe keratoconjunctivitis sicca secondary to chronic graft-versus-host disease. Biol Blood Marrow Transplant. (2007) 13:1016–21. doi: 10.1016/j.bbmt.2007.05.006
154. DeLoss, KS, Le, HG, Gire, A, Chiu, GB, Jacobs, DS, and Carrasquillo, KG. PROSE treatment for ocular chronic graft-versus-host disease as a clinical network expands. Eye Contact Lens. (2016) 42:262–6. doi: 10.1097/ICL.0000000000000186
155. Luo, ZK, Domenech-Estarellas, EA, Han, A, Lee, D, Khatri, R, Wahl, JL, et al. Efficacy and safety of 1% progesterone gel to the forehead for ocular chronic graft-versus-host disease. Transplant Cell Ther. (2021) 27:433.e1–8. doi: 10.1016/j.jtct.2021.02.008
156. Russo, PA, Bouchard, CS, and Galasso, JM. Extended-wear silicone hydrogel soft contact lenses in the management of moderate to severe dry eye signs and symptoms secondary to graft-versus-host disease. Eye Contact Lens. (2007) 33:144–7. doi: 10.1097/01.icl.0000244154.76214.2d
157. Inamoto, Y, Sun, YC, Flowers, ME, Carpenter, PA, Martin, PJ, Li, P, et al. Bandage soft contact lenses for ocular graft-versus-host disease. Biol Blood Marrow Transplant. (2015) 21:2002–7. doi: 10.1016/j.bbmt.2015.07.013
158. Smith, VA, and Cook, SD. Doxycycline-a role in ocular surface repair. Br J Ophthalmol. (2004) 88:619–25. doi: 10.1136/bjo.2003.025551
159. Horwitz, ME, and Sullivan, KM. Chronic graft-versus-host disease. Blood Rev. (2006) 20:15–27. doi: 10.1016/j.blre.2005.01.007
160. Wolff, D, Fatobene, G, Rocha, V, Kröger, N, and Flowers, ME. Steroid-refractory chronic graft-versus-host disease: treatment options and patient management. Bone Marrow Transplant. (2021) 56:2079–87. doi: 10.1038/s41409-021-01389-5
161. Wolff, D, Hilgendorf, I, Wagner-Drouet, E, Jedlickova, Z, Ayuk, F, Zeiser, R, et al. Changes in immunosuppressive treatment of chronic graft-versus-host disease: comparison of 2 surveys within allogeneic hematopoietic stem cell transplant centers in Germany, Austria, and Switzerland. Biol Blood Marrow Transplant. (2019) 25:1450–5. doi: 10.1016/j.bbmt.2019.03.003
162. Yalniz, FF, Murad, MH, Lee, SJ, Pavletic, SZ, Khera, N, Shah, ND, et al. Steroid refractory chronic graft-versus-host disease: cost-effectiveness analysis. Biol Blood Marrow Transplant. (2018) 24:1920–7. doi: 10.1016/j.bbmt.2018.03.008
163. Flowers, ME, Apperley, JF, van Besien, K, Elmaagacli, A, Grigg, A, Reddy, V, et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood. (2008) 112:2667–74. doi: 10.1182/blood-2008-03-141481
164. Zeiser, R, Polverelli, N, Ram, R, Hashmi, SK, Chakraverty, R, Middeke, JM, et al. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med. (2021) 385:228–38. doi: 10.1056/NEJMoa2033122
165. Cutler, C, Lee, SJ, Arai, S, Rotta, M, Zoghi, B, Lazaryan, A, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar study. Blood. (2021) 138:2278–89. doi: 10.1182/blood.2021012021
166. Miklos, D, Cutler, CS, Arora, M, Waller, EK, Jagasia, M, Pusic, I, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. (2017) 130:2243–50. doi: 10.1182/blood-2017-07-793786
167. Copsel, SN, Lightbourn, CO, Barreras, H, Lohse, I, Wolf, D, Bader, CS, et al. BET Bromodomain inhibitors which permit Treg function enable a combinatorial strategy to suppress GVHD in pre-clinical allogeneic HSCT. Front Immunol. (2018) 9:3104. doi: 10.3389/fimmu.2018.03104
168. Poe, JC, Jia, W, di Paolo, JA, Reyes, NJ, Kim, JY, Su, H, et al. SYK inhibitor entospletinib prevents ocular and skin GVHD in mice. JCI Insight. (2018) 3:122430. doi: 10.1172/jci.insight.122430
169. Hida, RY, Takano, Y, Okada, N, Dogru, M, Satake, Y, Fukagawa, K, et al. Suppressive effects of tranilast on eotaxin-1 production from cultured conjunctival fibroblasts. Curr Eye Res. (2008) 33:19–22. doi: 10.1080/02713680701817366
170. Chihara, E, Dong, J, Ochiai, H, and Hamada, S. Effects of tranilast on filtering blebs: a pilot study. J Glaucoma. (2002) 11:127–33. doi: 10.1097/00061198-200204000-00008
171. Peris-Martínez, C, Menezo, JL, Díaz-Llopis, M, Aviñó-Martínez, JA, Navea-Tejerina, A, and Risueño-Reguillo, P. Multilayer amniotic membrane transplantation in severe ocular graft versus host disease. Eur J Ophthalmol. (2001) 11:183–6. doi: 10.1177/112067210101100215
172. Murube, J, and Murube, E. Treatment of dry eye by blocking the lacrimal canaliculi. Surv Ophthalmol. (1996) 40:463–80. doi: 10.1016/S0039-6257(96)82013-3
173. Benson, DR, Hemmady, PB, and Snyder, RW. Efficacy of laser punctal occlusion. Ophthalmology. (1992) 99:618–21. doi: 10.1016/S0161-6420(92)31928-1
174. Vrabec, MP, Elsing, SH, and Aitken, PA. A prospective, randomized comparison of thermal cautery and argon laser for permanent punctal occlusion. Am J Ophthalmol. (1993) 116:469–71. doi: 10.1016/S0002-9394(14)71406-0
175. Yang, HY, Fujishima, H, Toda, I, Shimazaki, J, and Tsubota, K. Lacrimal punctal occlusion for the treatment of superior limbic keratoconjunctivitis. Am J Ophthalmol. (1997) 124:80–7. doi: 10.1016/S0002-9394(14)71647-2
176. Liu, D, and Sadhan, Y. Surgical punctal occlusion: a prospective study. Br J Ophthalmol. (2002) 86:1031–4. doi: 10.1136/bjo.86.9.1031
177. Law, RW, Li, RT, Lam, DS, and Lai, JS. Efficacy of pressure topical anaesthesia in punctal occlusion by diathermy. Br J Ophthalmol. (2005) 89:1449–52. doi: 10.1136/bjo.2005.066969
178. Arocker-Mettinger, E, Skorpik, F, Grabner, G, Hinterberger, W, and Gadner, H. Manifestations of graft-versus-host disease following allogenic bone marrow transplantation. Eur J Ophthalmol. (1991) 1:28–32. doi: 10.1177/112067219100100106
179. Adams, GG, Kirkness, CM, and Lee, JP. Botulinum toxin a induced protective ptosis. Eye (Lond). (1987) 1:603–8. doi: 10.1038/eye.1987.93
180. Yeh, PT, Hou, YC, Lin, WC, Wang, IJ, and Hu, FR. Recurrent corneal perforation and acute calcareous corneal degeneration in chronic graft-versus-host disease. J Formos Med Assoc. (2006) 105:334–9. doi: 10.1016/s0929-6646(09)60125-x
181. Portnoy, SL, Insler, MS, and Kaufman, HE. Surgical management of corneal ulceration and perforation. Surv Ophthalmol. (1989) 34:47–58. doi: 10.1016/0039-6257(89)90129-X
182. Lee, SH, and Tseng, SC. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. (1997) 123:303–12. doi: 10.1016/S0002-9394(14)70125-4
183. Solomon, A, Meller, D, Prabhasawat, P, John, T, Espana, EM, Steuhl, KP, et al. Amniotic membrane grafts for nontraumatic corneal perforations, descemetoceles, and deep ulcers. Ophthalmology. (2002) 109:694–703. doi: 10.1016/S0161-6420(01)01032-6
184. Schuerch, K, Baeriswyl, A, Frueh, BE, and Tappeiner, C. Efficacy of amniotic membrane transplantation for the treatment of corneal ulcers. Cornea. (2020) 39:479–83. doi: 10.1097/ICO.0000000000002179
185. Abdulhalim, BE, Wagih, MM, Gad, AA, Boghdadi, G, and Nagy, RR. Amniotic membrane graft to conjunctival flap in treatment of non-viral resistant infectious keratitis: a randomised clinical study. Br J Ophthalmol. (2015) 99:59–63. doi: 10.1136/bjophthalmol-2014-305224
186. Solomon, A, Rosenblatt, M, Monroy, D, Ji, Z, Pflugfelder, SC, and Tseng, SC. Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br J Ophthalmol. (2001) 85:444–9. doi: 10.1136/bjo.85.4.444
187. Kim, JS, Kim, JC, Hahn, TW, and Park, WC. Amniotic membrane transplantation in infectious corneal ulcer. Cornea. (2001) 20:720–6. doi: 10.1097/00003226-200110000-00010
188. Ikarashi, H, Aketa, N, Shimizu, E, Takano, Y, Kawakita, T, Uchino, Y, et al. Two case reports of continued progression of chronic ocular graft-versus-host disease without concurrent systemic comorbidities treated by amniotic membrane transplantation. BMC Ophthalmol. (2021) 21:164. doi: 10.1186/s12886-021-01925-3
189. Mohammadpour, M, Maleki, S, Hashemi, H, and Beheshtnejad, AH. Recurrent corneal perforation due to chronic graft versus host disease; a Clinicopathologic report. J Ophthalmic Vis Res. (2016) 11:108–11. doi: 10.4103/2008-322X.180705
190. Lavaris, A, Elanwar, MFM, Al-Zyiadi, M, Xanthopoulou, PT, and Kopsachilis, N. Glueless and Sutureless multi-layer amniotic membrane transplantation in a patient with pending corneal perforation. Cureus. (2021) 13:e16678. doi: 10.7759/cureus.16678
191. Xu, Y, Wang, YM, Sun, ZT, Yang, XL, Zhuang, XY, Ren, YR, et al. Corneal perforation associated with ocular graft-versus-host disease. Front Oncol. (2022) 12:962250. doi: 10.3389/fonc.2022.1049305
192. Pellegrini, M, Bernabei, F, Barbato, F, Arpinati, M, Giannaccare, G, Versura, P, et al. Incidence, risk factors and complications of ocular graft-versus-host disease following hematopoietic stem cell transplantation. Am J Ophthalmol. (2021) 227:25–34. doi: 10.1016/j.ajo.2021.02.022
193. Sinha, S, Singh, RB, Dohlman, TH, Taketani, Y, Yin, J, and Dana, R. Prevalence and risk factors associated with corneal perforation in chronic ocular graft-versus-host-disease. Cornea. (2021) 40:877–82. doi: 10.1097/ICO.0000000000002526
194. Plattner, K, Goldblum, D, Halter, J, Kunz, C, Koeppl, R, and Gerber-Hollbach, N. Osteo-Odonto-Keratoprosthesis in severe ocular graft versus host disease. Klin Monatsbl Augenheilkd. (2017) 234:455–6. doi: 10.1055/s-0042-123148
195. Liu, C, Okera, S, Tandon, R, Herold, J, Hull, C, and Thorp, S. Visual rehabilitation in end-stage inflammatory ocular surface disease with the osteo-odonto-keratoprosthesis: results from the UK. Br J Ophthalmol. (2008) 92:1211–7. doi: 10.1136/bjo.2007.130567
196. Orive Bañuelos, A, Arana Larrea, B, Crnej, A, Arce Soto, A, Andollo Victoriano, N, and Etxebarria, EJ. Transscleral Cyclophotocoagulation for the treatment of uncontrolled glaucoma in a Boston Keratoprosthesis type II patient. Case Rep Ophthalmol. (2022) 13:158–65. doi: 10.1159/000522440
197. Tung, CI. Graft versus host disease: what should the oculoplastic surgeon know. Curr Opin Ophthalmol. (2017) 28:499–504. doi: 10.1097/ICU.0000000000000400
198. Komai, S, Inatomi, T, Nakamura, T, Ueta, M, Horiguchi, G, Teramukai, S, et al. Long-term outcome of cultivated oral mucosal epithelial transplantation for fornix reconstruction in chronic cicatrising diseases. Br J Ophthalmol. (2022) 106:1355–62. doi: 10.1136/bjophthalmol-2020-318547
199. Dulz, S, Wagenfeld, L, Richard, G, Schrum, J, Muschol, N, and Keserü, M. A case of a bilateral Cicatricial upper eyelid entropion after hematopoietic stem cell transplantation in Mucopolysaccharidosis type I. Ophthalmic Plast Reconstr Surg. (2017) 33:S75–7. doi: 10.1097/IOP.0000000000000592
200. Kheirkhah, A, Ghaffari, R, Kaghazkanani, R, Hashemi, H, Behrouz, MJ, and Raju, VK. A combined approach of amniotic membrane and oral mucosa transplantation for fornix reconstruction in severe symblepharon. Cornea. (2013) 32:155–60. doi: 10.1097/ICO.0b013e318247983d
201. Saboo, U, Shikari, H, and Dana, R. Cataract prevalence and cataract surgery outcomes in patients with ocular graft-versus-host disease (GVHD). Invest Ophthalmol Vis Sci. (2013) 54:3001–1.
202. Bae, SS, Iovieno, A, and Yeung, SN. Long-term outcomes of cataract surgery in patients with chronic ocular graft-versus-host disease. Cornea. (2022) 41:587–92. doi: 10.1097/ICO.0000000000002779
203. Saboo, US, Amparo, F, Shikari, H, Jurkunas, UV, and Dana, R. Outcomes of phacoemulsification in patients with chronic ocular graft-versus-host disease. Graefes Arch Clin Exp Ophthalmol. (2015) 253:901–7. doi: 10.1007/s00417-015-2940-3
204. Gehlsen, U, Faust, C, Blecha, C, Dietrich-Ntoukas, T, Eberwein, P, Issleib, S, et al. Outcomes and complications of cataract surgery in patients with chronic ocular graft-versus-host-disease-a multicenter, retrospective analysis. Graefes Arch Clin Exp Ophthalmol. (2022) 260:2613–22. doi: 10.1007/s00417-022-05613-w
205. Balaram, M, and Dana, MR. Phacoemulsification in patients after allogeneic bone marrow transplantation. Ophthalmology. (2001) 108:1682–7. doi: 10.1016/S0161-6420(01)00675-3
206. Penn, EA, and Soong, HK. Cataract surgery in allogeneic bone marrow transplant recipients with graft-versus-host disease(1). J Cataract Refract Surg. (2002) 28:417–20. doi: 10.1016/S0886-3350(01)01165-8
207. de Melo, FR, Kron-Gray, MM, De la Parra-Colin, P, et al. Outcomes of cataract surgery in graft-versus-host disease. Cornea. (2015) 34:506–11. doi: 10.1097/ICO.0000000000000395
208. Schroeder, MA, and DiPersio, JF. Mouse models of graft-versus-host disease: advances and limitations. Dis Model Mech. (2011) 4:318–33. doi: 10.1242/dmm.006668
209. Ogawa, Y, Morikawa, S, Okano, H, Mabuchi, Y, Suzuki, S, Yaguchi, T, et al. MHC-compatible bone marrow stromal/stem cells trigger fibrosis by activating host T cells in a scleroderma mouse model. elife. (2016) 5:e09394. doi: 10.7554/eLife.09394
210. Yamane, M, Sato, S, Shimizu, E, Shibata, S, Hayano, M, Yaguchi, T, et al. Senescence-associated secretory phenotype promotes chronic ocular graft-vs-host disease in mice and humans. FASEB J. (2020) 34:10778–800. doi: 10.1096/fj.201900218R
211. Kheirkhah, A, Di Zazzo, A, Satitpitakul, V, Fernandez, M, Magilavy, D, and Dana, R. A pilot randomized trial on safety and efficacy of a novel topical combined inhibitor of Janus kinase 1/3 and spleen tyrosine kinase for GVHD-associated ocular surface disease. Cornea. (2017) 36:799–804. doi: 10.1097/ICO.0000000000001206
212. Kwon, J, Surenkhuu, B, Raju, I, Atassi, N, Mun, J, Chen, YF, et al. Pathological consequences of anti-citrullinated protein antibodies in tear fluid and therapeutic potential of pooled human immune globulin-eye drops in dry eye disease. Ocul Surf. (2020) 18:80–97. doi: 10.1016/j.jtos.2019.10.004
Keywords: ocular graft-versus-host disease (GVHD), dry eye, ocular surface inflammation, diagnosis, treatment
Citation: Tappeiner C, Heiligenhaus A, Halter JP, Miserocchi E, Bandello F and Goldblum D (2023) Challenges and concepts in the diagnosis and management of ocular graft-versus-host disease. Front. Med. 10:1133381. doi: 10.3389/fmed.2023.1133381
Edited by:
Georgios Panos, Nottingham University Hospitals NHS Trust, United KingdomReviewed by:
Daniel Wolff, University Hospital Regensburg, GermanyBowen Wang, Zhongshan Ophthalmic Center, Sun Yat-sen University, China
Victor L. Perez, Duke University, United States
Copyright © 2023 Tappeiner, Heiligenhaus, Halter, Miserocchi, Bandello and Goldblum. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christoph Tappeiner, ✉ christoph.tappeiner@pallas-kliniken.ch
 Christoph Tappeiner
Christoph Tappeiner