
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 30 March 2023
Sec. Obstetrics and Gynecology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1133042
This article is part of the Research Topic Saving Mothers and Babies for the New World View all 17 articles
Adenomyosis is a diffuse or localized organic disease caused by benign invasion of endometrial glands and stroma into the myometrium. It is a common disease that seriously affects reproductive health of women in childbearing age. Due to the unknown etiology and pathophysiological mechanism, and the lack of unified diagnostic criteria and effective treatment methods, total or subtotal hysterectomy has become a radical treatment for adenomyosis, which will lead to the complete loss of fertility. With the continuous exploration of the treatment to adenomyotic patients who have infertility or fertility intentions, new drugs, surgical methods and treating concepts appears. Adopt individualized conservative therapeutic strategies for patients with different conditions, preserve the uterus as much as possible and protect the patient’s fertility, which will play an important role on the follow-up assisted reproductive treatment and long-term management of adenomyosis.
Adenomyosis is a benign invasion of endometrial glands and stroma into the myometrium, causing myofibrillar connective tissue hypertrophy and hyperplasia, and forming diffuse or localized lesions of the uterus (1). Its main clinical manifestations are progressive dysmenorrhea, uterine enlargement, abnormal uterine bleeding (including increased menstrual volume and prolonged menstrual cycle) and infertility (2). Its occurrence in the infertility population can reach to more than 30%, and it seriously affects the patients’ quality of life. As adenomyosis is more common in women of childbearing age, it has gradually become one of the important clinical problems impairing human fertility, and its incidence rate range from 5 to 70% (3). Severe adenomyosis may be associated with adverse pregnancy and delivery outcomes (4).
The pathogenesis of adenomyosis is an important basis for treatment, but it has not been fully clarified. There are two main hypotheses about the pathogenesis of adenomyosis (5). One is the endometrial metaplastic hypothesis: adenomyosis lesions are generated directly by the differentiation of ectopic Mullerian tube residual cells, especially pluripotent stem cells with embryonic pluripotent (6). This hypothesis can partly explain the reason why many unmarried young women without pregnancy suffer from adenomyosis.
The other is the endometrial metastatic hypothesis, which believes that the enhancement and disorder of endometrial peristaltic waves, intrauterine infection (including Mycobacterium tuberculosis) or surgical procedures lead to the damage of endometrial-myometrial interface, namely the endometrial myometrium junction zone (JZ); then the glandular cells and stromal cells in the endometrium pass through the endometrial basal layer at the damaged site and invade the myometrium (7, 8). Endometrial stem cells may be recruited and self-repairing mechanism may be initiated in the local lesion. At the same time, the lesion can induce immune imbalance and aseptic inflammation, with increasing proglandin E2 (PGE2), proglandin H2 (PGH2), cyclooxygenase-2 (COX-2) and transforming growth factor β1 (TGF-β1), vascular endothelial growth factor (VEGF) and other factors (5). The increased aromatase activity promotes the synthesis of estradiol, which will up-regulate oxytocin and its receptor, as well as enhance peristalsis and damage of the endometrium (9). In this way, a vicious circle will be created, which eventually caused the cracking of the myometrium, endometrial basal cells invading into the myometrium, and the formation of adenomyosis lesions locally. This hypothesis can explain the clinical manifestations of elevated estrogen, menstrual abdominal pain, and increased menstruation in patients with adenomyosis (5).
In addition, there are other hypotheses, such as the hypothesis of retrograde menstruation, mesothelial metaplasia, etc. (6, 10). So far, no single hypothesis can fully explain all the pathological mechanisms and clinical manifestations of adenomyosis. Recently, investigations on the relationship between colors of human iris and hair and the characteristics of endometriosis may provide new inroads into the molecular aspects of the pathological mechanisms of these complex diseases (11).
At present, there is no consensus on the diagnostic criteria of adenomyosis by imaging methods. The criteria for the diagnosis of adenomyosis based on transvaginal ultrasonography (TVUS) images developed by the Federation International of Gynecology and Obstetrics (FIGO) in 2018 included eight ultrasound features (12, 13): asymmetric thickening of the myometrium, cystic lesions of the myometrium, island hyperechoic signals, fan-shaped shadows, linear or punctate echoes of the endometrium, streaked blood flow signals passing through the lesions, irregular shape and discontinuous JZ. If there are 2 or more of the above signs, the diagnosis can be made in combination with the patient’s clinical manifestations.
MRI features of adenomyosis are of great significance for diagnosis (13, 14). On T2-weighted imaging, localized adenomyosis showed ovoid, irregular or quasi-circular mass in the myometrium, with unclear boundary, low signal similar to the JZ, and scattered dotted or flaky high signal in the lesion, showing cystic expansion or hemorrhage in the lesion. Diffuse adenomyosis showed equal signal on T1-weighted image (T1WI), and dotted high signal in some lesions of T2WI showed that JZ was damaged, showing diffuse thickening. When the thickness of JZ > 12 mm, adenomyosis was highly suspected. If the thickness of JZ is 8–12 mm, combined with high signal spots or irregular boundary, it is proposed to be diagnosed as adenomyosis. Of course, pathological examination through surgery or biopsy is the gold standard for the diagnosis of adenomyosis.
The general therapeutic principles to be followed in clinical practice include reducing and removing lesions, relieving and eliminating pain, improving and promoting fertility, and reducing and avoiding recurrence. Adenomyosis has been regarded as a chronic disease requiring long-term management (15). Most premenopausal women need to receive long-term drug treatment, even after conservative surgery or interventional therapy (16). The individualized treating plan should be made according to the patient’s age, symptoms, ovarian reserve, severity of disease, fertility desire and previous treatments. The main purpose of individualized treatment plan is to solve the problems including chronic pelvic pain, secondary dysmenorrhea, excessive menstruation and infertility.
For patients with the main symptom of pelvic or menstrual pain, non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used if the pain is mild, no fertility requirements or the menopause is approaching. NSAIDs are cyclooxygenase inhibitors, which can reduce the synthesis of prostaglandins in the endometrium and relieve dysmenorrhea (17). However, it cannot prevent the progression of endometriosis and adenomyosis. Adenomyosis relapses easily after NSAIDs withdrawal, and about 18% of patients have no response to NSAIDs (17, 18). Therefore, NSAIDs, such as ibuprofen, is usually used in combination with other treatment methods. It should be noted that long-term use may result to adverse reactions such as peptic ulcer, liver and kidney injury (19).
Compound oral contraceptives (COC), such as drospirenone and ethinylestradiol tablets or cyproterone acetate and ethinylestradiol tablets, can reduce the amount of menstruation and relieve dysmenorrhea by inhibiting follicular development and ovulation, reducing estrogen level, inhibiting endometrial proliferation, and promoting endometrial atrophy (19, 20). COC is an important second-line treatment for menorrhagia patients due to adenomyosis, especially for those who need contraception (20). However, because COC has the potential to increase the risk of venous thrombosis and pulmonary embolism, patients over 40 years old or with high-risk factors (such as hypertension, diabetes, thrombosis and smoking history) should be very cautious.
Androgen derivatives including danazol, gestrinone, etc., can lead to endometrial atrophy through double effects (21). On one hand, they inhibit the secretion of follicle stimulating hormone (FSH) and luteinizing hormone (LH), and inhibit ovarian synthesis of steroid hormones (16). On the other hand, they combine with estrogen and progesterone receptors of the endometrium, and finally cause temporary amenorrhea in patients (22). Androgen derivatives have certain effects on dysmenorrhea symptoms and abnormal menstruation (21). However, there is a problem of recurrence after drug withdrawal, and the incidence of androgen like adverse reactions, such as acne and seborrheic dermatitis, weight gain and liver injury.
Mifepristone is a progesterone receptor antagonist with high affinity. It is a derivative of 9-demethyltestosterone, which can be used to terminate pregnancy, soften the cervix, and also cause amenorrhea (23). There have been some studies on the short-term use of low-dose mifepristone in the treatment of adenomyosis with leiomyoma, which has a significant effect on the relief of dysmenorrhea (19). Long term use of mifepristone can result in continuous stimulation of the endometrium by estrogen, so its safety needs further verification.
Dienogest is a new generation of synthetic progesterone, which has high affinity with progesterone receptor, and has little effect on androgen, glucocorticoid and mineralocorticoid receptor (24). Therefore, it has little effect on organ function and metabolism. Dienogest moderately inhibits the hypothalamus-pituitary-ovary (HPO) axis, and moderately reduces the estrogen level and endometrial proliferation, which prevents the expansion of uterine lesions. The incidence of perimenopausal symptoms after long-term use is low. At the same time, dienogest has anti-inflammatory and anti-angiogenesis effects, as well as promoting the reduction of nerve growth factor expression and nerve fiber density in tissues, which can effectively prevent recurrence and relieve pain (25). Dienogest is mainly suitable for the patients who are in or near the perimenopause with pelvic pain. In addition, the number of invasive NK cells in the endometrial layer increased after dienogest treatment, which may be conducive to the follow-up assisted reproductive treatment of patients with infertility (25, 26). Common adverse reactions include irregular bleeding, breast discomfort, headache, etc., so patients with adenomyosis accompanied by menorrhagia and anemia should be cautious.
The molecular structure of dydrogesterone is similar to that of natural progesterone, but its activity is much higher. Because it has no androgen, estrogen and mineralocorticoid like effects, dydrogesterone does not inhibit ovulation and has no inhibitory effect on normal endometrium. The patient can be pregnant during the medication of dydrogesterone, so it is especially suitable for adenomyosis patients with a recent pregnancy plan (27). Dydrogesterone is rapidly absorbed by oral administration with short half-life, and has obvious effect on inhibiting endometrial proliferation without causing low estrogen symptoms or affecting lipid metabolism and blood system (28). Thus it is safe for long-term use and suitable for patients above 40 years old, or those with high risks of metabolic and thrombotic diseases. Dydrogesterone is also often used in combination with other treatments (29). Due to the widespread use of didroxyprogesterone in other fields of gynecology and reproductive medicine, patients with adenomyosis have higher acceptance and compliance with it (29, 30).
Traditional Chinese medicine has a long history in dealing with various symptoms of adenomyosis. From the perspective of traditional Chinese medicine, the basic pathogenesis of adenomyosis is due to Blood-stasis blocking Chong-ren and Bao-gong, which belongs to internal syndrome (31). The disease location of adenomyosis is in Xia-jiao, so the general treatment principle is to promote blood circulation and remove Blood-stasis (Blood-stasis, Chong-ren, Bao-gong and Xia-jiao are all anatomical and pathological terms of traditional Chinese medicine). Traditional Chinese medicine therapies, such as Guizhi Fuling Capsule, Danhuang Quyu Capsule, Zhitong Huazheng Capsule, Sanjie Zhentong Capsule, Shaofu Zhuyu Decoction Modified, as well as catgut embedding, acupuncture and acupoint application, all have certain effects on relieving the clinical symptoms of adenomyosis (31, 32).
Traditional Chinese medicine is not only beneficial to the treatment of clinical symptoms of adenomyosis, but also provides a completely individualized treatment concept more importantly (33). In fact, traditional Chinese medical treatment is highly individualized for all diseases, including adenomyosis. The types of Chinese herbal medicine and measures used vary from person to person, time to time, and place to place. There is no unified treatment standard, which is instructive for the diagnosis and treatment in modern medicine. There will be a bright future to treat adenomyosis with the concept of combining traditional Chinese and modern medicine.
Gonadotropin releasing hormone agonist (GnRH-a) is an category of GnRH analog, whose affinity with GnRH receptor is much higher than that of natural GnRH (34). Long acting GnRH-a binds to a GnRH receptor in the pituitary gland to produce a transient increase in gonadotropin. Its continued binding to the receptor with subsequent movement into the pituitary cell prevents the pituitary gland from responding to endogenous GnRH. In this way, GnRH-a thoroughly inhibits the HPO axis of patients with adenomyosis and induces a sharp decrease in ovarian synthetic steroids, endometrial atrophy, decreased bleeding and even temporary amenorrhea (35). Long-acting GnRH-a can reduce uterine volume of patients with adenomyosis, and dysmenorrhea is also significantly relieved. However, continuous use for several months may lead to perimenopausal symptoms and osteoporosis, and estrogen should be added as appropriate. Because of the high price and obvious recurrence after drug withdrawal, GnRH-a is rarely used alone (36). Although GnRH-a is applicable to a wide range of patients, it is generally used as a pretreatment for other long-term treatments (35).
After levonorgestrel releasing intrauterine system (LNG-IUS) is implanted into the uterus, it can sustain a constant sustained release of 20 μg per day for 5 years. LNG-IUS mainly acts on the endometrium directly, and inhibits the synthesis of estrogen receptor in the endometrium which induces the sensitivity of the endometrium to estradiol and strongly antagonizes the proliferation of the endometrium (37). Therefore, it can be used not only for contraception, but also to treat adenomyosis, reduce the amount of menstruation, shrink the uterine volume, and relieve dysmenorrhea. LNG-IUS is effective for adenomyosis with dysmenorrhea and menorrhagia, especially recommended for the latter (38, 39). LNG rarely enters the peripheral circulation, so there are few systemic side effects except for minor vaginal bleeding and amenorrhea. Thus LNG-IUS can also be used for patients with a history of systemic diseases (such as hypercoagulable state, liver injury) or high risk, but patients must be fully informed of the advantages and disadvantages before use (20). LNG-IUS is applicable to adenomyosis patients who wish to conserve the uterus and fertility. As uterine cavity deformation, poor closure and excessive menstruation are common in adenomyosis patients, LNG-IUS is easy to fall off or move down (40). Accurate positioning under hysteroscopy, fixation to uterine wall, using GnRH-a pretreatment and other measures will help reduce the rate of LNG-IUS shedding and save medical expenses.
Xiao et al. found that LNG-IUS had a good therapeutic effect on patients with small lesions or small uterine volumes, while it had a poor therapeutic effect on patients with large lesions or large uterine volumes, or even no effect (41). This suggests that LNG-IUS is selective for lesion size in the treatment of adenomyosis, that is the phenomenon of ‘lesion threshold’ for LNG-IUS to effectively treat adenomyosis. This may be related to the pharmacological mechanism that LNG directly acts on the endometrium and diffuses to the myometrium. A large number of clinical data shows that the lesion threshold for LNG-IUS to take effect is the lesion range (LR: mean diameter of adenomyoma lesions or thickness of unilateral uterine muscle wall) ≤ 40 mm, and the corresponding threshold of uterine volume is (140.0 ± 35.4) ml. The lesion threshold for marked therapeutic effect is LR < 30 mm, with corresponding uterine volume (117.4 ± 34.1) ml, so that LNG-IUS had the most obvious effect in relieving symptoms and reversing the condition. When LR is 30 ~ 40 mm, LNG-IUS can reduce menstruation and dysmenorrhea, but the effect on reducing the uterine volume is not satisfactory. When LR > 40 mm, LNG-IUS alone has nearly no effect on reducing symptoms (41, 42). Lee et al. also showed that the effect of LNG-IUS on adenomyosis was poor if the uterine volume was >150 ml (43). We hope that there will be more in vivo and in vitro researches on the LR of LNG-IUS in the future, which will provide a research basis for the wider clinical application of LNG-IUS.
The main component of subdermal implant contraceptives is also synthetic potent progestogen which can achieve a theoretical effect similar to LNG, but its clinical application is limited (44).
High intensity focused ultrasound (HIFU) is a kind of physical therapy, which has the advantages of no operation, no bleeding, no ionizing radiation and repeatability (38). It is a more accurate treatment for localized adenomyosis with good effect, and preserves the integrity of the uterus. It is suitable for patients who have fertility planning and desire to conserve the uterus. The principle of HIFU ablation is to focus the ultrasound on the target tissue through the instrument. The thermal effect, cavitation effect and other physical effects of the ultrasound instantly raise the temperature of the target tissue to 65°C ~ 100°C, and cause tissue protein denaturation, coagulative necrosis. The tissue finally is dissolved, absorbed, calcified or fibrotic (41). With the continuous improvement of technology, the popularity and indications of HIFU treatment are gradually expanding, but it is easy to recur. In most cases it needs to be used in combination with other methods (45).
The uterus is not only a reproductive organ, but also plays a role in maintaining the normal anatomical structure of the pelvic floor as well as the blood supply of pelvic organs, especially ovary. Radical surgery, including total hysterectomy or subtotal hysterectomy, is the most thorough and effective method to treat adenomyosis, but it will deprive the fertility and have adverse effects on patients’ pelvic floor function, ovarian function, sexual life quality and self-cognition (46). Therefore, more and more attention has been paid to the conservative surgery.
Uterine artery embolization (UAE) and laparoscopic uterine artery occlusion on patients with adenomyosis both aim to block the blood supply of the lesion, but may lead to obstruction of ovarian blood supply or endometrial necrosis, resulting to secondary infertility (47). Transcervical endometrial resection (TCER) can lessen the symptoms of adenomyosis but cannot interfere with its progress. TCER removes the material basis of embryo implantation, so it cannot be used for patients with fertility requirements (48).
Resection of uterine lesion (RUL) is a very complex and challenging operation, and to achieve a balance between removing adenomyosis and retaining normal myometrium and the integrity of the uterus (49). RUL is often applied to adenomyosis patients with fertility requirements or young age. Therefore, there is no completely rigid surgical procedure, and flexible operations should be carried out according to factors such as the shape of the uterus, the size and location of the lesions. Common operational methods include wedge resection, asymmetric resection, and partial resection of muscle wall lesions (50). The repair of uterine myometrium and the reconstruction of uterine body are crucial for the uterus to withstand pregnancy, including H-shaped and, U-shaped suture, muscle overlapping-flap suture and triple-flap suture proposed by Osada, etc. (51, 52). In recent years, Xiao et al. have created the major uterine wall resection and reconstruction of the uterus (MURU) on the basis of RUL, which provides a new surgical method for patients with severe adenomyosis to preserve the uterus (42). MURU destroys the structure of the uterus, and the uterine scar is large with the high risk of uterine rupture during pregnancy. Therefore we are modifying the procedures to meet the fertility requirements of patients after operation. Of course, multi-center clinical research and more verification are needed before large-scale promotion of MURU. However, the recurrence, low natural pregnancy rate and the risk of uterine rupture during pregnancy are the main problems after conservative surgery (53). It is very important to treat as early as possible and develop an individual combined therapy to preserve the uterus according to the degree of adenomyosis.
Different therapies for adenomyosis have their own advantages and disadvantages, which causes great confusions for doctors and patients and probably leads to insufficient or excessive treatments (Table 1). Xiao et al. proposed a personalized therapeutic strategy based on the concept of grading, which provides a new option for patients with adenomyosis who wish to preserve fertility (41, 42). It divides adenomyosis into three grades according to the LR: mild (LR ≤ 30 mm), medium (LR between 30-40 mm) and severe (LR ≥ 40 mm). The corresponding processing methods for each grade are as follows:
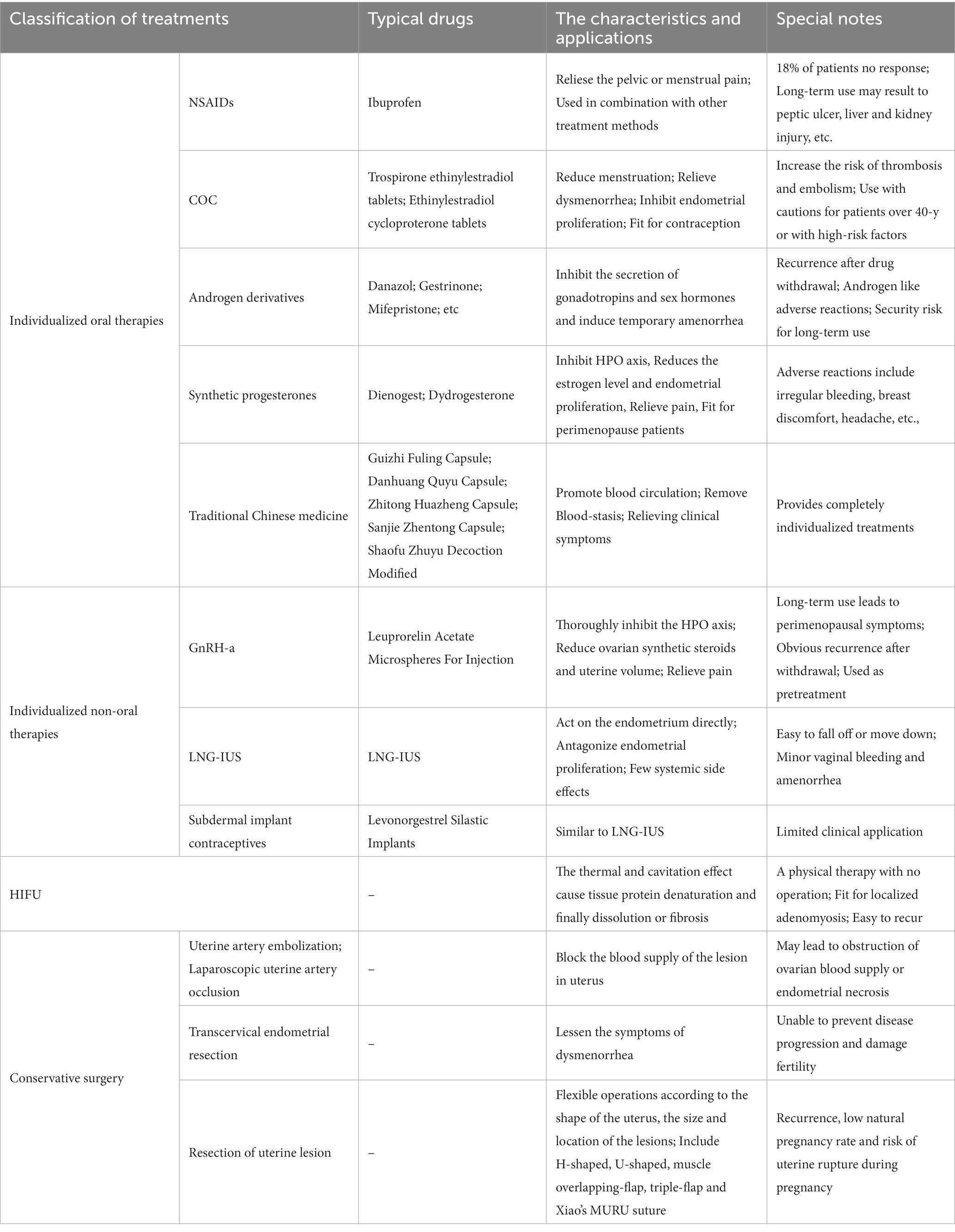
Table 1. The characteristics and application of individualized fertility preserving treatments for adenomyosis.
(1) For patients of mild grade, the long-term management by LNG-US is the main method. If there are still symptoms such as dysmenorrhea after that, NSAIDs, progesterone and COC should be given. Remove LNG-US before receiving assisted reproductive therapy.
(2) For patients of moderate grade, GnRH-a can be used for 3–6 months firstly. If LR reduces to ≤30 mm, the patients should be treated according to the principle of mild grade. If LR does not reduce obviously, HIFU can be used for to reduce LR as combined treatment.
(3) For patients of severe grade, GnRH-a should also be used for 3–6 months firstly. RUL or modified MURU should be performed after GnRH-a, and LNG-US is placed into the uterine cavity during the surgery. Remove LNG-US before receiving assisted reproductive therapy.
This grading of adenomyosis is mainly based on the LR in imaging, which lacks evaluation of clinical symptoms of patients, and needs further development to be widely used in practice. Anyway, this grading strategy is a great promotion to the treatment of adenomyosis.
Adenomyosis is a disease that tends to occur in childbearing aged women. The treatments are diverse but lack specificity. The basic treating principles for the adenomyosis patients with fertility requirements should include protecting physical and mental health, preserving reproductive organs and functions. It is crucial to allow patients the full advantages of each method and choose an individualized strategy according to the grading and needs of each patient. Follow up should be insisted, and combined or continuous therapy should be used to relieve symptoms and prevent disease progression if necessary.
LH and YL determined the topic and write the manuscript. KL and JJ retrieved literatures. CZ made the table. CZ and YW analyzed data and revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Scientific Research Fund of Binzhou Medical University (BY2021KYQD34); Scientific Research Initiation Fund of Affiliated Hospital of Binzhou Medical University Hospital (2021-03); Science and Technology Plan Project of Honghuagang District in Zunyi City (Zun Hong Ke He She[2020] No. 07); Science and Technology Plan Project of Zunyi City (Zun Yi Ke He HZ[2020] No. 271).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Moawad, G, Kheil, MH, Ayoubi, JM, Klebanoff, JS, Rahman, S, and Sharara, FI. Adenomyosis and infertility. J Assist Reprod Genet. (2022) 39:1027–31. doi: 10.1007/s10815-022-02476-2
2. Khan, KN, Fujishita, A, and Mori, T. Pathogenesis of human Adenomyosis: current understanding and its association with infertility. J Clin Med. (2022) 11:4057. doi: 10.3390/jcm11144057
3. Upson, K, and Missmer, SA. Epidemiology of Adenomyosis. Semin Reprod Med. (2020) 38:89–107. doi: 10.1055/s-0040-1718920
4. Berlanda, N, Alio, W, Angioni, S, Bergamini, V, Bonin, C, Boracchi, P, et al. Endometriosis treatment Italian, impact of endometriosis on obstetric outcome after natural conception: a multicenter Italian study. Arch Gynecol Obstet. (2022) 305:149–57. doi: 10.1007/s00404-021-06243-z
5. Zhai, J, Vannuccini, S, Petraglia, F, and Giudice, LC. Adenomyosis: mechanisms and pathogenesis. Semin Reprod Med. (2020) 38:129–43. doi: 10.1055/s-0040-1716687
6. Garcia-Solares, J, Donnez, J, Donnez, O, and Dolmans, MM. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril. (2018) 109:371–9. doi: 10.1016/j.fertnstert.2017.12.030
7. Xie, T, Xu, X, Yang, Y, Wu, C, Liu, X, Zhou, L, et al. The role of abnormal uterine junction zone in the occurrence and development of Adenomyosis. Reprod Sci. (2022) 29:2719–30. doi: 10.1007/s43032-021-00684-2
8. Guo, SW. The pathogenesis of Adenomyosis Vis-a-Vis endometriosis. J Clin Med. (2020) 9:485. doi: 10.3390/jcm9020485
9. Yen, CF, Huang, SJ, Lee, CL, Wang, HS, and Liao, SK. Molecular characteristics of the endometrium in uterine Adenomyosis and its biochemical microenvironment. Reprod Sci. (2017) 24:1346–61. doi: 10.1177/1933719117691141
10. Maruyama, S, Imanaka, S, Nagayasu, M, Kimura, M, and Kobayashi, H. Relationship between adenomyosis and endometriosis; different phenotypes of a single disease? Eur J Obstet Gynecol Reprod Biol. (2020) 253:191–7. doi: 10.1016/j.ejogrb.2020.08.019
11. Salmeri, N, Ottolina, J, Bartiromo, L, Schimberni, M, Dolci, C, Ferrari, S, et al. 'Guess who'? An Italian multicentric study on pigmentation traits prevalence in endometriosis localizations. Eur J Obstet Gynecol Reprod Biol. (2022) 274:5–12. doi: 10.1016/j.ejogrb.2022.05.001
12. Bazot, M, and Darai, E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril. (2018) 109:389–97. doi: 10.1016/j.fertnstert.2018.01.024
13. Munro, MG, Critchley, HOD, and Fraser, IS, F.M.D. Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. (2018) 143:393–408. doi: 10.1002/ijgo.12666
14. Agostinho, L, Cruz, R, Osorio, F, Alves, J, Setubal, A, and Guerra, A. MRI for adenomyosis: a pictorial review. Insights Imaging. (2017) 8:549–56. doi: 10.1007/s13244-017-0576-z
15. Matsushima, T, Akira, S, Asakura, H, and Takeshita, T. Low-dose gonadotropin-releasing hormone agonist therapy (draw-back therapy) for successful long-term management of adenomyosis associated with cerebral venous and sinus thrombosis from low-dose oral contraceptive use. Clin Exp Obstet Gynecol. (2017) 44:143–5. doi: 10.12891/ceog3214.2017
16. Vannuccini, S, Luisi, S, Tosti, C, Sorbi, F, and Petraglia, F. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. (2018) 109:398–405. doi: 10.1016/j.fertnstert.2018.01.013
17. Oladosu, FA, Tu, FF, and Hellman, KM. Nonsteroidal antiinflammatory drug resistance in dysmenorrhea: epidemiology, causes, and treatment. Am J Obstet Gynecol. (2018) 218:390–400. doi: 10.1016/j.ajog.2017.08.108
18. Streuli, I, Dubuisson, J, Santulli, P, de Ziegler, D, Batteux, F, and Chapron, C. An update on the pharmacological management of adenomyosis. Expert Opin Pharmacother. (2014) 15:2347–60. doi: 10.1517/14656566.2014.953055
19. Cope, AG, Ainsworth, AJ, and Stewart, EA. Current and future medical therapies for Adenomyosis. Semin Reprod Med. (2020) 38:151–6. doi: 10.1055/s-0040-1719016
20. Benetti-Pinto, CL, Mira, TAAD, Yela, DA, Teatin-Juliato, CR, and Brito, LGO. Pharmacological treatment for symptomatic Adenomyosis: a systematic review. Rev Bras Ginecol Obstet. (2019) 41:564–74. doi: 10.1055/s-0039-1695737
21. Szubert, M, Kozirog, E, Olszak, O, Krygier-Kurz, K, Kazmierczak, J, and Wilczynski, J. Adenomyosis and infertility-review of medical and surgical approaches. Int J Environ Res Public Health. (2021) 18:1235. doi: 10.3390/ijerph18031235
22. Pontis, A, D'Alterio, MN, Pirarba, S, de Angelis, C, Tinelli, R, and Angioni, S. Adenomyosis: a systematic review of medical treatment. Gynecol Endocrinol. (2016) 32:696–700. doi: 10.1080/09513590.2016.1197200
23. Che, X, Wang, J, He, J, Yu, Q, Sun, W, Chen, S, et al. A new trick for an old dog: the application of mifepristone in the treatment of adenomyosis. J Cell Mol Med. (2020) 24:1724–37. doi: 10.1111/jcmm.14866
24. Yang, S, Liu, Y, Wen, J, Sun, Y, and Ren, F. Clinical efficacy of Dienogest versus Levonorgestrel-releasing intrauterine system for Adenomyosis. Evid Based Complement Alternat Med. (2022) 2022:1995472. doi: 10.1155/2022/1995472
25. Prathoomthong, S, Tingthanatikul, Y, Lertvikool, S, Rodratn, N, Waiyaput, W, Dittharot, K, et al. The effects of Dienogest on macrophage and natural killer cells in Adenomyosis: a randomized controlled study. Int J Fertil Steril. (2018) 11:279–86. doi: 10.22074/ijfs.2018.5137
26. Murji, A, Biberoglu, K, Leng, J, Mueller, MD, Romer, T, Vignali, M, et al. Use of dienogest in endometriosis: a narrative literature review and expert commentary. Curr Med Res Opin. (2020) 36:895–907. doi: 10.1080/03007995.2020.1744120
27. Dasrilsyah, RA, Shan, LP, Kwang, NB, Shafiee, MN, and Omar, MH. Spontaneous conception following GnRHa and progestogen therapy in adenomyosis. Horm Mol Biol Clin Investig. (2016) 27:77–9. doi: 10.1515/hmbci-2015-0061
28. Schindler, AE. Progestational effects of dydrogesterone in vitro, in vivo and on the human endometrium. Maturitas. (2009) 65:S3–S11. doi: 10.1016/j.maturitas.2009.10.011
29. Sun, S, Zhang, H, Zhong, P, and Xu, Z. The effect of Letrozole combined with Dydrogesterone for endometriosis in China: a meta-analysis. Biomed Res Int. (2021) 2021:9946060. doi: 10.1155/2021/9946060
30. Nadarajah, R, Rajesh, H, Wong, KY, Faisal, F, and Yu, SL. Live birth rates and safety profile using dydrogesterone for luteal phase support in assisted reproductive techniques. Singap Med J. (2017) 58:294–7. doi: 10.11622/smedj.2016080
31. Wang, J, Zhai, T, Sun, X, Du, X, Zhang, X, Shi, X, et al. Efficacy and safety of acupuncture for adenomyosis: a protocol for systematic review and meta-analysis. Medicine (Baltimore). (2021) 100:e28080. doi: 10.1097/MD.0000000000028080
32. Ying, P, Li, H, Jiang, Y, Yao, Z, Lu, S, Yang, H, et al. Qiu's Neiyi recipe regulates the inflammatory action of Adenomyosis in mice via the MAPK signaling pathway. Evid Based Complement Alternat Med. (2021) 2021:9791498. doi: 10.1155/2021/9791498
33. Wang, Q. Individualized medicine, health medicine, and constitutional theory in Chinese medicine. Front Med. (2012) 6:1–7. doi: 10.1007/s11684-012-0173-y
34. Qin, Z, Dong, Z, Tang, H, Zhang, S, Wang, H, Bao, M, et al. Application of modified subtotal resection of adenomyosis combined with LNG-IUS and GnRH-a sequential therapy in severe adenomyosis: a case series. Front Surg. (2022) 9:914725. doi: 10.3389/fsurg.2022.914725
35. Donnez, J, Stratopoulou, CA, and Dolmans, MM. Uterine Adenomyosis: from disease pathogenesis to a new medical approach using GnRH antagonists. Int J Environ Res Public Health. (2021) 18:9941. doi: 10.3390/ijerph18199941
36. Pang, LL, Mei, J, Fan, LX, Zhao, TT, Li, RN, and Wen, Y. Efficacy of high-intensity focused ultrasound combined with GnRH-a for Adenomyosis: a systematic review and meta-analysis. Front Public Health. (2021) 9:688264. doi: 10.3389/fpubh.2021.688264
37. Ota, I, Taniguchi, F, Ota, Y, Nagata, H, Wada, I, Nakaso, T, et al. A controlled clinical trial comparing potent progestins, LNG-IUS and dienogest, for the treatment of women with adenomyosis. Reprod Med Biol. (2021) 20:427–34. doi: 10.1002/rmb2.12408
38. Peng, Y, Dai, Y, Yu, G, Yang, X, Wen, C, and Jin, P. Clinical evaluation of HIFU combined with GnRH-a and LNG-IUS for adenomyosis patients who failed to respond to drug therapies: two-year follow-up results. Int J Hyperth. (2021) 38:1271–5. doi: 10.1080/02656736.2021.1967467
39. Bahamondes, L, Valeria Bahamondes, M, and Shulman, LP. Non-contraceptive benefits of hormonal and intrauterine reversible contraceptive methods. Hum Reprod Update. (2015) 21:640–51. doi: 10.1093/humupd/dmv023
40. Haiyan, S, Lin, W, Shuhua, H, and Wang, W. High-intensity focused ultrasound (HIFU) combined with gonadotropin-releasing hormone analogs (GnRHa) and levonorgestrel-releasing intrauterine system (LNG-IUS) for adenomyosis: a case series with long-term follow up. Int J Hyperth. (2019) 36:1179–85. doi: 10.1080/02656736.2019.1679892
41. Sun, C, Ren, XY, Gao, Y, Liang, ZG, Mou, M, Gu, HF, et al. Clinical efficacy and safety of major Uterine Wall resection and reconstruction of the uterus combined with LNG-IUS for the treatment of severe Adenomyosis. Geburtshilfe Frauenheilkd. (2020) 80:300–6. doi: 10.1055/a-0995-2200
42. Xiao, YB, Mou, M, Gu, HF, Li, HX, You, MY, Liang, ZG, et al. The impact of major uterine wall resection and reconstruction of the uterus (MURU) on uterine hemodynamics and ovarian function in patients with adenomyosis: a clinical study. Clin Exp Obstet Gynecol. (2021) 48:132–7. doi: 10.31083/j.ceog.2021.01.5495
43. Lee, KH, Kim, JK, Lee, MA, Ko, YB, Yang, JB, Kang, BH, et al. Relationship between uterine volume and discontinuation of treatment with levonorgestrel-releasing intrauterine devices in patients with adenomyosis. Arch Gynecol Obstet. (2016) 294:561–6. doi: 10.1007/s00404-016-4105-y
44. Wu, J, Huang, Y, Chen, L, Hu, J, and Zou, Y. Treatment of Adenomyosis with subcutaneous Etonogestrel implants: a clinical observational study in 17 patients. Med Sci Monit. (2018) 24:6085–92. doi: 10.12659/MSM.908979
45. Zhang, X, and Duan, H. One-time high-intensity focused ultrasound ablation of abdominal wall endometriosis with concurrent uterine fibroids or adenomyosis: two cases and literature review. Quant Imaging Med Surg. (2020) 10:511–7. doi: 10.21037/qims.2019.12.07
46. Koebele, SV, Palmer, JM, Hadder, B, Melikian, R, Fox, C, Strouse, IM, et al. Hysterectomy uniquely impacts spatial memory in a rat model: a role for the nonpregnant uterus in cognitive processes. Endocrinology. (2019) 160:1–19. doi: 10.1210/en.2018-00709
47. Lohle, PNM, Higue, D, and Herbreteau, D. Uterine artery embolisation in women with symptomatic adenomyosis. Presse Med. (2019) 48:435–9. doi: 10.1016/j.lpm.2019.03.013
48. Rasmussen, CK, Hansen, ES, Al-Mashadi Dahl, S, Ernst, E, and Dueholm, M. The effect of transcervical endometrial resection on clinical symptoms related to intrinsic adenomyosis and junctional zone changes. Eur J Obstet Gynecol Reprod Biol X. (2019) 3:100029. doi: 10.1016/j.eurox.2019.100029
49. Li, JJ, Chung, JPW, Wang, S, Li, TC, and Duan, H. The investigation and Management of Adenomyosis in women who wish to improve or preserve fertility. Biomed Res Int. (2018) 2018:6832685. doi: 10.1155/2018/6832685
50. Otsubo, Y, Nishida, M, Arai, Y, and Ichikawa, R. Spontaneous conception and two successful pregnancies in patient who underwent conservative surgical management of diffuse uterine adenomyosis. J Obstet Gynaecol. (2016) 36:830–2. doi: 10.3109/01443615.2016.1157685
51. Osada, H, Silber, S, Kakinuma, T, Nagaishi, M, Kato, K, and Kato, O. Surgical procedure to conserve the uterus for future pregnancy in patients suffering from massive adenomyosis. Reprod Biomed Online. (2011) 22:94–9. doi: 10.1016/j.rbmo.2010.09.014
52. Osada, H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. (2018) 109:406–17. doi: 10.1016/j.fertnstert.2018.01.032
Keywords: adenomyosis, individualized treatment, uterus sparing operation, levonorgestrel-releasing intrauterine system, gonadotrophin releasing hormone agonist
Citation: Han L, Liu Y, Lao K, Jiang J, Zhang C and Wang Y (2023) Individualized conservative therapeutic strategies for adenomyosis with the aim of preserving fertility. Front. Med. 10:1133042. doi: 10.3389/fmed.2023.1133042
Received: 28 December 2022; Accepted: 10 March 2023;
Published: 30 March 2023.
Edited by:
Zaleha Abdullah Mahdy, National University of Malaysia, MalaysiaReviewed by:
Brunella Zizolfi, University of Naples Federico II, ItalyCopyright © 2023 Han, Liu, Lao, Jiang, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caiying Zhang, b3JjaGlkODdAMTYzLmNvbQ==; Yanlin Wang, ZG9jeWx3YW5nMjAwM0AxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.