- 1Department of Nephrology, The First Affiliated Hospital of Shenzhen University, Shenzhen Second People's Hospital, Shenzhen, China
- 2Medical College, Shantou University, Shantou, China
- 3École Doctorale Interdisciplinaire Sciences-Santé, Université Claude Bernard Lyon 1, Lyon, France
A patient complaining of edema of the face and lower extremities was admitted to the nephrology department for nephrotic syndrome. Renal biopsy revealed findings of minimal change disease (MCD). Thyroid ultrasound showed a hypoechoic 16 × 13 mm nodule in the right lobe, suspicious of malignancy. Later, total thyroidectomy confirmed the diagnosis of papillary thyroid carcinoma (PTC). After surgery, MCD remitted rapidly and completely, strongly suggesting the diagnosis of MCD secondary to PTC. We report here the first adult case of the paraneoplastic finding of MCD secondary to PTC. Additionally, we discuss the possible role of the BRAF gene in the pathophysiology of PTC-associated MCD in this case and highlight the importance of tumor screening.
Introduction
Minimal change disease (MCD) is a major cause of nephrotic syndrome (NS), characterized by severe proteinuria and a good response to steroid treatment. MCD can be triggered by drugs, infections, allergies, tumors, and lymphoproliferative disorders. To date, there is limited clinical experience with tumor-related MCD. We herein report the first adult case of MCD secondary to papillary thyroid carcinoma (PTC) and discuss the possible role of the BRAF gene in the pathogenesis of PTC-associated MCD in this case.
Case report
A 38-year-old woman was admitted to our nephrology department with facial and lower extremities edema and a weight gain of 4.5 kg for 3 days. Her medical history was remarkable for angina pectoris and chronic hepatitis B. She denied allergy, recent infection, COVID vaccination, occupational exposure to heavy metals, or family history of renal diseases. Physical examination revealed bilateral pretibial edema and a 15 × 10 mm, hard, painless, irregular nodule on the right side of her neck.
Laboratory tests (Supplementary Table 1) showed hypoalbuminemia (serum albumin 19.9 g/L) with hyperlipidemia (total cholesterol 8.52 mmol/L, low-density lipoprotein 6.45 mmol/L). Complete blood count, C-reactive protein and renal function were unremarkable. HbsAg, HbeAb and HbcAb were positive, but HBV viral load was low (HBV DNA < 500 IU/ml). Test results for HCV, HIV, and autoimmune antibodies were negative. Serum and urine protein immunofixation electrophoresis revealed no monoclonal proteins. Serum total thyroxine (T4) levels were slightly decreased (35.4 ng/ml), while triiodothyronine (T3), free T4, free T3, and thyroid-stimulating hormone levels were within the normal range. Macroalbuminuria was noted, with a urinary protein-creatinine ratio (UPCR) of 16.26 g/g and a protein excretion rate of 8.6 g/d. Ultrasonography revealed a 16 × 13 mm hypoechoic nodule in the right thyroid lobe with irregular shape and ill-defined margin. Color Doppler flow imaging showed abundant blood flow to the lesion, highly suggestive of thyroid malignancy (Figure 1). An enlarged lymph node (22 × 5 mm) was also noted in the right neck.
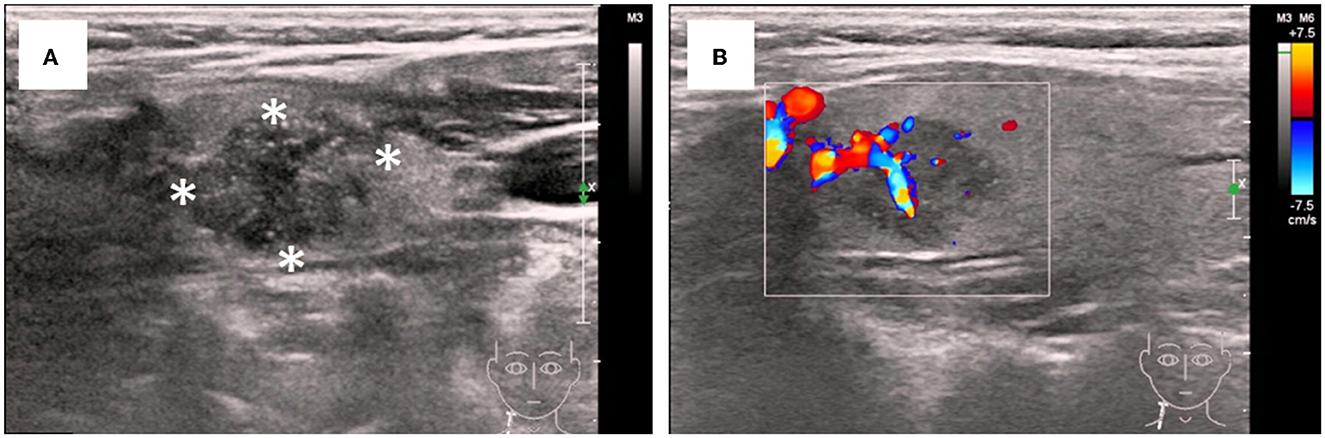
Figure 1. Ultrasonographic features of the thyroid nodular. (A) Irregular and ill-defined solid nodule with hypoisoechogenicity and microcalcifications (asterisks). (B) Increased vascular flow insides and around the nodular on color Doppler sonography.
We performed a renal biopsy and an ultrasound-guided fine-needle aspiration (FNA) biopsy for the thyroid nodule. The specimen obtained by FNA suggested thyroid papillary carcinoma. Renal biopsy specimens on light microscopy showed no evident alterations in the glomeruli, tubulointerstitium or vessels. Immunofluorescence microscopy was negative for immune complexes but revealed weak deposits of IgM in the mesangium and focal staining for reabsorbed albumin particles in the tubules. Electron microscopy exhibited extensive podocyte injury, as evidenced by diffuse foot processes effacement, cytoplasmic vacuoles, and microvillous transformation (Figures 2A–H). Therefore, the patient was diagnosed with MCD and suspected thyroid papillary carcinoma.
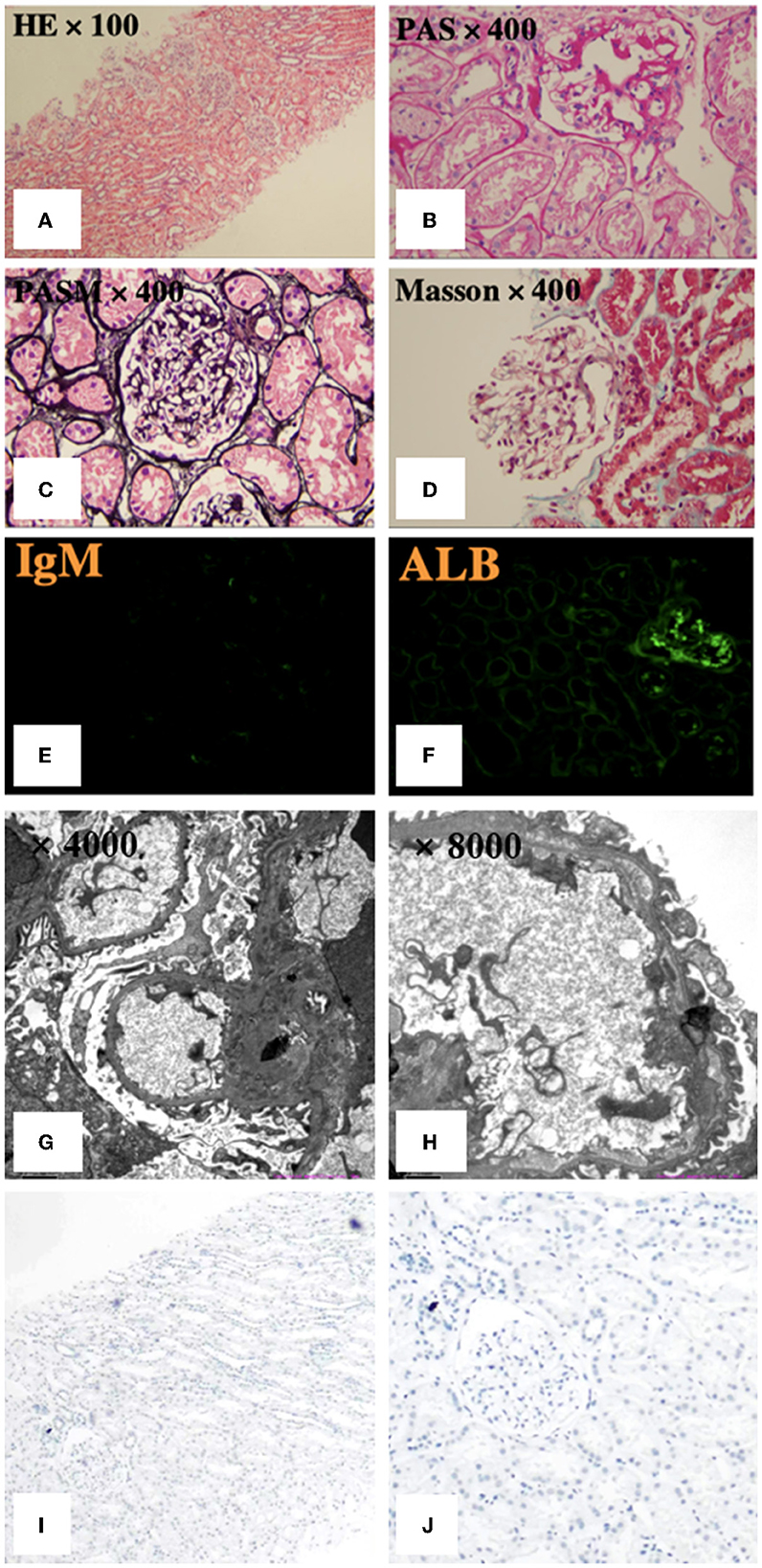
Figure 2. Pathological features of renal biopsy. (A–D) Light microscopy showed no apparent glomerular, tubulointerstitial or vascular alterations. (E, F) Immunofluorescence revealed weak deposits of IgM in the mesangium and focal staining for reabsorbed albumin particles in the tubules. (G, H) Electron microscopy exhibited extensive foot processes effacement, with no segmental sclerosis or electron-dense deposits, normal mesangial cellularity and matrix, and normal glomerular basement membrane thickness. PASM: periodic acid-silver methenamine, PAS: periodic acid-Schiff. (I, J) Immunohistochemistry staining for BRAF was negative.
Considering the presence of a malignant thyroid nodule, corticosteroids and other immunosuppressive therapies were deferred. Before the planned thyroidectomy, perindopril, an angiotensin receptor blocker (ARB), was used to decrease proteinuria. However, the patient discontinued perindopril after surgery. After a total thyroidectomy with a recurrent laryngeal nerve exploration, postoperative pathology confirmed the diagnosis of PTC (Figures 3A, B) with 6 level VI metastatic lymph nodes. The tumor cells were immunopositive for the BRAF (V-raf murine sarcoma viral oncogene homolog B1) protein (Roche Diagnostics) (Figures 3C, D). The surgical team ordered a BRAF mutation analysis using a quantitative fluorescent PCR with a human BRAF V600E mutation detection kit (Amoy Diagnostics, 1799T>A), which detected the BRAF V600E mutation. Surprisingly, proteinuria and serum albumin gradually normalized after surgery. At 6 months postoperatively, the patient achieved complete remission of MCD [proteinuria < 0.3 g/d or UPCR < 300 mg/g, serum albumin >3.5 g/dl according to the 2021 KDIGO guideline (1)], and no relapse occurred during a follow-up period of up to 30 months (Figure 4). The patient was satisfied with her treatment process and the clinical recovery. The case was finally diagnosed as PTC-associated MCD.
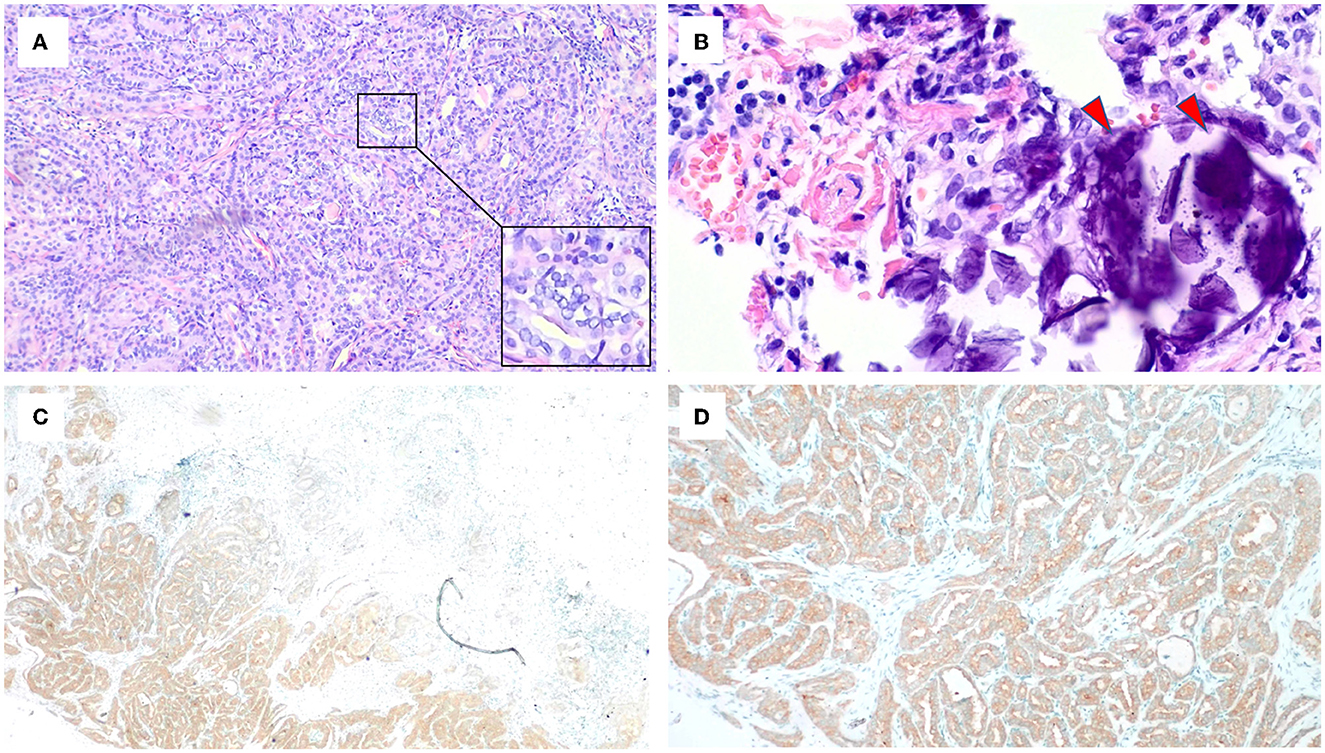
Figure 3. Microscopic appearance of papillary thyroid carcinoma. (A) Pathologic features reveal papillae lined by cuboidal to columnar cells with predominant nuclear changes. A magnification box on the right shows ground-glass nuclei with thick nuclear membranes, giving rise to what has been described as Orphan Annie Eye nuclei. Nuclear grooves are also noticeable (HE, ×10). (B) The presence of psammoma bodies shown by red arrows (HE, ×40). (C, D) Immunohistochemistry staining for BRAF was positive.
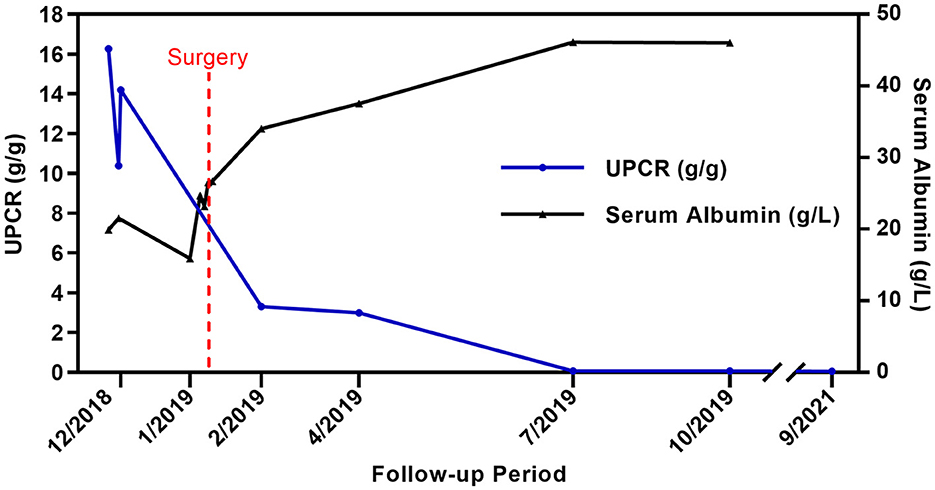
Figure 4. Clinical course of the patient. UPCR(blue line) and serum albumin (black line) changes before and after total thyroidectomy. Both UPCR and serum albumin normalized 6 months after the surgery (There was no spot urine or 24-h urine sample test performed in January 2019).
Discussion
To our knowledge, this is the first reported adult case of MCD secondary to PTC. Although approximately 50% to 60% of untreated MCD spontaneously remit over 2 to 3 years (1), in our case, the patient diagnosed with MCD was found to have PTC. Moreover, the patient achieved remission of MCD soon after tumor excision, strongly suggesting a link between MCD and PTC.
Paraneoplastic glomerulopathy is a condition of glomerular injury induced by products of tumor cells such as hormones, growth factors, cytokines, and tumor antigens (2). Compared with solid cancer-associated membranous nephropathy (MN) and Hodgkin lymphoma-associated MCD, the pathogenesis of solid cancer-associated MCD is less clear. In solid cancers, paraneoplastic MCD has been observed in lung cancer, colorectal cancer, renal cell carcinoma, and thymoma and less frequently in pancreatic, bladder, prostate, breast, and ovarian cancers (3, 4). In thyroid cancer, PTC has been associated with MN (5) and membranoproliferative glomerulonephritis (MPGN) (6). Liu et al. reported a case of minimal-change NS complicated with thyroid carcinoma in a pediatric case (7). However, it had a different clinical course compared to our own. The child with MCD was found to have co-existing thyroid cancer and his condition did not improve after surgery; instead, he had multiple recurrences of NS. Fortunately, the child responded well to steroid treatment. In contrast, our patient achieved remission of MCD soon after tumor excision, strongly supporting the link between MCD and PTC in our case.
Interestingly, there is a strong correlation between renal manifestation and thyroid disorder. Physiologically, thyroid hormones are essential for renal development and growth, while the kidney plays an important role in the metabolism and elimination of these hormones (8). When thyroid dysfunction occurs, there can be remarkable changes in glomerular and tubular functions, as demonstrated by the association between hypothyroidism /hyperthyroidism and nephropathy, tubulointerstitial nephritis. Likewise, renal disease can affect thyroid function, for instance, a higher prevalence of hypothyroidism has been observed in chronic kidney disease (CKD) (9). Furthermore, a reciprocal association between thyroid and renal cancers has been reported, suggesting that shared genetic and environmental risk factors may be at play (10).
In PTC, 40–45% of patients have a BRAF mutation, which is associated with an advanced stage and poorer prognosis. BRAF is a proto-oncogene that encodes the BRAF protein, a key component of the mitogen-activated protein kinase (MAPK) pathway. This pathway, more specifically the RAS-RAF-MEK-ERK pathway, regulates cell growth, proliferation, and apoptosis, and its alteration is one of the major contributors to tumorigenesis. Of the reported BRAF mutations, 98–99% are V600E mutations, which arise from the substitution of valine by glutamate at amino acid 600, and it is the most common mechanism of MAPK signaling activation in PTC (11).
In murine NS model, gene enrichment analysis in podocytes with foot processes effacement revealed upregulation of the BRAF/MAPK signaling genes (RAF1, MAPK1, BRAF as the leading genes), suggesting a podocyte-specific BRAF/MAPK signaling pathway (12). Analysis of a large gene database of human kidney diseases showed that the BRAF gene expression was elevated in several kidney diseases associated with podocyte damage, such as focal segmental glomerular sclerosis, lupus nephritis and MCD, and correlated with the severity of proteinuria (12). Moreover, the BRAF V600E inhibitor GDC-0879 has been shown to rescue podocyte foot processes effacement and promote podocyte survival in vivo and in vitro (13). However, there are also studies demonstrating that podocytes are protected when the MAPK pathway is activated (14), and podocytes are damaged when the BRAF gene is inhibited (15). Although the specific role of the BRAF gene in podocyte pathology remains controversial, these studies suggest that the abnormal expression of BRAF is implicated in podocyte dysfunction.
The relationship between the BRAF expression in tumor cells of PTC and podocytes is unclear. A common trigger, possibly molecular signals such as cytokines and microRNA released by tumor cells or microenvironment cells that affect the BRAF expression in these two distinct cell types, needs further investigation. Further study of the BRAF/MAPK pathway in podocytes before and after tumor removal might help to identify the upstream factors. In addition, there is dysregulation of T cells in both PTC and MCD (16–18), especially abnormal Th2 and related signaling pathways. Notably, the BRAF mutation in PTC is usually associated with the induction of immune tolerance and evasion (19, 20). Focusing on T cells and their signaling pathways by analyzing peripheral T helper cell subsets, cytokine levels, and Treg function may provide new insights.
Due to the close physiological and pathological relationships between the thyroid and the kidney, and reports of podocyte dysfunction caused by BRAF mutation, we explored the co-occurring gene events of thyroid and kidney in the patient. We performed immunohistochemistry staining (Roche Diagnostics) for BRAF in kidney tissues, which was immuno-negative (Figures 2I, J). There was no evidence of BRAF abnormality in the podocytes in our case, while the thyroid tumors was BRAF immuno-positive. Given the full recovery of podocyte dysfunction after tumor removal, there may be no intrinsic abnormalities in podocytes, and the podocyte dysfunction was related to PTC. Therefore, secondary MCD was considered for diagnosis.
In summary, we present here a rare adult case of PTC-associated MCD. The patient was successfully treated with thyroidectomy, which cured both PTC and MCD. Further studies are needed to elucidate the underlying mechanism of paraneoplastic MCD. From this case, we learn that individualized cancer screening in newly diagnosed nephropathy is reasonable in view of the possible, albeit rare, malignancy-associated nephropathy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethical Committees of Shenzhen Second People's Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
XC wrote the first draft of the manuscript. YW and XZ contributed to the revisions of the manuscript. XC, QW, and XZ contributed to conception and design of the study. All authors read and approved the final manuscript.
Funding
This study was supported by Shenzhen Key Medical Discipline Construction Fund (Grant no. SZXK009). These funders had no role in data collection, analysis, reporting, and manuscript revision.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1132259/full#supplementary-material
References
1. Kidney Disease: Improving Global Outcomes (KDIGO). Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. (2021) 100:S1–S276. doi: 10.1016/j.kint.2021.05.021
2. Ronco PM. Paraneoplastic glomerulopathies: new insights into an old entity. Kidney Int. (1999) 56:355–77. doi: 10.1046/j.1523-1755.1999.00548.x
3. Bacchetta J, Juillard L, Cochat P, Droz J-P. Paraneoplastic glomerular diseases and malignancies. Crit Rev Oncol Hematol. (2009) 70:39–58. doi: 10.1016/j.critrevonc.2008.08.003
4. Lionaki S, Marinaki S, Panagiotellis K, Tsoumbou I, Liapis G, Vlahadami I, et al. Glomerular diseases associated with malignancies: histopathological pattern and association with circulating autoantibodies. Antibodies (Basel). (2020) 9:18. doi: 10.3390/antib9020018
5. Zirino F, Gembillo G, Lamanna F, Calabrese V, Longhitano E, Siligato R, et al. SAT-367 A case of thyroid papillary carcinoma associated with membranous anti-PLA2R positive glomerulonephritis. ISN World Congress of Nephrology (WCN) 2020 Abstracts. Kidney Int. (2020) 5:S154–S155. doi: 10.1016/j.ekir.2020.02.389
6. Pattanashetti N, Kapatia G, Nada R, Gupta KL, Ramachandran R. Association of membranoproliferative glomerulonephritis with papillary carcinoma thyroid. Indian J Nephrol. (2019) 29:368–9. doi: 10.4103/ijn.IJN_215_18
7. Liu P, Tian M, Wei L, Cao GH, Zhang SF et al. A case of minimal-change nephrotic syndrome complicated with thyroid carcinoma in children. Zhonghua er ke za zhi. (2019) 57:714–5. doi: 10.3760/cma.j.issn.0578-1310.2019.09.014
8. Simeoni M, Cerantonio A, Pastore I, Liguori R, Greco M, Foti D, et al. The correct renal function evaluation in patients with thyroid dysfunction. J Endocrinol Invest. (2016) 39:495–507. doi: 10.1007/978-3-319-29456-8
9. Dousdampanis P, Trigka K, Vagenakis GA, Fourtounas C. The thyroid and the kidney: a complex interplay in health and disease. Int J Artif Organs. (2014) 37:1–12. doi: 10.5301/ijao.5000300
10. Bellini MI, Lori E, Forte F, Lauro A, Tripodi D, Amabile MI, et al. Thyroid and renal cancers: a bidirectional association. Front Oncol. (2022) 12:951976. doi: 10.3389/fonc.2022.951976
11. Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. (2011) 7:569–80. doi: 10.1038/nrendo.2011.142
12. Sidhom E-H, Kim C, Kost-Alimova M, Ting MT, Keller K, Avila-Pacheco J, et al. Targeting a Braf/Mapk pathway rescues podocyte lipid peroxidation in CoQ-deficiency kidney disease. J Clin Invest. (2021) 131:e141380. doi: 10.1172/JCI141380
13. Sieber J, Wieder N, Clark A, Reitberger M, Matan S, Schoenfelder J, et al. GDC-0879, a BRAF(V600E) inhibitor, protects kidney podocytes from death. Cell Chem Biol. (2018) 25:175–84. doi: 10.1016/j.chembiol.2017.11.006
14. Li X, Ma A, Liu K. Geniposide alleviates lipopolysaccharide-caused apoptosis of murine kidney podocytes by activating Ras/Raf/MEK/ERK-mediated cell autophagy. Artif Cells Nanomed Biotechnol. (2019) 47:1524–32. doi: 10.1080/21691401.2019.1601630
15. Perico L, Mandalà M, Schieppati A, Carrara C, Rizzo P, Conti S, et al. BRAF Signaling pathway inhibition, podocyte injury, and nephrotic syndrome. Am J Kidney Dis. (2017) 70:145–50. doi: 10.1053/j.ajkd.2016.12.013
16. Bertelli R, Bonanni A, Caridi G, Canepa A, Ghiggeri GM. Molecular and cellular mechanisms for proteinuria in minimal change disease. Front Med. (2018) 5:170. doi: 10.3389/fmed.2018.00170
17. Xi C, Zhang G-Q, Sun Z-K, Song H-J, Shen C-T, Chen X-Y, et al. Interleukins in thyroid cancer: from basic researches to applications in clinical practice. Front Immunol. (2020) 11:1124. doi: 10.3389/fimmu.2020.01124
18. Simonovic SZ, Mihaljevic O, Majstorovic I, Djurdjevic P, Kostic I, Djordjevic OM, et al. Cytokine production in peripheral blood cells of patients with differentiated thyroid cancer: elevated Th2/Th9 cytokine production before and reduced Th2 cytokine production after radioactive iodine therapy. Cancer Immunol Immunother. (2015) 64:75–82. doi: 10.1007/s00262-014-1619-7
19. Zhi J, Zhang P, Zhang W, Ruan X, Tian M, Guo S, et al. Inhibition of BRAF sensitizes thyroid carcinoma to immunotherapy by enhancing tsMHCII-mediated Immune Recognition. J Clin Endocrinol Metab. (2021) 106:91–107. doi: 10.1210/clinem/dgaa656
Keywords: minimal change disease, secondary nephropathy, paraneoplastic glomerulopathy, thyroid cancer, BRAF
Citation: Cai X, Wu Y, Wan Q and Zhang X (2023) Minimal change disease associated with thyroid cancer: a case report. Front. Med. 10:1132259. doi: 10.3389/fmed.2023.1132259
Received: 27 December 2022; Accepted: 20 April 2023;
Published: 10 May 2023.
Edited by:
Sree Bhushan Raju, Nizam's Institute of Medical Sciences, IndiaReviewed by:
Jing Miao, Mayo Clinic, United StatesMariadelina Simeoni, University of Campania Luigi Vanvitelli, Italy
Copyright © 2023 Cai, Wu, Wan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiuli Zhang, WmhhbmcyMDEzXzEyQDE2My5jb20=
 Xiaoyi Cai
Xiaoyi Cai Yuenv Wu
Yuenv Wu Qijun Wan
Qijun Wan Xiuli Zhang1*
Xiuli Zhang1*