
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 17 July 2023
Sec. Gastroenterology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1128766
 Xi Wu1†
Xi Wu1† Ming-Chuang Zhu2†
Ming-Chuang Zhu2† Guo-Liang Li3
Guo-Liang Li3 Peng Xiong4
Peng Xiong4 Wei Sun1
Wei Sun1 Ni Zhang1
Ni Zhang1 Bo Zhao1
Bo Zhao1 Le-Qun Li1
Le-Qun Li1 Xiang-Ning Fu1
Xiang-Ning Fu1 Min Zhu1*
Min Zhu1*Background: Upper esophageal cancer (UEC) is rare in both Eastern and Western countries. The epidemiological characteristics and long-term survival of UEC patients are less known. In addition, the choice of optimal treatment for UEC has been controversial.
Methods: Cases of UEC (C15.3 and C15.0) arising during the period from 1973 to 2013 were identified and selected using the SEER database. Student's t-test and Pearson's chi-square test were used to compare the differences in parameters among different groups. Esophageal cancer-specific survival (ECSS) and overall survival (OS) rates were calculated by using the Kaplan–Meier method. Cox proportional hazard regression was used to analyze predictive factors.
Results: In the past 40 years, the cases of UEC have gradually increased, and the proportion of adenocarcinoma (AD) has gradually increased (from 3.6% to 11.8%, p < 0.001). There has been a significant increase (1973–1982 vs. 2004–2013) in median OS (7 months vs. 10 months, p < 0.001) and median ECSS (7 months vs. 11 months, p < 0.001) among UEC patients from 1973 to 2013. For the impact of different treatments, the results showed that the ECSS and OS of surgery without radiation (SWR) and radiation plus surgery (R+S) were superior to those of radiation without surgery (RWS). Subgroup analysis showed that ECSS and OS were highest among patients treated with SWR compared with R+S and RWS for patients with localized disease. For regional disease, ECSS and OS were highest among patients with R+S compared with SWR or RWS. Among patients with regional-stage squamous cell carcinoma (SCC), OS was higher with neoadjuvant radiotherapy or adjuvant radiotherapy compared with SWR. Multivariate analysis showed that radiotherapy sequence was dependently associated with OS among patients with regional-stage SCC.
Conclusion: Although the long-term survival of UEC remains poor, it has gradually increased since 1973. This should be closely related to the improvement of medical care over the past 40 years. Different treatment methods have a great influence on the long-term survival of UEC. For localized diseases, surgery may be a better choice. For regional disease, surgery plus adjuvant or neoadjuvant radiotherapy may be more beneficial to improve the long-term prognosis of UEC patients.
Esophageal cancer is one of the most common malignant tumors in the world, ranking sixth in morbidity and eighth in mortality (1). Esophageal cancer rarely affects the upper esophagus, including the cervical and upper thoracic esophagus, which accounts for only 5–10% of all cases of esophageal cancer (2–4). Compared with carcinoma affecting the middle or lower segments of the esophagus, carcinoma of the upper esophagus is challenging. Multidisciplinary treatment is always required because of the complicated anatomy of the upper esophagus, and because carcinoma affecting the upper esophagus is typically advanced at the time of diagnosis, with a tendency to invade surrounding anatomical structures when being diagnosed (3–6). Thus, UEC is associated with a poorer prognosis than any other type of esophageal cancer (6).
In general, treatment of UEC includes surgery, radiotherapy (RT), chemotherapy, or a combination of these approaches. Surgery was once the major management of UEC. The surgical method used most commonly by UEC is the McKeown approach (tri-incisional esophagectomy) (7), which always requires cervical or total esophagectomy. A pharyngo-laryngo-esophagectomy (PLE) is required in the case of a high disease burden (5). Therefore, postoperative complications are common, and the 5-year overall survival (OS) for surgical resection is low (12–33%) (8, 9).
Previous reports have found similar OS after surgery, radiotherapy (RT), and definitive chemoraidotherapy (CRT) (5, 10). RT and CRT gradually became the preferred treatments for UEC in many countries and regions, including the United States (3, 11, 12). However, among patients with resectable tumors, long-term outcomes were significantly improved for those who underwent surgery compared with those who received only received definitive CRT (13). Surgery may also result in improvements in prognosis, quality of life, and post-treatment dysphagia symptoms (10, 14). The centers included in these studies continue to rely on surgery as the primary treatment for UEC (5, 8, 13).
The surgical procedure for esophageal cancer has changed dramatically in recent years with the development of medical skills and instruments. For example, many centers have adopted minimally invasive esophagectomy. The advantages of minimally invasive esophagectomy compared with open esophagectomy include decreased postoperative pain, decreased length of hospital stay, and fewer complications (15, 16). Furthermore, surgical robots have been used widely to perform esophageal cancer operations, with positive clinical outcomes (17, 18). Technological advancement has also brought numerous improvements to RT and chemotherapy (2, 12).
However, esophageal carcinoma of the upper segment is a rare type of esophageal cancer. Due to its small number of cases, it accounts for a very small proportion of clinical trials of esophageal cancer compared with middle- and lower-segment esophageal cancer. In addition, clinical studies on upper esophageal carcinoma are relatively lacking (19). Upper esophageal cancer is not well understood, and the choice of treatment for upper esophageal carcinoma is still controversial.
The National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) database is a cancer database covering ~26% of the total US population. This database includes information on the incidence of cancer in 18 areas of the US (20). This study aimed to use information from the SEER database to analyze the epidemiological characteristics and long-term survival trends of UEC over the past 40 years. In addition, the effects of different treatment strategies on the survival of patients with UEC were also analyzed to make people have a clearer and deeper understanding of UEC.
This study was performed using data from the SEER database. Data were collected during the period from 1973 to 2013 (available at: www.seer.cancer.gov) based on the November 2015 submission using SEER*Stat software, version 8.3.5.
The outcomes of interest in this study were OS and esophageal cancer-specific survival (ECSS), according to specific codes. We collected information for patients with UEC diagnosed during the period from 1973 to 2013. All patients included in the study had a primary site-labeled recode diagnosis of “C15.0- Cervical esophagus and C15.3-Upper third of esophagus” (from the lower margin of the sixth cervical vertebra to the superior margin of the sixth thoracic vertebrae). Exclusion criteria were multiple primary carcinomas, an unknown number of survival months, and diagnosis < 1 month prior to death. Tumors were classified as squamous cell carcinoma (SCC) (8050-8082), adenocarcinoma (AC) (8140-8573), or “other” pathological type. Criteria for SEER historical stage A (localized, regional, and distant) were adopted in order to comply with a unified tumor staging system across all years of the study.
Continuous variables were compared with Student's t-test; categorical variables were compared with Pearson's chi-square test. The Kaplan–Meier (KM) methods were used to estimate survival time for time-to-event endpoints, OS, and ECSS. The log-rank test and Cox proportional hazards models were used to conduct univariate and multivariate analyses, respectively. Multivariate analysis included only those variables that were significantly associated with survival in univariate analysis. All p-values were two-sided, and p < 0.05 was considered statistically significant. All analyses were performed using IBM SPSS version 20.0.
The study flow chart is shown in Supplementary Figure 1. A total of 4424 patients who met our inclusion criteria were included in the study (Supplementary Table 1). The mean age was 66.8 years. Men accounted for 66.5% of all patients. Most patients had regional (37.6%) or distant (31.4%) stages of disease at the time of diagnosis. First, patients were divided into four groups according to the year of diagnosis (Supplementary Table 2). Although SCC (85.3%) remained the most common pathological type of UEC, the proportion of AC gradually increased over the study period (from 3.6% to 11.8%, p < 0.001). Most patients had undergone RT (75.7%) and only a few (13.3%) had been treated with surgery. While patients with localized or regional-stage diseases were more likely to receive surgery (p < 0.001). RT was more commonly used in patients with regional-stage disease (p < 0.001 Supplementary Table 3). The rate of surgical resection was higher for AC compared with SCC. Patients with SCC were more likely to receive RT than patients with AC (p < 0.001 for all, Supplementary Table 4).
Patients were divided into four groups based on the type of treatment received (Supplementary Table 5). Patients with localized diseases were more likely to undergo SWR, while patients with regional-stage tumors tended to choose R+S (p < 0.001). Patients who chose neither surgery nor radiotherapy tended to have advanced-stage diseases (p < 0.001).
In order to evaluate the effect of RT sequence on survival among patients who underwent surgery, we divided patients into three groups (Supplementary Table 6): SWR, neoadjuvant radiotherapy (NRT), and adjuvant radiotherapy (ART). The proportion of SCC was lower in the SWR group compared with the ART and NRT (p < 0.001). The number of patients who elect to undergo NRT has increased over recent decades (p < 0.001). SWR was most likely to be performed for patients with localized disease (p < 0.001). While ART was most commonly used in the treatment of patients with regional stage (p < 0.001), NRT was most commonly used in patients with distant stage (p < 0.001).
Median OS was ~9.0 months (95% CI: 8.65–9.35). Overall, ECSS was also ~9.0 months (95% CI: 8.60–9.40). OS at 1, 3, and 5 years was 37.0%, 13.2%, and 10.0%, respectively; ECSS at 1, 3, and 5 years was 40.8%, 17.9%, and 14.1%, respectively.
Overall survival values at 1, 2, 3, and 5 years, respectively, increased further for each year that elapsed between 1973 and the time of diagnosis (Figure 1A). OS and ECSS differed significantly among these four groups (p < 0.05 for all, Figures 1B, C). These results indicate significant increases (1973–1982 vs. 2004–2013) in median OS (7 months vs. 10 months, p < 0.001) and median ECSS (7 months vs. 11 months, p < 0.001) since 1973.
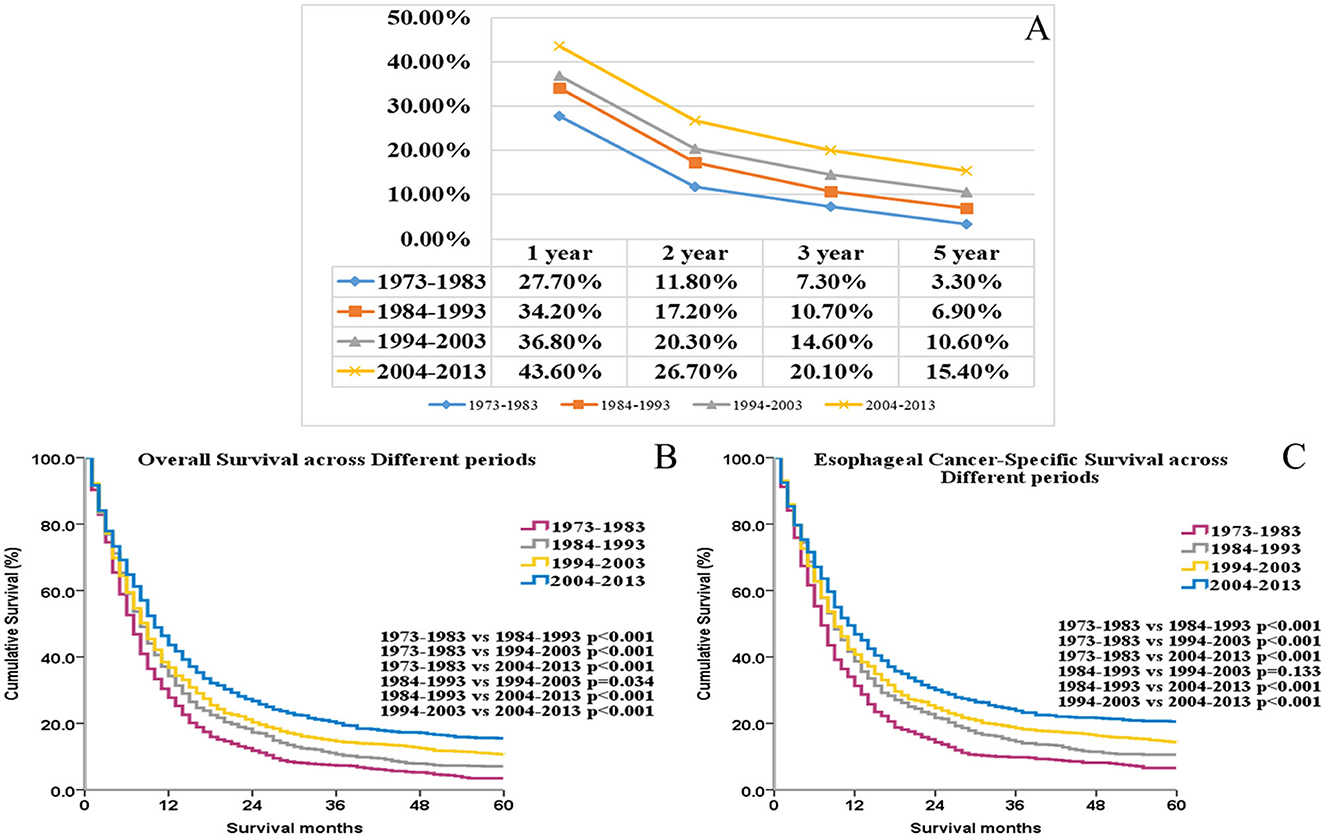
Figure 1. The trends of the 1-, 2-, 3-, and 5-year overall survival rate for UEC patients (A). The OS and ECSS of UEC patients across different periods (B, C).
The OS and ECSS were greater for AC than for SCC (p < 0.001 for all, Figures 2A, B). OS was higher among women compared with men (p < 0.001; Figure 2C). ECSS was also higher among women compared with men (p < 0.001; Figure 2D). Univariate (Supplementary Table 7) and multivariate (Supplementary Table 8) Cox analyses identified the following independent factors associated with ECSS as well as OS: date of diagnosis, ethnicity, sex, age, marital status, histologic subtype, SEER historic stage, surgical treatment, and RT.
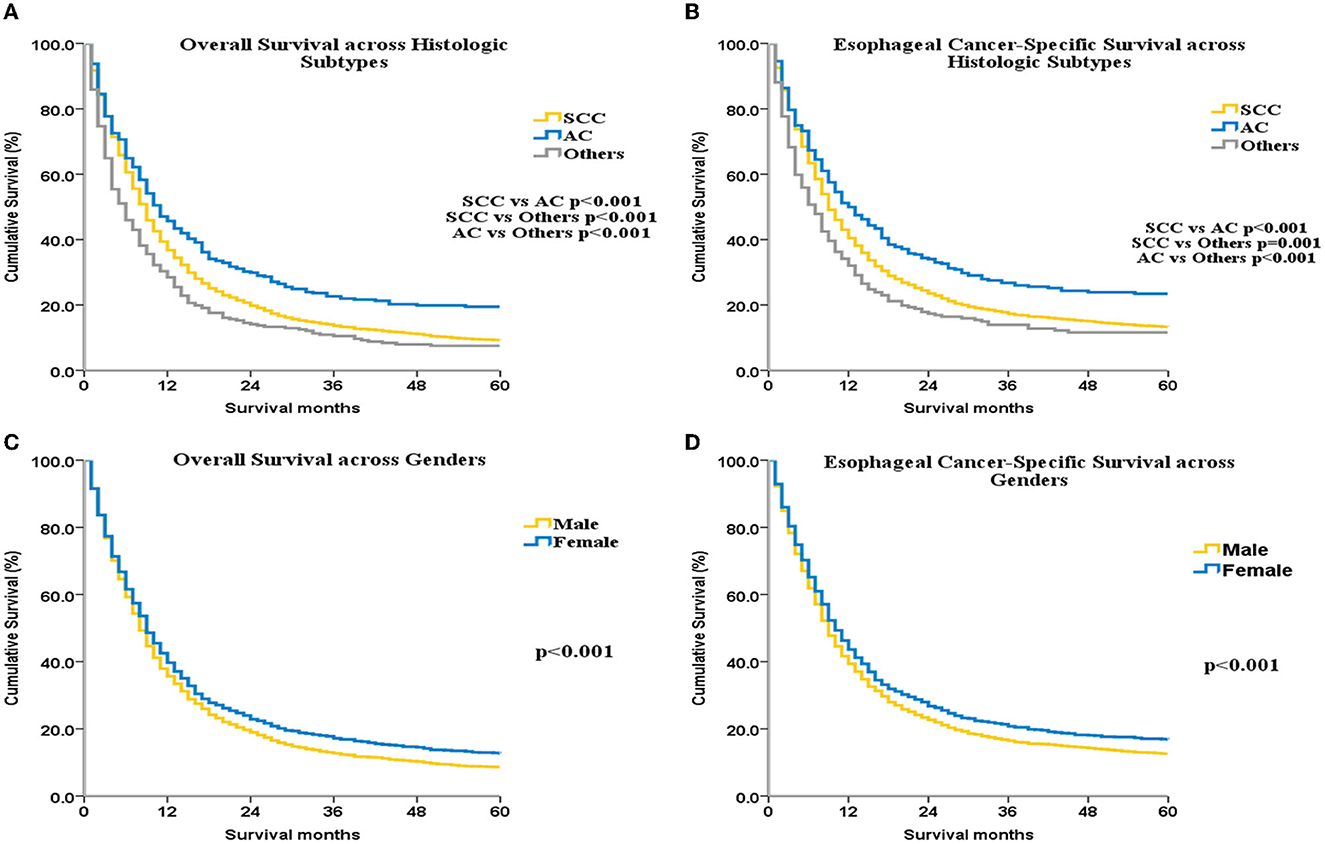
Figure 2. OS and ECSS for UEC patients across different histological subtypes (A, B) and different genders (C, D).
Median OS for the control, RWS, SWR, and R+S groups was 3 months, 9 months, 15 months, and 15 months, respectively. ECSS and OS were improved among patients who underwent RT or surgery compared with patients who did not receive treatment (p < 0.001 for all, Figures 3A, B). ECSS and OS were lower in the RWS group compared with the SWR and R+S groups (p < 0.001 for all, Figures 3A, B).
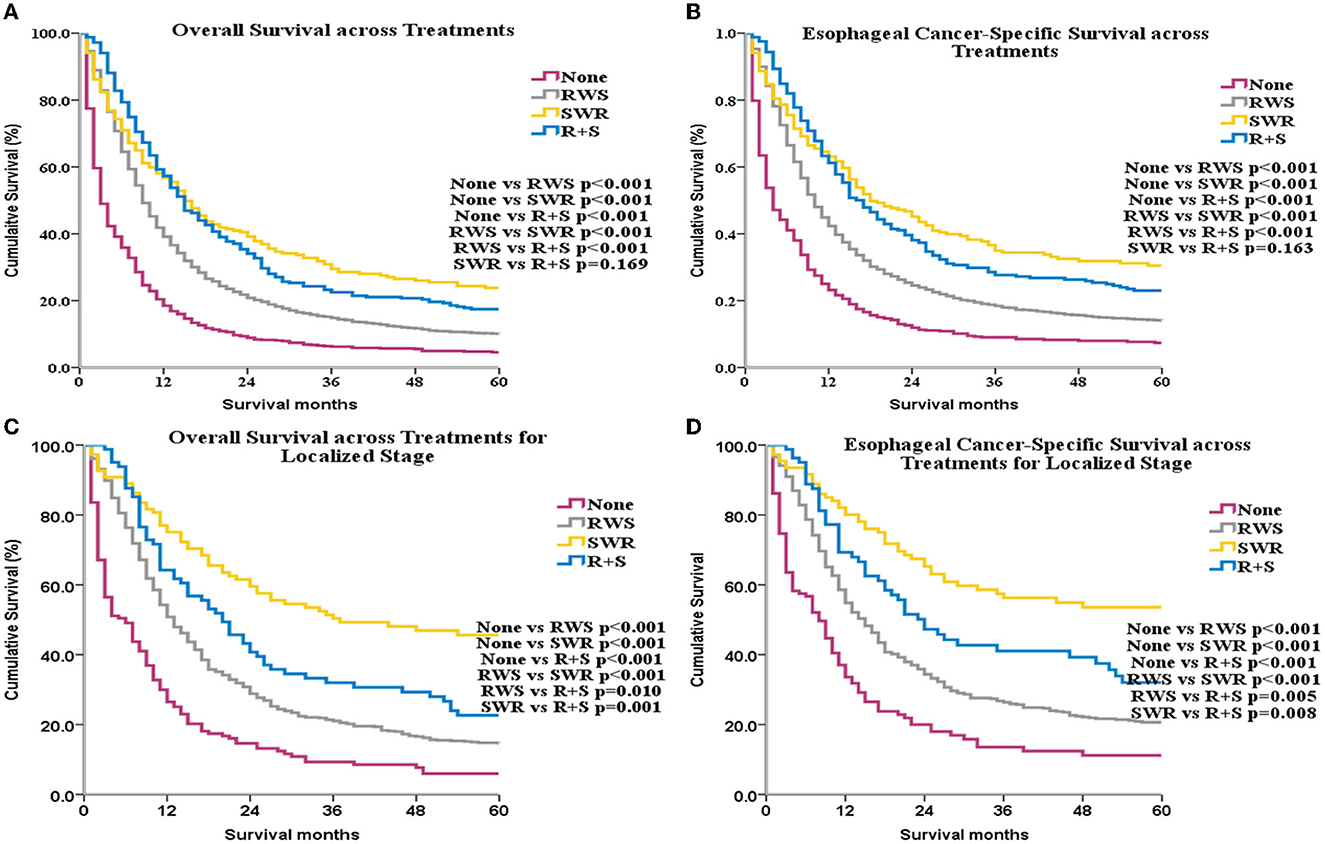
Figure 3. OS and ECSS across different treatments for all patients (A, B) and for patients with localized stage disease (C, D).
Subgroup analyses by SEER historical stage A revealed that, for patients with localized disease, ECSS and OS were greatest in the SWR group (Figures 3C, D). For patients with regional disease, ECSS and OS were highest in the R+S group (Figures 4A, B). Univariate (Supplementary Table 9) and multivariate (Table 1) analyses demonstrated that treatment strategy was independently associated with both ECSS and OS. For patients with distant stage disease, OS and ECSS were higher than None and RWS group (p < 0.05, Figures 4C, D). However, the fluctuation of OS and ECSS curves are relatively large in SWR group for patients with distant stage disease (Figures 4C, D).
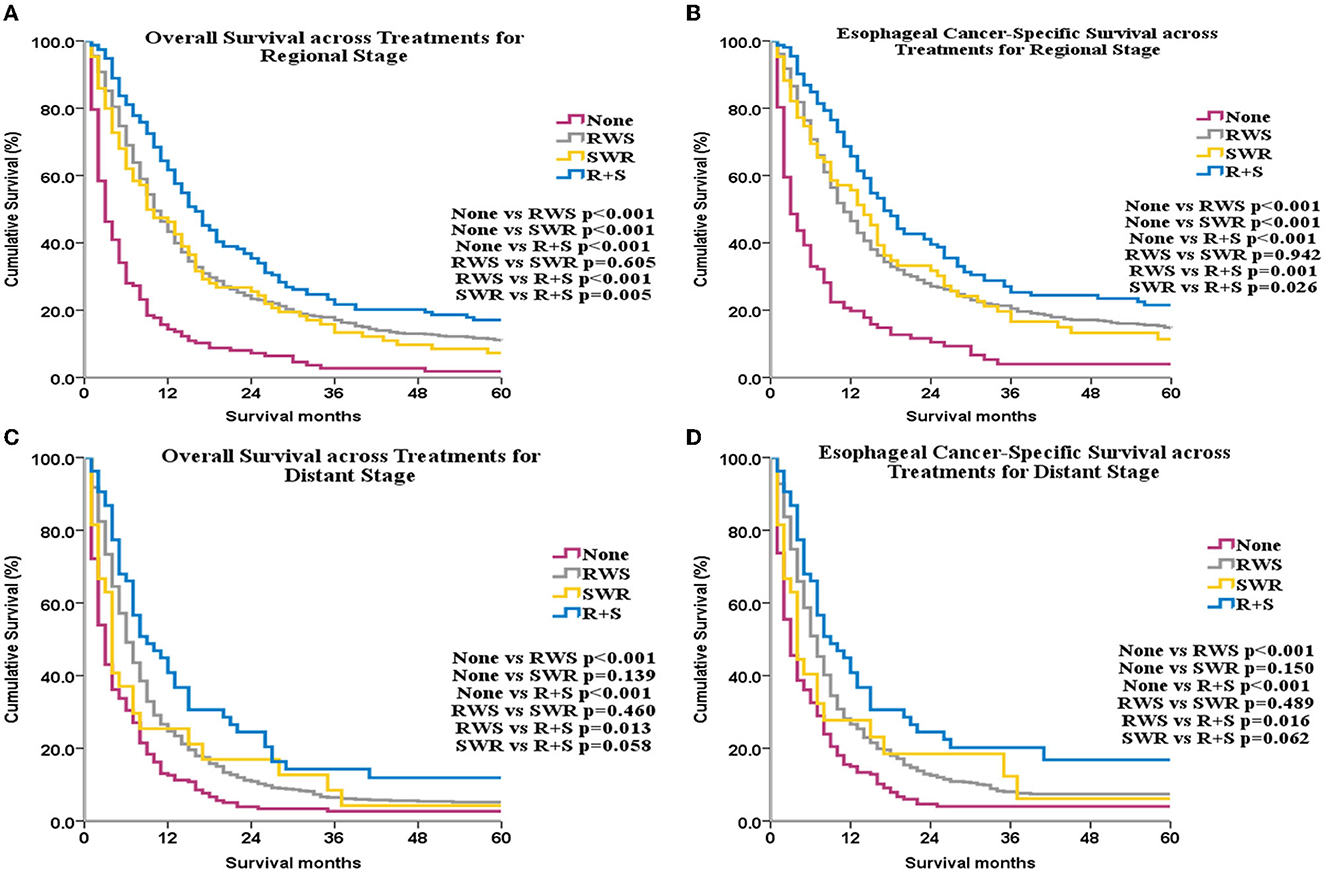
Figure 4. OS and ECSS across different treatments for patients with regional stage disease (A, B) and for patients with distant stage disease (C, D).
No significant difference in ECSS or OS was found among the SWR, NRT, and ART groups (p > 0.05 for all, Figures 5A, B).
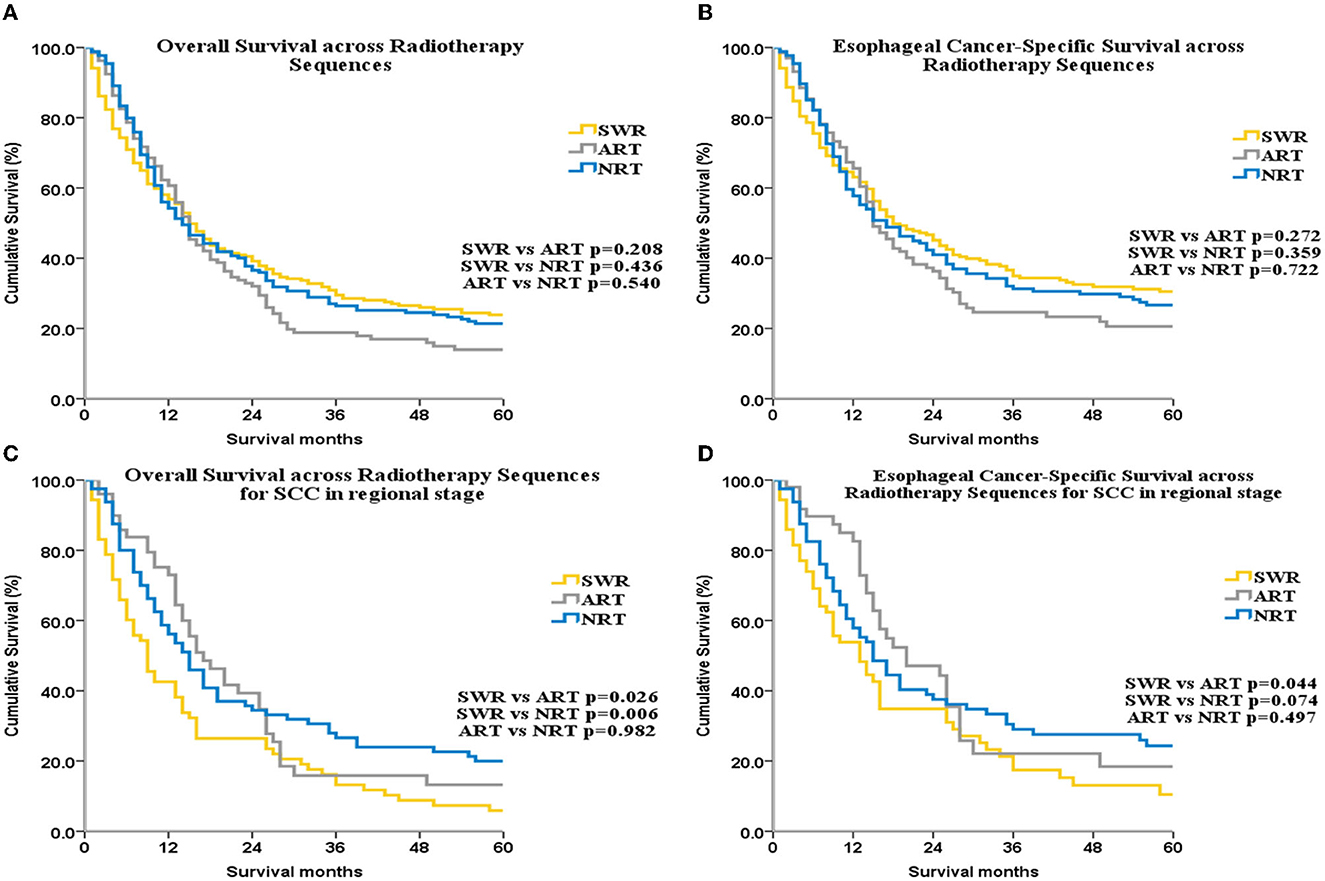
Figure 5. OS and ECSS across radiotherapy sequences for all patients (A, B) and for patients with SCC patients with regional stage disease (C, D).
Sub-group analysis by SEER stage showed that, for patients with localized disease, OS was highest in the SWR group. Among patients with regional disease, OS was lowest in the SWR group compared with the ART and NRT groups. However, multivariate analysis did not reveal a significant difference in OS in localized or regional sub-groups.
Next, we performed sub-group analysis by histologic subtype. For the SCC subgroup, among patients with regional disease, OS was lower with SWR (median OS: 9 months, 95% CI: 6.34–11.67) compared with NRT (median OS: 17 months, 95% CI: 11.79–22.21) and ART (median OS: 15 months, 95% CI: 11.42–18.59; Figure 5C). Multivariate analysis for this subgroup also demonstrated that RT sequence was an independent factor for OS (SWR as reference, HR of NRT: 0.633, 95% CI: 0.427–0.938, p = 0.023; HR of ART: 0.635, 95% CI: 0.453–0.889, p = 0.008, Supplementary Table 10). No other subgroup analysis yielded statistically significant results. ECSS was higher in the ART group compared with SWR group for SCC patients with regional disease (p = 0.044, Figure 5D). There were no significant difference for ECSS between ART group and NRT group (p = 0.479, Figure 5D). Although the trend of ECSS in ART group was better than that in SWR group, it was not statistically significant (p = 0.074, Figure 5D).
Although the long-term survival of patients with UEC remains extremely low, this figure gradually increased in the US during the period from 1973 to 2013. This trend may reflect advancements in medical equipment, surgical technique, and related adjuvant therapy. OS and ECSS were higher among women compared with men, perhaps due to gender-based differences in lifestyle. The use of alcohol and cigarette smoking is more common among men, and both are common causes of esophageal cancer (1).
Recent decades have also seen a dramatic rise in the incidence of esophageal adenocarcinoma (EAC) in Western countries (21, 22). The results of our study indicate a similar rise in the prevalence of UEC. Notably, AC was rarely found in the upper esophagus (23, 24). And the histogenesis of AC in the upper esophagus remains unclear. The pathogenesis of AC in the middle and/or lower esophagus is typically related to gastroesophageal reflux or Barrett's esophagus. However, the pathogenesis of AC in the upper esophagus is different from that of AC in the middle or lower esophagus. Previous studies have revealed that AC in the upper esophagus may arise from esophageal glands or heterotopic gastric mucosa (HGM), the latter of which may be related to infection with helicobacter pylori (24). Our results presented above identified histologic type as an independent prognostic factor in UEC. Survival was higher for AC than for SCC, as reported previously (25). Among patients with regional or distant disease, SCC was more common than AC (Supplementary Table 4). This finding is concordant with the results described above, which found that lymph node metastasis was more commonly associated with SCC than with AC (25).
In most countries and regions including the US, first-line strategies for UEC are CRT and RT (2, 3, 11, 12). Our study revealed that RT was commonly used. For SCC, this proportion reached 70.6%. Previous studies have reported no significant difference in outcomes after combined surgery and RT compared with CRT alone (5). However, other studies have shown that among patients with resectable UEC, long-term outcomes appear to be better with surgery plus CRT compared with definitive CRT alone. Patients who underwent surgery also had improvements in prognosis, quality of life, and post-treatment dysphagia (10, 14). In our study, OS and ECSS were higher among the SWR and R+S groups compared with the group of patients who underwent RT alone. Among patients with localized disease, survival was highest with surgery alone compared with RWS and R+S. Notably, RT may decrease the patient's autoimmunity and increase the risk for postoperative complications (26, 27). Furthermore, the use of RT alone was likely insufficient to entirely eliminate the primary lesion. For patients with regional disease, the highest OS was found in the R+S group. This finding is similar to those published in a previous report (13). Importantly, R+S, in addition to removing the lesion, eliminated any potential unresected lesions.
Surgery may afford reasonable OS and improve the quality of life for UEC patients (28). Surgery may also significantly reduce local tumor recurrence and improve dysphagia (14). The surgical method used most commonly to treat UEC is the McKeown approach (tri-incisional esophagectomy) (7). Surgery is not recommended for tumors < 20 cm from the incisors, because UEC at this site always requires a pharyngo-laryngo-esophagectomy, which is associated with poor quality of life post-operatively because of the loss of vocal function (29). Previous studies also showed that long-term survival was similar in the CRT and surgery groups for patients with lesions that were positioned more superiorly (14, 30). Thus, CRT gradually became the main strategy for these patients. However, the prognosis for these UEC patients who underwent definitive CRT alone was always unsatisfactory because of insufficient local disease control; salvage surgery was always needed for these patients (29). In recent years, studies have sought to elaborate on a larynx-preserving surgery that would preserve vocal function in patients with UEC (29). Furthermore, larynx-preserving surgery compared with non-preserving procedures was associated with improved prognosis and decreased complications (29). However, a comparison of larynx-preserving surgery and CRT alone showed no significant difference in long-term survival (28). Compared with CRT alone, surgery decreases the risk of local recurrence and improves dysphagia symptoms (14). Therefore, larynx-preserving surgery may be an acceptable surgical approach for UEC proximal to the larynx.
Nowadays, the combination of surgery and chemoradiotherapy or immunotherapy is increasingly preferred for the treatment of resectable esophageal cancer (31, 32). Clinical practice has recently seen the increased use of adjuvant and neoadjuvant therapies for the treatment of esophageal cancer. However, the role of adjuvant and neoadjuvant therapies remains controversial. For neoadjuvant radiotherapy (NRT), previous studies reported that NRT compared with surgery alone, may improve 5-year OS and increase the curative resectability of tumors (33). However, other researchers found no increase in resectable rate or OS among patients treated with NRT (34). For neoadjuvant chemotherapy (NCT), some researchers believed that patients were more tolerant to chemotherapy response before the operation. Ando N et al. found that the 5-year OS rate was 55.00% in the NCT group and 43.00% in the postoperative chemotherapy group (P = 0.04) of 330 patients with stage II/III (35). Another study showed that the NCT group had a better R0 removal rate (60.00% vs. 50.00%) and a better 5-year OS (23.00% vs. 17.10%, HR = 0.84; 95% CI: 0.72 ~0.98, P = 0.003) compared with surgery alone (36). The other study also showed that NCT can improve the radical resection rate, PFS, and OS of patients with esophageal adenocarcinoma (37). However, the study results of Kelsen et al. (38) showed that NCT could not improve postoperative OS in patients with locally advanced esophageal cancer, but patients with tumor response in the NCT group had improved OS. This study also found that only R0 resection could bring significant survival benefits. There was no significant difference in the median OS among R1, R2, and unresected patients (38). Patients receiving neoadjuvant chemoradiotherapy (NCRT) had a higher pathological complete response (pCR) rate and greatly improve the tumor resection rate. Compared with surgery alone, patients who received NCRT plus surgery had better OS and PFS. In addition, the R0 rate was also higher than surgery alone (39–41).
In addition, several studies are ongoing or have shown results regarding the role of immunotherapy as adjuvant or neoadjuvant therapy for esophageal cancer (42, 43). Notably, none of these studies studied UEC alone. In our study, NRT was mainly used in patients with advanced disease (Supplementary Table 5, p < 0.001), suggesting that NRT may improve tumor resection rate. However, for patients undergoing surgery, there was no significant effect of NRT or ART on OS or ECSS (p > 0.05 for all, Figures 5A, B). OS for patients with regional disease was higher in the ART and NRT groups compared with the SWR group. This finding was expected because lymph node metastasis is observed in almost all patients with regional disease. NRT and ART may eliminate potentially metastatic lesions and, thus, reduce tumor recurrence.
The radiotherapy sequence was not an independent factor in the NRT and ART subgroups. In a previous analysis of the SEER database, NRT was beneficial to long-term survival and an independent factor for OS (33). However, this previous analysis did not study UEC alone. In our study, which did investigate UEC alone, RT sequence was an independent factor for OS among patients with regional SCC.
Current treatment for UEC typically includes CRT or surgery plus CRT. However, we could not assess the effect of chemotherapy on patent survival, as these data were not available in the SEER database. RT methods, radiation dose, and surgical methodology are also reported to have significant effects on patient prognosis (2, 12, 15). However, the SEER database does not include these data. The database did not register information related to smoking, drinking, or postoperative complications. Finally, we used SEER historical stage A (localized: confined to the primary site; regional: spread to regional lymph nodes; distant: cancer metastasis) in order to comply with unified tumor staging criteria across all years of the study. SEER historical stage A differs from the staging criteria provided by the American Joint Committee on Cancer (4). Any of the factors mentioned above may have resulted in a deviation in the research results.
Although the long-term survival of UEC patients remains poor, it has gradually increased from 1973 to 2013. This should be closely related to the improvement of medical care over the past 40 years. Our results also showed that different treatment methods have a great influence on the long-term survival of UEC patients. For patients with localized disease, surgery may be a better choice. For patients with regional disease, surgery plus adjuvant or neoadjuvant radiotherapy may be more beneficial to improve the long-term prognosis of UEC patients. Additional clinical studies are needed to identify the optimal treatment strategies for UEC.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
MZ conceived, designed, and supervised the study. XW, M-CZ, and G-LL collected, analyzed, visualized the data, and wrote the draft of the manuscript. PX, WS, and NZ provided support for the statistical analysis and results interpretation. MZ, BZ, L-QL, and X-NF revised and edited the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the National Natural Science Foundation of China (Grant No. 81172786), the Health Commission of Hubei Province scientific research project (No. WJ2019M146), and Funding from Tongji Hospital (No. 2016hgryzm).
The authors appreciated the SEER database for providing the original study data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1128766/full#supplementary-material
Supplementary Figure 1. The flow chart of this study.
UEC, upper esophageal cancer; SEER database, Surveillance, Epidemiology, and End Results database; ECSS, esophageal cancer-specific survival; OS, overall survival; SWR, surgery without radiation; R+S, radiation plus surgery; RWS, radiation without surgery; AD, adenocarcinoma; SCC, squamous cell carcinoma; RT, radiotherapy; CRT, chemoradiotherapy; PLE, pharyngo-laryngo-esophagectomy; KM, Kaplan–Meier; NRT, neoadjuvant radiotherapy; ART, adjuvant radiotherapy; HR, hazard ratio; CI, credibility interval.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Tu L, Sun L, Xu Y, Wang Y, Zhou L, Liu Y, et al. Paclitaxel and cisplatin combined with intensity-modulated radiotherapy for upper esophageal carcinoma. Radiat Oncol. (2013) 8:75. doi: 10.1186/1748-717X-8-75
3. Wang S, Liao Z, Chen Y, Chang JY, Jeter M, Guerrero T, et al. Esophageal cancer located at the neck and upper thorax treated with concurrent chemoradiation: a single-institution experience. J Thorac Oncol. (2006) 1:252–9. doi: 10.1016/S1556-0864(15)31576-8
4. Hoeben A, Polak J, Van De Voorde L, Hoebers F, Grabsch HI, de Vos-Geelen J. Cervical esophageal cancer: a gap in cancer knowledge. Ann Oncol. (2016) 27:1664–74. doi: 10.1093/annonc/mdw183
5. Grass GD, Cooper SL, Armeson K, Garrett-Mayer E, Sharma A. Cervical esophageal cancer: a population-based study. Head Neck. (2015) 37:808–14. doi: 10.1002/hed.23678
6. Esmati E, Safaei AM, Ghalehtaki R, Mousavi N, Saraee E, Shirouei S, et al. Outcomes of definitive chemoradiotherapy for cervical and upper thoracic esophageal cancers: a single-institution experience of a rare cancer. J Gastrointest Cancer. (2019) 50:380–5. doi: 10.1007/s12029-018-0081-8
7. Napier KJ, Scheerer M, Misra S. Esophageal cancer: a Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. (2014) 6:112–20. doi: 10.4251/wjgo.v6.i5.112
8. Daiko H, Hayashi R, Saikawa M, Sakuraba M, Yamazaki M, Miyazaki M, et al. Surgical management of carcinoma of the cervical esophagus. J Surg Oncol. (2007) 96:166–72. doi: 10.1002/jso.20795
9. Homma A, Nakamaru Y, Hatakeyama H, Mizumachi T, Kano S, Furusawa J, et al. Early and long-term morbidity after minimally invasive total laryngo-pharyngo-esophagectomy with gastric pull-up reconstruction via thoracoscopy, laparoscopy and cervical incision. Eur Arch Otorhinolaryngol. (2015) 272:3551–6. doi: 10.1007/s00405-014-3420-9
10. Chiu P.W, et al. Multicenter prospective randomized trial comparing standard esophagectomy with chemoradiotherapy for treatment of squamous esophageal cancer: early results from the Chinese University research group for esophageal cancer (CURE). J Gastrointest Surg. (2005) 9:794–802. doi: 10.1016/j.gassur.2005.05.005
11. Tong DKH, Law S, Wan Kwong DL, Wei WI, Ng RWM, Wong KH. Current management of cervical esophageal cancer. World J Surg. (2011) 35:600–7. doi: 10.1007/s00268-010-0876-7
12. Fu Y, Deng M, Zhou X, Lin Q, Du B, Tian X, et al. Dosimetric effect of beam arrangement for intensity-modulated radiation therapy in the treatment of upper thoracic esophageal carcinoma. Med Dosim. (2017) 42:47–52. doi: 10.1016/j.meddos.2016.11.002
13. Chen SB, Yang XH, Weng HR, Liu DT, Li H, Chen YP. Clinicopathological features and surgical treatment of cervical oesophageal cancer. Sci Rep. (2017) 7:3272. doi: 10.1038/s41598-017-03593-0
14. Chou SH, Li HP, Lee JY, Huang MF, Lee CH, Lee KW, et al. Radical resection or chemoradiotherapy for cervical esophageal cancer? World J Surg. (2010) 34:1832–9. doi: 10.1007/s00268-010-0595-0
15. Yerokun BA, Sun Z, Yang C-FJ, Gulack BC, Speicher PJ, Adam MA, et al. Minimally invasive versus open esophagectomy for esophageal cancer: a population-based analysis. Ann Thorac Surg. (2016) 102:416–23. doi: 10.1016/j.athoracsur.2016.02.078
16. Gottlieb-Vedi E, Kauppila JH, Mattsson F, Lindblad M, Nilsson M, Lagergren P, et al. Long-term survival in esophageal cancer after minimally invasive esophagectomy compared to open esophagectomy. Ann Surg. (2022) 276:e744–8. doi: 10.1097/SLA.0000000000004645
17. van der Horst S, Weijs TJ, Ruurda JP, Mohammad NH, Mook S, Brosens LA, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy for esophageal cancer in the upper mediastinum. J Thorac Dis. (2017) 9(Suppl 8):S834–42. doi: 10.21037/jtd.2017.03.151
18. Merboth F, Nebelung H, Wotschel N, Liebscher H, Eckert F, von Renesse J, et al. Robotic esophagectomy compared with open esophagectomy reduces sarcopenia within the first postoperative year: a propensity score-matched analysis. J Thorac Oncol. (2023) 18:232–44. doi: 10.1016/j.jtho.2022.10.018
19. de Vos-Geelen J, Geurts SM, van Putten M, Valkenburg-van Iersel LB, Grabsch HI, Mohammad NH, et al. Trends in treatment and overall survival among patients with proximal esophageal cancer. World J Gastroenterol. (2019) 25:6835–46. doi: 10.3748/wjg.v25.i47.6835
20. Thumallapally N, Meshref A, Mousa M, Hendawi M, Lan M, Salem AI, et al. Survival benefit of neoadjuvant versus adjuvant radiotherapy in lymph node positive esophageal cancer: a population based analysis. J Gastrointest Oncol. (2017) 8:825–32. doi: 10.21037/jgo.2017.06.19
21. Murphy CC, Yang YC, Shaheen NJ, Hofstetter WL, Sandler RS. An age-period-cohort analysis of obesity and incident esophageal adenocarcinoma among white males. Dis Esophagus. (2017) 30:1–8. doi: 10.1111/dote.12526
22. Hur C, Miller M, Kong CY, Dowling EC, Nattinger KJ, Dunn M, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. (2013) 119:1149–58. doi: 10.1002/cncr.27834
23. Lauwers GY, Scott GV, Vauthey JN. Adenocarcinoma of the upper esophagus arising in cervical ectopic gastric mucosa: rare evidence of malignant potential of so-called “inlet patch”. Dig Dis Sci. (1998) 43:901–7. doi: 10.1023/A:1018855223225
24. Chatelain D, de Lajarte-Thirouard AS, Tiret E, Flejou JF. Adenocarcinoma of the upper esophagus arising in heterotopic gastric mucosa: common pathogenesis with Barrett's adenocarcinoma? Virchows Arch. (2002). 441:406–11. doi: 10.1007/s00428-002-0697-7
25. Siewert JR, Stein HJ, Feith M, Bruecher BL, Bartels H, Fink U. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the Western world. Ann Surg. (2001) 234:360–7. doi: 10.1097/00000658-200109000-00010
26. Heidecke C-D, Weighardt H, Feith M, Fink U, Zimmermann F, Stein HJ, et al. Neoadjuvant treatment of esophageal cancer: immunosuppression following combined radiochemotherapy. Surgery. (2002) 132:495–501. doi: 10.1067/msy.2002.127166
27. Kumagai K, Rouvelas I, Tsai JA, Mariosa D, Klevebro F, Lindblad M, et al. Meta-analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro-oesophageal junctional cancers. Br J Surg. (2014) 101:321–38. doi: 10.1002/bjs.9418
28. Wang HW, Kuo KT, Wu YC, Huang BS, Hsu WH, Huang MH, et al. Surgical results of upper thoracic esophageal carcinoma. J Chin Med Assoc. (2004) 67:447–57.
29. Makino T, Yamasaki M, Miyazaki Y, Takahashi T, Kurokawa Y, Nakajima K, et al. Short- and long-term outcomes of larynx-preserving surgery for cervical esophageal cancer: analysis of 100 consecutive cases. Ann Surg Oncol. (2016) 23(Suppl 5):858–65. doi: 10.1245/s10434-016-5511-x
30. Takebayashi K, Tsubosa Y, Matsuda S, Kawamorita K, Niihara M, Tsushima T, et al. Comparison of curative surgery and definitive chemoradiotherapy as initial treatment for patients with cervical esophageal cancer. Dis Esophagus. (2017) 30:1–5. doi: 10.1111/dote.12502
31. Lewis S, Lukovic J. Neoadjuvant therapy in esophageal cancer. Thorac Surg Clin. (2022) 32:447–56. doi: 10.1016/j.thorsurg.2022.06.003
32. Lee Y, Samarasinghe Y, Lee MH, Thiru L, Shargall Y, Finley C, et al. Role of adjuvant therapy in esophageal cancer patients after neoadjuvant therapy and esophagectomy: a systematic review and meta-analysis. Ann Surg. (2022) 275:91–8. doi: 10.1097/SLA.0000000000005227
33. Schwer AL, Ballonoff A, McCammon R, Rusthoven K, Jr RBD, Schefter TE. Survival effect of neoadjuvant radiotherapy before esophagectomy for patients with esophageal cancer: a surveillance, epidemiology, and end-results study. Int J Radiat Oncol Biol Phys. (2009) 73:449–55. doi: 10.1016/j.ijrobp.2008.04.022
34. Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: a SEER database analysis. J Gastroenterol Hepatol. (2016) 31:1141–6. doi: 10.1111/jgh.13289
35. Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. (2012) 19:68–74. doi: 10.1245/s10434-011-2049-9
36. Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. (2009) 27:5062–7. doi: 10.1200/JCO.2009.22.2083
37. Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. (2011) 29:1715–21. doi: 10.1200/JCO.2010.33.0597
38. Kelsen DP, Winter KA, Gunderson LL, Mortimer J, Estes NC, Haller DG, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. (2007) 25:3719–25. doi: 10.1200/JCO.2006.10.4760
39. van Hagen P, Hulshof MCCM, van Lanschot JJB, Steyerberg EW, Henegouwen MIv, Wijnhoven BPL, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. (2012) 366:2074–84. doi: 10.1056/NEJMoa1112088
40. Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. (2008) 26:1086–92. doi: 10.1200/JCO.2007.12.9593
41. Mariette C, Dahan L, Mornex F, Maillard E, Thomas PA, Meunier B, et al. Surgery alone vs. chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. (2014) 32:2416–22. doi: 10.1200/JCO.2013.53.6532
42. Wang Z, Shao C, Wang Y, Duan H, Pan M, Zhao J, et al. A commentary on efficacy and safety of neoadjuvant immunotherapy in surgically resectable esophageal cancer: a systematic review and meta-analysis. Int J Surg. (2022) 106:106929. doi: 10.1016/j.ijsu.2022.106929
43. Mamdani H, Schneider B, Perkins SM, Burney HN, Kasi PM, Abushahin LI, et al. A Phase II trial of adjuvant durvalumab following trimodality therapy for locally advanced esophageal and gastroesophageal junction adenocarcinoma: a big ten cancer research consortium study. Front Oncol. (2021) 11:736620. doi: 10.3389/fonc.2021.736620
Keywords: upper esophageal cancer, long-term survival, surgery, radiotherapy, the surveillance, epidemiology, end results database
Citation: Wu X, Zhu M-C, Li G-L, Xiong P, Sun W, Zhang N, Zhao B, Li L-Q, Fu X-N and Zhu M (2023) Treatment and survival analysis for 40-year SEER data on upper esophageal cancer. Front. Med. 10:1128766. doi: 10.3389/fmed.2023.1128766
Received: 21 December 2022; Accepted: 21 April 2023;
Published: 17 July 2023.
Edited by:
Jacopo Troisi, University of Salerno, ItalyReviewed by:
Haibo Sun, The Affiliated Cancer Hospital of Zhengzhou University, ChinaCopyright © 2023 Wu, Zhu, Li, Xiong, Sun, Zhang, Zhao, Li, Fu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Zhu, bXpodUB0amgudGptdS5lZHUuY24=; bWluemh1dGpAYWxpeXVuLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.