
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Med., 02 March 2023
Sec. Gastroenterology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1123576
This article is part of the Research TopicIrritable Bowel Syndrome: What is Known and What is Missing in Daily PracticeView all 9 articles
Several dietary adaptions have been suggested to successfully reduce irritable bowel syndrome (IBS) symptoms; prebiotics, probiotics, gluten-free diet and low FODMAP (Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols) diet (1–3). The low FODMAP diet has evolved as the most promising diet therapy (3). Against a theoretical background, the low FODMAP diet is accepted as a treatment strategy in healthcare (4) and efforts are made to implement foods low in FODMAPs on the market (5). However, current evidence for the low FODMAP diet is weak (3): Most reported studies are small and lack double or even single blinding and a majority of the studies have focused on FODMAP eliminations rather than provocations. Trials eliminating FODMAPs from the diet have consistently been shown to reduce IBS symptoms (3). However, removing foods from the diet poses a risk to confound the effect of the intervention with that of placebo, since the blinding is lost. The low FODMAP diet is well known among IBS patients, hence there is a high risk that prior knowledge will shape the clinical responses to a sizable extent, potentially even greater than the actual intervention (6). Another well-known situation is the Hawthorne effect, i.e., change of behavior in response to being observed, which may affect the outcome of the study (6).
Recently our research group performed a double-blind, placebo-controlled, randomized 3-way crossover study with a large number of subjects with IBS (7). After introduction of a diet low in FODMAPs excluding gluten, participants were exposed to week-long provocations with high doses of either FODMAPs and gluten or placebo. The exposure dose was 1.5 times the daily intake for an average person. Despite provocation with such high doses, IBS symptoms were only modestly elevated after the FODMAP intervention, and no effect of the gluten intervention was measured by the IBS severity scoring system (IBS-SSS).
An interesting observation from this trial was that the symptom severity was much worse at the screening visit [mean (SD); 308 ± 50] than after any of the week-long provocations with FODMAPs, gluten or placebo (240 ± 91, 208 ± 91, 198 ± 91) (Figure 1). Our study is not the first to observe this phenomenon. Hustoft et al. (8) found similar results after introducing a low FODMAP diet and thereafter provoking with one specific FODMAP constituent, i.e., fructo-oligosaccharides (FOS). By comparing baseline (freeliving conditions) to a low FODMAP diet during the 3 week run-in period, the authors concluded that a low FODMAP diet was effective since IBS symptoms were drastically reduced. The limited increase in IBS symptoms after the FOS provocation was hypothesized to relate to provoking with one FODMAP component only. However, also in our trial, the effect of FODMAP provocation was minor, even though the FODMAP composition reflected that of the general population but at a higher dose. It seems that symptoms from everyday life, including food habits, by far outweigh symptoms exclusively related to the provocations. Therefore, FODMAPs seem to have a minor effect on IBS symptoms.
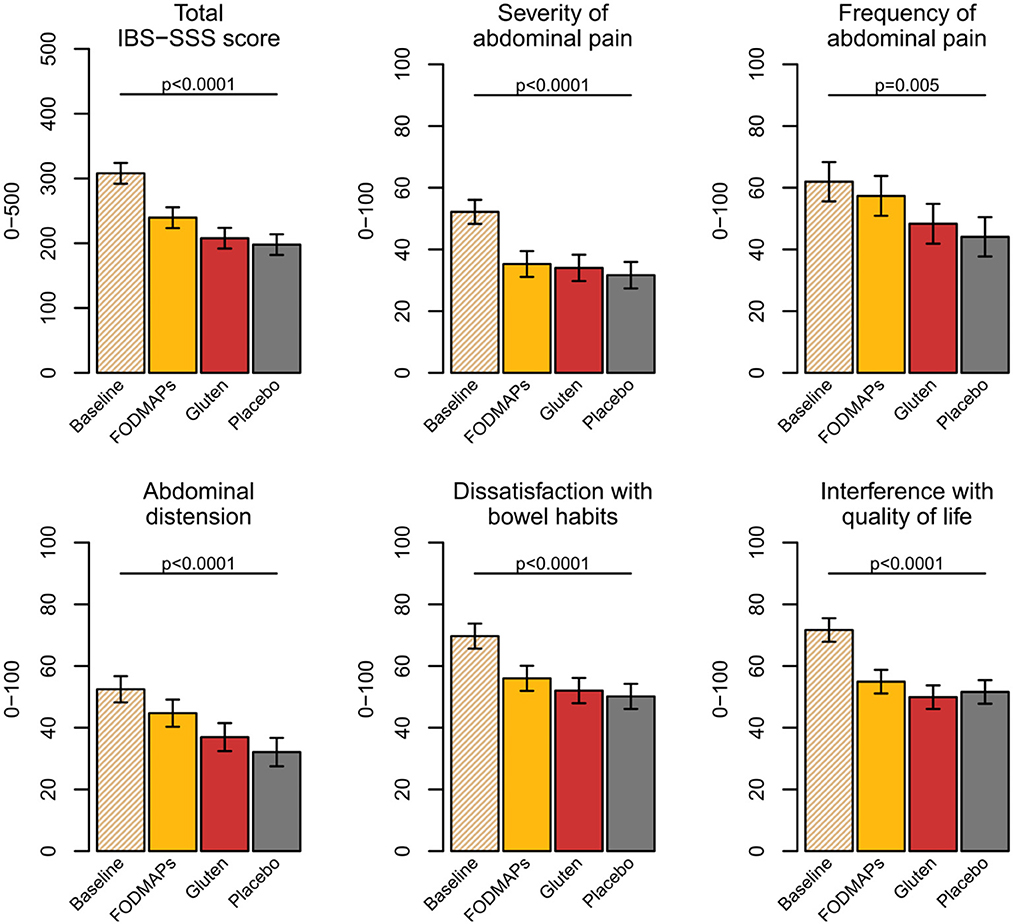
Figure 1. IBS-SSS scores at baseline and after the FODMAPs, gluten, and placebo interventions. Data presented as estimated marginal means, confidence interval (%) and p-value from type 3 test. FODMAPs, fermentable oligo-, di-, monosaccharides, and polyols; IBS-SSS, Irritable bowel syndrome Severity Scoring System. This figure from doctoral thesis by Elise Nordin Chalmers, University Technology 2023.
A systematic review and metanalysis concluded that a low FODMAP diet reduces IBS symptoms by 45 points on the IBS-SSS scale (9). In our trial, the FODMAP diet challenge increased the IBS-SSS to a similar extent (42 points), suggesting it to be a reasonable estimate. All studies from the systematic review concluded that a low FODMAP diet alleviates gastrointestinal symptoms, with no major discussion about the effect size despite the consensus recommendation that 50 IBS-SSS points are required for a clinical improvement (10). Thus, neither our study, nor the meta-analysis support that FODMAPs have an effect > 50 IBS-SSS units.
Increased awareness is needed considering that the low FODMAP diet has been remarkably effective in comparison to baseline (8, 11, 12). However, in fact, comparison to baseline is strongly discouraged (13). On the other hand, there is only a small or no difference in the effectiveness in reducing IBS symptoms following the low FODMAP diet, general dietary advice, traditional dietary advice, or a gluten-free diet (12, 14–16), hence, diets with a large difference in FODMAP content. In line with these findings, FODMAP provocations have been performed with a large range of doses, from 1.7 to 19 g per day (8, 17, 18) in IBS patients, and 5–20 g FOS per day (19, 20) in healthy, with only mild increases of gastrointestinal symptoms, mainly flatulence, bloating and abdominal pain along with a lack of apparent dose-response. The few available double-blind studies that have included both healthy and people with IBS (21–23), have suggested that FODMAPs cause more severe symptoms in people with IBS, although with some inconsistencies in results: Abdominal pain was higher in IBS subjects compared to healthy, although it did not differ between fructan and control exposure (22). Moreover, those studies were small and employed high doses of fructan. Effect sizes at doses commonly consumed need to be further evaluated. Before drawing conclusions, both people with IBS and healthy need to be studied within the same trial with an adequate control (i.e., not baseline), including evaluation of dose-response.
In light of our findings and the reported, but largely neglected, findings from previous studies (8, 11, 12, 14, 15, 24), we do question the practice of conducting clinical trials to evaluate effects of dietary components such as FODMAPs or gluten in IBS if we cannot assure adequate study conditions. IBS symptoms are clearly related to other factors beyond diet, such as psychological factors (4). Given such factors together with the high placebo response in IBS (25, 26), the importance of a randomized, double-blind, controlled study design has long since been raised (25, 26). In addition, dietary confounding is a major challenge when supplementing foods (6). Therefore, as a basic requirement a double-blind design should substitute the same food(s) in each intervention, effectively ensuring that outcome differences should be purely related to the interventions.
It is known that IBS is a complex condition, and general guidelines recommend that IBS should be treated from a holistic perspective, integrating medical treatment, lifestyle and dietary adaptations and behavioral therapy (4). Several trials have in fact shown that other interventions can be effective in reducing IBS symptoms, for example acupuncture, cognitive behavioral therapy, hypnotherapy, meditation, and yoga (4), although strong evidence for their efficacy is lacking due to methodological concerns such as lack of blinding (4). Recently, an interesting study (27) concluded that the low FODMAP diet is effective, but at the same time exposure-based cognitive therapy, i.e., the consumption of FODMAPs to target the fear of inducing IBS symptoms is also considered effective (27). A suggested explanation for this was that these two treatments should attract different type of subgroups of people with IBS. However, randomized control trials have shown that both hypnotherapy and yoga were equally effective as a low FODMAP diet (28, 29), indicating that the effectiveness of these different treatment regimen is not related to specific subtypes of IBS.
To conclude, even though elimination of FODMAPs could be part of a holistic IBS treatment, it should be noted that they are complex dietary fibers which are part of a healthy diet and in line with official dietary guidelines (30, 31). This calls for justification based on stronger objective evidence than the theories of saccharide fermentation in IBS that are presently at hand. To gain robust evidence, large double-blind dietary studies with an adequate comparator group are needed. Furthermore, effect size in response to interventions needs to be further discussed and dose-response studies in subjects with and without IBS are highly warranted.
EN drafted the text. CB, RL, and PH revised the text critically. All authors contributed to the article and approved the submitted version.
This study was funded by Formas (Grant No. 2016-00314) and Swedish Research Council (Grant No. 2017-05840).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wilson B, Rossi M, Dimidi E, Whelan K. Prebiotics in irritable bowel syndrome and other functional bowel disorders in adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. (2019) 109:1098–111. doi: 10.1093/ajcn/nqy376
2. Dale HF, Rasmussen SH, Asiller ÖÖ, Lied GA. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients. (2019) 11:2048. doi: 10.3390/nu11092048
3. Dionne J, Ford AC, Yuan Y, Chey WD, Lacy BE, Saito YA, et al. A systematic review and meta-analysis evaluating the efficacy of a gluten-free diet and a low FODMAPS diet in treating symptoms of irritable bowel syndrome. Am J Gastroenterol. (2018) 113:1290–300. doi: 10.1038/s41395-018-0195-4
4. Chey WD, Keefer L, Whelan K, Gibson PR. Behavioral and diet therapies in integrated care for patients with irritable bowel syndrome. Gastroenterology. (2021) 160:47–62. doi: 10.1053/j.gastro.2020.06.099
5. Ispiryan L, Zannini E, Arendt EK. FODMAP modulation as a dietary therapy for IBS: scientific and market perspective. Compr Rev Food Sci Food Saf. (2022) 21:1491–516. doi: 10.1111/1541-4337.12903
6. Staudacher HM, Yao CK, Chey WD, Whelan K. Optimal design of clinical trials of dietary interventions in disorders of gut-brain interaction - PubMed. Am J Gastroenterol. (2022) 117:973–84. doi: 10.14309/ajg.0000000000001732
7. Nordin E, Brunius C, Landberg R, Hellström PM. Fermentable oligo-, di-, monosaccharides, and polyols (FODMAPs), but not gluten, elicit modest symptoms of irritable bowel syndrome: a double-blind, placebo-controlled, randomized three-way crossover trial. Am J Clin Nutr. (2022) 115:344–52. doi: 10.1093/ajcn/nqab337
8. Hustoft TN, Hausken T, Ystad SO, Valeur J, Brokstad K, Hatlebakk JG, et al. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol Motility. (2017) 29:e12969. doi: 10.1111/nmo.12969
9. van Lanen AS, de Bree A, Greyling A. Efficacy of a low-FODMAP diet in adult irritable bowel syndrome: a systematic review and meta-analysis. Eur J Nutr. (2021) 60:3505–22. doi: 10.1007/s00394-020-02473-0
10. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. (1997) 11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x
11. McIntosh K, Reed DE, Schneider T, Dang F, Keshteli AH, de Palma G, et al. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut. (2017) 66:1241–51. doi: 10.1136/gutjnl-2015-311339
12. Zahedi MJ, Behrouz V, Azimi M. Low fermentable oligo-di-mono-saccharides and polyols diet vs. general dietary advice in patients with diarrhea-predominant irritable bowel syndrome: a randomized controlled trial. J Gastroenterol Hepatol. (2018) 33:1192–9. doi: 10.1111/jgh.14051
13. Bland JM, Altman DG. Comparisons against baseline within randomised groups are often used and can be highly misleading. Trials. (2011) 12:1–7. doi: 10.1186/1745-6215-12-264
14. Paduano D, Cingolani A, Tanda E, Usai P. Effect of three diets (low-FODMAP, gluten-free and balanced) on irritable bowel syndrome symptoms and health-related quality of life. Nutrients. (2019) 11:566. doi: 10.3390/nu11071566
15. Rej A, Sanders DS, Shaw CC, Buckle R, Trott N, Agrawal A, et al. Efficacy and acceptability of dietary therapies in non-constipated irritable bowel syndrome: a randomized trial of traditional dietary advice, the low FODMAP diet, and the gluten-free diet. Clin Gastroenterol Hepatol. (2022) 20:2876–87. doi: 10.1016/j.cgh.2022.02.045
16. Böhn L, Störsrud S, Liljebo T, Collin L, Lindfors P, Törnblom H, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. (2015) 149:1399–407.e2. doi: 10.1053/j.gastro.2015.07.054
17. Laatikainen R, Koskenpato J, Hongisto SM, Loponen J, Poussa T, Hillilä M, et al. Randomised clinical trial: low-FODMAP rye bread vs. regular rye bread to relieve the symptoms of irritable bowel syndrome. Aliment Pharmacol Ther. (2016) 44:460–70. doi: 10.1111/apt.13726
18. Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. (2008) 6:765–71. doi: 10.1016/j.cgh.2008.02.058
19. Bonnema AL, Kolberg LW, Thomas W, Slavin JL. Gastrointestinal tolerance of chicory inulin products. J Am Diet Assoc. (2010) 110:865–8. doi: 10.1016/j.jada.2010.03.025
20. Bruhwyler J, Carreer F, Demanet E, Jacobs H. Digestive tolerance of inulin-type fructans: a double-blind, placebo-controlled, cross-over, dose-ranging, randomized study in healthy volunteers. Int J Food Sci Nutr. (2009) 60:165–75. doi: 10.1080/09637480701625697
21. Major G, Pritchard S, Murray K, Alappadan JP, Hoad CL, Marciani L, et al. Colon hypersensitivity to distension, rather than excessive gas production, produces carbohydrate-related symptoms in individuals with irritable bowel syndrome. Gastroenterology. (2017) 152:124–33.e2. doi: 10.1053/j.gastro.2016.09.062
22. Masuy I, van Oudenhove L, Tack J, Biesiekierski JR. Effect of intragastric FODMAP infusion on upper gastrointestinal motility, gastrointestinal, and psychological symptoms in irritable bowel syndrome vs. healthy controls. Neurogastroenterol Motil. (2018) 30:13167. doi: 10.1111/nmo.13167
23. Wu J, Masuy I, Biesiekierski JR, Fitzke HE, Parikh C, Schofield L, et al. Gut-brain axis dysfunction underlies FODMAP-induced symptom generation in irritable bowel syndrome. Aliment Pharmacol Ther. (2022) 55:670–82. doi: 10.1111/apt.16812
24. Böhn L, Störsrud S, Törnblom H, Bengtsson U, Simrén M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. (2013) 108:634–41. doi: 10.1038/ajg.2013.105
25. Elsenbruch S, Enck P. Placebo effects and their determinants in gastrointestinal disorders. Nat Rev Gastroenterol Hepatol. (2015) 12:472–85. doi: 10.1038/nrgastro.2015.117
26. Ford AC, Moayyedi P. Meta-analysis: factors affecting placebo response rate in the irritable bowel syndrome. Aliment Pharmacol Ther. (2010) 32:144–58. doi: 10.1111/j.1365-2036.2010.04328.x
27. Biesiekierski JR, Manning LP, Murray HB, Vlaeyen JWS, Ljótsson B, van Oudenhove L. Review article: exclude or expose? The paradox of conceptually opposite treatments for irritable bowel syndrome. Aliment Pharmacol Ther. (2022) 56:592–605. doi: 10.1111/apt.17111
28. Peters SL, Yao CK, Philpott H, Yelland GW, Muir JG, Gibson PR. Randomised clinical trial: the efficacy of gut-directed hypnotherapy is similar to that of the low FODMAP diet for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. (2016) 44:447–59. doi: 10.1111/apt.13706
29. Schumann D, Langhorst J, Dobos G, Cramer H. Randomised clinical trial: yoga vs. a low-FODMAP diet in patients with irritable bowel syndrome. Aliment Pharmacol Ther. (2018) 47:203–11. doi: 10.1111/apt.14400
30. Anderson JW, Baird P, Davis RH, Ferreri S, Knudtson M, Koraym A, et al. Health benefits of dietary fiber. Nutr Rev. (2009) 67:188–205. doi: 10.1111/j.1753-4887.2009.00189.x
Keywords: irritable bowel syndrome, FODMAPs, double-blind, control, baseline
Citation: Nordin E, Brunius C, Landberg R and Hellström PM (2023) FODMAPs—Do they really affect IBS symptoms? Front. Med. 10:1123576. doi: 10.3389/fmed.2023.1123576
Received: 14 December 2022; Accepted: 14 February 2023;
Published: 02 March 2023.
Edited by:
David Sanders, The University of Sheffield, United KingdomReviewed by:
Azita Hekmatdoost, National Nutrition and Food Technology Research Institute, IranCopyright © 2023 Nordin, Brunius, Landberg and Hellström. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elise Nordin, ZWxpc2Uubm9yZGluQGNoYWxtZXJzLnNl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.